Abstract
Study Objectives:
The aim of the study is to verify a possible association between arterial blood pressure and obstructive sleep apnea (OSA) severity in a group of non-hypertensive patients.
Methods:
This is a retrospective study of 1,171 consecutive patients referred to the sleep laboratory with complaints suggestive of OSA who underwent standard diagnostic polysomnography. In total, 454 patients with no history of arterial hypertension nor had received any such treatment were selected from this group.
Results:
Patients with severe OSA (apnea-hypopnea index [AHI] ≥ 30 events/h) presented with higher diastolic blood pressure (DBP) in the morning than healthy subjects (AHI < 5 events/h) or those suffering from mild (15 < AHI ≥ 5 events/h) or moderate OSA (30 < AHI ≥ 15 events/h): 86.2 ± 11.3 versus 79.2 ± 8.5, 80.3 ± 10.2 and 81.4 ± 9.6 mmHg, P < .01, respectively. In a linear regression model, a rise in morning DBP was predicted by AHI (ß = 0.14, P < .001) and body mass index (BMI) (ß = 0.22, P < .01), but not by age (ß = 0.01, P = .92), male sex (ß = −0.06, P = .19), or smoking (ß = 0.01, P = .86). In contrast, no association existed between morning systolic blood pressure (SBP) and AHI independently of BMI, sex, age, or smoking. High blood pressure (ie, SBP ≥ 140 mmHg or DBP ≥ 90 mmHg on each of three measurements on different occasions) was predicted by age of 42 years or older, BMI of at least 29 kg/m2, and severe OSA.
Conclusions:
High AHI, independent of obesity, age and sex, was associated with elevated DBP in the morning. Thus, elevated morning DBP may be one of the symptoms related to OSA that warrants specific diagnostics.
Commentary:
A commentary on this article appears in this issue on page 861.
Citation:
Mokros Ł, Kuczyński W, Franczak Ł, Białasiewicz P. Morning diastolic blood pressure may be independently associated with severity of obstructive sleep apnea in non-hypertensive patients: a cross-sectional study. J Clin Sleep Med. 2017;13(7):905–910.
Keywords: apnea-hypopnea index, body mass index, morning blood pressure
INTRODUCTION
Obstructive sleep apnea (OSA) is characterized by recurrent episodes of asphyxia (apnea) or shallow breathing (hypopnea) during sleep, leading to intermittent hypoxemia and arousals. These episodes are responsible for the disruption of sleep structure and related symptoms (ie, excessive daytime sleepiness and unrefreshing sleep). The two most deleterious consequences of OSA are increased risk for traffic accidents because of excessive daytime sleepiness, and increased cardiovascular risk, partly due to arterial hypertension (HT).1–3 It is estimated that OSA affects from 2% to 4% of the middle-aged population,4 although new data suggest mild to severe sleep-disordered breathing may affect up to 50% of men and 23% of women.5
Both OSA and HT are comorbidities of obesity and are related to metabolic syndrome.6,7 In some patients, HT may be solely secondary to OSA. It is presumed that the arterial blood pressure (BP) is elevated by intermittent hypoxemia during the night, which activates the sympathetic system via peripheral and central chemoreceptors. This mechanism may underlie the resistance of HT to treatment.8
BRIEF SUMMARY
Current Knowledge/Study Rationale: Obesity and other interrelated comorbidities of obstructive sleep apnea (OSA) makes it difficult to reveal associations between clinical variables. Although OSA is known to influence blood pressure, this effect is rarely investigated independently of obesity, especially in non-hypertensive patients.
Study Impact: The effect on morning diastolic blood pressure of a 1 kg/m2 increment of body mass index seems to be similar to the effect of a 1 event/h increment of apnea-hypopnea index. Therefore, we suggest that elevated morning diastolic blood pressure may be one of the symptoms related to OSA that warrants specific diagnostics.
During sleep, a trend toward lower BP, known as a “dipping” phenomenon, is observed. This dipping is characteristically attenuated in patients with OSA (nondippers) and may inversely correlate with the severity of disease.9–11 The sequelae of obesity are difficult to separate from the effects of OSA on blood pressure, given the association between the two.12 Male sex, obesity, and age are known risk factors for both cardiovascular diseases and OSA. The nondipping phenomenon may indicate that OSA affects BP directly. Lee and Jeong found that OSA was associated with increased diastolic blood pressure (DBP) but not systolic blood pressure (SBP), and this was only true in men.13
Therefore, the current study examines whether morning BP is related to the severity of OSA, independently of sex, obesity, and age, in a group of patients who underwent polysomnography (PSG) due to presumptive diagnosis of OSA.
METHODS
Study Design and Subjects
The study group consisted of 1,171 consecutive patients of the Sleep and Respiratory Disorders Centre (880 men, 75.2%) who underwent PSG from the beginning of 2009 to the end of 2011. They were referred for suspected OSA, due to typical not mutually exclusive complaints: snoring (78.6%), witnessed apneas (72.3%), excessive daytime sleepiness (32.7%), or unrefreshing sleep (83.3%). All patients gave their written informed consent prior to PSG. This study was conducted in accordance with the Declaration of Helsinki and the study protocol was approved by the Ethics Committee of Medical University of Lodz (RNN/23/15/KE).
Patients who had been treated for or had a history of HT (n = 660), and those who slept fewer than 3 hours during PSG (n = 27) were excluded. A group of 454 subjects were therefore included in the analysis of association between BP and selected OSA variables.
Study Procedures
Patients were admitted to the sleep laboratory at 9:00 PM (± 0.5 hour) and underwent physical examination (measurement of body mass, height, heart rate, and BP). A standard nocturnal PSG was performed. The following channels were recorded: electroencephalography (C4-A1, C3-A2), chin muscles and anterior tibialis electromyography, electrooculography, measurements of oronasal air flow (a thermistor gauge), snoring, body position, respiratory movements of chest and abdomen (piezoelectric gauges), unipolar electrocardiogram, and hemoglobin oxygen saturation (SaO2) (Sleep Lab, Jaeger-Viasys, Hoechberg, Germany). Sleep stages were scored according to the criteria based on the 30-second epoch standard.14,15 Apnea was attained with the reduction of airflow to less than 10% of the baseline for at least 10 seconds. Hypopnea was defined as at least 30% reduction of airflow for at least 10 seconds, accompanied by 4% or greater decrease in SaO2 or an arousal. Electroencephalography arousals were scored according to American Academy of Sleep Medicine guidelines.16
Measurement of Blood Pressure
BP was measured on three occasions: at the first ambulatory visit (by a consulting physician), in the evening before polysomnography, and in the morning afterward. The morning measurement was performed according to the operating procedure of our sleep clinic by a physician on duty (ie, within 15 minutes of awakening). It was performed before detachment of the polysomnographic sensors and electrodes and thus, before ambulation. All BP measurements were performed once on the randomly chosen arm of a sitting patient with sphygmomanometer (Riester, Jungingen, Germany).
Arbitrary Cutoffs
Standard cutoffs of OSA severity based on apnea-hypopnea index (AHI) were applied: no disease (< 5 events/h, no-OSA); mild (≥ 5 and < 15 events/h, mi-OSA); moderate (≥ 15 and < 30 events/h, mo-OSA), and severe (≥ 30 events/h, se-OSA).
For the purpose of analysis, “high BP” was defined as SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg on all three measurements: at the first ambulatory visit, on the night before PSG, and the morning afterward. “At least 15 pack years” was adopted as an arbitrary cutoff point for smoking.
Statistics
Different methods for hypotheses verification were used depending on the type of variables. These included analysis of variance with post hoc Tukey test, Kruskal-Wallis test with post hoc multiple comparisons test, multivariate linear regression, and multivariate logistic regression.17 Linear regression models were adjusted to assess the influence of variables of interest on BP. An analysis of residuals was performed for each model to assess the validity of assumptions of normality, homoscedasticity, and independence between observations (with the Durbin-Watson test). The tolerance indices were analyzed to check for possible multicollinearity.18 The linear regression results were reported as standardized ß parameter values with 95% confidence intervals (95% CI) to present the size of effects.19 A logistic regression model was used for the high BP prediction analysis, and its results were shown as odds ratios (OR) with 95% CI.17 The best cutoff values for age and body mass index (BMI) were chosen based on receiver operating characteristic curves and the largest sum of sensitivity and specificity.20 Results were considered statistically significant when P < .05. STATISTICA 12 PL with Medical Pack (Stat-soft, Tulsa, Oklahoma, United States) was used for all analyses.
RESULTS
Differences Between OSA Severity Groups
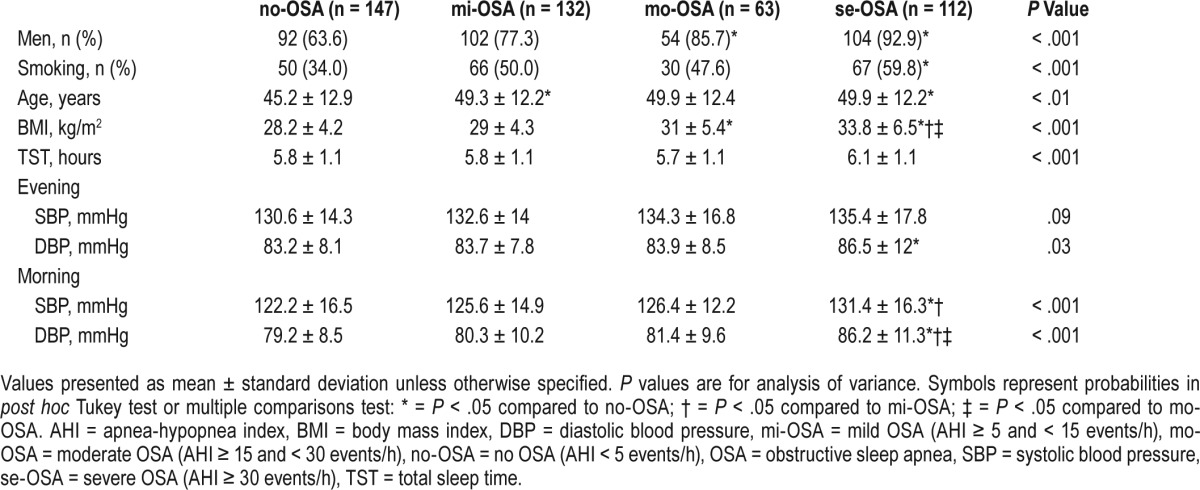
A higher proportion of men were found in the mo-OSA group (85.7%) or se-OSA group (92.9%) than those in the no-OSA group (63.6%, P < .05). A higher proportion of those in the se-OSA group than the no-OSA group were smokers, 59.8% versus 34.0%, respectively (P < .01).
The differences between the OSA severity groups with regard to sex, age, BMI, and BP are summarized in Table 1.
Table 1.
Comparison of selected variables between study groups based on OSA severity.
Multivariate Linear Regression Models for Morning BP Prediction
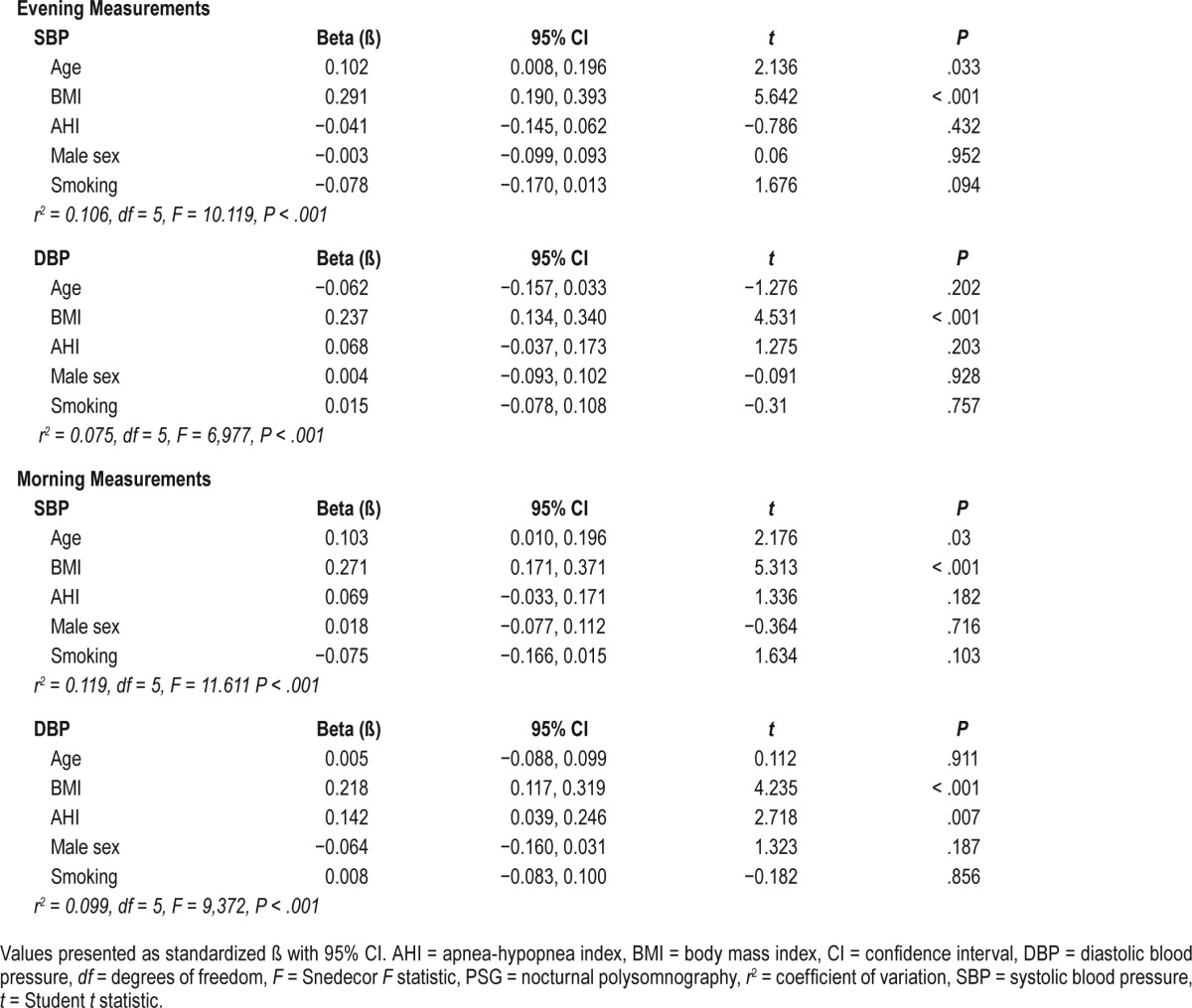
In the linear regression model, a rise in the morning SBP was predicted by BMI (ß = 0.27, 95% CI 0.17–0.37, P < .001) and age (ß = 0.10, 95% CI 0.01–0.20, P < .05), but not AHI (ß = 0.07, 95% CI −0.03 to 0.17, P = 0.18), male sex (ß = 0.02, 95% CI −0.08 to 0.11, P = 0.72), or smoking (ß = −0.08, 95% CI −0.17 to 0.02, P = .10). This model accounted for 13% of the variability in the morning SBP (r2 = 0.13, P < .001).
A rise in morning DBP was predicted by AHI (ß = 0.14, 95% CI 0.04–0.25, P < .01) and BMI (ß = 0.14, 95% CI 0.12–0.32, P < .01), but not by age (ß = 0.01, 95% CI −0.09 to 0.10, P = .92), male sex (ß = −0.06, 95% CI −0.16 to 0.03, P = .19), or smoking (ß = 0.01, 95% CI −0.08 to 0.10, P = .86). The model found a 1 event/h increment of AHI to have a similar effect to the 1 kg/m2 increment of BMI. This model explained 11% of the variability in the morning DBP (r2 = 0.11, P < .001). These results, and those concerning results on evening BP are summarized in Table 2.
Table 2.
Parameters of linear regression models for prediction of the evening (before PSG) and the morning (after PSG) arterial blood pressure.
Logistic regression model for high BP prediction
High BP was predicted by an age of 42 years or older (OR 2.09, 95% CI 1.19–3.65, P = .01), BMI of at least 29 kg/m2 (OR 1.98, 95% CI 1.21–3.26, P < .01), and by se-OSA (OR 1.74, 95% CI 1.04–2.92, P = .04). In the proposed logistic regression model, male sex was not found to predict high BP (OR 1.40, 95% CI 0.76–2.59, P = .29).
DISCUSSION
OSA is one of the acknowledged risk factors for HT.2,3 Our findings indicate that morning DBP was associated with the severity of disordered breathing during sleep in apparently non-hypertensive patients (ie, those without any previous diagnosis, nor treated for HT, with a presumptive diagnosis of OSA). This effect was independent of age, degree of obesity, or sex. However, our results may have been biased by the fact that some patients in the non-hypertensive group were actually naïve-hypertensive according to the standard diagnostic criteria.3
In a recent study, Ma et al.21 showed that BP 24-hour profiles were associated with the severity of OSA in a nonobese population. Similarly, the results of a linear regression model used in the current study suggest that the effect of OSA severity on morning BP may be independent of obesity, defined by high BMI. In addition, the effect of an increment of BMI by 1 kg/m2 seems to be similar to the effect of an increment of AHI by 1 event/h (Table 2). The current study is one of the first to report such a relationship, and to directly compare the influence of obesity and OSA on morning DBP.
The assumption that the severity of OSA caused the gain in morning DBP was based on two premises. Namely, no relationship was found between evening DBP and AHI, and no association was found between the morning SBP and AHI. Generally, high SBP is attributed to noncompliant, stiff arteries, whereas elevated DBP is related to the activation of the sympathetic autonomic nervous system.22–24 This augmented activity may be related to activation of chemoreceptors by intermittent hypoxia caused by the occurrence of apnea or hypopnea prior to the morning BP measurement.25,26 Furthermore, microarousals that usually end apnea events have also been implicated in the stimulation of sympathetic system, leading to BP elevation.27 Moreover, this effect may be sustained and present during the day.25 However, this assumption was contradicted by the results of our study, as neither evening SBP nor DBP was associated with SDB. Arterial stiffness, which has also been associated with OSA, may rather reflect a chronic proarteriosclerotic effect of sleep-disordered breathing, whereas sympathetic system activation reflects the acute form.3,28 This pathogenetic hypothesis is consistent with our results; also, it is in agreement with the findings of a study on a Korean population.13 Likewise, in the large cohort of a Sleep Heart Health Study, SDB was associated with systolic/diastolic hypertension, but not with isolated systolic hypertension and this association was age dependent (ie, observed only in the patient group younger than 60 years).29 This is partially in line with our results, as age was found to be linked with morning SBP but not with DBP. Consequently, as we did not restrict the analyses to subjects younger than 60 years, the observed association between SDB and SBP may have been diluted by the subgroup of patients older than 60 years (n = 77). Similarly, the continuous positive airway pressure treatment arm examined in a recent SAVE (Sleep Apnea Cardiovascular Endpoints) trial report demonstrated a small reduction only in DBP.30
Elevated morning DBP may be a reflection of a nondipping phenomenon observed in patients with OSA11; however, because of the large number of subjects included in the study and the limited technical resources, BP was not measured during sleep.
In addition, the relationship between morning DBP and AHI was found to be independent of sex. This appears to contradict the findings of Lee and Jeoung13; however, this discrepancy may be explained by the fact that in this study, men tended to reach higher AHI levels than women and these higher scores may be responsible for the observed association.
There is some evidence for a relationship between BP and severity of OSA among patients without hypertension. One example is in the study by Lee and Jeoung,13 which used a group similar to ours and similar methodology (ie, single time point BP measurement, PSG results from all patients, a retrospective design) and their results seem in line with ours. Also, there are a few studies employing ambulatory 24-hour BP monitoring (ABPM) instead of single time-point BP measurement, but these typically included a number of subjects two orders lower than in the current study. In a group of 47 consecutive patients, Sekizuka et al.31 report a significantly higher morning BP measured minutes after awakening and a higher nocturnal BP in se-OSA than in non se-OSA groups. Similarly, Cho et al.32 note that both morning SBP and DBP positively correlated with AHI, with a trend toward slightly higher correlation quotient for DBP, in a group of 58 patients with naïve hypertension. In addition, in a group of 22 non-hypertensive, nondiabetic patients with OSA, Kallianos et al.33 report a positive correlation between AHI and 24-hour mean DBP, but not SBP; however, they did not specifically investigate the morning BP. Our findings on the relationship between BP and AHI may be weakened by the occurrence of masked hypertension in patients with OSA (ie, some patients with normal clinical BP actually may have had an elevated ABPM, which would otherwise strengthen this relationship).34
However, these three studies did not correct for the possible confounding effect of sex, age, or BMI on the associations, although Sekizuka et al.31 mentioned a possible interplay between high BP, OSA, and obesity. Yet, future studies should adopt this approach (ie, combining PSG and ABPM, when examining larger groups of normotensive OSA patients) to deepen the understanding of the relationship between breathing disturbances during sleep and BP circadian variability.
The retrospective character of the study is its major limitation. Another lesser limitation relates to the method of BP measurement, which was performed routinely by a physician as a part of the physical examination: BP was measured only once per time point and on a randomly chosen arm. Nevertheless, the large number of study subjects seems to overly attenuate the potential variability of measurement.
BP regulation is a complex and heterogenous process and depends on numerous mechanisms. Thus, when modeling the predictive factors of BP, confounding factors should be recognized and included. Due to its retrospective design, the study does not include potentially confounding variables such as shift work, which is considered a risk factor for hypertension, insulin resistance, obesity, and dyslipidaemia.35–37 It has been suggested that shift work may cause a rise in BP or cause progression of hypertension38–42; however, this is not supported by more recent studies.43–45 Nevertheless, not including shift work as a confounding factor may be considered as a limitation and we recognize the need for controlling for shift work in future research on the subject. It is estimated that up to 20% of the population may work a shift system.46 This percentage might be higher among patients with presumptive OSA diagnosis, because shift-work sleep disorder and sleep-disordered breathing share some manifestations, although no direct link between these two has been found.47,48
In the recently-published results from MAPEC, a longitudinal ABPM study, Hermida et al. showed that when studying long-term cardiovascular and metabolic consequences, the only important BP recording appears to be during sleep.49 However, from the clinical perspective, ABPM may still be far less accessible than single time-point BP measurement. Despite not being as significant a prognostic marker of cardiovascular risk as mean overnight BP, elevated morning BP (especially DBP) may warrant specific hypertension diagnosis, including PSG and ABPM.34,50
Our findings also show that of the examined groups, the members of the se-OSA group had significantly higher BP. An AHI score of over 30 events/h predicted high BP, along with obesity and age of at least 42 years. These results suggest that patients with severe OSA are at increased risk of HT and require BP monitoring by frequent measurements, especially in the morning right after awakening. Nevertheless, to support our findings we recognize the need for further research employing a prospective design.
DISCLOSURE STATEMENT
The study was funded by Medical University of Lodz institutional grant no 503/0-079-06/ 503-01-002. Work for this study was performed at the Sleep and Respiratory Disorders Centre, Łódź, Poland. All authors have seen and approved the manuscript. The authors have indicated no financial conflicts of interest.
ABBREVIATIONS
- ABPM
ambulatory blood pressure monitoring
- AHI
apnea-hypopnea index
- BMI
body mass index
- BP
blood pressure
- DBP
diastolic blood pressure
- HT
arterial hypertension
- mi-OSA
mild obstructive sleep apnea
- mo-OSA
moderate obstructive sleep apnea
- OSA
obstructive sleep apnea
- PSG
polysomnography
- SBP
systolic blood pressure
- SDB
sleep-disordered breathing
- se-OSA
severe obstructive sleep apnea
- TST
total sleep time
REFERENCES
- 1.Karimi M, Hedner J, Lombardi C, et al. Driving habits and risk factors for traffic accidents among sleep apnea patients - a European multi-centre cohort study. J Sleep Res. 2014;23(6):689–699. doi: 10.1111/jsr.12171. [DOI] [PubMed] [Google Scholar]
- 2.Furlan SF, Braz CV, Lorenzi-Filho G, Drager LF. Management of hypertension in obstructive sleep apnea. Curr Cardiol Rep. 2015;17(12):108. doi: 10.1007/s11886-015-0663-z. [DOI] [PubMed] [Google Scholar]
- 3.Parati G, Lombardi C, Hedner J, et al. Position paper on the management of patients with obstructive sleep apnea and hypertension: joint recommendations by the European Society of Hypertension, by the European Respiratory Society and by the members of European COST (COoperation in Scientific and Technological research) ACTION B26 on obstructive sleep apnea. J Hypertens. 2012;30(4):633–646. doi: 10.1097/HJH.0b013e328350e53b. [DOI] [PubMed] [Google Scholar]
- 4.Jennum P, Riha RL. Epidemiology of sleep apnoea/hypopnoea syndrome and sleep-disordered breathing. Eur Respir J. 2009;33(4):907–914. doi: 10.1183/09031936.00180108. [DOI] [PubMed] [Google Scholar]
- 5.Heinzer R, Marti-Soler H, Haba-Rubio J. Prevalence of sleep apnoea syndrome in the middle to old age general population. Lancet Respir Med. 2017;4(2):e5–e6. doi: 10.1016/S2213-2600(16)00006-0. [DOI] [PubMed] [Google Scholar]
- 6.Xu S, Wan Y, Xu M, et al. The association between obstructive sleep apnea and metabolic syndrome: a systematic review and meta-analysis. BMC Pulm Med. 2015;15(1):105. doi: 10.1186/s12890-015-0102-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mesarwi OA, Sharma EV, Jun JC, Polotsky VY. Metabolic dysfunction in obstructive sleep apnea: a critical examination of underlying mechanisms. Sleep Biol Rhythms. 2015;13(1):2–17. doi: 10.1111/sbr.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siddiqui M, Dudenbostel T, Calhoun DA. Resistant and refractory hypertension: antihypertensive treatment resistance vs treatment failure. Can J Cardiol. 2016;32(5):603–606. doi: 10.1016/j.cjca.2015.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolf J, Hering D, Narkiewicz K. Non-dipping pattern of hypertension and obstructive sleep apnea syndrome. Hypertens Res. 2010;33(9):867–871. doi: 10.1038/hr.2010.153. [DOI] [PubMed] [Google Scholar]
- 10.Sasaki N, Ozono R, Edahiro Y, et al. Impact of non-dipping on cardiovascular outcomes in patients with obstructive sleep apnea syndrome. Clin Exp Hypertens. 2015;37(6):449–453. doi: 10.3109/10641963.2015.1057833. [DOI] [PubMed] [Google Scholar]
- 11.Hla KM, Young T, Finn L, Peppard PE, Szklo-Coxe M, Stubbs M. Longitudinal association of sleep-disordered breathing and nondipping of nocturnal blood pressure in the Wisconsin Sleep Cohort Study. Sleep. 2008;31(6):795–800. doi: 10.1093/sleep/31.6.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peppard PE. Is obstructive sleep apnea a risk factor for hypertension? -Differences between the wisconsin sleep cohort and the sleep heart health study. J Clin Sleep Med. 2009;5(5):404–405. [PMC free article] [PubMed] [Google Scholar]
- 13.Lee Y-JG, Jeong D-U. Obstructive sleep apnea syndrome is associated with higher diastolic blood pressure in men but not in women. Am J Hypertens. 2014;27(3):325–330. doi: 10.1093/ajh/hpt280. [DOI] [PubMed] [Google Scholar]
- 14.Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques, and Scoring System for Sleep Stages of Human Subjects. Los Angeles, CA: Brain Information Service, Brain Research Institute, UCLA; 1968. [Google Scholar]
- 15.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15(2):173–184. [PubMed] [Google Scholar]
- 17.Altman DG. Practical Statistics for Medical Research. London, UK: Chapman & Hall; 1991. [Google Scholar]
- 18.Slinker BK, Glantz SA. Multiple linear regression: accounting for multiple simultaneous determinants of a continuous dependent variable. Circulation. 2008;117(13):1732–1737. doi: 10.1161/CIRCULATIONAHA.106.654376. [DOI] [PubMed] [Google Scholar]
- 19.Nieminen P, Lehtiniemi H, Vähäkangas K, Huusko A, Rautio A. Standardised regression coefficient as an effect size index in summarising findings in epidemiological studies. Epidemiol Biostat Public Heal. 2013;10(4):e8854-1–e8854-15. [Google Scholar]
- 20.Altman DG, Bland JM. Diagnostic tests 3: receiver operating characteristic plots. Br Med J. 1994;309(6948):188. doi: 10.1136/bmj.309.6948.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma Y, Sun S, Peng C, Fang Y, Thomas RJ. Ambulatory blood pressure monitoring in Chinese patients with obstructive sleep apnea. J Clin Sleep Med. 2017;13(3):433–439. doi: 10.5664/jcsm.6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones A, Vennelle M, Connell M, et al. Arterial stiffness and endothelial function in obstructive sleep apnoea/hypopnoea syndrome. Sleep Med. 2013;14(5):428–432. doi: 10.1016/j.sleep.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Esler M, Rumantir M, Kaye D, et al. Sympathetic nerve biology in essential hypertension. Clin Exp Pharmacol Physiol. 2001;28(12):986–989. doi: 10.1046/j.1440-1681.2001.03566.x. [DOI] [PubMed] [Google Scholar]
- 24.Lakatta E, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107(1):139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 25.Tkacova R, McNicholas WT, Javorsky M, et al. Nocturnal intermittent hypoxia predicts prevalent hypertension in the European Sleep Apnoea Database cohort study. Eur Respir J. 2014;44(4):931–941. doi: 10.1183/09031936.00225113. [DOI] [PubMed] [Google Scholar]
- 26.Wang J, Yu W, Gao M, et al. Impact of obstructive sleep apnea syndrome on endothelial function, arterial stiffening, and serum inflammatory markers: an updated meta-analysis and metaregression of 18 studies. J Am Heart Assoc. 2015;4(11):e002454. doi: 10.1161/JAHA.115.002454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chouchou F, Pichot V, Pépin JL, et al. Sympathetic overactivity due to sleep fragmentation is associated with elevated diurnal systolic blood pressure in healthy elderly subjects: the PROOF-SYNAPSE study. Eur Heart J. 2013;34(28):2122–2131. doi: 10.1093/eurheartj/eht208. [DOI] [PubMed] [Google Scholar]
- 28.Dewan NA, Nieto FJ, Somers VK. Intermittent hypoxemia and OSA implications for comorbidities. Chest. 2015;147(1):266–274. doi: 10.1378/chest.14-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haas DC, Foster GL, Nieto FJ, et al. Age-dependent associations between sleep-disordered breathing and hypertension: importance of discriminating between systolic/diastolic hypertension and isolated systolic hypertension in the sleep heart health study. Circulation. 2005;111(5):614–621. doi: 10.1161/01.CIR.0000154540.62381.CF. [DOI] [PubMed] [Google Scholar]
- 30.McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive dleep spnea. N Engl J Med. 2016;375(10):919–931. doi: 10.1056/NEJMoa1606599. [DOI] [PubMed] [Google Scholar]
- 31.Sekizuka H, Kida K, Akashi YJ, et al. Relationship between sleep apnea syndrome and sleep blood pressure in patients without hypertension. J Cardiol. 2010;55(1):92–98. doi: 10.1016/j.jjcc.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 32.Cho JS, Ihm SH, Kim CJ, et al. Obstructive sleep apnea using Watch-PAT 200 is independently associated with an increase in morning blood pressure surge in never-treated hypertensive patients. J Clin Hypertens. 2015;17(9):675–681. doi: 10.1111/jch.12581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kallianos A, Trakada G, Papaioannou T, et al. Glucose and arterial blood pressure variability in obstructive sleep apnea syndrome. Eur Rev Med Pharmacol Sci. 2013;17(14):1932–1937. [PubMed] [Google Scholar]
- 34.Baguet J-P, Lévy P, Barone-Rochette G, et al. Masked hypertension in obstructive sleep apnea syndrome. J Hypertens. 2008;26(5):885–892. doi: 10.1097/HJH.0b013e3282f55049. [DOI] [PubMed] [Google Scholar]
- 35.Proper KI, van de Langenberg D, Rodenburg W, et al. The relationship between shift work and metabolic risk factors. Am J Prev Med. 2017;50(5):e147–e157. doi: 10.1016/j.amepre.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 36.Guo Y, Rong Y, Huang X, et al. Shift work and the relationship with metabolic syndrome in chinese aged workers. PLoS One. 2015;10(3):e0120632. doi: 10.1371/journal.pone.0120632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lajoie P, Aronson KJ, Day A, Tranmer J. A cross-sectional study of shift work, sleep quality and cardiometabolic risk in female hospital employees. BMJ Open. 2015;5(3):e007327. doi: 10.1136/bmjopen-2014-007327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elliott JL, Lal S. Blood pressure, sleep quality and fatigue in shift working police officers: effects of a twelve hour roster system on cardiovascular and sleep health. Int J Environ Res Public Health. 2016;13(2):172. doi: 10.3390/ijerph13020172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morris CJ, Purvis TE, Hu K, Scheer FAJL. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc Natl Acad Sci U S A. 2016;113(10):E1402–E1411. doi: 10.1073/pnas.1516953113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cassat M, Wuerzner G, Burnier M. [Shift work and night work: what effect on blood pressure?] Rev Med Suisse. 2015;11(485):1648–1654. [PubMed] [Google Scholar]
- 41.Virkkunen H, Härmä M, Kauppinen T, Tenkanen L. Shift work, occupational noise and physical workload with ensuing development of blood pressure and their joint effect on the risk of coronary heart disease. Scand J Work Environ Heal. 2007;33(6):425–434. doi: 10.5271/sjweh.1170. [DOI] [PubMed] [Google Scholar]
- 42.Oishi M, Suwazono Y, Sakata K, et al. A longitudinal study on the relationship between shift work and the progression of hypertension in male Japanese workers. J Hypertens. 2005;23(12):2173–2178. doi: 10.1097/01.hjh.0000189870.55914.b3. [DOI] [PubMed] [Google Scholar]
- 43.Sfreddo C, Fuchs SC, Merlo ÁR, Fuchs FD. Shift work is not associated with high blood pressure or prevalence of hypertension. PLoS One. 2010;5(12):3–7. doi: 10.1371/journal.pone.0015250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gholami Fesharaki M, Kazemnejad A, Zayeri F, Rowzati M, Akbari H. Historical cohort study of shift work and blood pressure. Occup Med (Chic Ill) 2014;64(2):109–112. doi: 10.1093/occmed/kqt156. [DOI] [PubMed] [Google Scholar]
- 45.Esquirol Y, Perret B, Ruidavets JB, et al. Shift work and cardiovascular risk factors: new knowledge from the past decade. Arch Cardiovasc Dis. 2011;104(12):636–668. doi: 10.1016/j.acvd.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 46.Antoniak M. [Working conditions in Poland against a background of other EU member states - European Working Conditions Survey results] Bezpieczeństwo Pr. 2011;9:26–28. [Google Scholar]
- 47.Belcher R, Gumenyuk V, Roth T. Insomnia in shift work disorder relates to occupational and neurophysiological impairment. J Clin Sleep Med. 2015;11(4):457–465. doi: 10.5664/jcsm.4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walia HK, Hayes AL, Przepyszny KA, Karumanchi P, Patel SR. Clinical presentation of shift workers to a sleep clinic. Sleep Breath. 2012;16(2):543–547. doi: 10.1007/s11325-011-0540-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hermida RC, Ayala DE, Mojón A, Fernández JR. Influence of circadian time of hypertension treatment on cardiovascular risk: results of the MAPEC study. Chronobiol Int. 2010;27(8):1629–1651. doi: 10.3109/07420528.2010.510230. [DOI] [PubMed] [Google Scholar]
- 50.Hermida RC, Ayala DE, Ríos MT, Fernández JR, Mojón A, Smolensky MH. Around-the-clock ambulatory blood pressure monitoring is required to properly diagnose resistant hypertension and assess associated vascular risk. Curr Hypertens Rep. 2014;16(7):445. doi: 10.1007/s11906-014-0445-9. [DOI] [PubMed] [Google Scholar]


