Abstract
Study Objectives:
This study examined empirically derived symptom cluster profiles among patients who present with insomnia using clinical data and polysomnography.
Methods:
Latent profile analysis was used to identify symptom cluster profiles of 175 individuals (63% female) with insomnia disorder based on total scores on validated self-report instruments of daytime and nighttime symptoms (Insomnia Severity Index, Glasgow Sleep Effort Scale, Fatigue Severity Scale, Beliefs and Attitudes about Sleep, Epworth Sleepiness Scale, Pre-Sleep Arousal Scale), mean values from a 7-day sleep diary (sleep onset latency, wake after sleep onset, and sleep efficiency), and total sleep time derived from an in-laboratory PSG.
Results:
The best-fitting model had three symptom cluster profiles: “High Subjective Wakefulness” (HSW), “Mild Insomnia” (MI) and “Insomnia-Related Distress” (IRD). The HSW symptom cluster profile (26.3% of the sample) reported high wake after sleep onset, high sleep onset latency, and low sleep efficiency. Despite relatively comparable PSG-derived total sleep time, they reported greater levels of daytime sleepiness. The MI symptom cluster profile (45.1%) reported the least disturbance in the sleep diary and questionnaires and had the highest sleep efficiency. The IRD symptom cluster profile (28.6%) reported the highest mean scores on the insomnia-related distress measures (eg, sleep effort and arousal) and waking correlates (fatigue). Covariates associated with symptom cluster membership were older age for the HSW profile, greater obstructive sleep apnea severity for the MI profile, and, when adjusting for obstructive sleep apnea severity, being overweight/obese for the IRD profile.
Conclusions:
The heterogeneous nature of insomnia disorder is captured by this data-driven approach to identify symptom cluster profiles. The adaptation of a symptom cluster-based approach could guide tailored patient-centered management of patients presenting with insomnia, and enhance patient care.
Citation:
Crawford MR, Chirinos DA, Iurcotta T, Edinger JD, Wyatt JK, Manber R, Ong JC. Characterization of patients who present with insomnia: is there room for a symptom cluster-based approach? J Clin Sleep Med. 2017;13(7):911–921.
Keywords: insomnia disorder, latent profile analysis, symptom profile, symptom clusters
INTRODUCTION
Insomnia is the experience of the difficulty falling asleep, difficulty staying asleep, or early morning awakenings. About one-third to one-half of adults complains of these symptoms.1 Frequently, other complaints, such as sleepiness, fatigue, and hyperarousal, will occur with nocturnal sleep disturbance. An insomnia disorder is defined in the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5)2 as the combination of the nocturnal sleep disturbance with one of these waking complaints at least 3 nights a week for at least 3 months. Approximately 8% to 10% of the adult population meets these criteria.3,4
Insomnia disorder is a heterogeneous condition.5 This can pose a challenge for optimal patient care, because a one-size-fits-all approach does not provide treatment that is tailored to the patient's unique combination of symptoms. The field of oncology has made great strides toward personalized/precision medicine6 by acknowledging the heterogeneity within the disease and matching treatment based on the individual's symptom/genetic profiles. Many other areas have followed suit, and we believe that there is room for the same type of personalized medicine in insomnia and that this could greatly improve patient care. A management approach to insomnia disorder that is not confined to diagnostic boundaries and instead considers symptom cluster profiles might offer a more targeted management by treating the most relevant symptoms. This would translate to assessment and treatment decisions informed by a profile based on the level of severity of each symptom.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Current management of insomnia disorder relies primarily on diagnostic boundaries; however, there might be merit in a symptom-based approach. This study used sophisticated, data-driven statistical models to elucidate possible symptom cluster profiles in a sample of patients with insomnia disorder.
Study Impact: The results may offer a clinical guide for those who present to sleep clinics with insomnia, which may lead to a more patient-centered approach and enhanced patient care.
This hypothesis is based partly on results from our previous mixed-methods study,7 which revealed that patients with multiple sleep symptoms tend to understand the symptoms and consequences better than diagnostic categories of sleep disorders. Yet, we as clinicians make decisions largely based on diagnostic categories. Empirically deriving symptom clusters for those who present with insomnia complaints might yield a model for a patient-centered approach. There have been previous attempts to identify nighttime and daytime symptom cluster profiles in patients with insomnia disorder using data-driven approaches.8–13 Similar attempts have been published characterizing the heterogeneity of obstructive sleep apnea.14,15 However, most of these studies used cluster analysis to characterize this heterogeneity. In contrast, mixture models, such as latent class or profile analysis, have certain advantages over cluster analysis as described further in the statistical analysis section.
In general, the primary aim of latent profile analysis is to classify individuals into symptom profiles reflecting symptom clusters that consist of homogeneous individuals with regard to continuous observed variables being studied.16 While ensuring homogeneity within a symptom profile, the different profiles are distinct from each other and are viewed as representing the unobserved heterogeneity across individuals. Therefore, this person-centered analytic technique uses actual empirical data, and not arbitrary dichotomization (such as diagnostic categories), to create quantitatively and qualitatively distinct profiles of individuals based on their dimensional presentation of daytime and nighttime symptoms of insomnia. Another strength of the analyses is the ability to examine covariates of symptom cluster membership. These may be tested in association with distinct outcome variables, such as treatment response, relapse risk, or obstructive sleep apnea (OSA) risk, in future reports.
To our knowledge, only two studies have used mixture models, such as latent profile or class analysis, to identify symptom cluster profiles within insomnia.17,18 Those studies did not explore symptom profiles among patients who met criteria for an insomnia disorder.17,18 The purpose of this study was to examine whether distinct symptom profiles could be identified across a heterogeneous sample of insomnia patients who are representative of those presenting to a sleep clinic, including those with comorbidities such as periodic limb movement disorder or OSA. We hypothesized that distinct symptom cluster profiles would emerge. We believe that empirically derived symptom profiles can provide an impetus for a dimensional profile of sleep health,19 which might be useful for reducing the gap between patient understanding and clinical decision making based on categories.
METHODS
Study Sample
Baseline assessments from two independent projects were used for this analysis. Individuals underwent a structured interview for sleep disorders20 and had to meet quantitative criteria for insomnia21 as determined by a 7-day sleep diary. Eligible participants had to be psychologically and medically stable, as evaluated by a structured interview for clinical disorders (Structured Clinical Interview for DSM-IV-TR Axis I Disorders22) and medical examination by a physician (study 2 only), respectively. Last, individuals who were not fluent in English were excluded. Individuals who were taking sedative-hypnotic medications were only eligible if they stopped the medication under supervision of their prescribing physician.
There were minor differences in the inclusion criteria for both studies: study 1 targeted individuals older than 21 years with psychophysiological insomnia,23 and study 2 targeted individuals older than 18 years with insomnia disorder and comorbid OSA.24 For study 1, insomnia had to be present for at least 6 months to meet criteria for chronicity,25 whereas for study 2 insomnia had to be present for at least 3 months (International Classification of Sleep Disorders26 and DSM-52 criteria). For study 2, unless agreeing not to drive, individuals who were excessively sleepy were also excluded. Excessive sleepiness was defined by scores on the Epworth Sleepiness Scale (ESS)27 greater than 16 or a score of 3 (high chance) on the ESS question about risk of dozing “In a car, while stopped for a few minutes in traffic” or a report of falling asleep at the wheel, a motor vehicle accident, or near-miss accident due to sleepiness in the past 24 months, which in the judgment of the study physician was not attributable to acute sleep loss. All participants also had to be naïve to continuous positive airway pressure therapy and cognitive behavioral therapy for insomnia.
By merging data from these two studies collected at the last step of the screening process (in-laboratory polysomnography [PSG], see next section) we were able to capitalize on the homogeneity with regard to inclusion criteria (both studies included individuals with insomnia disorder) while retaining some heterogeneity with regard to exclusion criteria (in this analysis we included those who were excluded in both studies—individuals with comorbid OSA [in study 1] and comorbid periodic limb movement [study 1 and 2]).
Procedures
The standard baseline assessment for both studies was designed to mimic common clinic procedures for new patients' evaluations at a sleep clinic. The baseline assessment consisted of a brief phone screening, an in-person interview, and an in-laboratory PSG. All individuals provided written informed consent. The institutional review board at Rush University Medical Center approved both studies. Data from participants who had successfully completed the baseline assessment were merged into one dataset.
Measures
At baseline, individuals completed a range of self-report questionnaires, a 7-day sleep diary, and a screening PSG.
Insomnia Severity Index
The Insomnia Severity Index (ISI)28 is a brief seven-item scale assessing nocturnal and daytime symptoms of insomnia, which has been used as both a screening and outcome measure in treatment research. Total scores range from 0 to 28, with higher scores indicative of increase insomnia severity. The ISI has adequate internal consistency with evidence supporting concurrent, predictive, and content validity.29,30
Glasgow Sleep Effort Scale
The Glasgow Sleep Effort Scale (GSES)31 measures sleep-related effort as experienced in the past week. The seven items are reverse coded so that a higher score (range 0–14) indicates increased sleep effort (eg, “I feel I should be able to control my sleep at night”). Adequate reliability and validity of this measure has been established.31
Beliefs and Attitudes about Sleep Scale
The Beliefs and Attitudes about Sleep Scale (BAS)32 is a 30-item measure of sleep-related dysfunctional thinking. Individuals are asked to indicate the level of agreement on statements related to sleep; a strong endorsement of these statements is suggestive of dysfunctional beliefs and attitudes about sleep. The total scores were computed by summing all items, thus scores ranged from 0 to 300. The short-form version has acceptable internal consistency (Cronbach alphas of around 0.8) and adequate test-retest reliability across a 2-week interval (r = 0.8).33
Pre-Sleep Arousal Scale
The Pre-Sleep Arousal Scale (PSAS)34 is a 16-item questionnaire that assesses both cognitive and somatic arousal typically experienced during the sleep onset period. The total score ranges from 16 to 80, with a higher score reflective of increased arousal at bedtime.
Fatigue Severity Scale
The Fatigue Severity Scale (FSS)35 is a nine-item measure providing a global score of the intensity of an individual's fatigue and has good internal consistency (α = 0.8–0.9). Scores range from 9 to 63, with increased scores reflective of increased fatigue.
Epworth Sleepiness Scale
The ESS27 is a brief eight-item questionnaire measuring the propensity for drowsiness or falling asleep in eight common situations and correlates moderately with sleep latency at night and during daytime naps.27 Total scores range from 0 to 24, with higher scores indicating increased subjective sleepiness.
Sleep Diary
Prospective sleep diaries were completed daily across a 7-day period. The following variables were derived for analyses and were included in the analytic model (see statistical analysis): sleep onset latency (SOL), wake after sleep onset (WASO) and sleep efficiency (SE, computed as the percent of time asleep relative to the time in bed). Study 1 used an in-house sleep diary that is similar to the consensus sleep diary, but did not separate WASO from early morning awakenings (EMA); study 2 used the consensus core sleep diary.36 For study 2, EMA was added to WASO, so that this measure was comparable to study 1.
Polysomnography
Each participant completed a technician-monitored, in-laboratory PSG to collect objective measures of sleep and respiratory events. Each study was scored by a registered polysomnography technologist and reviewed by a board-certified sleep medicine physician in accordance with The American Academy of Sleep Medicine Manual for the Scoring of Sleep and Associated Events.37 For our analyses the following variables were extracted: total sleep time (TST) and apnea-hypopnea index (AHI).
Statistical Analysis
Preliminary statistical analyses included descriptive statistics and assessment of normality of distributions. Data for continuous variables are presented as means and standard deviations and were compared between profiles using independent t-tests. Categorical variables are presented as percentages and were compared with the chi-square test. The Statistical Package for the Social Sciences (SPSS) version 19.0 was used for all preliminary analyses.
Latent profile analysis (LPA) was used to characterize insomnia symptom profiles. LPA is an empirically driven approach, which uses continuous variables (or indicators) to derive latent clusters of individuals with a particular symptom profile. Symptom cluster membership is inferred by examining the patterns of interrelationships among individuals with the goal of maximizing homogeneity within class (or symptom cluster profile) and heterogeneity between classes. Therefore, underlying this method is an emphasis on differentiating individuals (individual-based approach) based on scores on various indicators, rather than on one particular variable (variable-based approach). The following continuous indicators were used to characterize insomnia symptom profiles: total scores on the (1) ISI, (2) GSES, (3) FSS, (4) BAS, (5) ESS, and (6) PSAS; as well as mean self-reported (7) SOL, (8) WASO, and (9) sleep efficiency from a 7-day sleep diary; and (10) PSG-measured TST. We used TST derived from the PSG rather than the sleep diary because of mounting evidence that insomnia with objective short sleep may form a distinct subtype of insomnia.10,38–42 The optimal number of symptom cluster profiles was determined after examination of the following fit indices: the Akaike information criteria (AIC), the Bayesian information criteria (BIC), the sample-size adjusted BIC (aBIC), log-likelihood (LL), entropy, the adjusted likelihood ratio test (ALRT), and the parametric bootstrapped likelihood ratio test (BLRT).43
Important advantages of LPA over standard cluster techniques have been identified in the literature.43 These include, for example, the ability to simultaneously include varying scales data in the same model, formal statistical criteria for selecting best-fitting models, and most relevant to this study: the ability to examine associations between covariates and emerging profiles. Given this advantage, analyses were conducted in a two-step manner. First, the aforementioned continuous indicators were included in the model to identify insomnia symptom cluster profiles. Second, covariates of symptom profiles were added to the model to examine cross-sectional associations between relevant covariates and symptom cluster profiles, using multinomial logistic regression. The following variables were entered as covariates: age, sex, education, race, ethnicity, body mass index (BMI), and AHI. Odds ratios (OR) of belonging to a symptom cluster with a specific symptom profile were estimated for each covariate. All tests were two-sided and α < 0.05 was considered to be statistically significant. Mplus version 6.0 was used for all LPA analyses.
RESULTS
Descriptive Characteristics
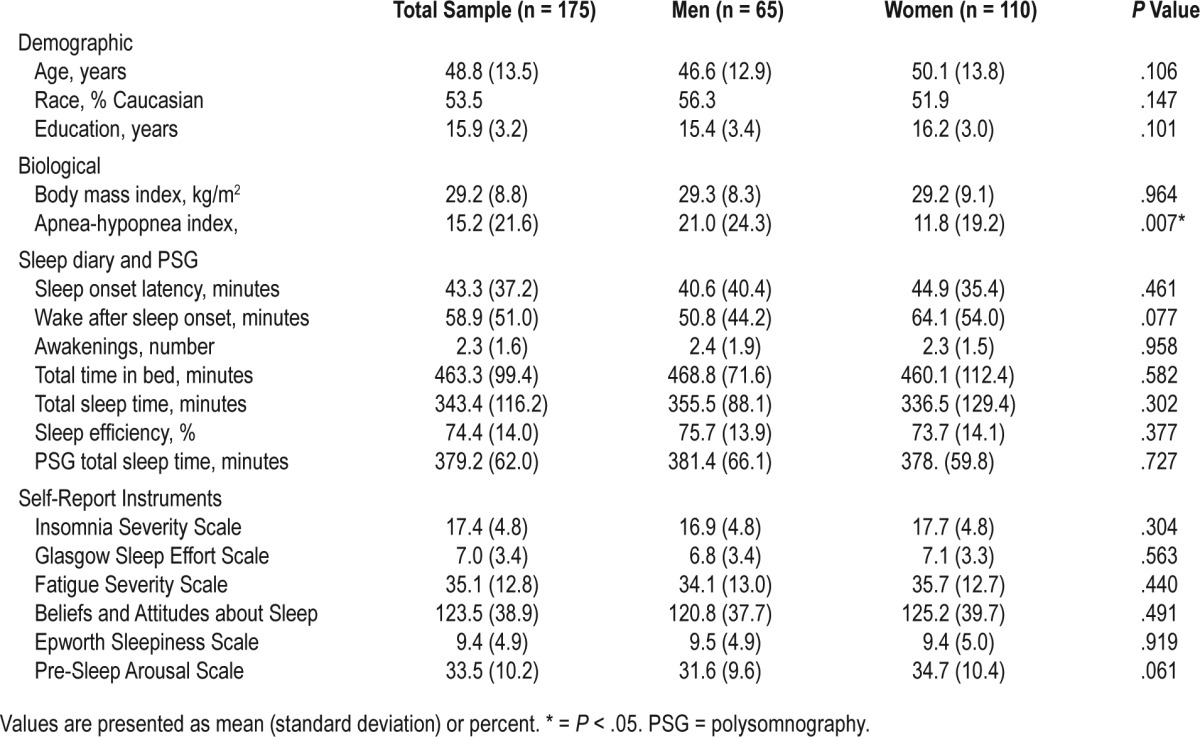
Our sample was composed of 175 individuals (n = 110 female). Approximately 52.60% of individuals in our study identified as Caucasian, whereas 34.9%, 5.8%, 1.2%, and 1.2% of the sample were African American, Asian, American Indian, and Native Hawaiian, respectively. In regard to ethnicity, 8.8% of the sample identified as Hispanic/Latino. Mean age and education were 48.8 (standard deviation [SD] = 13.5) and 15.9 (SD = 3.2) years, respectively. Mean AHI was significantly higher among men (mean = 21.0, SD = 24.3) when compared with that of women (mean = 11.8, SD = 19.2). No other significant differences in study variables were found across sex. In regard to AHI categories, 25.1% of the sample had mild OSA (AHI ≥ 5 and < 15), 20.6% had moderate OSA (AHI ≥ 15 and < 30), and 14.3% had severe OSA (AHI ≥ 30). Detailed descriptive characteristics of the study sample are presented in Table 1.
Table 1.
Descriptive characteristics of the study sample.
Characterization of Insomnia Symptom Profiles
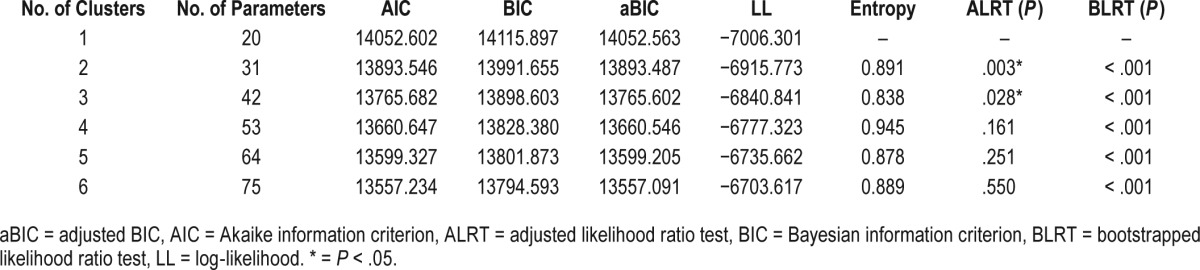
Multiple LPA models were examined with the number of symptom profiles (or latent clusters) ranging from 1 to 6. Fit indexes for all models are presented in Table 2. The AIC, BIC, aBIC, and log-likelihood values decreased as the number of classes increased, which suggests that a greater number of clusters fit the data progressively better. Similarly, the bootstrapped likelihood ratio test was significant across comparisons of progressively greater number of clusters. Entropy values for the three- to six-cluster solution ranged from 0.838 to 0.945, indicating good fit with the data across all clusters. The ALRT test, however, suggested that the three-cluster solution was the best fitting model as it was shown to perform significantly better than the two-cluster solution (P = .028). Further, the ALRT indicated that the four-cluster solution was not significantly better than the three-cluster solution (P = .161). In fact, proportion of individuals belonging to each cluster pronouncedly decreased as the number of clusters increased, and the four-cluster solution included one symptom cluster profile comprised of only eight individuals (5% of total sample). After collectively accounting for model fit indexes, as well as the size of each cluster, the three-cluster solution was selected as best representing the data.
Table 2.
Fit indexes for latent profile analysis.
Based on visual examination of the severity and presentation of symptoms within the different profiles and discussion among the authors (MRC, DAC, JCO), the three latent symptom profiles were labeled the “High Subjective Wakefulness” (HSW), “Mild Insomnia” (MI), and “Insomnia-Related Distress” (IRD). The MI symptom cluster profile was the largest comprising 79 individuals (45.1%), followed by the IRD and the HSW symptom cluster profiles with 50 (28.6%) and 46 (26.3%) individuals, respectively.
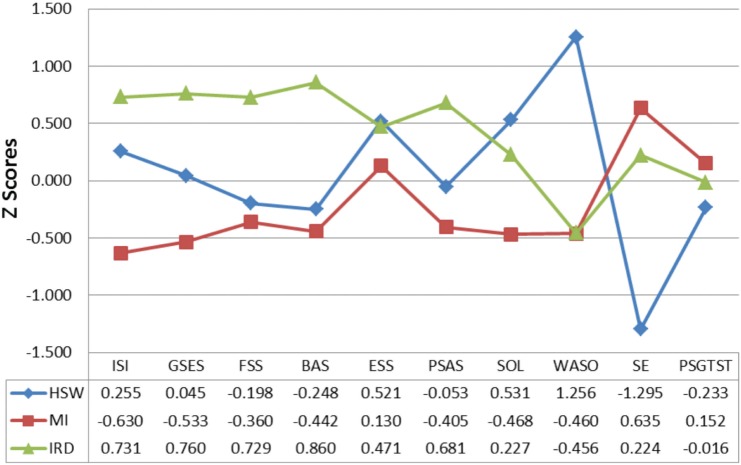
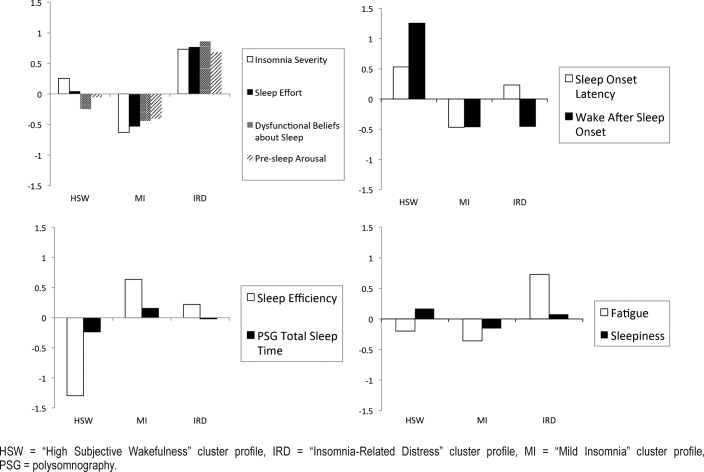
Means and SD of all indicators (self-report scales, 7-day sleep diary variables, and PSG TST) across each symptom cluster profile are presented in Table 3. As shown in Figure 1 and Figure 2—the graphical representations of the three symptom profiles—the HSW symptom cluster profile had the highest levels of daytime sleepiness (mean = 10.2; Z score = 0.5), and WASO, lasting on average 144 minutes, (Z score = 1.3), high SOL (mean = 36.0, Z score = 0.5), and the lowest SE (56.3%; Z score = −1.3) in spite of a relatively comparable objective TST to the other two profiles (mean = 364.8, Z score = −0.2). In contrast, the MI symptom cluster profile presented with relative low means across most self-report and sleep diary variables and the highest diary-based SE of all three symptom cluster profiles (mean = 83.3%; Z score = 0.6). Finally, the IRD symptom cluster profile was characterized by the highest overall means on self-report instruments measuring sleep arousal (PSAS mean = 40.4; Z score = 0.7), effort (GSES mean = 9.5; Z score = 0.8) and symptomatic severity (ISI mean = 20.9; Z score = 0.7), as well as cognitions about sleep (BAS mean = 156.86, Z score = 0.86) and daytime fatigue (FSS mean = 44.4; Z score = 0.7).
Table 3.
Indicators and unadjusted predictors means by symptom cluster profile.
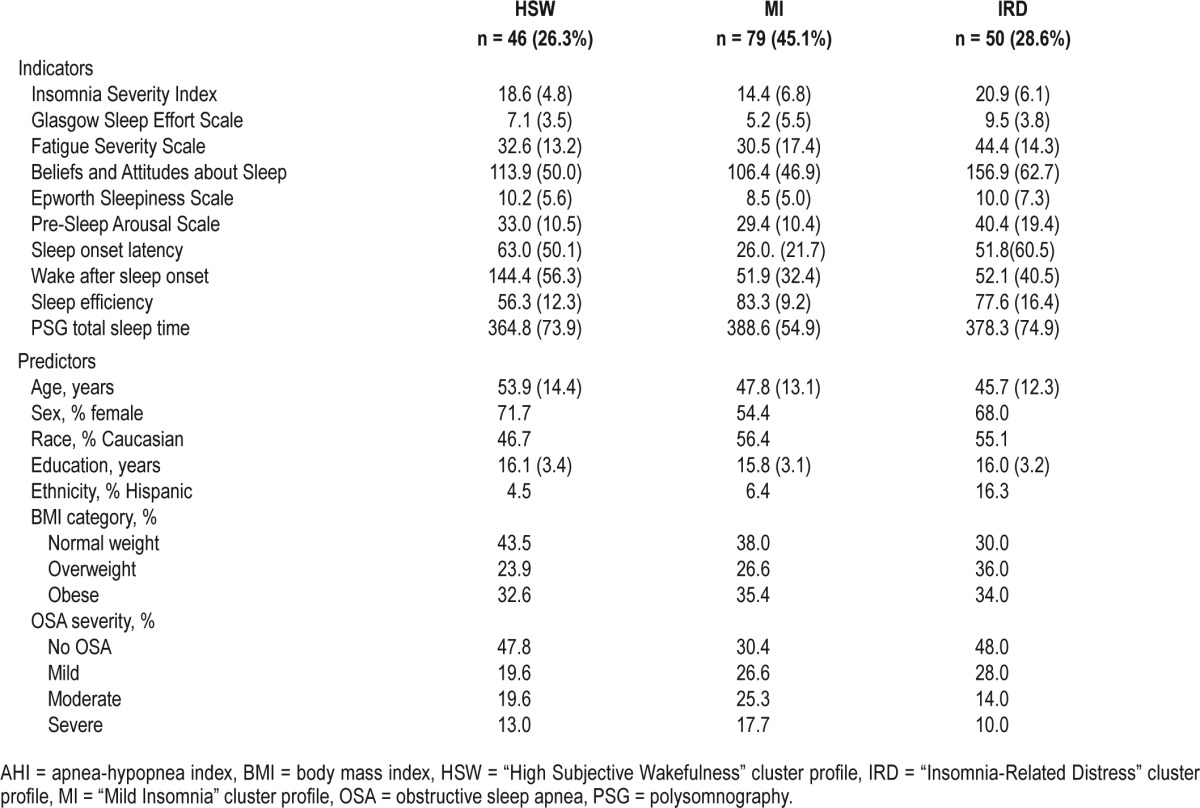
Figure 1. Latent profiles of symptoms.
BAS = Beliefs and Attitudes about Sleep Scale, ESS = Epworth Sleepiness Scale, FSS = Fatigue Severity Scale, GSES = Glasgow Sleep Effort Scale, HSW = “High Subjective Wakefulness” cluster profile, IRD = “Insomnia-Related Distress” cluster profile, ISI = Insomnia Severity Index, MI = “Mild Insomnia” cluster profile, PSGTST = total sleep time derived from polysomnography, PSAS = Pre-Sleep Arousal Scale, SE = sleep efficiency, SOL = sleep onset latency, WASO = wake after sleep onset.
Figure 2. Visualization of Z scores for all variables across subgroups.
HSW = “High Subjective Wakefulness” cluster profile, IRD = “Insomnia-Related Distress” cluster profile, MI = “Mild Insomnia” cluster profile, PSG = polysomnography.
Symptom Cluster Membership Covariates
The inclusion of covariates to the model (age, sex, education, race, ethnicity, AHI category, and BMI category) did not significantly alter the indicator mean scores for each symptom cluster profile, which further confirms the stability of the three-cluster solution. Unadjusted mean values and percentages for each predictor by the three insomnia symptom cluster profiles are presented in Table 3.
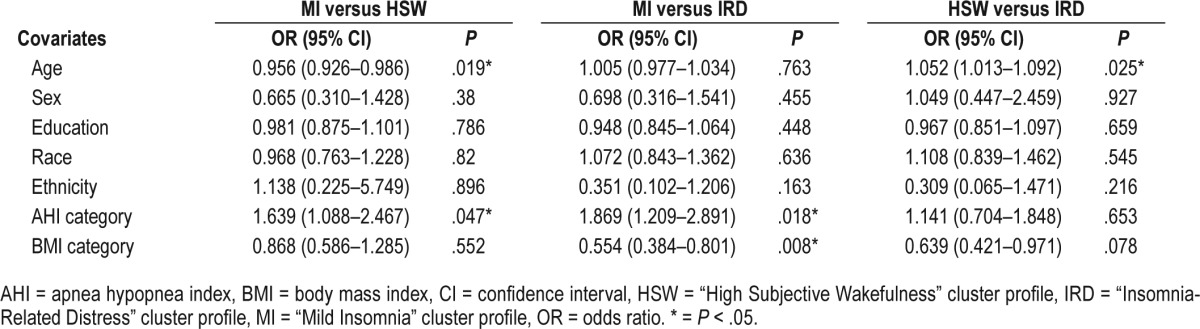
Participants across all insomnia symptom cluster profiles were comparable in terms of sex, education, race, and ethnicity. However, significant predictors of symptom cluster membership included age, OSA severity, and BMI category (see Table 4 for unstandardized OR for all variables). In terms of age, older participants were significantly more likely to belong to the HSW profile than the IRD profile (OR = 1.052, P = .025) or the MI profile (OR = 1.046, P = .019). This indicates that for every 1-year increase in age participants were 5% more likely to belong to the HSW profile when compared with the IRD or MI profile. No significant differences in age were found between the MI and IRD profiles. In regard to AHI, participants with higher degree of OSA severity were significantly more likely to belong to the MI profile when compared to the IRD (OR = 1.870, P = .018) or the HSW (OR = 1.639, P = .047) profiles. In fact, for every progressive increase in OSA severity category (no OSA versus mild versus moderate versus severe), there was an 87% increase in the odds of belonging to the MI as compared with the IRD profile. Similarly, for every progressive increase in OSA severity category, there was a 64% increase in participants' odds of belonging to the MI profile as compared to the HSW profile. Finally, when adjusting for OSA severity, participants with greater degree of obesity were more likely to belong to the IRD than the MI (OR = 1.804, P = .008) profile. This indicates that overweight/obese participants had an 80% increase in their odds to belong to the IRD profile when compared with the MI profile. No significant differences in BMI category were found between the HSW profiles and the other two symptom cluster profiles.
Table 4.
Unstandardized odds ratios for symptom cluster membership.
DISCUSSION
Considering the heterogeneity of insomnia disorder, a symptom-based approach is a timely consideration. The aim of our study was to generate symptom cluster profiles, which could guide development of models of patient-centered care. A symptom cluster-based approach might provide a more personalized, precise management of the patient's primary complaints. Unique patterns nested within the symptom cluster would otherwise be lost.44 To do this, we used a different data-driven approach (LPA) in a sample of individuals who represent patients presenting to a sleep clinic for insomnia symptoms.
Compared with most previous studies, we used data-driven methods here to characterize the heterogeneity, rather predetermined categories as used in other studies. For example, predetermined categories have included insomnia disorder sub-types, such as psychophysiological, paradoxical, or idiopathic insomnia, insomnia related to a mental disorder, which has been associated with different disease characteristics,45–47 treatment perceptions,47,48 and treatment responses.47,49 Nightly insomnia symptoms (sleep onset or sleep maintenance problems or EMA) have also been associated with different disease characteristics50–56 and treatment responses.57,58 More recently, the heterogeneity driven by objective TST has garnered attention. A number of studies have highlighted differential outcomes associated with short versus long objective sleep.38–42,59 In contrast to these top-down approaches, data-driven methods, such as cluster analysis, have been applied to this area and have revealed that daytime symptoms such as sleepiness, fatigue, mood and sleep hygiene practices,9 nighttime symptoms such as objective sleep parameters,10 night-to-night variability and longitudinal development of subjective sleep variables,11,17 and dysfunctional beliefs about sleep12 uniquely fall together in identifiable and meaningful clusters. Others have used both sleep and psychiatric history, and daytime and nighttime symptoms to identify symptom clusters.8
To our knowledge, only two studies to date have used sophisticated mixture models to derive symptom cluster profiles in individuals with sleep disturbances18 or from a population-based sample.17 Foley and colleagues18 identified four symptom clusters (“weekly sleep disturbance and distressed,” “transient sleep disturbances,” “early morning awakenings” and “comorbid & non-restorative sleep”). Also using latent class analysis, Green and colleagues derived four symptom profiles, “healthy with low reports of sleep problems,” “episodic reports of sleep problems,” “developing over the 20 years” and “chronic problems of both sleep onset and maintenance problems.”17 Our latent profile analysis reported here, builds on these previous studies. The symptom cluster profiles that emerged in our study were characterized by the following symptoms: increased self-reported wakefulness (HSW), low reporting of insomnia symptoms (MI), and high distress about sleeplessness and its consequences (IRD).
“High Subjective Wakefulness” Symptom Cluster Profile
The HSW symptom cluster profile was best characterized by the significant subjective sleep disruption as reported on sleep diary (high SOL and WASO, and low SE). This symptom cluster profile shares similarities with one of Foley et al.'s symptom profiles18: the “difficulty maintaining sleep” group also reported high rates of sleep maintenance problems. Interestingly, PSG-derived TST of the HSW symptom cluster profile did not vary greatly from the other two profiles (10- to 20-minute difference), yet the HSW group reported taking 30 minutes longer to fall asleep than those with the MI profile and 90 minutes more wakefulness in the middle of the night than the other two symptom cluster profiles, which suggest potential overestimation of subjective wakefulness in the HSW symptom cluster profile. It is noteworthy, however, that this study used 1 night of PSG to determine objective TST, compared to an average 7-day sleep diary; hence, measurement bias reduces the ability to estimate the true extent of the sleep misperception. In addition, the use of only 1 night of PSG raises the potential influence of “first-night effects.” Future replications, ideally with multiple consecutive nights, are needed at this stage; however, it is worth mentioning that recent evidence emerged indicating the validity of 1 night of PSG in the classification of short versus long objective total sleep time for individuals with insomnia.60
The statistical method of LPA enabled us to examine predictors of symptom cluster membership. Because participants of older age were more likely to belong to the HSW symptom cluster profile than to the other two symptom cluster profiles, it is possible that the observed elevation in subjective wakefulness might be explained in part by age-related increase in WASO.61 The average objective TST for this symptom cluster profile—just above 6 hours—was below the sample's overall average; thus, individuals with this symptom profile might benefit from therapeutic approaches that lead to rapid sleep consolidation—for example, sleep restriction, stimulus control, or sedative-hypnotics. Whether some of these individuals present with similar characteristics and sequelae as the symptom cluster profile “insomnia with objective short sleep”10,38–40,42,59 remains to be elucidated in future studies.
“Mild Insomnia” Symptom Cluster Profile
The MI symptom cluster profile had less severe insomnia, less fatigue, more consolidated sleep, and the least sleep-interfering mental activity (dysfunctional beliefs, sleep-related effort, and presleep arousal) of all profiles. There are similarities between this symptom cluster profile and Sánchez-Ortuño and Edinger's “low endorsement” symptom profile.12 Sánchez-Ortuño and Edinger's profile scored low on all subscales of the beliefs and attitudes about sleep scale, reported the fewest number of nights with insomnia complaints, and had the lowest insomnia severity score.
The results in the current study indicated that this symptom profile had the highest percentage of cases with OSA overall and per severity level among the three symptom profiles. This symptom cluster profile might represent individuals with co-morbid OSA and insomnia, who may not identify insomnia as their chief complaint. In a previous study on OSA and comorbid insomnia, we found that a quarter of the sample (24.1%) identified OSA (rather than insomnia) as their primary complaint.7 This finding has important clinical implications and could improve the precision and cost-effectiveness of evaluations conducted at sleep disorders clinics. Specifically, whereas PSG is not currently recommended for the routine assessment of insomnia,62 a patient presenting for the treatment of insomnia whose symptom profile fits the MI symptom cluster profile might benefit from a PSG to evaluate the possible presence of OSA. The danger of OSA going undetected among insomnia patients has been previously documented. Krakow and colleagues found that in patients endorsing insomnia but no sleep-disordered breathing (SDB) symptoms, most nighttime awakenings actually followed respiratory events, unbeknownst to the patient,63 and 50% of the sample met criteria for OSA. Fung et al. found that almost half of study participants with insomnia suffered from occult SDB (AHI ≥ 15), and the presence of excessive daytime sleepiness was the distinguishing factor between occult and nonexistent SDB.64 In the primary care setting, insomnia was found to predict OSA irrespective of age.65 Cronlein and colleagues found that occult OSA was most likely to be found on PSG in older and overweight individuals with insomnia, alluding to the possible necessity for PSG in these patients.66 Men with insomnia who frequently reported dry mouths were likely to have occult sleep apnea even after been screened for possible OSA.67 Our findings complement these studies by highlighting the risks of occult OSA in insomnia patients, and this might be particularly prominent in those with the MI symptom profile. For this profile, treatment of insomnia using brief behavioral therapy68–70 might suffice, and these patients might require concomitant treatment for both insomnia disorder and OSA.
We did not include participants who were excluded prior to the PSG screening evaluation (eg, those who had a high [study 1] or low risk [study 2] for OSA based on subjective symptoms such as snoring or witness apneas or based on medical examination). This selection method might have biased our results, as we are left with two distinct samples at two extremes of the continuum: insomnia + no OSA for study 1, and insomnia + OSA for study 2. This selection bias might lead to an overrepresentation of the MI profile. In contrast, our analysis did include participants who underwent a screening PSG, even if they were excluded post-PSG from each individual parent study, which increases the selection of insomnia patients with comorbidities to a greater extent that most previous studies have done.
“Insomnia-Related Distress” Symptom Cluster Profile
Finally, the IRD symptom cluster profile was characterized by the highest reports of sleep-interfering mental activity and of fatigue. This profile is very similar to previously reported clusters. Sánchez-Ortuño and colleagues' “worried and symptom focused” and “worried and medication biased” clusters12; Edinger and colleagues' “bedtime arousal” cluster8; and Foley et al.'s “distressed” cluster.18 These clusters are all characterized by presleep arousal, distress, worry, or dysfunctional beliefs about sleep. Interestingly, in the current study, those belonging to the IRD symptom cluster profile were more likely to have no or mild OSA. Thus PSG evaluation would not likely be indicated, unless other risk factors such as snoring or witness apneas have been reported. Particularly noteworthy are the high rates of fatigue in this cluster, despite relatively comparable levels of daytime sleepiness to the other profiles (see Table 3, Figure 1, and Figure 2); this dichotomy is not present in other symptom cluster profiles. The dissociation between fatigue and sleepiness ratings has been highlighted previously, with the former more frequently reported in insomnia.71,72
Controlling for OSA severity, overweight/obese participants were more likely to belong to the IRD than the MI symptom cluster profile. Our findings report on cross-sectional data, and thus preclude inference about causal relationships between insomnia-related distress and obesity; however, these results might suggest avenues for further investigation. Others have reported an association between obesity, insomnia, and emotional stress,73 and psychological stress has been associated with changes in the production of appetite regulating hormones, such as ghrelin.74
Patients who present with a symptom profile consistent with the IRD symptom cluster profile might benefit from a treatment plan that includes cognitive therapy, mindfulness or relaxation strategies to reduce the arousal, and distress and a mixture of cognitive and behavioral components to address fatigue.
One notable limitation that is relevant to this symptom profile is that parent study 1 specifically recruited individuals with psychophysiological insomnia; thus, individuals with heightened somatic and cognitive arousal at bedtime were over-represented in this sample. This sample selection might have contributed to an overrepresentation of this symptom profile.
Implications for Clinical Practice
With our analysis we supplement previous data-driven attempts to characterize the symptom cluster profiles within insomnia disorder. Symptom cluster profiles add important clinical data that may be lost when individuals are grouped within one single diagnostic boundary. These symptom cluster profiles might also transcend diagnostic boundaries: in clinical practice, insomnia patients often share symptoms with other medical, psychiatric, or sleep disorders, and so treatment decisions that are guided by symptom clusters will not be biased by symptom overlap across comorbidities. We hope that these results, along with other attempts, will inform clinical practice by guiding patient-centered care. We can see the success of these approaches in other areas such as oncology,75 asthma,76 and various psychiatric disorders.77 The heterogeneity within insomnia disorder lends itself to such an approach.
We currently define insomnia disorder as a distinct entity, and our management of insomnia disorder is a one-size-fits-all approach, simply because we do not have sufficient evidence for (1) valid and meaningful symptom cluster profiles, and (2) whether treatments can be tailored to these symptom cluster profiles. The attempts to date, including ours and future studies, will help to move the needle toward a more patient-centered approach, which is gaining popularity in the United States. With established and validated symptom clusters, we envision the practitioner can make assessment and treatment decisions based on the symptom cluster each individual patient reports. These findings are hopefully a catalyst for a dimensional profile of sleep health19 that might be useful for reducing the gap between patient experiences, and clinical decision making based on categories. A symptom profile-based approach represents the middle ground on the dimension from very individualized medicine on the one side, and a one-size-fits-all approach on the other side. This approach would be more effective than the onesize-fits-all approach, because symptoms specific to the individual's symptom profile are targeted, but more feasible in our current health system (particularly in the United States) than an entirely individualized one, where time and cost limitations play a considerable role. We envision that established and validated symptom profiles will offer the practitioner with a model for patient-centered management of insomnia disorder.
Summary
Our results revealed three different symptom clusters in a group of individuals presenting with insomnia complaints, highlighting the symptom heterogeneity within insomnia disorder. Hopefully, these results provide an impetus for a symptom-based approach to the management of insomnia disorder. We intentionally selected self-reported variables for the profile analysis that have been recommended for the clinical evaluation of insomnia78 so that these results can easily translate to clinical practice. The vision is that the patient's profile from these clinical measures could guide clinical decision-making when treating insomnia. Undoubtedly though, before this approach can be translated into a model for interdisciplinary sleep clinics, further research is needed.
DISCLOSURE STATEMENT
Work for this study was performed at Rush University Medical Center, Chicago, Illinois. Research reported in this publication was supported by the National Heart Lung and Blood Institute (NHLBI) of the National Institutes of Health under award number R01HL114529 and by the National Center for Complementary and Integrative Health (NCCIH) of the National Institutes of Health under award number K23AT003678. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Jack Edinger has received research support from Merck and Philips Respironics; Jason Ong is a consultant for Big Health Inc. and receives royalties from APA Books. This article is not related to these relationships. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors acknowledge the contributions of the study coordinators and research assistants for both projects: David Sholtes, Christina Khou, Sarah Snyder, and Christine Smith. The study physicians M. Isabel Crisostomo, MD and Bantu Chhangani, MD provided assistance in the physical evaluations and reviewed the PSGs. Finally, we are grateful for the time and energy each participant sacrificed to help us successfully conduct these studies.
ABBREVIATIONS
- aBIC
sample-size adjusted Bayesian information criteria
- AHI
apnea-hypopnea index
- AIC
Akaike information criteria
- ALRT
adjusted likelihood ratio test
- BAS
Beliefs and Attitudes about Sleep Scale
- BIC
Bayesian information criteria
- BLRT
bootstrapped likelihood ratio test
- BMI
body mass index
- CI
confidence intervals
- DSM
Diagnostic and Statistical Manual of Mental Disorders
- EMA
early morning awakening
- ESS
Epworth Sleepiness Scale
- FSS
Fatigue Severity Scale
- GSES
Glasgow Sleep Effort Scale
- HSW
“High Subjective Wakefulness” cluster profile
- IRD
“Insomnia-Related Distress” cluster profile
- ISI
Insomnia Severity Index
- LL
log-likelihood
- LPA
latent profile analysis
- MI
“Mild Insomnia” cluster profile
- OR
odds ratio
- OSA
obstructive sleep apnea
- PSAS
Pre-Sleep Arousal Scale
- PSG
polysomnography
- PSGTST
total sleep time derived from polysomnography
- SDB
sleep-disordered breathing
- SE
sleep efficiency
- SOL
sleep onset latency
- TST
total sleep time
- WASO
wake after sleep onset
REFERENCES
- 1.Roth T. Insomnia: definition, prevalence, etiology, and consequences. J Clin Sleep Med. 2007;3(5):S7–S10. [PMC free article] [PubMed] [Google Scholar]
- 2.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 3.Uhlig BL, Sand T, Odegard SS, Hagen K. Prevalence and associated factors of DSM-V insomnia in Norway: the Nord-Trøndelag Health Study (HUNT 3) Sleep Med. 2014;15(6):708–713. doi: 10.1016/j.sleep.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 4.Chung KF, Yeung WF, Ho FY, Yung KP, Yu YM, Kwok CW. Cross-cultural and comparative epidemiology of insomnia: the Diagnostic and Statistical Manual (DSM), International Classification of Diseases (ICD) and International Classification of Sleep Disorders (ICSD) Sleep Med. 2015;16(4):477–482. doi: 10.1016/j.sleep.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 5.Benjamins JS, Migliorati F, Dekker K, et al. Insomnia heterogeneity: characteristics to consider for data-driven multivariate subtyping. Sleep Med Rev. doi: 10.1016/j.smrv.2016.10.005. doi: 10.1016/j.smrv.2016.10.005. In press. [DOI] [PubMed] [Google Scholar]
- 6.Millner LM, Strotman LN. The future of precision medicine in oncology. Clin Lab Med. 2016;36(3):557–573. doi: 10.1016/j.cll.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Ong JC, Crawford MR, Kong A, et al. Management of obstructive sleep apnea and comorbid insomnia: a mixed-methods evaluation. Behav Sleep Med. 2017;15(3):180–197. doi: 10.1080/15402002.2015.1087000. [DOI] [PubMed] [Google Scholar]
- 8.Edinger JD, Fins AI, Goeke JM, et al. The empirical identification of insomnia subtypes: a cluster analytic approach. Sleep. 1996;19(5):398–411. [PubMed] [Google Scholar]
- 9.Sánchez-Ortuño MM, Edinger JD, Wyatt JK. Daytime symptom patterns in insomnia sufferers: is there evidence for subtyping insomnia? J Sleep Res. 2011;20(3):425–433. doi: 10.1111/j.1365-2869.2010.00905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller CB, Bartlett DJ, Mullins AE, et al. Clusters of insomnia disorder: an exploratory cluster analysis of objective sleep parameters reveals differences in neurocognitive functioning, quantitative EEG, and heart rate variability. Sleep. 2016;39(11):1993–2004. doi: 10.5665/sleep.6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vallieres A, Ivers H, Bastien CH, Beaulieu-Bonneau S, Morin CM. Variability and predictability in sleep patterns of chronic insomniacs. J Sleep Res. 2005;14(4):447–453. doi: 10.1111/j.1365-2869.2005.00480.x. [DOI] [PubMed] [Google Scholar]
- 12.Montserrat Sánchez-Ortuño M, Edinger JD. A penny for your thoughts: patterns of sleep-related beliefs, insomnia symptoms and treatment outcome. Behav Res Ther. 2010;48(2):125–133. doi: 10.1016/j.brat.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edinger JD, Carney CE, Wohlgemuth WK. Pretherapy cognitive dispositions and treatment outcome in cognitive behavior therapy for insomnia. Behav Ther. 2008;39(4):406–416. doi: 10.1016/j.beth.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Ye L, Pien GW, Ratcliffe SJ, et al. The different clinical faces of obstructive sleep apnoea: a cluster analysis. Eur Respir J. 2014;44(6):1600–1607. doi: 10.1183/09031936.00032314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vavougios GD, George DG, Pastaka C, Zarogiannis SG, Gourgoulianis KI. Phenotypes of comorbidity in OSAS patients: combining categorical principal component analysis with cluster analysis. J Sleep Res. 2016;25(1):31–38. doi: 10.1111/jsr.12344. [DOI] [PubMed] [Google Scholar]
- 16.Vermunt JK, Magidson J. Latent class cluster analysis. In: Hagenaars JA, McCutcheon AL, editors. Applied Latent Class Analysis. Cambridge, UK: Cambridge University Press; 2002. pp. 89–106. [Google Scholar]
- 17.Green MJ, Espie CA, Benzeval M. Social class and gender patterning of insomnia symptoms and psychiatric distress: a 20-year prospective cohort study. BMC Psychiatry. 2014;14:152. doi: 10.1186/1471-244X-14-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foley KA, Sarsour K, Kalsekar A, Walsh JK. Subtypes of sleep disturbance: associations among symptoms, comorbidities, treatment, and medical costs. Behav Sleep Med. 2010;8(2):90–104. doi: 10.1080/15402001003622842. [DOI] [PubMed] [Google Scholar]
- 19.Buysse DJ. Sleep health: can we define it? Does it matter? Sleep. 2014;37(1):9–17. doi: 10.5665/sleep.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edinger J, Kirby A, Lineberger MD, Loiselle M, Wohlgemuth WK, Means MK. The Duke Structured Interview for Sleep Disorders. Durham, NC: Duke University Medical Center; 2004. [Google Scholar]
- 21.Lichstein KL, Durrence HH, Taylor DJ, Bush AJ, Riedel BW. Quantitative criteria for insomnia. Behav Res Ther. 2003;41(4):427–445. doi: 10.1016/s0005-7967(02)00023-2. [DOI] [PubMed] [Google Scholar]
- 22.First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV-TR Axis I Disorders. Hoboken, NJ: Biometrics Research, New York State Psychiatric Institute; 2002. Research Version. Non-Patient ed. [Google Scholar]
- 23.Ong JC, Manber R, Segal Z, Xia Y, Shapiro S, Wyatt JK. A randomized controlled trial of mindfulness meditation for chronic insomnia. Sleep. 2014;37(9):1553–1563. doi: 10.5665/sleep.4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crawford MR, Turner AD, Wyatt JK, Fogg LF, Ong JC. Evaluating the treatment of obstructive sleep apnea comorbid with insomnia disorder using an incomplete factorial design. Contemp Clin Trials. 2016;47:146–152. doi: 10.1016/j.cct.2015.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 2000. text rev. [Google Scholar]
- 26.American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 27.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 28.Morin CM. Insomnia: Psychological Assessment and Management. New York, NY: The Guilford Press; 1993. [Google Scholar]
- 29.Morin CM, Belleville G, Belanger L, Ivers H. The insomnia severity index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–608. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bastien CH, Vallieres A, Morin CM. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 31.Broomfield NM, Espie CA. Towards a valid, reliable measure of sleep effort. J Sleep Res. 2005;14(4):401–407. doi: 10.1111/j.1365-2869.2005.00481.x. [DOI] [PubMed] [Google Scholar]
- 32.Morin CM, Stone J, Trinkle D, Mercer J, Remsberg S. Dysfunctional beliefs and attitudes about sleep among older adults with and without insomnia complaints. Psychol Aging. 1993;8(3):463–467. doi: 10.1037//0882-7974.8.3.463. [DOI] [PubMed] [Google Scholar]
- 33.Morin CM, Vallieres A, Ivers H. Dysfunctional beliefs and attitudes about sleep (DBAS): validation of a brief version (DBAS-16) Sleep. 2007;30(11):1547–1554. doi: 10.1093/sleep/30.11.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicassio PM, Mendlowitz DR, Fussell JJ, Petras L. The phenomenology of the pre-sleep state: the development of the pre-sleep arousal scale. Behav Res Ther. 1985;23(3):263–271. doi: 10.1016/0005-7967(85)90004-x. [DOI] [PubMed] [Google Scholar]
- 35.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46(10):1121–1123. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 36.Carney CE, Buysse DJ, Ancoli-Israel S, et al. The consensus sleep diary: standardizing prospective sleep self-monitoring. Sleep. 2012;35(2):287–302. doi: 10.5665/sleep.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iber C, Ancoli-Israel S, Chesson AL, Quan SF for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 38.Vgontzas AN, Liao D, Bixler EO, Chrousos GP, Vela-Bueno A. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep. 2009;32(4):491–497. doi: 10.1093/sleep/32.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, Bixler EO. Insomnia with objective short sleep duration is associated with type 2 diabetes: a population-based study. Diabetes Care. 2009;32(11):1980–1985. doi: 10.2337/dc09-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vgontzas AN, Liao D, Pejovic S, et al. Insomnia with short sleep duration and mortality: the Penn State cohort. Sleep. 2010;33(9):1159–1164. doi: 10.1093/sleep/33.9.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vgontzas AN, Fernandez-Mendoza J, Bixler EO, et al. Persistent insomnia: the role of objective short sleep duration and mental health. Sleep. 2012;35(1):61–68. doi: 10.5665/sleep.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bathgate CJ, Edinger JD, Wyatt JK, Krystal AD. Objective but not subjective short sleep duration associated with increased risk for hypertension in individuals with insomnia. Sleep. 2016;39(5):1037–1045. doi: 10.5665/sleep.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muthen B. Latent variable mixture modeling. In: Marcoulides GA, Schumacker RE, editors. New Developments and Techniques in Structural Equation Modeling. Mahwah, NJ: Lawrence Erlbaum Associates; 2009. pp. 1–33. [Google Scholar]
- 44.Boschloo L, van Borkulo CD, Rhemtulla M, Keyes KM, Borsboom D, Schoevers RA. The network structure of symptoms of the diagnostic and statistical manual of mental disorders. PLoS One. 2015;10(9):e0137621. doi: 10.1371/journal.pone.0137621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim KW, Kang SH, Yoon IY, et al. Prevalence and clinical characteristics of insomnia and its subtypes in the Korean elderly. Arch Gerontol Geriatr. 2017;68:68–75. doi: 10.1016/j.archger.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 46.Krystal AD, Edinger JD, Wohlgemuth WK, Marsh GR. NREM sleep EEG frequency spectral correlates of sleep complaints in primary insomnia subtypes. Sleep. 2002;25(6):630–640. [PubMed] [Google Scholar]
- 47.Borkovec TD, Grayson JB, O'Brien GT, Weerts TC. Relaxation treatment of pseudoinsomnia and idiopathic insomnia: an electroencephalographic evaluation. J Appl Behav Anal. 1979;12(1):37–54. doi: 10.1901/jaba.1979.12-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Espie CA, Barrie LM, Forgan GS. Comparative investigation of the psychophysiologic and idiopathic insomnia disorder phenotypes: psychologic characteristics, patients' perspectives, and implications for clinical management. Sleep. 2012;35(3):385–393. doi: 10.5665/sleep.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krystal AD, Edinger JD, Wohlgemuth WK, Marsh GR. The effects of behavioral therapy on Non-REM sleep EEG spectral amplitude in primary insomnia subtypes. Poster presented at: 15th Annual Meeting of the Associated Professional Sleep Societies; June 5-10, 2001; Chicago, IL. [Google Scholar]
- 50.Pillai V, Roth T, Drake CL. The nature of stable insomnia phenotypes. Sleep. 2015;38(1):127–138. doi: 10.5665/sleep.4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Espie CA, Kyle SD, Hames P, Cyhlarova E, Benzeval M. The daytime impact of DSM-5 insomnia disorder: comparative analysis of insomnia subtypes from the great British sleep survey. J Clin Psychiatry. 2012;73(12):e1478–e1484. doi: 10.4088/JCP.12m07954. [DOI] [PubMed] [Google Scholar]
- 52.Hara C, Stewart R, Lima-Costa MF, et al. Insomnia subtypes and their relationship to excessive daytime sleepiness in Brazilian community-dwelling older adults. Sleep. 2011;34(8):1111–1117. doi: 10.5665/SLEEP.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yokoyama E, Kaneita Y, Saito Y, et al. Association between depression and insomnia subtypes: a longitudinal study on the elderly in Japan. Sleep. 2010;33(12):1693–1702. doi: 10.1093/sleep/33.12.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Palagini L, Faraguna U, Mauri M, Gronchi A, Morin CM, Riemann D. Association between stress-related sleep reactivity and cognitive processes in insomnia disorder and insomnia subgroups: preliminary results. Sleep Med. 2016;19:101–107. doi: 10.1016/j.sleep.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 55.Suh S, Ong JC, Steidtmann D, et al. Cognitions and insomnia subgroups. Cognit Ther Res. 2012;36(2):120–128. doi: 10.1007/s10608-011-9415-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walsh JK, Coulouvrat C, Hajak G, et al. Nighttime insomnia symptoms and perceived health in the America Insomnia Survey (AIS) Sleep. 2011;34(8):997–1011. doi: 10.5665/SLEEP.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bjornsdottir E, Janson C, Sigurdsson JF, et al. Symptoms of insomnia among OSA patients before and after 2 years of PAP treatment. Sleep. 2013;36(12):1901–1909. doi: 10.5665/sleep.3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Waters WF, Hurry MJ, Binks PG, et al. Behavioral and hypnotic treatments for insomnia subtypes. Behav Sleep Med. 2003;1(2):81–101. doi: 10.1207/S15402010BSM0102_2. [DOI] [PubMed] [Google Scholar]
- 59.Fernandez-Mendoza J, Calhoun S, Bixler EO, et al. Insomnia with objective short sleep duration is associated with deficits in neuropsychological performance: a general population study. Sleep. 2010;33(4):459–465. doi: 10.1093/sleep/33.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gaines J, Vgontzas AN, Fernandez-Mendoza J, et al. Short- and long-term sleep stability in insomniacs and healthy controls. Sleep. 2015;38(11):1727–1734. doi: 10.5665/sleep.5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bliwise DL, Scullin MK. Normal aging. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 6th ed. Philadelphia, PA: Elsevier; 2016. p. 25. [Google Scholar]
- 62.Kushida CA, Littner MR, Morgenthaler T, et al. Practice parameters for the indications for polysomnography and related procedures: an update for 2005. Sleep. 2005;28(4):499–521. doi: 10.1093/sleep/28.4.499. [DOI] [PubMed] [Google Scholar]
- 63.Krakow B, Romero E, Ulibarri VA, Kikta S. Prospective assessment of nocturnal awakenings in a case series of treatment-seeking chronic insomnia patients: a pilot study of subjective and objective causes. Sleep. 2012;35(12):1685–1692. doi: 10.5665/sleep.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fung CH, Martin JL, Dzierzewski JM, et al. Prevalence and symptoms of occult sleep disordered breathing among older veterans with insomnia. J Clin Sleep Med. 2013;9(11):1173–1178. doi: 10.5664/jcsm.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Glidewell RN, Roby EK, Orr WC. Is insomnia an independent predictor of obstructive sleep apnea? J Am Board Fam Med. 2012;25(1):104–110. doi: 10.3122/jabfm.2012.01.110123. [DOI] [PubMed] [Google Scholar]
- 66.Cronlein T, Geisler P, Langguth B, et al. Polysomnography reveals unexpectedly high rates of organic sleep disorders in patients with prediagnosed primary insomnia. Sleep Breath. 2012;16(4):1097–1103. doi: 10.1007/s11325-011-0608-8. [DOI] [PubMed] [Google Scholar]
- 67.Lichstein KL, Riedel BW, Lester KW, Aguillard RN. Occult sleep apnea in a recruited sample of older adults with insomnia. J Consult Clin Psychol. 1999;67(3):405–410. doi: 10.1037//0022-006x.67.3.405. [DOI] [PubMed] [Google Scholar]
- 68.Troxel WM, Germain A, Buysse DJ. Clinical management of insomnia with brief behavioral treatment (BBTI) Behav Sleep Med. 2012;10(4):266–279. doi: 10.1080/15402002.2011.607200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Germain A, Moul DE, Franzen PL, et al. Effects of a brief behavioral treatment for late-life insomnia: preliminary findings. J Clin Sleep Med. 2006;2(4):403–406. [PubMed] [Google Scholar]
- 70.Buysse DJ, Germain A, Moul DE, et al. Efficacy of brief behavioral treatment for chronic insomnia in older adults. Arch Intern Med. 2011;171(10):887–895. doi: 10.1001/archinternmed.2010.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chambers MJ, Keller B. Alert insomniacs: are they really sleep deprived? Clin Psychol Rev. 1993;13(7):649–666. [Google Scholar]
- 72.Bailes S, Libman E, Baltzan M, Amsel R, Schondorf R, Fichten CS. Brief and distinct empirical sleepiness and fatigue scales. J Psychosom Res. 2006;60(6):605–613. doi: 10.1016/j.jpsychores.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 73.Vgontzas AN, Lin HM, Papaliaga M, et al. Short sleep duration and obesity: the role of emotional stress and sleep disturbances. Int J Obes. 2008;32(5):801–809. doi: 10.1038/ijo.2008.4. [DOI] [PubMed] [Google Scholar]
- 74.Kristenssson E, Sundqvist M, Astin M, et al. Acute psychological stress raises plasma ghrelin in the rat. Regul Pept. 2006;134(2-3):114–117. doi: 10.1016/j.regpep.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 75.Miaskowski C, Aouizerat BE, Dodd M, Cooper B. Conceptual issues in symptom clusters research and their implications for quality-of-life assessment in patients with cancer. J Natl Cancer Inst Monogr. 2007;(37):39–46. doi: 10.1093/jncimonographs/lgm003. [DOI] [PubMed] [Google Scholar]
- 76.Howard R, Rattray M, Prosperi M, Custovic A. Distinguishing asthma phenotypes using machine learning approaches. Curr Allergy Asthma Rep. 2015;15(7):38. doi: 10.1007/s11882-015-0542-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Marquand AF, Wolfers T, Mennes M, Buitelaar J, Beckmann CF. Beyond lumping and splitting: a review of computational approaches for stratifying psychiatric disorders. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1(5):433–447. doi: 10.1016/j.bpsc.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Buysse DJ, Ancoli-Israel S, Edinger JD, Lichstein KL, Morin CM. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29(9):1155–1173. doi: 10.1093/sleep/29.9.1155. [DOI] [PubMed] [Google Scholar]