Abstract
Idiopathic pulmonary fibrosis (IPF) is a devastating disease with few available treatment options. Recently, the involvement of purinergic receptor subtypes in the pathogenesis of different lung diseases has been demonstrated. Here we investigated the role of the purinergic receptor subtype P2Y2 in the context of fibrotic lung diseases.
The concentration of different nucleotides was measured in the broncho-alveolar lavage (BAL) fluid derived from IPF patients and animals with bleomycin-induced pulmonary fibrosis. In addition expression of P2Y2 receptors by different cell types was determined. To investigate the functional relevance of P2Y2 receptors for the pathogenesis of the disease the bleomycin model of pulmonary fibrosis was used. Finally, experiments were performed in pursuit of the involved mechanisms.
Compared to healthy individuals or vehicle treated animals, extracellular nucleotide levels in the BAL fluid were increased in patients with IPF and in mice after bleomycin administration, paralleled by a functional up-regulation of P2Y2R expression. Both bleomycin-induced inflammation and fibrosis were reduced in P2Y2R-deficient compared to wild type animals. Mechanistic studies demonstrated that recruitment of neutrophils into the lungs, proliferation and migration of lung fibroblasts as well as IL6 production are key P2Y2R mediated processes.
Our results clearly demonstrate the involvement of P2Y2R subtypes in the pathogenesis of fibrotic lung diseases in humans and mice and hence support the development of selective P2Y2R antagonists for the treatment of IPF.
Keywords: ATP, chemotaxis, neutrophils, nucleotides, pulmonary fibrosis
INTRODUCTION
Although some progress has been made recently idiopathic pulmonary fibrosis (IPF) is still a devastating disease with few available treatment options, at least partly due to our lack of knowledge about IPF pathophysiology. Currently, it is supposed that the fibrotic response is driven by mediators secreted by abnormally activated alveolar epithelial cells type II after epithelial cell injury. The excessive deposition of collagen and other components of the extracellular matrix eventually results in scarring and destruction of the lung architecture [1–3].
Nucleotides such as adenosine-5′-triphosphate (ATP), uridine-5′-triphosphate (UTP) or uridine-5′-diphosphate (UDP) are released into the extracellular space following tissue injury. Consecutively, elevated levels of extracellular nucleotides have been measured under inflammatory or hypoxic conditions [4–6]. The effects of extracellular nucleotides are mediated via P2 purinergic receptors (P2Rs) which can be subdivided into metabotropic P2Y (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11-P2Y14) and ionotropic P2X receptors (P2X1-P2X7) [7]. Expression of P2R has been shown on both immune and lung structural cells [5, 8]. Activation of P2Rs is associated with different cellular responses, including migration, release of cytokines or growth factors, production of reactive oxygen species, or apoptosis [7]. In addition, the involvement of specific P2R subtypes in the pathophysiology of different lung disorders has been shown extensively [4, 6, 8–10]. Recently, it has been demonstrated that ATP is released into the extracellular space upon bleomycin administration and that P2X7R-deficiency is associated with reduced pulmonary inflammation and fibrosis in different animal models [11, 12]. However, due to the above mentioned widespread expression of purinergic receptors the involvement of more than one receptor subtype is likely.
Functional expression of P2Y2 receptors, which are preferably activated by ATP or UTP, has been observed on different inflammatory cells including neutrophils, eosinophils, alveolar macrophages, and dendritic cells [13–16]. Previous studies demonstrated the involvement of this receptor subtype in allergic airway inflammation and chronic obstructive pulmonary disease [6, 16]. However, the precise role of P2Y2 receptors in the context of fibrotic lung disease has not been investigated yet.
RESULTS
Increased extracellular ATP levels in idiopathic pulmonary fibrosis
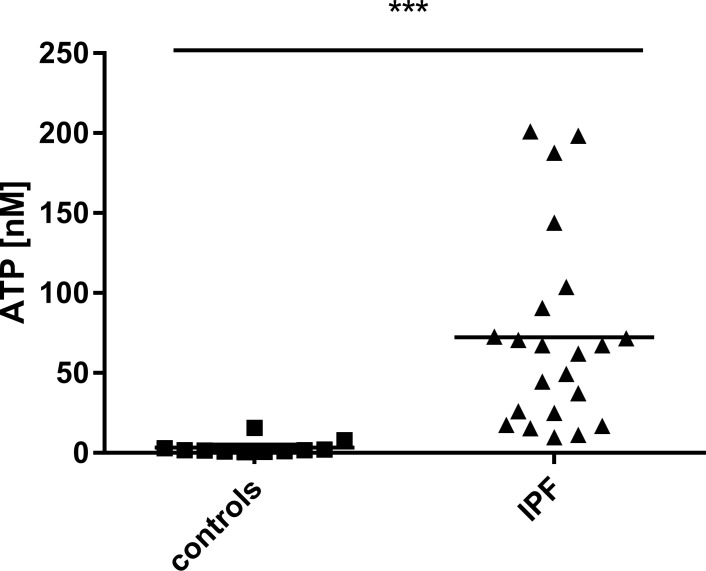
Broncho-alveolar lavage (BAL) fluid obtained during bronchoscopy was used to measure extracellular ATP levels. As shown in Figure 1, ATP levels in the BAL fluid derived from IPF patients were significantly increased compared to healthy subjects (for patient characteristics see Table 1).
Figure 1. Elevated ATP levels in patients suffering from pulmonary fibrosis.
ATP levels in BAL fluid obtained during bronchoscopy from patients with IPF (n = 22) and healthy volunteers (n = 11) were determined using a luminometric assay. ***p < 0.001.
Table 1. Patient characteristics.
| FEV1 | TLC | DLCOc | Age | sex | treatment for ILD | |
|---|---|---|---|---|---|---|
| 1 | 82 | 65 | 43 | 71 | m | no |
| 2 | 54 | 56 | 31 | 49 | m | no |
| 3 | 77 | 58 | 36 | 89 | m | no |
| 4 | 55 | 69 | N/A | 84 | m | no |
| 5 | 94.2 | 82 | 94.5 | 61 | m | no |
| 6 | 72.9 | 76.4 | 61.8 | 73 | f | no |
| 7 | 55 | 75 | N/A | 55 | m | no |
| 8 | 76.5 | 68.3 | 49.7 | 82 | m | no |
| 9 | 88 | 66 | 50 | 80 | m | prednisone |
| 10 | 54.7 | 62.5 | 17.7 | 51 | m | no |
| 11 | 39.4 | 35.1 | 9 | 59 | m | prednisone |
| 12 | 86.1 | 75.3 | N/A | 86 | m | no |
| 13 | 56.2 | 58.2 | 33.1 | 75 | m | prednisone |
| 14 | 51.9 | 61.5 | 52.5 | 55 | m | prednisone |
| 15 | 65.9 | 55.5 | 35.5 | 73 | f | study drug |
| 16 | 50.4 | 44.1 | 31.6 | 61 | m | Prednisone study drug |
| 17 | 43 | 62.5 | 47.8 | 63 | m | no |
| 18 | 59.9 | 66.5 | 29.8 | 58 | m | prednisone |
| 19 | 41.3 | 48.8 | N/A | 65 | f | prednisone azathioprine |
| 20 | 53.1 | 65.7 | 39.7 | 73 | f | no |
| 21 | 68.7 | 83.3 | 31.7 | 88 | f | no |
| 22 | 33.7 | 37.3 | 17.2 | 59 | m | prednisone cyclophosphamide |
Functional up-regulation of P2Y2R subtypes in idiopathic pulmonary fibrosis
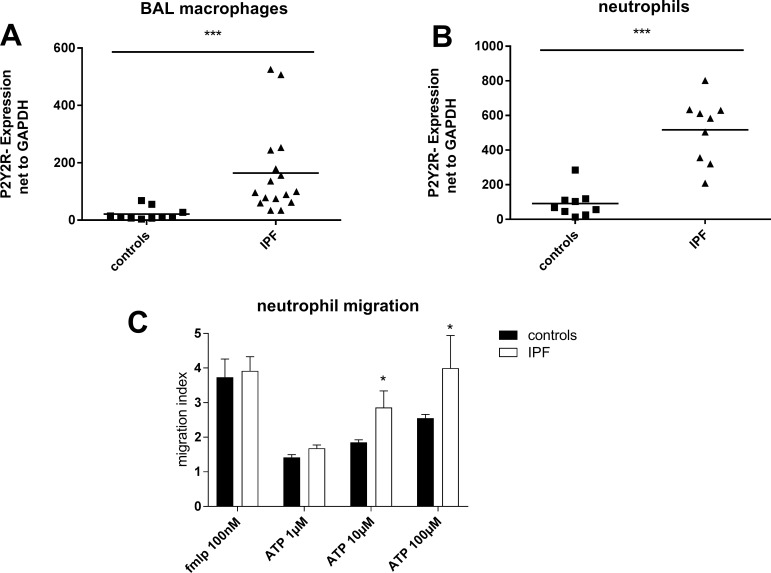
A strong up-regulation of P2Y2R expression on BAL fluid macrophages and blood neutrophils derived from IPF patients compared to cells isolated from healthy individuals was observed (Figure 2A, 2B). ATP is known as a strong chemoattractant for neutrophils via P2Y2 receptors [17]. Hence, the functional relevance of increased P2Y2R expression was investigated by migration assay. The chemotaxis towards ATP of neutrophils isolated from IPF patients was stronger compared to neutrophils isolated from healthy individuals (Figure 2C). In line with this finding, ATP levels correlated with the number of leukocytes in the BAL fluid (r = 0.70; p = 0.03; data not shown).
Figure 2. Up-regulation of P2Y2R expression in idiopathic pulmonary fibrosis.
(A) Expression of P2Y2 receptor subtypes on BAL cells derived from IPF patients (n = 16) and healthy individuals (n = 10) was determined by quantitative RT-PCR. (B) Neutrophils were isolated from whole blood of IPF patients (n = 9) and healthy individuals (n = 9) and P2Y2R expression was measured by quantitative RT-PCR. (C) Neutrophils were isolated from whole blood derived from IPF patients (n = 5) or healthy volunteers (n = 6). Migration in response to ATP or fMLP as a positive control was assessed using the Boyden chamber. *p < 0.05; ***p < 0.001.
Increased extracellular nucleotide levels and P2Y2R expression in experimental lung fibrosis
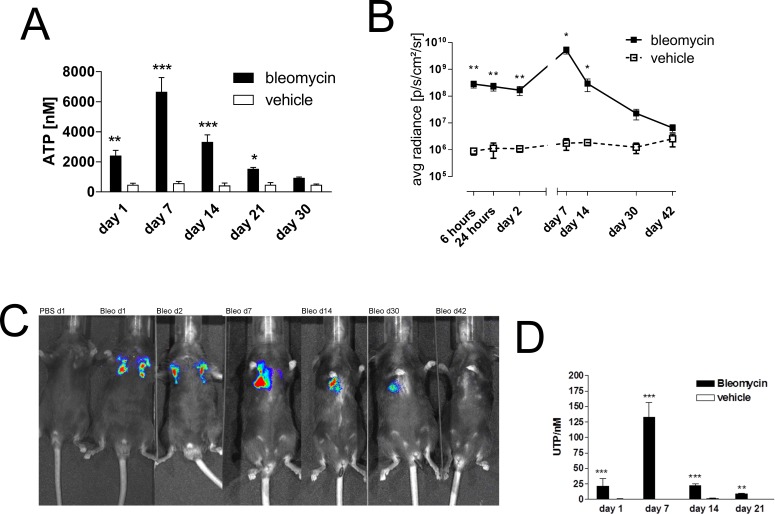
To confirm the pathophysiological relevance of these results in vivo we switched to the well characterized animal model of bleomycin-induced pulmonary fibrosis [18]. Extracellular ATP was significantly increased in the BAL fluid following bleomycin administration and maximal ATP concentration was observed at day 7 with a decline at later time points (Figure 3A), extracellular ATP was significantly increased in the BAL fluid following bleomycin administration and maximal ATP concentration was observed at day 7 with a decline at later time points (Figure 3A). Similar results were obtained when ATP release was visualized in vivo by ATP dependent luciferin-induced bioluminescence from HEK293-pmeLUC cells (Figure 3B, 3C) [19]. In addition, BAL fluid UTP levels were also increased in a time-dependent manner after intratracheal bleomycin administration (Figure 3D).
Figure 3. Elevated intrapulmonary nucleotide levels following bleomycin exposure in mice.
(A) Male C57Bl/6 mice received an i. t. injection of BLM or vehicle on day 0. Animals were killed at the indicated time points and ATP levels were measured (n = 4–5 per group). (B–C) Mice received ATP-dependent luciferase-expressing HEK293 cells, followed by an i. t. injection of BLM or vehicle on day 0. Bioluminescence was quantified at the indicated time points (n = 3–6 per group). (D) Male C57Bl/6 mice received an i. t. injection of BLM or vehicle on day 0. Animals were killed at the indicated time points and UTP levels were measured (n = 4–5 per group). *p < 0.05; **p < 0.01; ***p < 0.001.
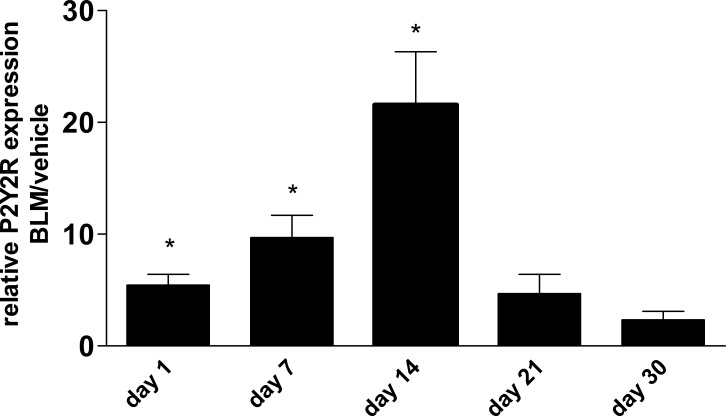
Apart from extracellular nucleotide levels, there was also an up-regulation of P2Y2R expression in whole lung tissue after bleomycin instillation followed by a decline at later time points (Figure 4).
Figure 4. P2Y2R expression in bleomycin induced pulmonary fibrosis.
Male C57Bl/6 mice received an i. t. injection of BLM or vehicle on day 0. Animals were killed at the indicated time points and P2R expression in lung tissue was analysed by quantitative RT-PCR (n = 3–5 per group). *p < 0.05.
P2Y2R-deficiency is associated with reduced inflammation and fibrosis after bleomycin administration
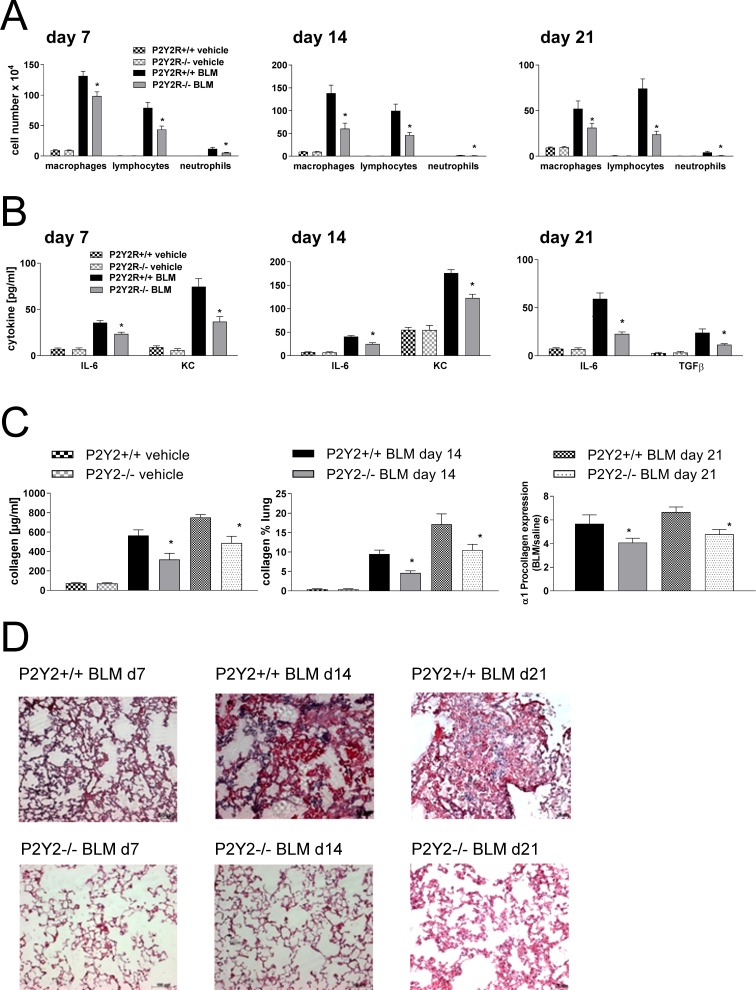
We next questioned whether P2Y2R-deficiency could alter the course of fibrotic lung disease in vivo. Therefore, P2Y2R-deficient or wild type animals received an intratracheal injection of bleomycin or vehicle as a negative control at day 0. The degree of inflammation and fibrosis was determined at different time points (day 7, day 14 and day 21 after bleomycin). As shown in Figure 5, pulmonary inflammation was reduced in P2Y2R-deficient animals compared to wild type animals at all time points demonstrated by a decreased number of inflammatory cells in the BAL fluid (Figure 5A), decreased concentration of the pro-inflammatory cytokines keratinocyte-derived chemokine (KC) and IL-6 (Figure 5B), and by reduced tissue infiltration by inflammatory cells (Figure 5D). Reduced fibrotic tissue remodelling was confirmed by reduced collagen content in the BAL fluid and on histological lung slides (Figure 5C). Furthermore, BAL fluid levels of the pro-fibrotic cytokine TGF-β (Figure 5B, right panel) and α1 pro-collagen expression (Figure 5C) were significantly lower in P2Y2R-deficient animals.
Figure 5. P2Y2R-deficient animals are partially protected from bleomycin induced lung injury and fibrosis.
P2Y2R-deficient or wild type mice received an i. t. injection of BLM or vehicle on day 0 and were killed 7, 14 or 21 days later. (A) Total and differential cell counts of the BALF were performed. (B) BALF cytokines were measured by ELISA. (C) Collagen contents were quantified in the BAL fluid by Sircol assay (left) and on histological lung slides (middle). α1 procollagen expression was determined by quantitative real time PCR (right). (D) Histological staining of lung slides (H&E). (n = 4–14 per group). *p < 0.05 compared to BLM-treated wt animals at the same time point.
P2Y2R activation is associated with multiple pro-inflammatory and pro-fibrotic effects
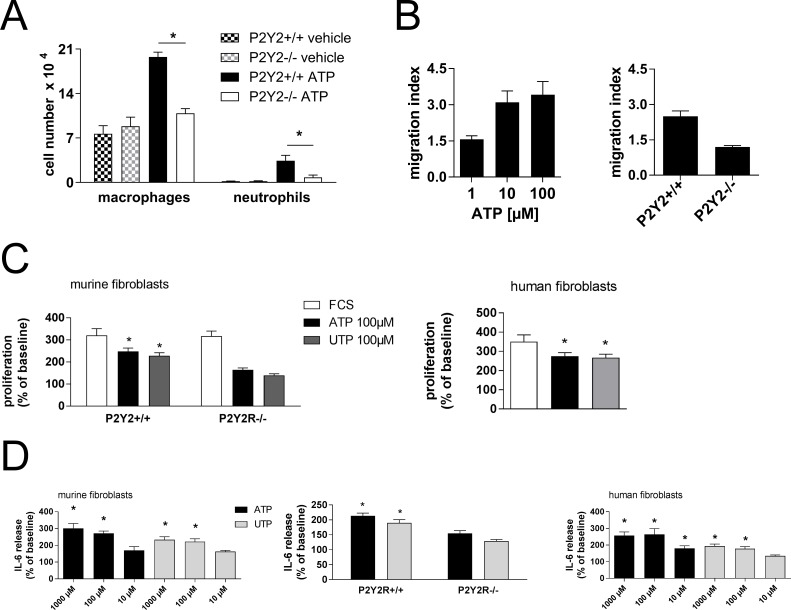
Recruitment of neutrophils into the lungs has been demonstrated to play a pivotal role in the pathogenesis of fibrotic lung disease [20]. Naïve wild type and P2Y2R-deficient animals received an intratracheal injection of ATP and the number of inflammatory cells in the BAL fluid was assessed after 24 h. As shown in Figure 6A, ATP increased the numbers of neutrophils and macrophages in the lungs of wild type animals whereas no changes were seen in P2Y2R-deficient mice.
Figure 6. Mechanisms in P2Y2R-induced inflammation and fibrosis.
(A) Wild type or P2Y2R-deficient (P2Y2R−/−) animals received an i. t. injection of ATP. BAL was performed after 24 h, and the number of BALF neutrophils and macrophages was determined. *p < 0.05 compared to ATP-treated wild type animals. (B) Primary lung fibroblasts were generated from wild type or P2Y2R-deficient animals. Migration towards ATP (100 μM) was analysed via Boyden chamber (n = 3 per group). (C) Proliferation rate of primary lung murine fibroblasts derived from wild type or P2Y2R-deficient animals (left) or human fibroblasts (right) after stimulation with ATP or UTP. (n = 3–5 per group). *p < 0.05 compared to vehicle stimulated cells. (D) IL6 release of primary murine lung fibroblasts (left and middle) or human lung fibroblasts (right) stimulated with different nucleotides was measured by ELISA (n = 3–7 per group). *p < 0.05 compared to vehicle-stimulated fibroblasts.
Increased migration of lung fibroblasts to the site of tissue injury is essential for the development of pulmonary fibrosis [1]. ATP induced migration of fibroblasts isolated from wild type but not from P2Y2R-deficient animals (Figure 6B).
Proliferation of lung fibroblasts is also crucial for the pathogenesis of pulmonary fibrosis [1]. ATP and UTP increased the proliferation rate of both primary murine and human lung fibroblasts (Figure 6C). The involvement of P2Y2R subtypes was confirmed by experiments with fibroblasts derived from P2Y2R-deficient animals (Figure 6C, left).
The importance of the cytokine IL6 in the context of tissue remodelling has been demonstrated previously [21]. Stimulation of both murine and human lung fibroblasts with ATP or UTP resulted in a dose-dependent increase in IL6 production (Figure 6D). The increase in IL6 secretion was weaker but not absent in fibroblasts derived from P2Y2R-deficient animals (Figure 6D, middle).
DISCUSSION
Signalling via purinergic receptors, e. g. P2Y2R subtypes, has been demonstrated to play an important role in the pathogenesis of various inflammatory disorders including bronchial asthma and COPD [7]. In this manuscript we investigated the involvement of extracellular nucleotides and the purinergic receptor P2Y2 in the pathogenesis of idiopathic pulmonary fibrosis in humans and in the animal model of bleomycin induced lung fibrosis.
We found elevated ATP concentrations in the BAL fluid in patients suffering from IPF compared to healthy controls. Additionally, a time dependent increase in BAL fluid ATP and UTP levels, peaking at day 7 after bleomycin administration in mice was observed. These data are in contrast to a previous study claiming that BAL fluid ATP levels are only increased very early (day 1) after bleomycin application in mice [11]. A possible explanation might be that Riteau and co-workers used a higher dosage of bleomycin [11]. Nevertheless, we think that our animal model with a chronic increase in extracellular ATP is much closer to the situation in humans with chronically elevated BAL fluid ATP levels in IPF patients.
Bleomycin-induced pulmonary fibrosis is known to be self-limiting with maximal fibrotic response around day 14 and a resolution of the fibrotic changes at later time points [18]. Hence, higher nucleotide levels in the extracellular space are paralleled by increased inflammation and fibrosis, in accordance with previous studies demonstrating increased extracellular ATP levels in inflammatory lung diseases such as bronchial asthma or COPD [4–6, 22]. Thereby, ATP release can occur via lytic and non-lytic pathways, e. g. via pannexins or connexin hemichannels or via exocytosis [7].
Apart from elevated extracellular nucleotide levels, an increased expression of P2Y2 receptors on cells derived from IPF patients and in the lung tissue of animals with bleomycin induced pulmonary fibrosis was observed. Of note in the animal model, maximal P2Y2R expression was found around day 14 when the maximal fibrotic response can be expected. The functional relevance of increased P2Y2 receptor expression on blood neutrophils was demonstrated by increased chemotaxis in response to ATP. In line with previous studies, the functional relevance was further supported by the finding that the ATP-induced recruitment of neutrophils into the lungs was highly dependent on P2Y2 receptor expression [6, 17].
P2Y2R-deficient animals were found to be partially protected from bleomycin-induced pulmonary inflammation and fibrosis. Whereas the involvement of P2Y2 receptors in the pathogenesis of different inflammatory disorders (e. g. bronchial asthma, COPD or hepatitis) has been demonstrated previously, much less is known about the role of this receptor subtype in tissue remodelling and fibrosis [6, 16, 23–25]. The effects following P2Y2R-activation can be attributed to different mechanisms. Intrapulmonary ATP levels were increased in both IPF patients and animals after intratracheal bleomycin administration paralleled by increased P2Y2R expression. Consecutively, given the fact that P2Y2 receptors are essential for nucleotide-induced migration of neutrophils, elevated ATP levels in the alveolar space might lead to an influx of blood neutrophils into the lungs during the initiation and progression of fibrotic lung disease [6, 17]. Though the exact role of neutrophils in the pathophysiology of IPF is still a matter of debate, the release of proteolytic enzymes, reactive oxygen species, and profibrotic cytokines by neutrophils can contribute to tissue destruction and fibrosis [26–28]. In accordance, an increased percentage of neutrophils in the BAL fluid has been shown to be a predictor of early mortality in IPF [29]. Activation, proliferation and migration of lung fibroblasts eventually lead to enhanced deposition of extracellular matrix components [28]. Our results clearly show that P2Y2R activation is associated with enhanced proliferation and migration of primary lung fibroblasts. Interestingly, nucleotides released upon tissue injury have been demonstrated to be involved in dermal wound healing and cardiac fibrosis via P2Y2R subtypes [24, 25]. Finally, P2Y2 receptor activation increased IL-6 secretion by lung fibroblasts, a cytokine linked with tissue remodelling and fibrosis apart from its well characterized role in acute inflammation [8, 21, 30].
However, it is very likely that even more mechanisms might be of importance in this context. Alveolar macrophages have been found to play a pivotal role in the pathogenesis of fibrotic lung diseases. Interestingly, P2Y2 receptor activation on this cell type has been shown to be associated with chemotaxis and secretion of pro-inflammatory cytokines [31–33].
In summary, we were able to demonstrate that the purinergic receptor subtype P2Y2R contributes to the pathogenesis of pulmonary fibrosis via different mechanisms including recruitment of neutrophils as well as migration, proliferation, and cytokine secretion of lung fibroblasts. Hence, targeting P2Y2R might be a new treatment option for idiopathic pulmonary fibrosis.
MATERIALS AND METHODS
Patient materials
Broncho-alveolar lavage (BAL) fluids were collected from patients undergoing bronchoscopy during the diagnostic workup of IPF or from healthy volunteers. IPF was diagnosed according to published criteria [34]. The study was approved by the local ethics committee (Ethikkommission des Universitätsklinikums Freiburg).
Animals
P2Y2R-deficient and wild type animals (both on C57BL/6 background) were bred at the University Freiburg. All experiments were approved by the local animal ethics committee (Regierungspraesidium Freiburg).
Bleomycin model of pulmonary fibrosis
Male C57BL/6 or P2Y2R-deficient animals were anaesthetized and received an i.t. injection of bleomycin (80 μl; 0.5mg/ml). Animals were killed at different time points via i. p. injection of pentobarbital (5 mg in 0.5 ml). BAL was performed with 3 × 1 ml of Ca2+ and Mg2+ free PBS supplemented with 0.1 mM sodium EDTA, followed by lung resection and storage in OCT freezing medium. BAL cells were counted, differential cell counts were done by FACS analysis, as described previously [35]. Frozen lung sections were stained with hematoxylin / eosin for histological analysis.
Mediator measurements in BALF
ATP levels in BALF were measured using ATPLite kit (Perkin Elmer), as previously described [4]. BALF cytokine contents were determined by ELISA (R&D Systems, Minneapolis, USA), as described by the manufacturer. BALF collagen content was measured by Sircol assay (Biocolor, Carrickfergus, U.K.).
Collagen quantification in histological lung slides
Frozen lung sections were incubated in picrosirius red solution (0, 2 gr of Picosirius Red diluted in 100 ml of 1, 2% picric acid, both Sigma-Aldrich), for one hour. After washing with water, tissue sections were stained with hematoxylin for 5–10 seconds. Slides were washed with running tap water and dehydrated in 70%, 90% and absolute ethanol, followed by xylene. Entellan (Merck) was used to mount the coverslip. Images were obtained using Axio Lab.A1 microscope (Zeiss) with 200 × magnification and AxioCam ICc1 (Zeiss). Collagen quantification was made with ImageJ.
PCR
Total RNA from human or murine lung tissue was isolated using RNeasy mini-kits (Qiagen, Hilden, Germany) according to the manufacturer's recommendations followed by reverse transcription using Stratascript reverse transcriptase (Stratagene, La Jolla, CA) and random primers (Invitrogen, Karlsruhe, Germany). Quantitative PCR was performed with Taqman Universal PCR Mastermix (Applied Biosystems, Foster City, CA) and pre-formulated primers and probe mixes (Assay on Demand; Applied Biosystems). PCR was performed on a thermal cycler (iCycler; Bio-Rad, Hercules, CA). PCR amplification of the housekeeping gene encoding glyceraldehyde 3-phosphate dehydrogenase was performed during each run for each sample to allow normalization between samples.
In vivo detection of extracellular ATP release
For this imaging, 2 × 106 cells with membrane-targeted luciferase (PME cells) were injected intravenously via tail vein and recipient mice were examined consecutively after bleomycin administration [19, 36].
In vitro migration of lung fibroblasts or blood neutrophils
Blood neutrophils from whole blood of control subjects or IPF patients as well as human and murine fibroblasts were isolated as described previously [5, 37].
Migration was investigated using transwell chambers (Corning, Lowell, USA). Nucleotides or vehicle were added into the lower compartment wells. Cells (5 × 104 cells/well) were added to the upper compartment and incubated at 37°C for 3 h min in a humidified atmosphere; cells in the lower compartment were counted. Results are shown as chemotactic index, calculated as the number of cells in the lower chamber containing the different stimuli divided by the number of cells in the chamber containing medium alone.
Proliferation of lung fibroblasts
Fibroblasts (2 × 105 cells/well) were seeded into cell culture plates and stimulated with the indicated nucleotides or vehicle. Higher concentrations of FCS (20%) were used as a positive control. After 48 h cells were trypsinized and counted. Results are shown as proliferation index, calculated as the number of nucleotide-stimulated cells divided by the number of vehicle treated cells.
Acknowledgments
The authors thank Kelly A Volcik for English language editing.
Abbreviations
- ATP
adenosine-5′-triphosphate
- BAL
broncho-alveolar lavage
- COPD
chronic obstructive pulmonary disease
- IL
interleukin
- IPF
idiopathic pulmonary fibrosis
- UTP
uridine-5′-triphosphate
Footnotes
Authors’ contributions
Experiments were planned, performed and analyzed by TM, SF, RPV, SC, TG and KA. HKQ, GZ and MI helped with the analysis of the experiments. The manuscript was written by TM, HKQ, GL, DF, FDV, BR, JMB, MB and MI.
CONFLICTS OF INTEREST
All authors declare that they have no competing interests.
FUNDING
This work was supported by grants from the German Research Foundation (DFG) to TM (grant ID MU 3194/2-1) and to MI (grant ID 7/4-1), and by a Boehringer Ingelheim Foundation 3-Plus program to MI.
REFERENCES
- 1.King TE, Jr, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011;378:1949–61. doi: 10.1016/S0140-6736(11)60052-4. [DOI] [PubMed] [Google Scholar]
- 2.King TE, Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, Gorina E, Hopkins PM, Kardatzke D, Lancaster L, Lederer DJ, Nathan SD, Pereira CA, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2083–92. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 3.Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, Cottin V, Flaherty KR, Hansell DM, Inoue Y, Kim DS, Kolb M, Nicholson AG, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2071–82. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 4.Idzko M, Hammad H, van Nimwegen M, Kool M, Willart MA, Muskens F, Hoogsteden HC, Luttmann W, Ferrari D, Di Virgilio F, Virchow JC, Jr, Lambrecht BN. Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nat Med. 2007;13:913–19. doi: 10.1038/nm1617. [DOI] [PubMed] [Google Scholar]
- 5.Lommatzsch M, Cicko S, Müller T, Lucattelli M, Bratke K, Stoll P, Grimm M, Dürk T, Zissel G, Ferrari D, Di Virgilio F, Sorichter S, Lungarella G, et al. Extracellular adenosine triphosphate and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;181:928–34. doi: 10.1164/rccm.200910-1506OC. [DOI] [PubMed] [Google Scholar]
- 6.Cicko S, Lucattelli M, Müller T, Lommatzsch M, De Cunto G, Cardini S, Sundas W, Grimm M, Zeiser R, Dürk T, Zissel G, Boeynaems JM, Sorichter S, et al. Purinergic receptor inhibition prevents the development of smoke-induced lung injury and emphysema. J Immunol. 2010;185:688–97. doi: 10.4049/jimmunol.0904042. [DOI] [PubMed] [Google Scholar]
- 7.Idzko M, Ferrari D, Eltzschig HK. Nucleotide signalling during inflammation. Nature. 2014;509:310–17. doi: 10.1038/nature13085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vieira RP, Müller T, Grimm M, von Gernler V, Vetter B, Dürk T, Cicko S, Ayata CK, Sorichter S, Robaye B, Zeiser R, Ferrari D, Kirschbaum A, et al. Purinergic receptor type 6 contributes to airway inflammation and remodeling in experimental allergic airway inflammation. Am J Respir Crit Care Med. 2011;184:215–23. doi: 10.1164/rccm.201011-1762OC. [DOI] [PubMed] [Google Scholar]
- 9.Müller T, Vieira RP, Grimm M, Dürk T, Cicko S, Zeiser R, Jakob T, Martin SF, Blumenthal B, Sorichter S, Ferrari D, Di Virgillio F, Idzko M. A potential role for P2×7R in allergic airway inflammation in mice and humans. Am J Respir Cell Mol Biol. 2011;44:456–64. doi: 10.1165/rcmb.2010-0129OC. [DOI] [PubMed] [Google Scholar]
- 10.Lucattelli M, Cicko S, Müller T, Lommatzsch M, De Cunto G, Cardini S, Sundas W, Grimm M, Zeiser R, Dürk T, Zissel G, Sorichter S, Ferrari D, et al. P2×7 receptor signaling in the pathogenesis of smoke-induced lung inflammation and emphysema. Am J Respir Cell Mol Biol. 2011;44:423–29. doi: 10.1165/rcmb.2010-0038OC. [DOI] [PubMed] [Google Scholar]
- 11.Riteau N, Gasse P, Fauconnier L, Gombault A, Couegnat M, Fick L, Kanellopoulos J, Quesniaux VF, Marchand-Adam S, Crestani B, Ryffel B, Couillin I. Extracellular ATP is a danger signal activating P2×7 receptor in lung inflammation and fibrosis. Am J Respir Crit Care Med. 2010;182:774–83. doi: 10.1164/rccm.201003-0359OC. [DOI] [PubMed] [Google Scholar]
- 12.Monção-Ribeiro LC, Faffe DS, Santana PT, Vieira FS, da Graça CL, Marques-da-Silva C, Machado MN, Caruso-Neves C, Zin WA, Borojevic R, Takiya CM, Coutinho-Silva R. P2×7 receptor modulates inflammatory and functional pulmonary changes induced by silica. PLoS One. 2014;9:e110185. doi: 10.1371/journal.pone.0110185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inoue Y, Chen Y, Hirsh MI, Yip L, Junger WG. A3 and P2Y2 receptors control the recruitment of neutrophils to the lungs in a mouse model of sepsis. Shock. 2008;30:173–77. doi: 10.1097/shk.0b013e318160dad4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Idzko M, Dichmann S, Ferrari D, Di Virgilio F, la Sala A, Girolomoni G, Panther E, Norgauer J. Nucleotides induce chemotaxis and actin polymerization in immature but not mature human dendritic cells via activation of pertussis toxin-sensitive P2y receptors. Blood. 2002;100:925–32. doi: 10.1182/blood.v100.3.925. [DOI] [PubMed] [Google Scholar]
- 15.Myrtek D, Müller T, Geyer V, Derr N, Ferrari D, Zissel G, Dürk T, Sorichter S, Luttmann W, Kuepper M, Norgauer J, Di Virgilio F, Virchow JC, Jr, Idzko M. Activation of human alveolar macrophages via P2 receptors: coupling to intracellular Ca2+ increases and cytokine secretion. J Immunol. 2008;181:2181–88. doi: 10.4049/jimmunol.181.3.2181. [DOI] [PubMed] [Google Scholar]
- 16.Müller T, Martin SF, Di Virgilio F, Boeynaems JM, Virchow JC, Idzko M. The purinergic receptor P2Y2 receptor mediates chemotaxis of dendritic cells and eosinophils in allergic lung inflammation. Allergy. 2010;65:1545–53. doi: 10.1111/j.1398-9995.2010.02426.x. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–95. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- 18.Moore BB, Hogaboam CM. Murine models of pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2008;294:L152–60. doi: 10.1152/ajplung.00313.2007. [DOI] [PubMed] [Google Scholar]
- 19.Pellegatti P, Raffaghello L, Bianchi G, Piccardi F, Pistoia V, Di Virgilio F. Increased level of extracellular ATP at tumor sites: in vivo imaging with plasma membrane luciferase. PLoS One. 2008;3:e2599. doi: 10.1371/journal.pone.0002599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bringardner BD, Baran CP, Eubank TD, Marsh CB. The role of inflammation in the pathogenesis of idiopathic pulmonary fibrosis. Antioxid Redox Signal. 2008;10:287–301. doi: 10.1089/ars.2007.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saito F, Tasaka S, Inoue K, Miyamoto K, Nakano Y, Ogawa Y, Yamada W, Shiraishi Y, Hasegawa N, Fujishima S, Takano H, Ishizaka A. Role of interleukin-6 in bleomycin-induced lung inflammatory changes in mice. Am J Respir Cell Mol Biol. 2008;38:566–71. doi: 10.1165/rcmb.2007-0299OC. [DOI] [PubMed] [Google Scholar]
- 22.Koeppen M, Di Virgilio F, Clambey ET, Eltzschig HK. Purinergic regulation of airway inflammation. Subcell Biochem. 2011;55:159–93. doi: 10.1007/978-94-007-1217-1_7. [DOI] [PubMed] [Google Scholar]
- 23.Ayata CK, Ganal SC, Hockenjos B, Willim K, Vieira RP, Grimm M, Robaye B, Boeynaems JM, Di Virgilio F, Pellegatti P, Diefenbach A, Idzko M, Hasselblatt P. Purinergic P2Y receptors promote neutrophil infiltration and hepatocyte death in mice with acute liver injury. Gastroenterology. 2012;143:1620–1629. doi: 10.1053/j.gastro.2012.08.049. e4. [DOI] [PubMed] [Google Scholar]
- 24.Lu D, Soleymani S, Madakshire R, Insel PA. ATP released from cardiac fibroblasts via connexin hemichannels activates profibrotic P2Y2 receptors. FASEB J. 2012;26:2580–91. doi: 10.1096/fj.12-204677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin H, Seo J, Eun SY, Joo YN, Park SW, Lee JH, Chang KC, Kim HJ. P2Y2 R activation by nucleotides promotes skin wound-healing process. Exp Dermatol. 2014;23:480–85. doi: 10.1111/exd.12440. [DOI] [PubMed] [Google Scholar]
- 26.Hunninghake GW, Gadek JE, Lawley TJ, Crystal RG. Mechanisms of neutrophil accumulation in the lungs of patients with idiopathic pulmonary fibrosis. J Clin Invest. 1981;68:259–69. doi: 10.1172/JCI110242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maher TM, Wells AU, Laurent GJ. Idiopathic pulmonary fibrosis: multiple causes and multiple mechanisms? Eur Respir J. 2007;30:835–39. doi: 10.1183/09031936.00069307. [DOI] [PubMed] [Google Scholar]
- 28.Bagnato G, Harari S. Cellular interactions in the pathogenesis of interstitial lung diseases. Eur Respir Rev. 2015;24:102–14. doi: 10.1183/09059180.00003214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kinder BW, Brown KK, Schwarz MI, Ix JH, Kervitsky A, King TE., Jr Baseline BAL neutrophilia predicts early mortality in idiopathic pulmonary fibrosis. Chest. 2008;133:226–32. doi: 10.1378/chest.07-1948. [DOI] [PubMed] [Google Scholar]
- 30.Grbic DM, Degagné E, Langlois C, Dupuis AA, Gendron FP. Intestinal inflammation increases the expression of the P2Y6 receptor on epithelial cells and the release of CXC chemokine ligand 8 by UDP. J Immunol. 2008;180:2659–68. doi: 10.4049/jimmunol.180.4.2659. [DOI] [PubMed] [Google Scholar]
- 31.Kronlage M, Song J, Sorokin L, Isfort K, Schwerdtle T, Leipziger J, Robaye B, Conley PB, Kim HC, Sargin S, Schön P, Schwab A, Hanley PJ. Autocrine purinergic receptor signaling is essential for macrophage chemotaxis. Sci Signal. 2010;3:ra55. doi: 10.1126/scisignal.2000588. [DOI] [PubMed] [Google Scholar]
- 32.Eun SY, Seo J, Park SW, Lee JH, Chang KC, Kim HJ. LPS potentiates nucleotide-induced inflammatory gene expression in macrophages via the upregulation of P2Y2 receptor. Int Immunopharmacol. 2014;18:270–76. doi: 10.1016/j.intimp.2013.11.026. [DOI] [PubMed] [Google Scholar]
- 33.Kawamura H, Kawamura T, Kanda Y, Kobayashi T, Abo T. Extracellular ATP-stimulated macrophages produce macrophage inflammatory protein-2 which is important for neutrophil migration. Immunology. 2012;136:448–58. doi: 10.1111/j.1365-2567.2012.03601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, Lynch DA, Ryu JH, Swigris JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cicko S, Grimm M, Ayata K, Beckert J, Meyer A, Hossfeld M, Zissel G, Idzko M, Müller T. Uridine supplementation exerts anti-inflammatory and anti-fibrotic effects in an animal model of pulmonary fibrosis. Respir Res. 2015;16:105. doi: 10.1186/s12931-015-0264-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilhelm K, Ganesan J, Müller T, Dürr C, Grimm M, Beilhack A, Krempl CD, Sorichter S, Gerlach UV, Jüttner E, Zerweck A, Gärtner F, Pellegatti P, et al. Graft-versus-host disease is enhanced by extracellular ATP activating P2×7R. Nat Med. 2010;16:1434–38. doi: 10.1038/nm.2242. [DOI] [PubMed] [Google Scholar]
- 37.Prasse A, Pechkovsky DV, Toews GB, Jungraithmayr W, Kollert F, Goldmann T, Vollmer E, Müller-Quernheim J, Zissel G. A vicious circle of alveolar macrophages and fibroblasts perpetuates pulmonary fibrosis via CCL18. Am J Respir Crit Care Med. 2006;173:781–92. doi: 10.1164/rccm.200509-1518OC. [DOI] [PubMed] [Google Scholar]