Figure 8. NMU signaling modulates the WNT receptor pathway in NMUR2-positive SKBR3 breast cancer cells.
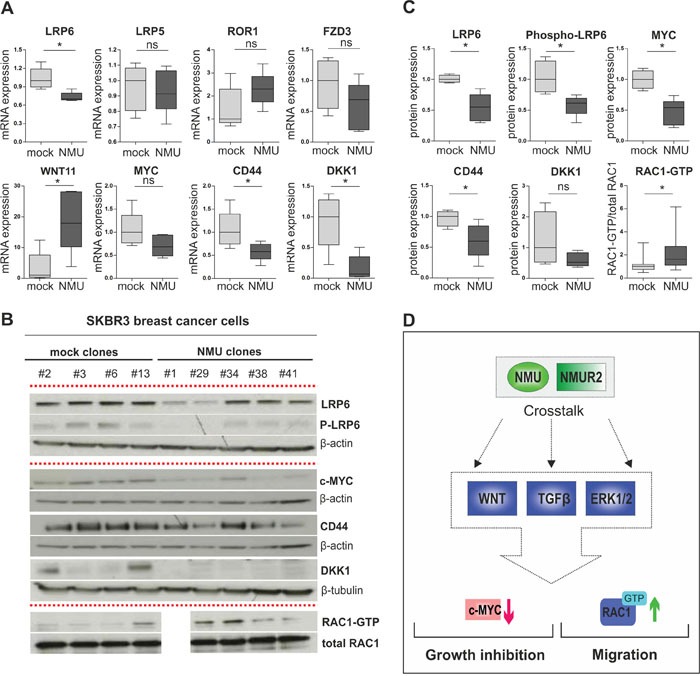
(A) Real-time PCR-based validation of candidate NMU downstream genes in independent stably transfected SKBR3 NMU (n=5) and mock clones (n=5). * P < 0.05; ns: not significant (Mann-Whitney-U test). (B) Representative western blots showing differential protein expression of candidate NMU downstream genes in independent stably transfected SKBR3 NMU and mock clones. Loading controls: β-actin, β-tubulin, total RAC1. All experiments were performed in triplicate. (C) Densitometrical evaluation of the western blot results shown in B depicted as box plots. Box plot showing RAC1-GTP in relation to total RAC1 amounts in SKBR3 NMU (n=4) and mock clones (n=4), combines data of three independent experiments. * P < 0.05; ns: not significant (Mann-Whitney-U test). (D) Hypothetical model of NMU's oncogenic role in dependency of NMUR2 in breast cancer: crosstalk of NMU signaling with WNT, TGFβ and ERK cascade results in decreased expression of the canonical WNT target MYC and enhanced activation of the non-canonical WNT/planar cell polarity (PCP) pathway effector RAC1 among others, contributing to growth inhibition and promotion of cell migration.
