Abstract
Purpose
Lung cancer (LC) is the leading cause of cancer-related deaths for both male and female worldwide. Early detection of LC could improve five-year survival rate up to 48.8% compared to 3.3% of late/distant stage. Autoantibodies to tumor-associated antigens (TAAs) have been described as being present before clinical symptoms in lung and other cancers. We aimed to identify more TAAs to improve the performance for discovering non-small cell lung cancer (NSCLC) patients from healthy individuals.
Methods
Two independent sets were included in this study. Serological proteome analysis (SERPA) was used to identify TAAs from NSCLC cell line H1299 in a discovery set. In validation study, anti-ENO1 autoantibody was examined by immunoassay in sera from 242 patients with NSCLC and 270 normal individuals.
Results
A 47 KDa protein was identified to be alpha-enolase (ENO1) by using SERPA. Analysis of sera from 512 participants by ELISA showed significantly higher frequency of anti-ENO1 autoantibodies in NSCLC sera compared with the sera from normal individuals, with AUC (95%CI) of 0.589 (0.539-0.638, P=0.001). There was no significant difference in frequency of anti-ENO1 in different stages, histological or metastasis status of NSCLC. When anti-ENO1 detection was combined with other two tumor protein biomarkers (CEA and CYFRA 21-1), the sensitivity of NSCLC increased to 84%.
Conclusions
ENO1 can elicit humoral immune response in NSCLC and its autoantibody has association with the tumorigenesis of NSCLC. Furthermore, these intriguing results suggest the possibility of autoantibody against ENO1 serving as a potential diagnostic biomarker in NSCLC and have implications for defining novel histological determinants of NSCLC.
Keywords: alpha-enolase (ENO1), non-small cell lung cancer (NSCLC), tumor associated-antigens (TAAs), autoantibody, serological proteome analysis (SERPA)
INTRODUCTION
Lung cancer (LC) is the leading cause of cancer-related deaths for both male and female worldwide. In 2015, it was estimated more than 220,000 would be diagnosed as LC and 158,000 would die from LC [1]. Until 2007, the five-year overall survival rate for LC patients is only 16% among all cancers [2]. Early detection of LC could improve five-year survival rate up to 48.8% compared to 3.3% of late/distant stage [3]. Currently, low dose spiral computed tomography (LDCT) is the limited approach to screen LC in at-risk individuals in early detection of LC [4, 5]. LDCT offers mortality benefit in high risk individuals [6], nevertheless, this technique has poor specificity, needs plenty of costs [7], requiring individuals to have unnecessary follow-up examinations and unnecessary surgery therapy [8]. Therefore, the identification and validation of a cost-effective early stage blood test to complement LDCT screening is essential. Serum biomarker detection, which possesses advantages such as easy operation, low cost, noninvasiveness, accessibility of samples, is a high-profile topic for detection of early LC [9]. An growing number of studies have demonstrated autoantibodies to tumor-associated antigens (TAAs), and these humoral autoimmune responses have been described as being present before clinical symptoms in lung and other cancers [10–13]. We have recently reported that elevated autoantibody levels could be detected in patients’ sera at least four years before the diagnosis of LC [13].
In the present study, we investigated the possibility of defining novel TAAs by serological proteome analysis (SERPA), which could improve the performance characteristics for discovering the NSCLC patients from the healthy individuals. To achieve this goal, we used a differential immunoproteomic strategy based on two-dimensional Gel Electrophoresis (2-DE) coupled with mass spectrometry to identify TAAs in whole cell lysates prepared from the non-small cell carcinoma (NSCLC) cell line H1299 and sera pools from NSCLC patients at early stage as well as normal human individuals. The identified TAAs were subsequently validated using NSCLC patients in different stages and normal individuals to determine whether they are specific markers to differentiate NSCLC from normal population.
RESULTS
A 47 KDa autoantigen was identified as ENO1 by serum autoantibody from patients with NSCLC at early stage
The objective of this study was to identify the specific autoantibodies and targeted antigens as biomarkers in lung cancer. An immunoproteomic approach known as SERPA has been well established in our lab to identify TAAs as biomarkers in cancers in our previous studies [14, 15]. The advantage of this method is based on the accurate identification of protein by mass spectrometry analysis. In initial study, 20 sera from patients with NSCLC at stage I and 20 matched normal individuals in discovery set were screened for the presence of autoantibodies against total protein extractions from NSCLC cell line H1299 by using Western blotting. Of interest, 5 of 20 (25%) NSCLC sera were observed containing antibodies against protein bands around 47 KDa. As shown in Figure 1A, no reactivity with the 47 KDa protein was detected in 20 normal human sera. Subsequently, proteins extracted from H1299 cells were separated by 2-DE (Figure 1B) and transferred onto the nitrocellulose membranes. Figure 1C shows two positive protein spots around 47 KDa after reacted with sera from NSCLC patients, while no corresponding spots were observed in Western blotting results for normal sera (Figure 1D). These two spots were cut out and analyzed by LC-MS/MS analysis. As shown in Table 1, proteomic analysis demonstrated that these two reactive protein spots matched with alpha-enolase (ENO1).
Figure 1. Western blotting analysis.
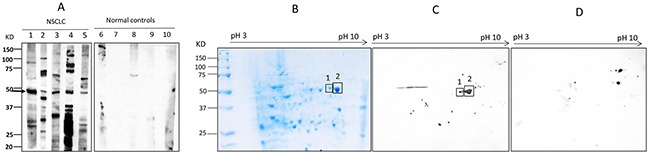
(A) Western blotting of representative serum samples with H1299 lung cancer cell line. Lanes 1-5 are lung cancer sera showing the common reactive bands with 47 KDa molecular weight, while lanes 6-10 (normal controls) are not observed corresponding bands in the same size. (B-C) Identification of the 47 KDa lung cancer protein by immunoproteomics. (B): 2-DE protein profile of H1299 cells. (C): 2-DE Western blotting analysis was probed with five lung cancer sera pool which contain antibodies to the 47 KDa protein. (D) 2-DE Western blotting of five normal human sera.
Table 1. Mass spectrum result for 47 KDa protein.
| No. in gel | Identified protein | Official Symbol | score | expected MW | function |
|---|---|---|---|---|---|
| 1 | alpha-enolase isoform X1 | ENO1 | 220 | 47.3 | a structural lens protein (tau-crystallin) in the monomeric form |
| 2 | alpha-enolase isoform X3 | ENO1 | 170 | 47.3 |
Validation of anti-ENO1 autoantibody as biomarker in NSCLC detection
To validate the potential of autoantibody against ENO1 as biomarker in NSCLC detection, the purified recombinant ENO1 protein was used as coating antigen in quantitative ELISA for detection of anti-ENO1 concentration in sera from 242 patients with NSCLC and 270 normal individuals in a validation set of samples. The cutoff value of anti-ENO1 (6.43 ng/ml) designating positive reaction was established as the optimal Youden's Index with regarding to ROC analysis. The autoantibody level of anti-ENO1 was significantly higher in sera from NSCLC patients (median ± IQR: 5.57 ± 3.38 ng/ml) than that in normal individuals (median ± IQR: 4.86 ± 2.50 ng/ml) (P=0.001, Figure 2A). The frequency of anti-ENO1 in sera form patients with NSCLC (35.1%) was higher than that in normal individuals (19.3%) (P<0.001, Figure 2B). ROC analysis showed that anti-ENO1 can differentiate NSCLC patients from normal individuals with AUC (95%CI) of 0.589 (0.539-0.638), sensitivity of 35.1%, specificity of 80.7%, positive predict value (PPV) of 62.0% and negative predictive value (NPV) of 58.0% (Figure 2C). In the subgroup analysis of autoantibody level, all of the subgroups including stages (P value for I+II and III+IV: 0.008 and 0.006, Figure 2D), histological (P value for AD and SCC: 0.003 and 0.005, Figure 2E) and metastasis (P value for yes or no: 0.002 and 0.011, Figure 2F), were observed to have similar results compared to normal control group. Table 2 shows that the median and frequency of anti-ENO1 antibody were not found to be significantly different in every comparison group (histology: AD vs SCC, stage: I+II vs III+IV, metastasis: yes vs no, smoking: yes vs no, gender: male vs female, age: ≤60 y vs >60 y). However, the ability in distinguishing NSCLC from normal individuals was found to have statistical significance in stage III+IV (AUC: 0.584, P=0.008) and in patients with age >60 y (AUC: 0.626, P=0.001), but boundary significance in stage I+II (AUC: 0.596, P=0.073) and in age ≤60 y (AUC: 0.562, P=0.067) (Table 2).
Figure 2. Distribution and ROC analysis of anti-ENO1 antibody in sera from patients with NSCLC and normal control individuals.
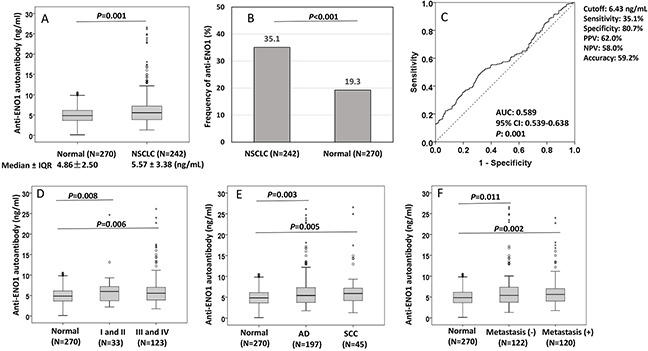
(A, D-F) Comparison of autoantibody level of anti-ENO1 in NSCLC patients and subgroups (stage, histology and metastasis) compared to normal individuals. Box and Whisker plots for serum level of anti-ENO1 in patients and normal individuals. The line within the box marks the median, and the 25th and 75th percentiles are presented by the edges of the area, which is known as inter-quartile range (IQR). The bars indicate 1.5 times of the IQR from upper or lower percentiles. P: Mann-Whitney test. (B) Frequency of anti-ENO1 autoantibody in NSCLC patients and normal individuals. P: χ2 test. (C): ROC curve of autoantibody to ENO1.
Table 2. Anti-ENO1 level and area under the curve in different characteristics in validation set.
| N | median | IQR | Pa | Frequency (%) | Pb | AUC | 95% CI | P | |
|---|---|---|---|---|---|---|---|---|---|
| Histology | |||||||||
| AD | 197 | 5.42 | 3.63 | 0.334 | 69 (35.0) | 0.946 | 0.579 | 0.526-0.633 | 0.003 |
| SCC | 45 | 5.91 | 2.98 | 16 (35.6) | 0.630 | 0.538-0.721 | 0.005 | ||
| Stage | |||||||||
| I and II | 33 | 6.00 | 3.58 | 0.786 | 12 (36.4) | 0.881 | 0.596 | 0.484-0.707 | 0.073 |
| III and IV | 123 | 5.55 | 3.17 | 43 (35.0) | 0.584 | 0.520-0.647 | 0.008 | ||
| Metastasis | |||||||||
| No | 122 | 5.4 | 3.76 | 0.799 | 41 (33.6) | 0.618 | 0.580 | 0.516-0.645 | 0.011 |
| Yes | 120 | 5.62 | 3.02 | 44 (36.7) | 0.598 | 0.534-0.661 | 0.002 | ||
| Smoking | |||||||||
| No | 157 | 5.71 | 4.11 | 0.486 | 57 (36.3) | 0.601 | 0.599 | 0.541-0.657 | 0.001 |
| Yes | 85 | 5.19 | 3.07 | 28 (32.9) | 0.570 | 0.497-0.643 | 0.051 | ||
| Gender | |||||||||
| Male | 144 | 5.38 | 3.3 | 0.685 | 49 (34.0) | 0.665 | 0.580 | 0.52-0.64 | 0.007 |
| Female | 98 | 5.75 | 4.38 | 36 (36.7) | 0.602 | 0.533-0.671 | 0.003 | ||
| Age | |||||||||
| ≤ 60 y | 138 | 5.78 | 3.7 | 0.390 | 50 (36.2) | 0.677 | 0.562 | 0.495-0.629 | 0.067 |
| > 60 y | 104 | 5.34 | 3.23 | 35 (33.7) | 0.626 | 0.552-0.700 | 0.001 |
AD: Adenocarcinoma; SCC: Squamous Cell Carcinoma; a: Mann-Whitney test; b: χ2 test
In addition, multivariable logistic regression analyses revealed that anti-ENO1 antibody could be used as potential diagnostic biomarker for the identification of patients with NSCLC, AD or SCC after adjustment for age and gender (P<0.05 for all) (Table 3).
Table 3. Multivariable logistic analyses for anti-ENO1 and various diagnostic factors in NSCLC patients.
| Comparison | Variables | OR (95%CI) | P |
|---|---|---|---|
| NSCLC vs Normal | |||
| Age (≤60 y vs >60 y) | 1.03 (0.72-1.48) | 0.870 | |
| Gender (Female vs Male) | 1.09 (0.76-1.56) | 0.650 | |
| anti-ENO1 (≥ 6.43 vs < 6.43 ng/ml) | 2.27 (1.52-3.40) | <0.001 | |
| AD vs Normal | |||
| Age (≤60 y vs >60 y) | 0.97 (0.66-1.42) | 0.880 | |
| Gender (Female vs Male) | 1.41 (0.96-2.06) | 0.080 | |
| anti-ENO1 (≥ 6.43 vs < 6.43 ng/ml) | 2.27 (1.49-3.46) | <0.001 | |
| SCC vs Normal | |||
| Age (≤60 y vs >60 y) | 1.31 (0.68-2.53) | 0.420 | |
| Gender (Female vs Male) | 0.25 (0.10-0.63) | 0.003 | |
| anti-ENO1 (≥ 6.43 vs < 6.43 ng/ml) | 2.39 (1.19-4.81) | 0.015 |
AD: Adenocarcinoma; SCC: Squamous Cell Carcinoma
Combinational use anti-ENO1 antibody and protein biomarkers (CEA and CYFRA 21-1) can improve sensitivity in diagnosis of lung cancer
There have been many reports concerning the use of CEA and CYFRA 21-1 in LC detection [16–20], and the results suggested that these markers may be useful in diagnosing LC [16–18, 20, 21]. Of 242 NSCLC patients, information of CEA level was available for 115 patients (median ± IQR: 9.90 ± 28.64 ng/ml), and CYFRA 21-1 level was available for 85 patients (median ± IQR: 3.75 ± 4.46 ng/ml). The detection of serum CEA and CYFRA 21-1 level were carried out by using the Electo-chemiluminescence immunoassay (ECLIA) kit followed the manufacturer's manual (Roche, USA). The threshold values for CEA and CYFRA 21-1 were setup at ≥10 ng/ml [22] and 3.3 ng/ml [23]. Firstly, we investigated the correlation of anti-ENO1 level with CEA (R=-0.029, P=0.755) or CYFRA 21-1 (R=-0.065, P=0.552) level and did not found statistical significance (Figure 3A and 3B), which suggests that anti-ENO1 and CEA or CYFRA 21-1 are independent biomarkers in patients sera. The positive frequency in NSCLC patients for each single biomarker was 35.1%, 48.7% and 61.2% for anti-ENO1, CEA and CYFRA 21-1, respectively (Figure 3C). When we combined anti-ENO1 with CEA or CYFRA 21-1, the frequency increased to 68.7% and 76.5%, respectively. The frequency reached at 84.0% when combined detection of anti-ENO1, CEA and CYFRA 21-1 was used (Figure 3C).
Figure 3. Correlation of anti-ENO1 level with CEA, and CYFRA 21-1 in sera from patients with NSCLC.
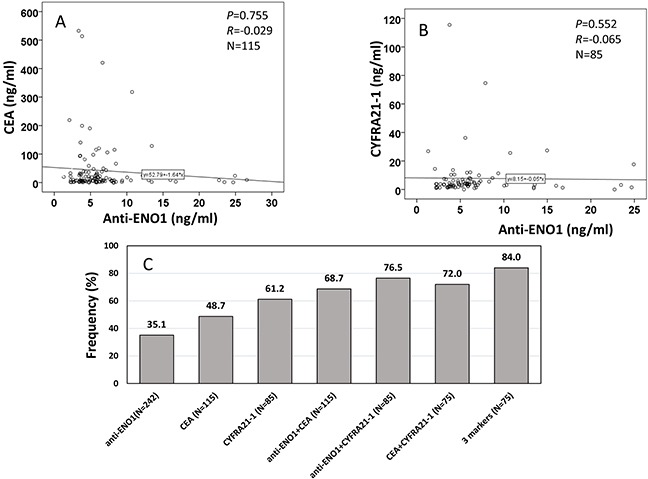
(A and B) show autoantibody level of anti-ENO1 has no correlation with CEA (A) and CYFRA 21-1 (B) in NSCLC patients. P: Spearman's test, R: Correlation coefficient in Spearman's test. (C): frequency/sensitivity of anti-ENO1, CEA, CYFRA 21-1 and their combination in NSCLC patients.
DISCUSSION
In order to identify specific LC biomarker, we initially screened 20 serum samples from patients with NSCLC and 20 normal individuals in a discovery set, and found that 20% of the patient sera contained autoantibodies against a cellular protein with molecular weight around 47 KDa in Western blotting analysis. Therefore, an immunoproteomic approach was used to identify this protein and found this 2DE-Western blotting-positive spot corresponded to ENO1. This approach was named as SERPA and successfully applied to identify and character many kinds of autoantigens in various cancers, such as eukaryotic elongation factor 2 (EEF2), enolase1(ENO1), aldolase A (ALDOA), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and heterogeneous nuclear ribonucleoproteins (HNRNP A2B1) in Melanoma [24], peroxiredoxin 6 in esophageal cancer, triophosphatase isomerase (Tim) [25], superoxide dismutase (MnSOD) in lung cancer [26], RS/DJ-1 in breast cancer [27]. In our previous study, alpha-enolase was identified as an autoantigen in liver fibrosis by using this approach [14]. Although SERPA has some drawbacks associated with limits of 2-DE, it remains a very robust method for evaluation of the humoral response to cancer.
Enolase was originally characterized as an enzyme involved in glycolytic metabolism. There are three isoforms in mammalian cells: ENO1 (α), ENO2 (β) and ENO3 (γ). ENO1 was found to exist on the cell surface functioning as one of the plasminogen receptors [28], and was responsible for NSCLC proliferation and metastasis through FAK-mediated PI3K/AKT pathway [29]. Chang et al identified ENO1 as LC associated antigen using similar serological approach and showed that increasing expression of ENO1 is a prevailing phenomenon in patients with NSCLC and its expression status is tightly correlated with disease recurrence and survival [30]. The autoimmune responses to ENO1 have been previously described in cancer patients and it has been demonstrated that ENO1 autoantibody may serve as a prognostic marker to monitor disease progression of these patients [31, 32]. Our previous study indicated that the presence of autoantibody against ENO1 may be a predictive marker for better prognosis of liver diseases [14]. To validate our proteomic results, anti-ENO1 antibody was examined in sera from 242 NSCLC patients and 270 normal controls. It was showed that the level and frequency of anti-ENO1 in sera from patients with NSCLC are significantly higher than that in sera from normal individuals, and detection of anti-ENO1 could differentiate NSCLC from normal individuals with AUC (95%CI) of 0.589 (0.539-0.638). There is no significant difference in frequency of anti-ENO1 in different stage, histology or metastasis status of NSCLC. This may suggest that the appearance of anti-ENO1 may have association with the development of NSCLC, but may have less association with the progression of NSCLC, and likely indicate that the antigen identified early- as well as late-stage disease.
Potential noninvasive lung cancer biomarkers in biological fluids have recently been discovered [33]. To date, a variety of NSCLC biomarkers have been identified and the most extensively studied circulating protein markers include CEA, CYFRA 21-1, NSE and CA-125 [16, 18, 19, 34, 35]. Because their sensitivity and specificity are far from satisfactory, their clinical applicability is limited and they have not been generally recommended as a tool for the early detection of LC [36]. Doseeva et al recently confirmed the value if using a mixed panel of tumor antigens (CEA, CA-125, and CYFRA 21-1) and one autoantibody (NY-ESO-1) in the early detection of NSCLC in high-risk individuals, and they found the 4-biomarker panel was able to discriminate NSCLC cases from controls with 74% sensitivity, 80% specificity, and 0.81 AUC in the training set and with 77% sensitivity, 80% specificity, and 0.85 AUC in the independent validation set [37]. In the present study, we combined detection of autoantibodies against ENO1, CEA and CYFRA 21-1, and found it could enhance sensitivity for the diagnosis of NSCLC. These results suggest that autoantibody against ENO1 could potentially act as a complementary clinical biomarker with tumor proteins, such as CEA and CYFRA 21-1, for serological detection of NSCLC.
In conclusion, in the present study, we identified ENO1 as autoantigen using SERPA. ENO1 can elite humoral immune response in NSCLC and have association with the tumorigenesis of NSCLC. Furthermore, autoantibody against ENO1 could be a potential diagnostic biomarker and improve the sensitivity of CEA and CYFRA 21-1 in the diagnosis of NSCLC. Further large-scale validation studies will be needed to determine the sensitivity, specificity and positive predictive value of this marker in real-world screening scenarios.
MATERIALS AND METHODS
Serum samples
Two independent sample sets (discovery set and validation set) were used in this study. The discovery set including 20 patients with NSCLC at stage I and 20 normal individuals as control matched by age, gender and smoking were collected from the New York University (NYU) Lung Cancer Biomarker Center, a member of the National Cancer Institute-sponsored Early Detection Research Network (NCI-EDRN). Blood samples from 242 patients with NSCLC in validation set were obtained from the First Affiliated Hospital of Zhengzhou University between March 2013 and April 2014. Control serum samples from 270 healthy individuals in validation set, matched to patients by age and gender, were selected from a census of angiocardiopathy diseases carried out in Zhengzhou City. All patients have been newly pathological diagnosed as primary NSCLC and have not received radiotherapy or chemotherapy before sample collection. All of the control participants had no evidence of cancer history and lung diseases. The characteristics of patients and controls are shown in Table 4. All subjects included in the study provided written informed consent. The study protocol was approved by the Medical Ethics Committee of Zhengzhou University (Zhengzhou, China).
Table 4. Characteristics of participants.
| Discovery set | Validation set | |||
|---|---|---|---|---|
| Group | NSCLC (N=20) | Normal (N=20) | NSCLC (N=242) | Normal (N=270) |
| Age, mean ± SD (range) | 63.6 ± 4.5 (53-75) | 64.5 ± 5.3 (55-78) | 58.5 ± 10.5 (27-84) | 58.5 ±10.7 (31-84) |
| ≤ 60 y | 4 (20.0) | 5 (25.0) | 138 (57.0) | 153 (56.7) |
| >60 y | 16 (80.0) | 15 (75.0) | 104 (43.0) | 117 (43.3) |
| Gender | ||||
| Male | 6 (30.0) | 6 (30.0) | 144 (59.5) | 166 (61.5) |
| Female | 14 (70.0) | 14 (70.0) | 98 (40.5) | 104 (38.5) |
| Smoking | ||||
| No | 0 (0.0) | 0 (0.0) | 157 (64.9) | 168 (62.2) |
| Yes | 20 (100.0) | 20 (100.0) | 85 (35.1) | 102 (37.8) |
| Histology | ||||
| AD | 18 (90.0) | 197 (81.4) | ||
| SCC | 2 (10.0) | 45 (18.6) | ||
| Stage | ||||
| I | 20 (100.0) | 20 (8.3) | ||
| II | 0 | 13 (5.4) | ||
| III | 0 | 29 (12.0) | ||
| IV | 0 | 94 (38.8) | ||
| unknown | 0 | 86 (35.5) | ||
| Metastasis | ||||
| No | 16 (80.0) | 122 (50.4) | ||
| Yes | 4 (20.0) | 120 (49.6) | ||
AD: Adenocarcinoma; SCC: Squamous Cell Carcinoma; SD: standard deviation
Cell culture
The NSCLC cell line H1299 was purchased from American Type Culture Collection (ATCC, Manassas, VA), and cultured in DMEM (Dulbecco's modified Eagle's medium, Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS), 100 units/ml penicillin and 100 units/ml streptomycin. Cells grown in 75-cm2 Falcon tissue culture flasks were allowed to reach 95% confluence. Then, cells were rinsed once with DMEM without FBS and removed from the flask by incubating them with a solution containing trypsin-EDTA (Gibco, Carlsbad, CA), and harvested in a 15 ml centrifuge tube.
Two-dimensional gel electrophoresis (2-DE) analysis
Briefly, total protein extractions of H1299 cells was directly lysed in rehydration sample buffer (8 M Urea, 50 mM dithiothreitol (DTT), 4% 3-[(3-cholamidopropyl) dimethylammonio] -1-propanesulfonate (CHAPS), 0.2% carrier ampholytes) as provided by Bio-Rad Laboratories (Hercules, CA) and were vortexed vigorously for 1 h at room temperature (RT). Insoluble substances were removed by centrifuge at 16,000 × g for 30 min at 4°C. Supernatant was collected and protein concentration was measured by the Bradford assay (Bio-Rad, Hercules, CA). A total of 200 μg protein was applied on a pH 3–10, 11-cm isoelectric focusing (IEF) strip (Bio-Rad, Hercules, CA). IEF was performed at a current of 50 mA per gel, 300 V for 30 min, followed by 3,500 V for 2.5 h, and additional 8,000 V for 5 h. Strips were immediately stored at −80°C for the second dimensional gel electrophoresis (2-DE) analysis. For the second dimensional electrophoresis, 10% SDS-polyacrylamide gels (SDS-PAGE) were used. Proteins were transferred onto nitrocellulose membrane (Osmonics Inc., MA) for subsequent Western blotting analysis or stained with 0.1% Coomassie blue R-250 prepared in 40% methanol/10% acetic acid. The spots were visualized using PDQuest 2-DE analysis software as described in the manufacturer's manual (Bio-Rad, Hercules, CA).
One- and two-dimensional western blotting and proteomic analysis
In order to screen the autoantibody-positive sera, H1299 cells were lysed directly in Laemmli's sample buffer and loaded onto 10% SDS-PAGE gel, which is then transferred onto nitrocellulose membrane (Osmonics Inc., MA) for Western blotting. The membrane was then cut into 0.5-cm wide stripes. After blocking with 5% nonfat milk prepared in Tris-buffered saline (TBS), containing 0.05% Tween-20 (TBST), for 1h at RT, the nitrocellulose membrane strips were incubated with sera at a dilution of 1:200. Horseradish peroxidase-conjugated goat anti-human IgG (Caltag Laboratories, San Francisco, CA) was used as secondary antibody with a dilution of 1:10,000 for 1h at RT. The positive bands were detected with Enhanced Chemiluminescence (ECL) kit (Amersham, Arlington Heights, IL). For 2-DE Western blotting, the proteins on 2-DE gel are directly transferred onto nitrocellulose membrane and incubated with two pools of five sera from patients with NSCLC and five normal individuals in the discovery set at a dilution of 1:500.
Mass spectrometry analysis
After identifying the interesting protein spots, protein spots from the 2-DE reference gel were excised and digested to perform liquid chromategraphytandem mass spectrometry (LC-MS/MS) analysis. MS/MS spectra derived from peptides were submitted for database search using TurboSequest (available in Bioworks version 3.3.1) against the human IPI database (v3.48), in both correct and reverse orientations to enable false-discovery rate (FDR) calculation. The following filters were applied in Bioworks: DCn ≥ 0.85; consensus score ≥ 10.0; protein probability ≤ 1 × 10−3; and Xcorr ≥ 1.5, 2.0 and 2.5, for singly-, doubly- and triply charged peptides, respectively.
Expression and purification of ENO1 recombinant protein
cDNA encoding human ENO1 was amplified by PCR from a commercial plasmid PMD18T-ENO1 (Sino Biological Inc., Beijing, China). For the expression and purification of recombinant ENO1 protein, the full length ENO1 cDNA was subcloned into expression vector pET-30a which was designed to produce a fusion protein with N-terminal 6× histidine and T7 epitope tags. Recombinant ENO1 protein was further expressed in E.coli BL21 (DE3) cells and purified using nickel column chromatography. The protocol used for high-level expression and purification of 6× His-tagged proteins were performed as described (QIAGEN Inc., Valencia, CA, USA). Elution buffer (8M urea, 0.1M NaH2PO4, 0.01M Tris, pH4.5) was used to elute the recombinant protein. The purified recombinant proteins were further analyzed by electrophoresis on SDS-PAGE, and identified by Western blotting using commercial monoclonal anti-ENO1 antibody.
Autoantibody measurement by quantitative Enzyme-linked immunosorbent assay (ELISA)
Serum IgG-type ENO1 autoantibody was detected by ELISA, and human IgG antigen was used as standard reference. Briefly, ENO1 protein was diluted in coating buffer (50mM sodium carbonate/bicarbonate pH9.6) to a final concentration of 0.5 μg/ml, and human IgG antigen (Beijing Dingguo Changsheng Biotechnology) was diluted in coating buffer to final concentrations of 300, 250, 200, 150, 100, 50, 10 and 0 ng/ml to generate standard curve for each plate. 100 ul diluted antigens were added into each well for coating at 4°C for overnight. After being blocked each well with 2% Bovine Serum Albumin (BSA, Sigma, USA) for overnight at 4°C, the plates were washed three times by PBST. Human serum samples at 1: 200 dilutions were added to the ENO1 coated wells and incubated for 2h at RT followed by washing three times by PBST. Horseradish peroxidase-conjugated goat anti-human IgG (Santa Cruz Biotechnology Inc., Dallas, TX, USA) at 1:10,000 dilution and the substrate 3,3′,5,5′-Tetramethylbenzidine (Sigma-Aldrich, St. Louis, MO, USA) were used as detecting reagents. Finally, 50 ul stopping solution (2M H2SO4) was added into each well and the optical density (OD) values were obtained by using a microplate reader (Thermo Fisher Scientific) at dual wavelength of 450 and 620 nm. The relative expression of autoantibodies was calculated and adjusted based on the standard curve of each plate. A positive and negative control were set in each plate to ensure the accuracy of the results. Each sample was tested in duplicate and the average value was used for the further analysis. All the ELISA positive serum samples were confirmed by using Western blotting analysis further.
Statistical analyses
Due to the sera autoantibody against ENO1 was not normally distributed (Shapiro Wilk's test), nonparametric Mann-Whitney U tests were used to compare differences of antibody levels between two groups. χ2 tests were used to compare the differences of frequency between two groups. A multivariable logistic regression model was used to calculate odds ratios (ORs) for age- and sex-adjusted cases associated with NSCLC, AD or SCC according to serum anti-ENO1 levels. Spearman's test was used to evaluate the correlation between anti-ENO1 autoantibody level and concentration of carcinoembryonic antigen (CEA) or cytokeratin 19 fragments (CYFRA 21-1). The receiver operating characteristic (ROC) analysis of anti-ENO1 for the distinguishing of NSCLC from controls, leading to estimates of area under the curve (AUC) with 95% confidence interval (CI). The optimal cutoff thresholds for designating positive reaction were determined at the point on the ROC curve at which Youden's index (sensitivity + specificity -1) was maximal. Differences were considered statistically significant when P < 0.05. Statistical analyses were performed using SPSS software (version 18.0).
Acknowledgments
We thank for Dr. William N. Rom and Dr. Jun-Chieh J. Tsay from New York University for providing serum samples to this study.
Abbreviations
- AD
adenocarcinoma
- ATCC
American Type Culture Collection
- AUC
area under the curve
- CEA
carcinoembryonic antigen
- CHAPS
3-[(3-cholamidopropyl) dimethylammonio] -1-propanesulfonate
- CI
confidence interval
- CYFRA 21-1
cytokeratin 19 fragments
- DMEM
Dulbecco's modified Eagle's medium
- DTT
dithiothreitol
- ENO1
alpha-enolase
- FBS
fetal bovine serum
- FDR
false-discovery rate
- IEF
isoelectric focusing
- IQR
inter-quartile range
- LC
lung cancer
- LC-MS/MS
liquid chromategraphytandem mass spectrometry
- LDCT
low dose spiral computed tomography
- NCI-EDRN
National Cancer Institute-sponsored Early Detection Research Network
- NSCLC
non-small cell lung cancer
- OD
optical density
- OR
odds ratios
- PPV
positive predict value
- ROC
receiver operating characteristic
- RT
room temperature
- SCC
squamous cell carcinoma
- SDS-PAGE
SDS-polyacrylamide gels
- SERPA
serological proteome analysis
- TAA
tumor-associated antigen
- TBS
Tris-buffered saline
- TBST
TBS containing 0.05% Tween-20
- 2-DE
two-dimensional gel electrophoresis.
Footnotes
Author's contributions
L.P.D. and J.Y.Z. contributed to designing research study. L.P.D. contributed to writing the manuscript. Y.H.Q. and J.T.L. contributed to conducting experiments. X.W., K.J.W. and P.W. contributed to providing serum samples. B.H.J. contributed to technical support.
CONFLICTS OF INTEREST
The authors have declared that no conflict of interest exists.
FINANCIAL SUPPORT
This work was supported by grant from National Natural Science Foundation of China (No.81672917 and No.81372371) and the Major Project of Science and Technology in Henan Province (No. 161100311400).
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Clegg LX, Ward E, Ries LA, Wu X, Jamison PM, Wingo PA, Howe HL, Anderson RN, Edwards BK. Annual report to the nation on the status of cancer, 1975-2001, with a special feature regarding survival. Cancer. 2004;101:3–27. doi: 10.1002/cncr.20288. [DOI] [PubMed] [Google Scholar]
- 4.Field JK, Duffy SW. Lung cancer screening: the way forward. Br J Cancer. 2008;99:557–62. doi: 10.1038/sj.bjc.6604509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swensen SJ, Jett JR, Hartman TE, Midthun DE, Mandrekar SJ, Hillman SL, Sykes AM, Aughenbaugh GL, Bungum AO, Allen KL. CT screening for lung cancer: five-year prospective experience. Radiology. 2005;235:259–65. doi: 10.1148/radiol.2351041662. [DOI] [PubMed] [Google Scholar]
- 6.National Lung Screening Trial Research T. Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, Gareen IF, Gatsonis C, Marcus PM, Sicks JD. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berrington de Gonzalez A, Mahesh M, Kim KP, Bhargavan M, Lewis R, Mettler F, Land C. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169:2071–7. doi: 10.1001/archinternmed.2009.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sone S, Li F, Yang ZG, Honda T, Maruyama Y, Takashima S, Hasegawa M, Kawakami S, Kubo K, Haniuda M, Yamanda T. Results of three-year mass screening programme for lung cancer using mobile low-dose spiral computed tomography scanner. Br J Cancer. 2001;84:25–32. doi: 10.1054/bjoc.2000.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du ZY, Shi MH, Ji CH, Yu Y. Serum pleiotrophin could be an early indicator for diagnosis and prognosis of non-small cell lung cancer. Asian Pac J Cancer Prev. 2015;16:1421–5. doi: 10.7314/apjcp.2015.16.4.1421. [DOI] [PubMed] [Google Scholar]
- 10.Zhong L, Coe SP, Stromberg AJ, Khattar NH, Jett JR, Hirschowitz EA. Profiling tumor-associated antibodies for early detection of non-small cell lung cancer. J Thorac Oncol. 2006;1:513–9. [PubMed] [Google Scholar]
- 11.Zhang JY, Casiano CA, Peng XX, Koziol JA, Chan EK, Tan EM. Enhancement of antibody detection in cancer using panel of recombinant tumor-associated antigens. Cancer Epidemiol Biomarkers Prev. 2003;12:136–43. [PubMed] [Google Scholar]
- 12.Negm OH, Hamed MR, Schoen RE, Whelan RL, Steele RJ, Scholefield J, Dilnot EM, Shantha Kumara HM, Robertson JF, Sewell HF. Human Blood Autoantibodies in the Detection of Colorectal Cancer. PLoS One. 2016;11:e0156971. doi: 10.1371/journal.pone.0156971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dai L, Tsay JC, Li J, Yie TA, Munger JS, Pass H, Rom WN, Zhang Y, Tan EM, Zhang JY. Autoantibodies against tumor-associated antigens in the early detection of lung cancer. Lung Cancer. 2016;99:172–9. doi: 10.1016/j.lungcan.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 14.Peng B, Huang X, Nakayasu ES, Petersen JR, Qiu S, Almeida IC, Zhang JY. Using immunoproteomics to identify alpha-enolase as an autoantigen in liver fibrosis. J Proteome Res. 2013;12:1789–96. doi: 10.1021/pr3011342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Looi KS, Nakayasu ES, Diaz RA, Tan EM, Almeida IC, Zhang JY. Using proteomic approach to identify tumor-associated antigens as markers in hepatocellular carcinoma. J Proteome Res. 2008;7:4004–12. doi: 10.1021/pr800273h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grunnet M, Sorensen JB. Carcinoembryonic antigen (CEA) as tumor marker in lung cancer. Lung Cancer. 2012;76:138–43. doi: 10.1016/j.lungcan.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 17.Tomita M, Shimizu T, Ayabe T, Yonei A, Onitsuka T. Prognostic significance of tumour marker index based on preoperative CEA and CYFRA 21-1 in non-small cell lung cancer. Anticancer Res. 2010;30:3099–102. [PubMed] [Google Scholar]
- 18.Hanagiri T, Sugaya M, Takenaka M, Oka S, Baba T, Shigematsu Y, Nagata Y, Shimokawa H, Uramoto H, Takenoyama M, Yasumoto K, Tanaka F. Preoperative CYFRA 21-1 and CEA as prognostic factors in patients with stage I non-small cell lung cancer. Lung Cancer. 2011;74:112–7. doi: 10.1016/j.lungcan.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Veronesi G, Pelosi G, Sonzogni A, Leon ME, D'Aiuto M, Gasparri R, De Braud F, De Pas T, Sandri M, Spaggiari L. Tumour CEA as predictor of better outcome in squamous cell carcinoma of the lung. Lung Cancer. 2005;48:233–40. doi: 10.1016/j.lungcan.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 20.Schneider J. Tumor markers in detection of lung cancer. Adv Clin Chem. 2006;42:1–41. doi: 10.1016/s0065-2423(06)42001-1. [DOI] [PubMed] [Google Scholar]
- 21.Edelman MJ, Hodgson L, Rosenblatt PY, Christenson RH, Vokes EE, Wang X, Kratzke R. CYFRA 21-1 as a prognostic and predictive marker in advanced non-small-cell lung cancer in a prospective trial: CALGB 150304. J Thorac Oncol. 2012;7:649–54. doi: 10.1097/JTO.0b013e31824a8db0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicholson BD, Shinkins B, Pathiraja I, Roberts NW, James TJ, Mallett S, Perera R, Primrose JN, Mant D. Blood CEA levels for detecting recurrent colorectal cancer. Cochrane Database Syst Rev. 2015:CD011134. doi: 10.1002/14651858.CD011134.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang YX, Hu D, Yan X. Diagnostic accuracy of Cyfra 21-1 for head and neck squamous cell carcinoma: a meta-analysis. Eur Rev Med Pharmacol Sci. 2013;17:2383–9. [PubMed] [Google Scholar]
- 24.Zhang J, Song M, Wang J, Sun M, Wang B, Li R, Huang Y, Hou L, Jin Y, Wang M, Tang J. Enoyl coenzyme A hydratase 1 is an important factor in the lymphatic metastasis of tumors. Biomed Pharmacother. 2011;65:157–62. doi: 10.1016/j.biopha.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 25.Mayeda A, Munroe SH, Caceres JF, Krainer AR. Function of conserved domains of hnRNP A1 and other hnRNP A/B proteins. EMBO J. 1994;13:5483–95. doi: 10.1002/j.1460-2075.1994.tb06883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abdul-Manan N, Williams KR. hnRNP A1 binds promiscuously to oligoribonucleotides: utilization of random and homo-oligonucleotides to discriminate sequence from base-specific binding. Nucleic Acids Res. 1996;24:4063–70. doi: 10.1093/nar/24.20.4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munro TP, Magee RJ, Kidd GJ, Carson JH, Barbarese E, Smith LM, Smith R. Mutational analysis of a heterogeneous nuclear ribonucleoprotein A2 response element for RNA trafficking. J Biol Chem. 1999;274:34389–95. doi: 10.1074/jbc.274.48.34389. [DOI] [PubMed] [Google Scholar]
- 28.Redlitz A, Fowler BJ, Plow EF, Miles LA. The role of an enolase-related molecule in plasminogen binding to cells. Eur J Biochem. 1995;227:407–15. doi: 10.1111/j.1432-1033.1995.tb20403.x. [DOI] [PubMed] [Google Scholar]
- 29.Fu QF, Liu Y, Fan Y, Hua SN, Qu HY, Dong SW, Li RL, Zhao MY, Zhen Y, Yu XL, Chen YY, Luo RC, Li R, et al. Alpha-enolase promotes cell glycolysis, growth, migration, and invasion in non-small cell lung cancer through FAK-mediated PI3K/AKT pathway. J Hematol Oncol. 2015;8:22. doi: 10.1186/s13045-015-0117-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang GC, Liu KJ, Hsieh CL, Hu TS, Charoenfuprasert S, Liu HK, Luh KT, Hsu LH, Wu CW, Ting CC, Chen CY, Chen KC, Yang TY, et al. Identification of alpha-enolase as an autoantigen in lung cancer: its overexpression is associated with clinical outcomes. Clin Cancer Res. 2006;12:5746–54. doi: 10.1158/1078-0432.CCR-06-0324. [DOI] [PubMed] [Google Scholar]
- 31.Shih NY, Lai HL, Chang GC, Lin HC, Wu YC, Liu JM, Liu KJ, Tseng SW. Anti-alpha-enolase autoantibodies are down-regulated in advanced cancer patients. Jpn J Clin Oncol. 2010;40:663–9. doi: 10.1093/jjco/hyq028. [DOI] [PubMed] [Google Scholar]
- 32.Hsiao KC, Shih NY, Chu PY, Hung YM, Liao JY, Chou SW, Yang YY, Chang GC, Liu KJ. Anti-alpha-enolase is a prognostic marker in postoperative lung cancer patients. Oncotarget. 2015;6:35073–86. doi: 10.18632/oncotarget.5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.I H, Cho JY. Lung Cancer Biomarkers. Adv Clin Chem. 2015;72:107–70. doi: 10.1016/bs.acc.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 34.Cedres S, Nunez I, Longo M, Martinez P, Checa E, Torrejon D, Felip E. Serum tumor markers CEA, CYFRA21-1, and CA-125 are associated with worse prognosis in advanced non-small-cell lung cancer (NSCLC) Clin Lung Cancer. 2011;12:172–9. doi: 10.1016/j.cllc.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 35.Isgro MA, Bottoni P, Scatena R. Neuron-Specific Enolase as a Biomarker: Biochemical and Clinical Aspects. Adv Exp Med Biol. 2015;867:125–43. doi: 10.1007/978-94-017-7215-0_9. [DOI] [PubMed] [Google Scholar]
- 36.Okamura K, Takayama K, Izumi M, Harada T, Furuyama K, Nakanishi Y. Diagnostic value of CEA and CYFRA 21-1 tumor markers in primary lung cancer. Lung Cancer. 2013;80:45–9. doi: 10.1016/j.lungcan.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 37.Doseeva V, Colpitts T, Gao G, Woodcock J, Knezevic V. Performance of a multiplexed dual analyte immunoassay for the early detection of non-small cell lung cancer. J Transl Med. 2015;13:55. doi: 10.1186/s12967-015-0419-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


