Abstract
Transcribing exogenous RNA in eukaryotic cells requires delivering DNA to their nuclei and changing their genome. Nuclear delivery is often inefficient, limiting the potential scope of gene therapy and synthetic biology. These challenges may be overcome by techniques that allow for extranucleate transcription within eukaryotic cells. Protocells have been developed that enable transcription inside of liposomes; however, it has not yet been demonstrated whether this technology can be extended for use within eukaryotic cells. Here we show RNA-synthesizing nanoliposomes allow transcription of exogenous RNA inside anucleate cells. To accomplish this, components of transcription were encapsulated into liposomes and delivered to platelets. These liposomes were capable of light-induced transcription in platelets, providing proof-of-concept that protocell technology can be adapted for use within mammalian cells.
INTRODUCTION
Transcription in healthy eukaryotic cells typically occurs only in nuclei and mitochondria. This presents challenges when modifying mammalian cells to transcribe RNA from exogenous DNA. Efficient nuclear gene delivery is a major challenge in developing effective non-viral vectors,[1] and questions remain about the long-term safety of viral vectors.[2] Engineering anucleate mammalian cells, such as platelets, to synthesize exogenous RNA with these methods is impossible. Cell- and nuclei-free systems have been developed for synthesizing RNA and proteins using phage RNA polymerases and translational machinery extracted from cells. These systems have been encapsulated within lipid bilayers to form “protocells”, liposomes capable of protein expression.[3] Protocells have been used to model early cellular life,[4, 5] and significant advances have been made in studying and maximizing protein expression within nano-[6] and microsized liposomes.[7, 8] Several applications for protocells in synthetic biology and drug delivery have been explored. These include using protein-synthesizing vesicles as synthetic vaccines,[9] reactors for directed evolution,[10] stimuli-responsive vehicles toward in vivo drug delivery,[11] and for modifying bacterial cell behavior.[12]
Can existing protocell technology be adapted to function in mammalian cells? This would enable transcription of exogenous RNA in cells without requiring delivery of DNA to nuclei. We hypothesized that RNA-synthesizing nanoliposomes could function within platelets, anucleate cells found in blood (Scheme 1). To test this hypothesis, we used liposomes capable of light-induced RNA synthesis,[11] allowing transcription to be initiated only after liposomes were internalized by platelets. Components of a transcription reaction, consisting of T7 RNA polymerase (T7RNAP), a linear DNA template, and ribonucleotide triphosphates (rNTPs), including photocaged adenosine triphosphate (caged-ATP), were encapsulated into nanoliposomes. While active protocells have previously been injected into mice,[11] their ability to function within eukaryotic cells has not been conclusively demonstrated. Protocells typically contain coupled RNA and protein synthesis, but we focused on transcription to bypass the difficulties in co-encapsulating components of translation,[13] while maintaining a wide range of potential applications in gene and RNAi therapy.[14]
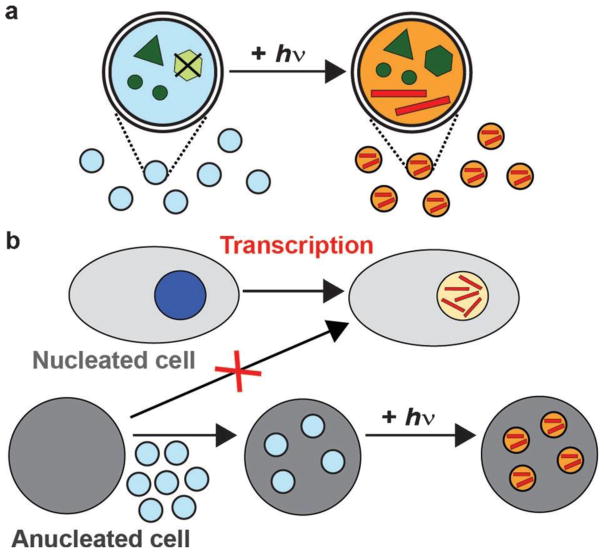
Scheme 1.
Transcription in nanoliposomes allows exogenous RNA to be synthesized in anucleate cells. a) Transcriptional components (dark green), including caged-ATP (light green), were encapsulated into nanoliposomes (light blue and orange), which synthesized RNA (red lines) following irradiation. b) Transcription of RNA is a basic function of nuclei (dark blue and yellow), but anucleate cells are incapable of de novo RNA synthesis. RNA-synthesizing nanoliposomes allow transcription to occur in anucleate cells.
RESULTS and DISCUSSION
To control transcription in platelets, we first tested whether transcription in purified nanoliposomes (220 ± 110 nm; mean ±s.d.) could be initiated using light. Liposomes were irradiated for 30 s with white light (λ > 300 nm) to release ATP from caged-ATP (Amax = 360 nm), and incubated for one hour at 37 °C for transcription to occur. GFP mRNA increased by 2300-fold, measured using quantitative polymerase chain reaction (qPCR). Only a 6-fold increase occurred in control samples without irradiation (Figure 1a). To determine if mRNA was made in liposomes of different sizes, two batches of liposomes with average diameters of 270 ± 50 nm and 430 ± 120 nm were prepared. The amount of RNA synthesized in these two populations was not significantly different (Supporting Information, Figure S1). Another DNA template, for firefly luciferase (FLuc) mRNA, was controllably transcribed in liposomes. A 12- fold increase in FLuc mRNA occurred in irradiated samples while there was no significant increase in samples that were not irradiated (Figure 1b). To confirm that mRNA transcribed within liposomes was functional, a cell-free expression system was used to translate FLuc mRNA isolated from liposomes. A 6-fold increase in luminescence occurred when a substrate for the FLuc enzyme was added, indicating that functional FLuc mRNA was synthesized in liposomes (Figure 1c). The lower RNA yield observed with the FLuc template (2 kb) compared to the GFP template (1 kb) suggests optimization is needed to maximize RNA synthesis of larger templates. Taken together, these data demonstrate that transcription in nanoliposomes can be suppressed and specifically initiated using light.
Figure 1.
Transcription of GFP and FLuc mRNA in nanoliposomes is controlled by light. a,b) The amount of GFP (n = 6) and FLuc mRNA (n = 3) increased only when irradiated with light (+ hv), measured using qPCR. c) FLuc mRNA extracted from liposomes produces functional enzyme that activates a luminescent substrate in a cell-free translation system (n = 3). Error bars represent standard error of the mean (SEM), * p < 0.05, n.s. indicates not significant compared to the results at 0 h.
Photoinduced transcription in liposomes has previously been achieved in liposomes as small as 170 nm, but the composition and purification of these liposomes was not optimized for use in cells.[11] To optimize the liposomes for transcriptional activity and delivery to cells, modifications to the composition and preparation of liposomes were made. A lipid functionalized with polyethylene glycol (PEG) was incorporated, which has previously been shown to enhance protein expression within liposomes.[7] This led to a 10-fold increase in RNA synthesis (Supporting Information, Figure S2). Liposomes were also purified using an anionic exchange column to remove unencapsulated T7RNAP and DNA (Supporting Information, Figure S3). This quick, single- step method for purification is an alternative to published methods of adding ethylenediaminetetraacetic acid (EDTA) or nucleases to inhibit transcription outside of liposomes,[5, 15] which are likely unsuitable for subsequent cellular delivery.
To determine whether RNA-synthesizing nanoliposomes could be delivered to cells, the ability of platelets to take up liposomes was first assessed using flow cytometry and confocal microscopy. Empty, fluorescently tagged liposomes were incubated with isolated platelets in a buffered solution. Excess liposomes were removed, and platelets were stained with a fluorescent antibody against CD42b, a cell-surface marker expressed only on platelets and megakaryocytes.[16] Liposomes co-localized with 35 to 65 % of platelets, with variation between platelet donors (Figure 2a). Confocal microscopy of liposome-treated platelets confirmed that the majority of co-localization corresponded to internalized liposomes (Figure 2b). Uptake was reduced by inhibitors of endocytosis, further confirming that liposomes were internalized, measured using flow cytometry (Figure 2c). Uptake was decreased by over 60 % by cytochalasin D, an inhibitor of actin polymerization, as well as by sodium azide (NaN3), a general metabolic inhibitor. Using inhibitors to specific endocytotic pathways, uptake was reduced by over 60 % by dynasore and 35 % by phenylarsine oxide (PAO) while amiloride did not significantly reduce uptake. Dynasore inhibits dynamin-dependent endocytosis pathways, including caveolae-and clathrin-mediated endocytosis, PAO inhibits clathrin-mediated endocytosis, and amiloride inhibits phagocytosis and micropinocytosis.[17] These results suggest platelets take up liposomes through multiple dynamin-dependent endocytotic pathways. Platelets have been reported to internalize nanoparticles through the open canalicular system (OCS), a surface-connected system of channels within the core of the platelet, and by cell engulfment and trafficking to storage vacuoles.[18] This is consistent with the results observed here, including the inability of any single inhibitor to completely abrogate uptake.
Figure 2.
Liposomes were internalized by platelets. a) Flow cytometry histograms of platelets depict an increase in fluorescence from liposomes, corresponding to liposome internalization (black curve).
b) Confocal images of platelets (green) and internalized liposomes (red). Scale bar: 10 mm. c) Uptake of liposomes was reduced when platelets were pre-treated with a metabolic inhibitor and inhibitors of endocytosis, quantified by flow cytometry. Error bars represent standard error of the mean (n = 3).
* p < 0.01, ** p < 0.05, n.s. indicates not significant compared to no inhibitor.
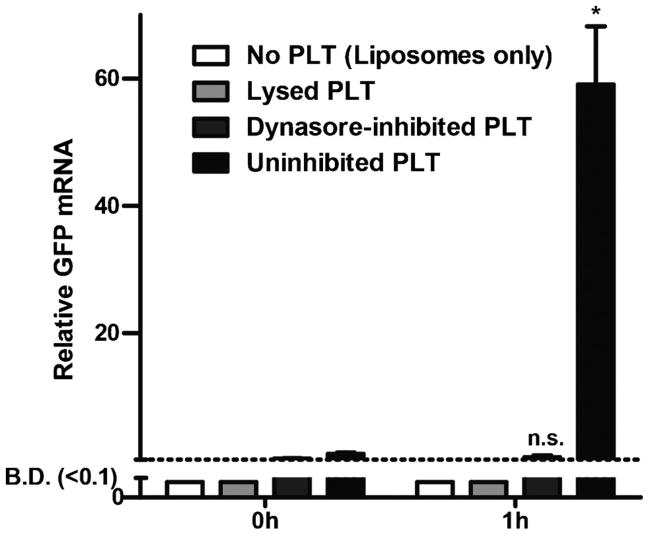
To test if protocells could function in platelets, light- inducible RNA-synthesizing liposomes were incubated with platelets. A 59-fold increase in mRNA was detected in irradiated platelets, compared to only a 2-fold increase when platelets were first treated with dynasore. To confirm all excess liposomes were removed, two control samples, consisting of liposomes without platelets, or liposomes incubated with platelets that were first lysed by freeze-thawing, were purified in the same manner as samples containing intact platelets. In both of these control samples, no significant increase in mRNA occurred, indicating efficient removal of all liposomes not internalized by platelets (Figure 3). This data demonstrates that RNA was synthesized in liposomes within intact platelets.
Figure 3.
Liposomes internalized by intact platelets (PLT) synthesized RNA. A significant increase in RNA occurred one hour after irradiation only in uninhibited, intact platelets. When platelets were lysed or absent, no RNA was detected. In dynasore-inhibited platelets, no significant increase in RNA was detected. Error bars represent standard error of the mean (n = 3), * p < 0.01, n.s. indicates not significant compared to the respective 0h sample, B.D. indicates below detection by qPCR.
This work shows, for the first time, that controlled transcription of exogenous RNA can occur in an anucleate cell. The goal of this work was to demonstrate proof-of-concept that protocells could be adapted for use in mammalian cells to express exogenous RNA, although applications of this system with other mammalian cells remain to be tested. The scope of the experiments described here did not include testing whether the newly synthesized RNA can be utilized by the cells, although this is an important next step toward using this technology in molecular biology, gene therapy, or RNAi-based therapeutics. To answer this, it will be important to determine whether the newly synthesized RNA escapes the OCS or endosomal compartments in platelets to enter the cytoplasm. Even with these limitations, the system described here is a first step towards engineering extranucleate transcription in mammalian cells for the expression of exogenous RNA, without modifying their genome.
METHODS
Whole blood collection
Informed, signed consent was obtained from healthy volunteers prior to collecting whole blood from donors. Approval for the study was given by the research ethics boards of the University of British Columbia. Whole blood was collected into tubes containing sodium citrate (0.105 m).
Preparing RNA-synthesizing liposomes
Liposomes were prepared using a published procedure, with minor modifications.[15] Liposomes were then purified with an anionic exchange column, irradiated for 30 s and incubated at 378C. RNA was measured using qPCR. To measure protein expression a rabbit reticulocyte lysate (RRL) system was used to translate FLuc mRNA. d-luciferin was added and luminescence was measured in a microplate reader. Statistical significance was determined using two-tailed Student’s t-test.
Measuring liposome uptake in isolated platelets
Platelets were isolated from citrated whole blood, washed, and resuspended in buffer. Liposomes were added and incubated for 30 min at 37 °C. Excess liposomes were removed by washing platelets with buffer. Samples used for qPCR were further purified with an ultrafiltration spin column, irradiated for 30 s, and incubated at 37 °C. To inhibit uptake, platelets were pre-incubated with inhibitors for 30 min before adding liposomes, and inhibitors were present in samples throughout the experiment. Statistical significance was determined using a two-tailed Student’s t-test.
Supplementary Material
Acknowledgments
The work was funded by Natural Sciences and Engineering Research Council (418652–2012), Canadian Institutes of Health Research (MOP-119426, MSH-130166, and GSD-134865), Canadian Foundation for Innovation (31928), Canadian Blood Services Graduate Fellowship Program, and Health Canada. We thank P. Schubert, D. Devine, and A. Schroeder for their helpful suggestions, E. Jan for the pEJ3 construct, C. Guo for help with microscopy, and the UBC Centre for Blood Research for support with blood collection.
Footnotes
References
- 1.Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ, Dorkin JR, Anderson DG. Nat Rev Genet. 2014;15:541–555. doi: 10.1038/nrg3763. [DOI] [PubMed] [Google Scholar]
- 2.Kay MA. Nat Rev Genet. 2011;12:316–328. doi: 10.1038/nrg2971. [DOI] [PubMed] [Google Scholar]
- 3.Yu W, Sato K, Wakabayashi M, Nakaishi T, Ko-Mitamura EP, Shima Y, Urabe I, Yomo T. J Biosci Bioeng. 2001;92:590–593. doi: 10.1263/jbb.92.590. [DOI] [PubMed] [Google Scholar]
- 4.Monnard PA, Luptak A, Deamer DW. Philos Trans R Soc London Ser B. 2007;362:1741–1750. doi: 10.1098/rstb.2007.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]; Fischer A, Franco A, Oberholzer T. ChemBioChem. 2002;3:409–417. doi: 10.1002/1439-7633(20020503)3:5<409::AID-CBIC409>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]; Noireaux V, Libchaber A. Proc Natl Acad Sci USA. 2004;101:17669–17674. doi: 10.1073/pnas.0408236101. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ishikawa K, Sato K, Shima Y, Urabe I, Yomo T. Febs Lett. 2004;576:387–390. doi: 10.1016/j.febslet.2004.09.046. [DOI] [PubMed] [Google Scholar]; Murtas G, Kuruma Y, Bianchini P, Diaspro A, Luisi PL. Biochem Biophys Res Commun. 2007;363:12–17. doi: 10.1016/j.bbrc.2007.07.201. [DOI] [PubMed] [Google Scholar]; Kuruma Y, Stano P, Ueda T, Luisi PL. Biochim Biophys Acta Biomembr. 2009;1788:567–574. doi: 10.1016/j.bbamem.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 5.Stano P, Carrara P, Kuruma Y, de Souza TP, Luisi PL. J Mater Chem. 2011;21:18887–18902. [Google Scholar]
- 6.Pereira de Souza T, Stano P, Luisi PL. ChemBioChem. 2009;10:1056–1063. doi: 10.1002/cbic.200800810. [DOI] [PubMed] [Google Scholar]
- 7.Amidi M, de Raad M, de Graauw H, van Ditmarsch D, Hennink WE, Crommelin DJA, Mastrobattista E. J Liposome Res. 2010;20:73–83. doi: 10.3109/08982100903402954. [DOI] [PubMed] [Google Scholar]
- 8.Nourian Z, Roelofsen W, Danelon C. Angew Chem Int Ed. 2012;51:3114–3118. doi: 10.1002/anie.201107123. [DOI] [PubMed] [Google Scholar]; Angew Chem. 2012;124:3168–3172. [Google Scholar]; Saito H, Kato Y, Le Berre M, Yamada A, Inoue T, Yosikawa K, Baigl D. ChemBioChem. 2009;10:1640–1643. doi: 10.1002/cbic.200900205. [DOI] [PubMed] [Google Scholar]
- 9.Amidi M, de Raad M, Crommelin DJA, Hennink WE, Mastrobattista E. Syst Biol Synth Biol. 2011;5:21–31. doi: 10.1007/s11693-010-9066-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sunami T, Sato K, Matsuura T, Tsukada K, Urabe I, Yomo T. Anal Biochem. 2006;357:128–136. doi: 10.1016/j.ab.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 11.Lentini R, Santero SP, Chizzolini F, Cecchi D, Fontana J, Marchioretto M, Del Bianco C, Terrell JL, Spencer AC, Martini L, Forlin M, Assfalg M, Dalla Serra M, Bentley WE, Mansy SS. Nat Commun. 2014;5:4012. doi: 10.1038/ncomms5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schroeder A, Goldberg MS, Kastrup C, Wang Y, Jiang S, Joseph BJ, Levins CG, Kannan ST, Langer R, Anderson DG. Nano Lett. 2012;12:2685–2689. doi: 10.1021/nl2036047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Nies P, Nourian Z, Kok M, van Wijk R, Moeskops J, Westerlaken I, Poolman JM, Eelkema R, van Esch JH, Kuruma Y, Ueda T, Danelon C. ChemBioChem. 2013;14:1963–1966. doi: 10.1002/cbic.201300449. [DOI] [PubMed] [Google Scholar]
- 14.Giacca M, Zacchigna S. J Controlled Release. 2012;161:377–388. doi: 10.1016/j.jconrel.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Sunami T, Matsuura T, Suzuki H, Yomo T. Cell-Free Protein Prod: Methods and Protocols. 2010;607:243–256. doi: 10.1007/978-1-60327-331-2_20. [DOI] [PubMed] [Google Scholar]
- 16.Procopio Evagrio George N, Wei Q, Shin PK, Konstanto-poulos K, Ross JM. Arterioscler Thromb Vasc Biol. 2006;26:2394–2400. doi: 10.1161/01.ATV.0000237606.90253.94. [DOI] [PubMed] [Google Scholar]
- 17.von Kleist L, Haucke V. Traffic. 2012;13:495–504. doi: 10.1111/j.1600-0854.2011.01292.x. [DOI] [PubMed] [Google Scholar]; Linares J, Concepcion Matesanz M, Vila M, Jose Feito M, Goncalves G, Vallet-Regi M, Marques PAAP, Teresa Portoles M. ACS Appl Mater Interfaces. 2014;6:13697–13706. doi: 10.1021/am5031598. [DOI] [PubMed] [Google Scholar]
- 18.Male R, Vannier WE, Baldeschwieler JD. Proc Natl Acad Sci USA. 1992;89:9191–9195. doi: 10.1073/pnas.89.19.9191. [DOI] [PMC free article] [PubMed] [Google Scholar]; White JG. Platelets. 2005;16:121–131. doi: 10.1080/09537100400007390. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.