Abstract
Indigenous microbes inside the host intestine maintain a complex self-regulating community. The mechanisms by which gut microbes interact with intestinal pathogens remain largely unknown. Here we identify a commensal Escherichia coli strain whose expansion predisposes mice to infection by Vibrio cholerae, a human pathogen. We refer to this strain as ‘atypical’ E. coli (atEc) because of its inability to ferment lactose. The atEc strain is resistant to reactive oxygen species (ROS) and proliferates extensively in antibiotic-treated adult mice. V. cholerae infection is more severe in neonatal mice transplanted with atEc compared with those transplanted with a typical E. coli strain. Intestinal ROS levels are decreased in atEc-transplanted mice, favouring proliferation of ROS-sensitive V. cholerae. An atEc mutant defective in ROS degradation fails to facilitate V. cholerae infection when transplanted, suggesting that host infection susceptibility can be regulated by a single gene product of one particular commensal species.
 The interactions between gut bacteria and enteric pathogens are poorly
understood. Here, Yoon et al. show that subinhibitory antibiotic treatment in a
mouse model leads to overgrowth of an E. coli strain carrying a catalase-encoding
gene that enhances infection with the human pathogen Vibrio cholerae.
The interactions between gut bacteria and enteric pathogens are poorly
understood. Here, Yoon et al. show that subinhibitory antibiotic treatment in a
mouse model leads to overgrowth of an E. coli strain carrying a catalase-encoding
gene that enhances infection with the human pathogen Vibrio cholerae.
Commensal microbes, collectively termed gut microbiota, are considered to exist as a symbiotic community in the mucus layer lining the intestinal epithelium1,2. Due to the lack of means to isolate and preserve intestinal tissues, how these microbes maintain a dynamic and permanent co-evolutionary relationship with the host is not clearly understood. In some imaging studies, however, biofilm-like structures were successfully observed in animal intestines and surgically removed human appendices3,4. This suggests that (i) the resident microbes can act as a barrier against invading pathogens5 and (ii) enteric infections are the outcomes of multifaceted interactions between commensals, pathogens, and the host intestinal tissue.
Infectivity of pathogenic E. coli strains is controlled by the composition of commensal E. coli strains that can metabolize specific carbohydrates, thereby reducing their availability for consumption by pathogenic strains6. Anaerobic growth of both E. coli and Salmonella enteria serovar typhimurium can be supported by respiration using nitrate or tetrathionate, which are byproducts of the host inflammatory response7,8. Certain bacterial pathogens can increase their survival fitness inside the host intestine by catabolizing host-derived carbohydrates, the production of which is mediated by Bacteroides thetaiotaomicron, a distinct member of the gut microbiota9. The alteration of gut microbiota composition by antibiotic treatment has been shown to increase host susceptibility to intestinal infections by S. enteria10,11 and Clostridium difficile12. Furthermore, individuals for whom faecal transplantation resulted in restored microbiota have been shown to exhibit improved resistance to recurrent C. difficile infection13,14,15. However, due to the high degree of diversity of commensal microbes and the difficulty in culturing these strains, it has been difficult to correlate host susceptibility to infection with changes in the relative abundance of a defined subgroup of commensal microbes.
In this study, we seek to identify a commensal species and its genetic factor(s) that specifically influence host resistance to enteric infection. An E. coli strain with unusual features was significantly propagated during antibiotic treatment in mice and found to be responsible for modulating host susceptibility to infection by V. cholerae (Vc). We sequenced the whole genome of the E. coli strain and identified a novel catalase gene, disruption of which abrogated the infection-facilitating effects. This report provides novel insights into the role of gut microbiota in regulating the extent of intestinal infections.
Results
Antibiotic-treated mice became sensitive to Vc colonization
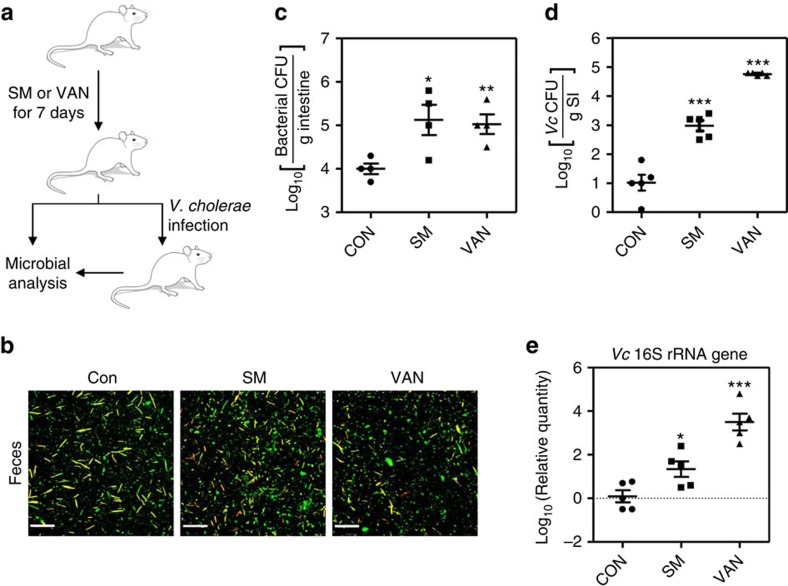
Commensal microbes play protective roles in host immunity against enteropathogenic infections16. To examine whether host susceptibility to intestinal infection is influenced by altered gut microbiota composition, we treated adult mice with low concentrations of streptomycin (SM, 1 mg per day) or vancomycin (VAN, 250 μg per day) daily for 7 days (Fig. 1a). Fluorescence staining of microbial cells recovered from mouse faeces revealed varying numbers of bacterial cells with differential shapes, with slightly more bacterial cells found in faeces obtained from SM-treated mice (Fig. 1b). Aerobic cultivation of mouse intestinal homogenates in plate count agar medium revealed that more bacterial colonies grew from the faeces recovered from SM-treated and VAN-treated mice compared with nontreated mice (Fig. 1c). These results suggest that our experimental conditions induced a change in the gut microbiota composition, rather than eliminating considerable portions of commensal microbes.
Figure 1. Antibiotic treatment induces changes in the gut microbiota composition and increases susceptibility to Vc infection.
(a) Schematic diagram of the experimental procedure. Female CD-1 mice (5 to 6 weeks old) were treated with streptomycin (SM) and vancomycin (VAN) daily by oral gavage for 7 days. The daily doses administered were 1 mg for SM and 250 μg for VAN. At day 7 post-treatment, a subset of each treatment group was challenged with Vc for 2 days. (b) After antibiotic treatment, mouse faeces were collected and homogenized in PBS. The microbial cells in each suspension were visualized using a Live/Dead bacterial staining kit. Scale bar, 20 μm. (c) Mice (n=4) were killed and intestinal tissue lysates were prepared by homogenization. Bacterial cells that grew aerobically on plate count agar medium were enumerated and data are presented on a log scale. Values are displayed as means±s.e.m. for each treatment group. *P<0.05, **P<0.01 versus bacterial CFUs detected in the control group. (d) Antibiotic-treated mice (n=5) were infected with N16961 by oral gavage (∼107 cells). At 2 days post-infection, mice were killed and the number of Vc cells recovered from the small intestine (SI) of each mouse was determined. Values are presented as means±s.e.m. and are displayed on a log scale. ***P<0.001 versus Vc CFUs detected in the control group. (e) Relative quantities of the Vc 16S rRNA gene in SI tissue homogenates (n=5) as determined by real-time PCR. Values were normalized to those of the gapdh gene. The ratios of the Vc 16S rRNA genes to the host gapdh gene are displayed on a log scale (means±s.e.m.). *P<0.05, ***P<0.001 versus the control group.
The adult mouse is not a natural host for Vc, the causative agent of the pandemic human disease cholera17. When control mice were infected with Vc via oral gavage, no significant Vc colonization was observed (Fig. 1d). However, a marked increase in Vc colonization was observed in antibiotic-treated mice; bacterial colonization was increased ∼100-fold and ∼10,000-fold in SM-treated and VAN-treated mice, respectively (Fig. 1d). We then measured the relative amount of the Vc 16S ribosomal RNA (rRNA) gene in the small intestine of each group. Consistent with the increased Vc colonization, a higher level of the Vc gene was detected in antibiotic-treated mice and this increase was most significant in VAN-treated mice (Fig. 1e). Altogether, these results demonstrate that administration of low concentrations of antibiotics alters the mouse gut microbiota composition, which subsequently results in increased host vulnerability to Vc colonization.
An atEc strain proliferated on antibiotic treatment
We then quantitated the relative abundance of different commensal microbe phylogenetic groups in each treatment group by real-time PCR using group-specific primer sets (Supplementary Table 1). Genomic DNA extracted from small intestine tissue lysates was subjected to amplification and normalized to the level of the host gapdh gene. The relative quantity of the γ-proteobacteria 16S rRNA gene was markedly increased in VAN-treated mice (Supplementary Fig. 1a). Subsequent family-level and species-level examinations indicated that the levels of the Enterobacteriaceae and E. coli 16S rRNA genes were remarkably increased in VAN-treated mice and were also increased, albeit to a lesser extent, in the SM-treated group (Supplementary Fig. 1b,c). In contrast, the relative quantities of the marker genes for selective detection of Bifidobacterium, Lactobacillus and Bacteroides were similar between all groups (Supplementary Fig. 1d–f). These results demonstrate that (i) the abundance of E. coli was specifically increased during treatment with SM or VAN, and (ii) this increase likely accounted for the elevated levels of Enterobacteriaceae and γ-proteobacteria class bacteria in the antibiotic-treated groups.
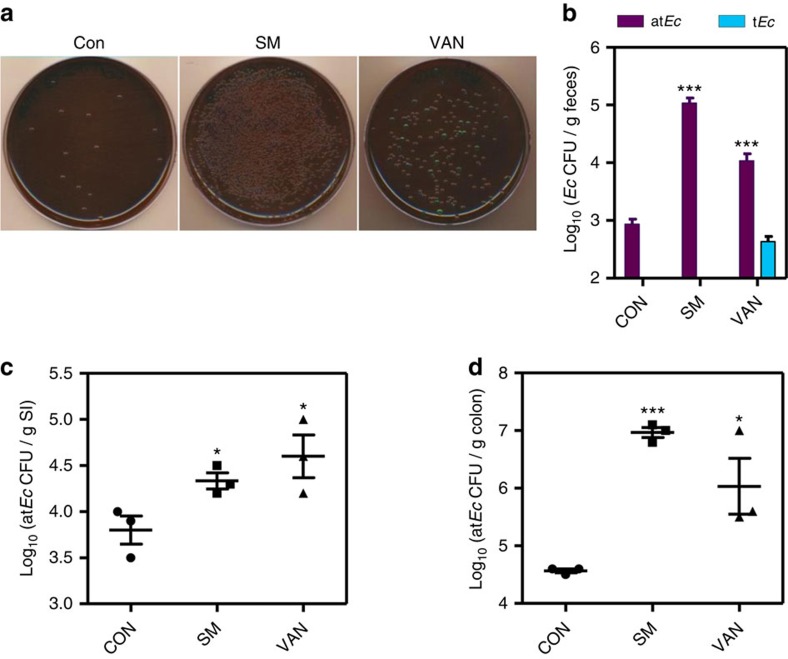
Next, suspensions of mouse faeces were cultivated on eosin methylene blue (EMB) agar, a selective medium for Gram-negative bacteria, especially those belonging to the Enterobacteriaceae family18. Consistent with our DNA-based assay results, more colonies grew from faeces obtained from SM-treated and VAN-treated mice (Fig. 2a). Species identification was performed on several colonies and revealed that all were E. coli. This finding further supports the results shown in Supplementary Fig. 1. Intriguingly, however, a majority of the bacterial cells grew as colourless colonies on the EMB plates (Fig. 2a). Typical E. coli strains can ferment lactose, thereby yielding colonies with a distinctive metallic green sheen when grown on EMB plates19. As shown in Fig. 2b, the numbers of atypical (that is, colourless) E. coli (termed atEc) colonies increased to ∼105 and ∼2.5 × 104 per g of faeces in SM-treated and VAN-treated mice, respectively. Notably, typical green colonies (termed tEc) were also recovered in the VAN-treated group (Fig. 2b). We then performed an RAPD assay to examine the extent of genomic diversity among these E. coli isolates. Sixteen strains (14 colourless, 1 green and 1 intermediate green) were included in this analysis. Although the amplification products were almost identical among the atEc strains, these products were distinct from those obtained from the typical green and intermediate green strains (Supplementary Fig. 2). This result suggests that (i) atEc strains possess a distinct genome, and (ii) the antibiotic-induced propagation of atEc strains was likely mediated by clonal expansion. The numbers of atEc strains were also significantly increased in the small intestine and the colon of SM-treated and VAN-treated mice (Fig. 2c,d). In each group, atEc strains were more prevalent in the colon than in the small intestine, indicating that the colon is a more suitable habitat for this strain.
Figure 2. Atypical E. coli cells proliferate rapidly in response to antibiotic treatment.
(a) Representative images of EMB plates inoculated with aliquots of mouse faecal suspensions. Mouse faeces were collected and homogenized in PBS before inoculation. (b) Quantification of atEc and tEc strains recovered from mouse faeces collected from each treatment group. The CFUs of atEc and tEc cells are shown in purple and blue bars, respectively. Values are expressed as means±s.e.m. and are displayed on a log scale. ***P<0.001 versus atEc CFUs detected in the control group. (c) Quantification of atEc colonies grown from the small intestine homogenates of each group. Values are expressed as means±s.e.m. and are displayed on a log scale. *P<0.05 versus the control group. (d) Quantification of atEc colonies grown from the mouse colon homogenates of each group. Values are expressed as means±s.e.m. and are displayed on a log scale. ***P<0.001, *P<0.05 versus the control group.
We next asked whether propagation of the atEc strain was invariably observed in mice that received a different antibiotic regimen (Supplementary Fig. 3a). Treatment of adult mice for 4 weeks with a mixture of four different antibiotics in the drinking water revealed a similar increase in the number of colourless colonies, which were later identified as E. coli, on EMB plates (Supplementary Fig. 3b). Of particular note, the mean mouse colon weight was increased in the antibiotic-treated group (∼0.2 g) versus the control group (∼0.16 g), an apparent host phenotypic change in response to antibiotic treatment (Supplementary Fig. 3c). On subsequent Vc infection, antibiotic-treated mice exhibited significantly increased fluid accumulation (FA) in their small intestines (Supplementary Fig. 3d). The extent of FA has been shown to depend on the degree of Vc infectivity of the host20. Most importantly, ∼50-fold more Vc cells were recovered from the intestines of antibiotic-treated mice (Supplementary Fig. 3e). Altogether, these findings further suggest that the atEc strain is highly proliferative under antibiotic stress and that Vc infection is facilitated by the increased abundance of the atEc strain.
The atEc strain possesses an extra catalase gene
We next sought to understand the molecular basis underlying the positive effect of the atEc strain on Vc colonization. To this end, we sequenced the genomes of the atEc and tEc strains and compared them with the E. coli K12 genome. The atEc strain was found to have the largest genome (5.24 Mbp), whereas the tEc and K12 genomes were 4.72 Mbp and 4.64 Mbp, respectively (Supplementary Fig. 4a). Alignment of the atEc and tEc genomes using the Maximal Unique Matcher algorithm21 revealed that most of the regions overlapped with each other (Supplementary Fig. 4b, red diagonal line). However, many similar genetic elements were found to be highly scattered over the entire genomes, revealing a high degree of dissimilarity between the two genomes (Supplementary Fig. 4b). Of particular note, the 3′ region of the lacY gene (which encodes lactose permease) was deleted in the atEc strain, whereas the full-length lacY gene was detected in the tEc and K12 strains (Supplementary Fig. 4c). The presence of a defective lacY gene explains why the atEc strain formed colourless colonies on the lactose-containing EMB plates.
We then carried out genome-based clustering analysis using two isolates and representative E. coli/Shigella strains. In a dendrogram constructed based on the average nucleotide identity (ANI), the tEc and atEc strains were quite distant from E. coli K12 (Supplementary Fig. 5). Futhermore, tEc and atEc were relatively distant from each other, with ANI of 99.1%, indicating that the two strains belong to different phylogenetic lineages within the E. coli group (Supplementary Fig. 5).
Importantly, the comparison with the tEc genome showed that the atEc genome contains an extra gene encoding a catalase, a critical enzyme that protects cells from ROS-mediated oxidative damage (Supplementary Fig. 6a). Activity-based catalase assays indicated that the atEc strain produced three distinct catalases, including two that were also detected in the tEc strain (Supplementary Fig. 6b, black arrowheads). The extra catalase (termed eKatE) expressed in the atEc strain was only ∼61% identical to KatE on the basis of amino-acid sequence (Supplementary Fig. 6c). Among 4,045 Escherichia and Shigella genomes available in public databases, only one strain, E. coli K02, has a gene of identical sequence to that of the atEc strain. Outside the Escherichia/Shigella group, an eKatE-like gene (with >90% nucleotide sequence identity) was found only in Serratia (γ-proteobacteria) and Frankia (Actinobacteria) species (Supplementary Fig. 6d). This information suggests that the eKatE gene might have been horizontally transferred from another species with a different degree of relatedness.
To provide supporting evidence for horizontal transfer of the eKatE gene, we performed several bioinformatics analyses. First, we constructed two different phylogenetic trees based on amino-acid sequences of KatG and KatE proteins, respectively (Supplementary Fig. 7). In the KatG-based tree, KatG proteins from tEc and atEc strains were clustered together with those of other E. coli strains (Supplementary Fig. 7a, black arrows). Likewise, evolutionary distance was not detected between KatE proteins from both strains (Supplementary Fig. 7b, black arrows). In contrast, eKatE-encoded catalase was distinctly clustered with proteins from unrelated species (for example, of the genera Serratia, Frankia, Lonsdalea and Rouxiella; Supplementary Fig. 7b, red arrow). Second, tetranucleotide frequency was analysed for the katE genes found in 33 representative E. coli strains. The tetranucleotide frequency of the eKatE gene exhibited the lowest correlation coefficient when compared with other values in the matrix table (Supplementary Table 2). In contrast, katE genes of the atEc or tEc strain were similar to other katE genes of E. coli origin in terms of the tetranucleotide frequency. Third, we also measured codon adaptation index (CAI) of three catalase genes (katG, katE and eKatE) of the atEc strain. The CAI of the eKatE gene clearly deviated from that of the other two genes (Supplementary Table 3). Moreover, GC content of the eKatE gene was significantly lower than that of katG and katE genes. Fourth, shared synteny was observed for genes encoding transposase in atEc and E. coli K02 strains (Supplementary Fig. 8). Genes encoding transposases are also detected in Serratia and Frankia species (Supplementary Fig. 8). More importantly, ccdBA genes are present near the eKatE gene in the atEc chromosome (Supplementary Fig. 9). These two genes are known to be plasmid-borne and produce the CcdA/CcdB toxin-antitoxin module that is involved in plasmid maintenance in E. coli22. Altogether, these analyses strongly suggest that the atEc strain acquired the eKatE gene by horizontal gene transfer from an outside mobile genetic source.
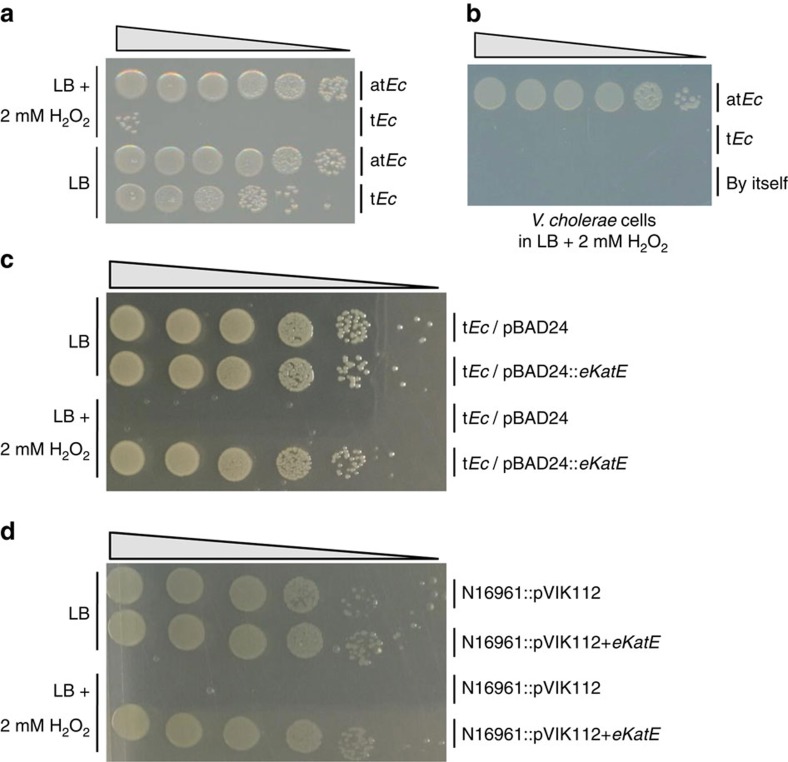
The atEc strain is highly resistant to H2O2
On the basis of our native gel-based activity assay (Supplementary Fig. 6b), eKatE-encoded catalase appeared to have a stronger enzymatic activity than KatG or KatE, suggesting that the atEc strain might be more resistant to H2O2 than the tEc strain. In LB supplemented with 2 mM H2O2, atEc cells grew completely normally, as they did in plain LB (Fig. 3a). In contrast, growth of the tEc strain was significantly inhibited in the presence of H2O2 (Fig. 3a), indicating that the production of eKatE may render the atEc strain resistant to H2O2. Vc cells grew normally in LB+2 mM H2O2 that had been preinoculated with atEc for 2 h, whereas no growth was observed in the same medium that had been pretreated with tEc for the same period of time (Fig. 3b). These results suggest that atEc cells can detoxify H2O2, thereby helping Vc cells proliferate in the presence of ROS stress. An additional 18 strains that formed colourless colonies on the EMB plates also produced significant levels of eKatE, further supporting the idea that the atEc strain had clonally expanded during antibiotic treatment (Supplementary Fig. 10). The relative quantity of the eKatE gene in small intestine tissue lysates was increased in the SM-treated group and to an even greater extent in the VAN-treated group (Supplementary Fig. 11). The increase in the level of the eKatE gene was proportional to that of the E. coli 16S rRNA gene (Supplementary Fig. 1c). This result further indicates that the increased population of E. coli in antibiotic-treated mice can be attributed to the proliferation of atEc strains.
Figure 3. The atEc strain is resistant to H2O2.
(a) Viable cell numbers of atEc and tEc strains after growth in LB for 3 h in the presence (top two rows) or absence (bottom two rows) of 2 mM H2O2. Serial dilutions of bacterial cultures were spot-inoculated onto LB plates. (b) Viable cell numbers of Vc N16961. Overnight cultures of N16961 cells were diluted 100-fold in LB+2 mM H2O2 that had been precultured for 2 h with atEc or tEc. N16961 was grown for a further 4 h. The Vc CFUs in each culture were determined by growing serial dilutions on LB+200 μg ml−1 SM plates. (c) Viable cell numbers of tEc strains harbouring pBAD24 or pBAD24::eKatE after growth in LB for 3 h in the absence (top two rows) or presence (bottom two rows) of 2 mM H2O2. Serial dilutions of bacterial cultures were spot-inoculated onto LB plates. (d) Viable cell numbers of Vc N16961 strains with chromosomally integrated pVIK112 or pVIK112+eKatE plasmid after growth in LB for 3 hrs in the absence (top two rows) or presence (bottom two rows) of 2 mM H2O2. Serial dilutions of bacterial cultures were spot-inoculated onto LB+200 μg ml−1 SM plates.
The tEc strain also produces KatG and KatE, two distinct catalases (Supplementary Fig. 6b). To further verify the role of eKatE in H2O2 resistance, we constructed recombinant tEc and Vc strains that express a plasmid-borne or a chromosomally inserted eKatE gene, respectively. A DNA element encompassing both the eKatE gene open reading frame and its endogenous promoter was used for cloning. The recombinant tEc strain harbouring the pBAD24::eKatE plasmid was found to be resistant to 2 mM H2O2 and grew completely normally in the presence of 2 mM H2O2, as in plain LB media (Fig. 3c). Moreover, a Vc N16961 strain expressing the eKatE gene was equally resistant to 2 mM H2O2 (Fig. 3d). These results demonstrate that the eKatE-encoded catalase is responsible for the ROS resistance detected in atEc cells. Besides the unique presence of eKatE gene in the atEc strain, genetic repertoires for oxidative stress responses are almost identical between the two strains. In each strain, 57 genes were identified that are known or presumed to be involved in oxidative stress response. Among these, 56 genes are found in both genomes, except for atEc_0417 (eKatE) and tEc_2780 genes (Supplementary Table 4).
Vc infection is severe in the atEc-transplanted infant mice
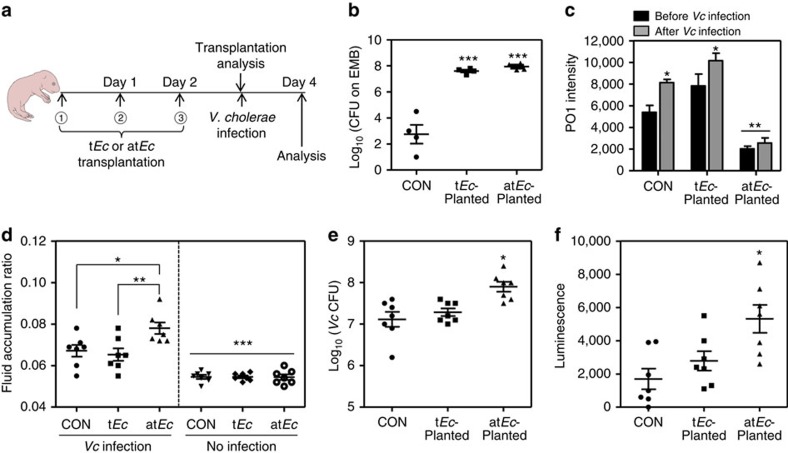
Infant mice have been widely used as a surrogate host to study Vc infection in vivo23,24. We found that antibiotic-treated mice exhibited a substantially altered gut microbiota composition and increased susceptibility to Vc colonization. To define causality in the relationship between the increased intestinal population of atEc and increased host sensitivity to Vc infection, infant mice were challenged with Vc following daily transplantation with 107 atEc or tEc cells for 3 days (Fig. 4a). Transplantation of tEc or atEc was efficiently achieved, as demonstrated by the recovery of ∼4 × 107 tEc and ∼9 × 107 atEc cells from mouse intestines at 3 days post transplantation (Fig. 4b). Significantly lower bacterial loads were detected in the control group, indicating that bacterial species belonging to the Enterobacteriaceae family represent only a minor proportion of the total commensal microbes in the infant mouse (Fig. 4b). Importantly, when mouse intestinal tissue homogenates were stained with PO1, a ROS-sensitive fluorescent dye25, a marked decrease in the fluorescence signal was observed in the atEc-transplanted group but not in its tEc-transplanted counterpart (Fig. 4c). The PO1-specific signal was somewhat increased in the intestines of tEc-transplanted mice compared with those from the control group (Fig. 4c). This finding indicates that atEc cells can also readily degrade host-derived ROS in vivo. The PO1-specific signals were increased in control and tEc-transplanted groups after Vc infection (Fig. 4c), suggesting that Vc infection stimulates ROS production in the mouse intestine. Of particular importance, such an increase was not observed in the atEc-transplated group (Fig. 4c), further suggesting that an abundant population of atEc cells can control intestinal ROS levels in the host.
Figure 4. Infant mice transplanted with atEc exhibit increased susceptibility to Vc infection.
(a) Schematic diagram of the experimental procedure. Five-day-old infant mice were orogastrically transplanted three times with either the atEc or tEc strain (107 cells). On day 3 post-transplant, mice were infected with 106 N16961 cells. (b) To assess the transplantation efficiency, a subset of the mice in each group (n=4) were killed and intestinal tissue homogenates were obtained. These homogenates were inoculated into EMB medium to determine the number of ingested bacterial cells. Values are expressed as means±s.e.m. and are displayed on a log scale. ***P<0.001 versus the number of bacterial colonies from the control group. (c) Mouse intestinal tissue lysates obtained from each group (n=4) were stained with 100 μM PO1 for 30 min to detect ROS. Values are expressed as means±s.e.m. and are displayed in each bar. *P<0.05 versus PO1 signals from the ‘before Vc infection’ groups. **P<0.01 versus the signals of all the other groups. (d) Each group of mice was challenged with Vc (n=7, left panel) or left uninfected (n=7, right panel). The fluid accumulation (FA) ratio of each group was measured and plotted on a linear scale. *P<0.05 versus the FA ratio of the Vc-infected control group. **P<0.01 versus the FA ratio of the Vc-infected tEc-transplanted mice. *** indicates the FA ratios of the noninfected groups were significantly different from those of all Vc-infected groups (P<0.005). (e) The number of Vc cells that colonized the mouse intestine (n=7 in each group) was determined by CFU counting. Values are expressed as means±s.e.m. and are displayed on a log scale. *P<0.05 versus Vc CFUs of the control or tEc-transplanted group. (f) Infant mice (n=7 per group) were infected with a Vc ctxAB promoter fusion reporter strain. The level of bioluminescence was measured in each mouse intestinal tissue homogenate. Values are expressed as means±s.e.m. and are displayed on a linear scale. *P<0.05 versus the bioluminescence level in control mice.
Subsequent Vc infection resulted in a noticeable increase in the level of intestinal FA in atEc-transplanted mice (Fig. 4d), whereas the FA ratios of the tEc-transplanted and control groups were comparable. Significantly active Vc colonization and high levels of the ctxAB promoter were also detected in mice transplanted with atEc (Fig. 4e,f). These results demonstrate that high loads of atEc cells inside the host intestine generate conditions that facilitate Vc infection.
Increased Vc infection is due to eKatE-encoded catalase
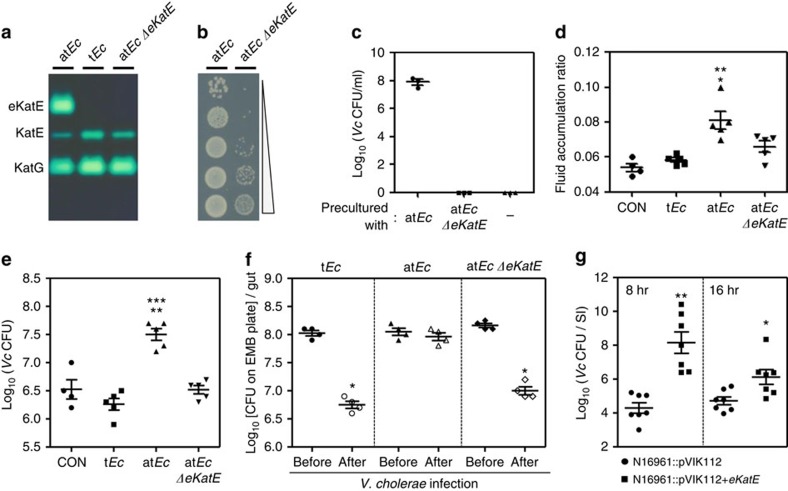
We then examined whether the catalase activity of the eKatE gene product plays a role in atEc-mediated enhancement of Vc infection. To address this, we constructed an eKatE in-frame deletion mutant of atEc strain. The data shown in Fig. 5a clearly demonstrate the lack of eKatE-encoded catalase activity of the mutant. Disruption of the eKatE gene abrogated the H2O2 resistance of the atEc strain (Fig. 5b). The atEc ΔeKatE mutant failed to protect Vc cells against H2O2 stress in our in vitro co-culture system (Fig. 5c). Importantly, the extent of Vc infection-mediated FA induction was significantly reduced in ΔeKatE-transplanted infant mice compared with atEc-transplanted infant mice (Fig. 5d). Moreover, the facilitatory effect of atEc transplantation on Vc colonization disappeared when the eKatE gene was deleted. As shown in Fig. 5e, 10-fold higher numbers of Vc cells were recovered after 24-h infection in atEc-transplanted infant mice, compared with tEc- or ΔeKatE-transplanted groups. It is of particular interest that atEc remained colonized during the 24-h Vc infection period, whereas the abundance of tEc and the ΔeKatE mutant substantially decreased in response to Vc infection (Fig. 5f). These findings suggest that (i) the atEc strain possesses better ‘colonization fitness’ under Vc infection-induced host stress conditions and (ii) Vc in vivo colonization likely occurs as a consequence of co-operative interaction with atEc cells in a catalase-dependent manner.
Figure 5. The eKatE-encoded catalase plays a critical role in atEc-mediated enhancement of Vc infectivity.
(a) Construction of an atEc eKatE deletion mutant. Bacterial extracts were loaded on a 7.5% nondenaturing polyacrylamide gel, electrophoresed to allow protein separation and then stained for catalase activity. (b) Serial dilutions of bacterial cultures (atEc strain and its ΔeKatE mutant) were inoculated onto LB plates after growth in LB+2 mM H2O2 for 3 h. (c) An overnight culture of N16961 was diluted 100-fold into LB+2 mM H2O2 that had been precultured for 2 h with atEc or its ΔeKatE counterpart. N16961 cells were grown for a further 4 h. Vc viability was determined by CFU counting. (d) Vc-induced FA ratios in groups of infant mice (n=5 per group) that had been transplanted with tEc, atEc or atEc ΔeKatE cells. Non-transplanted mice (n=4) were used for the control infection. **P<0.005 versus the FA ratio of the control or tEc-transplanted group. *P<0.05 versus the FA ratio of ΔeKatE-transplanted mice. (e) The numbers of Vc N16961 cells recovered from the intestines of each group are expressed as means±s.e.m. and are displayed on a log scale. **P<0.005 versus Vc CFUs from the control group. ***P<0.001 versus Vc CFUs from tEc-transplanted or ΔeKatE-transplanted mice. (f) The number of each E. coli strain recovered from infant mice (n=4 per group) before (solid) and after (open) Vc infection was determined; values are expressed as means±s.e.m. and are displayed on a log scale. *P<0.01 versus CFUs from the ‘before’ group. (g) Adult mice (n=14 per group) were infected with either Vc N16961::pVIK112 (circles) or N16961::pVIK112+eKatE (squares) by oral gavage. At 8 and 16 h post-infection, seven mice in each infection group were killed and bacterial numbers present in small intestine were determined. Values are expressed as means±s.e.m. **P<0.001, *P<0.05 versus control infection. Bacterial suspensions were prepared from mid-log phase cultures at ∼1014 per ml and 200 μl of suspensions were used for oral gavage.
Finally, we examined whether the H2O2-resistant Vc strain shown in Fig. 3d exhibited a superior colonization capability in conventional adult mice. The N16961::pVIK112+eKatE strain colonized significantly better than the control strain; at 8 h post-infection ∼10,000-fold more bacterial cells remained colonized in the mouse small intestine (Fig. 5g). Although lower numbers of bacterial cells were recovered after 16 h of infection, the eKatE-expressing cells still colonized ∼13.8-fold better than the control cells (Fig. 5g). This result demonstrates that Vc colonization also occurs more readily when Vc alone can handle ROS stress.
Discussion
The gut microbiota and products encoded by its genome (that is, the gut microbiome) play critical roles in human health. Commensal bacteria that reside in the host intestine contribute to the development of a functional intestinal immune system26. The gut microbiome, which is considered to supplement the human genome with >100-fold more genes, contains genes that produce digestive enzymes lacking in humans27. Furthermore, phylum-level changes in their composition account for the differential propensity of individuals to develop obesity28. Along with these important functions, commensal microbes also participate in regulating host defenses against the invasion of pathogenic bacteria16,29. In this study, we isolated a commensal E. coli strain that exhibits exceptional resistance to ROS and demonstrated that such an atypical E. coli strain, when abundantly present in the host intestine, can enhance host susceptibility to enteric infection.
Our results suggested that the atEc strain was slightly more resistant to SM than the tEc strain; in the presence of SM (32 μg ml−1), atEc cells grew slightly better than tEc cells (red arrow, Supplementary Fig. 12). However, both atEc and tEc strains were equally sensitive to ampicillin and tetracycline (data not shown). In addition, both strains showed no sensitivity to VAN, an effective antibiotic against Gram-positive organisms (data not shown). On the basis of these results, it appears that the atEc strain is not more resistant to antibiotics and therefore the abundant population of atEc in antibiotic-treated mice is not caused by its superior capability to survive antibiotic treatment.
On the other hand, host-mediated changes may play a critical role in creating an environment that stimulates the propagation of atEc cells in antibiotic-treated mice. Accumulating evidence suggests that an increase in LPS inside the host intestine stimulates ROS production30,31,32. During antibiotic treatment, the leve of Enterobacteriaceae, a large family of Gram-negative bacteria, was greatly increased. Therefore, it is likely that the amount of LPS released from those Gram-negative species may also be significantly increased. Consistent with this notion, the data in Fig. 4c show that the PO1 signal was noticeably increased in tEc-transplanted mice, indicating that the increased population of tEc cells inside the mouse intestine resulted in elevated ROS levels. Stable transplantation of the atEc strain, however, resulted in a significant decrease in the level of ROS inside the host intestine (Fig. 4c). This change in the intestinal environment presumably helps Vc, which is known to be ROS-sensitive33, colonize and exert pathogenic effects. Recently, Lupp et al.34 reported that the relative abundance of nonpathogenic E. coli was increased during chemically induced intestinal inflammation, a process that generates excessive ROS. However, no basis was provided for such a population change. It will be very interesting to see whether the E. coli strain described in their study shares common phenotypes with the atEc strain that we identified in the current study. The robust activity of the eKatE-encoded catalase helped the atEc strain overcome surrounding environmental changes, which likely involved the accumulation of high levels of ROS. Although it remains unclear how much ROS was actually produced during antibiotic treatment in our model system, the atEc strain must have competitive survival fitness under conditions of host-mediated ROS production.
Vc colonization and cholera toxin-induced intestinal fluid accumulation were increased in atEc-transplanted infant mice, but not in mice transplanted with atEc cells lacking the eKatE gene. These results indicated that the pathogenesis of Vc infection was critically influenced by gut microbiota composition and a microbiome gene product that regulates intestinal ROS level in vivo. Diverse virulence determinants have been reported to play distinct roles in Vc pathogenesis in different animal models. For example, persistent colonization of Vc in adult mice depends on accessory toxins (that is, hemolysin and RTX toxin), but not on toxin co-regulated pilus35. Toxin co-regulated pilus, however, is essential for bacterial colonization in infant mice and infant rabbits36,37. In addition, cholera toxin promotes bacterial colonization in adult rabbits38, but not in infant rabbits36. These previous results strongly suggest that host age is an important factor that affects susceptibility to Vc infection. Since changes in microbiota composition are clearly observed with aging39,40,41, how Vc infectivity is modulated by age-dependent changes in microbiota composition must be further addressed.
The development of cholera vaccines has been hampered, in part, by the difficulty in assessing the efficacy of a candidate vaccine economically using adult mice. Our results suggest that adult mice with an increased population of atEc cells can develop cholera-like symptoms. The activity of catalase encoded by the eKatE gene appears to be significantly higher than that of other well-characterized catalases42. We showed that a recombinant Vc strain that produces eKatE-encoded catalase was fully resistant to 2 mM H2O2 and this ROS-resistant Vc strain exhibited enhanced colonization capability in a normal adult mouse. Provided that virulence is unaffected by eKatE gene expression, this recombinant Vc strain may have potential application as a challenge strain in future vaccine development.
As shown in Supplementary Data 1, 269 different katE-encoded catalases were identified in the human gut microbiome catalogue. This indicates that at least 269 bacterial species can produce proteins identical or similar to the KatE catalase in human intestine. Of note, KatE proteins produced by the species belonging to the Proteobacteria phylum exhibit the highest sequence similarities with that of the atEc strain. The genomes of 50 bacterial species, mostly in the same phylum, encode KatG catalases. Meanwhile, in the mouse gut microbiome catalogue, 93 and seven katE- and katG-encoded catalases were identified, respectively. Interestingly, Proteobacteria is not a major group that produces KatE catalase in mouse intestine. Altogether, these results suggest that (i) KatE might be produced in larger quantities than KatG in both human and mouse intestines, and (ii) different bacterial species contribute to the production of KatE catalases in human versus mouse intestine. Currently, detailed information is lacking in regards to the relative abundances of individual catalase producers in the gut microbiota, and more information is needed to more definitively compare overall catalase activity in human versus mouse intestines. We expect that such information would prove useful in identifying the mechanisms of human-restricted tropism of bacterial infections, including that by V. cholerae.
The commensal species that modulate host susceptibility to disease are beginning to be defined9,43. However, the underlying genetic determinants that modulate this susceptibility have not yet been identified. The ability of atEc to facilitate Vc infection was completely abrogated by the deletion of a single gene, eKatE. This finding demonstrates that the level of ROS inside the host intestine, which must be carefully regulated for protection against pathogenic invaders, can be controlled by a single-microbiome gene product. We anticipate that our results (summarized in Fig. 6) will stimulate the assembly of a database of microbiota-associated genes with defined functions, to better understand the roles such genes play in the complicated ecosystem of the host intestine.
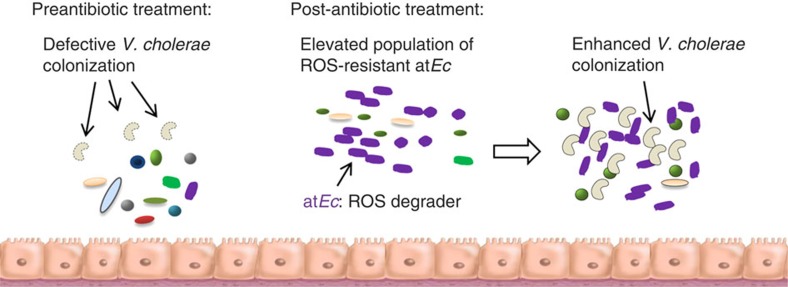
Figure 6. Summary of antibiotic-induced proliferation of atEc and its impact on host susceptibility to Vc infection.
Phylogenetically diverse bacterial cells that were originally present in the untreated host are depicted in different colours and shapes. Defective and enhanced colonization of Vc cells are denoted with dotted and solid lines (comma-shaped), respectively. The increased population density of atEc is indicated by the increased number of purple cells.
Methods
Bacterial strains
The indigenous E. coli strains termed typical E. coli (tEc) and atypical E. coli (atEc) were isolated from CD-1 mouse intestines. V. cholerae (Vc) O1 serotype N16961 (ref. 44) was used as a model pathogen in all experiments. All strains were routinely grown aerobically in Luria-Bertani (LB) broth (10 g tryptone, 5 g yeast extract, and 10 g NaCl per l) or on LB agar plates (15 g l−1 agar) at 37 °C. Streptomycin (200 μg ml−1) was added to selectively cultivate N16961.
Construction of mutant and reporter strains
The atEc ΔekatE knockout mutant was created by allelic replacement as described previously45. The 500-base pair flanking sequences located at both ends were amplified by PCR with the primer sets listed in Supplementary Table 1 and used to introduce the mutation by homologous recombination. The primers used to construct the deletion mutant were designed according to the atEc genomic sequence. A transcriptional luxCDABE reporter construct was constructed by PCR amplification of a 500-bp EcoRI–XbaI ctxAB promoter fragment from N16961 genomic DNA. This fragment was subsequently cloned into the EcoRI–XbaI sites of pVIK112, thereby generating pVIK112::PctxAB. The transcriptional fusion reporter was then constructed by cloning the SalI–SacI promoterless luxCDABE fragment from pBBR-lux into pVIK112::PctxAB digested with the same enzymes, thus generating pVIK112::PctxAB::luxCDABE. Chromosomal integration of the resultant fusion construct was performed as previously described20.
Mouse models and housing conditions
Experiments were performed with CD-1 and Balb/c mice purchased from Orient Bio (Seoungnam, Korea). Pregnant Balb/c mice were purchased from Central Lab. Animal Inc. (Seoul, Korea) and raised for neonatal delivery. Mice were provided with irradiated food and sterile water. All animal studies were performed in compliance with the guidelines provided by the Department of Animal Resources of Yonsei Biomedical Research Institute. The Committee on the Ethics of Animal Experiments at the Yonsei University College of Medicine approved this study (permit number 2011-0166). For antibiotic treatment, 5- to 6-week-old CD-1 female mice were orally treated with streptomycin (1 mg), vancomycin (250 μg), or PBS as a control once a day for 7 days. Antibiotic doses were chosen to induce alteration of the gut microbiota composition without eliminating commensal microbes11. Bacterial counts were determined by plating serial dilutions of small intestine homogenates and faecal suspensions on plate count agar (Difco), LB agar, and Eosin Methylene Blue (EMB) agar plates. Fluorescence images of faecal suspensions were acquired with a LIVE/DEAD BacLight staining kit (Invitrogen)46. For Vc infection, antibiotic-treated CD-1 mice were orally inoculated with 107 CFU of LB-grown N16961 cells after 18 h of food and water starvation. At 48 h postinfection, bacterial cells were recovered by homogenizing the mouse intestines in 2 ml of PBS containing 10% glycerol. The homogenates were diluted and plated on solid medium containing 200 μg ml−1 streptomycin. Luminescence was measured using a Victor X4 plate reader (Perkin Elmer). For experiments involving antibiotic cocktail treatment, mice were treated for 4 weeks with drinking water supplemented with four different antibiotics (ampicillin, 100 μg ml−1; vancomycin, 10 μg ml−1; metronidazole, 50 μg ml−1; and neomycin, 30 μg ml−1). For mouse intestinal transplantation, neonatal mice (4–5 days old) were treated orally once daily with 107 CFU of tEc or atEc cells for 3 days. During the transplantation period, neonates were housed with their mothers.
DNA purification from mouse faeces, small intestine and colon
Fresh stool pellets were collected before mice were killed. Faecal samples were stored at −80 °C before DNA purification. Immediately after the mice were euthanized, their small intestines and colons were recovered manually. Total genomic DNA was extracted from the faecal samples using a QIAamp DNA Stool Mini Kit (Qiagen) according to the manufacturer’s protocol. DNA was precipitated with ethanol and resuspended in 50 μl of TE buffer with 100 μg ml−1 RNase. Total genomic DNA was extracted from intestinal samples using the QIAamp DNA Stool Mini Kit (Qiagen) with a minor modification. Briefly, the recovered mouse organs were homogenized in 2 ml of PBS and centrifuged at low speed to remove tissue debris. The resultant supernatants were subjected to the purification procedures outlined in the QIAamp DNA Stool Mini Kit.
16S rRNA gene quantification by qRT–PCR
The relative abundance of each specific bacterial group was analysed by quantitative real-time PCR. Two microlitres of 10-fold diluted genomic DNA samples obtained from mouse faeces, small intestines, or colons served as the templates for SYBR Green-based quantitative PCR with reverse transcription (qRT–PCR) reactions. qRT–PCR was performed using a SYBR Premix Ex Taq kit (Takara Bio Inc., Japan) and gene-specific primers. All primers used in this study are listed in Supplementary Table 1. For each sample, at least three qRT–PCR replicates were performed. The total volume of each reaction was 25 μl. Each reaction contained DNA template (diluted 10- or 100-fold), 0.2 mM dNTPs, 1.5 mM MgCl2, 1.25 U SYBR premix Ex Taq DNA polymerase, 2.5 μl of 10 × PCR buffer, and 0.2 μM of each species-specific primer. Primers were designed to amplify the 16 S region as previously described8,47,48,49,50,51. Thermocycling conditions were as follows: 94 °C for 3 min, followed by 35 cycles (faecal samples) or 40 cycles (small intestine or colon samples) of 94 °C for 30 s, 60 or 62 °C for 45 s, and 72 °C for 1 min. The level of each gene was normalized to that of the host housekeeping gene gapdh. Results are expressed relative to the 16S rRNA level obtained with species-specific primers from an intestinal sample isolated from control mice.
RAPD assay
E. coli strains isolated from antibiotic-treated mice were genotyped by random amplified polymorphic DNA (RAPD) fingerprinting as described elsewhere52. Briefly, genomic DNA was extracted using a G-Spin Genomic DNA Extraction Kit (iNtRON Biotechnology Inc.) following procedures provided by the manufacturer. A dendrogram was generated with XLSTAT software (Addinsoft USA, USA) based on the unweighted pair group method using an arithmetic algorithm (UPGMA).
Whole-genome sequence and annotation
The tEc and atEc strains were grown in LB medium at 37 °C for 15–16 h, with shaking under aerobic conditions. Genomic DNA was extracted from bacteria using the G-Spin Genomic DNA Extraction Kit (iNtRON Biotechnology Inc.). Bacterial genomes were sequenced on an Illumina MiSeq system (Illumina, Inc., USA). Sequence reads (tEc: 7,156,532 reads with a total read length of 2,281,359,270 bp; atEc: 6,496,870 reads with a total read length of 1,721,860,260 bp) were assembled de novo using GS Assembler v. 2.6 (Roche Diagnostics) and CLC genomics workbench 6.0 (CLC bio, Denmark). This assembly resulted in 62 and 152 contigs for tEc and atEc, respectively. The contigs and PCR-based long reads were combined through manual curation using CodonCode Aligner 3.7.1 (CodonCode Corp., Dedham, MA, USA). The gaps within and between contigs were filled by custom primer walking and long-distance PCR amplification, followed by DNA sequencing with an ABI 3730XL sequencer. Coding sequences (CDSs) were predicted by Prodigal53 and tRNAs were searched using tRNAscan-SE54. The rRNAs and other noncoding RNAs were searched by a covariance model search with Rfam 12.0 database55. Basic genetic information such as GC content, number of ORFs, and prediction of ORF function was analysed by CLgenomics software (http://www.chunlab.com). The CDSs were classified into different groups based on their roles, with reference to orthologous groups (EggNOG 4.1; http://eggnogdb.embl.de)56. For more functional annotation, the predicted CDSs were compared with KEGG57 and SEED58 databases (BLASTP). The complete genome sequence of tEc was 4,726,216 bp and its G+C content was 50.65%. Gene prediction identified 4,403 putative CDSs. The total genome length and G+C content of atEc were 5,243,781 bp and 50.56%, respectively; 5,019 putative CDSs were identified in the atEc genome.
Bioinformatic analyses
Tetranucleotide frequency and CAI were calculated following procedures described elsewhere59,60. The pairwise overall similarity between genome sequences was calculated using the OrthoANI method61 and the dendrogram (shown in Supplementary Fig. 5) was generated using the UPGMA method. Sequences for phylogenetic analysis of KatG and KatE were retrieved from the GenBank database using the BLAST algorithm. Sequences were aligned with MUSCLE v3.8.31 (ref. 62) and aligned positions with >50% gaps were removed using GBLOCKS v0.91 (ref. 63). The phylogenetic relationships were inferred with RAxML v8.2.4 (ref. 64) and the trees (shown in Supplementary Fig. 7) were visualized using Dendroscope v3.2.2 (ref. 65). The trees were rooted by proteins that belong to the basal clade of each catalase group66.
Catalase activity assays
A native gel-based catalase activity assay was performed as previously reported67,68. Briefly, proteins in bacterial lysates were resolved on a 7.5% nondenaturing acrylamide gel and stained with 3,3′-diaminobenzidine (Sigma). Lysates were prepared by sonicating bacterial cell suspensions in 50 mM Tris-HCl, pH 7.4.
Construction of recombinant tEc and Vc strains
A DNA element encompassing the eKatE gene and its own promoter was PCR amplified and cloned into the multicloning site of pBAD24 plasmid. The resultant plasmid, named pBAD24::eKatE, was transformed into the tEc strain. PCR primers used for cloning are listed in Supplementary Table 1. A 600 bp non-functional region between the VC0512 and VC0513 genes in the genome of Vc N16961 strain was PCR amplified and ligated into pVIK112 plasmid. The resultant plasmid, pVIK112-600 bp, was digested with SacI and ligated with the SacI-digested PCR product that amplified the eKatE gene locus in the pBAD24::eKatE plasmid. The final plasmid, named pVIK112+eKatE, was conjugated into N16961. Transconjugants, in which pVIK112+eKatE was integrated in the 600 bp noncoding region via homologous recombination, were selected and verified by sequencing. pVIK112 with only the 600 bp non-functional sequence was used as a control. PCR primers used are listed in Supplementary Table 1.
Measurement of ROS levels in intestinal extracts
Five to six neonatal mice were orally inoculated with 1 × 109 CFU of tEc and atEc cells once a day for 3 days. The mice were killed and their intestinal contents were homogenized and resuspended in 2 ml of 0.1 M Tris/HCl, pH 7.5. Large particulates were removed by centrifugation at 1,000 r.p.m. for 5 min at 4 °C, after which the supernatants were harvested and incubated with 10 μM of the hydrogen peroxide-specific dye Peroxy Orange 1 (PO1, Tocris Bioscience) for 30 min in the dark. The level of orange fluorescence, which is indicative of the level of H2O2, was determined with a Victor X4 plate reader.
BLASTp analyses of catalase proteins
To provide further information about the distribution of catalase proteins among commensal microbes in the human gut, we BLAST searched amino-acid sequences obtained from an integrated catalogue of reference genes in the human gut microbiome69. This comprehensive catalogue is composed of 9,879,896 non-redundant genes, which is a near complete set of genes found in almost all human gut bacteria. We also searched against an integrated gene catalogue of the mouse gut metagenome70 to outline the relative abundance of catalase proteins in human versus mouse intestine. The mouse metagenome catalogue comprises 2,572,074 non-redundant bacterial genes present in faecal samples collected from 184 mice of diverse backgrounds. We downloaded the amino-acid sequences listed in the companion web-servers and used those for pairwise sequence alignments using the BLASTp algorithm.
Statistical analysis
Data are expressed as mean±standard error of the mean (SEM). Unpaired Student’s t-test and ANOVA (for Fig. 4d and Supplementary Fig. 1d-f) were used to determine whether differences between groups were significant. A P value<0.05 was considered to indicate statistical significance. All experiments were repeated for reproducibility.
Data availability
The genomic sequences of the tEc and atEc strains have been deposited in the NCBI genome database with accession codes LRAB00000000 and LRBX00000000, respectively. The authors declare that all other data supporting the findings of this study are available within the article and its Supplementary Information files.
Additional information
How to cite this article: Yoon, M. Y. et al. A single gene of a commensal microbe affects host susceptibility to enteric infection. Nat. Commun. 7:11606 doi: 10.1038/ncomms11606 (2016).
Supplementary Material
Supplementary Figures 1-12 and Supplementary Tables 1-4
Metagenome catalog search results
Acknowledgments
This work was supported by grants from the National Research Foundation (NRF) of Korea, funded by the Korean Government (MSIP), 2014R1A2A2A01002861, 2014R1A4A1008625, 2015M3C9A2054020 and 2014R1A1A1006403. This work was also supported by a grant from the Korean Ministry of Health & Welfare, HI14C1324. We appreciate the assistance of Dr. Byung Kwon Kim (OmicsPia, Inc., Taejun, Korea) for bioinformatics analyses.
Footnotes
The authors declare no competing financial interests.
Author contributions M.Y.Y. and S.S.Y. conceived, designed and coordinated the study. M.Y.Y., K.B.M., K.M.L, Y.Y, Y.K., Y.T.O and K.L. performed the experiment and acquired the data. J.C., B.Y.K., S.H.Y., I.L. and C.Y.K. performed bioinformatic analyses. S.S.Y. and M.Y.Y. wrote the paper. All the authors participated in discussions of the results and reviewed the final draft.
References
- Vesterlund S., Paltta J., Karp M. & Ouwehand A. C. Adhesion of bacteria to resected human colonic tissue: quantitative analysis of bacterial adhesion and viability. Res. Microbiol. 156, 238–244 (2005). [DOI] [PubMed] [Google Scholar]
- Collado M. C., Gueimonde M., Hernandez M., Sanz Y. & Salminen S. Adhesion of selected Bifidobacterium strains to human intestinal mucus and the role of adhesion in enteropathogen exclusion. J. Food Prot. 68, 2672–2678 (2005). [DOI] [PubMed] [Google Scholar]
- Palestrant D. et al. Microbial biofilms in the gut: visualization by electron microscopy and by acridine orange staining. Ultrastruct. Pathol. 28, 23–27 (2004). [PubMed] [Google Scholar]
- Bollinger R. R., Barbas A. S., Bush E. L., Lin S. S. & Parker W. Biofilms in the normal human large bowel: fact rather than fiction. Gut 56, 1481–1482 (2007). [PMC free article] [PubMed] [Google Scholar]
- Buffie C. G. & Pamer E. G. Microbiota-mediated colonization resistance against intestinal pathogens. Nat. Rev. Immunol. 13, 790–801 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltby R., Leatham-Jensen M. P., Gibson T., Cohen P. S. & Conway T. Nutritional basis for colonization resistance by human commensal Escherichia coli strains HS and Nissle 1917 against E. coli O157:H7 in the mouse intestine. PLoS ONE 8, e53957 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter S. E. et al. Host-derived nitrate boosts growth of E. coli in the inflamed gut. Science 339, 708–711 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter S. E. et al. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature 467, 426–429 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng K. M. et al. Microbiota-liberated host sugars facilitate post-antibiotic expansion of enteric pathogens. Nature 502, 96–99 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croswell A., Amir E., Teggatz P., Barman M. & Salzman N. H. Prolonged impact of antibiotics on intestinal microbial ecology and susceptibility to enteric Salmonella infection. Infect. Immun. 77, 2741–2753 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekirov I. et al. Antibiotic-induced perturbations of the intestinal microbiota alter host susceptibility to enteric infection. Infect. Immun. 76, 4726–4736 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassis C. M., Theriot C. M. & Young V. B. Alteration of the murine gastrointestinal microbiota by tigecycline leads to increased susceptibility to Clostridium difficile infection. Antimicrob. Agents. Chemother. 58, 2767–2774 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough E., Shaikh H. & Manges A. R. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin. Infect. Dis. 53, 994–1002 (2011). [DOI] [PubMed] [Google Scholar]
- van Nood E. et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N. Engl. J. Med. 368, 407–415 (2013). [DOI] [PubMed] [Google Scholar]
- Fuentes S. et al. Reset of a critically disturbed microbial ecosystem: faecal transplant in recurrent Clostridium difficile infection. ISME J. 8, 1621–1633 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon M. Y., Lee K. & Yoon S. S. Protective role of gut commensal microbes against intestinal infections. J. Microbiol. 52, 983–989 (2014). [DOI] [PubMed] [Google Scholar]
- Ritchie J. M. & Waldor M. K. Vibrio cholerae interactions with the gastrointestinal tract: lessons from animal studies. Curr. Top. Microbiol. Immunol. 337, 37–59 (2009). [DOI] [PubMed] [Google Scholar]
- Parisi J. T. & Marsik F. J. Atypical reactions of Escherichia coli on eosin methylene blue agar. Appl. Microbiol. 18, 948–949 (1969). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesser H. W., Muller-Hill B. & Overath P. Characterization of beta-galactosidase--lactose-permease chimaeras of Escherichia coli. Eur. J. Biochem. 137, 567–572 (1983). [DOI] [PubMed] [Google Scholar]
- Lee K.-M. et al. Activation of cholera toxin production by anaerobic respiration of trimethylamine N-oxide in Vibrio cholerae. J. Biol. Chem. 287, 39742–39752 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delcher A. L., Phillippy A., Carlton J. & Salzberg S. L. Fast algorithms for large-scale genome alignment and comparison. Nucleic Acids Res. 30, 2478–2483 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam J. E. & Kline B. C. Control of the ccd operon in plasmid F. J. Bacteriol. 171, 2353–2360 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baselski V. S., Upchurch S. & Parker C. D. Isolation and phenotypic characterization of virulence-deficient mutants of Vibrio cholerae. Infect. Immun. 22, 181–188 (1978). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D. X., Mekalanos J. J. & Taylor R. K. Antibodies directed against the toxin-coregulated pilus isolated from Vibrio cholerae provide protection in the infant mouse experimental cholera model. J. Infect. Dis. 161, 1231–1236 (1990). [DOI] [PubMed] [Google Scholar]
- Gough D. R. & Cotter T. G. Hydrogen peroxide: a Jekyll and Hyde signalling molecule. Cell Death Dis. 2, e213 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly D., King T. & Aminov R. Importance of microbial colonization of the gut in early life to the development of immunity. Mutat. Res. 622, 58–69 (2007). [DOI] [PubMed] [Google Scholar]
- Flint H. J., Scott K. P., Duncan S. H., Louis P. & Forano E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes 3, 289–306 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Million M., Lagier J. C., Yahav D. & Paul M. Gut bacterial microbiota and obesity. Clin. Microbiol. Infect. 19, 305–313 (2013). [DOI] [PubMed] [Google Scholar]
- Shi H. N. & Walker A. Bacterial colonization and the development of intestinal defences. Can. J. Gastroenterol. 18, 493–500 (2004). [DOI] [PubMed] [Google Scholar]
- Hsu H.-Y. & Wen M.-H. Lipopolysaccharide-mediated reactive oxygen species and signal transduction in the regulation of interleukin-1 gene expression. J. Biol. Chem. 277, 22131–22139 (2002). [DOI] [PubMed] [Google Scholar]
- Bulua A. C. et al. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS). J. Exp. Med. 208, 519–533 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLeo F. R. et al. Neutrophils exposed to bacterial lipopolysaccharide upregulate NADPH oxidase assembly. J. Clin. Invest. 101, 455–463 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. et al. Catalases promote resistance of oxidative stress in Vibrio cholerae. PLoS ONE 7, e53383 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupp C. et al. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe 2, 204 (2007). [DOI] [PubMed] [Google Scholar]
- Olivier V., Salzman N. H. & Satchell K. J. Prolonged colonization of mice by Vibrio cholerae El Tor O1 depends on accessory toxins. Infect. Immun. 75, 5043–5051 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie J. M., Rui H., Bronson R. T. & Waldor M. K. Back to the future: studying cholera pathogenesis using infant rabbits. MBio 1, e00047–10 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhine J. A. & Taylor R. K. TcpA pilin sequences and colonization requirements for O1 and O139 vibrio cholerae. Mol. Microbiol. 13, 1013–1020 (1994). [DOI] [PubMed] [Google Scholar]
- Pierce N. F., Kaper J. B., Mekalanos J. J. & Cray W. C. Jr. Role of cholera toxin in enteric colonization by Vibrio cholerae O1 in rabbits. Infect. Immun. 50, 813–816 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins M. J., Sharp R. & Macfarlane G. T. Variation in human intestinal microbiota with age. Dig. Liver Dis. 34, S12–S18 (2002). [DOI] [PubMed] [Google Scholar]
- Woodmansey E. J. Intestinal bacteria and ageing. J. Appl. Microbiol. 102, 1178–1186 (2007). [DOI] [PubMed] [Google Scholar]
- Enck P. et al. The effects of ageing on the colonic bacterial microflora in adults. Z. Gastroenterol. 47, 653–658 (2009). [DOI] [PubMed] [Google Scholar]
- Switala J., Triggs-Raine B. L. & Loewen P. C. Homology among bacterial catalase genes. Can. J. Microbiol. 36, 728–731 (1990). [DOI] [PubMed] [Google Scholar]
- Garrett W. S. et al. Enterobacteriaceae act in concert with the gut microbiota to induce spontaneous and maternally transmitted colitis. Cell Host Microbe 8, 292–300 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon S. S. & Mekalanos J. J. 2,3-butanediol synthesis and the emergence of the Vibrio cholerae El Tor biotype. Infect. Immun. 74, 6547–6556 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippe N., Alcaraz J.-P., Coursange E., Geiselmann J. & Schneider D. Improvement of pCVD442, a suicide plasmid for gene allele exchange in bacteria. Plasmid 51, 246–255 (2004). [DOI] [PubMed] [Google Scholar]
- Berney M., Hammes F., Bosshard F., Weilenmann H.-U. & Egli T. Assessment and interpretation of bacterial viability by using the LIVE/DEAD baclight kit in combination with flow cytometry. Appl. Environ. Microbiol. 73, 3283–3290 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armougom F., Henry M., Vialettes B., Raccah D. & Raoult D. Monitoring bacterial community of human gut microbiota reveals an increase in Lactobacillus in obese patients and Methanogens in anorexic patients. PLoS ONE 4, e7125 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X. et al. Development of a real-time PCR method for Firmicutes and Bacteroidetes in faeces and its application to quantify intestinal population of obese and lean pigs. Lett. Appl. Microbiol. 47, 367–373 (2008). [DOI] [PubMed] [Google Scholar]
- Malinen E., Kassinen A., Rinttila T. & Palva A. Comparison of real-time PCR with SYBR Green I or 5'-nuclease assays and dot-blot hybridization with rDNA-targeted oligonucleotide probes in quantification of selected faecal bacteria. Microbiology 149, 269–277 (2003). [DOI] [PubMed] [Google Scholar]
- Rinttilä T., Kassinen A., Malinen E., Krogius L. & Palva A. Development of an extensive set of 16S rDNA-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR. J. Appl. Microbiol. 97, 1166–1177 (2004). [DOI] [PubMed] [Google Scholar]
- Walter J. et al. Detection of Lactobacillus, Pediococcus, Leuconostoc, and Weissella species in human feces by using group-specific pcr primers and denaturing gradient gel electrophoresis. Appl. Environ. Microbiol. 67, 2578–2585 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon M. Y., Lee K. M., Jeong S. H., Kim J. & Yoon S. S. Heterogeneous virulence potential and high antibiotic resistance of Pseudomonas aeruginosa strains isolated from Korean pneumonia patients. J. Microbiol. 48, 518–525 (2010). [DOI] [PubMed] [Google Scholar]
- Hyatt D. et al. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics 11, 119 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schattner P., Brooks A. N. & Lowe T. M. The tRNAscan-SE, snoscan and snoGPS web servers for the detection of tRNAs and snoRNAs. Nucleic Acids Res. 33, W686–W689 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrocki E. P. & Eddy S. R. Infernal 1.1: 100-fold faster RNA homology searches. Bioinformatics. 29, 2933–2935 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell S. et al. eggNOG v4.0: nested orthology inference across 3686 organisms. Nucleic Acids Res. 42, D231–D239 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M. et al. Data, information, knowledge and principle: back to metabolism in KEGG. Nucleic Acids Res. 42, D199–D205 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbeek R. et al. The subsystems approach to genome annotation and its use in the project to annotate 1000 genomes. Nucleic Acids Res. 33, 5691–5702 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon M. Y. et al. Functional screening of a metagenomic library reveals operons responsible for enhanced intestinal colonization by gut commensal microbes. Appl. Environ. Microbiol. 79, 3829–3838 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puigbo P., Bravo I. G. & Garcia-Vallve S. CAIcal: a combined set of tools to assess codon usage adaptation. Biol. Direct. 3, 38 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I., Kim Y. O., Park S. C. & Chun J. OrthoANI: an improved algorithm and software for calculating average nucleotide identity. Int. J. Syst. Evol. Microbiol doi:10.1099/ijsem.0.000760 (2015). [DOI] [PubMed] [Google Scholar]
- Edgar R. C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castresana J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 17, 540–552 (2000). [DOI] [PubMed] [Google Scholar]
- Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 30, 1312–1313 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huson D. H. et al. Dendroscope: An interactive viewer for large phylogenetic trees. BMC Bioinformatics 8, 460 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamocky M., Gasselhuber B., Furtmuller P. G. & Obinger C. Molecular evolution of hydrogen peroxide degrading enzymes. Arch. Biochem. Biophys. 525, 131–144 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne L. G. & Diaz G. A. A double staining method for differentiating between two classes of mycobacterial catalase in polyacrylamide electrophoresis gels. Anal. Biochem. 157, 89–92 (1986). [DOI] [PubMed] [Google Scholar]
- Hassett D. J. et al. Quorum sensing in Pseudomonas aeruginosa controls expression of catalase and superoxide dismutase genes and mediates biofilm susceptibility to hydrogen peroxide. Mol. Microbiol. 34, 1082–1093 (1999). [DOI] [PubMed] [Google Scholar]
- Li J. et al. An integrated catalog of reference genes in the human gut microbiome. Nat. Biotechnol. 32, 834–841 (2014). [DOI] [PubMed] [Google Scholar]
- Xiao L. et al. A catalog of the mouse gut metagenome. Nat. Biotechnol. 33, 1103–1108 (2015). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1-12 and Supplementary Tables 1-4
Metagenome catalog search results
Data Availability Statement
The genomic sequences of the tEc and atEc strains have been deposited in the NCBI genome database with accession codes LRAB00000000 and LRBX00000000, respectively. The authors declare that all other data supporting the findings of this study are available within the article and its Supplementary Information files.