Abstract
To facilitate development of CSC-targeting therapies, we analyzed publicly available breast cancer datasets to identify genes co-expressed with SOX10, a neural crest marker, and PROM1/CD133, a common CSC marker. The conserved SOX10/PROM1 gene signature that we characterized supports the existence in basal breast cancers of SOX10+/CD133+ cells with neural stem-like features exposing clinically relevant CSC markers and signaling networks.
Background
We previously characterized in salivary adenoid cystic carcinoma (ACC) a novel population of CSC marked by co-expression of two stemness genes, SOX10 and CD133. We also reported that in ACC and basal-like breast carcinoma (BBC), a triple-negative breast cancer subtype, expression of SOX10 similarly demarcates a highly conserved gene signature enriched with neural stem cell genes. Based on these findings, we hypothesized that BBC may be likewise driven by SOX10+/CD133+ cells with neural stem cell properties.
Methods
To validate our hypothesis on clinical data, we used a novel approach to meta-analysis that merges gene expression data from independent breast cancer studies and ranks genes by statistical significance of their co-expression with the gene of interest. Genes that showed strong association with both CD133/PROM1 and SOX10 were validated across different platforms and datasets and analyzed for enrichment with genes involved in neurogenesis.
Results
We identified in clinical breast cancer datasets a highly conserved SOX10/PROM1 gene signature that contains neural stem cell markers common for Schwann cells, ACC, BBC, and melanoma. Identification of TRIM2, TRIM29, MPZL2, KCNN4, and VTCN1/B7-H4 within this signature provides insight into molecular mechanisms of CSC maintenance.
Conclusion
Our results suggest that BBC is driven by SOX10+/CD133+ cells that express NSC-specific markers and share molecular similarities with CSC of neural crest origin. Our study provides clinically relevant information on possible drivers of these cells that may facilitate development of CSC-targeting therapies against this cancer distinguished with poor prognosis and resistance to conventional therapies.
Keywords: SOX10, CD133, basal-like breast carcinoma, triple-negative breast cancers, adenoid cystic salivary carcinoma, cancer stem cells, neural stem cells, FABP7, TRIM2, TRIM29, MPZL2, KCNN4, MPZL2, B7-H4
BACKGROUND
CSC are attractive therapeutic targets due to their abilities to maintain tumor growth, invade, metastasize, withstand cytotoxic therapies, and recur1. However, isolation of CSC from tumor specimens is challenging, especially when information on clinically relevant cell surface markers is lacking. The great majority of published CSC studies relies on in vitro studies, but significance of CSC markers derived from cell lines is questionable, especially when their expression in clinical specimens is not confirmed2–6. We believe that molecular profiling of clinical specimens could help the pursuit for clinically relevant CSC. If CSC with similar properties are shared across tumor specimens, such cells can be tracked by gene expression patterns conserved between patients. It can be also argued that stemness markers related to cell of origin can be shared by cancers with similar histogenesis and exposed via gene expression profiling. A remarkable example of such a marker is SOX10 (SRY-related HMG box-containing factor 10), which plays pivotal roles in gliogenesis, melanogenesis, differentiation of myoepithelial cells and other lineages that originate from neural crest, and maintenance of adult neural stem cells (NSC) that reside in the subventricular CNS zone7–12 8, 13–15. Recent studies that characterized SOX10 as a melanoma driver16, and a key breast stem cell regulator17 raised the important question about SOX10 relevance to stem cell maintenance in a certain group of cancers.
Our previously performed expression analysis of clinical specimens of adenoid cystic carcinoma (ACC), a highly lethal neuroinvasive cancer of salivary glands, identified in 94% of ACC transcripts of SOX10, NOTCH1, FABP7, and other NSC genes18 whose co-expression was remarkably consistent from patient to patient. The most plausible explanation for this phenomenon was the existence in the majority of ACC of a previously uncharacterized population of CSC cells with NSC properties. Indeed, our recent experiments performed on cultured ACC cells and PDX models demonstrated that SOX10 marks a previously unrecognized population of CD133+ stem cells that express stemness genes and form tumors with ACC histology when injected into nude mice19. Overall, these experiments validated SOX10 as a valuable cell lineage marker and key ACC-CSC regulator suggesting that identification of genes associated with SOX10 activation may provide important insights into molecular features of previously uncharacterized CSC in SOX10-positive cancers.
Interestingly, we and others previously demonstrated that SOX10 expression is also diagnostic for basal-like breast carcinoma (BBC), a breast cancer subtype distinguished with myoepithelial-like gene expression profile and myoepithelial differentiation akin to ACC 8, 13, 14, 17. BBC as a triple-negative breast cancer (TNBC) subtype was described 15 years ago based on a characteristic gene expression signature found in ~15% of breast cancer specimens20–22. This finding was the first molecular insight into BBC that urgently requires targeted therapies due to the absence of estrogen, progesterone, and ERBB2 receptors, shows poor prognosis, and affects young women23. Based on the observed similarities between ACC and BBC, we hypothesized that SOX10 promotes in both cancers CD133+ cells with stem cell properties that could be tracked in clinical tumor specimens via genes that are co-expressed with CD133 and SOX10.
Tracking conserved elements of gene expression patterns across multiple specimens and datasets requires a reliable noise-resistant and straightforward algorithm for meta-analysis. Here, using Multi Experiment Matrix (MEM) meta-analysis that merges information from different Affymetrix datasets into a single global ordering and ranks genes by their co-expression24, we confirm that CD133 and SOX10 co-expression in breast cancers is highly statistically significant, intrinsic to BBC specimens, and clinically and biologically relevant. This observation supports our hypothesis on the existence in BBC of SOX10+/CD133+ cells with neural stem properties and provides a blueprint for identification of true CSC and their targeting.
MATERIALS AND METHODS
Meta-analysis
of publicly available breast cancer datasets was performed using Multi Experiment Matrix (MEM) software and datasets produced on Affymetrix U133 plus 2.0 platform. MEM ranks Affymetrix probes/genes by statistical score assigned based on co-expression of these probes with the gene of interest across multiple datasets. To improve biological and clinical aspects of the analysis, MEM allows access to individual datasets, performs stratification of output data, and builds heat maps to identify clinical groups with differential gene expression 24, 25.
Cross-validation
of meta-data was done with cBioPortal software on a TCGA Breast Invasive Carcinoma dataset at http://www.cbioportal.org/study?id=brca_tcga#summary. This dataset was produced on 1105 clinical specimens collected from 1098 patients and combines mutational analysis with DNA copy number variation, gene expression array, and RPPA data.
Breast cancer PDX models
were profiled using E-GEOD-46106 dataset and software available at BioGPS (www.biogps.org). This dataset was produced using U133 Plus 2.0 Affymetrix platform on xenografts established from 25 breast cancer patients.
Analysis of discriminators
for breast cancer groups was done via http://www.ncbi.nlm.nih.gov/geo/geo2r/, a web tool available via Gene Expression Omnibus for comparative statistical analysis between two or more groups for identification of differentially expressed genes.
Gene ontology analysis
was done via http://software.broadinstitute.org/gsea/, a computational method that determines whether a priori defined sets of genes show statistically significant, concordant differences between two biological states (e.g. phenotypes).
Gene expression analysis of human primary cells
was performed using Primary Cell Atlas dataset (http://biogps.org/dataset/BDS_00013/) and software available at BioGPS. This dataset combines 745 samples from over 100 studies performed on human primary cells.
RESULTS
Expression of PROM1/CD133 in breast cancers strongly correlates with SOX10, its core signature elements, and BBC-intrinsic stem cell markers
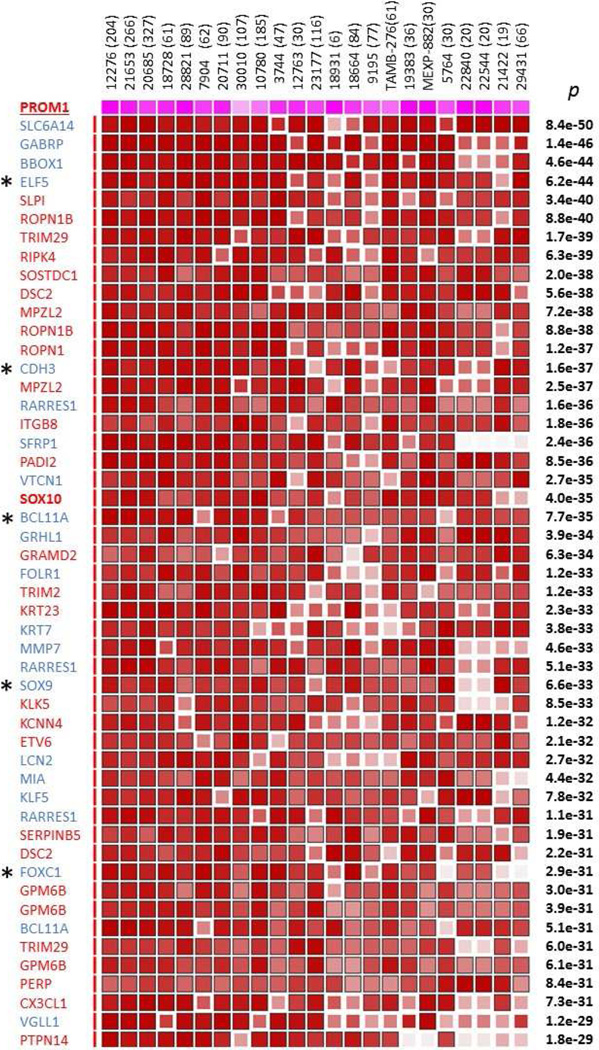
We previously demonstrated that PROM1/CD133 is co-expressed with SOX10 in the great majority of clinical ACC specimens and in a novel population of SOX10+/CD133+ stem cells, ACC-CSC, that we recently characterized19. Furthermore, we showed that BBC is molecularly similar to ACC as it expresses SOX10 with its core gene signature elements ROPN1, ROPN1B, TRIM2, and GPM6B14, 26–28 suggesting that BBC may contain a similar population of SOX10+/CD133+ cells with neural stem cell properties. Here, we interrogated publicly available clinical breast cancer datasets to determine if PROM1/CD133 expression is also BBC-intrinsic and correlates with SOX10 expression. Using MEM server (http://biit.cs.ut.ee/mem/) that uses a novel statistical rank aggregation algorithm that ranks genes by their co-expression with the gene of interest25, we analyzed 23 publicly available datasets to identify genes whose expression closely correlates with PROM1. In line with our expectation, SOX10 and core SOX10 signature genes ROPN1, ROPN1B, TRIM2, and GPM6B14 showed statistically significant association with PROM1 across these 23 datasets (FIG. 1). We then asked if this PROM1 gene signature contains previously published BBC/TNBC markers. Remarkably, we found that almost half (19/41) of genes with highest p values (e-50<p<e-28) have been previously linked to BBC (FIG. 1, blue font). Interestingly, GABRP, which encodes the enigmatic π subunit of gamma-aminobutyric acid type A receptor, has been previously linked to CD133-expressing hepatic progenitors29 and reported as a BBC marker in several studies30–32. VGLL1, one of four human orthologs of Drosophila vestigial, is an intriguing but poorly studied component of Wnt and TEAD signaling whose expression in BBC was previously linked to CD133/PROM1 and GABRP30, 33. While VGLL1 relation to stem cell maintenance in BBC remains unclear, its paralog VGLL4 promotes survival of human embryonic stem cells34 and regulates Hippo-YAP signaling in postnatal cardiomyocytes35. Other previously published BBC markers that we found in the PROM1 signature were transcription factors ELF5, BCL11A, and SOX9, all of them linked to promotion of progenitor/stem cells in breast cancer and normal breast36–39, and CDH3/P-cadherin, a breast basal stem cell marker40. Thus, our analysis of the PROM1/CD133 MEM signature was consistent with the existence in BBC, but not other breast cancer types, SOX10+/CD133+ cells that are molecularly similar to ACC-CSC and express stem cell genes previously linked to both ACC and BBC.
Figure 1. Top 44 genes ranked by their co-expression with PROM1 in Affymetrix U133 Plus 2.0 datasets using aggregation MEM meta-analysis.
Novel genes that we associated with BBC and SOX10 are shown in red font, while previously published BBC-intrinsic genes are in blue. All genes show highly statistically significant co-expression with PROM1 and each other (p values shown at the right edge of matrix). Some genes are represented by 2 or 3 probes. Dataset IDs and their sizes (in brackets) are shown on top. Dark brown color signifies highest ranks of association by co-expression. More information on how to read MEM output can be found at http://biit.cs.ut.ee/mem/help/help.html.
CD133 expression in breast cancer is BBC-selective
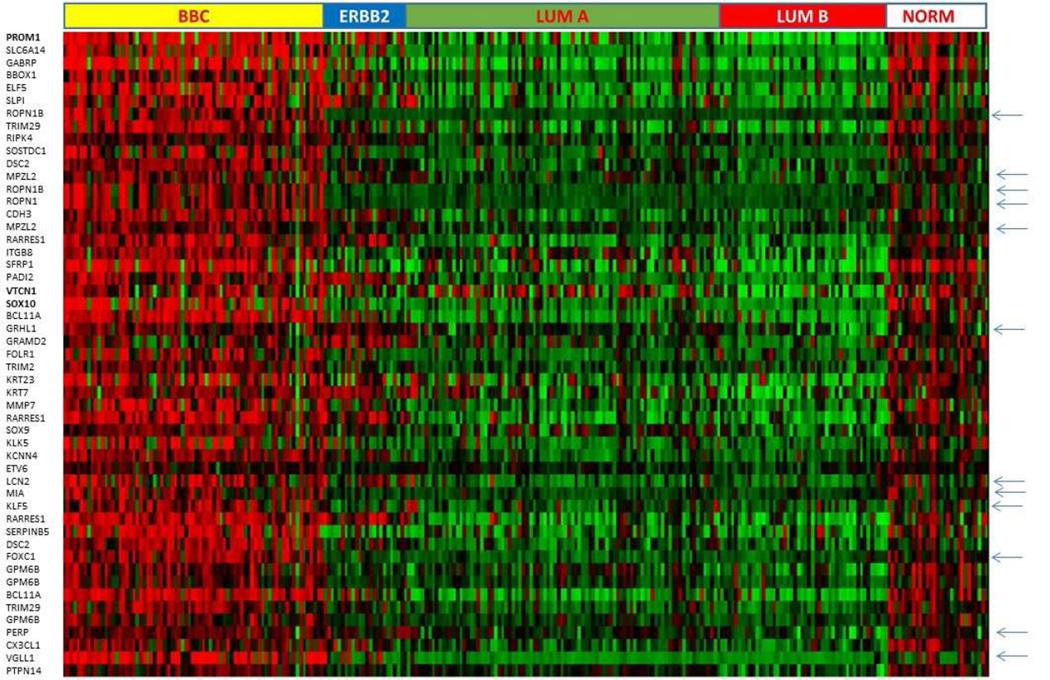
Our hypothesis that the CD133/PROM1 gene signature is BBC-selective was then validated on one of the 23 datasets used in the MEM analysis, E-GEOD-21653, which stratified clinical specimens into five major groups: ERBB2, BBC, luminal A, luminal B, and normal-like subtypes (N total=266). The heat map built for this study at http://biit.cs.ut.ee/mem/ revealed that PROM1 is strongly and consistently expressed in the great majority of BBC (~80%), in a fraction of ERBB2+ tumors, and in the normal-like tumor group, but to a much lesser extent in the luminal subtypes (FIG. 2). In line with this finding, CD133/PROM1 was among top 250 discriminators of the BBC subtype in our analysis of distribution of gene expression across five breast cancer groups (http://www.ncbi.nlm.nih.gov/geo/geo2r/, Padj =7.7E-29). We also observed expression of PROM1 in normal-like breast specimens included in E-GEOD-21653 (FIG. 2) as well as in normal breast specimens represented in other datasets that we analyzed (data not shown), which was in line with previously published reports on PROM1 expression in normal breast 41. Interestingly though, some PROM1 signature genes, such as ROPN1, ROPN1B, MPZL2, GRHL1, LCN2, MIA, KLF5, FOXC1, PERP, and VGLL1 (shown with arrows in FIG. 2), appeared to be more selective to cancerous tissue that PROM1. Overall, our analysis strongly supports a major role for SOX10+/CD133+ cells in BBC providing a set of novel molecular markers for these prospective CSC and a new insight into signaling pathways involved in their maintenance.
Figure 2. Heat map of the E-GEOD-21653 dataset produced by MEM on clinical specimens sorted into 5 breast cancer subtypes illustrates BBC-selective expression of top 44 genes ranked by co-expression with PROM1 (red – high expression, green –low expression levels).
Genes whose expression is strongly selective to cancer specimens are shown with arrows.
Cross-validation of the PROM1 gene signature
As MEM rank aggregation analysis has not been previously used for CSC tracking, we validated our PROM1 gene signature on a large breast cancer dataset (Breast Invasive Carcinoma, TCGA Provisional, N=1093, RNA-Seq and RPPA data available from http://www.cbioportal.org). In this dataset, statistically significant associations found between SOX10, PROM1, and core SOX10 signature genes supported the results of our MEM analysis (Table 1). Using standard Pearson and Spearman score calculation, a list of genes positively and negatively correlating with PROM1 in the TCGA dataset was generated (r cutoff 0.5, Suppl. Table 1, N=197) and compared with the MEM-generated PROM1 gene signature shown in FIG. 1. Remarkably, 80% of these PROM1 signature genes (33 of 41) were reproduced in this validation study as positively correlating with PROM1 (Suppl. Table 1, grey rows). On the other hand, estrogen receptor (ESR1, r=−0.62), as well as FOXA1 (r=−0.55) and GATA3 (r=−0.56), two transcription master regulators and molecular markers of sex hormone receptor-positive breast cancers42, were at the very bottom of the list of genes with the highest negative correlation with PROM1.
Table 1.
Statistically significant associations between expression of SOX10/PROM1 signature genes in the Breast Invasive Carcinoma TCGA dataset.
| Gene A | Gene B | p-Value | Log Odds Ratio |
|---|---|---|---|
| SOX10 | PROM1 | 2.34E-13 | 2.22 |
| SOX10 | GPM6B | 3.79E-21 | 3.00 |
| SOX10 | ROPN1 | 2.37E-42 | 4.02 |
| SOX10 | ROPN1B | 3.72E-42 | 4.14 |
| SOX10 | TRIM2 | 1.56E-26 | 3.20 |
| PROM1 | GPM6B | 1.06E-09 | 2.01 |
| PROM1 | ROPN1 | 7.60E-23 | 2.84 |
| PROM1 | ROPN1B | 3.45E-21 | 2.83 |
| PROM1 | TRIM2 | 8.63E-12 | 2.11 |
| GPM6B | ROPN1 | 9.64E-21 | 2.95 |
| GPM6B | ROPN1B | 8.29E-20 | 2.97 |
| GPM6B | TRIM2 | 6.61E-23 | 3.13 |
| ROPN1 | ROPN1B | 1.14E-70 | 5.81 |
| ROPN1 | TRIM2 | 2.83E-33 | 3.56 |
| ROPN1B | TRIM2 | 5.21E-36 | 3.82 |
We then used RPPA data available with the TCGA dataset and demonstrated that PROM1-expressing specimens were mostly ESR1, PGR, and ERBB2/HER2-negative (e-10<p<e-27) and overexpressed NOTCH1 (p~e-7), CDH3 (p~e-3), and previously published BBC markers (Suppl. Table 2).
Overall, cross-validation of our MEM meta-analysis on an independent cohort at both RNA and protein levels strengthened clinical relevance of our findings and produced new information of their molecular identity.
Identification of TRIM2, TRIM29, MPZL2, KCNN4, and VTCN1/B7-H4 within the PROM1 gene signature suggests strategies for CSC eradication
Within the PROM1 signature, we found genes that, to our knowledge, have not been previously associated with BBC/TNBC (FIG. 1, red font). Two of these genes were represented by TRIM proteins implicated in neurodegenerative disorders, axonopathies and ataxia43–45. The TRIM2 function in cancers has been associated with its E3 ubiquitin ligase activity towards BIM/BCL2L11, its pro-apoptotic substrate46, 47. Pro-tumorigenic activities of TRIM29 appear to be mediated via multiple mechanisms as its overexpression in cancers was linked with suppression of p53, PTEN, and promotion of radioresistance and other stem cell properties 48–51. Other potentially useful therapeutic targets within the PROM1 signature were MPZL2/EVA1, which has been recently linked to maintenance of glioblastoma stem cells through activation of non-canonical NFkB pathway52, and KCNN4 that encodes potassium channel KCa3.1 involved in regulation of motility in glioblastoma stem cells53. One of the most intriguing genes that we associated with PROM1 expression was VTCN1, which encodes B7-H4, a checkpoint receptor in immune tolerance regulation54. Interestingly, expression of B7-H4 has been previously demonstrated in CSC in neuroectodermal tumors and linked to stimulation of CSC in renal cell carcinoma where it was essential for tumor growth, chemoresistance, and immune evasion55, 56. Supporting their clinical relevance, all these five genes passed cross-validation on the TCGA dataset (Suppl. Table 1). Thus, tracking CSC in clinical specimens by their molecular markers may provide valuable information on genes and signaling pathways intrinsically linked to poor prognosis, which may facilitate development of CSC-targeting therapies for BBC and other cancers.
Expression of PROM1 in BBC is paralleled by activation of neural stem genes
Our previous studies associated SOX10 and CD133 expression in ACC with expression of neural stem cell genes and markers14, 18, 19. To find out if this is also true for BBC, we performed enrichment analysis of genes listed in Suppl. Table 1. Our GEO analysis performed using software available from http://software.broadinstitute.org/gsea/index.jsp revealed that the list of 149 genes co-expressed with PROM1 show has highly statistically significant overlaps with CNS Development and Neurogenesis GO sets (Suppl. Table 3). SMO, ID4, FABP7, NFIB, FZD9, CX3CL1, and LMO4 that have been previously characterized as key signaling factors involved in NSC regulation were the most interesting genes in these overlaps with therapeutic potential57–63.
Schwann cell markers reveal similarities between BBC and neural progenitors
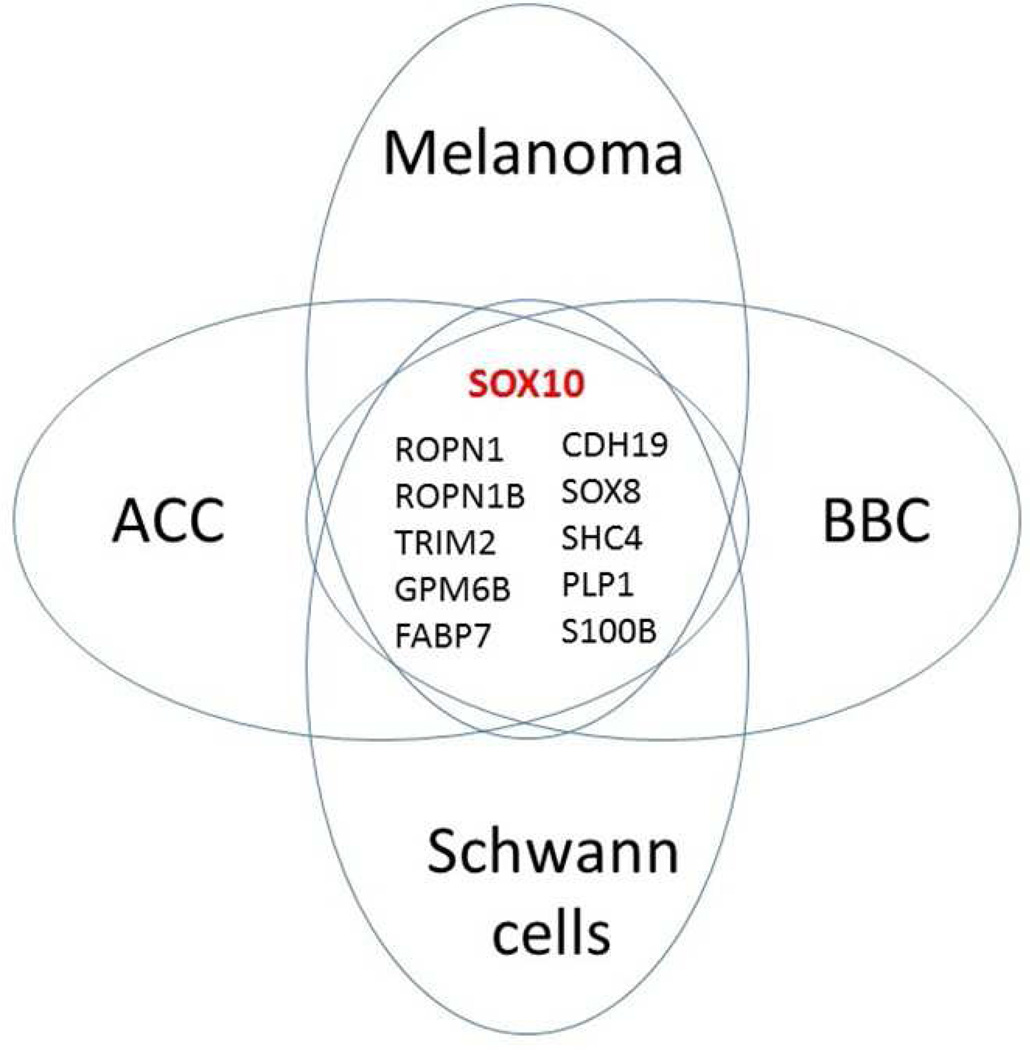
SOX10 has been previously described as a highly selective marker of Schwann cells8, 14. This finding was in line with the key SOX10 role in neural crest development and its differentiation into glial cells and melanocytes64. To obtain more information on the potential link between SOX10/PROM1-expressing BBC cells and Schwann cells, we inspected distribution of ROPN1, ROPN1B, MIA, GPM6B, and other genes co-expressed in BBC with either PROM1 or SOX10 across normal human cell lineages using the Primary Cell Atlas dataset (http://biogps.org/dataset/BDS_00013/). Remarkably, in addition to SOX10 and 4 genes listed above, this analysis identified six more genes as selectively expressed in Schwann cells and BBC (FIG. 3 and Suppl. FIG. 3). Of note, MIA, S100B, FABP7, and SHC4/RaLP have been previously linked to melanoma65–68 and glioma69–71. Overall, we identified 10 basic cell lineage markers shared between ACC, BBC, melanoma, and Schwann cells that strongly supported our hypothesis on the origin of these three cancers from Schwann cell precursors (FIG. 3). Based on these associations, it is reasonable to propose that all these novel genes/markers, along with SOX10, are CSC-intrinsic factors that play important roles in aggressive cancer behavior and may be used for tracking, isolation, and selective elimination of CSC as well as for diagnostics, and prognostication of SOX10-positive cancers.
Figure 3. Candidate CSC markers shared between ACC, BBC, melanoma, and Schwann cells.
Expression of 10 Schwann cell markers/genes is conserved in three cancers suggesting that they all originate from neural crest.
The PROM1/SOX10 gene signature is partially maintained in PDX models of BBC
Patient-derived xenografts (PDX) are becoming common tools in preclinical studies as they maintain tumor heterogeneity, support CSC, and faithfully mimic tumor response to therapy72. To determine if PDX models of BBC can be used for tracking and isolation of SOX10/PROM1-expressing cells, we asked if a publicly available TNBC PDX dataset, E-GEOD-47079, N=7, maintains expression of genes shown in FIG.1. This comparison identified in the TNBC dataset 56% (23/41) of the clinical PROM1 gene signature as close PROM1 correlates (r>0.65) suggesting that expression of at least half of the PROM1 signature genes is maintained in PDX models (data not shown). Overall, this analysis supported these models as adequate tools for CSC isolation, but also underlined differences in gene expression, which are most likely caused by model limitations, such as murine tissue environment and lack of interaction with immune system.
DISCUSSION
CSC have taken center stage in clinical research as they are considered the root of aggressive tumor behavior and resistance to cytotoxic therapies. However, identification of true CSC is challenging as CD24, CD44, CD133, and other cell surface markers used for their detection are not cell lineage- or cancer-specific. Thus, inclusion of more definitive markers for reliable and clinically relevant CSC identification is required4, 5.
Molecular profiling separated clinical breast cancer specimens into four major types: luminal, ERB2-positive, basal-like (BBC), and normal breast73. BBC, which comprises ~15–20% of all breast cancers, is a molecularly and clinically distinct cancer type distinguished with a myoepithelial cell-like expression profile, poor prognosis, and resistance to cytotoxic and hormone receptor-targeting therapies74. We recently demonstrated that SOX10, a master regulator of neural stem cells and highly specific marker of Schwann cells and melanocytes, is overexpressed in clinical ACC and BBC specimens as a part of conserved gene signature. Furthermore, we identified substantial overlaps between the SOX10 gene signatures in BBC, ACC, and neuroectodermal cancers (melanoma, neuroblastoma, and glioma) suggesting that SOX10 and its gene signature demarcate CSC with common properties in cancers with similar cell origin14, 75. Subsequently, we focused on ACC to demonstrate that SOX10+ cells purified from ACC tissue express CD133, a neural and cancer stem cell surface marker 76–79, and activate signaling pathways and genes characteristic for NSC, such as NOTCH1, FABP7, etc19, 75. This novel SOX10+/CD133+ CSC population, which we named ACC-CSC, possessed stem cell properties, such as the ability to form spheroids in culture and initiate tumors with ACC histology in nude mice19. We then asked if tracking SOX10 and CD133 expression in clinical breast cancer specimens may similarly provide information on CSC in BBC.
In this study, using publicly available clinical data, we were able to track genes co-expressed with PROM1/CD133 and SOX10 across different breast cancer specimens and datasets. Moreover, we identified based on this straightforward approach a conserved gene signature highly enriched with novel and previously reported stem cell markers. Overall, gene co-expression data strongly suggested the existence in BBC of a previously unknown population of CSC cells with molecular traits similar to NSC and cancers of neural crest origin. The validity of our conclusion is strengthened by 1) successful application of similar approach to tracking and isolation of CSC from ACC; 2) cross-validation of breast cancer MEM data on an independent dataset using a different platform and calculation algorithm; 3) overlap found between our data and previously published BBC stem cell markers; and 4) identification of cell markers shared between BBC, ACC, melanoma, and Schwann cells. As we also demonstrate, PDX models produced from clinical BBC specimens further support the existence of SOX10+/CD133+ cells. It can be argued, however, that gene expression patterns derived from mixed tumor specimens by themselves cannot define cancer stem cells or any cellular subpopulations, and direct isolation of such cells from grafted or fresh tumor tissue is required. While this is true and we already initiated such experiments, it is important to note that our analysis of available BBC cell lines for SOX10 expression demonstrated that HCC1569, HCC38, and MX-1 retain expression of SOX10 and its signature elements14 supporting the existence of cells with suggested core phenotype. MX-1, one of the most aggressive triple-negative breast cancer cell lines, was especially informative as it consisted entirely of SOX10+/CD133+ cells that also expressed GABRP, FABP7, VTCN1, activated NOTCH1, and many other genes listed in FIG. 1 (our unpublished data). Thus, experiments with MX-1, one of the oldest cancer cell line/xenograft model which is available from NCI-Frederick (https://frederick.cancer.gov/services/tumorcatalog.aspx), may provide an important insight into molecular signaling of SOX10+/CD133+ cells related to poor prognosis and help with designing CSC-targeting therapies.
In summary, our study provides a blueprint for BBC studies focused on CSC isolation, modeling, and targeting as well as for validation of already available and newly derived cell lines and PDX as adequate tools for CSC research. The results of our analysis are consistent with the existence in BBC of SOX10+/CD133+ cells with NSC properties and major role proposed for SOX10 in breast stem cell maintenance17. Tracking CSC in BBC using novel markers that we identified offers new research tools and approaches to improve BBD diagnostics, prognostication, and treatment.
CONCLUSION
Our study suggests that most of clinical BBC specimens contain a population of SOX10+/CD133+ CSC that coordinately express genes and markers characteristic for NSC. Highly conserved gene expression patterns associated with SOX10 and CD133/PROM1 expression suggest that these CSC can be tracked in clinical tumor specimens and PDX models via a large set of coordinately expressed NSC markers. Overall, the results of our study provide a new approach for identification and isolation of true CSC for basic research as well as diagnostics, prognostication, and pre-clinical studies.
Supplementary Material
CLINICAL PRACTICE POINTS.
While CSC eradication may be an effective solution for treating cytotoxic therapy-resistant cancers, tracking CSC in clinical specimens and their isolation from tumor tissue and patient-derived xenografts is impossible without reliable CSC markers. Our approach to CSC identification in clinical specimens is based on analysis of massive gene expression data with the goal to track potential CSC by their conserved gene signatures. We recently demonstrated that SOX10, a marker of neural crest stem cells and their derivatives, is overexpressed as a part of conserved gene signature in two histologically similar cancers resistant to radio- and chemotherapies, salivary adenoid cystic carcinoma (ACC) and basal-like breast carcinoma (BBC). Consequently, we showed that SOX10 is a reliable and specific CSC marker in ACC as it marks a novel population of tumor-initiating cells with the SOX10+/CD133+ phenotype. Here, we test our hypothesis that co-expression of these two markers may help to identify a similar population of CSC in BBC. Our results produced on clinical breast cancer gene expression datasets strongly support our hypothesis that BBC, similar to ACC and other SOX10-expressing cancers, is driven by a population of SOX10+/CD133+ cells with neural stem properties that have not been previously recognized in breast cancer. Our study suggests that SOX10+/CD133+ cells can be tracked via a large group of coordinately expressed markers in clinical tumor specimens and PDX models validating PDX models as adequate tools for CSC research and pre-clinical studies aimed at CSC eradication.
Acknowledgments
This study was supported by funds from the Adenoid Cystic Carcinoma Research Foundation and by grants 5R21DE023228 (WGY) and 5R21DE022641 (SVI) from the National Institute of Dental and Craniofacial Research. This work was also supported in part by funds from the Department of Surgery, Yale School of Medicine, by an endowment to the Barry Baker Laboratory for Head and Neck Oncology, by Laura and Isaac Perlmutter Cancer Center Support Grant NIH/NCI P30CA016087, and the National Institutes of Health S10 Grants NIH/ORIP S10OD01058 and S10OD018338.
List of abbreviations
- ACC
adenoid cystic salivary carcinoma
- BBC
basal-like breast carcinoma
- TNBC
triple-negative breast cancer
- CSC
cancer stem cells
- NSC
neural stem cells
- PDX
patient-derived xenografts
- RPPA
reverse phase protein array
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interests.
REFERENCES
- 1.Borah A, Raveendran S, Rochani A, Maekawa T, Kumar DS. Targeting self-renewal pathways in cancer stem cells: clinical implications for cancer therapy. Oncogenesis. 2015;4:e177. doi: 10.1038/oncsis.2015.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gedye C, Sirskyj D, Lobo NC, Meens J, Hyatt E, Robinette M, Fleshner N, Hamilton RJ, Kulkarni G, Zlotta A, Evans A, Finelli A, Jewett MA, Ailles LE. Cancer stem cells are underestimated by standard experimental methods in clear cell renal cell carcinoma. Sci Rep. 2016;6:25220. doi: 10.1038/srep25220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams SA, Anderson WC, Santaguida MT, Dylla SJ. Patient-derived xenografts, the cancer stem cell paradigm, and cancer pathobiology in the 21st century. Lab Invest. 2013;93:970–982. doi: 10.1038/labinvest.2013.92. [DOI] [PubMed] [Google Scholar]
- 4.Medema JP. Cancer stem cells: the challenges ahead. Nat Cell Biol. 2013;15:338–344. doi: 10.1038/ncb2717. [DOI] [PubMed] [Google Scholar]
- 5.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y, Nenutil R, Appleyard MV, Murray K, Boylan M, Thompson AM, Coates PJ. Lack of correlation of stem cell markers in breast cancer stem cells. Br J Cancer. 2014;110:2063–2071. doi: 10.1038/bjc.2014.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bondurand N, Sham MH. The role of SOX10 during enteric nervous system development. Dev Biol. 2013;382:330–343. doi: 10.1016/j.ydbio.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 8.Nonaka D, Chiriboga L, Rubin BP. Sox10: a pan-schwannian and melanocytic marker. Am J Surg Pathol. 2008;32:1291–1298. doi: 10.1097/PAS.0b013e3181658c14. [DOI] [PubMed] [Google Scholar]
- 9.Kim J, Lo L, Dormand E, Anderson DJ. SOX10 maintains multipotency and inhibits neuronal differentiation of neural crest stem cells. Neuron. 2003;38:17–31. doi: 10.1016/s0896-6273(03)00163-6. [DOI] [PubMed] [Google Scholar]
- 10.Nait-Oumesmar B, Picard-Riera N, Kerninon C, Baron-Van Evercooren A. The role of SVZ-derived neural precursors in demyelinating diseases: from animal models to multiple sclerosis. J Neurol Sci. 2008;265:26–31. doi: 10.1016/j.jns.2007.09.032. [DOI] [PubMed] [Google Scholar]
- 11.Pozniak CD, Langseth AJ, Dijkgraaf GJ, Choe Y, Werb Z, Pleasure SJ. Sox10 directs neural stem cells toward the oligodendrocyte lineage by decreasing Suppressor of Fused expression. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:21795–21800. doi: 10.1073/pnas.1016485107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris ML, Baxter LL, Loftus SK, Pavan WJ. Sox proteins in melanocyte development and melanoma. Pigment cell & melanoma research. 2010;23:496–513. doi: 10.1111/j.1755-148X.2010.00711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cimino-Mathews A, Subhawong AP, Elwood H, Warzecha HN, Sharma R, Park BH, Taube JM, Illei PB, Argani P. Neural crest transcription factor Sox10 is preferentially expressed in triple-negative and metaplastic breast carcinomas. Hum Pathol. 2013;44:959–965. doi: 10.1016/j.humpath.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ivanov SV, Panaccione A, Nonaka D, Prasad ML, Boyd KL, Brown B, Guo Y, Sewell A, Yarbrough WG. Diagnostic SOX10 gene signatures in salivary adenoid cystic and breast basal-like carcinomas. Br J Cancer. 2013;109:444–451. doi: 10.1038/bjc.2013.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohtomo R, Mori T, Shibata S, Tsuta K, Maeshima AM, Akazawa C, Watabe Y, Honda K, Yamada T, Yoshimoto S, Asai M, Okano H, Kanai Y, Tsuda H. SOX10 is a novel marker of acinus and intercalated duct differentiation in salivary gland tumors: a clue to the histogenesis for tumor diagnosis. Mod Pathol. 2013;26:1041–1050. doi: 10.1038/modpathol.2013.54. [DOI] [PubMed] [Google Scholar]
- 16.Shakhova O, Zingg D, Schaefer SM, Hari L, Civenni G, Blunschi J, Claudinot S, Okoniewski M, Beermann F, Mihic-Probst D, Moch H, Wegner M, Dummer R, Barrandon Y, Cinelli P, Sommer L. Sox10 promotes the formation and maintenance of giant congenital naevi and melanoma. Nat Cell Biol. 2012;14:882–890. doi: 10.1038/ncb2535. [DOI] [PubMed] [Google Scholar]
- 17.Dravis C, Spike BT, Harrell JC, Johns C, Trejo CL, Southard-Smith EM, Perou CM, Wahl GM. Sox10 Regulates Stem/Progenitor and Mesenchymal Cell States in Mammary Epithelial Cells. Cell Rep. 2015;12:2035–2048. doi: 10.1016/j.celrep.2015.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ivanov SV, Panaccione A, Brown B, Guo Y, Moskaluk CA, Wick MJ, Brown JL, Ivanova AV, Issaeva N, El-Naggar AK, Yarbrough WG. TrkC signaling is activated in adenoid cystic carcinoma and requires NT-3 to stimulate invasive behavior. Oncogene. 2013;32:3698–3710. doi: 10.1038/onc.2012.377. [DOI] [PubMed] [Google Scholar]
- 19.Panaccione A, Chang MT, Carbone BE, Guo Y, Moskaluk CA, Virk RK, Chiriboga L, Prasad ML, Judson B, Mehra S, Yarbrough WG, Ivanov SV. NOTCH1 and SOX10 are Essential for Proliferation and Radiation Resistance of Cancer Stem-Like Cells in Adenoid Cystic Carcinoma. Clin Cancer Res. 2016;22:2083–2095. doi: 10.1158/1078-0432.CCR-15-2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 21.Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Lonning PE, Borresen-Dale AL. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, Demeter J, Perou CM, Lonning PE, Brown PO, Borresen-Dale AL, Botstein D. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003;100:8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dawood S. Triple-negative breast cancer: epidemiology and management options. Drugs. 2010;70:2247–2258. doi: 10.2165/11538150-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 24.Adler P, Kolde R, Kull M, Tkachenko A, Peterson H, Reimand J, Vilo J. Mining for coexpression across hundreds of datasets using novel rank aggregation and visualization methods. Genome Biol. 2009;10:R139. doi: 10.1186/gb-2009-10-12-r139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kolde R, Laur S, Adler P, Vilo J. Robust rank aggregation for gene list integration and meta-analysis. Bioinformatics. 2012;28:573–580. doi: 10.1093/bioinformatics/btr709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leong C, Zhai D, Kim B, Yun SW, Chang YT. Neural stem cell isolation from the whole mouse brain using the novel FABP7-binding fluorescent dye, CDr3. Stem Cell Res. 2013;11:1314–1322. doi: 10.1016/j.scr.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Alvarez-Saavedra M, De Repentigny Y, Lagali PS, Raghu Ram EV, Yan K, Hashem E, Ivanochko D, Huh MS, Yang D, Mears AJ, Todd MA, Corcoran CP, Bassett EA, Tokarew NJ, Kokavec J, Majumder R, Ioshikhes I, Wallace VA, Kothary R, Meshorer E, Stopka T, Skoultchi AI, Picketts DJ. Snf2h-mediated chromatin organization and histone H1 dynamics govern cerebellar morphogenesis and neural maturation. Nat Commun. 2014;5:4181. doi: 10.1038/ncomms5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao XL, Zhang X, Zhang YF, Zhang YZ, Song CG, Liu F, Hu YY, Zheng MH, Han H. Expression and purification of mouse Ttyh1 fragments as antigens to generate Ttyh1-specific monoclonal antibodies. Protein Expr Purif. 2016;130:81–89. doi: 10.1016/j.pep.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 29.Yovchev MI, Grozdanov PN, Joseph B, Gupta S, Dabeva MD. Novel hepatic progenitor cell surface markers in the adult rat liver. Hepatology. 2007;45:139–149. doi: 10.1002/hep.21448. [DOI] [PubMed] [Google Scholar]
- 30.Castilla MA, Lopez-Garcia MA, Atienza MR, Rosa-Rosa JM, Diaz-Martin J, Pecero ML, Vieites B, Romero-Perez L, Benitez J, Calcabrini A, Palacios J. VGLL1 expression is associated with a triple-negative basal-like phenotype in breast cancer. Endocr Relat Cancer. 2014;21:587–599. doi: 10.1530/ERC-13-0485. [DOI] [PubMed] [Google Scholar]
- 31.Sizemore GM, Sizemore ST, Seachrist DD, Keri RA. GABA(A) receptor pi (GABRP) stimulates basal-like breast cancer cell migration through activation of extracellular-regulated kinase 1/2 (ERK1/2) The Journal of biological chemistry. 2014;289:24102–24113. doi: 10.1074/jbc.M114.593582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Symmans WF, Fiterman DJ, Anderson SK, Ayers M, Rouzier R, Dunmire V, Stec J, Valero V, Sneige N, Albarracin C, Wu Y, Ross JS, Wagner P, Theriault RL, Arun B, Kuerer H, Hess KR, Zhang W, Hortobagyi GN, Pusztai L. A single-gene biomarker identifies breast cancers associated with immature cell type and short duration of prior breastfeeding. Endocr Relat Cancer. 2005;12:1059–1069. doi: 10.1677/erc.1.01051. [DOI] [PubMed] [Google Scholar]
- 33.Landin-Malt A, Benhaddou A, Zider A, Flagiello D. An evolutionary, structural and functional overview of the mammalian TEAD1 and TEAD2 transcription factors. Gene. 2016;591:292–303. doi: 10.1016/j.gene.2016.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tajonar A, Maehr R, Hu G, Sneddon JB, Rivera-Feliciano J, Cohen DE, Elledge SJ, Melton DA. Brief report: VGLL4 is a novel regulator of survival in human embryonic stem cells. Stem cells. 2013;31:2833–2841. doi: 10.1002/stem.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin Z, Guo H, Cao Y, Zohrabian S, Zhou P, Ma Q, VanDusen N, Guo Y, Zhang J, Stevens SM, Liang F, Quan Q, van Gorp PR, Li A, Dos Remedios C, He A, Bezzerides VJ, Pu WT. Acetylation of VGLL4 Regulates Hippo-YAP Signaling and Postnatal Cardiac Growth. Dev Cell. 2016 doi: 10.1016/j.devcel.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khaled WT, Choon Lee S, Stingl J, Chen X, Raza Ali H, Rueda OM, Hadi F, Wang J, Yu Y, Chin SF, Stratton M, Futreal A, Jenkins NA, Aparicio S, Copeland NG, Watson CJ, Caldas C, Liu P. BCL11A is a triple-negative breast cancer gene with critical functions in stem and progenitor cells. Nat Commun. 2015;6:5987. doi: 10.1038/ncomms6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rios AC, Fu NY, Lindeman GJ, Visvader JE. In situ identification of bipotent stem cells in the mammary gland. Nature. 2014;506:322–327. doi: 10.1038/nature12948. [DOI] [PubMed] [Google Scholar]
- 38.Chakrabarti R, Wei Y, Romano RA, DeCoste C, Kang Y, Sinha S. Elf5 regulates mammary gland stem/progenitor cell fate by influencing notch signaling. Stem Cells. 2012;30:1496–1508. doi: 10.1002/stem.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ye X, Tam WL, Shibue T, Kaygusuz Y, Reinhardt F, Ng Eaton E, Weinberg RA. Distinct EMT programs control normal mammary stem cells and tumour-initiating cells. Nature. 2015;525:256–260. doi: 10.1038/nature14897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vieira AF, Ricardo S, Ablett MP, Dionisio MR, Mendes N, Albergaria A, Farnie G, Gerhard R, Cameselle-Teijeiro JF, Seruca R, Schmitt F, Clarke RB, Paredes J. P-cadherin is coexpressed with CD44 and CD49f and mediates stem cell properties in basal-like breast cancer. Stem Cells. 2012;30:854–864. doi: 10.1002/stem.1075. [DOI] [PubMed] [Google Scholar]
- 41.Visvader JE. Keeping abreast of the mammary epithelial hierarchy and breast tumorigenesis. Genes Dev. 2009;23:2563–2577. doi: 10.1101/gad.1849509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakshatri H, Badve S. FOXA1 in breast cancer. Expert Rev Mol Med. 2009;11:e8. doi: 10.1017/S1462399409001008. [DOI] [PubMed] [Google Scholar]
- 43.Ylikallio E, Poyhonen R, Zimon M, De Vriendt E, Hilander T, Paetau A, Jordanova A, Lonnqvist T, Tyynismaa H. Deficiency of the E3 ubiquitin ligase TRIM2 in early-onset axonal neuropathy. Hum Mol Genet. 2013;22:2975–2983. doi: 10.1093/hmg/ddt149. [DOI] [PubMed] [Google Scholar]
- 44.Balastik M, Ferraguti F, Pires-da Silva A, Lee TH, Alvarez-Bolado G, Lu KP, Gruss P. Deficiency in ubiquitin ligase TRIM2 causes accumulation of neurofilament light chain and neurodegeneration. Proc Natl Acad Sci U S A. 2008;105:12016–12021. doi: 10.1073/pnas.0802261105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hatakeyama S. Early evidence for the role of TRIM29 in multiple cancer models. Expert Opin Ther Targets. 2016;20:767–770. doi: 10.1517/14728222.2016.1148687. [DOI] [PubMed] [Google Scholar]
- 46.Chen X, Dong C, Law PT, Chan MT, Su Z, Wang S, Wu WK, Xu H. MicroRNA-145 targets TRIM2 and exerts tumor-suppressing functions in epithelial ovarian cancer. Gynecol Oncol. 2015;139:513–519. doi: 10.1016/j.ygyno.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 47.Thompson S, Pearson AN, Ashley MD, Jessick V, Murphy BM, Gafken P, Henshall DC, Morris KT, Simon RP, Meller R. Identification of a novel Bcl-2-interacting mediator of cell death (Bim) E3 ligase, tripartite motif-containing protein 2 (TRIM2), and its role in rapid ischemic tolerance-induced neuroprotection. J Biol Chem. 2011;286:19331–19339. doi: 10.1074/jbc.M110.197707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palmbos PL, Wang L, Yang H, Wang Y, Leflein J, Ahmet ML, Wilkinson JE, Kumar-Sinha C, Ney GM, Tomlins SA, Daignault S, Kunju LP, Wu XR, Lotan Y, Liebert M, Ljungman ME, Simeone DM. ATDC/TRIM29 Drives Invasive Bladder Cancer Formation through miRNA-Mediated and Epigenetic Mechanisms. Cancer Res. 2015;75:5155–5166. doi: 10.1158/0008-5472.CAN-15-0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang L, Yang H, Palmbos PL, Ney G, Detzler TA, Coleman D, Leflein J, Davis M, Zhang M, Tang W, Hicks JK, Helchowski CM, Prasad J, Lawrence TS, Xu L, Yu X, Canman CE, Ljungman M, Simeone DM. ATDC/TRIM29 phosphorylation by ATM/MAPKAP kinase 2 mediates radioresistance in pancreatic cancer cells. Cancer Res. 2014;74:1778–1788. doi: 10.1158/0008-5472.CAN-13-2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yuan Z, Villagra A, Peng L, Coppola D, Glozak M, Sotomayor EM, Chen J, Lane WS, Seto E. The ATDC (TRIM29) protein binds p53 and antagonizes p53-mediated functions. Mol Cell Biol. 2010;30:3004–3015. doi: 10.1128/MCB.01023-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xiao Z, Jiang Q, Willette-Brown J, Xi S, Zhu F, Burkett S, Back T, Song NY, Datla M, Sun Z, Goldszmid R, Lin F, Cohoon T, Pike K, Wu X, Schrump DS, Wong KK, Young HA, Trinchieri G, Wiltrout RH, Hu Y. The pivotal role of IKKalpha in the development of spontaneous lung squamous cell carcinomas. Cancer Cell. 2013;23:527–540. doi: 10.1016/j.ccr.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ohtsu N, Nakatani Y, Yamashita D, Ohue S, Ohnishi T, Kondo T. Eva1 Maintains the Stem-like Character of Glioblastoma-Initiating Cells by Activating the Noncanonical NF-kappaB Signaling Pathway. Cancer Res. 2016;76:171–181. doi: 10.1158/0008-5472.CAN-15-0884. [DOI] [PubMed] [Google Scholar]
- 53.Ruggieri P, Mangino G, Fioretti B, Catacuzzeno L, Puca R, Ponti D, Miscusi M, Franciolini F, Ragona G, Calogero A. The inhibition of KCa3.1 channels activity reduces cell motility in glioblastoma derived cancer stem cells. PLoS One. 2012;7:e47825. doi: 10.1371/journal.pone.0047825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guo Y, Wang AY. Novel Immune Check-Point Regulators in Tolerance Maintenance. Front Immunol. 2015;6:421. doi: 10.3389/fimmu.2015.00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yao Y, Wang X, Jin K, Zhu J, Wang Y, Xiong S, Mao Y, Zhou L. B7-H4 is preferentially expressed in non-dividing brain tumor cells and in a subset of brain tumor stem-like cells. J Neurooncol. 2008;89:121–129. doi: 10.1007/s11060-008-9601-x. [DOI] [PubMed] [Google Scholar]
- 56.Zhang L, Wu H, Lu D, Li G, Sun C, Song H, Li J, Zhai T, Huang L, Hou C, Wang W, Zhou B, Chen S, Lu B, Zhang X. The costimulatory molecule B7-H4 promote tumor progression and cell proliferation through translocating into nucleus. Oncogene. 2013;32:5347–5358. doi: 10.1038/onc.2012.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stasiulewicz M, Gray SD, Mastromina I, Silva JC, Bjorklund M, Seymour PA, Booth D, Thompson C, Green RJ, Hall EA, Serup P, Dale JK. A conserved role for Notch signaling in priming the cellular response to Shh through ciliary localisation of the key Shh transducer Smo. Development. 2015;142:2291–2303. doi: 10.1242/dev.125237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ochoa SD, Salvador S, LaBonne C. The LIM adaptor protein LMO4 is an essential regulator of neural crest development. Dev Biol. 2012;361:313–325. doi: 10.1016/j.ydbio.2011.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.De Rosa A, Pellegatta S, Rossi M, Tunici P, Magnoni L, Speranza MC, Malusa F, Miragliotta V, Mori E, Finocchiaro G, Bakker A. A radial glia gene marker, fatty acid binding protein 7 (FABP7), is involved in proliferation and invasion of glioblastoma cells. PLoS One. 2012;7:e52113. doi: 10.1371/journal.pone.0052113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yun K, Mantani A, Garel S, Rubenstein J, Israel MA. Id4 regulates neural progenitor proliferation and differentiation in vivo. Development. 2004;131:5441–5448. doi: 10.1242/dev.01430. [DOI] [PubMed] [Google Scholar]
- 61.Rolando C, Erni A, Grison A, Beattie R, Engler A, Gokhale PJ, Milo M, Wegleiter T, Jessberger S, Taylor V. Multipotency of Adult Hippocampal NSCs In Vivo Is Restricted by Drosha/NFIB. Cell Stem Cell. 2016 doi: 10.1016/j.stem.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 62.Van Raay TJ, Wang YK, Stark MR, Rasmussen JT, Francke U, Vetter ML, Rao MS. frizzled 9 is expressed in neural precursor cells in the developing neural tube. Dev Genes Evol. 2001;211:453–457. doi: 10.1007/s004270100174. [DOI] [PubMed] [Google Scholar]
- 63.Erreni M, Solinas G, Brescia P, Osti D, Zunino F, Colombo P, Destro A, Roncalli M, Mantovani A, Draghi R, Levi D, Rodriguez YBR, Gaetani P, Pelicci G, Allavena P. Human glioblastoma tumours and neural cancer stem cells express the chemokine CX3CL1 and its receptor CX3CR1. Eur J Cancer. 2010;46:3383–3392. doi: 10.1016/j.ejca.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 64.Schreiner S, Cossais F, Fischer K, Scholz S, Bosl MR, Holtmann B, Sendtner M, Wegner M. Hypomorphic Sox10 alleles reveal novel protein functions and unravel developmental differences in glial lineages. Development. 2007;134:3271–3281. doi: 10.1242/dev.003350. [DOI] [PubMed] [Google Scholar]
- 65.Riechers A, Bosserhoff AK. Melanoma inhibitory activity in melanoma diagnostics and therapy - a small protein is looming large. Exp Dermatol. 2014;23:12–14. doi: 10.1111/exd.12281. [DOI] [PubMed] [Google Scholar]
- 66.Fagiani E, Giardina G, Luzi L, Cesaroni M, Quarto M, Capra M, Germano G, Bono M, Capillo M, Pelicci P, Lanfrancone L. RaLP, a new member of the Src homology and collagen family, regulates cell migration and tumor growth of metastatic melanomas. Cancer Res. 2007;67:3064–3073. doi: 10.1158/0008-5472.CAN-06-2301. [DOI] [PubMed] [Google Scholar]
- 67.Hartman KG, McKnight LE, Liriano MA, Weber DJ. The evolution of S100B inhibitors for the treatment of malignant melanoma. Future Med Chem. 2013;5:97–109. doi: 10.4155/fmc.12.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goto Y, Matsuzaki Y, Kurihara S, Shimizu A, Okada T, Yamamoto K, Murata H, Takata M, Aburatani H, Hoon DS, Saida T, Kawakami Y. A new melanoma antigen fatty acid-binding protein 7, involved in proliferation and invasion, is a potential target for immunotherapy and molecular target therapy. Cancer Res. 2006;66:4443–4449. doi: 10.1158/0008-5472.CAN-05-2505. [DOI] [PubMed] [Google Scholar]
- 69.Wang H, Zhang L, Zhang IY, Chen X, Da Fonseca A, Wu S, Ren H, Badie S, Sadeghi S, Ouyang M, Warden CD, Badie B. S100B promotes glioma growth through chemoattraction of myeloid-derived macrophages. Clin Cancer Res. 2013;19:3764–3775. doi: 10.1158/1078-0432.CCR-12-3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Morihiro Y, Yasumoto Y, Vaidyan LK, Sadahiro H, Uchida T, Inamura A, Sharifi K, Ideguchi M, Nomura S, Tokuda N, Kashiwabara S, Ishii A, Ikeda E, Owada Y, Suzuki M. Fatty acid binding protein 7 as a marker of glioma stem cells. Pathol Int. 2013;63:546–553. doi: 10.1111/pin.12109. [DOI] [PubMed] [Google Scholar]
- 71.Wills MK, Tong J, Tremblay SL, Moran MF, Jones N. The ShcD signaling adaptor facilitates ligand-independent phosphorylation of the EGF receptor. Mol Biol Cell. 2014;25:739–752. doi: 10.1091/mbc.E13-08-0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Whittle JR, Lewis MT, Lindeman GJ, Visvader JE. Patient-derived xenograft models of breast cancer and their predictive power. Breast Cancer Res. 2015;17:17. doi: 10.1186/s13058-015-0523-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bosch A, Eroles P, Zaragoza R, Vina JR, Lluch A. Triple-negative breast cancer: molecular features, pathogenesis, treatment and current lines of research. Cancer Treat Rev. 2010;36:206–215. doi: 10.1016/j.ctrv.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 74.Prat A, Pineda E, Adamo B, Galvan P, Fernandez A, Gaba L, Diez M, Viladot M, Arance A, Munoz M. Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast. 2015;24(Suppl 2):S26–S35. doi: 10.1016/j.breast.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 75.Yarbrough WG, Pannaccione A, Chang MT, Ivanov SV. Clinical and Molecular Insights Into Adenoid Cystic Carcinoma: Neural Crest-Like Stemness as a Target. Laryngoscope Investigative Otolaryngology. 2016 doi: 10.1002/lio2.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Peh GS, Lang RJ, Pera MF, Hawes SM. CD133 expression by neural progenitors derived from human embryonic stem cells and its use for their prospective isolation. Stem Cells Dev. 2009;18:269–282. doi: 10.1089/scd.2008.0124. [DOI] [PubMed] [Google Scholar]
- 77.Coskun V, Wu H, Blanchi B, Tsao S, Kim K, Zhao J, Biancotti JC, Hutnick L, Krueger RC, Jr, Fan G, de Vellis J, Sun YE. CD133+ neural stem cells in the ependyma of mammalian postnatal forebrain. Proc Natl Acad Sci U S A. 2008;105:1026–1031. doi: 10.1073/pnas.0710000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu Q, Nguyen DH, Dong Q, Shitaku P, Chung K, Liu OY, Tso JL, Liu JY, Konkankit V, Cloughesy TF, Mischel PS, Lane TF, Liau LM, Nelson SF, Tso CL. Molecular properties of CD133+ glioblastoma stem cells derived from treatment-refractory recurrent brain tumors. J Neurooncol. 2009;94:1–19. doi: 10.1007/s11060-009-9919-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lai CY, Schwartz BE, Hsu MY. CD133+ melanoma subpopulations contribute to perivascular niche morphogenesis and tumorigenicity through vasculogenic mimicry. Cancer Res. 2012;72:5111–5118. doi: 10.1158/0008-5472.CAN-12-0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.