Abstract
Anxious temperament (AT) is an early life disposition that markedly increases the risk to develop stress related psychopathology such as anxiety and depressive disorders. Since anxiety and depression are common, and frequently have their onset early in life, a better understanding of the factors related to their childhood onset will facilitate the development of new more effective neurally informed interventions. A nonhuman primate (NHP) developmental model of childhood AT has been established, which has provided an understanding of the neural systems and molecular mechanisms mediating the development of AT. Multimodal neuroimaging studies reveal altered brain metabolism across prefrontal, limbic (e.g. central nucleus of the amygdala (Ce) and anterior hippocampus), and brainstem regions, as well as altered functional connectivity involving the Ce. Heritability studies demonstrate that individual variation in AT is heritable, and genetic correlational analyses demonstrate that metabolism in the posterior orbital frontal cortex, the bed nucleus of the stria terminalis, and the periaqueductal gray share a genetic substrate with AT. On a molecular level, the finding of reduced expression of Ce neuroplasticity genes provides the basis for a neurodevelopmental hypothesis focused on the Ce. Viral vector methods for altering gene expression in the Ce of young NHPs are currently being used as a prelude to conceptualizing novel molecularly targeted early life interventions.
Understanding mechanisms underlying the development of anxiety disorders: importance of nonhuman primate studies
Anxiety disorders (ADs) frequently begin during childhood (Copeland et al., 2014; Costello et al., 2005; Merikangas et al., 2010; Pine, 2007), are in part heritable (Hettema et al., 2005; Shimada-Sugimoto et al., 2015), and if not effectively treated can result in long-term disability and suffering (Connolly and Bernstein, 2007; Ezpeleta et al., 2001; Flannery-Schroeder, 2004; Hirshfeld-Becker et al., 2008; Kessler et al., 2008; Massion et al., 1993; Pine et al., 1998; Roy-Byrne and Katon, 1997). ADs are among the most common psychiatric illnesses, with lifetime prevalences in adults and adolescents of 29% and 25%, respectively (Kessler et al., 2005; Kessler et al., 2012; Merikangas et al., 2010). Existing treatments are helpful for some patients with ADs but many patients fail to significantly improve (Roy-Byrne, 2015; Walkup et al., 2008), highlighting the need for new treatment approaches (Hyman, 2014). In addition, anxiety is highly comorbid with other disorders and its presence is generally a negative predictor of treatment outcome for both psychiatric and medical illnesses. To improve the care and treatment of patients with ADs, and other disorders, psychiatry must pursue the understanding of the basic brain mechanisms that underlie psychopathology. Focus should be on translating these basic research findings with the goal of facilitating the development of improved early detection and treatment strategies in children.
Research using animal models is a critical component of new treatment development efforts and nonhuman primate (NHP) models provide an unparalleled opportunity to translate findings from rodent mechanistic studies to humans suffering from ADs and other psychiatric illnesses (Kalin and Shelton, 2003; Machado and Bachevalier, 2003). We have developed an evolutionarily conserved model of ADs in young rhesus monkeys (Macaca mulatta) that has proved highly valuable in elucidating the neural circuitry that underlies ADs as well as its molecular underpinnings (Fox et al., 2015; Kalin and Shelton, 1989). Rhesus monkeys are ideally suited for modeling human anxiety as they are very similar to humans in the expression of their social and emotional behaviors, have rearing and early cognitive developmental patterns similar to humans and are affected by the same types of stressors as are humans (Fox and Kalin, 2014). These similarities are consistent with the relatively recent evolutionary divergence of humans and rhesus monkeys, which also accounts for their similarities in brain structure and function (i.e., prefrontal cortex and amygdala) and in factors influencing brain development.
Behavioral Inhibition and Anxious Temperament as a risk phenotype
Because a risk phenotype for ADs can be identified early in life, ADs provide a unique opportunity for establishing the feasibility of new neuroscientifically-informed early intervention and prevention strategies. The now classic behavioral inhibition studies led by Jerome Kagan provided the groundwork for establishing an AD risk phenotype, as he found that approximately 15% of young children have an anxiety-prone trait. This trait, termed behavioral inhibition, is characterized by an extreme inhibitory response that is evident when children are confronted with novel situations and/or strangers (Kagan, 1997; Kagan et al., 1987). Extreme behavioral inhibition is frequently accompanied by excessive physiological arousal including increased pituitary-adrenal and sympathetic nervous system activity (Kagan et al., 1988). Later studies clearly demonstrated the strong linkage between extreme behavioral inhibition and the development of anxiety and depression. It is estimated that up to 50% of children with extreme behavioral inhibition will later develop stress-related psychopathology with the greatest risk for developing social anxiety disorder (Biederman et al., 2001; Clauss and Blackford, 2012).
Our early work in young rhesus monkeys focused on characterizing adaptive and maladaptive anxiety-related behavioral responses to threat. As part of this effort, we developed the Human Intruder Paradigm (HIP, see Figure 1A), which reliably elicits specific threat-related responses that are associated with three different contexts: attachment disruption or separation (Alone condition, A), the potential threat of a human being present but avoiding eye contact with the subject (No Eye Contact condition, NEC), and the more certain and immediate threat of a human directly staring at the test monkey (Stare condition, ST) (Kalin, 1993; Kalin and Shelton, 1989). During the Alone condition, the monkey is separated from its mother or partner and placed in a test cage by itself. Monkeys respond by locomoting and emitting “coo” vocalizations. Together these behaviors function to elicit conspecific support. During the No Eye Contact condition, a human intruder enters the room, stands still, and presents her/his profile to the monkey. In the absence of eye contact, the adaptive response is to remain inconspicuous, which is accomplished by reducing locomotion and vocalizations such that monkeys stay very still or even freeze in one position. The Stare condition is similar to NEC except that the intruder stares directly at the monkey while maintaining a neutral face. In contrast to the behavioral inhibition induced by NEC, direct eye contact elicits active fight and flight behaviors characterized by monkeys engaging in a combination of threatening and submissive behaviors directed towards the staring human intruder. Exposure to each of these conditions also results in activation of the pituitary-adrenal axis as evidenced by increased plasma levels of ACTH and cortisol. It is important to note that while the A, NEC, and ST conditions reliably elicit different adaptive behavioral repertoires, monkeys demonstrate marked individual variation in their responses to each of these stressful and potentially threatening conditions (Kalin and Shelton, 1998).
Figure 1.
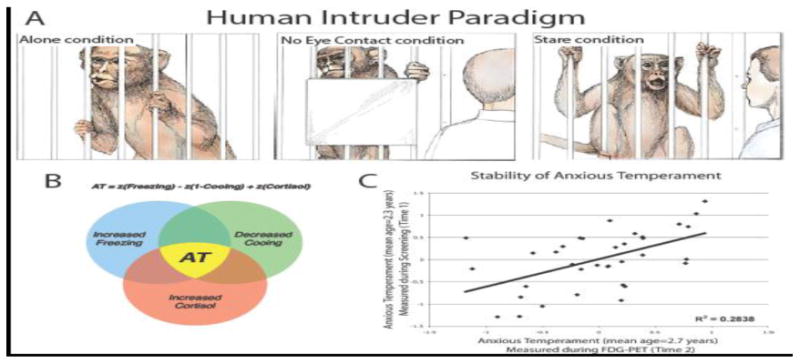
(A) The three experimental conditions of the human intruder paradigm elicit distinct fear and anxiety-related behaviors in young rhesus monkeys. When alone and separated from their cagemate (Alone condition, left), young monkeys actively explore the test cage and emit “coo” calls, thought to reflect an attempt to attract help from their mothers or other conspecifics. In the next condition, a human intruder presents his or her profile, while avoiding direct eye contact with the monkey (No Eye Contact condition, center). In this situation, the monkeys typically orient their focus to the intruder, trying to evade discovery by remaining completely still (freezing) and reducing their coo volcalizations. In the third condition, the human intruder enters the room and stares at the animal (Stare condition, right). This direct threat condition elicits aggressive and submissive behaviors (e.g., barking, threatening gestures, lip smacking, and cage rattling). From Kalin (1993). Copyright 2002 by Scientific American, Inc. Reprinted by permission. (B) Anxious Temperament (AT) is calculated as the mean z-scores of NEC-induced freezing, coo vocalizations, and plasma cortisol levels. (C) AT is a relatively stable trait. In this example, taken from Fox et al. (2008), AT assessed at two time points with an approximate 4-month interval was significantly correlated. Reprinted with permission.
The striking similarities between children with extreme behavioral inhibition and young monkeys that engage in prolonged NEC-induced freezing prompted us to further develop a NHP developmental model of early anxiety relevant to the risk to develop stress-related psychopathology (Kalin et al., 1998a; Kalin et al., 1998b; Kalin et al., 1991). Building on the concept of behavioral inhibition, we used the term anxious temperament (AT) to denote the early life behavioral and physiological traits that when extreme confer the risk to develop anxiety and depressive disorders (Fox et al., 2008). In the monkeys, we defined AT (Figure 1B) as the combination of an individual's freezing duration, vocal reductions, and cortisol responses that occur in response to NEC (Fox et al., 2008; Oler et al., 2010; Shackman et al., 2013). As in children, our studies demonstrated a wide range of individual differences in AT that were trait-like, as they were relatively stable over time (Fox et al., 2012)(see Figure 1C). In field studies on the island of Cayo Santiago, we demonstrated that our laboratory measure of AT was relevant to primate social behavior and dysfunction occurring in a naturalistic setting (Fox and Kalin, 2014). As predicted from human anxiety, we found that young monkeys with high levels of AT did not venture far from their mothers and interacted less with their peers. In the laboratory, we also found that monkeys with extreme threat-induced freezing behavior have a greater fear of snakes and unlike their low freezing peers continue to display anxiety after threat cessation (Shackman et al., In Press). We also validated the AT model pharmacologically by demonstrating that benzodiazepines decrease the behavioral and physiological components of AT (Kalin, 2003; Kalin and Shelton, 1989) whereas administration of the anxiogenic, inverse benzodiazepine agonist, beta-carboline, increases the expression of these parameters (Kalin et al., 1992).
Identifying the neural substrates underlying childhood anxiety and primate AT
To understand the neural substrates underlying AT and to directly link the monkey studies to childhood ADs, we engaged in a series of studies using similar imaging methods in young monkeys and children. While many neuroimaging studies have been performed in adults with ADs, few have been done in adolescents and even less have been performed in preadolescent children. Understanding the brain alterations associated with the emergence and initial presentations of ADs is critical to provide a neuroscientific basis for the development of early intervention strategies. Therefore, our strategy has been to use multimodal neuroimaging methods to explore the neural substrates underlying childhood ADs and to use these findings to guide mechanistic studies that can be carried out in our nonhuman primate model.
In an initial fMRI study, we demonstrated that AD children are particularly sensitive to uncertainty in that they showed increased activation of the amygdala and anterior insula when they viewed fear faces that were preceded by an uncertain cue (Figure 2A). In every day life, children with ADs become symptomatic when confronted with uncertain predicaments and these data provide an initial glimpse into understanding the neurobiological basis underlying their sensitivity (Williams et al., 2015). To further explore amygdala alterations in preadolescent AD children, we used resting state fMRI to examine possible alterations in functional coupling between the amygdala and other brain regions. Based on our NHP findings described below, we selected the, central nucleus of the amygdala (Ce), the critical outflow region of the amygdala, as our focus and discovered that AD children had decreased coupling between the Ce and the dorsolateral prefrontal cortex (dlPFC). This relation between reduced Ce-dlPFC functional coupling and anxiety is evolutionarily conserved as we also found a similar relation between increased anxiety and decreased Ce-dlPFC coupling in our primate model (Birn et al., 2014).
Figure 2.
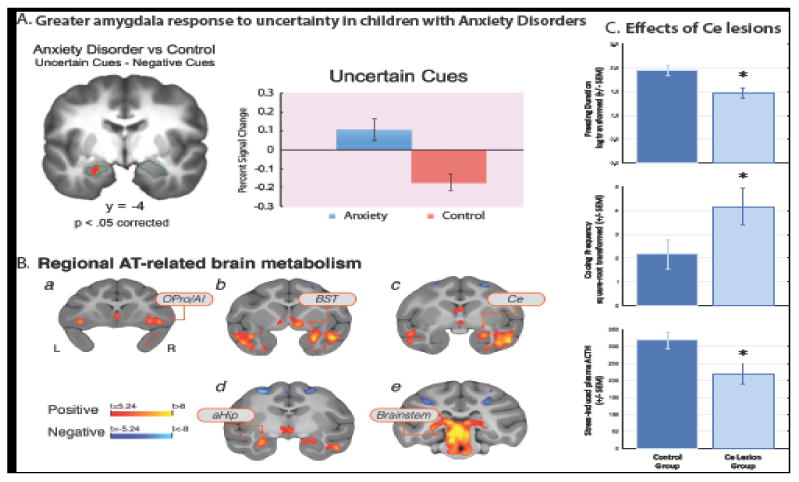
(A) During uncertain anticipation, relative to the certain anticipation of fear faces viewed inside the MRI scanner, children with anxiety disorders exhibited greater activation in the left amygdala (red cluster). The bar graph displays the percent signal change extracted from the cluster, and shows that the group difference is driven by a higher amygdala response to the uncertain cue in children with anxiety. Error bars represent SEM. Region of interest (face-responsive voxels within the amygdala) outlined in green. Modified from Williams et al. (2015), with permission. (B) Regions where brain metabolism was significantly associated with individual differences in rhesus monkey AT (p<.05, Šidák corrected for multiple comparisons across the whole-brain). Regions include ortibal proisocortex/anterior insula (OPro/AI; shown in [a]), subgenual anterior cingulate, temporal cortex, bed nucleus of the stria terminalis (BST; shown in [b]), central nucleus of the amygdala (Ce; shown in [c]), anterior hippocampus (aHip; shown in [d]) and brainstem regions including the periaqueductal gray (PAG; shown in [e]). Reprinted with permission from Fox et al. (2015). (C) The effects of central nucleus (Ce) lesions on components of AT. Monkeys with Ce lesions displayed less freezing (top), emitted more coo calls (middle), and released less adrenocorticotropic hormone (ACTH, bottom) during exposure to the human intruder paradigm. Adapted by permission from Kalin, Shelton, and Davidson (2004).
These findings, along with those described in adolescents and adults (Etkin et al., 2010; Etkin and Schatzberg, 2011; Prater et al., 2013; Roy et al., 2013; Shin et al., 2005), suggest that anxiety disorder related alterations in amygdala function and its prefrontal regulation likely have their origins early in life.
We used 18fluoro-deoxyglucose positron emission tomography (FDG-PET) in young monkeys to examine the possibility that alterations in brain metabolism are associated with early life anxiety. Regional brain metabolism studies provide important functional information that differs from that gleaned from fMRI, but because of the need to administer radioactive FDG, PET should only be performed in children for necessary diagnostic purposes. Our strategy was to identify brain regions in which metabolism predicted individual differences in AT (Fox et al., 2005; Kalin et al., 2005). In these studies, FDG was administered; monkeys were exposed to NEC for 30min and then were anesthetized and placed in the microPET scanner. Because of the kinetics and properties of FDG, these studies allowed us to assess individual differences in AT at the same time brain metabolism was assessed. The early findings from smaller samples of young monkeys revealed associations between increased metabolism in the amygdala and bed nucleus of the stria terminalis (BST) with increased levels of NEC-induced freezing behavior (Fox et al., 2008; Kalin et al., 2005). Interestingly, work performed primarily in rodents suggested that the BST is involved in longer-term responses to threat (Gungor and Pare, 2016; Walker et al., 2009). Later studies in very large sample sizes of periadolescent monkeys (n=238; n=592 that included the 238 in the first study) confirmed and extended these findings identifying a network of regions including the orbitofrontal cortex (OFC), central nucleus of the amygdala (Ce), BST, and the periaqueductal gray (PAG) that predicted individual differences in AT (Fox et al., 2015; Oler et al., 2010)(see Figure 2B). The PAG is important because it is a brain stem region that in rodent studies has been demonstrated to be involved with stress-induced freezing behavior and pain processing (Bandler and Shipley, 1994).
Because AT has trait-like qualities, we also examined whether individual differences in AT's underlying neural substrate exhibited trait-like features (Fox et al., 2012; Fox et al., 2008). In a subset of monkeys, we assessed NEC-related metabolism 3 times over a 6-18 month period and found that among other regions, individual differences in Ce metabolism were stable over time (Fox et al., 2012). In another experiment, we assessed the extent to which the relation between brain metabolism and AT was stable regardless of the context in which brain metabolism was assessed (Fox et al., 2008). In this study, FDG was administered to the same monkeys four times: 2 stressful contexts (NEC and Alone conditions of the Human Intruder Paradigm) and 2 nonstressful contexts (in home cage with partner and in home cage without partner). Results demonstrated that while brain metabolism changed between stressful and nonstressful contexts, the relation between brain metabolism in the amygdala, BST, anterior hippocampus, and PAG with individual differences in AT remained regardless of the context in which brain metabolism was measured. Taken together, these findings demonstrate that AT's underlying neural substrate is stable over time and across contexts underscoring the ever-present nature of the brain alterations that underlie early life extreme anxiety.
While very informative, the human and monkey imaging data is correlational in nature and does not allow for interpretations of causality. It is critical to recognize that the value of animal models lies in the ability to investigate underlying mechanisms. Therefore, in addition to the FDG-PET studies, we performed lesioning experiments focused on understanding the roles of the Ce and OFC in mediating AT. Using a neurotoxin to selectively lesion Ce neurons, we found that bilateral Ce lesions had anxiolytic effects resulting in a reduction in components of AT, including freezing behavior and pituitary-adrenal activation (Kalin et al., 2004)(see Figure 2C). To lesion the OFC, we used an aspiration technique targeted at the OFC regions that are most interconnected with the amygdala. In addition to removing cortex from these regions, the aspiration lesions also interrupt fibers that are passing through the OFC, to and from other prefrontal regions as well as to and from limbic regions such as the amygdala. Similar to the Ce neurotoxic lesions, the OFC aspiration lesions resulted in decreased threat-induced freezing behavior (Kalin et al., 2007). Furthermore, by combining the lesioning strategy with FDG-PET imaging, we found that the OFC lesions decreased NEC-induced metabolism in the BST (Fox et al., 2010). Taken together these findings point to a mechanistic role for the Ce and its extended amygdala partner, the BST, in mediating AT and also provide evidence for a regulatory role of the OFC in modulating extended amygdala function.
Establishing the heritability of AT and its underlying neural substrate
We have conceptualized AT as temperamental trait that is evident early in life and when extreme is a prominent risk factor for the later development of stress-related psychopathology. To understand the factors contributing to the early life expression of AT, and to provide a basis for further genetic studies, we explored the extent to which AT is influenced by heritable and non-heritable (e.g., environmental, rearing, attachment, stress, etc.) factors. We were able to accomplish this because our fully phenotyped (i.e. behavior, pituitary-adrenal activation, functional and structural imaging) periadolescent rhesus monkey sample is large (n=592), constitutes a multigenerational pedigree, and has a structure of familial relationships that increases confidence in estimates of heritability. An important aspect of this study, was our ability to also estimate the heritability of AT's intermediate phenotype, the regional brain metabolism that is associated with AT. Consistent with human anxiety and depressive disorders, we found that monkey AT was estimated to be 29% heritable (Fox et al., 2015). Using the FDG-PET data and performing voxel-wise analyses, we found that metabolism in many regions of the AT circuit, including OFC, amygdala, hippocampus and brain stem regions, was also significantly heritable with heritability estimates that varied depending on the region (Fox et al., 2015). However, demonstrating heritability of brain function alone does not directly link the heritability of that brain region to AT. To accomplish this it is necessary to perform genetic correlation analyses, which provide heritability estimates of the extent to which 2 traits (i.e. regional brain metabolism and AT) fall through the family tree in the same pattern. These analyses revealed that metabolism in 3 of the AT-related regions was significantly coheritable with AT (see Figure 3A); in the OFC, the critical region was the orbital proisocortex abutting on the anterior insula (Opro/AI, Figure 3B), in the extended amygdala it was the BST (Figure 3C), and in the brain stem it was the PAG (Figure 3D) (Fox et al., 2015). These findings suggest that the intergenerational transfer of AT may in part be mediated by the underlying heritability of brain metabolism in a circuit that comprises regulatory OFC regions, core extended amygdala regions such as the BST, and effector brain stem regions, for example the PAG.
Figure 3.
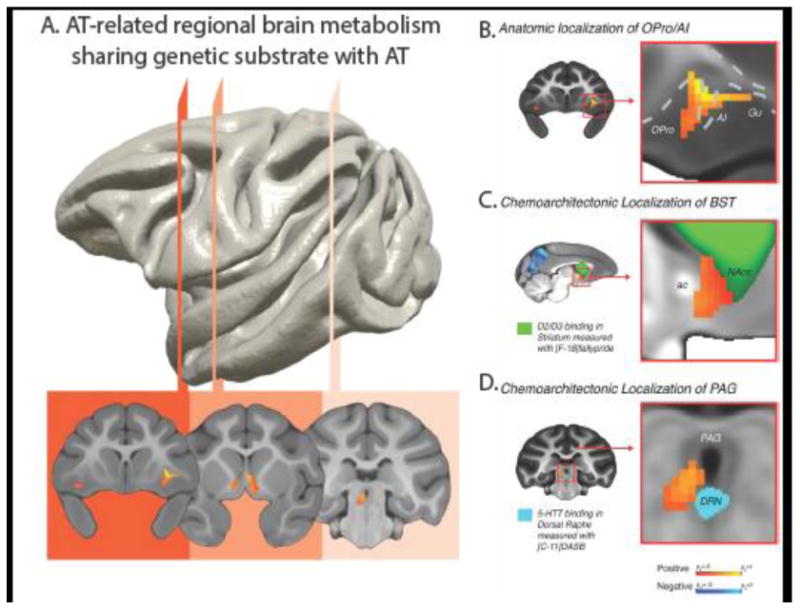
A tripartite prefrontal-limbic-midbrain circuit involved in the genetic transmission of AT. (A) Regions where brain metabolism demonstrated a significant genetic correlation with AT, thus sharing a genetic substrate, include: the ortibal proisocortex/anterior insula (OPro/AI, red), bed nucleus of the stria terminalis (BST, orange), and the periaqueductal gray, (PAG, peach). (B-D) Using high-resolution anatomical and chemoarchitectonic imaging in a separate group of monkeys, regions were precisely localized as: agranular OPro/AI (B); the BST region lying between the anterior commissure (ac) and the [18-F] fallypride identified dopamine receptor-rich ventral striatum that includes the nucleus accumbens (green; NAcc) (C); and the vlPAG-region in the gray matter surrounding the ventricle, superior to the [11-C] DASB identified serotonin transporter-rich dorsal raphe nucleus, DRN (D). Modified from Fox et al. (2015), and reproduced with permission.
We further examined structural alterations in candidate genes; the serotonin transporter gene (SLC6A4), corticotropin releasing hormone receptor 1 (CRHR1), and corticotropin releasing hormone receptor 2 (CRHR2), each of which was potentially relevant to AT1. While the short allele of the repeat polymorphism in the promoter region of SLC6A4 (known as 5-HTTLPR) has been associated with neuroticism and is also suggested to interact with the influences of early trauma (Caspi et al., 2003), we found no indication that the 5-HTTLPR influenced nonhuman primate threat-induced freezing behavior or behavioral inhibition (Oler et al., 2010), although detecting such effects may depend on the context within which they are assessed (Kalin et al., 2008). The CRHR1 receptor gene is of interest as structural variation in CRHR1 has been demonstrated to interact with early trauma (Bradley et al., 2008), similar to the 5HTTLPR findings (Caspi et al., 2003). Additionally, altered function of the CRF system is associated with anxiety and depression, and activation of brain CRF1 receptors is frequently anxiogenic (Binder and Nemeroff, 2010; Nemeroff and Vale, 2005). We found that SNPs in exon 6 of the rhesus CRHR1 were not only significantly associated with AT, but also were associated with hippocampal and amygdala brain metabolism (Rogers et al., 2013). This may be particularly important since exon 6 is specific to, and conserved in, primate species (Rogers et al., 2013). Other research suggests that the predominant isoform of the CRF1 receptor excludes exon 6, but when present exon 6 encodes an intracellular loop that reduces the efficacy of CRF1 signal transduction (Markovic et al., 2006; Teli et al., 2008). Interestingly, one of the exon 6 SNPs that we identified is in a splice site region while another is in an amino acid sequence-altering region. The CRF2 receptor has also been implicated in modulating anxiety (Bakshi et al., 2002; Bale et al., 2000) and in primates has a more diverse brain distribution than in rodent species (Sanchez et al., 1999). While influences of genetic variation in the CRHR2 gene and the function of its receptor have been relatively understudied in humans, we have been interested in this receptor as a potential therapeutic target. Our study identified a series of synonymous and non-synonymous SNPs in CRHR2 that were significantly associated with AT and brain metabolism in the hippocampus (unpublished data). Taken together, these findings suggest that alterations in both CRHR1 and CRHR2 genes may affect the function of AT's neural substrate with subsequent influences on the expression of the AT phenotype.
Identifying and testing molecular substrates underlying AT
To further characterize the molecular substrates mediating AT and its neural circuit, we embarked on studies examining transcriptome-wide alterations in the expression of genes within the Ce (Fox et al., 2012; Roseboom et al., 2013). In addition, we developed the ability to directly, and site specifically, manipulate gene expression in nonhuman primates using viral vector strategies, allowing us to test the causal role for leads identified in the RNA studies (Kalin et al., 2016). In our initial gene expression study, we collected brains from 24 fully phenotyped and imaged young monkeys and assessed individual differences in Ce gene expression. It is important to underscore that measuring RNA transcripts from a specific tissue reflects the combined influences of genetics and epigenetic factors (e.g., environment) on the expression of a specific gene within that particular tissue. While the expression of numerous Ce genes predicted individual differences in AT, of particular interest was the finding that the expression of a number of neuroplasticity and neurotrophic genes were negatively associated with AT. For example, we found that NTRK3 (also known as the TrkC receptor) gene expression was negatively correlated with AT, such that individuals with lower levels of Ce NTRK3 expression were more anxious (Fox et al., 2012)(Figure 4A). Lower levels of NTRK3 expression also predicted increased threat-induced Ce metabolism (Fox et al., 2012)(Figure 4B). In addition to the NTRK3 finding, other members of the intracellular cascade downstream of NTRK3 activation negatively predicted AT and brain metabolism (see Figure 4C). Based on these findings, we performed a “proof of concept” study to test whether targeting the NTRK3 pathway would provide a mechanistic rationale for new treatment development. Accordingly, we overexpressed the TrkC receptor's cognate ligand, neurotrophin 3 (NT3), in the Ce to test whether increased signaling through this pathway would result in a reduction in anxiety. While the data are not yet available, this experiment demonstrates how site-specific molecular RNA expression data can be leveraged to test mechanistic hypotheses and new treatment strategies in primates.
Figure 4.
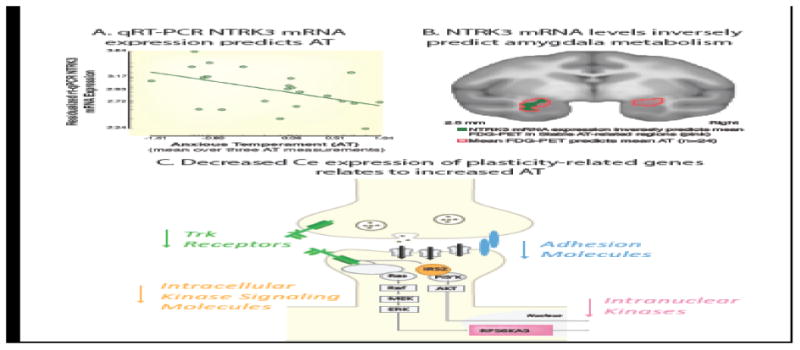
Ce expression of mRNA for the neurotrophic receptor, NTRK3, negatively predicts AT. (A) Microarray data showed that individuals with higher levels of Ce NTRK3 mRNA expression exhibited lower AT. Quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR) confirmed the negative relationship between Ce NTRK3 mRNA expression levels and AT (r = - 0.49; P = 0.029). (B) Individuals showing higher levels of NTRK3 mRNA expression, indexed by qRT-PCR, show reduced amygdala metabolism in vivo (green) [FDR-corrected within the stable AT-related region (outlined in red)]. (C) Schematic of the neuroplasticity-associated NTRK3 (tropomyosin receptor kinase [Trk]) pathway. A similar pattern in relation to AT was found for IRS2, an intracellular kinase signaling molecule (orange) and RPS6KA3 (pink), two downstream mediators of NTRK3 activation. Other molecules in the NTRK3 pathway are also depicted in light gray. Adapted with permission from Fox et al. (2012) and from Fox & Kalin (2014).
Using the viral vector strategy we also tested the role of the CRF system in mediating AT (see Figure 5A-E). As mentioned earlier, CRF is thought to be anxiogenic and involved in mediating anxiety and depressive symptomatology. Additionally, polymorphisms in the genes encoding the CRF receptors (i.e., CRHR1 and CRHR2) are associated with increased levels of anxiety and AT. As predicted, we found that Ce CRF overexpression resulted in increased AT (Figure 5F) as well as increased metabolism in the amygdala and other components of the AT neural circuit (Figure 5G). These findings are the first demonstration in a primate of the potential pathophysiological role for Ce CRF in mediating maladaptive anxiety and its neural substrates (Kalin et al., 2016).
Figure 5.
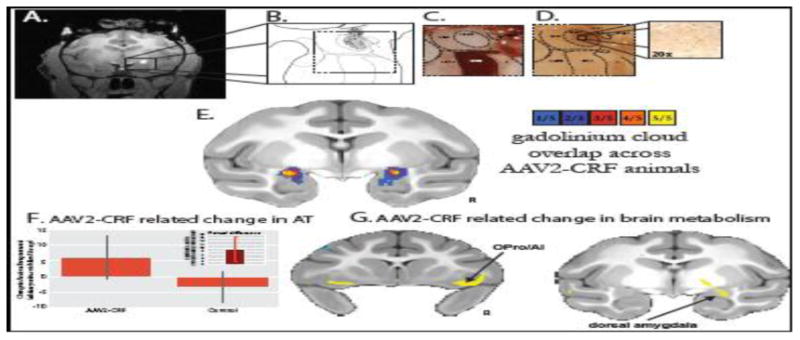
In vivo estimation and postmortem verification of dorsal amygdala corticotropin-releasing factor (CRF) overexpression. (A) The gadolinium cloud in the dorsal amygdala, central nucleus (Ce) region, during and immediately following adeno-associated virus type 2 (AAV2)-CRF delivery provided an estimate of the location and extent of the infusions. (B) Camera lucida drawings of CRF expression from postmortem tissue reflected the extent of viral infusion as estimated from the intraoperative gadolinium signal. Gray regions represent neuropil staining and the black dots represent CRF overexpressing cells. (C) Acetylcholinesterase staining defined the boundaries of the amygdalar nuclei. (D) Adjacent sections were used for CRF immunohistochemistry demonstrating marked overexpression in the dorsal amygdala, Ce region. (E) Based on the intraoperative gadolinium images, we estimated the infusion extent in standard space to examine the overlap of the gadolinium injection clouds across the five experimental animals. The colors represent the number of animals with gadolinium signal at each voxel. Note the bilateral overlap across all experimental animals within the Ce region (yellow). (F) Compared with their matched control animals, the CRF overexpressing animals demonstrated increased postsurgical levels of AT (mean +/- SEM). Significance was determined using a paired-samples t-test comparing dorsal amygdala CRF animals and their cagemate control animals (CRF group [post-pre] – control group [post-pre]) (p<, .05, one-tailed; see inset and Kalin et al., (2016) for details). (G) Compared with their matched control animals, the CRF overexpressing animals demonstrated increased [post-pre] change in metabolism within the dorsal amygdala, OPro/AI, and hippocampus (yellow, p <, .01, two-tailed, uncorrected). ABmc, accessory basal nucleus, magnocellular subdivision; Astr, amygdalostriatal transition zone; Bmc, basal nucleus, magnocellular subdivision; CeLpc, central nucleus, lateral central subdivision; CeM, central nucleus, medial subdivision; L, lateral nucleus; R, right.
Conclusions
This work demonstrates the important translational value of using nonhuman primates to understand mechanisms underling human psychopathology. Our studies provide new insights into AT, the early life risk phenotype for the development of anxiety and depressive disorders. Using imaging methods routinely used in humans, we demonstrated that the AT neural circuit is distributed and that the Ce is a core component of the circuit. We found that AT is heritable and that the intergenerational transfer of AT may be via heritability of metabolism in select prefrontal, extended amygdala, and brain stem regions. At a molecular level, we identified alterations in the expression of neuroplasticity genes within the Ce that may be responsible for the emergence and maintenance of AT and anxiety disorders. We also developed new methods to alter the expression of specific genes in select brain regions so that primate models of psychopathology can be used to test mechanistic molecular hypotheses as well as the feasibility of new treatment targets. It is our hope that this work will drive the development of new neuro-molecularly informed strategies aimed at early intervention and perhaps prevention of the long-term suffering experienced by individuals with anxiety and depressive disorders.
Acknowledgments
This work was supported by the following National Institutes of Health Grants: R01-MH046729, R01-MH081884, R01-MH107563, P50-MH100031, P51-OD011106. Thanks go to the dedicated personnel of the Harlow Center for Biological Psychology, the Lane Imaging Laboratory at the HealthEmotions Research Institute, the Waisman Laboratory for Brain Imaging and Behavior, the Wisconsin National Primate Research Center, the Wisconsin Institutes for Medical Research, and members of the Kalin laboratory. Thanks also to the children and their parents who participated in the pediatric imaging studies.
Footnotes
The nomenclature for corticotropin-releasing factor follows the official terminology of IUPHAR (Hauger et al., 2003). Note that while the peptide and its receptors are referred to as CRF, CRF1 and CRF2, the HUGO Gene names are CRH, CRHR1 and CRHR2.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bakshi VP, Smith-Roe S, Newman SM, Grigoriadis DE, Kalin NH. Reduction of stress-induced behavior by antagonism of corticotropin-releasing hormone 2 (CRH2) receptors in lateral septum or CRH1 receptors in amygdala. Journal of Neuroscience. 2002;22:2926–2935. doi: 10.1523/JNEUROSCI.22-07-02926.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Contarino A, Smith GW, Chan R, Gold LH, Sawchenko PE, Koob GF, Vale WW, Lee KF. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat Genet. 2000;24:410–414. doi: 10.1038/74263. [DOI] [PubMed] [Google Scholar]
- Bandler R, Shipley MT. Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? Trends Neurosci. 1994;17:379–389. doi: 10.1016/0166-2236(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Biederman J, Hirshfeld-Becker DR, Rosenbaum JF, Herot C, Friedman D, Snidman N, Kagan J, Faraone SV. Further evidence of association between behavioral inhibition and social anxiety in children. The American journal of psychiatry. 2001;158:1673–1679. doi: 10.1176/appi.ajp.158.10.1673. [DOI] [PubMed] [Google Scholar]
- Binder EB, Nemeroff CB. The CRF system, stress, depression and anxiety-insights from human genetic studies. Molecular psychiatry. 2010;15:574–588. doi: 10.1038/mp.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn RM, Shackman AJ, Oler JA, Williams LE, McFarlin DR, Rogers GM, Shelton SE, Alexander AL, Pine DS, Slattery MJ, Davidson RJ, Fox AS, Kalin NH. Evolutionarily conserved prefrontal-amygdalar dysfunction in early-life anxiety. Molecular psychiatry. 2014;19:915–922. doi: 10.1038/mp.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley RG, Binder EB, Epstein MP, Tang Y, Nair HP, Liu W, Gillespie CF, Berg T, Evces M, Newport DJ, Stowe ZN, Heim CM, Nemeroff CB, Schwartz A, Cubells JF, Ressler KJ. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Arch Gen Psychiatry. 2008;65:190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Clauss JA, Blackford JU. Behavioral inhibition and risk for developing social anxiety disorder: a meta-analytic study. J Am Acad Child Adolesc Psychiatry. 2012;51:1066–1075. doi: 10.1016/j.jaac.2012.08.002. e1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly SD, Bernstein GA. Practice parameter for the assessment and treatment of children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2007;46:267–283. doi: 10.1097/01.chi.0000246070.23695.06. [DOI] [PubMed] [Google Scholar]
- Copeland WE, Angold A, Shanahan L, Costello EJ. Longitudinal patterns of anxiety from childhood to adulthood: the Great Smoky Mountains Study. J Am Acad Child Adolesc Psychiatry. 2014;53:21–33. doi: 10.1016/j.jaac.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello EJ, Egger HL, Angold A. The developmental epidemiology of anxiety disorders: phenomenology, prevalence, and comorbidity. Child Adolesc Psychiatr Clin N Am. 2005;14:631–648. doi: 10.1016/j.chc.2005.06.003. vii. [DOI] [PubMed] [Google Scholar]
- Etkin A, Prater KE, Hoeft F, Menon V, Schatzberg AF. Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder. American Journal of Psychiatry. 2010;167:545–554. doi: 10.1176/appi.ajp.2009.09070931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Schatzberg AF. Common abnormalities and disorder-specific compensation during implicit regulation of emotional processing in generalized anxiety and major depressive disorders. The American journal of psychiatry. 2011;168:968–978. doi: 10.1176/appi.ajp.2011.10091290. [DOI] [PubMed] [Google Scholar]
- Ezpeleta L, Keeler G, Erkanli A, Costello EJ, Angold A. Epidemiology of psychiatric disability in childhood and adolescence. Journal of child psychology and psychiatry, and allied disciplines. 2001;42:901–914. doi: 10.1111/1469-7610.00786. [DOI] [PubMed] [Google Scholar]
- Flannery-Schroeder EC. Generalized Anxiety Disorder. In: Morris TL, March JS, editors. Anxiety disordes in children and adolescents. 2. The Guilford Press; New York: 2004. pp. 125–140. [Google Scholar]
- Fox AS, Kalin NH. A Translational Neuroscience Approach to Understanding the Development of Social Anxiety Disorder and Its Pathophysiology. The American journal of psychiatry. 2014 doi: 10.1176/appi.ajp.2014.14040449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AS, Oakes TR, Shelton SE, Converse AK, Davidson RJ, Kalin NH. Calling for help is independently modulated by brain systems underlying goal-directed behavior and threat perception. Proc Natl Acad Sci U S A. 2005;102:4176–4179. doi: 10.1073/pnas.0409470102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AS, Oler JA, Shackman AJ, Shelton SE, Raveendran M, McKay DR, Converse AK, Alexander A, Davidson RJ, Blangero J, Rogers J, Kalin NH. Intergenerational neural mediators of early-life anxious temperament. Proc Natl Acad Sci U S A. 2015;112:9118–9122. doi: 10.1073/pnas.1508593112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AS, Oler JA, Shelton SE, Nanda SA, Davidson RJ, Roseboom PH, Kalin NH. Central amygdala nucleus (Ce) gene expression linked to increased trait-like Ce metabolism and anxious temperament in young primates. Proc Natl Acad Sci U S A. 2012;109:18108–18113. doi: 10.1073/pnas.1206723109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AS, Shelton SE, Oakes TR, Converse AK, Davidson RJ, Kalin NH. Orbitofrontal cortex lesions alter anxiety-related activity in the primate bed nucleus of stria terminalis. J Neurosci. 2010;30:7023–7027. doi: 10.1523/JNEUROSCI.5952-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AS, Shelton SE, Oakes TR, Davidson RJ, Kalin NH. Trait-like brain activity during adolescence predicts anxious temperament in primates. PLoS ONE. 2008;3:e2570. doi: 10.1371/journal.pone.0002570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gungor NZ, Pare D. Functional Heterogeneity in the Bed Nucleus of the Stria Terminalis. J Neurosci. 2016;36:8038–8049. doi: 10.1523/JNEUROSCI.0856-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauger RL, Grigoriadis DE, Dallman MF, Plotsky PM, Vale WW, Dautzenberg FM. International Union of Pharmacology. XXXVI. Current status of the nomenclature for receptors for corticotropin-releasing factor and their ligands. Pharmacol Rev. 2003;55:21–26. doi: 10.1124/pr.55.1.3. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Prescott CA, Myers JM, Neale MC, Kendler KS. The structure of genetic and environmental risk factors for anxiety disorders in men and women. Archives of General Psychiatry. 2005;62:182–189. doi: 10.1001/archpsyc.62.2.182. [DOI] [PubMed] [Google Scholar]
- Hirshfeld-Becker DR, Micco JA, Simoes NA, Henin A. High risk studies and developmental antecedents of anxiety disorders. Am J Med Genet C Semin Med Genet. 2008;148C:99–117. doi: 10.1002/ajmg.c.30170. [DOI] [PubMed] [Google Scholar]
- Hyman SE. Revitalizing psychiatric therapeutics. Neuropsychopharmacology. 2014;39:220–229. doi: 10.1038/npp.2013.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan J. Galen's prophecy: Temperament in human nature. Perseus; NY: 1997. [Google Scholar]
- Kagan J, Reznick JS, Snidman N. The physiology and psychology of behavioral inhibition in children. Child development. 1987;58:1459–1473. [PubMed] [Google Scholar]
- Kagan J, Reznick JS, Snidman N. Biological bases of childhood shyness. Science. 1988;240:167–171. doi: 10.1126/science.3353713. [DOI] [PubMed] [Google Scholar]
- Kalin NH. The neurobiology of fear. Sci Am. 1993;268:94–101. doi: 10.1038/scientificamerican0593-94. [DOI] [PubMed] [Google Scholar]
- Kalin NH. Nonhuman primate studies of fear, anxiety, and temperament and the role of benzodiazepine receptors and GABA systems. J Clin Psychiatry. 2003;64(Suppl 3):41–44. [PubMed] [Google Scholar]
- Kalin NH, Fox AS, Kovner R, Riedel MK, Fekete EM, Roseboom PH, Tromp DP, Grabow BP, Olsen ME, Brodsky EK, McFarlin DR, Alexander AL, Emborg ME, Block WF, Fudge JL, Oler JA. Overexpressing Corticotropin-Releasing Hormone in the Primate Amygdala Increases Anxious Temperament and Alters Its Neural Circuit. Biol Psychiatry. 2016 doi: 10.1016/j.biopsych.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Larson C, Shelton SE, Davidson RJ. Asymmetric frontal brain activity, cortisol, and behavior associated with fearful temperament in rhesus monkeys. Behav Neurosci. 1998a;112:286–292. doi: 10.1037//0735-7044.112.2.286. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE. Defensive behaviors in infant rhesus monkeys: environmental cues and neurochemical regulation. Science. 1989;243:1718–1721. doi: 10.1126/science.2564702. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE. Ontogeny and stability of separation and threat-induced defensive behaviors in rhesus monkeys during the first year of life. Am J Primatol. 1998;44:125–135. doi: 10.1002/(SICI)1098-2345(1998)44:2<125::AID-AJP3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE. Nonhuman primate models to study anxiety, emotion regulation, and psychopathology. Ann N Y Acad Sci. 2003;1008:189–200. doi: 10.1196/annals.1301.021. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. J Neurosci. 2004;24:5506–5515. doi: 10.1523/JNEUROSCI.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ. Role of the primate orbitofrontal cortex in mediating anxious temperament. Biol Psychiatry. 2007;62:1134–1139. doi: 10.1016/j.biopsych.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Fox AS, Oakes TR, Davidson RJ. Brain regions associated with the expression and contextual regulation of anxiety in primates. Biological Psychiatry. 2005;58:796–804. doi: 10.1016/j.biopsych.2005.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Fox AS, Rogers J, Oakes TR, Davidson RJ. The serotonin transporter genotype is associated with intermediate brain phenotypes that depend on the context of eliciting stressor. Molecular psychiatry. 2008;13:1021–1027. doi: 10.1038/mp.2008.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Rickman M, Davidson RJ. Individual differences in freezing and cortisol in infant and mother rhesus monkeys. Behav Neurosci. 1998b;112:251–254. doi: 10.1037//0735-7044.112.1.251. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Takahashi LK. Defensive behaviors in infant rhesus monkeys: Ontogeny and context-dependent selective expression. Child development. 1991;62:1175–1183. [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Turner JG. Effects of beta-carboline on fear-related behavioral and neurohormonal responses in infant rhesus monkeys. Biol Psychiatry. 1992;31:1008–1019. doi: 10.1016/0006-3223(92)90094-g. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Gruber M, Hettema JM, Hwang I, Sampson N, Yonkers KA. Co-morbid major depression and generalized anxiety disorders in the National Comorbidity Survey follow-up. Psychol Med. 2008;38:365–374. doi: 10.1017/S0033291707002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen HU. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21:169–184. doi: 10.1002/mpr.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J. Non-human primate models of childhood psychopathology: the promise and the limitations. J Child Psychol Psychiatry. 2003;44:64–87. doi: 10.1111/1469-7610.00103. [DOI] [PubMed] [Google Scholar]
- Markovic D, Papadopoulou N, Teli T, Randeva H, Levine MA, Hillhouse EW, Grammatopoulos DK. Differential responses of corticotropin-releasing hormone receptor type 1 variants to protein kinase C phosphorylation. J Pharmacol Exp Ther. 2006;319:1032–1042. doi: 10.1124/jpet.106.107441. [DOI] [PubMed] [Google Scholar]
- Massion AO, Warshaw MG, Keller MB. Quality of life and psychiatric morbidity in panic disorder and generalized anxiety disorder. The American journal of psychiatry. 1993;150:600–607. doi: 10.1176/ajp.150.4.600. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, He JP, Burstein M, Swanson SA, Avenevoli S, Cui L, Benjet C, Georgiades K, Swendsen J. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication–Adolescent Supplement (NCS-A) J Am Acad Child Adolesc Psychiatry. 2010;49:980–989. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff CB, Vale WW. The neurobiology of depression: inroads to treatment and new drug discovery. J Clin Psychiatry. 2005;66(Suppl 7):5–13. [PubMed] [Google Scholar]
- Oler JA, Fox AS, Shelton SE, Rogers J, Dyer TD, Davidson RJ, Shelledy W, Oakes TR, Blangero J, Kalin NH. Amygdalar and hippocampal substrates of anxious temperament differ in their heritability. Nature. 2010;466:864–868. doi: 10.1038/nature09282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine DS. Research review: a neuroscience framework for pediatric anxiety disorders. Journal of child psychology and psychiatry, and allied disciplines. 2007;48:631–648. doi: 10.1111/j.1469-7610.2007.01751.x. [DOI] [PubMed] [Google Scholar]
- Pine DS, Cohen P, Gurley D, Brook J, Ma Y. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Archives of General Psychiatry. 1998;55:56–64. doi: 10.1001/archpsyc.55.1.56. [DOI] [PubMed] [Google Scholar]
- Prater KE, Hosanagar A, Klumpp H, Angstadt M, Phan KL. Aberrant amygdala-frontal cortex connectivity during perception of fearful faces and at rest in generalized social anxiety disorder. Depress Anxiety. 2013;30:234–241. doi: 10.1002/da.22014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J, Raveendran M, Fawcett GL, Fox AS, Shelton SE, Oler JA, Cheverud J, Muzny DM, Gibbs RA, Davidson RJ, Kalin NH. CRHR1 genotypes, neural circuits and the diathesis for anxiety and depression. Molecular psychiatry. 2013;18:700–707. doi: 10.1038/mp.2012.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseboom PH, Nanda SA, Fox AS, Oler JA, Shackman AJ, Shelton SE, Davidson RJ, Kalin NH. Neuropeptide Y Receptor Gene Expression in the Primate Amygdala Predicts Anxious Temperament and Brain Metabolism. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy AK, Fudge JL, Kelly C, Perry JS, Daniele T, Carlisi C, Benson B, Xavier Castellanos F, Milham MP, Pine DS, Ernst M. Intrinsic functional connectivity of amygdala-based networks in adolescent generalized anxiety disorder. J Am Acad Child Adolesc Psychiatry. 2013;52:290–299. doi: 10.1016/j.jaac.2012.12.010. e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy-Byrne P. Treatment-refractory anxiety; definition, risk factors, and treatment challenges. Dialogues Clin Neurosci. 2015;17:191–206. doi: 10.31887/DCNS.2015.17.2/proybyrne. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy-Byrne PP, Katon W. Generalized anxiety disorder in primary care: the precursor/modifier pathway to increased health care utilization. The Journal of clinical psychiatry. 1997;58(Suppl 3):34–38. discussion 39-40. [PubMed] [Google Scholar]
- Sanchez MM, Young LJ, Plotsky PM, Insel TR. Autoradiographic and in situ hybridization localization of corticotropin-releasing factor 1 and 2 receptors in nonhuman primate brain. J Comp Neurol. 1999;408:365–377. [PubMed] [Google Scholar]
- Shackman AJ, Fox AS, Oler JA, Shelton SE, Davidson RJ, Kalin NH. Neural mechanisms underlying heterogeneity in the presentation of anxious temperament. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:6145–6150. doi: 10.1073/pnas.1214364110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Fox AS, Oler JA, Shelton SE, Oakes TR, Davidson RJ, Kalin NH. Heightened extended amygdala metabolism following threat characterizes the early phenotypic risk to develop anxiety-related psychopathology. Molecular psychiatry. doi: 10.1038/mp.2016.132. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada-Sugimoto M, Otowa T, Hettema JM. Genetics of anxiety disorders: Genetic epidemiological and molecular studies in humans. Psychiatry Clin Neurosci. 2015;69:388–401. doi: 10.1111/pcn.12291. [DOI] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, Macklin ML, Lasko NB, Cavanagh SR, Krangel TS, Orr SP, Pitman RK, Whalen PJ, Rauch SL. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Archives of General Psychiatry. 2005;62:273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- Teli T, Markovic D, Hewitt ME, Levine MA, Hillhouse EW, Grammatopoulos DK. Structural domains determining signalling characteristics of the CRH-receptor type 1 variant R1beta and response to PKC phosphorylation. Cell Signal. 2008;20:40–49. doi: 10.1016/j.cellsig.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Walker DL, Miles LA, Davis M. Selective participation of the bed nucleus of the stria terminalis and CRF in sustained anxiety-like versus phasic fear-like responses. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1291–1308. doi: 10.1016/j.pnpbp.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkup JT, Albano AM, Piacentini J, Birmaher B, Compton SN, Sherrill JT, Ginsburg GS, Rynn MA, McCracken J, Waslick B, Iyengar S, March JS, Kendall PC. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359:2753–2766. doi: 10.1056/NEJMoa0804633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LE, Oler JA, Fox AS, McFarlin DR, Rogers GM, Jesson MA, Davidson RJ, Pine DS, Kalin NH. Fear of the unknown: uncertain anticipation reveals amygdala alterations in childhood anxiety disorders. Neuropsychopharmacology. 2015;40:1428–1435. doi: 10.1038/npp.2014.328. [DOI] [PMC free article] [PubMed] [Google Scholar]


