Abstract
Mechanistic target of rapamycin (mTOR) is a conserved serine/threonine kinase that plays a critical role in the control of cellular growth and metabolism. Hyperactivation of mTOR pathway is common in human cancers, driving uncontrolled proliferation. MicroRNA (miRNA) is a class of short noncoding RNAs that regulate the expression of a wide variety of genes. Deregulation of miRNAs is a hallmark of cancer. Recent studies have revealed interplays between miRNAs and the mTOR pathway during cancer development. Such interactions appear to provide a fine-tuning of various cellular functions and contribute qualitatively to the behavior of cancer. Here we provide an overview of current knowledge regarding the reciprocal relationship between miRNAs and mTOR pathway: regulation of mTOR signaling by miRNAs and control of miRNA biogenesis by mTOR. Further research in this area may prove important for the diagnosis and therapy of human cancer.
Keywords: mTOR, MicroRNA, Cancer, Cell signaling pathway, Biomarker
Introduction
Mechanistic target of rapamycin (mTOR) is an evolutionarily conserved serine/threonine kinase belonging to the phosphoinositide 3-kinase (PI3K)-related kinase family. It lies at the nexus of a regulatory network and integrates extracellular and intracellular events, coordinating processes in growth and proliferation (summarized in Fig. 1). mTOR forms two distinct complexes called mTOR complex 1 (mTORC1), which is composed of raptor, binds to the FKBP12–rapamycin complex, and is inhibited by rapamycin. On the other hand, mTOR complex 2 (mTORC2) contains rictor but does not bind to FKBP12–rapamycin or is directly inhibited by rapamycin [1].
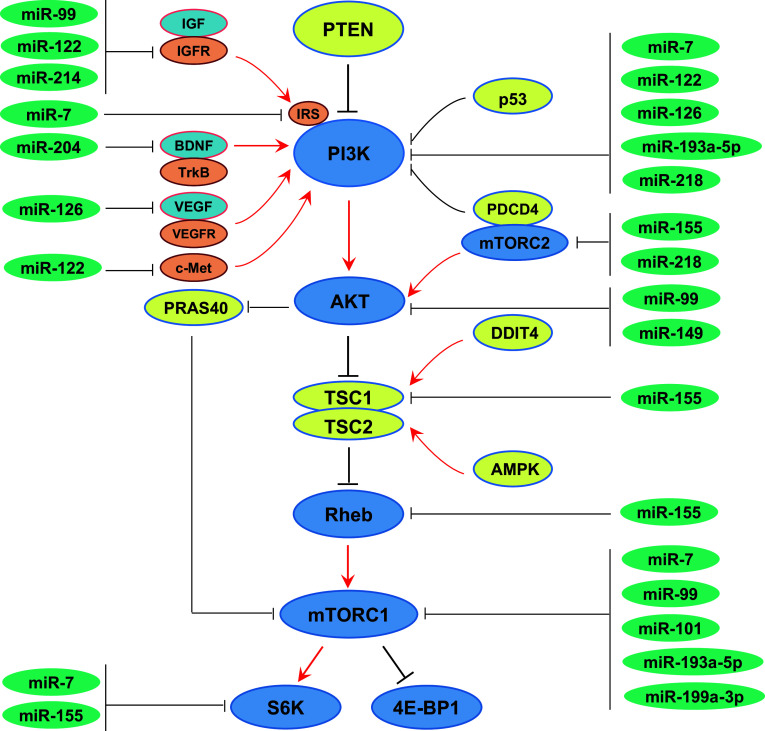
Fig. 1.
PI3K/AKT/mTOR signaling pathway. mTOR pathway integrates signals from growth factors and nutrients to regulate diverse cellular processes, including translation, autophagy, and transcription of genes involved in ribosome biogenesis and metabolism
mTORC1 couples signals from diverse nutritional and environmental cues to promote cellular growth and metabolism. It achieves this by promoting anabolic processes such as mRNA translation and transcription of genes that involved in ribosome biogenesis and metabolism, or limiting catabolic processes such as autophagy. Rheb (Ras homology enriched in brain) is a Ras-related small GTPase. When present in the GTP-bound form, it acts as a potent activator of mTORC1, which is negatively regulated by its GAP, the tuberous sclerosis complex (TSC) heterodimer. TSC2 integrates diverse upstream signals through phosphorylation by kinases such as AKT, ERK, and p38, which causes either activation or inhibition of mTORC1, depending on the specific serine/threonine residues [2]. Proline-rich AKT substrate 40 kDa (PRAS40) is a negative regulator of mTORC1 by preventing the binding of mTOR to its substrates. Both AKT and mTORC1 can phosphorylate PRAS40, which results in PRAS40 dissociation from mTORC1, enhancing mTORC1 activity. mTORC2 regulates cellular survival, cytoskeletal dynamics, ion transport, and growth by phosphorylating and activating AGC kinase family members, including AKT, SGK1, and PKC [3].
mTOR acts downstream of class IA PI3Ks in response to tyrosine kinase receptors. Class IA PI3Ks are heterodimeric proteins consisting of a p110 catalytic subunit associated with a p85, p55, or p50 regulatory subunit. The major regulatory subunit p85α is a multi-functional component that has both positive and negative roles in PI3K regulation. As a positive regulator, p85α participates in trafficking of p110 to the cell membrane as an initial step in the PI3K-AKT signaling cascade. As a negative regulator, p85α mediates basal inhibition of the p110 subunit, to keep PI3K activity low under non-stimulatory conditions. Furthermore, monomeric p85α competes with p85-p110 dimers for activated receptor binding [4, 5]. Upon PI3K activation, PDK1 phosphorylates AKT at Thr-308 to expose the Ser-473 residue, and mTORC2 phosphorylates AKT at Ser-473 for its full activation [6] (summarized in Fig. 1). Diverse upstream growth factors and cytokines enhance the PI3K/AKT/mTOR pathway, including IGF-1 [7], VEGF [8], c-Met [9], and BDNF [10]. mTOR pathway is antagonized by negative regulators such as PTEN [11, 12], GSK3β [13], LKB1/AMPK [14], p53 [15], DDIT4 [16], PDCD4 [17], and IKKβ [18]. Aberrant mTOR signaling pathway is known to be involved in many disease states particularly cancer. Previous studies from several laboratories, including our own, have shown that hyperactive mTOR signaling represents one of the major mechanisms that promote the pathogenesis and therapeutic resistance of various solid tumors and hematological malignancies [19, 20].
The discovery of microRNAs (miRs or miRNAs) has added another layer of complexity to the control of gene expression. miRs are a class of short (18–22 nucleotides) noncoding RNAs that regulate the expression of a wide variety of genes. Conservative estimate shows that approximately 60% of all human mRNAs are regulated by miRNAs, covering virtually all cellular functions. Through base pairing with the 3′-untranslated region (3′UTR) of target genes, miRNAs were found to enhance mRNA degradation or inhibit post-transcriptional translation [21]. At least 50% of miRNA genes are located in cancer-associated genomic regions or in fragile sites and are aberrantly expressed in cancer, suggesting that these miRNAs have an important role in the pathogenesis of cancers. Overall, cancer is characterized by many upregulated and repressed miRNAs, potentially exerting oncogenic and tumor-suppressive functions, respectively. While a subset of these miRNAs are overexpressed in cancers and shown to target tumor suppressor genes, hence termed oncogenic miRNAs or oncomiRs, some other miRNAs that target oncogenes are underexpressed in cancers, which are often called tumor suppressor-like miRNAs. Certain miRNAs are known to have a dual role, either oncogenic or tumor-suppressive, depending on the cancer-specific context [22].
Accumulating evidence indicates that miRNAs contribute to tumorigenesis and cancer metastasis by targeting different steps of mTOR pathway. While some miRNAs target mTOR directly, others act on mTOR signaling by targeting other components of the pathway such as PI3K and AKT. A comprehensive list of miRNAs targeting mTOR pathway is listed (Table 1). Much like mTOR, miRNAs regulate broad cellular functions, including proliferation, differentiation, apoptosis, and autophagy. This review intends to provide an overview of current knowledge regarding the miRNA profiles that intercept mTOR pathway, with special emphasis on the regulatory mechanism of miRNAs for mTOR signaling and the link between mTOR and miRNA biogenesis. A detailed understanding of their relationship has implications in cancer diagnosis, therapy, and prognosis.
Table 1.
miRNAs target different steps of mTOR signaling pathway
| Component | Direct Target | miRNA | Cancer Types | Function |
|---|---|---|---|---|
| PTEN | PTEN | miR-21 | 1.Breast cancer 2.NSCLC 3.RCC 4.CML 5.HCC 6.Gastric cancer 7.ALL(Ph+) 8.DLBCL | Oncogenic |
| miR-93 | 1.Osteosarcoma 2.Ovarian cancer 3.Breast cancer 4.Glioma 5.HCC | Oncogenic | ||
| miR-193a-3p | 1.Gastric cancer | Oncogenic | ||
| PI3K | IGF/IGFR | miR-99 | 1.HNSCC 2.HCC | Tumor-suppressive |
| miR-122 | 1.Breast cancer | Tumor-suppressive | ||
| miR-214 | 1.RCC | Tumor-suppressive | ||
| IRS | miR-7 | 1.Glioblastoma 2.Adrenocortical carcinoma | Tumor-suppressive | |
| BDNF | miR-204 | 1.Ovarian cancer 2.Pediatric renal tumor 3.Peripheral nerve sheath tumor 4.CRC 5.Breast cancer 6.Endometrial cancer 7.Papillary thyroid carcinoma 8.Gastric cancer | Tumor-suppressive | |
| VEGF | miR-126 | 1.Gastric cancer | Tumor-suppressive | |
| c-Met | miR-122 | 1.HCC | Tumor-suppressive | |
| PDCD4 | miR-21 | 1.Renal cancer | Oncogenic | |
| P53 | miR-125b | 1.Breast cancer | Oncogenic | |
| PI3K | miR-7 | 1.HCC 2.Adrenocortical carcinoma | Tumor-suppressive | |
| miR-21 | 1.Pancreatic ductal adenocarcinoma 2.Breast cancer | Oncogenic | ||
| miR-122 | 1.Breast cancer | Tumor-suppressive | ||
| miR-126 | 1.CRC | Tumor-suppressive | ||
| miR-193a-5p | 1.NSCLC | Tumor-suppressive | ||
| miR-218 | 1.CRC 2.ESCC | Tumor-suppressive | ||
| AKT | AKT | miR-99 | 1.Endometrial carcinoma 2.NSCLC 3.Cervical cancer | Tumor-suppressive |
| miR-149 | 1.Neuroblastoma 2.Cervical cancer 3.Glioma 4.HCC | Tumor-suppressive | ||
| TSC1 | PDCD4 | miR-21 | 1.Renal cancer 2. NSCLC | Oncogenic |
| TSC1 | miR-155 | 1.Breast cancer | Tumor-suppressive | |
| miR-451 | 1.Multiple myeloma | Oncogenic | ||
| TSC2 | DDIT4 | miR-221 | 1.Pancreatic cancer 2.Thyroid papillary carcinoma 3.Breast cancer 4.Glioblastoma 5.NSCLC 6.SCLC 7.HCC | Oncogenic |
| CAB39 | miR-451 | 1.Glioma | Oncogenic | |
| AMPK | miR-451 | 1.CRC | Oncogenic | |
| Rheb | Rheb | miR-155 | 1.Nasopharyngeal cancer 2.Cervical cancer | Tumor-suppressive |
| mTORC1/ mTORC2 | PRAS40 | miR-96 | 1.Breast cancer 2.Pancreatic cancer 3.Prostate cancer 4.HCC | Oncogenic |
| mTOR | miR-7 | 1.HCC 2.Adrenocortical carcinoma | Tumor-suppressive | |
| miR-99 | 1.Metastatic CRC 2.Clear cell ovarian cancer 3.Cervical cancer 4.Breast cancer 5.Prostate cancer 6.HCC 7.ESCC 8.Childhood adrenocortical tumor 9.Osteosarcoma 10.Endometrial carcinoma 11.NSCLC 12.HNSCC 13.Bladder cancer | Tumor-suppressive | ||
| miR-101 | 1.Osteosarcoma 2.Anaplastic large-cell lymphoma | Tumor-suppressive | ||
| miR-193a-5p | 1.NSCLC | Tumor-suppressive | ||
| miR-199a | 1.Endometrioid adenocarcinoma 2.Glioma 3.Osteosarcoma 4.HCC | Tumor-suppressive | ||
| Rictor | miR-155 | 1.Nasopharyngeal cancer 2.Cervical cancer 3.Breast cancer | Tumor-suppressive | |
| miR-218 | 1. Cervical cancer 2. OSCC | Tumor-suppressive | ||
| S6K1 | S6K1 | miR-7 | 1.HCC 2.Adrenocortical carcinoma | Tumor-suppressive |
| miR-155 | 1.Nasopharyngeal cancer 2.Cervical cancer | Tumor-suppressive |
NSCLC non-small cell lung cancer, RCC renal cell carcinoma, CML chronic myeloblastic leukemia, HCC hepatic cellular cancer, ALL acute lymphoblastic leukemia, Ph+ Philadelphia chromosome positive, DLBCL diffuse large B-cell lymphoma, HNSCC head and neck squamous cell carcinoma, CRC colorectal cancer, ESCC esophageal squamous cell carcinoma, SCLC small cell lung cancer, OSCC oral squamous cell carcinoma)
miRNAs that suppress upstream of mTOR pathway
There is a large number of miRNAs acting to oppose mTOR signaling. For instance, cancer development often associates with increased mTOR activity [23], but with a loss of many miRNAs targeting this pathway [24]. In particular, mTOR and miRNAs play opposite roles in regulating protein translation and mRNA stability, raising the possibility of close relationship between them [25] (summarized in Fig. 2).
Fig. 2.
Tumor-suppressive miRNAs that inhibit mTOR pathway. A large number of miRNAs display tumor-suppressive activity by directly targeting mTOR and/or upstream positive regulators of mTOR
miR-7
Mature miRNA-7 (miR-7) is derived from three miRNA precursors in human genome, miR-7-1 (9q21.32), miR-7-2 (15q26.1), and miR-7-3 (19p13.3). It has been implicated as tumor suppressor in multiple cancer types. miR-7 suppresses glioblastoma partially by directly inhibiting IRS-2 expression and subsequent AKT-mTOR signaling [26]. In adrenocortical carcinomas, systemic delivery of miR-7 by nanoparticle delivery reduces growth of both cell line- and patient-derived xenograft tumors [27]. In hepatocellular carcinoma, miR-7 was shown to inhibit tumorigenesis and metastasis of HCC through direct targeting PIK3CD (encoding PI3K catalytic subunitp110δ), mTOR, and p70S6K [28].
miR-99 family
The miR-99 family, consisting of miR-99a (21q21.1), miR-99b (19q13.41), and miR-100 (11q24.1), is one of the most ancient miRNA families. It plays a critical role in developmental timing and maintenance of tissue identity. Recent studies suggest that miR-99 also regulates various physiological processes in adult tissues, such as dermal wound healing, and a number of disease processes, including viral infection and cancer [29]. For example, miR-100 inhibits human cytomegalovirus (HCMV) replication by targeting both mTOR and raptor during HCMV infection [30]. miR-99 has been reported to be a tumor suppressor whose expression is frequently lost or reduced in various human cancers. Deregulation of the miR-99 family contributes to tumorigenesis at least partially, by direct and indirect targeting at multiple points in mTOR signaling pathway. In some cancer types, including metastatic CRC [31], esophageal squamous cell carcinoma (ESCC) [32, 33], clear cell ovarian cancer [34], cervical cancer [35], breast cancer [36], prostate cancer [12, 37], bladder cancer [38], childhood adrenocortical tumor [39], osteosarcoma [40], and c-Src-transformed cells [41], miR-99 family members suppress mTOR expression via direct targeting of its 3′-UTR in a post-transcriptional manner. Ectopic overexpression of miR-99 family members in these cancers reduces cell proliferation and migration, induces apoptosis, and enhances sensitivity to the rapamycin analog RAD001 (everolimus). In some cancers, the miR-99 family regulates mTOR signaling by simultaneously targeting multiple genes. In HNSCC [42] and HCC cells [43], restoration of miR-99a and miR-100 reduces cell proliferation and migration, and induces G1 cell cycle arrest and apoptosis by dual suppression of IGF1R and mTOR. In endometrial carcinoma [44], NSCLC [45], and cervical cancer [35], miR-99a inhibits two major members of the PI3K/AKT/mTOR signaling pathway, AKT, and mTOR. Loss of miR-99a appears to at least partially mediate hyperactivation of PI3K/AKT/mTOR signaling through AKT and mTOR, thereby inducing tumor initiation and progression. Restoration of miR-99a in these cancer cells induces a complex phenotype including blockage of the G1/S phase transition, induction of cell apoptosis, and suppression of cell proliferation, invasion, and tumor metastasis.
miR-101
miR-101 (1p31.3 for miR-101-1, 9p24.1 for miR-101-2) is downregulated in several cancer cell lines and cancer tissues. miR-101 functions as a tumor suppressor that is associated with the growth and apoptosis of various human cancers [46]. mTOR gene is a direct target of miR-101 and plays an important role in mediating miR-101’s effects in cancer. Overexpression of miR-101 significantly decreases mTOR expression at both mRNA and protein levels in osteosarcoma cells, resulting in cell proliferation inhibition and apoptosis [47]. miR-101 is downregulated in anaplastic large-cell lymphoma (ALCL). Ectopic expression of miR-101 attenuates cell proliferation in anaplastic lymphoma kinase (ALK)-positive ALCL through direct repression of mTOR [48].
miR-122
miR-122 (18q21.31) expression is restricted to the liver. Reduced miR-122 level was first noticed to be associated with primary HCC [49], and the c-Met/AKT/mTOR cascade axis seems to be involved in miR-122’s functional mechanism [50]. A recent study showed that miR-122 is a tumor suppressor in breast cancer, and overexpression of miR-122 inhibits BC cell growth both in vitro and in vivo. miR-122 regulates BC cell proliferation through the PI3K/AKT/mTOR signaling pathway, by concurrently targeting at least two major signaling molecules upstream of mTOR signaling, IGF1R and PI3CG (encoding PI3K catalytic subunitp110γ) [7].
miR-126
miR-126 (9q34.3), encoded by intron 7 of the epidermal growth factor-like domain 7 (egfl7) gene, plays an essential role in vascular integrity and angiogenesis. miR-126 acts as a tumor suppressor through modulation of signaling pathways that control tumor cell proliferation, migration, invasion, and survival [51]. In colorectal cancer (CRC), miR-126 target sites in the promoter of p85β, a PI3K subunit that stabilizes and propagates PI3K signal, which is involved in the carcinogenesis of CRC [52, 53]. miR-126 is frequently lost in CRC, whereas p85β is commonly overexpressed, resulting in the hyperactivation of the downstream AKT/mTOR pathway. In gastric cancer, miR-126 regulates AKT/mTOR pathway by targeting VEGF-A. Downregulation of miR-126 expression increases the expression of VEGF-A and its downstream mTOR signaling. In contrast, restoration of miR-126 expression reduces the expression of VEGF-A and suppresses the activation of its downstream AKT and mTOR [54].
miR-149
miR-149-3p (miR-149*) and miR-149-5p are processed from the 3-arm and 5-arm ends of pre-miRNA149 (2q37.3), respectively; miR-149-5p is the most abundant form. The role of miR-149-5p/miR149-3p in cancer progression is controversial and varies in different types of tumors, functioning as either a tumor suppressor or an oncogene [55]. In some tumors, miR-149-5p/miR149-3p expression is downregulated and acts as a tumor suppressor by inhibiting mTOR signaling. miR-149-5p/miR149-3p induces apoptosis, and inhibits proliferation and invasion in Be2C, HeLa, glioma, and HCC cells by directly targeting AKT1 and subsequently inhibiting the downstream signaling events [56, 57]. Low miR-149 expression is associated with enhanced AKT/mTOR signaling in glioma and HCC tissues, and predicts tumor aggressiveness and unfavorable prognosis [58]. Reintroduction of miR-149 significantly inhibits HCC cell proliferation and tumorigenicity by regulating the AKT/mTOR pathway [59]. Because the sequences of miR-149-5p and miR-149-3p are different, it will be important to further characterize their unique roles in this context.
miR-155
miR-155 (21q21.3) is involved in various physiological and pathological processes, including malignant growth, viral infections, and cardiovascular diseases [60]. It has been shown to target multiple aspects of mTOR pathway. miR-155 inhibits mTORC1 signaling through suppression of Rheb in macrophages, thus promoting autophagy to eliminate intracellular mycobacteria [61]. miR-155 also interferes with mTORC2 signaling by targeting Rictor and TSC1 in the estrogen receptor (ER) α + breast cancer cells, which controls ER function and renders RAD001 sensitivity [62]. In human nasopharyngeal cancer and cervical cancer cells, miR-155 targets multiple players in mTOR pathway, including Rheb, Rictor, and RPS6KB2 (encoding P70S6K2), by directly interacting with their 3′-UTRs. By downregulating mTOR signaling, miR-155 acts as a potent inducer of hypoxia-induced autophagy, attenuates cell proliferation, and induces G1/S cell cycle arrest in nasopharyngeal cancer and cervical cancer cells [63].
miR-193a-5p
The miR-193a gene is mapped to chromosome 17q11.2. It has been reported to negatively regulate mTOR pathway. miR-193a-5p functions as a tumor suppressor and has an important role in NSCLC metastasis through the PI3K/AKT/mTOR pathway. PIK3R3 (encoding PI3K regulatory subunit p55γ and p50γ) and mTOR are direct targets of miR-193a-5p in NSCLC. Decreased miR-193a-5p expression is significantly associated with NSCLC tumor-node-metastasis (TNM) stage and lymph node metastasis. Reintroduction of miR-193a-5p inhibits NSCLC cell migration, invasion, epithelial-mesenchymal transition (EMT) in vitro, and lung metastasis formation in vivo [64].
miR-199a-3p
miR-199a-3p (1q24.3), a member of the miR-199 family, is located on the opposite strand of the orthologous intron of Dynamin genes (Dnm3os). The downregulation of miR-199a-3p occurs in various malignancies and is accompanied by enhanced activity of the mTOR signaling pathway. MiR-199a-3p inhibits mTOR signaling through direct binding of the 3′UTR of mTOR. Restoration of miR-199a-3p leads to downregulation of mTOR and p-mTOR, and increases cell populations at the G1-phase, resulting in restrained cellular growth, proliferation and migration in endometrial endometrioid adenocarcinomas [65], glioma cells [66], and osteosarcoma cells [67]. In HCC cells, restoration of miR-199a-3p results in arresting G1-phase cell cycle and increasing sensitivity to doxorubicin-induced apoptosis [68].
miR-204
miR-204 (9q21.12), also known as RDICC, plays important roles in smooth muscle cell calcification as well as the endoplasmic reticulum stress response in trabecular meshwork cells [69, 70]. Recent reports have shown that genomic loci encoding miR-204 are frequently lost in ovarian cancers, pediatric renal tumors, breast cancers, peripheral nerve sheath tumors, endometrial cancer cell lines, papillary thyroid carcinomas, gastric cancers and CRCs, suggesting that is potentially a tumor suppressor [71]. The BDNF (brain-derived neurotrophic factor)/AKT/mTOR/Rac1 signaling cascade is a major target of miR-204. Genomic loss of miR-204 results in BDNF/TrkB (tyrosine kinase receptor of BDNF) overexpression, promoting BDNF-induced cancer cell migration and invasion by activating AKT/mTOR signaling, leading to Rac1 translocation and act in reorganization [10].
miR-214
miR-214 (1q24.3) is encoded by Dnm3os, the same gene that also encodes miR-199a-3p. miR-214 is often dysregulated in various cancers and its functions vary considerably, depending on the tissue types [72]. IGF-1R is a direct targets of miR-214 and reduced miR-214 expression contributes to increased IGF-1R levels in renal cancer cells. Increased IGF-1/IGF-1R level in turn results in phosphorylation and inactivation of PRAS40 in an AKT-dependent manner, leading to activation of mTORC1 signal transduction to increase phosphorylation of p70S6K and 4E-BP1. Reintroduction of miR-214 significantly inhibits the AKT/PRAS40/mTORC1 axis in the IGF-1R signaling cascade, which dampens renal cancer cell proliferation [73].
miR-218
Two distinct genes, miR-218-1 (4p15.31) and miR-218-2 (5q34), encode the same mature miR-218 sequence. miR-218 acts as a tumor suppressor and plays a pivotal role in tumorigenesis and progression [74]. miR-218 inhibits the invasion and migration of CRC cells through regulation of PI3K/AKT/mTOR signaling by targeting PIK3C2A (encoding PI3K C2 subunit α) and PIK3R1(encoding PI3K regulatory subunit p85α) [75]. PI3K/AKT/mTOR signaling is also involved in miR-218 suppression of ESCC growth and miR-218 enhancement of the chemosensitivity of ESCC cells to cisplatin [76]. In cervical cancer [77] and oral squamous cell carcinoma (OSCC) [78], miR-218 targets Rictor, which directly regulates the phosphorylation of AKT at Ser-473 independently of the PI3K-AKT signaling pathway. Silencing of miR-218 and activation of mTORC2-AKT signaling is an important mechanism of carcinogenesis and cancer progression in OSCC. Ectopic expression of miR-218 reduces OSCC and cervical cancer cell growth in vitro and in vivo, and increases the chemosensitivity of cervical cancer to cisplatin.
miRNAs that activate mTOR pathway
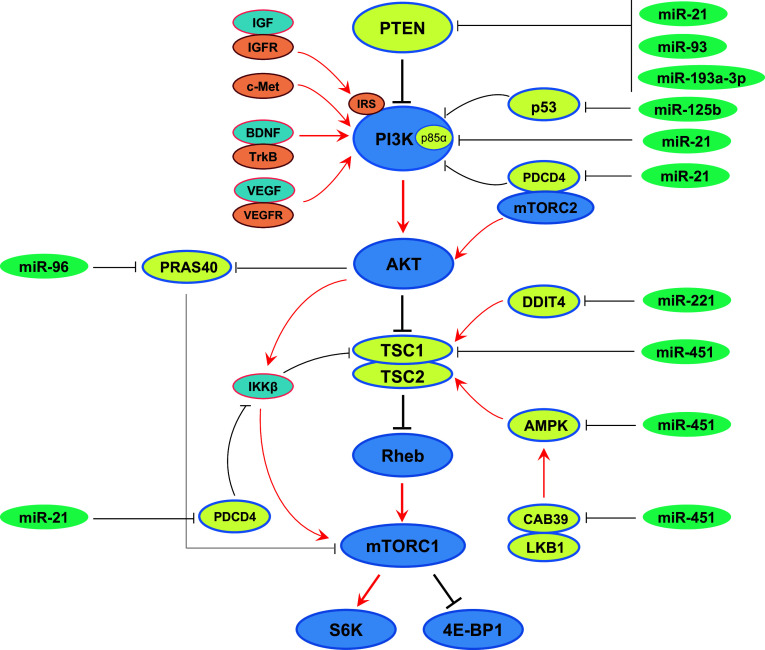
Besides the miRNAs mentioned above that act as tumor suppressors by targeting mTOR and/or positive regulators upstream of mTOR signaling pathway, some other miRNAs are always overexpressed in cancers and are able to promote the development and/or progression of tumors by stimulating the mTOR signaling pathway. These are termed oncogenic miRNAs, or oncomiRs. Molecules that suppress mTOR pathway are always targeted by these oncomiRs (summarized in Fig. 3).
Fig. 3.
Oncogenic miRNAs that activate mTOR pathway. Many oncogenic miRNAs are overexpressed in human cancers that promote the development and/or progression of tumors by stimulating mTOR signaling through downregulation of negative regulators of mTOR
miR-21
miR-21 (17q23.2) is overexpressed under many pathological conditions, including many types of cancers [79]. Overexpression of miR-21 is correlated with enhanced cancer cell proliferation and survival, reduced apoptosis and autophagy, poor differentiation, lymph node metastasis, advanced TNM stage, and unfavorable clinical outcomes. As an oncomiR, miR-21 affects major events of tumor development and progression. In particular, miR-21 targets multiple genes in mTOR pathway, including PTEN, p85α, and PDCD4. Besides promoting malignant growth, the miR-21/PTEN/PI3K/AKT/mTOR axis influences anti-cancer effects of zoledronic acid [80], triptolide [81], Metformin [82], Matrine [83], and curcumin [84]; modulates radiosensitivity [85, 86]; and chemosensitivity to tamoxifen, fulvestrant [87], sorafenib [88], cisplatin [89, 90], imatinib [91], gefitinib [92], doxorubicin [93], daunorubicin [94], anti-EGFR tyrosine kinase inhibitors (TKI) [95], and the CHOP chemotherapy regimen [96]. miR-21 also influences PI3K/AKT/mTOR signaling via PIK3R1 that negatively regulates p110 subunit under certain circumstances. This miR-21/PI3K/AKT/mTOR axis has been shown to affect chemosensitivity of pancreatic ductal adenocarcinomas to gemcitabine [97]; modulate tumor cell migration, invasion, and EMT; and predict clinical outcomes of breast cancer [98]. PDCD4 levels are decreased as a result of direct targeting of its 3′-UTR by miR-21, which regulates AKT and IKKβ activation and contributes to activation of mTORC1 signaling. Furthermore, overexpression of miR-21 decreases the association between PDCD4 and Rictor, thereby stimulating mTORC2 signaling and subsequently AKT activity. Both mechanisms enhance renal cancer cell migration and invasion, contributing to metastatic fitness of renal cancer cells [17, 99].
miR-93
miR-93 (7q22.1) is encoded within the minichromosome maintenance 7 (MCM7) gene. As an oncomiR, miR-93 is overexpressed and contributes to the pathogenesis of osteosarcomas, ovarian cancers, breast cancers, and others. PTEN is a putative target of miR-93. By directly targeting PTEN in osteosarcoma [100], gliomas [101], and HCC [102], miR-93 promotes cancer cell proliferation, migration, and invasion, while inhibiting apoptosis. In ovarian cancer [11, 103] and HCC [102], miR-93 is known to affect cisplatin and 5-FU chemosensitivity through suppression of PTEN expression.
miR-96
miR-96(7q32.2) is processed from a conserved polycistronic transcript. miR-96 functions as an oncomiR by promoting cellular growth, invasiveness and metastasis in breast cancer [104], pancreatic cancer [105], and HCC [106]. miR-96 directly targets AKT1S1 that encodes for PRAS40, a negative regulator of mTOR kinase, which prevents the binding of mTOR to its substrates [107]. miR-96 upregulation in prostate cancer cells also leads to the downregulation of the AKT1S1, thereby unleashing mTOR signaling to promote prostate bone metastasis [108].
miR-125b
MiR-125b belongs to the miR-125 family and is transcribed from two loci located on chromosomes 11q23 and 21q21. It acts as an oncogene in several cancer types [109]. MiR-125b directly targets the p53 tumor suppressor gene, which negatively regulates the expression of PI3KCA (encoding PI3Kcatalytic subunit p110α). Elevated miR-125b expression leads to upregulation of PI3KCA and constitutive activation of PI3K/AKT/mTOR pathway and thus pathophysiology of cancer [110, 111]. The miR-125b/PI3K/AKT/mTOR axis contributes to confer resistance to anastrozole and letrozole in breast cancer cells [112].
miR-193-3p
miR-193-3p plays a critical role in regulating human gastric cancer through direct targeting of PTEN, which inhibits mTOR signaling. Downregulation of miR-193-3p inhibits tumor proliferation, migration, and chemoresistance in human gastric cancer, by upregulating the PTEN gene [113].
miR-221
miR-221 (Xp11.3) overexpression has been observed in a number of advanced malignancies, including pancreatic cancer, thyroid papillary carcinomas, breast cancers, glioblastomas, HCCs, and lung cancers [114]. miR-221 directly targets DNA damage-inducible transcript 4 (DDIT4), which is an essential negative regulator of mTOR kinase through stimulation of the tuberous sclerosis tumor suppressor TSC1/2 complex [115]. In this fashion, the miR-221/DDIT4/TSC/mTOR axis contributes to HCC cell proliferation and tumor development [16].
miR-451
miR-451 is located on chromosome 17q11.2, which is in close proximity to ERBB2 (17q12), a region frequently amplified in human cancer [116, 117]. miR-451 has attracted increasing attention due to its critical role in several types of cancers. In multiple myeloma, miR-451 regulates stemness of side population cells via the PI3K/AKT/mTOR signaling pathway, by specifically targeting TSC1, the upstream negative regulator of mTORC1. Inhibition of miR-451 potentiated the anti-myeloma effect by increasing apoptosis, and decreasing clonogenicity and MDR1 mRNA expression [118]. In CRC cells, overexpression of miR-451 inhibits AMPK activation, causing mTORC1 hyperactivation, and hence enhanced migration and proliferation of cancer cells [119]. In glioma cells, miR-451 regulates LKB1/AMPK/mTOR signaling through direct targeting of CAB39, a component of the active LKB1 complex, which acts as a conditional switch controlling glioma cell proliferation and migration. Under conditions of abundant energy, miR-451 expression is high, which suppresses LKB1/AMPK signaling that allows cells to maintain elevated proliferation via unrestrained mTOR signaling. In contrast, under conditions of glucose limitation, miR-451 is downregulated, leading to AMPK activation and suppression of cell proliferation as well as increase in cell survival and migration. This miR-451/LKB1/AMPK/mTOR axis may represent a common mechanism that controls cellular adaptation to glucose availability [120].
Regulation of miRNA expression by mTOR pathway
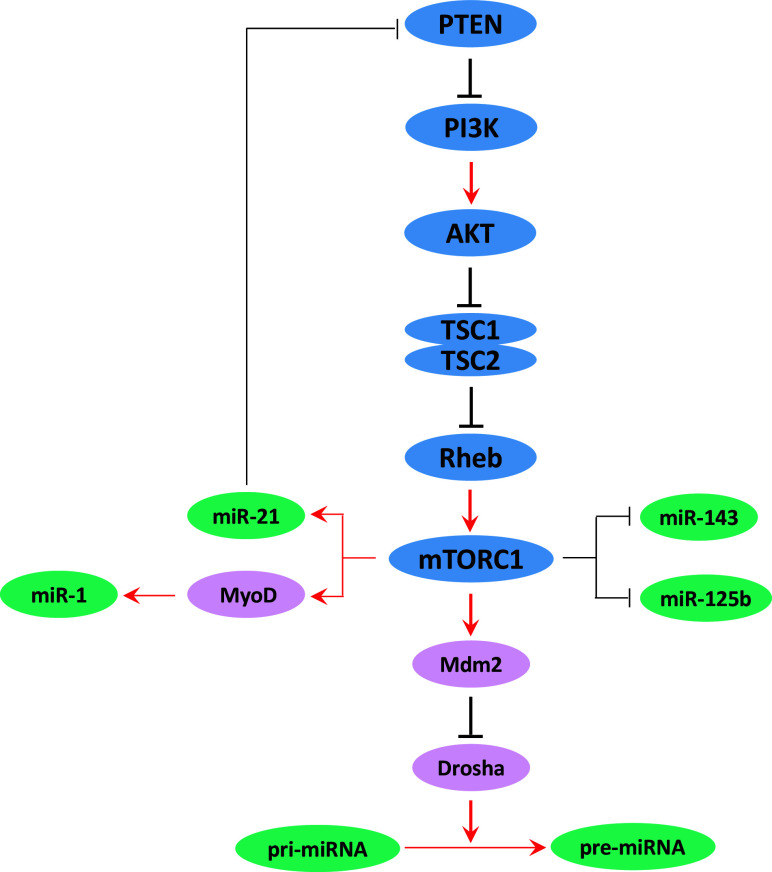
Although the biological importance of miRNAs has been well recognized a decade ago, the regulatory mechanism for miRNA expression remains elusive. Previous studies have largely focused on individual miRNAs that regulate mTOR pathway. However, whether and how mTOR regulates miRNA biogenesis is not well understood. It has been shown in cancer cells that chronic rapamycin treatment leads to significant alterations in miRNA profiles, and these changes correlate with rapamycin resistance [121]. Inactivation of TSC complex, an essential repressor of mTOR activation, also causes broad downregulation of precursor and mature microRNA processing in mouse and human cells. Consistently, targeted mutation of Raptor, an essential component of mTORC1, increases miRNA biogenesis [25]. mTOR activation broadly downregulates miRNA biogenesis through Mdm2-dependent ubiquitination of Drosha, an essential RNase dedicated to processing primary miRNA (pri-miRNA) to produce precursor miRNA (pre-miRNA) [25]. These studies provide a missing link between mTOR and miRNA biogenesis, and given the broad function of miRNAs, these data raise the intriguing possibility that a significant portion of mTOR function may act through its ability to control miRNA biogenesis. Recently, several individual miRNAs have been confirmed to be regulated by mTOR signaling, including miR-21, miR-143, miR-125b, and miR-1, which are known to be involved in cancer and other physiological conditions (summarized in Fig. 4).
Fig. 4.
mTOR pathway controls expression of miRNAs. mTOR broadly regulates miRNA biogenesis in mammals through Mdm2-dependent ubiquitination and degradation of Dorsha
miR-21
Positive feedback has evolved between miR-21 and mTOR signaling. While miR-21 targets multiple genes in the mTOR signaling pathway as discussed above, miR-21 expression is modulated by mTOR and Stat3 in spontaneous tumors. The upregulated miR-21 level in cancer is decreased by mTOR or Stat3 inhibitors. Active mTOR and Stat3 signaling augments miR-21 expression, conferring increased aggressiveness and metastatic properties to tumor keratinocytes, both in vitro and in vivo [15].
miR-143
miR-143 (5q32) is encoded by a fragile site of chromosome 5, which is frequently deleted in various types of cancers. miR-143 is downregulated following mTOR activation, via an unknown mechanism. mTOR-miR-143 signaling regulates cancer glycolysis by targeting hexokinase 2 (HK2), an enzyme that phosphorylates glucose to produce glucose-6-phosphate, the first step in glucose metabolism pathway. The mTOR-miR-143/HK2 axis has been shown to enhance cancer cell proliferation and tumor formation by promoting glucose metabolism in human lung cancer [122].
Conclusions and future perspectives
miRNAs have been clearly demonstrated to have close relationships with mTOR signaling pathway. Current evidence shows that the interactions between miRNAs and mTOR signaling occur in most cancer types. In addition, their interaction has been reported in many other diseases and physiological conditions. For instances, mTORC1 regulates miR-125b and miR-1 biogenesis, which is a mechanism to govern skeletal myogenesis [123, 124]. The Let-7/mTOR axis regulates glucose metabolism [125], neurodegenerative diseases [126], and organ regeneration [127]. Let-7 coordinately suppresses components of an amino acid sensing pathway to repress mTORC1 and induce autophagy [128]. These findings suggest that miRNA interaction with the mTOR signaling pathway is a general mechanism involved in broad biological processes in eukaryotes.
Dysregulation of miRNAs is a key characteristic of cancer. Expression profiling of miRNAs has been extensively used to understand the development, invasion, and progression in different types of cancers. By targeting mTOR signaling, miRNA interferes with many different cellular processes and, according to the cellular context, acts as a tumor suppressor or oncogene, to promote uncontrolled growth, metabolism, and metastasis. Because mTOR is a validated therapeutic target for cancer, targeting miRNAs may provide a novel approach to facilitate an integrated anti-cancer therapy. Two therapeutic strategies may be used: inhibition or replacement. Through the delivery of antagomiRs to silence endogenous oncomiRs, or via the ectopic replacement of tumor-suppressive miRNAs by delivery primary miRNA or miRNA synthetic mimics to restore miRNA levels in cancer cells and tissues, it is possible to achieve blockage of key mTOR signaling event that drives oncogenesis [129].
To date, a large body of evidence demonstrates that miRNA expression correlates well with various aspects of pathological features of human cancer. Therefore, miRNAs are also promising biomarkers for cancer diagnosis and prognosis, as well as predictive biomarkers for therapeutic response. Compared with other molecular markers such as proteins, miRNAs, particularly when used as signatures, can provide unparalleled accuracy and sensitivity. Moreover, circulating miRNAs may be captured for liquid biopsy. The specificity and sensitivity may be further improved by combining miRNAs with key protein molecules in mTOR signaling pathway. For instance, osteosarcoma patients with miR-99a-high/mTOR-low expression have better outcomes, while the miR-99a-low/mTOR-high patients have worse outcomes. The miR-99a-low/mTOR-high co-expression may be used as an independent prognostic indicator for osteosarcoma [40]. As several clinical investigations are ongoing, there is a great expectation for the power of integrating miRNAs with cancer signaling cascades to fulfill clinical needs.
Acknowledgements
Related work in authors’ laboratories was supported by NIH R01 Grants CA123391, CA166575, and CA173519, the National Natural Science Foundation of China 81672354, 81372600, 81572440, and Shanghai Pujiang Program 15PJ1404900.
Abbreviations
- AMPK
Adenosine 5′-monophosphate-activated protein kinase
- BDNF
Brain-derived neurotrophic factor
- DDIT4
DNA damage-inducible transcript 4
- 4E-BP1
eIF4E-binding protein 1
- IGF-1/IGF1R
Insulin-like growth factor 1/insulin-like growth factor 1 receptor
- IKKβ
IkB kinaseβ
- IRS
Insulin receptor substrate
- LKB1
Liver kinase B1
- MiRNA
microRNA
- mTOR
Mechanistic/mammalian target of rapamycin
- mTORC1/2
mTOR complex 1/2
- p70S6K
70 kDa ribosomal protein S6 kinase
- PDCD4
Programmed cell death 4
- PI3K
Phosphoinositide 3-kinase
- PRAS40
Proline-rich AKT substrate 40 kDa
- PTEN
Phosphatase and tensin homolog
- Rheb
Ras homology enriched in brain
- Stat3
Signal transducer and activator of transcription 3
- TSC1/2
Tuberous sclerosis 1/2
- VEGF
Vascular Endothelial Growth Factor
Contributor Information
Yanjie Zhang, Email: zhangyanjie@shsmu.edu.cn.
X. F. Steven Zheng, Email: zhengst@cinj.rutgers.edu.
References
- 1.Meng L-H, Zheng XS. Toward rapamycin analog (rapalog)-based precision cancer therapy. Acta Pharmacol Sin. 2015;36(10):1163–1169. doi: 10.1038/aps.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Y, et al. PP2AC level determines differential programming of p38-TSC-mTOR signaling and therapeutic response to p38-targeted therapy in colorectal cancer. EBioMedicine. 2015;2(12):1944–1956. doi: 10.1016/j.ebiom.2015.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsang CK, et al. Targeting mammalian target of rapamycin (mTOR) for health and diseases. Drug Discov Today. 2007;12(3):112–124. doi: 10.1016/j.drudis.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 4.Luo J, Cantley LC. Then negative regulation of phosphoinositide 3-kinase signaling by p85 and its implication in cancer. Cell Cycle. 2005;4(10):1309–1312. doi: 10.4161/cc.4.10.2062. [DOI] [PubMed] [Google Scholar]
- 5.Yu J, et al. Regulation of the p85/p110 phosphatidylinositol 3′-kinase: stabilization and inhibition of the p110α catalytic subunit by the p85 regulatory subunit . Mol Cell Biol. 1998;18(3):1379–1387. doi: 10.1128/MCB.18.3.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiao L, et al. Protein phosphatase-1 regulates Akt1 signal transduction pathway to control gene expression, cell survival and differentiation. Cell Death Differ. 2010;17(9):1448–1462. doi: 10.1038/cdd.2010.16. [DOI] [PubMed] [Google Scholar]
- 7.Wang B, Wang H, Yang Z. MiR-122 inhibits cell proliferation and tumorigenesis of breast cancer by targeting IGF1R. PLoS ONE. 2012;7(10):e47053. doi: 10.1371/journal.pone.0047053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.SK P, et al. Novel therapies for metastatic renal cell carcinoma: efforts to expand beyond the VEGF/mTOR signaling paradigm. Mol Cancer Ther. 2012;11(3):526–537. doi: 10.1158/1535-7163.MCT-11-0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma PC, et al. A selective small molecule c-MET Inhibitor, PHA665752, cooperates with rapamycin. Clinical cancer research. 2005;11(6):2312–2319. doi: 10.1158/1078-0432.CCR-04-1708. [DOI] [PubMed] [Google Scholar]
- 10.Imam JS, et al. Genomic loss of tumor suppressor miRNA-204 promotes cancer cell migration and invasion by activating AKT/mTOR/Rac1 signaling and actin reorganization. PLoS ONE. 2012;7(12):e52397. doi: 10.1371/journal.pone.0052397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu X, et al. Involvement of microRNA-93, a new regulator of PTEN/Akt signaling pathway, in regulation of chemotherapeutic drug cisplatin chemosensitivity in ovarian cancer cells. FEBS Lett. 2012;586(9):1279–1286. doi: 10.1016/j.febslet.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Leite KR, et al. MicroRNA 100: a context dependent miRNA in prostate cancer. Clinics. 2013;68(6):797–802. doi: 10.6061/clinics/2013(06)12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang D, et al. Leptin regulates proliferation and apoptosis of colorectal carcinoma through PI3K/Akt/mTOR signalling pathway. J Biosci. 2012;37(1):91–101. doi: 10.1007/s12038-011-9172-4. [DOI] [PubMed] [Google Scholar]
- 14.Dienstmann R, et al. Picking the point of inhibition: a comparative review of PI3K/AKT/mTOR pathway inhibitors. Mol Cancer Ther. 2014;13(5):1021–1031. doi: 10.1158/1535-7163.MCT-13-0639. [DOI] [PubMed] [Google Scholar]
- 15.Bornachea O, et al. EMT and induction of miR-21 mediate metastasis development in Trp53-deficient tumours. Sci Rep. 2012;2:434. doi: 10.1038/srep00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pineau P, et al. miR-221 overexpression contributes to liver tumorigenesis. Proc Natl Acad Sci USA. 2010;107(1):264–269. doi: 10.1073/pnas.0907904107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bera A, et al. microRNA-21-induced dissociation of PDCD4 from rictor contributes to Akt-IKKbeta-mTORC1 axis to regulate renal cancer cell invasion. Exp Cell Res. 2014;328(1):99–117. doi: 10.1016/j.yexcr.2014.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee DF, et al. IKKβ suppression of TSC1 function links the mTOR pathway with insulin resistance. Int J Mol Med. 2008;22(5):633–638. doi: 10.3892/ijmm_00000065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas JD, et al. Rab1A is an mTORC1 activator and a colorectal oncogene. Cancer Cell. 2014;26(5):754–769. doi: 10.1016/j.ccell.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y-J, Duan Y, Zheng XS. Targeting the mTOR kinase domain: the second generation of mTOR inhibitors. Drug Discov Today. 2011;16(7):325–331. doi: 10.1016/j.drudis.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewis BP, Burge CB, Bartel DP (2005) Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120(1):15–20 [DOI] [PubMed]
- 22.Banzhaf-Strathmann J, Edbauer D. Good guy or bad guy: the opposing roles of microRNA 125b in cancer. Cell Commun Signal. 2014;12(1):1. doi: 10.1186/1478-811X-12-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sato T, et al. Single amino-acid changes that confer constitutive activation of mTOR are discovered in human cancer. Oncogene. 2010;29(18):2746–2752. doi: 10.1038/onc.2010.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Volinia S, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci USA. 2006;103(7):2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ye P, et al. An mTORC1-Mdm2-Drosha axis for miRNA biogenesis in response to glucose- and amino acid-deprivation. Mol Cell. 2015;57(4):708–720. doi: 10.1016/j.molcel.2014.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kefas B, et al. microRNA-7 inhibits the epidermal growth factor receptor and the Akt pathway and is down-regulated in glioblastoma. Cancer Res. 2008;68(10):3566–3572. doi: 10.1158/0008-5472.CAN-07-6639. [DOI] [PubMed] [Google Scholar]
- 27.Glover AR, et al. microRNA-7 as a tumor suppressor and novel therapeutic for adrenocortical carcinoma. Oncotarget. 2015;6(34):36675–36688. doi: 10.18632/oncotarget.5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fang Y, et al. MicroRNA-7 inhibits tumor growth and metastasis by targeting the phosphoinositide 3-kinase/Akt pathway in hepatocellular carcinoma. Hepatology. 2012;55(6):1852–1862. doi: 10.1002/hep.25576. [DOI] [PubMed] [Google Scholar]
- 29.Jin Y, et al. MicroRNA-99 family targets AKT/mTOR signaling pathway in dermal wound healing . PLoS ONE. 2013;8(5):e64434. doi: 10.1371/journal.pone.0064434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang FZ, et al. Human cytomegalovirus infection alters the expression of cellular microRNA species that affect its replication. J Virol. 2008;82(18):9065–9074. doi: 10.1128/JVI.00961-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li W, et al. miRNA-99b-5p suppresses liver metastasis of colorectal cancer by down-regulating mTOR. Oncotarget. 2015;6(27):24448. doi: 10.18632/oncotarget.4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun J, et al. MicroRNA-99a/100 promotes apoptosis by targeting mTOR in human esophageal squamous cell carcinoma. Med Oncol. 2013;30(1):1–9. doi: 10.1007/s12032-012-0411-9. [DOI] [PubMed] [Google Scholar]
- 33.Zhang N, et al. MicroRNA-100 promotes migration and invasion through mammalian target of rapamycin in esophageal squamous cell carcinoma. Oncol Rep. 2014;32(4):1409–1418. doi: 10.3892/or.2014.3389. [DOI] [PubMed] [Google Scholar]
- 34.Nagaraja AK, et al. A link between mir-100 and FRAP1/mTOR in clear cell ovarian cancer. Mol Endocrinol. 2010;24(2):447–463. doi: 10.1210/me.2009-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang L, et al. miR-99a and – 99b inhibit cervical cancer cell proliferation and invasion by targeting mTOR signaling pathway. Med Oncol. 2014;31(5):1–8. doi: 10.1007/s12032-014-0934-3. [DOI] [PubMed] [Google Scholar]
- 36.Hu Y, Zhu Q, Tang L. MiR-99a antitumor activity in human breast cancer cells through targeting of mTOR expression. PLoS ONE. 2014;9(3):e92099. doi: 10.1371/journal.pone.0092099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun D, et al. miR-99 family of MicroRNAs suppresses the expression of prostate-specific antigen and prostate cancer cell proliferation. Cancer Res. 2011;71(4):1313–1324. doi: 10.1158/0008-5472.CAN-10-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu C, et al. miRNA-100 inhibits human bladder urothelial carcinogenesis by directly targeting mTOR. Mol Cancer Ther. 2013;12(2):207–219. doi: 10.1158/1535-7163.MCT-12-0273. [DOI] [PubMed] [Google Scholar]
- 39.Doghman M, et al. Regulation of insulin-like growth factor–mammalian target of rapamycin signaling by microRNA in childhood adrenocortical tumors. Cancer Res. 2010;70(11):4666–4675. doi: 10.1158/0008-5472.CAN-09-3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao J, et al. Aberrant expression of microrna-99a and its target gene mTOr associated with malignant progression and poor prognosis in patients with osteosarcoma. Onco Target Therapy. 2016;9:1589. doi: 10.2147/OTT.S102421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oneyama C, et al. MicroRNA-mediated downregulation of mTOR/FGFR3 controls tumor growth induced by Src-related oncogenic pathways. Oncogene. 2011;30(32):3489–3501. doi: 10.1038/onc.2011.63. [DOI] [PubMed] [Google Scholar]
- 42.Chen Z, et al. Down-regulation of the microRNA-99 family members in head and neck squamous cell carcinoma. Oral Oncol. 2012;48(8):686–691. doi: 10.1016/j.oraloncology.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li D, et al. MicroRNA-99a inhibits hepatocellular carcinoma growth and correlates with prognosis of patients with hepatocellular carcinoma. J Biol Chem. 2011;286(42):36677–36685. doi: 10.1074/jbc.M111.270561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Y, et al. A dual PI3K/AKT/mTOR signaling inhibitor miR-99a suppresses endometrial carcinoma. Am J Transl Res. 2016;8(2):719. [PMC free article] [PubMed] [Google Scholar]
- 45.Yu S, et al. miR-99a suppresses the metastasis of human non-small cell lung cancer cells by targeting AKT1 signaling pathway †. J Cell Biochem. 2015;116(2):268–276. doi: 10.1002/jcb.24965. [DOI] [PubMed] [Google Scholar]
- 46.Gui T, Shen K. miRNA-101: a potential target for tumor therapy. Cancer Epidemiol. 2012;36(6):537–540. doi: 10.1016/j.canep.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 47.Lin S, et al. Effect of microRNA-101 on proliferation and apoptosis of human osteosarcoma cells by targeting mTOR. J Huazhong Univ Sci Technol [Med Sci] 2014;34:889–895. doi: 10.1007/s11596-014-1369-y. [DOI] [PubMed] [Google Scholar]
- 48.Merkel O, et al. Identification of differential and functionally active miRNAs in both anaplastic lymphoma kinase (ALK) + and ALK–anaplastic large-cell lymphoma. Proc Natl Acad Sci. 2010;107(37):16228–16233. doi: 10.1073/pnas.1009719107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bai S, et al. MicroRNA-122 inhibits tumorigenic properties of hepatocellular carcinoma cells and sensitizes these cells to sorafenib. J Biol Chem. 2009;284(46):32015–32027. doi: 10.1074/jbc.M109.016774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang YM, et al. Gα12 overexpressed in hepatocellular carcinoma reduces microRNA-122 expression via HNF4α inactivation, which causes c-Met induction. Oncotarget. 2015;6(22):19055. doi: 10.18632/oncotarget.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meister J, Schmidt MH. miR-126 and miR-126*: new players in cancer. Sci World J. 2010;10:2090–2100. doi: 10.1100/tsw.2010.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Banerjee N, et al. Pomegranate polyphenolics suppressed azoxymethane-induced colorectal aberrant crypt foci and inflammation: possible role of miR-126/VCAM-1 and miR-126/PI3K/AKT/mTOR. Carcinogenesis. 2013;34(12):2814–2822. doi: 10.1093/carcin/bgt295. [DOI] [PubMed] [Google Scholar]
- 53.Guo C, et al. The noncoding RNA, miR-126, suppresses the growth of neoplastic cells by targeting phosphatidylinositol 3-kinase signaling and is frequently lost in colon cancers. Genes Chromosom Cancer. 2008;47(11):939–946. doi: 10.1002/gcc.20596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lei Y, et al. Reduced miR-126 expression facilitates angiogenesis of gastric cancer through its regulation on VEGF-A. RNA Dis. 2015;2(1):31. doi: 10.18632/oncotarget.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Y, et al. MicroRNA-149 inhibits proliferation and cell cycle progression through the targeting of ZBTB2 in human gastric cancer. PLoS ONE. 2012;7(10):e41693. doi: 10.1371/journal.pone.0041693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin RJ, Lin YC, Yu AL. miR-149* induces apoptosis by inhibiting Akt1 and E2F1 in human cancer cells. Mol Carcinog. 2010;49(8):719–727. doi: 10.1002/mc.20647. [DOI] [PubMed] [Google Scholar]
- 57.Pan S, et al. MicroRNA-149 inhibits proliferation and invasion of glioma cells via blockade of AKT1 signaling. Int J Immunopathol Pharmacol. 2012;25(4):871–881. doi: 10.1177/039463201202500405. [DOI] [PubMed] [Google Scholar]
- 58.Xue L, et al. Low MiR-149 expression is associated with unfavorable prognosis and enhanced Akt/mTOR signaling in glioma. Int J Clin Exp Pathol. 2015;8(9):11178. [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Y, et al. Comprehensive analysis of microRNA-regulated protein interaction network reveals the tumor suppressive role of microRNA-149 in human hepatocellular carcinoma via targeting AKT-mTOR pathway. Mol Cancer. 2014;13(1):1. doi: 10.1186/1476-4598-13-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Faraoni I, et al. miR-155 gene: a typical multifunctional microRNA. Biochimica et Biophysica Acta. 2009;1792(6):497–505. doi: 10.1016/j.bbadis.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 61.Wang J, et al. MicroRNA-155 promotes autophagy to eliminate intracellular mycobacteria by targeting Rheb. PLoS Pathog. 2013;9(10):e1003697. doi: 10.1371/journal.ppat.1003697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martin EC, et al. microRNA regulation of mammalian target of rapamycin expression and activity controls estrogen receptor function and RAD001 sensitivity. Mol Cancer. 2014;13(1):1. doi: 10.1186/1476-4598-13-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wan G, et al. Hypoxia-induced MIR155 is a potent autophagy inducer by targeting multiple players in the MTOR pathway. Autophagy. 2014;10(1):70–79. doi: 10.4161/auto.26534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yu T, et al. MicroRNA-193a-3p and-5p suppress the metastasis of human non-small-cell lung cancer by downregulating the ERBB4/PIK3R3/mTOR/S6K2 signaling pathway. Oncogene. 2015;34(4):413–423. doi: 10.1038/onc.2013.574. [DOI] [PubMed] [Google Scholar]
- 65.Wu D, et al. MicroRNA-199a-3p regulates endometrial cancer cell proliferation by targeting mammalian target of rapamycin (mTOR) Int J Gynecol Cancer. 2013;23(7):1191–1197. doi: 10.1097/IGC.0b013e31829ea779. [DOI] [PubMed] [Google Scholar]
- 66.Shen L, et al. MicroRNA-199a-3p suppresses glioma cell proliferation by regulating the AKT/mTOR signaling pathway. Tumor Biol. 2015;36(9):6929–6938. doi: 10.1007/s13277-015-3409-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Duan Z, et al. MicroRNA-199a-3p is downregulated in human osteosarcoma and regulates cell proliferation and migration. Mol Cancer Ther. 2011;10(8):1337–1345. doi: 10.1158/1535-7163.MCT-11-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fornari F, et al. MiR-199a-3p regulates mTOR and c-Met to influence the doxorubicin sensitivity of human hepatocarcinoma cells. Cancer Res. 2010;70(12):5184–5193. doi: 10.1158/0008-5472.CAN-10-0145. [DOI] [PubMed] [Google Scholar]
- 69.Li G, et al. Role of miR-204 in the regulation of apoptosis, endoplasmic reticulum stress response, and inflammation in human trabecular meshwork cells. Investig Ophthalmol Vis Sci. 2011;52(6):2999–3007. doi: 10.1167/iovs.10-6708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cui R-R, et al. MicroRNA-204 regulates vascular smooth muscle cell calcification in vitro and in vivo. Cardiovasc Res. 2012;2012:cvs258. doi: 10.1093/cvr/cvs258. [DOI] [PubMed] [Google Scholar]
- 71.Xia Z, et al. Decreased expression of MiRNA-204-5p contributes to glioma progression and promotes glioma cell growth, migration and invasion. PLoS ONE. 2015;10(7):e0132399. doi: 10.1371/journal.pone.0132399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yu X, et al. MiR-214 increases the sensitivity of breast cancer cells to tamoxifen and fulvestrant through inhibition of autophagy. Mol Cancer. 2015;14(1):1. doi: 10.1186/1476-4598-14-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Das F, et al. microRNA-214 reduces IGF-1 receptor expression and downstream mTORC1 signaling in renal carcinoma cells. J Biol Chem. 2016;2016:jbc. M115. 694331. doi: 10.1074/jbc.M115.694331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lu Y-f, et al. MiR-218 mediates tumorigenesis and metastasis: perspectives and implications. Exp Cell Res. 2015;334(1):173–182. doi: 10.1016/j.yexcr.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 75.Zhang X, et al. miR-218 inhibits the invasion and migration of colon cancer cells by targeting the PI3K/Akt/mTOR signaling pathway. Int J Mol Med. 2015;35(5):1301–1308. doi: 10.3892/ijmm.2015.2126. [DOI] [PubMed] [Google Scholar]
- 76.Tian H, et al. miR-218 suppresses tumor growth and enhances the chemosensitivity of esophageal squamous cell carcinoma to cisplatin. Oncol Rep. 2015;33(2):981–989. doi: 10.3892/or.2014.3657. [DOI] [PubMed] [Google Scholar]
- 77.Li J, Ping Z, Ning H. MiR-218 impairs tumor growth and increases chemo-sensitivity to cisplatin in cervical cancer. Int J Mol Sci. 2012;13(12):16053–16064. doi: 10.3390/ijms131216053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Uesugi A, et al. The tumor suppressive microRNA miR-218 targets the mTOR component Rictor and inhibits AKT phosphorylation in oral cancer. Cancer Res. 2011;2011:canres. 0368.2011. doi: 10.1158/0008-5472.CAN-11-0368. [DOI] [PubMed] [Google Scholar]
- 79.Shi J. Considering exosomal miR-21 as a biomarker for cancer. J Clin Med. 2016;5(4):42. doi: 10.3390/jcm5040042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fragni M, et al. The miR-21/PTEN/Akt signaling pathway is involved in the anti-tumoral effects of zoledronic acid in human breast cancer cell lines. Naunyn-Schmiedeberg’s Arch Pharmacol. 2016;389(5):529–538. doi: 10.1007/s00210-016-1224-8. [DOI] [PubMed] [Google Scholar]
- 81.Li X, et al. Triptolide reduces proliferation and enhances apoptosis of human non-small cell lung cancer cells through PTEN by targeting miR-21. Mol Med Rep. 2016;13(3):2763–2768. doi: 10.3892/mmr.2016.4844. [DOI] [PubMed] [Google Scholar]
- 82.Kalogirou C, et al. Metformin-derived growth inhibition in renal cell carcinoma depends on miR-21-mediated PTEN expression. Urol Int. 2015;96(1):106–115. doi: 10.1159/000441011. [DOI] [PubMed] [Google Scholar]
- 83.Li L-Q, et al. Matrine inhibits breast cancer growth via miR-21/PTEN/Akt pathway in MCF-7 cells. Cell Physiol Biochem. 2012;30(3):631–641. doi: 10.1159/000341444. [DOI] [PubMed] [Google Scholar]
- 84.Chen J, Xu T, Chen C. The critical roles of miR-21 in anti-cancer effects of curcumin. Ann Transl Med. 2015;3(21):330. doi: 10.3978/j.issn.2305-5839.2015.09.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhou L, et al. MicroRNA-21 is involved in X-ray irradiation resistance in K562 leukaemia cells. Hematology. 2014;20(6):343–348. doi: 10.1179/1607845414Y.0000000201. [DOI] [PubMed] [Google Scholar]
- 86.Ma Y, et al. Silencing miR-21 sensitizes non-small cell lung cancer A549 cells to ionizing radiation through inhibition of PI3K/Akt. Biomed Res Int. 2014;2014(2):617868–617868. doi: 10.1155/2014/617868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yu X, et al. Silencing of MicroRNA-21 confers the sensitivity to tamoxifen and fulvestrant by enhancing autophagic cell death through inhibition of the PI3K-AKT-mTOR pathway in breast cancer cells. Biomed Pharmacother. 2016;77:37–44. doi: 10.1016/j.biopha.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 88.He C, et al. MiR-21 mediates sorafenib resistance of hepatocellular carcinoma cells by inhibiting autophagy via the PTEN/Akt pathway. Oncotarget. 2015;6(30):28867. doi: 10.18632/oncotarget.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang Z, et al. Modulation of NF-κB/miR-21/PTEN pathway sensitizes non-small cell lung cancer to cisplatin. PLoS ONE. 2015;10(3):e0121547. doi: 10.1371/journal.pone.0121547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang S-m, et al. miR-21 confers cisplatin resistance in gastric cancer cells by regulating PTEN. Toxicology. 2013;306:162–168. doi: 10.1016/j.tox.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 91.Wang W-Z, et al. Targeting miR-21 sensitizes Ph + ALL Sup-b15 cells to imatinib-induced apoptosis through upregulation of PTEN. Biochem Biophys Res Commun. 2014;454(3):423–428. doi: 10.1016/j.bbrc.2014.10.107. [DOI] [PubMed] [Google Scholar]
- 92.Shen H, et al. Alteration in Mir-21/PTEN expression modulates gefitinib resistance in non-small cell lung cancer. PLoS ONE. 2014;9(7):e103305. doi: 10.1371/journal.pone.0103305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Go H, et al. MicroRNA-21 plays an oncogenic role by targeting FOXO1 and activating the PI3K/AKT pathway in diffuse large B-cell lymphoma. Oncotarget. 2015;6(17):15035. doi: 10.18632/oncotarget.3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bai H, et al. Involvement of miR-21 in resistance to daunorubicin by regulating PTEN expression in the leukaemia K562 cell line. FEBS Lett. 2011;585(2):402–408. doi: 10.1016/j.febslet.2010.12.027. [DOI] [PubMed] [Google Scholar]
- 95.Li B, et al. MiR-21 overexpression is associated with acquired resistance of EGFR-TKI in non-small cell lung cancer. Lung Cancer. 2014;83(2):146–153. doi: 10.1016/j.lungcan.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 96.Bai H, et al. MicroRNA-21 regulates the sensitivity of diffuse large B-cell lymphoma cells to the CHOP chemotherapy regimen. Int J Hematol. 2013;97(2):223–231. doi: 10.1007/s12185-012-1256-x. [DOI] [PubMed] [Google Scholar]
- 97.Toste PA, et al. p85α is a microRNA target and affects chemosensitivity in pancreatic cancer. J Surg Res. 2015;196(2):285–293. doi: 10.1016/j.jss.2015.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yan L-X, et al. PIK3R1 targeting by miR-21 suppresses tumor cell migration and invasion by reducing PI3K/AKT signaling and reversing EMT, and predicts clinical outcome of breast cancer. Int J Oncol. 2016;48(2):471–484. doi: 10.3892/ijo.2015.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhen Y, et al. Reduced PDCD4 expression promotes cell growth through PI3K/Akt signaling in non-small cell lung cancer. Oncol Res Featur Preclin Clin Cancer Ther. 2016;23(1–2):61–68. doi: 10.3727/096504015X14478843952861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kawano M, et al. microRNA-93 promotes cell proliferation via targeting of PTEN in Osteosarcoma cells. J Exp Clin Cancer Res. 2015;34(1):1. doi: 10.1186/s13046-015-0192-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jiang L, et al. miR-93 promotes cell proliferation in gliomas through activation of PI3K/Akt signaling pathway. Oncotarget. 2015;6(10):8286. doi: 10.18632/oncotarget.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ohta K, et al. MicroRNA-93 activates c-Met/PI3K/Akt pathway activity in hepatocellular carcinoma by directly inhibiting PTEN and CDKN1A. Oncotarget. 2015;6(5):3211. doi: 10.18632/oncotarget.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen Q, et al. Berberine sensitizes human ovarian cancer cells to cisplatin through mir-93/pten/akt signaling pathway. Cell Physiol Biochem. 2015;36(3):956–965. doi: 10.1159/000430270. [DOI] [PubMed] [Google Scholar]
- 104.Zhang W, et al. Autocrine/paracrine human growth hormone-stimulated MicroRNA 96-182-183 cluster promotes epithelial-mesenchymal transition and invasion in breast cancer. J Biol Chem. 2015;290(22):13812–13829. doi: 10.1074/jbc.M115.653261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Feng J, et al. HERG1 functions as an oncogene in pancreatic cancer and is downregulated by miR-96. Oncotarget. 2014;5(14):5832–5844. doi: 10.18632/oncotarget.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Leung WK, et al. Wnt/β-Catenin activates MiR-183/96/182 expression in hepatocellular carcinoma that promotes cell invasion. Cancer Lett. 2015;362(1):97–105. doi: 10.1016/j.canlet.2015.03.023. [DOI] [PubMed] [Google Scholar]
- 107.Chong ZZ. Targeting PRAS40 for multiple diseases. Drug Discov Today. 2016;21(8):1222–1231. doi: 10.1016/j.drudis.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 108.Siu M, et al. Transforming growth factor-β promotes prostate bone metastasis through induction of microRNA-96 and activation of the mTOR pathway. Oncogene. 2014;34(36):4767–4776. doi: 10.1038/onc.2014.414. [DOI] [PubMed] [Google Scholar]
- 109.Sun Y-M, Lin K-Y, Chen Y-Q. Diverse functions of miR-125 family in different cell contexts. J Hematol Oncol. 2013;6(1):1. doi: 10.1186/1756-8722-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Astanehe A, et al. Mechanisms underlying p53 regulation of PIK3CA transcription in ovarian surface epithelium and in ovarian cancer. J Cell Sci. 2008;121(5):664–674. doi: 10.1242/jcs.013029. [DOI] [PubMed] [Google Scholar]
- 111.Singh B, et al. p53 regulates cell survival by inhibiting PIK3CA in squamous cell carcinomas. Genes Dev. 2002;16(8):984–993. doi: 10.1101/gad.973602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vilquin P, et al. MicroRNA-125b upregulation confers aromatase inhibitor resistance and is a novel marker of poor prognosis in breast cancer. Breast Cancer Res. 2015;17(1):1. doi: 10.1186/s13058-015-0515-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jian B, et al. Downregulation of microRNA-193-3p inhibits tumor proliferation migration and chemoresistance in human gastric cancer by regulating PTEN gene. Tumor Biol. 2016;37:1–9. doi: 10.1007/s13277-015-4727-x. [DOI] [PubMed] [Google Scholar]
- 114.Garofalo M, et al. miR221/222 in cancer: their role in tumor progression and response to therapy. Curr Mol Med. 2012;12(1):27–33. doi: 10.2174/156652412798376170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.DeYoung MP, et al. Hypoxia regulates TSC1/2–mTOR signaling and tumor suppression through REDD1-mediated 14-3-3 shuttling. Genes Dev. 2008;22(2):239–251. doi: 10.1101/gad.1617608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mahlamäki EH, et al. Frequent amplification of 8q24, 11q, 17q, and 20q-specific genes in pancreatic cancer. Genes Chromosom Cancer. 2002;35(4):353–358. doi: 10.1002/gcc.10122. [DOI] [PubMed] [Google Scholar]
- 117.Varis A, et al. Targets of gene amplification and overexpression at 17q in gastric cancer. Cancer Res. 2002;62(9):2625–2629. [PubMed] [Google Scholar]
- 118.Du J, et al. MicroRNA-451 regulates stemness of side population cells via PI3K/Akt/mTOR signaling pathway in multiple myeloma. Oncotarget. 2015;6(17):14993. doi: 10.18632/oncotarget.3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen M-B, et al. MicroRNA-451 regulates AMPK/mTORC1 signaling and fascin1 expression in HT-29 colorectal cancer. Cell Signal. 2014;26(1):102–109. doi: 10.1016/j.cellsig.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 120.Godlewski J, et al. MicroRNA-451 regulates LKB1/AMPK signaling and allows adaptation to metabolic stress in glioma cells. Mol Cell. 2010;37(5):620–632. doi: 10.1016/j.molcel.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Totary-Jain H, et al. Reprogramming of the microRNA transcriptome mediates resistance to rapamycin. J Biol Chem. 2013;288(9):6034–6044. doi: 10.1074/jbc.M112.416446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fang R, et al. MicroRNA-143 (miR-143) regulates cancer glycolysis via targeting hexokinase 2 gene. J Biol Chem. 2012;287(27):23227–23235. doi: 10.1074/jbc.M112.373084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sun Y, et al. Mammalian target of rapamycin regulates miRNA-1 and follistatin in skeletal myogenesis. J Cell Biol. 2010;189(7):1157–1169. doi: 10.1083/jcb.200912093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ge Y, Sun Y, Chen J. IGF-II is regulated by microRNA-125b in skeletal myogenesis. J Cell Biol. 2011;192(1):69–81. doi: 10.1083/jcb.201007165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhu H, et al. The Lin28/let-7 axis regulates glucose metabolism. Cell. 2011;147(1):81–94. doi: 10.1016/j.cell.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kumar L, Haque R, Nazir A. Role of microRNA Let-7 in modulating multifactorial aspect of neurodegenerative diseases: an overview. Mol Neurobiol. 2016;53(5):2787–2793. doi: 10.1007/s12035-015-9145-y. [DOI] [PubMed] [Google Scholar]
- 127.Wu L, et al. Precise let-7 expression levels balance organ regeneration against tumor suppression. Elife. 2015;4:e09431. doi: 10.7554/eLife.09431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dubinsky AN, et al. Let-7 coordinately suppresses components of the amino acid sensing pathway to repress mTORC1 and induce autophagy. Cell Metab. 2014;20(4):626–638. doi: 10.1016/j.cmet.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Orellana EA, Kasinski AL. MicroRNAs in cancer: a historical perspective on the path from discovery to therapy. Cancers. 2015;7(3):1388–1405. doi: 10.3390/cancers7030842. [DOI] [PMC free article] [PubMed] [Google Scholar]