Abstract
Hypoxic-ischemic (HI) brain injury is recognized as a significant problem in the perinatal period, contributing to life-long language-learning and other cognitive impairments. Central auditory processing deficits are common in infants with hypoxic-ischemic encephalopathy and have been shown to predict language learning deficits in other at risk infant populations. Inter-alpha inhibitor proteins (IAIPs) are a family of structurally related plasma proteins that modulate the systemic inflammatory response to infection and have been shown to attenuate cell death and improve learning outcomes after neonatal brain injury in rats. Here, we show that systemic administration of IAIPs during the early HI injury cascade ameliorates complex auditory discrimination deficits as compared to untreated HI injured subjects, despite reductions in brain weight. These findings have significant clinical implications for improving central auditory processing deficits linked to language learning in neonates with HI related brain injury.
Keywords: neonatal brain injury, auditory temporal processing, pre-pulse inhibition, neurobehavioral protection
Introduction
Perinatal hypoxic-ischemic (HI) brain injury, prematurity, very-low-birth-weight (VLBW<1200g) and infectious disorders often place neonates at high risk for developmental language delays (Vohr 2014, Vohr 2016). Central auditory processing deficits have been reported to contribute to later language delays similar to delays observed in children with language learning impairments of unknown origin (Ortiz-Mantilla, Choudhury et al. 2008). Previous work has consistently reported an association between complex and/or rapid auditory processing impairments and later language outcomes in young children (Benasich and Tallal 2002). Further, infant auditory temporal processing thresholds for the discrimination of rapidly presented tone-pairs are highly predictive of the age at which language commences in both typical and at risk infants (Benasich and Tallal 2002, Cantiani, Riva et al. 2016). Likewise, experimental models in neonatal rodents after exposure to unilateral HI brain injury exhibit complex central auditory processing deficits and injury profiles similar to those observed in human full term and premature infants with brain injury (Downie, Jakobson et al. 2002, McClure, Threlkeld et al. 2006, McClure, Threlkeld et al. 2007).
Currently, hypothermia is the only approved therapy to attenuate brain damage in infants, but is only partially protective and can only be used in the presence of HI encephalopathy (HIE) in full term infants (Gunn, Gunn et al. 1997, Shankaran, Laptook et al. 2005, Gluckman, Gunn et al. 2006). Despite efforts to develop additional therapeutic strategies, there are currently no pharmacological agents available to treat human neonates at risk for brain injury (McClure, Threlkeld et al. 2006, McClure, Threlkeld et al. 2007, Xiong, Qu et al. 2011). Nonetheless, recent work has suggested a critical role for inflammation and immune reactivity in regulating the injury cascade after exposure to HI in neonates (Dammann and Leviton 1997, Becker 1998, Ferriero 2004, Iadecola and Anrather 2011).
Inter-alpha inhibitor proteins (IAIPs) are a family of structurally related serine protease inhibitors with immune modulating capabilities that have been shown to down-regulate pro-inflammatory cytokines, Interleukin (IL)-1β and Tumor Necrosis Factor (TNF)-α and upregulate anti-inflammatory cytokine IL-10, leading to improved outcomes in several different models of systemic inflammatory disorders (Baek, Brokat et al. 2003, Wakahara, Kobayashi et al. 2005, Chen, Rivard et al. 2016). The major forms if IAIPs are inter-alpha inhibitor (IαI), which consists of two heavy chairs (H1 & H2), and a single light chain (LC), and pre-alpha inhibitor (PαI) consisting of one heavy (H3) chain and one light chain (LC)(Fries and Blom 2000). Recently, blood plasma levels of this protein have been shown to accurately predict the development of sepsis in premature infants, as levels decrease during systemic inflammation (Chaaban, Singh et al. 2009). Furthermore, hepatic IAIP synthesis is down regulated during severe inflammation and levels of IAIPs have been shown to decrease during sepsis, (Chaaban, Singh et al. 2009) which is associated with an increased incidence of brain damage in premature infants (Stoll, Hansen et al. 2004, Shah, Doyle et al. 2008).
Recently, our group has shown that systemic IAIPs administration increases neocortical cell survival, prevents hippocampal and cortical tissue loss, and improves spatial and non-spatial learning and working memory performance in rats after exposure to neonatal HI brain injury (Threlkeld, Gaudet et al. 2014, Gaudet, Lim et al. 2016). Given the potential for IAIPs to attenuate neonatal brain injury, and the importance of delayed language development in infants with premature delivery and related brain injury (Vohr 2014, Vohr 2016), it is important to examine the effects of IAIPs on complex auditory processing in model species with neonatal HI injury.
In addition, investigations in humans and rodent models of neonatal brain injury have shown that deficits in learning and in auditory processing are increasingly detected with progressive demand of any given task (Threlkeld, McClure et al. 2006, Barde, Yeatman et al. 2012, Threlkeld, Gaudet et al. 2014, Gaudet, Lim et al. 2016). We have previously shown that as the demand of a specific task increases, the deficits become more apparent in juvenile and adult rodents exposed to neonatal HI brain injury relative to sham subjects, irrespective of the quantity of HI injury (Threlkeld, Hill et al. 2009, Gaudet, Lim et al. 2016). Therefore, given the above considerations, the present study sought to determine the efficacy of IAIPs to improve auditory processing using a modified acoustic startle paradigm with increasing levels of cue complexity (i.e., task demand; simple normal single tone detection, gap detection in white noise and complex oddball tone-pair discrimination) in adult rats exposed to neonatal HI.
Methods
Subjects and Surgical Procedures
Subjects were 34 male Wistar rats born to 7 time-mated dams (Charles River Laboratories; Wilmington, MA) at Rhode Island College. Dams arrived at the college on gestational day 7. Animals were housed using a 12-hour light/dark cycle with food and water available ad libitum. On postnatal day one (P1), pups were separated into litters of eight males and two females to control for litter size and sex ratio. Weighting for males produced five litters that were maintained up until weaning (P21), when subjects were pair housed. Prior to surgery on postnatal day (P) 7, male subjects, within each litter, were randomly assigned to one of three groups: Sham+vehicle (n=12), hypoxia-ischemic vehicle treated (HI + Vehicle, n=13), and hypoxia-ischemic IAIP treated (HI +IAIP, n=9). This distributed treatment assignment was designed to control for litter effects and reflected an even distribution of treatment groups for each dam. Male subjects were assessed given that rodent and human epidemiological data indicate greater behavioral deficits in males as compared to females with neonatal brain injury (Raz 1995, Abe, Sugino et al. 1996, Peiffer, Rosen et al. 2004, Hill, Threlkeld et al. 2011) – findings that are similar to the higher diagnostic rates of neurodevelopmental disorders including dyslexia, epilepsy, autism and intellectual disabilities that are detected in human males compared with females (Raz 1995, Rutter 2003, Liederman 2005).
Subjects were weighed and anesthetized using 3–4% isoflurane and maintained with 1% during the surgical procedure. Methods for HI brain injury in neonatal rodents have been extensively described elsewhere (Rice, Vannucci et al. 1981, McClure, Threlkeld et al. 2006, Threlkeld, Gaudet et al. 2014). Following a 1 cm midline incision of the neck, the right common carotid artery (RCCA) was located and completely cauterized (McClure, Threlkeld et al. 2006, McClure, Threlkeld et al. 2006). The incision was sutured and labeled with dermal paw India Ink (Higgins) injections (10μl) for later identification. Sham subjects underwent identical surgical procedures without cauterization of the RCCA. Body temperature was maintained at 37°C preoperatively, during surgery and during postoperative recovery. After surgery, the pups were returned to their dams and allowed to feed for 2–3 hours before exposure to hypoxia.
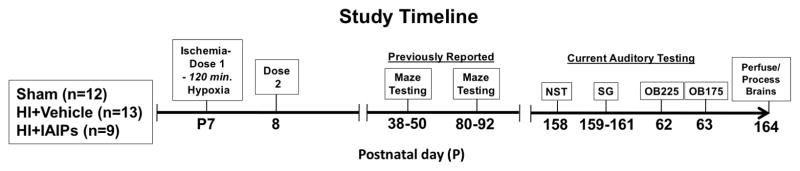
After recovery from surgery, as described above, subjects were removed from their home cage and received an intraperitoneal (IP) injection of either 30 mg/kg of human IAIP (HI+IAIPs, Pro Thera biologics, Providence, RI) or placebo (0.9% NaCl vehicle; sham and HI). The dose of IAIPs was selected based upon studies showing that the same dose of IAIPs reduced the incidence of death from sepsis in neonatal and adult rats and increased cortical neuronal survival after neonatal HI (Lim, Bendelja et al. 2003, Singh, Zhang et al. 2010, Threlkeld, Gaudet et al. 2014). After the IP injections, the HI groups were exposed to humidified 8% O2 and 92 % N2 for 120 minutes. Body temperature was maintained for all groups during hypoxia or room air exposure using isothermal heating pads (Braintree Scientific, Braintree, MA). Sham subjects received identical treatment but were maintained in a separate container exposed to room air for 120 minutes and received 0.9% NaCl vehicle injections. A second identical dose of IAIPs or vehicle was administered 24 hours after hypoxia. The 12-hour half-life of IAIPs was hypothesized to ensure that protein levels would remain high between the two dosing windows prior to clearance by the kidneys (Fries, 2000). Prior to auditory assessment subjects were tested for spatial and non-spatial learning as previously reported (see figure 1 for study timeline) (Threlkeld, Gaudet et al. 2014). All procedures were performed according to the National Institutes of Health guide for the care and use of laboratory animals and approved by the Rhode Island College Institutional Animal Care and Use Committee.
Figure 1.
Diagram showing treatment groups, age of hypoxic-ischemic (HI) surgery, IAIPs dose timing and ages of maze testing (previously reported, Threlkeld et al., 2014) and the auditory testing sequence.
Production and purification of IAIPs
The methods for IAIPs extraction and purification have been described previously (Threlkeld et al., 2014). Briefly, A monolithic anion-exchange chromatographic method was used to extract IAIPs from frozen human plasma (Rhode Island Blood Center, RI; US Patent #7,932,365,2011; (Opal et al, 2011, Spasova et al, 2014). After binding, the column was sequentially washed with buffer containing 200nM NaCl and 200nM acetate buffer, with pH 3.0. IAIPs were eluted from the column using a buffer containing 759 mM NaCl, and concentrated and buffer exchanged using a tangential flow filtration system (Labscale, Millipore). Purity analysis was performed by SDS-Page, Western immunoblot, competitive IAIP ELISA and standardized in-vitro trypsin inhibition assay (Lim, 2013, Opal et al., 2011 Spasova et al, 2014). As previously reported, the biological activity is based on the ability of IAIPs to inhibit the hydrolysis of the substrate N-benzoyl-L-arginine-p-nitroaniline HCl (BAPNA, Sigma, St. Louis, MO) by trypsin.
Brain Analyses
At the end of auditory testing, subjects were weighed, anesthetized with pentobarbital (Sleepaway, Fort Dodge, IA), and transcardially perfused with saline followed by 10% phosphate buffered formalin. Brains were extracted and weighed (see figure 2). Tissue was then sectioned, mounted on glass slides and processed for nissl substance. Regional brain volume measures (hippocampus, cortex, corpus callosum and striatum) were calculated with Cavalieri’s estimation of volume equation using a point counting procedure and these volume measures were reported previously (See Threlkeld et al, 2014 and the present discussion for implications of these combined analyses).
Figure 2.
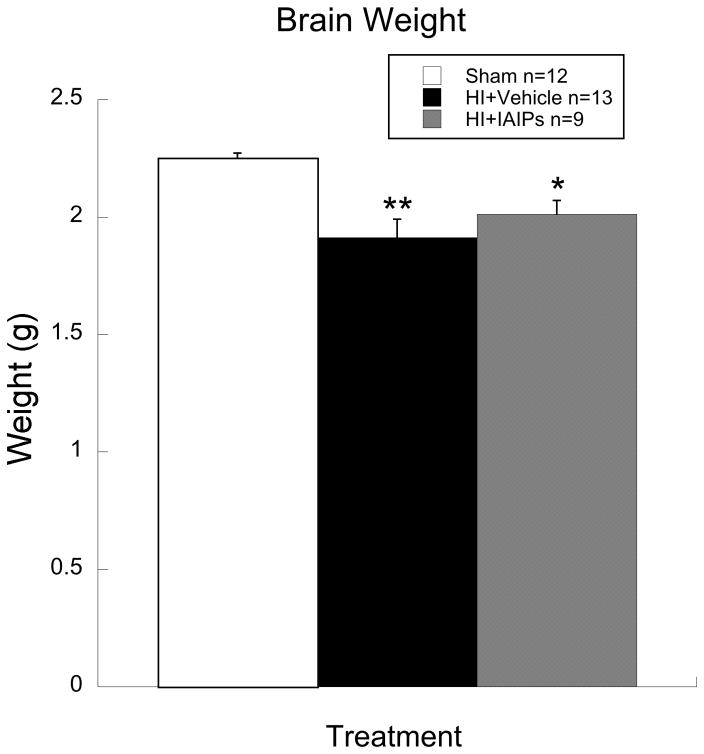
Histogram showing significant brain weight (Mean/SEM) differences between HI+vehicle (n=13) and Sham (n=12) subjects (**p=0.001) and HI+IAIPs (n=9) and Sham subjects (*p=0.028). The magnitude of the brain weight reduction in IAIPs treated HI subjects was less than that of untreated HI subjects and reflects the profile of hippocampal and cortical volume sparring reported previously for these same subjects (Threlkeld, Gaudet et al. 2014).
Behavioral Testing Startle Reduction
All behavioral testing was performed in adult subjects (postnatal day (P) 158–63) following a battery of previously reported maze testing (Threlkeld, Gaudet et al. 2014). Auditory testing involved a modified acoustic startle paradigm that has been discussed extensively elsewhere (also termed pre-pulse inhibition (PPI), see (Peiffer, Rosen et al. 2002, Peiffer, Rosen et al. 2004, Fitch, Threlkeld et al. 2008). Briefly, the startle modification paradigm involves the presentation of an auditory cue prior to a startle-eliciting stimulus (Opal, Lim et al.). The SES elicits an acoustic startle reflex (ASR) and if the preceding auditory cue is detected, the intensity of the ASR is reduced accordingly.
For all auditory assessments, subjects were placed on a load cell platform (Med Associates, Georgia, VT, USA), which measured the ballistic motor response to the SES in mV. Signals were acquired and passed through a linear load cell amplifier (PHM-250–60) into a Biopac MP150 acquisition system (Biopac Systems, Santa Barbra, CA) connected to two PC work stations, which recorded the subject’s movement and ASR as mV signals. The maximum peak value defining the ASR for each trial was extracted by algorithm from the 200 ms following the onset of the SES, and this ASR represents the dependent variable. Auditory stimuli were generated using a Pentium 4 Dell PC with custom programmed software and a Tucker Davis Technologies (RX6) real time processor. Stimulus files were played through a Pyle PT8000CH amplifier connected to four 200-Watt PCB4 Pyle speakers (Pyle Audio inc., Brooklyn, NY), with sound levels calibrated by sound-level meter (Peiffer, Rosen et al. 2002). Each pair of platforms had one speaker centered and mounted 50 cm above. Attenuated response scores (ATT) were calculated from the peak ASR using the formula ([mean cued response/mean uncued response] × 100). In this formula, absolute response scores (as measured by load-cell displacement for each subject’s startle response) for cued and uncued trials are expressed as a ratio and multiplied by 100, thus ATT scores represent a percentage. ATT scores were analyzed as a second dependent variable for all tasks.
Single Tone Procedure
Pre-pulse inhibition paradigms are commonly used to assess basic sensory-motor gating and auditory acuity (Fitch, Threlkeld et al. 2008). In the current paradigm, the single tone task was used to evaluate basic auditory acuity and pre-pulse inhibition prior to the assessment of more complex temporal processing (e.g., Silent gap and two-tone oddball discrimination; analogous to auditory temporal tasks used to test language learning impaired populations; (Tallal, 2004; Fitch, Threlkeld et al. 2008)). The normal single tone test (NST) session consisted of 104 trials (cued or uncued), presented in a pseudo-random order. Uncued trials included a silent background followed by the 105 dB, 50 ms SES. On cued trials a 75 dB, 7 ms, 2300 Hz tone was presented 50 ms prior to the SES. Trials were variable in duration (16 – 24 sec, 20 sec. on average).
Silent Gap Procedure
The silent gap procedure is a common tool (similar to single tone) utilized to evaluate basic auditory temporal processing in humans and rodent models (Threlkeld, McClure et al. 2006, Fournier and Hebert 2016). In contrast to the single tone procedure, the gap detection paradigm is a well-established means of assessing higher order rate specific processes that require at least thalamic level processing (Bowen GP 2003, Threlkeld, Penley et al. 2008). Silent gap testing began one day after the NST task. Subjects were given two days of testing on a long duration task (0, 2, 5, 10, 20, 30, 40, 50, 75, or 100 ms) and one day of a short duration task (0, 2, 3, 4, 5, 6, 7, 8, 9, 10 ms). Each session included 300 trials, each consisting of the presentation of a variable duration silent gap embedded in continuous 75 dB broadband white-noise. Each silent gap was presented 50 ms prior to a 105 dB burst of white noise. Uncued trials used a “gap” of 0 ms. The cue-burst interval for each task was maintained at 50 ms (Peiffer, Rosen et al. 2004).
Two-tone Oddball procedure
The two-tone oddball procedure reflects an increase in sensory demand, relative to the gap and normal single tone detection paradigms, and this task has been implicated in cortically dependent processes (Fitch et al, 2008, Threlkeld et al, 2008, Tallal 2004, Benasich, Choudhury et al. 2006). Each oddball session was comprised of 104 trials, with a total of two sessions (i.e., one per day over 2 days). This procedure involved the repeated presentation of a background 75 dB, high-low tone sequence (2300–1100 Hz, respectively) separated by a within-stimulus inter-stimulus interval (Suppiej, Cainelli et al. 2015) of variable duration (225 or 175 ms; one interval used per session). Each sequence was separated by a between sequence ISI, which was always 200 ms greater than the inter-stimulus interval to maintain perceptual contiguity of the tone-pair. On the uncued trials, the last tone sequence was followed by 50 ms of silence, then by the 105 dB/50 ms SES. On cued trials, a reversal of the tone sequence occurred (low-high, 1100–2300 Hz) followed by 50 ms of silence, and then the SES. Again, if stimuli were discriminated (high-low tone pair from low-high), animals would show inhibition of the startle response to the SES.
Statistics
For brain weight, Analysis of Variance (ANOVA) was used to assess main effects of HI injury. Post hoc analysis with Tukey’s HSD was used to assess simple effects across treatment groups. For all auditory testing procedures, ATT scores (dependent variable) were compared as a function of treatment. Analysis of variance (ANOVA) was used to assess treatment effects (Sham, HI+Vehicle and HI+IAIP) for the NST task. A repeated measures ANOVA was used to evaluate treatment across the 9 silent gap levels (2, 5, 10, 20, 30, 40, 50, 75, 100). A repeated measures ANOVA was used to compare treatment groups across the oddball levels (ISI, 275 ms and 225 ms). Tukey’s post hoc analysis was used to assess simple effects at each oddball ISI when main effects of treatment were observed.
Results
Brain Analysis
Results for analysis of brain weight using a one-way ANOVA, showed a significant effect of treatment [F(2, 31) = 9.34, p<0.01]. Post hoc analyses using Tukey’s HSD test revealed a significant decrease in brain weight for HI+vehicle subjects as compared to shams (p=0.001), and a significant decrease in brain weight for HI+IAIPs subjects as compared to shams (p=0.028). However, the relative degree of reduced brain weight, as compared to shams, was greater for HI+vehicle subjects (15.1% reduction) than for HI+IAIPs animals (10.6% reduction; see figure 2).
Normal Single Tone
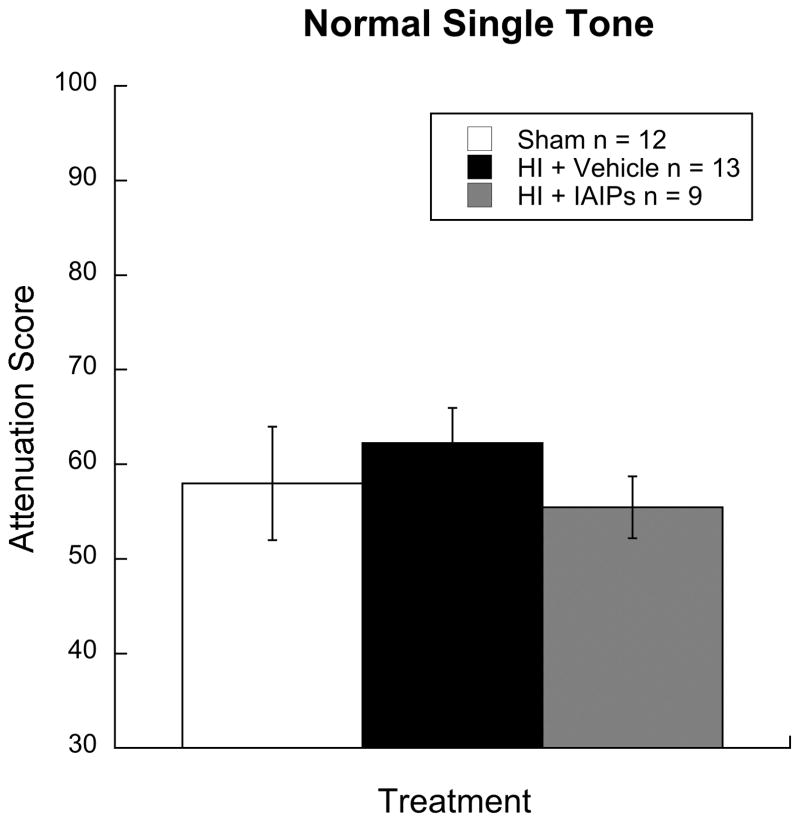
Significant differences were observed between cued and uncued peak response scores for all groups as shown by paired samples t-test (p< 0.05), indicating significant detection of the single tone across all groups. No difference in baseline startle amplitude was observed between the groups. Results for a one-way ANOVA showed no significant effect of treatment on the single tone task [F (2, 31) = 0.531, p = 0.59], indicating comparable hearing and pre-pulse inhibition function across all treatment conditions (see figure 3).
Figure 3.
Histogram showing attenuation performance (detection; Mean/SEM) on the single tone detection task indicating comparable pre-pulse inhibition and auditory acuity for all groups regardless of injury or treatment (Sham n=12; HI+Vehicle n=13; HI+IAIPs n=9).
Silent Gap
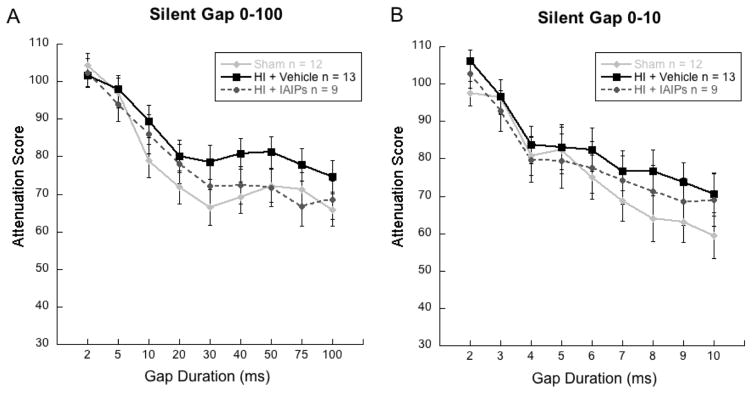
Results for a Treatment (3, Levels) by Day (2 levels) by Gap (9 Levels) repeated measures analysis of variance (ANOVA) for the long duration silent gap task (0–100 ms) revealed effects of Day [F (1) = 37.41, p < 0.001] indicating improved detection from day one to day two of the task. An effect of gap [F (8) = 80.58, p < 0.001] was also observed, indicated as gap duration (from 2–100 ms) increased, detection by all groups improved (see figure 4a). No effect of Treatment [F (2, 31) = 1.06, p is ns] was observed indicating that all treatment groups performed similarly. Results for a Treatment (3 Levels) by Gap (9 Levels) repeated measures ANOVA for the short duration silent gap task (0–10 ms) revealed an effect of Gap [F (8) = 61.66, p < 0.001], indicating that as gap duration increased overall detection improved. As with the long duration gap task, no effect of Treatment [F (2, 31) = .517, p is ns], was observed for the short silent gap task (0–10 ms), indicating that all treatment groups performed similarly on both long and short duration gap detection tasks (see figure 4b).
Figure 4.
Line graphs showing attenuation scores (detection; Mean/SEM) on moderately temporally demanding (a) long-duration (0–100ms) and (b) short-duration (0–10ms) silent gap detection tasks for all treatment groups, indicating comparable gap discrimination regardless of injury or treatment (Sham n=12; HI+Vehicle n=13; HI+IAIPs n=9).
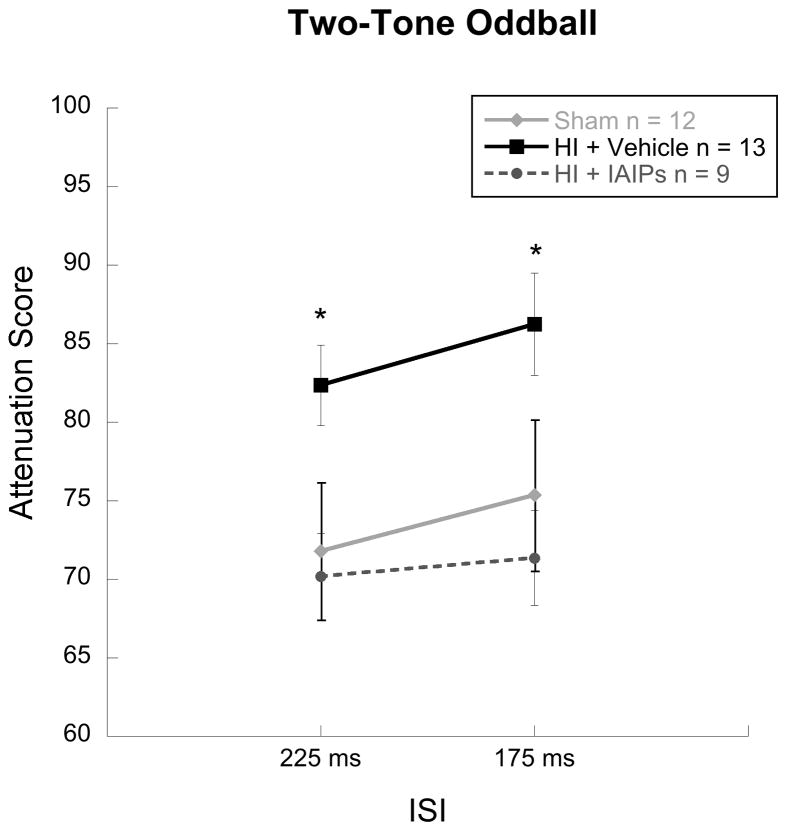
Two-Tone Oddball
Results for a Treatment (3 Levels) by Inter-Stimulus Interval (2 levels, ISI 275 ms and 175 ms) repeated measures ANOVA revealed a significant effect of Treatment [F (2, 31) = 5.89, p = 0.007] Power for this analysis was 0.842, reflecting adequate treatment group representation. Tukey’s post hoc analyses showed significant differences between sham and HI+vehicle subjects (p = 0.031) and between HI+IAIP and HI+vehicle (p = 0.011) subjects, indicating that both sham and IAIP treated hypoxic-ischemic rats had significantly better oddball tone pair detection (lower ATT scores) as compared to untreated HI injured animals across both 175 and 225 ms inter-stimulus intervals (see figure 4).
Discussion
Striking parallels have been observed between HI injury in humans and animal models (Rice, Vannucci et al. 1981, McClure, Threlkeld et al. 2006, Volpe 2009). These similarities extend to the disruption of multiple behavioral and neurosensory domains including the regulation of complex auditory processes necessary for the proper development of human language (Tallal 2004, Benasich, Choudhury et al. 2006). Results from the present study show that treated (IAIPs) and untreated HI injured rats maintain intact pre-pulse inhibition as compared to sham subjects as evidenced by comparable attenuation performance on the normal single tone detection task. These findings reflect those of other studies showing intact startle reactivity and basic hearing function in rats that have been exposed to HI brain injury as neonates (McClure, Peiffer et al. 2005, McClure, Threlkeld et al. 2006, Smith, Garbus et al. 2015). Likewise, regardless of treatment, HI injured subjects were able to discriminate both short (0–10 ms) and long (0–100 ms) duration silent gaps embedded in white noise at levels comparable to sham subjects. These findings indicate that basic auditory temporal discrimination was intact in all injury conditions regardless of treatment. In contrast, HI injured rats showed significant processing impairments on the complex two-tone oddball discrimination task, at both 275 and 175ms inter-stimulus interval durations, as compared to IAIPs treated HI animals and sham subjects. In fact, IAIPs treated subjects showed comparable performance to shams (no impairment). These findings indicate that IAIPs ameliorate complex tone-order discrimination deficits in rats with neonatal HI brain injury when tested as adults (postnatal day (P) 158–63).
Brain weight data presented here parallel the variable pattern of tissue sparing reported previously for the present IAIPs treated HI subjects, in that hippocampal and cortical volumes were not significantly reduced as compared to the same structures in the sham animals (Threlkeld, Gaudet et al. 2014). However, significant reductions in corpus callosum and striatal volumes were observed in the present IAIPs treated subjects in comparison to shams. In contrast, significant reductions in all four measured structures (hippocampus, cortex, corpus callosum and striatum) were seen in HI+vehicle subjects as compared to shams (see Threlkeld et al, 2014 for details on these measures). Therefore, the present brain weight data complements the variable pattern of brain tissue sparing previously reported for the current HI+IAIPs treated subjects. Further, despite the severe (120 min.) hypoxic exposure, and variable pattern of tissue sparing, IAIPs treatment appears to mitigate higher order complex auditory processes as compared to untreated HI subjects. It appears that global measures of brain integrity, such as brain weight, may be poor predictors of treatment outcome, requiring more sensitive domain specific tasks and/or measures to reveal improvements after experimental treatment.
It is important to point out that studies have repeatedly shown the predictive relationship between underlying sensory processing deficits and language performance in humans with neurodevelopmental disorders (Benasich and Tallal 2002, Benasich, Choudhury et al. 2006, Ortiz-Mantilla, Choudhury et al. 2008, Cantiani, Riva et al. 2016). Furthermore, both human and rodent studies have shown associations between complex auditory discrimination and neocortical processes that are highly vulnerable to brain injury during development (Downie, Jakobson et al. 2002, Bishop DV 2004, Benasich, Choudhury et al. 2006, Threlkeld, Penley et al. 2008, White-Schwoch, Woodruff Carr et al. 2015). In addition, studies in rodents have shown that as auditory temporal and spectral complexity increase, processes required for cue discrimination depend increasingly on complex cortical circuits (Bowen GP 2003, Threlkeld, Penley et al. 2008). Therefore, the two-tone oddball task employed in this study most likely reflects a sensitive measure of cortical circuity profoundly impacted by neonatal brain injury, which appears to be rescued by treatment of subjects with IAIPs after exposure of the neonates to hypoxic-ischemic conditions (Threlkeld, Penley et al., 2008). Future studies will investigate the extent of cellular sparing within the thalamo-cortical auditory circuit, which may underlie the processing improvements observed in IAIPs treated HI subjects (Rosen et al., 2006).
Recently, we have shown improvements in cognitive and behavioral performance after IAIPs treatment of neonatal rats exposed to HI brain injury (Threlkeld, Gaudet et al. 2014, Gaudet, Lim et al. 2016). Our group has shown that spatial and non-spatial learning performance in IAIPs treated and untreated HI injured rats is dependent on the age of testing (Threlkeld, Gaudet et al. 2014). Specifically, rats exposed to HI injury and IAIP treatment as neonates, showed impaired performance on the spatial Morris water maze task when tested as juveniles (P38). However, deficits were no longer observed when retested in adulthood (P80+). Whereas, untreated HI injured rats showed persistent impairments into adulthood. This finding suggests that treatment with IAIPs in neonates can have long-term beneficial effects well beyond the neonatal period when combined with an early training/experience paradigm. In contrast, sparing of non-spatial learning was observed for IAIPs treated HI rats in the juvenile period that was maintained into adulthood. In comparison, untreated HI injured rats showed juvenile deficits that did not persist when reassessed as adults (Threlkeld, Gaudet et al. 2014). More recently, we showed that improvements in high demand working memory performance require early training in combination with IAIPs treatment in order to produce improved outcomes for adult rats with neonatal HI injury (Gaudet, Lim et al. 2016). It is likely that presentation of the currently reported auditory tasks at an earlier age in HI+vehicle and IAIP treated subjects would result in a shifted pattern of acuity and impairment to the more basic gap detection tasks. Age dependent auditory temporal acuity has been reported extensively by our group and others in rodent models of neonatal brain injury (Friedman, Peiffer et al. 2004, Threlkeld, McClure et al. 2006). Future studies will investigate the effects of IAIPs treatment after HI injury across discrete developmental windows to determine if age of assessment interacts with treatment as seen in other cognitive domains (Threlkeld, Gaudet et al. 2014, Gaudet, Lim et al. 2016).
The above mentioned cognitive studies utilized the same dosing regimen as in the present experiments, emphasizing that HI injury and IAIPs treatment produce differential patterns of domain specific deficits, recovery and brain regional sparing. Thus, as previously reported, improved behavioral performance in HI injured animals is likely to interact with, the age of assessment, treatment dose, injury and test timing and the cognitive/sensory domain that is examined (Threlkeld, McClure et al. 2006, Threlkeld, Hill et al. 2012, Threlkeld, Gaudet et al. 2014, Gaudet, Lim et al. 2016). It is important to emphasize that these interacting factors have been reported in many other rodent models of neurodevelopmental disorders including those with neocortical microgiria, heterotopia and knockdown of candidate genes specific to human learning impairments (Threlkeld, McClure et al. 2007, Threlkeld, Hill et al. 2009, Threlkeld, Hill et al. 2009). These complexities are further supported by the present results showing profound differences in tone-order discrimination for adult rats that were treated with IAIPs as neonates at the time of Hypoxia-ischemia exposure, as compared to untreated subjects. The current findings reflect the efficacy and potential therapeutic application of IAIPs in the auditory processing domain, and have important implications for human clinical application. Given that auditory temporal acuity is highly predictive of later language performance in intact and at risk infants and that limited treatment options are available for perinatal brain injury, the present findings provide further support for the potential use of IAIPs as a candidate treatment for developmental HI related brain injury (Benasich and Tallal 2002, Ortiz-Mantilla, Choudhury et al. 2008, Cantiani, Riva et al. 2016).
Figure 5.
Line graph of attenuation scores (detection; Mean/SEM) on the high demand, two-tone oddball task showing significantly impaired tone order discrimination for HI-vehicle subjects as compared to both sham and IAIPs treated HI animals (*p<0.05; Sham n=12; HI+Vehicle n=13; HI+IAIPs n=9).
Highlights.
Neonatal HI is linked to auditory processing deficits in humans and rodent models
Subjects with HI injury and those with HI and IAIP had reductions in brain weight
HI subjects regardless of treatment showed basic auditory acuity (gap detection)
IAIP prevented complex tone-order discrimination impairments in HI subjects
Acknowledgments
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under grants 1R15HD077544 and 1R01-HD-057100 and by the National Institute of Neurological Disorders and Stroke under grants 1R21 NS095130-01 and R21NS096525. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe H, Sugino N, Matsuda T, Kanamaru T, Oyanagi S, Mori H. Effect of ulinastatin on delayed neuronal death in the gerbil hippocampus. Masui. 1996;45(1):38–43. [PubMed] [Google Scholar]
- Baek YW, Brokat S, Padbury JF, Pinar H, Hixson DC, Lim YP. Inter-alpha inhibitor proteins in infants and decreased levels in neonatal sepsis. J Pediatr. 2003;143(1):11–15. doi: 10.1016/S0022-3476(03)00190-2. [DOI] [PubMed] [Google Scholar]
- Barde LH, Yeatman JD, Lee ES, Glover G, Feldman HM. Differences in neural activation between preterm and full term born adolescents on a sentence comprehension task: implications for educational accommodations. Dev Cogn Neurosci. 2012;2(Suppl 1):S114–128. doi: 10.1016/j.dcn.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker KJ. Inflammation and acute stroke. Curr Opin Neurol. 1998;11(1):45–49. doi: 10.1097/00019052-199802000-00008. [DOI] [PubMed] [Google Scholar]
- Benasich AA, Choudhury N, Friedman JT, Realpe-Bonilla T, Chojnowska C, Gou Z. The infant as a prelinguistic model for language learning impairments: predicting from event-related potentials to behavior. Neuropsychologia. 2006;44(3):396–411. doi: 10.1016/j.neuropsychologia.2005.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benasich AA, Tallal P. Infant discrimination of rapid auditory cues predicts later language impairment. Behav Brain Res. 2002;136(1):31–49. doi: 10.1016/s0166-4328(02)00098-0. [DOI] [PubMed] [Google Scholar]
- Bishop DV, MG Immature cortical responses to auditory stimuli in specific langauge imapirment: evidence from ERPs to rapid tone sequences. Dev Sci. 2004;7:F11–F18. doi: 10.1111/j.1467-7687.2004.00356.x. [DOI] [PubMed] [Google Scholar]
- Bowen GP, LD, Taylor MK, Ison JR. Auditory cortex lesions in the rat impair both temporal acuity and noise increment thresholds, revealing a common neural substrate. Cerebral Cortex. 2003;13(8):815–822. doi: 10.1093/cercor/13.8.815. [DOI] [PubMed] [Google Scholar]
- Cantiani C, Riva V, Piazza C, Bettoni R, Molteni M, Choudhury N, Marino C, Benasich AA. Auditory discrimination predicts linguistic outcome in Italian infants with and without familial risk for language learning impairment. Dev Cogn Neurosci. 2016;20:23–34. doi: 10.1016/j.dcn.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaaban H, Singh K, Huang J, Siryaporn E, Lim YP, Padbury JF. The role of inter-alpha inhibitor proteins in the diagnosis of neonatal sepsis. J Pediatr. 2009;154(4):620–622. e621. doi: 10.1016/j.jpeds.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Chen X, Rivard L, Naqvi S, Nakada S, Padbury JF, Sanchez-Esteban J, Stopa EG, Lim YP, Stonestreet BS. Expression and localization of Inter-alpha Inhibitors in rodent brain. Neuroscience. 2016;324:69–81. doi: 10.1016/j.neuroscience.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dammann O, Leviton A. Maternal intrauterine infection, cytokines, and brain damage in the preterm newborn. Pediatr Res. 1997;42(1):1–8. doi: 10.1203/00006450-199707000-00001. [DOI] [PubMed] [Google Scholar]
- Downie AL, Jakobson LS, Frisk V, Ushycky I. Auditory temporal processing deficits in children with periventricular brain injury. Brain Lang. 2002;80(2):208–225. doi: 10.1006/brln.2001.2594. [DOI] [PubMed] [Google Scholar]
- Ferriero DM. Neonatal brain injury. N Engl J Med. 2004;351(19):1985–1995. doi: 10.1056/NEJMra041996. [DOI] [PubMed] [Google Scholar]
- Fitch RH, Threlkeld SW, McClure MM, Peiffer AM. Use of a modified prepulse inhibition paradigm to assess complex auditory discrimination in rodents. Brain Res Bull. 2008;76(1–2):1–7. doi: 10.1016/j.brainresbull.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier P, Hebert S. The gap-startle paradigm to assess auditory temporal processing: Bridging animal and human research. Psychophysiology. 2016;53(5):759–766. doi: 10.1111/psyp.12620. [DOI] [PubMed] [Google Scholar]
- Friedman JT, Peiffer AM, Clark MG, Benasich AA, Fitch RH. Age and experience-related improvements in gap detection in the rat. Brain Res Dev Brain Res. 2004;152(2):83–91. doi: 10.1016/j.devbrainres.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Fries E, Blom AM. Bikunin--not just a plasma proteinase inhibitor. Int J Biochem Cell Biol. 2000;32(2):125–137. doi: 10.1016/s1357-2725(99)00125-9. [DOI] [PubMed] [Google Scholar]
- Gaudet CM, Lim YP, Stonestreet BS, Threlkeld SW. Effects of age, experience and inter-alpha inhibitor proteins on working memory and neuronal plasticity after neonatal hypoxia-ischemia. Behav Brain Res. 2016;302(302):88–99. doi: 10.1016/j.bbr.2016.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, Gunn AJ, Wyatt JS. Hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2006;354(15):1643–1645. author reply 1643–1645. [PubMed] [Google Scholar]
- Gunn AJ, Gunn TR, de Haan HH, Williams CE, Gluckman PD. Dramatic neuronal rescue with prolonged selective head cooling after ischemia in fetal lambs. J Clin Invest. 1997;99(2):248–256. doi: 10.1172/JCI119153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CA, Threlkeld SW, Fitch RH. Early testosterone modulated sex differences in behavioral outcome following neonatal hypoxia ischemia in rats. Int J Dev Neurosci. 2011;29(4):381–388. doi: 10.1016/j.ijdevneu.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17(7):796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liederman JKL, Flannery K. Male vulnerability to reading disability is not likely to be a myth: a call for new data. Journal of learning disabilities. 2005;38(2):652–662. doi: 10.1177/00222194050380020201. [DOI] [PubMed] [Google Scholar]
- Lim YP, Bendelja K, Opal SM, Siryaporn E, Hixson DC, Palardy JE. Correlation between mortality and the levels of inter-alpha inhibitors in the plasma of patients with severe sepsis. J Infect Dis. 2003;188(6):919–926. doi: 10.1086/377642. Epub 2003 Aug 2026. [DOI] [PubMed] [Google Scholar]
- McClure MM, Peiffer AM, Rosen GD, Fitch RH. Auditory processing deficits in rats with neonatal hypoxic-ischemic injury. Int J Dev Neurosci. 2005;23(4):351–362. doi: 10.1016/j.ijdevneu.2004.12.008. [DOI] [PubMed] [Google Scholar]
- McClure MM, Threlkeld SW, Fitch RH. The effects of erythropoietin on auditory processing following neonatal hypoxic-ischemic injury. Brain Res. 2006;1087(1):190–195. doi: 10.1016/j.brainres.2006.03.016. [DOI] [PubMed] [Google Scholar]
- McClure MM, Threlkeld SW, Fitch RH. Auditory processing and learning/memory following erythropoietin administration in neonatally hypoxic-ischemic injured rats. Brain Res. 2007;1132(1):203–209. doi: 10.1016/j.brainres.2006.11.006. [DOI] [PubMed] [Google Scholar]
- McClure MM, Threlkeld SW, Rosen GD, Holly Fitch R. Rapid auditory processing and learning deficits in rats with P1 versus P7 neonatal hypoxic-ischemic injury. Behav Brain Res. 2006;172(1):114–121. doi: 10.1016/j.bbr.2006.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opal SM, Lim YP, Cristofaro P, Artenstein AW, Kessimian N, Delsesto D, Parejo N, Palardy JE, Siryaporn E. Inter-alpha inhibitor proteins: a novel therapeutic strategy for experimental anthrax infection. Shock. 2011;35(1):42–44. doi: 10.1097/SHK.0b013e3181e83204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Mantilla S, Choudhury N, Leevers H, Benasich AA. Understanding language and cognitive deficits in very low birth weight children. Dev Psychobiol. 2008;50(2):107–126. doi: 10.1002/dev.20278. [DOI] [PubMed] [Google Scholar]
- Peiffer AM, Rosen GD, Fitch RH. Sex differences in rapid auditory processing deficits in ectopic BXSB/MpJ mice. Neuroreport. 2002;13(17):2277–2280. doi: 10.1097/00001756-200212030-00021. [DOI] [PubMed] [Google Scholar]
- Peiffer AM, Rosen GD, Fitch RH. Sex differences in rapid auditory processing deficits in microgyric rats. Brain Res Dev Brain Res. 2004;148(1):53–57. doi: 10.1016/j.devbrainres.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Raz SL, MD, Hopkins TL, Glogowski BK, Porter CL, Riggs WW, Sander CJ. A female advantage in cognitive recovery from early cerebral insult. Dev Psychobiol. 1995;31(6):958–966. [Google Scholar]
- Rice JE, 3rd, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol. 1981;9(2):131–141. doi: 10.1002/ana.410090206. [DOI] [PubMed] [Google Scholar]
- Rosen GD, Mesples B, Hendrik M, Galaburda AM. Histometric changes and cell death in the thalamus aftter neonatal neocorteical injury in the rat. Neuroscience. 2006;141(2):875–88. doi: 10.1016/j.neuroscience.2006.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter MCA, Moffitt TE. Using sex differences in psychopathology to study causal mechanisms: unifying issues and research strategies. Journal of child psychology and psychiatry. 2003;44(8):109–129. doi: 10.1111/1469-7610.00194. [DOI] [PubMed] [Google Scholar]
- Shah DK, Doyle LW, Anderson PJ, Bear M, Daley AJ, Hunt RW, Inder TE. Adverse neurodevelopment in preterm infants with postnatal sepsis or necrotizing enterocolitis is mediated by white matter abnormalities on magnetic resonance imaging at term. J Pediatr. 2008;153(2):170–175. 175 e171. doi: 10.1016/j.jpeds.2008.02.033. [DOI] [PubMed] [Google Scholar]
- Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, Fanaroff AA, Poole WK, Wright LL, Higgins RD, Finer NN, Carlo WA, Duara S, Oh W, Cotten CM, Stevenson DK, Stoll BJ, Lemons JA, Guillet R, Jobe AH. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353(15):1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- Singh K, Zhang LX, Bendelja K, Heath R, Murphy S, Sharma S, Padbury JF, Lim YP. Inter-alpha inhibitor protein administration improves survival from neonatal sepsis in mice. Pediatr Res. 2010;68(3):242–247. doi: 10.1203/PDR.0b013e3181e9fdf0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AL, Garbus H, Rosenkrantz TS, Fitch RH. Sex differences in behavioral outcomes following temperature modulation during induced neonatal hypoxic ischemic injury in rats. Brain Sci. 2015;5(2):220–240. doi: 10.3390/brainsci5020220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll BJ, Hansen NI, Adams-Chapman I, Fanaroff AA, Hintz SR, Vohr B, Higgins RD. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. Jama. 2004;292(19):2357–2365. doi: 10.1001/jama.292.19.2357. [DOI] [PubMed] [Google Scholar]
- Suppiej A, Cainelli E, Cappellari A, Ermani M, Sartori S, Bisiacchi PS. Neonatal Cortical Auditory Evoked Potentials Are Affected by Clinical Conditions Occurring in Early Prematurity. J Clin Neurophysiol. 2015;32(5):419–423. doi: 10.1097/WNP.0000000000000182. [DOI] [PubMed] [Google Scholar]
- Tallal P. Improving language and literacy is a matter of time. Nat Rev Neurosci. 2004;5(9):721–728. doi: 10.1038/nrn1499. [DOI] [PubMed] [Google Scholar]
- Threlkeld SW, Gaudet CM, La Rue ME, Dugas E, Hill CA, Lim YP, Stonestreet BS. Effects of inter-alpha inhibitor proteins on neonatal brain injury: Age, task and treatment dependent neurobehavioral outcomes. Exp Neurol. 2014;261C:424–433. doi: 10.1016/j.expneurol.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlkeld SW, Gaudet CM, La Rue ME, Dugas E, Hill CA, Lim YP, Stonestreet BS. Effects of inter-alpha inhibitor proteins on neonatal brain injury: Age, task and treatment dependent neurobehavioral outcomes. Exp Neurol. 2014;261:424–433. doi: 10.1016/j.expneurol.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlkeld SW, Hill CA, Cleary CE, Truong DT, Rosen GD, Fitch RH. Developmental learning impairments in a rodent model of nodular heterotopia. J Neurodev Disord. 2009;1(3):237–250. doi: 10.1007/s11689-009-9026-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlkeld SW, Hill CA, Rosen GD, Fitch RH. Early acoustic discrimination experience ameliorates auditory processing deficits in male rats with cortical developmental disruption. Int J Dev Neurosci. 2009;27(4):321–328. doi: 10.1016/j.ijdevneu.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlkeld SW, Hill CA, Szalkowski CE, Truong DT, Rosen GD, Fitch RH. Effects of test experience and neocortical microgyria on spatial and non-spatial learning in rats. Behav Brain Res. 2012;235(2):130–135. doi: 10.1016/j.bbr.2012.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlkeld SW, McClure MM, Bai J, Wang Y, LoTurco JJ, Rosen GD, Fitch RH. Developmental disruptions and behavioral impairments in rats following in utero RNAi of Dyx1c1. Brain Res Bull. 2007;71(5):508–514. doi: 10.1016/j.brainresbull.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlkeld SW, McClure MM, Rosen GD, Fitch RH. Developmental timeframes for induction of microgyria and rapid auditory processing deficits in the rat. Brain Res. 2006;1109(1):22–31. doi: 10.1016/j.brainres.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Threlkeld SW, Penley SC, Rosen GD, Fitch RH. Detection of silent gaps in white noise following cortical deactivation in rats. Neuroreport. 2008;19(8):893–898. doi: 10.1097/WNR.0b013e3283013d7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vohr B. Speech and language outcomes of very preterm infants. Semin Fetal Neonatal Med. 2014;19(2):78–83. doi: 10.1016/j.siny.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Vohr BR. Language and hearing outcomes of preterm infants. Semin Perinatol. 2016 doi: 10.1053/j.semperi.2016.09.003. [DOI] [PubMed] [Google Scholar]
- Volpe JJ. The encephalopathy of prematurity--brain injury and impaired brain development inextricably intertwined. Semin Pediatr Neurol. 2009;16(4):167–178. doi: 10.1016/j.spen.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakahara K, Kobayashi H, Yagyu T, Matsuzaki H, Kondo T, Kurita N, Sekino H, Inagaki K, Suzuki M, Kanayama N, Terao T. Bikunin suppresses lipopolysaccharide-induced lethality through down-regulation of tumor necrosis factor- alpha and interleukin-1 beta in macrophages. J Infect Dis. 2005;191(6):930–938. doi: 10.1086/428134. [DOI] [PubMed] [Google Scholar]
- White-Schwoch T, Woodruff Carr K, Thompson EC, Anderson S, Nicol T, Bradlow AR, Zecker SG, Kraus N. Auditory Processing in Noise: A Preschool Biomarker for Literacy. PLoS Biol. 2015;13(7):e1002196. doi: 10.1371/journal.pbio.1002196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong T, Qu Y, Mu D, Ferriero D. Erythropoietin for neonatal brain injury: opportunity and challenge. Int J Dev Neurosci. 2011;29(6):583–591. doi: 10.1016/j.ijdevneu.2010.12.007. [DOI] [PubMed] [Google Scholar]