Abstract
Objectives
Autoimmune hepatitis (AIH) is a common pediatric liver disease and long-term remission is usually maintained with azathioprine (AZA). There is no consensus on the target range for AZA active metabolite 6-thioguanine (6-TGN) levels in pediatric AIH. The aim of this study was to characterize the outcomes of pediatric patients with AIH and determine correlations between AZA dosing or 6-TGN metabolite levels and biochemical remission.
Methods
A retrospective chart review was performed and data on presentation, laboratories including AZA metabolite levels, medication use and outcomes was collected.
Results
Between 2002–2013, 66 children with AIH were identified (mean age at diagnosis 9.6 ± 5.1 yrs.) with a mean follow-up period of 2.9 ± 3.2 yrs. Common presenting symptoms included jaundice, fatigue and abdominal pain. The majority of subjects received steroids for induction, and AZA for maintenance of remission. 79% achieved biochemical remission (mean time to remission 6.2 ± 9.2 mons.), 14% were in the induction phase of therapy, 6% required liver transplantation and 18% were weaned off of immunosuppression and remained in remission. 6-TGN levels ranging from 50–250 pmol/8×108 RBC were associated with biochemical remission (ALT levels of ≤ 50 U/L).
Conclusions
The vast majority of children with AIH maintain a sustained remission with AZA monotherapy. Biochemical remission was maintained with 6-TGN levels much lower than that recommended for inflammatory bowel disease. These findings suggest that patients should be maintained at the lowest AZA dose possible that is associated with biochemical remission.
Keywords: children, chronic hepatitis, autoimmune liver disease, 6-thioguanine
Introduction
The incidence of pediatric autoimmune hepatitis (AIH) is estimated to be bewteen 0.1 to 0.23 per 100,000 children.1,2 Autoimmune hepatitis is a common cause of liver disease in children, accounting for 12% of referrals to a liver clinic at a tertiary care center.3 Azathioprine (AZA), a corticosteroid-sparing agent, is commonly used for long-term disease control. Thiopurine methyltransferase (TPMT) metabolizes AZA into 6-thioguanine (6-TGN) and 6-mercaptopurine (6-MMP). 6-TGN levels correlate with drug activity and hematopoetic toxicity, while 6-MMP levels correlate with hepatotoxicity. A 6-MMP level of greater than 5,700 pmol/8 × 108 RBC (hereafter abbreviated “pmol”) is considered hepatotoxic. The appropriate AZA dosing and 6-TGN metabolite levels to maintain remission and limit toxicity in AIH in children have not been well eluciated in the literature.4 In contrast, in inflammatory bowel disease (IBD), 6-TGN levels between 250 and 450 pmol are recommended to maintain remission.5,6 When managing IBD, gastroenterologists routinely adjust AZA doses to achieve these levels.
Azathioprine dosing recommendations for children with AIH vary widely, with published dose ranges of 0.5 to 5 mg/kg/day.7–10 Adult guidelines for the treatment of AIH recommend a starting AZA dose of 50 mg per day, and checking TPMT enzyme activity for doses greater than 150 mg per day.4,11 Two previous studies attempted to quantitate AZA dosing and metabolites for pediatric AIH, one of which targeted 6-TGN levels similar to IBD treatment7 and the other study which could not conclusively recommend a specific goal 6-TGN.12 In our clinical practice, however, we have found that AIH remission can be maintained with modest AZA dosing and associated 6-TGN levels far below the values recommended to treat IBD. Based on the lack of a clear consensus on AZA dosing in children with AIH, we aimed to determine the correlation between AZA dosing or 6-TGN metabolite levels and biochemical remission in pediatric patients with AIH. We hypothesized that biochemical remission can be maintained in the majority of children with AIH with 6-TGN levels lower than 250 pmol, well below the level required in IBD. A second aim of this study was to describe the outcomes in pediatric AIH in our patient cohort at a non-tertiary referral institution.
Methods
A retrospective single-center chart review was performed with Colorado Institutional Review Board approval (IRB #13-2262). Records of AIH patients seen at Children’s Hospital Colorado Pediatric Liver Center from January 1, 2002 until September 1, 2013 were included. Identification of children with AIH occurred through a review of pathology records to identify subjects with a liver biopsy consistent with AIH and a review of an internal quality improvement database of AIH patients. Subjects with primary sclerosing cholangitis (PSC; 26 patients), IBD (12 patients), both PSC and IBD (10 patients), severe immunodeficiency (4 patients), post-transplant de novo or recurrent AIH, or other autoimmune diseases that required immunosuppression (6 patients) were excluded. Chart review was performed by a single reviewer and data was recorded in the REDCap database.
Demographic and clinical data from the time of presentation were collected, including age, sex, race, ethnicity, weight, presenting symptoms, physical exam (hepatomegaly, splenomegaly, ascites), laboratories [total protein, albumin, total bilirubin, direct bilirubin, alkaline phosphatase, aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyl transferase (GGT), lipase, prothrombin time (PT), international normalized ratio (INR), white blood cell count (WBC), hemoglobin (Hgb), hematocrit (Hct), platelet count, anti-nuclear antibody (ANA), liver kidney microsomal type 1 antibody (LKM-1), anti-smooth muscle antibody (ASMA), soluble liver antigen antibody (SLA), immunoglobulin G (IgG)], TPMT activity, ultrasound findings, and liver biopsy results. Data from each follow up visit at Children’s Hospital Colorado Liver Center was recorded, including weight, medications and dosing, compliance, symptoms, physical exam, laboratories (all labs described above as well as 6-TGN, and 6-MMP levels), ultrasound, and repeat liver biopsies.
For this study we defined biochemical remission as an ALT level of ≤ 50 U/L. Active hepatitis (including a “flare”) was defined as ALT > 50 U/L. Histologic remission was considered as ≤ grade 1 inflammation (interface hepatitis) and no fibrosis. Subjects were classified as having portal hypertension if they had presence of one or more of the following: splenomegaly on physical exam or ultrasound, portal venous hepatofugal flow, presence of varices on imaging or endoscopy, or platelet count of less than 100,000/uL. Positive autoantibody testing was defined as an ANA titer ≥ 1:40, an ASMA titer of ≥ 1:20 or a value ≥ 20 units and an LKM-1 value of ≥ 20 units. Type 1 AIH was defined as a positive ANA and/or ASMA titer and Type 2 AIH as a positive LKM. Non-compliance was classified as missing one or more doses of their medications per week, or a physician stating that the patient was noncompliant in the medical record.
Data is shown as the mean ± standard deviation. Comparison between groups (medication compliant vs. noncompliant; relapse vs. non-relapse) was performed using student t-test of unpaired samples. Kaplan-Meier plot was utilized to analyze time to biochemical remission and steroid cessation. Correlation analysis (blood chemistries vs. AZA dose or 6-TGN) was performed with Pearson correlation coefficient (GraphPad Prism6) and p values <0.05 were considered significant.
Results
Assessment of clinical presentation and treatment
Sixty-six subjects with AIH and without IBD or PSC were identified. Demographic and clinical data, including signs, symptoms and laboratory values at presentation are shown in Table 1. Twenty four percent of subjects met criteria for having portal hypertension and 11% presented with an INR > 2, meeting criteria for liver failure. Co-morbid conditions at the time of diagnosis of AIH are shown in Table 1 and included 2 patients that were 10 months and 18 months status post bone marrow transplant (BMT), respectively. Both patients were fully engrafted at the time of inclusion in the study and were off all other immunosuppression related to the previous BMT. Six percent of subjects had been on medications known to trigger AIH (minocycline, doxycycline, or tetracycline).13
Table 1.
Patient Characteristics
| Demographics (N=66; Age 9.6 ± 5.1 years) | Percentage |
|---|---|
| Female | 65 |
| Caucasian | 86 |
| Type 1 AIH (ANA and/or ASMA) | 66 |
| Type 2 AIH (LKM) | 17 |
| Seronegative AIH | 17 |
| Co-morbid conditions: Thyroid disease | 6 |
| *Other disease | 12 |
| Symptoms and Signs at Presentation (N=59) | Percentage |
| Jaundice | 66 |
| Fatigue | 54 |
| Abdominal pain | 39 |
| Abdominal distention | 15 |
| Fevers | 12 |
| Pruritus | 10 |
| Weight loss/gain | 10 |
| Asymptomatic | 10 |
| Hepatomegaly | 41 |
| Splenomegaly | 24 |
| Jaundice | 30 |
| Ascites | 6 |
| Normal exam | 39 |
| Laboratory Values at Presentation (N=62) |
Mean ± S.D. (Normal Range) |
| Total protein (g/dL) | 7.8 ± 1.3 (5–8) |
| Albumin (g/dL) | 3.5 ± 0.8 (3.5–5.5) |
| Total bilirubin (mg/dL) | 5.6 ± 6.2 (0.2–1.2) |
| Direct bilirubin (mg/dL) | 3.7 ± 4.8 (0–0.3) |
| Alkaline phosphatase (U/L) | 399 ± 365 (45–150) |
| AST (U/L) | 1071 ± 1120 (10–50) |
| ALT (U/L) | 1024 ± 989 (10–50) |
| GGT (U/L) | 123 ± 101 (5–25) |
| INR | 1.37 ± 0.44 (0.9–1.1) |
| Platelets (count × 103/μl) | 251 ± 97 (150–500) |
| Total IgG (mg/dL) (N=42) | 2385 ± 1593 (400–1600) |
1 each: status post bone marrow transplant for leukemia or sickle cell anemia, autoimmune hemolytic anemia, food-protein induced enterocolitis syndrome, spina bifida, protein C deficiency, recurrent pancreatitis, vitiligo.
Autoantibody test results at presentation are shown in Table 1. and Supplemental Figure 1, revealing that the majority of subjects had Type 1 AIH (51% both ANA and ASMA positive, 35% only ANA positive, and 14% only ASMA positive). Of the 4 patients who developed drug-induced AIH, 75% had Type 1 AIH, and 25% had seronegative AIH. In addition, SLA testing was sent in 7 subjects and was negative for all. Review of liver histology at the time of diagnosis showed that 92% of subjects had interface hepatitis, 80% had increased number of plasma cells, 61% had hepatocyte ballooning or drop out, 83% had grade 1–3 fibrosis and 17% had grade 4 fibrosis (cirrhosis; METAVIR scoring system).
Within the first month of presentation 96% of subjects were started on methylprednisolone or prednisone. The 2 subjects who had received a BMT were started on budesonide instead of prednisone. TPMT enzyme activity was checked in 82% of subjects, the vast majority (94%) of whom had normal enzyme activity (> 21.0 EU). Only 5.5% of subjects had intermediate enzyme activity (6.0–21.0 EU) and no subject had low/absent activity. Azathioprine was used as maintenance monotherapy for 80%, AZA plus corticosteroids for 6% and corticosteroids only for 5% of subjects. In the setting of persistent elevation of liver tests while on AZA, the standard management was to increase the dosing of AZA up to 2.5 mg/kg/day. If liver tests remained elevated, then patients were switched to alternative therapies at the discretion of the hepatologist. Subjects unable to maintain biochemical remission with AZA were treated with either mycophenolate mofetil (6% of subjects) or tacrolimus (3% of subjects).
Liver transplantation was the primary treatment modality in 6% (4 subjects) with a mean age at transplant of 13.8 ± 2.8 years. The time from presentation of AIH to transplant was 25 ± 23 days. Three of these subjects had type 1 AIH and presented with acute on chronic liver failure. One subject had type 2 AIH and presented in acute liver failure.14 Three of these subjects were started on corticosteroids at presentation but did not respond to therapy.
Patient outcomes
Overall outcomes
Sixty-six subjects were followed for an average of 2.9 ± 3.2 years and patient outcomes are summarized in Figure 1.: 79% achieved biochemical remission, 6% required liver transplantation and 15% had active hepatitis at the end of the study period. For those subjects with active hepatitis at the close of the study period, the average follow-up time from diagnosis was only 66 ± 35 days, suggesting that these subjects were still in the induction phase of treatment. An additional subject with chronic active hepatitis was noncompliant with medications for the length of the study period. The average time to achieve biochemical remission was 6.2 ± 9.2 months. The rate of biochemical remission was 28% at 3 months, 60% at 6 months, 80% at 9 months, and 88% at 1 year (Figure 2). Despite achieving biochemical remission, 35% of subjects had at least one flare (ALT > 50 U/L) in AIH within the study period. Treatment of the flares varied from administration of a course of steroids (30% of subjects), an increase in AZA dosing (45%) or counseling to be compliant with medications, without a change in AZA dosing (25%). 6-TGN levels were checked sporadically at the time of a flare and ~50% of reported 6-TGN levels at the time of a flare were ≥200 pmol.
Figure 1. Patient Outcomes.
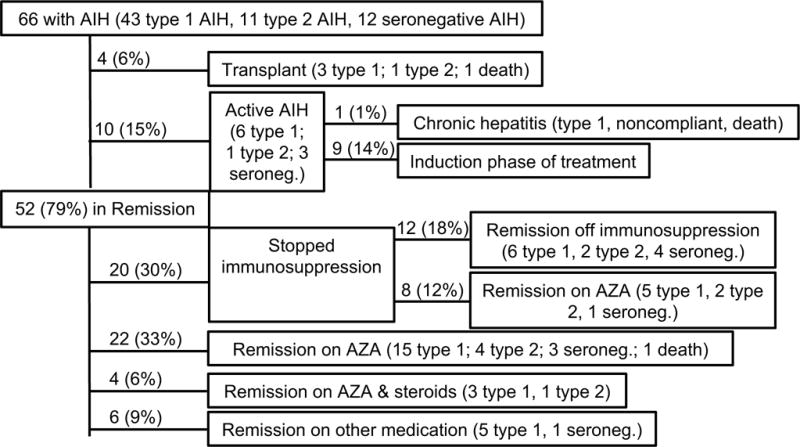
This diagram outlines the outcome of all 66 subjects in the cohort. Shown are the number of subjects in each category and the percent of total subjects.
Figure 2. Time to Biochemical Remission.
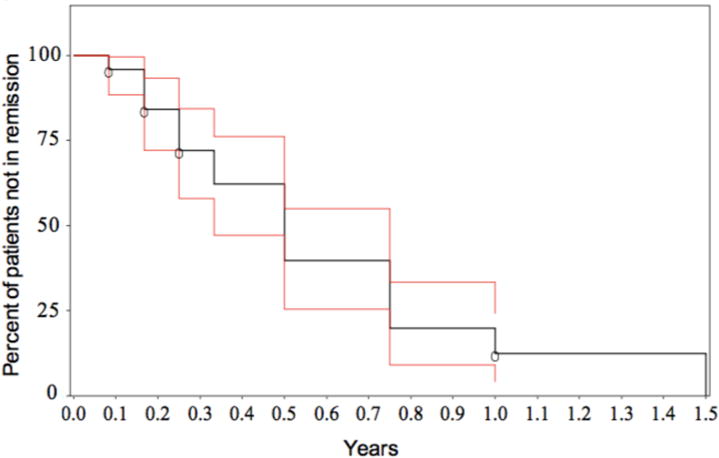
The percent of subjects who were not in remission following presentation with AIH is shown over time. Kaplan-Meier estimates of survival function is shown in black with the 95% confidence interval shown in red.
Previous literature suggests that patients with drug-induced AIH have a milder course and can often be effectively weaned off of immunosuppression. Outcomes of our cohort of 4 subjects with drug-induced AIH included remission off of immunosuppression in 3 subjects (2 type 1, 1 seronegative) and remission on prednisone in 1 subject (type 1) at the end of the study period. One subject who was successfully weaned off of immunosuppression died from a non-liver related cause.
Liver transplantation was performed in 4 subjects with acute liver failure. Three subjects are alive (median follow-up 5.2 years) and one died 5.7 years after transplantation due to medication noncompliance. Two additional deaths in the subject cohort consisted of acute on chronic liver failure due to medication noncompliance and a death unrelated to liver disease.
Medication outcomes
Corticosteroids
The average daily starting dose of corticosteroids was 1.42 mg/kg/day of prednisone (methylprednisolone was converted to prednisone equivalents for this calculation). The number of subjects on steroids over time is shown in Figure 3, with 40 subjects included for this calculation based on availability of complete data on steroid use over time. At 6 months, 61% of subjects remained on steroids; at 1 year, 25%, and at 2 years, 8% of subjects. Recurrent use of steroids occurred in 12% of subjects due to a flare (ALT > 50 U/L) in the AIH. Based on the medical record, complete data on exact steroid dosing over time was available for 29 subjects and the calculated cumulative steroid exposure was 5979 ± 3581 mg of prednisone or 191.8 ± 142.5 mg/kg (methylprednisolone was multiplied by 1.25 to equal the dose of prednisone.).
Figure 3. Time to Steroid Cessation.
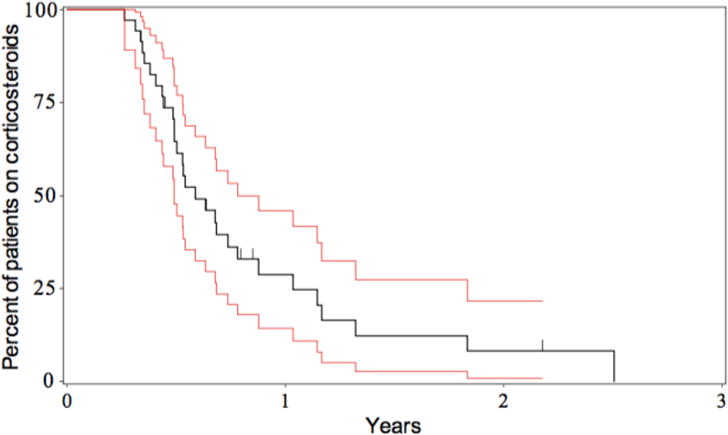
The percent of subjects remaining on steroids following presentation with AIH is shown over time. Kaplan-Meier estimates of survival function is shown in black with the 95% confidence interval shown in red.
Azathioprine
Eighty percent of subjects were on chronic AZA therapy for the duration of this study. There is no policy at the institution to target a specific AZA dose or 6-TGN level. The mean AZA dose at the start of therapy was 1.28 mg/kg/day and at end of follow-up was 1.67 ± 0.55 mg/kg/day. There was no correlation between AZA dose (Figure 4A.) or 6-TGN level (Figure 4B.) with either AST or ALT levels for all time points ≥ 6 months after AZA initiation. 6-TGN levels of 50–250 pmol (comprising 87% of all 6-TGN levels recorded) were associated with an 87% rate of biochemical remission. 6-TGN levels of ≥ 250 pmol (13% of all 6-TGN levels recorded) were associated with an incremental increase of 90% rate of biochemical remission (not statistically significant difference compared to 6-TGN levels < 250 pmol). In addition, AZA dose and 6-TGN level did not correlate with lipase or WBC levels and 6-MMP levels did not correlate with AST or ALT (data not shown). Only 3 subjects had 6-MMP levels that were considered in the hepatotoxic range (> 5,700 pmol) and all 3 had 6-TGN levels of > 250 pmol, with a median AZA dose of 2.8 mg/kg/day.
Figure 4. Lack of correlation of AZA dose or 6-TGN levels with AST and ALT levels.
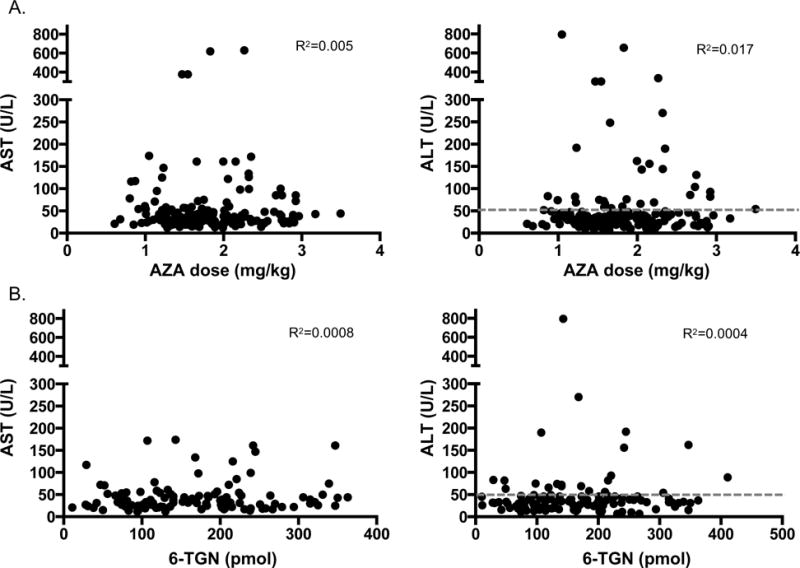
Shown here are correlation coefficient graphs between either AZA dose in mg/kg/day (A.) or 6-TGN levels in pmol/ 8 × 108 RBC (B.) and AST and ALT levels (U/L). No correlations between AZA dose or 6-TGN level and AST or ALT were found. Grey dotted line represents normal ALT level reflecting biochemical remission (≤ 50 U/L).
Medication compliance was tracked with every follow-up visit and 23% of subjects had at least one visit where non-compliance was documented. Compliance greatly affected both ALT and 6-TGN levels after 6 months of follow-up. Noncompliant subjects had a mean ALT of 131 ± 223 IU/L and compliant subjects had a mean ALT of 50 ± 93 (p=0.0003). 6-TGN levels were lower in noncompliant subjects (116 ± 108 pmol) compared to compliant subjects (170 ± 85 pmol; p=0.003).
Side effects of AZA were rare in this population. One subject had pancreatitis during the study time, but this was attributed to their pancreatic divisum. Five subjects (7.5% of total) had leukopenia while on AZA. Two subjects had serious bacterial illnesses while on AZA and corticosteroids: one had pneumocystis carini pneumonia and another had cellulitis that required hospitalization. No subjects developed lymphoma during the study period.
Histologic remission outcomes
It is our clinical practice to perform a repeat liver biopsy on all AIH patients in sustained biochemical remission after 2 years of maintenance therapy, in order to determine if histologic remission is present and if we can consider withdrawing immunosuppression. In this subject cohort, 20 follow-up liver biopsies were performed in 17 (27%) subjects with sustained biochemical remission for ≥ 2 years, in order to determine histologic remission (≤ grade 1 inflammation and no fibrosis). Based on biopsy results, 11 subjects (65% of the subjects biopsied), were in histologic remission and were weaned off of AZA. Seven of these 11 subjects (64%) remained off of AZA at the end of this study. Only 1 of the 7 subjects had a history of a mild flare in their AIH prior to stopping AZA. Of the 4 Type 2 AIH subjects that were weaned off of immunosuppression, 2 remained in remission at the last study follow-up (23 and 25 months respectively). A comparison of subjects who successfully weaned off AZA versus those who relapsed during weaning revealed that successfully weaned subjects had higher albumin levels (4.41 ± 0.27 mg/dL; relapsers: 3.90 ± 0.31 mg/dL; p=0.016) at initiation of weaning. There was no statistically significant differences in age at time of weaning, length of time with disease, AZA dose, total protein, total or direct bilirubin, AST, ALT, INR, GGT, alkaline phosphatase, or platelet count between groups.
In addition to the 11 subjects detailed above who were weaned off of AZA after confirmation of histologic remission, an additional 9 subjects (7 Type 1, 1 Type 2, 1 seronegative) had stopped AZA without being instructed to do so by the hepatologist. When accounting for all of the 20 subjects who were either instructed to stop AZA or stopped AZA on their own accord, 8 patients (40%) relapsed with an average time until relapse of 1.0 ± 1.4 years. The majority of subjects relapsed within the first two months after AZA discontinuation, but two subjects relapsed at 2.7 and 3.4 years after AZA cessation. Twelve subjects (60% of those weaned; 18% of total) were able to remain off of all medications at an average time of 2.8 ± 2.2 years from initial presentation. All subjects who successfully weaned off of AZA were instructed to do so by the hepatologist.
Discussion
This study revealed that children with AIH maintained high rates of sustained biochemical remission while on low-dose AZA. The vast majority (87%) of subjects had ALT levels of ≤ 50 U/L associated with low 6-TGN levels ranging from 50–250 pmol. Low-dose AZA has been previously shown to be effective in adults with AIH11, however this is the first study to document the efficacy of low-dose AZA and associated 6-TGN levels for AIH in children. Our study suggests that AZA dosing of approximately 1.2–1.6 mg/kg/day will achieve 6-TGN levels of 50–250 pmol, which is sufficient to maintain biochemical remission in the majority of patients.
Previous reports in the literature on AZA use in pediatric AIH suggest using higher AZA dosing to achieve 6-TGN levels similar to that necessary for IBD patients (6-TGN levels 250–450 pmol). Rumbo et al. assessed 25 pediatric AIH subjects without IBD who maintained biochemical remission (ALT ≤ 50 U/L) with a mean AZA dose of 1.83 mg/kg/day and 6-TGN level of 270 pmol at the end of the study.7 Our study, however, demonstrates similar outcomes in remission with lower AZA dosing and 6-TGN levels in a larger subject cohort. In addition, a study from France retrospectively analyzed 6-TGN levels and outcomes in 28 children with AIH.12 In their study, subjects in remission had similar 6-TGN levels (mean 6-TGN 436 pmol) as those with active disease (mean 6-TGN of 406 pmol). Furthermore, only 61% of subjects were in biochemical remission, suggesting that high levels of 6-TGN (IBD range) do not correlate with remission. Furthermore, leukopenia from AZA was observed in 21% of their cohort and correlated with 6-TGN levels, a rate much higher that the 7.5% observed in our cohort. As such, our study illustrates that remission can be maintained while minimzing drug toxicity with much lower AZA dosing and 6-TGN levels than previously reported.
In the setting of lower AZA dosing and 6-TGN levels, the outcomes described in our study are comparable to previous reports. Our overall biochemical remission rate of 79% is similar to the rates of 76–80% previously reported in the literature.15,16 Our relapse rate in 35% of subjects taken off AZA was similar to the 40% relapse rate previously reported.17 The presentation of acute liver failure (ALF) in AIH occurred in 11% of our subjects and of these, 57% required liver transplantation. These findings are consistent with recently published data showing that 56% of pediatric AIH patients with ALF required transplantation.18 Our overall rate of transplantation at 6.1% is similar to previously published rates of 9–10% of AIH pediatric patients requiring liver transplantation nationwide.18
This retrospective study has several limitations. First, certain data may have been missing for a given subject, impacting the overall results. Second, the frequency of liver transaminase and AZA metabolite assessment varied between hepatologists and this could have skewed the data toward the practice patterns of certain physicians. Third, a type 2 error in data analysis could have occurred whereby a relationship with AZA dose or 6-TGN level was present but not identified due to sample size. Fourth, targeting biochemical remission does not guarantee histologic remission, as shown by our liver biopsy data and supported by the AIH literature.15,19 Fifth, autoantibody testing relied on various clinical laboratories, and thus, different techniques utilized may have resulted in variability in the sensitivity of detection. Furthermore, the subjects who were seronegative did not consistently undergo further testing of ANCA or SLA, which could have increased the level of positivity of autoantibody detection.20 Sixth, MRCP was not routinely performed unless the subject was clinically suspected to have bile duct disease or if the liver tests remained elevated despite therapy. Thus, cases of autoimmune sclerosing cholangitis may have been missed. Finally, this paper focused on patients with only AIH and not with concurrent PSC or IBD, making our results difficult to extrapolate into these populations. Clearly, if the patient has concurrent IBD and the AZA is also being used to treat the IBD, then AZA dosing to achieve 6-TGN levels within the recommended IBD range should be sought.
In conclusion, this study illustrates that biochemical remission for children with AIH can be maintained with lower AZA dosing and 6-TGN levels than previously reported. Measurement of AZA metabolites is helpful to ensure modest levels of 6-TGN, medication compliance and to rule out toxicity.
Supplementary Material
Supplementary Figure 1: Level of Autoantibody Positivity. Shown here are the number of subjects who tested positive for an autoantibody at the various levels of detection. ANA and ASMA were measured based on titers (1:x) and ASMA (beginning in January 2013) and LKM were measured based on units (u.).
What is known: Long-term remission of pediatric AIH is usually sustained with AZA
Measurement of the AZA metabolite, 6-TGN, is useful in managing AIH
Previous reports suggest that 6-TGN levels in the ranges used for inflammatory bowel disease (IBD) are also necessary to achieve remission in AIH (250–450 pmol/8×108 RBC)
What is new: 6-TGN levels ranging from 50–250 pmol/8×108 RBC are associated with biochemical remission of AIH
Biochemical remission can be maintained with 6-TGN levels significantly lower than that recommended for IBD
Acknowledgments
Grant support: Dr. Sheiko received support through NIH grant 5 T32 DK067009-10. The REDCap database was used for data collection and storage, and this is supported through NIH/NCRR Colorado CTSI Grant Number UL1 TR000154.
Abbreviations
- AIH
Autoimmune hepatitis
- ALT
Alanine aminotransferase
- ANA
Anti-nuclear antibody
- ASMA
Anti-smooth muscle actin antibody
- AST
Aspartate aminotransferase
- IBD
Inflammatory bowel disease
- IgG
Immunoglobin G
- LKM-1
Liver kidney microsomal type 1 antibody
- 6-MMP
6-mercaptopurine
- 6-TGN
6-thioguanine
Footnotes
Disclosures: None of the authors have any financial disclosures to report.
Specific Author Contributions: Dr. Sheiko, Dr. Sundaram and Dr. Mack designed the study. Annette McCoy assisted in identifying subjects for this study. Dr. Sheiko performed all data collection and initial analysis. Dr. Pan and Dr. Mack performed the statistic analyses. Dr. Capocelli reviewed all follow up liver biopsies and performed the search through the pathology database for potential subjects. Dr. Sheiko and Dr. Mack wrote the manuscript, which was edited by other authors.
Contributor Information
Melissa A. Sheiko, University of Colorado School of Medicine, Department of Pediatrics, Digestive Health Institute, Children’s Hospital Colorado, 13123 E. 16th Ave, B290, Aurora, CO 80045.
Shikha S. Sundaram, University of Colorado School of Medicine, Department of Pediatrics, Digestive Health Institute, Children’s Hospital Colorado, 13123 E. 16th Ave, B290, Aurora, CO 80045.
Kelley E. Capocelli, University of Colorado School of Medicine, Department of Pathology, Department of Pathology, Children’s Hospital Colorado, 13123 E. 16th Ave, B120, Aurora, CO 80045.
Zhaoxing Pan, University of Colorado School of Public Health, Department of Biostatics and Informatics, 13001 E. 17th Place, B119, Building 500, Room W3112, Aurora, CO 80045.
Annette M. McCoy, University of Colorado School of Medicine, Department of Pediatrics, Digestive Health Institute, Children’s Hospital Colorado, 13123 E. 16th Ave, B290, Aurora, CO 80045.
Cara L. Mack, University of Colorado School of Medicine, Department of Pediatrics, Digestive Health Institute, Children’s Hospital Colorado, 13123 E. 16th Ave, B290, Aurora, CO 80045.
References
- 1.Jiménez-Rivera C, Ling SC, Ahmed N, et al. Incidence and characteristics of autoimmune hepatitis. Pediatrics. 2015;136(5):e1237–48. doi: 10.1542/peds.2015-0578. [DOI] [PubMed] [Google Scholar]
- 2.Deneau M, Jensen MK, Holmen J, et al. Primary sclerosing cholangitis, autoimmune hepatitis, and overlap in Utah children: Epidemiology and natural history. Hepatology. 2013;58:1392–1400. doi: 10.1002/hep.26454. [DOI] [PubMed] [Google Scholar]
- 3.Mieli-Vergani G, Vergani D. Autoimmune hepatitis in children: What is Ddifferent from adult autoimmune hepatitis? Semin Liv Dis. 2009;29(3):297–306. doi: 10.1055/s-0029-1233529. [DOI] [PubMed] [Google Scholar]
- 4.Czaja AJ. Current and prospective pharmacotherapy for autoimmune hepatitis. Exp Opin Pharmacother. 2014;15(12):1715–1736. doi: 10.1517/14656566.2014.931938. [DOI] [PubMed] [Google Scholar]
- 5.Derijks LJJ, Gilissen LPL, Hooymans PM, et al. Thiopurines in inflammatory bowel disease. Aliment Pharmacol Ther. 2006;24:715–729. doi: 10.1111/j.1365-2036.2006.02980.x. [DOI] [PubMed] [Google Scholar]
- 6.Gearry RB, Barclay ML. Azathioprine and 6-mercaptopurine pharmacogenetics and metabolite monitoring in inflammatory bowel disease. J Gastroenterol Hepatol. 2005;20:1149–1157. doi: 10.1111/j.1440-1746.2005.03832.x. [DOI] [PubMed] [Google Scholar]
- 7.Rumbo C, Emerick KM, Emre S, et al. Azathioprine metabolite measurements in the treatment of autoimmune hepatitis in pediatric patients: a preliminary report. J Pediatr Gastroenterol Nutr. 2002;35(3):391–8. doi: 10.1097/00005176-200209000-00032. [DOI] [PubMed] [Google Scholar]
- 8.Della Corte C, Sartorelli MR, Sindoni CD, et al. Autoimmune hepatitis in children: An overview of the disease focusing on current therapies. Eur Jrl Gastroenterol Hepatol. 2012;24(7):739–46. doi: 10.1097/MEG.0b013e328353750c. [DOI] [PubMed] [Google Scholar]
- 9.El-Shabrawi MH, Kamal NM. Medical management of chronic liver diseases in children (part I): focus on curable or potentially curable diseases. Paediatr Drugs. 2011;13(6):357–70. doi: 10.2165/11591610-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 10.Murray KF, Shah U, Mohan N, et al. Chronic hepatitis. J Pediatr Gastroenterol Nutr. 2008;47(2):225–33. doi: 10.1097/MPG.0b013e318181b08b. [DOI] [PubMed] [Google Scholar]
- 11.Manns MP, Czaja AJ, Gorham JD, et al. Diagnosis and management of autoimmune hepatitis. Hepatology. 2010;51(6):2193–213. doi: 10.1002/hep.23584. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen TM, Daubard M, Le Gall C, et al. Monitoring of azathioprine metabolites in pediatric patients with autoimmune hepatitis. Ther Drug Monit. 2010;32(4):433–7. doi: 10.1097/FTD.0b013e3181dbd712. [DOI] [PubMed] [Google Scholar]
- 13.Stine JG, Northup PG. Autoimmune-like drug-induced liver injury: A review and update for the clinician. Expert Opin Drug Metab Toxicol. 2016:1–11. doi: 10.1080/17425255.2016.1211110. [DOI] [PubMed] [Google Scholar]
- 14.Squires RH. Acute liver failure in children. Semin Liver Dis. 2008;28(2):153–66. doi: 10.1055/s-2008-1073115. [DOI] [PubMed] [Google Scholar]
- 15.Deneau M, Book LS, Guthery SL, et al. Outcome after discontinuation of immunosuppression in children with autoimmune hepatitis: A population-based study. J Pediatr. 2014;164(4):714–719. doi: 10.1016/j.jpeds.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 16.Manns MP, Lohse AW, Vergani D. Autoimmune hepatitis - Update 2015. J Hepatol. 2015;62(1S):S100–S111. doi: 10.1016/j.jhep.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 17.Longhi MS, Mieli-Vergani G, Vergani D. Autoimmune hepatitis. Curr Pediatr Rev. 2014;10(4):268–74. doi: 10.2174/1573396310666141114230147. [DOI] [PubMed] [Google Scholar]
- 18.Di Giorgio A, Bravi M, Bonanomi E, et al. Fulminant hepatic failure of autoimmune aetiology in children. J Pediatr Gastroenterol Nutr. 2015;60(2):159–64. doi: 10.1097/MPG.0000000000000593. [DOI] [PubMed] [Google Scholar]
- 19.Floreani A, Liberal R, Vergani D, Mieli-Vergani G. Autoimmune hepatitis: Contrasts and comparisons in children and adults- A comprehensive review. J Autoimmunity. 2013;46:7–16. doi: 10.1016/j.jaut.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Vergani D, Alvarez F, Bianchi FB, Cancado E, Mackay IR, Manns MP, Nishioka M, Penner E. Liver autoimmune serology: a consensus statement for the committee for autoimmune serology of the International Autoimmune Hepatitis Group. J Hepatol. 2004;41:677–683. doi: 10.1016/j.jhep.2004.08.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Level of Autoantibody Positivity. Shown here are the number of subjects who tested positive for an autoantibody at the various levels of detection. ANA and ASMA were measured based on titers (1:x) and ASMA (beginning in January 2013) and LKM were measured based on units (u.).


