Abstract
Patients with mild traumatic brain injury (mTBI) with associated intracranial injury, or complicated mTBI, are at risk of deterioration. Clinical management differs within and between institutions. We conducted an exploratory analysis to determine which of these patients are unlikely to have an adverse outcome and may be future targets for less resource intensive care.
This single center retrospective cohort study included patients presenting to the ED with blunt complicated mTBI between January 2001 and December 2010. Patients with a Glasgow coma score (GCS) of 15, an initial head CT with a traumatic abnormality, and a repeat head CT within 24 h were eligible. We defined the composite adverse outcome as death within two weeks, neurosurgical procedure within two weeks, hospitalization >48 h, and worsened second head CT. Classification and Regression Tree methodology was used to identify factors associated with adverse outcomes.
Of 1011 patients with two head CTs performed in a 24-h period, 240 (24%) had complicated mTBI and GCS 15. Of these, 56 (23%) experienced the composite adverse outcome defined above. Age, headache, and subarachnoid hemorrhage, correctly classified 93% of patients with an adverse outcome. No instance of death or neurosurgical procedure was missed.
Our analysis highlighted three factors associated with adverse outcomes in persons who have complicated mTBI but a GCS of 15. Absence of these risk factors suggests low risk of adverse outcomes, and may suggest that a patient is safe for discharge home. Additional research is required before utilizing these findings in clinical practice.
Keywords: Traumatic brain injury, Emergency department, Low risk TBI
1. Introduction
1.1 Background
Traumatic brain injuries (TBIs) may account for upwards of 1.8 million annual emergency department (ED) visits in the United States (US) [1]. In 2010, 0.7% of all ED visits were for TBI [2]. When the neurologic exam is normal and the initial head computed tomography (CT) is negative, it is considered safe to discharge patients home from the ED [3–5]. However, about six to 9% of patients with a Glasgow Coma Score (GCS) of 15 demonstrate traumatic intracranial hemorrhage on head CT [6], and are referred to as complicated mTBI [7]. There is great variability in the ED management of patients with GCS 15 complicated mTBI [8].
In 2002, the European Federation of Neurological Societies recommended that complicated mTBI patients should be routinely admitted to an intensive care unit (ICU) [9]. This practice, as well as the common practice of obtaining a repeat head CT, has been questioned [10], yet some centers still routinely admit all TBI patients with trauma-related intracranial abnormality to an ICU and obtain routine repeat imaging, regardless of GCS [11]. It is not uncommon for patients to be transferred to hospitals with neurosurgical coverage, further contributing to increased resource utilization. Conversely, about one in ten patients with complicated mTBI are discharged home from the ED [8].This does not include patients who had ED observational stays, however, as ED observation units are increasing in number, this may be an alternative approach for patients with complicated mTBI [12]. Given the lack of contemporary guidance for the management of patients with complicated mTBI with GCS 15, there is a critical need for research to help inform clinical decision-making.
1.2. importance
Up to 95% of TBI patients with complicated mTBI and GCS 16–15 who are admitted to the ICU do not require critical care or neurosurgical intervention [13]. We have previously reported that about two-thirds of TBI patients with GCS 14–15 with associated trauma-related intracranial abnormality can be safely discharged from the ED after monitoring for six hours followed by a stable repeat head CT. The 14-day mortality rate among patients with GCS 14 or 15 discharged in this way was <0.5%, and < 1% required a neurosurgical procedure [14]. Admitting all patients with complicated mTBI is a potential over-utilization of resources, particularly for those with GCS 15.
1.3. Goals of this investigation
It is possible that some patients with a normal neurological exam and GCS 15 can be safely discharged from the ED despite having traumatic intracranial findings on head CT. Identifying such a cohort of patients could reduce resource utilization without incurring harm for this common clinical condition. We sought to identify variables associated with higher risk of adverse short-term outcomes in patients with complicated mTBI with GCS 15. Identifying those factors associated with increased risk could allow patients with the absence of such risks to be safely discharged from the ED.
2. Methods
2.1. Study design
This was a secondary analysis of an existing data set. The data were originally collected as part of a retrospective cohort study describing the practice pattern of repeat head CTs after mild TBI. This study was approved by the local Institutional Review Board.
2.2. Study setting and population
Patients who presented to our tertiary academic ED between January 2001 and December 2010 were included. Potential study subjects were identified from the cohort of all adult patients who underwent two head CTs within 24 h with a traumatic intracranial abnormality detected on the first CT. The practice pattern during this time period was that all patients had a scheduled repeat head CT performed if the baseline CT revealed any trauma related abnormality (defined as traumatic subarachnoid hemorrhage, subdural hematoma, epidural hematoma, and intraparenchymal hemorrhage or traumatic contusion), so all patients presenting with complicated mTBI would have been included. Our institutional protocol is that all patients with a traumatic abnormality on initial head CT have a second head CT to determine stability.
Subjects who were on antiplatelet medications or warfarin with international normalized ratio (INR) < 1.4 were included. Subjects were excluded if they were < 18 years old, had no documented GCS, GCS < 15 based on first GCS upon arrival in the ED, unknown time of injury, had their head CT performed or interpreted at an outside hospital, were pregnant, had penetrating head injury, were intubated prior to ED evaluation, had abnormal ED vital signs (systolic blood pressure <89 mm Hg, respiratory rate >29 breaths per minute, pulse oximetry <92% on room air) at any point during the ED visit, had concomitant non-minor injuries (injuries for which a patient would require hospitalization) or had an inherited or acquired coagulopathy. Patients with polytrauma were excluded so that we could target a population of patients who may be safe for ED discharge on the basis of their head injury alone. Inherited coagulopathy was defined as hemophilia A or B, von Willebrand disease, Bernard-Soulier syndrome, Wiskott-Aldrich syndrome, or Glanzmann’s thrombasthenia. Acquired coagulopathies were defined as liver failure, therapeutic warfarin use (INR ≥ 1.4), heparin product use, and disseminated intravascular coagulopathy (INR ≥ 1.4, activated partial thromboplastin time (aPTT) >39 s, and platelets < 50 000/μl).
2.3. Study protocol
Chart review methods by which the data were originally obtained have been published previously [14].Briefly, a single data abstractor reviewed each identified case using explicitly defined inclusion and exclusion criteria. Two reviewers performed a second chart review to ascertain worsening or stability on the repeat head CT. Quality checks were performed on 10% of the chart abstractions. Missing data were minimal and were left missing.
2.4. Outcome measures
A composite adverse outcome was defined to capture the cohort of patients who had an adverse clinical outcome, and those for whom ready discharge from the ED may not be clinically feasible. The composite outcome was comprised of death within two weeks, neurosurgical intervention within two weeks (defined as a procedure performed by neurosurgery either at the bedside or in the operating room for head injury, including external ventricular drain (EVD) placement or intracranial pressure (ICP) monitor placement), length of stay >48 h [15],or worsening trauma related intracranial abnormality on the second head CT. Worsening traumatic abnormality was defined as a second head CT that was described as worsened hemorrhage by the attending neuroradiologist interpretation. Length of stay >48 h was chosen because previous studies have reported that any worsening after 48 h is unlikely due to the primary neurologic cause [16,17].Further, the time frame of 48 h is consistent with previously published guidelines [9] and likely captures a group of patients that would not be readily discharged from the ED. The social security death index was searched for patients who neither returned to the ED nor had a documented clinic visit at or beyond 14 days after their injury. This query was performed three years after the last patient was evaluated in the hospital for this retrospective review, leaving ample time for patients to be listed in the index. In all instances when this occurred, a social security number was available in our medical records system for query of the database. We did not attempt to contact patients to ascertain adverse outcomes.
2.5. Data analysis
For this analysis, we used Classification and Regression Trees (CART) to identify variables associated with risk of an adverse outcome. CART methodology is advantageous as it is able to accommodate nonlinear relationships, unexpected interactions, and missing values [18].To maximize model sensitivity for adverse outcomes, missing an adverse outcome was set to have a cost four times greater than missing a favorable outcome. The minimum number of cases for a parent node in the CART was set to ten, and the minimum number of cases for a child node was set to five. Splitting used the Gini criteria, with minimum improvement set to 0.0001. The tree was not pruned. The model has not yet been validated and external validation is planned.
Based on prior research, biological plausibility (i.e., contribution of blood thinning medications to worsening hemorrhage, age) [19–21], and clinical observation (i.e., TBI symptoms), the following variables were determined a priori as candidate variables for consideration: age [22]; use of any home medications that affect blood clotting; nausea or vomiting at any point during ED visit; headache of any severity documented by the provider; seizure; focal neurologic deficit; traumatic abnormality on head CT [15] defined as: subarachnoid hemorrhage, subdural hemorrhage, epidural hematoma, cerebral contusion or intraparenchymal hematoma, or any combination thereof. These variables, listed in Table 1, represent the universe of variables CART was given. Using CART, optimal cut-points for continuous variables are identified; for age, the cut points were suggested by the model and were rounded to the nearest 5 year interval for simplicity. Age was forced into first position to aid in potential clinical utility and ease of use of the model, as age is quite often readily available to the clinician. The variables associated with a favorable outcome and model performance were unchanged when age was not forced into the first position. All analyses were conducted using SPSS 23 (IBM Corporation, Armonk, NY) and R (3.1.1) base package and package epiR [23,24].The data and code for this model are available from the authors.
Table 1.
Demographic characteristics, injury characteristics, and outcomes of subjects.
| Favorable (n = 184) |
Non-favorable (n = 56) |
All patients (n = 240) |
|
|---|---|---|---|
| Age – mean (SD) | 41 (17) | 47(21) | 42 (19) |
| Caucasian – n (%) | 110 (59.8) | 33 (58.9) | 143 (59.6) |
| Male – n (%) | 131 (71.2) | 43 (76.8) | 174(72.5) |
| Home medication that affects clotting - n(%) | 12 (6.5) | 10 (17.9) | 22 (9.2) |
| Symptoms – n(%) | |||
| Altered memory or confusion | 113 (61.4) | 35 (62.5) | 148 (61.7) |
| Headache | 109 (59.2) | 37 (66.1) | 146 (60.8) |
| Nausea or vomiting | 23 (12.5) | 11 (19.6) | 34 (14.2) |
| Seizure | 14(7.6) | 2 (3.6) | 16 (6.7) |
| Focal neurologic deficit | 1 (0.5) | 0 (0.0) | 1 (0.4) |
| CT findings – n(%) | |||
| Subarachnoid hemorrhage | 80 (43.5) | 36 (64.3) | 116 (48.3) |
| Cerebral contusion or intraparenchymal hematoma | 65 (35.3) | 29 (51.8) | 94(39.2) |
| Subdural hematoma | 67 (36.4) | 25 (44.6) | 92 (38.3) |
| Isolated SAH | 50 (27.2) | 14(25.0) | 64(26.7) |
| Skull fracture | 19 (10.3) | 7 (12.5) | 26 (10.8) |
| Epidural hematoma | 6 (3.3) | 3 (5.4) | 9 (3.8) |
| Outcomes – n (%) | |||
| CT Worse | – | 40 (71.4) | 40 (16.7) |
| LOS > 48 h | – | 24 (43.6) | 24(10.1) |
| Patient died | – | 2 (3.6) | 2 (0.8) |
| Neurosurgical intervention | – | 1 (1.8) | 1 (0.4) |
N – number of patients. GCS – Glasgow Coma Scale. SD- standard deviation. CT- computed tomography. SAH - subarachnoid hemorrhage.
3. Results
Of 1011 patients screened for inclusion in the original analysis, 323 had a GCS of 14 or 15, all of whom were included in the original paper [14]. This analysis reported here only included patients with a GCS of 15. Of the 323 in the prior study, 19 had incomplete records available at the time of second chart review. There were 240 patients with a complicated mTBI and a GCS of 15 included for the CART analysis (Fig. 1). This available sample and proportion of outcomes exceed the generally accepted minimum sample size for conducting CART analysis [18]. Mean age of included patients was 42 (SD 19) years. Of these, 143 (60%) were Caucasian, and 174 (73%) were male. Complete demographic characteristics and injury characteristics are presented in Table 1.
Fig. 1.
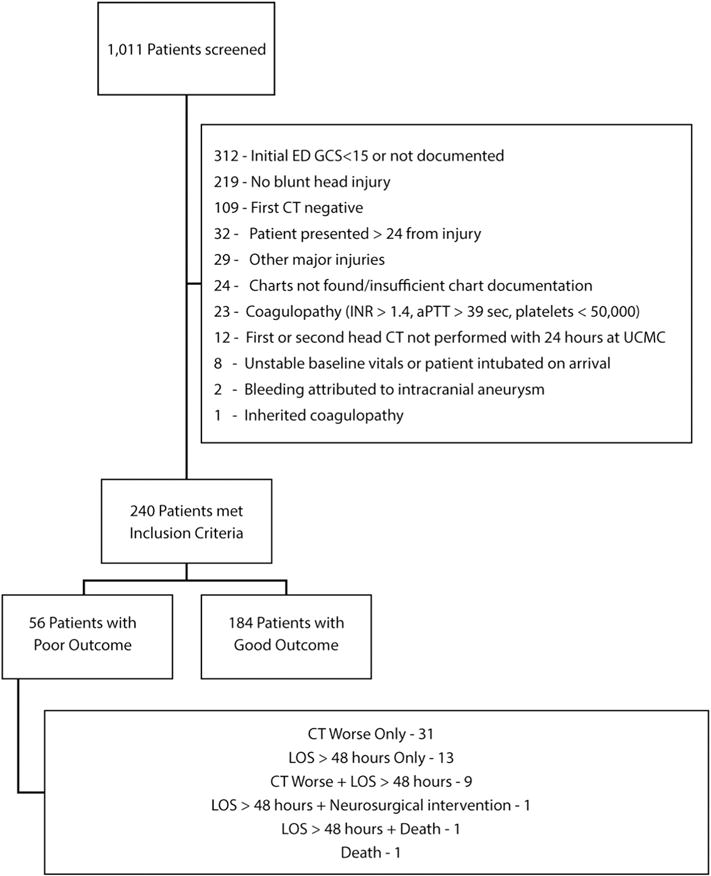
Flow diagram of reviewed patients.
Fifty six patients (23%) experienced the composite adverse outcome. Worsening head CT comprised 55% of all adverse outcomes. Table 1 and Fig. 1 show the rates of the individual components of the composite outcome.
The final model is shown in Fig. 2. The sensitivity of finding a patient who may have an adverse outcome 93% (95% CI 83%–98%); and the negative predictive value, or the proportion of patients who would have been discharged after application of the model and did not have an actual adverse outcome, was 91% (95% CI 89%–97%) (Table 2). Complete test characteristics are presented in Table 3. There were eight terminal nodes: one lower risk node (< 10%) and seven higher risk nodes (≥ 10%). Of the 44 out of 240 patients (18%) classified as lower risk, there were 0/44 (0%, 95% CI 0%–6%) with death or neurosurgical intervention. Of the four out of 44 (9%, 95% CI 3%–23%) with an adverse outcome, all of these had a worsening head CT only. The three variables that led to the lower risk node included age < 65, no subarachnoid hemorrhage, and no headache. The two patients who died were in node 5 or node 6. One died of unrelated urosepsis while the other died after sustaining a second fall and subsequent large intraparenchymal hemorrhage two days after the initial TBI. The one patient with neurosurgical intervention underwent placement of a lumbar drain for cerebrospinal fluid (CSF) diversion secondary to a CSF leak due to a basilar skull fracture and was in node 13.
Fig. 2.
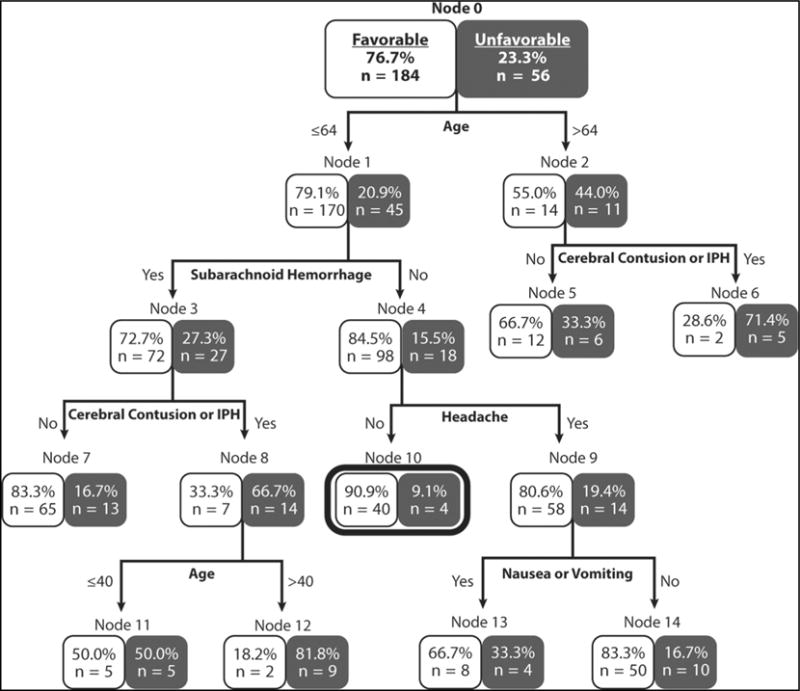
Classification and Regression Tree (CART) Model.
Table 2.
Test characteristics of the model.
| 95% CI
|
|||
|---|---|---|---|
| Lower | Upper | ||
| True positive | 52 | ||
| False positive | 144 | ||
| True negative | 40 | ||
| False negative | 4 | ||
| Sensitivity | 0.93 | 0.83 | 0.98 |
| Specificity | 0.22 | 0.16 | 0.28 |
| Positive predictive value | 0.27 | 0.20 | 0.33 |
| Negative predictive value | 0.91 | 0.78 | 0.97 |
| Positive likelihood ratio | 1.19 | 1.07 | 1.32 |
| Negative likelihood ratio | 0.33 | 0.12 | 0.88 |
| Area under the curve | 0.57 | 0.49 | 0.65 |
Table 3.
Components of the composite outcome (n = 56).
| N | % | |
|---|---|---|
| CT worse | 30 | (54.5) |
| LOSa > 48 h | 13 | (23.6) |
| CT worse + LOS > 48 h | 9 | (16.4) |
| LOS > 48 h + NSIb | 1 | (1.8) |
| CT worse + LOS > 48 h + death + NSIb | 0 | (0.0) |
| LOS > 48 h + death | 1 | (1.8) |
| CT worse + death | 0 | (0.0) |
| Death | 1 | (1.8) |
Length of stay.
Neurosurgical intervention.
4. Discussion
In this exploratory analysis to identify variables associated with adverse outcomes in complicated mild TBI patients with GCS 15, we found that patients under 65 years of age with no subarachnoid hemorrhage and no headache had a low risk of adverse outcome (Table 4). Risk factors for an adverse outcome have been reported and include advanced age (>70), coagulopathy, and GCS ≤8 [25–28]. To our knowledge, this is the first study to identify factors that, if absent, could identify a specific subset of patients with complicated mTBI who are GCS 15, and are at low risk of adverse outcomes. If these findings are confirmed, these patients may be considered for discharge from the ED after an initial head CT, brief period of observation, and possibly no repeat imaging. Overall, it is possible that 18% of patients with complicated mild TBI could be safely discharged from the ED. This would result in approximately 16,000 fewer CTs and hospital admissions every year in the US alone [6].
Table 4.
Patients must meet following criteria for potential for safe ED discharge without repeat head CT after a period of observation.
| < 65 years old |
| No traumatic subarachnoid hemorrhage on initial head CT |
| No headache |
Applicable for patients with blunt traumatic brain injury, >17 years old, GCS 15, and without coagulopathy (but may be taking antiplatelet medications).
The European Federation of Neurological Societies (EFNS) recommended in 2002 that all patients with trauma related intracranial abnormalities, even those with GCS 15 and a normal neurologic exam, be admitted to the hospital [9]. The American College of Emergency Physicians (ACEP) TBI policy statement was silent on this matter [29], and there are no guidelines from the Brain Trauma Foundation on this topic. A multicenter study of 888 patients recently found that 95% of complicated mild TBI patients with GCS 15 admitted to the ICU never require critical care intervention [13]. This study described admission practices of this patient population, and although the majority of patients were admitted to an ICU (63%), the study did not determine which patients could be safely discharged from the ED. Similarly, observational studies suggest that an adverse outcome in this patient population is rare in general, even when routine CT imaging demonstrates injury progression [25–28].Our findings are consistent with these prior reports as the lower risk node patients (44 of 240 total patients) had only worsened head CT findings as an adverse outcome.
The one patient with neurosurgical intervention underwent placement of a lumbar drain for cerebrospinal fluid (CSF) diversion secondary to a CSF leak due to a basilar skull fracture and was in node 13. We did not collect the exact date of the surgical procedure, so it is possible that this could have been placed between the two head CTs. Although beyond the scope of this paper, the lack of medical or surgical interventions after the baseline CT calls into question the routine use of a second CT within 24 h at our institution, at least for this particular group of patients.
Altogether, 55% of the patients with adverse outcomes in our study had a worsened repeat head CT only, without any other aspect of the composite outcome. Our composite outcome was designed to have a high sensitivity to identify any patient who may worsen. Our rationale for including worsened CT findings was that we would capture any patient with the potential to worsen as it would be less likely for a patient to worsen clinically without having a radiographic change. Our most common head CT finding was traumatic subarachnoid hemorrhage, and many patients had multiple traumatic findings on head CT. Furthermore, there is evidence to suggest that patients with complicated mild TBI who have isolated traumatic subarachnoid hemorrhage may not require as high an intensity of observation, such as the ICU setting, when compared to other traumatic head injuries [30]. Our data contradict this slightly, in that we found that patients with isolated traumatic subarachnoid hemorrhage were about equally likely to have a favorable or non-favorable outcome (Table 1). Given previous reports of low mortality and neurosurgical intervention event rates in similar cohorts of TBI patients [31], a composite outcome is necessary. Our composite outcome allowed us to investigate clinically meaningful adverse outcomes that would preclude ready discharge from the ED.
We found that age ≥ 65 years, subarachnoid hemorrhage, and presence of a headache were predictors for adverse outcome. Although no study has previously used our composite outcome as an endpoint, other series have attempted to predict which complicated mTBI patients may worsen. Schuster et al. used multivariable logistic regression in a cohort of 255 patients with TBI and found that elevated PT, age > 70 years, and antiplatelet medication were risk factors for the need for craniotomy after repeat head CT [25]. This cohort differed from ours in that patients of all GCS were included, however, the majority of patients were mild TBI, with GCS of 13–15. Age was also a risk factor for the need for neurosurgical intervention in a series of 692 patients with GCS of 13–15, in addition to multiple traumatic lesions on initial head CT, and interval < 90 min from injury to head CT [26]. Lastly a prospective series of 161 patients with complicated mTBI did not demonstrate the need for neurosurgical intervention based on repeat head CT findings without a change in the neurological exam [27]. Patients who have a GCS of 13–14 may be considered neurologically impaired, thus precluding their ready discharge from the ED. Thus, inclusion of these patients in these prior reports limits their utility for identifying patients with complicated mTBI that may be discharged from the ED.
5. Limitations
Limitations of our study include its exploratory nature and its retrospective design at a single center. We were unable to assess functional outcome or cognitive ability after mTBI. It is possible that patients who worsened but received care at other centers were missed. As our center is the only tertiary referral center for neurotrauma in the area, it is unlikely that a neurosurgical procedure would have been missed. A worsened head CT or admission >48 h would not have been missed based on our inclusion criteria. The study was not designed to determine the balance or harm of repeat imaging. Only patients getting repeat imaging were included, reflecting the local practice of repeat head CT in patients with complicated mTBI during the study period. Another limitation is that chart review was mostly performed by a single chart abstractor and abstractors were not blinded to the study hypothesis. However, dual abstraction was performed in a guided random sample of 50 charts during the initial abstraction period and during the second abstraction 10% of charts were selected for quality checks. No discrepancies were detected.
The initial head CT was obtained at the discretion of the ordering provider, and it is possible that patients in whom a head CT was never obtained may have been missed. We note that the outcome “admission >48 h” does not connote the harder endpoints of neurosurgical procedure or death. However, within the context of identifying patients who may be discharged from the ED, hospital admission for two days is a reasonable surrogate for patients that have either social, cognitive or other issues that the ED may not be able to readily resolve and discharge the patient. Further, the time frame of 48 h is consistent with previously published guidelines [9]. We do not know if any of the patients discharged before 48 h were sent to rehabilitation, skilled nursing facility, or an acute care facility rather than home. Although our study is of reasonable size, the available sample for certain subject characteristics or outcomes was small. For example, there was only one patient with a focal neurologic deficit, so it is difficult to estimate how the model may differ in a larger cohort of patients. While our model had a sensitivity of 93%, specificity was just 22% and the AUC was 0.57 (Table 3). The low specificity reflects our decision to weight the cost of missing an adverse outcome as being four times higher than the cost of missing a favorable outcome. Testing our findings in an independent cohort would facilitate translation into clinical care.
It is likely that some patients in our cohort with headache and nausea were treated for their symptoms. We did not record pain medication or antiemetics that were given to patients while they were in the ED. It is possible that treatment of these symptoms may have resulted in some persons not having these documented in their chart, but this is unlikely as we evaluated for any headache or nausea that were present at any point in the ED stay. It is possible that response to treatment of these symptoms may have been informative, but this was not recorded.
Another limitation of this study is that we had a small sample size and a low rate of adverse outcomes. Given the 95% confidence intervals, the death or neurosurgical intervention rate in patients with favorable characteristics may be as high as 6%. Validation studies in larger cohorts would increase confidence in our findings.
Lastly, nine patients in our cohort had epidural hemorrhage (EDH), a clinical scenario whereby a hemorrhage progression may be life-threatening. Three of these patients had hemorrhage progression seen on head CT, but none of these patients required neurosurgical intervention or died. Given the potential threat to life EDH may represent, careful consideration will need to be given before recommending discharge in such patients.
6. Conclusions
This exploratory analysis indicates that complicated mTBI patients who have a GCS of 15 are at low risk of adverse outcome requiring hospitalization provided they are under 65 years old, do not have subarachnoid hemorrhage, and do not report headache. Given the extensive resources currently used to monitor these patients, our findings suggest that there is an opportunity to refine the approach to managing lower risk patients. Testing the prognostic value of these variables in an independent cohort of TBI patients is needed to fully benefit from this opportunity.
Acknowledgments
This project was supported in part by an Institutional Clinical and Translational Science Award, NIH/NCRR Grant Number 5UL1RR026314-03.
Funding
This work was supported by an Institutional Clinical and Translational Science Award, NIH/NCRR Grant Number 8UL1-TR000077.
Footnotes
Meetings
This work was presented at the American College of Emergency Physicians Meeting in Boston, MA, on October 27, 2015.
Disclosures
No competing financial interests exist.
References
- 1.Centers for Disease Control and Prevention. The National Hospital Ambulatory Medical Care Survey (NHAMCS): 2012 Emergency Department Summary Tables — United States. 2012 Accessed January 3, 2017 https://www.cdc.gov/nchs/ahcd/index.htm.
- 2.Centers for Disease Control and Prevention. Rates of TBI-Related Emergency Department Visits, Hospitalizations, and Deaths — United States. 2001–2010 Accessed November 7, 2015, http://www.cdc.gov/traumaticbraininjury/data/rates.html.
- 3.Fortuna GR, Mueller EW, James LE, Shutter LA, Butler KL. The impact of preinjury antiplatelet and anticoagulant pharmacotherapy on outcomes in elderly patients with hemorrhagic brain injury. Surgery. 2008;144:598–603. doi: 10.1016/j.surg.2008.06.009. [discussion -5] [DOI] [PubMed] [Google Scholar]
- 4.Fainardi E, Chieregato A, Antonelli V, Fagioli L, Servadei F. Time course of CT evolution in traumatic subarachnoid haemorrhage: a study of 141 patients. Acta Neurochir. 2004;146:257–63. doi: 10.1007/s00701-003-0207-y. [discussion 63] [DOI] [PubMed] [Google Scholar]
- 5.Armin SS, Colohan AR, Zhang JH. Traumatic subarachnoid hemorrhage: our current understanding and its evolution over the past half century. Neurol Res. 2006;28:445–52. doi: 10.1179/016164106X115053. [DOI] [PubMed] [Google Scholar]
- 6.Kisat M, Zafar SN, Latif A, Villegas CV, Efron DT, Stevens KA, et al. Predictors of positive head CT scan and neurosurgical procedures after minor head trauma. J Surg Res. 2012;173:31–7. doi: 10.1016/j.jss.2011.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams DH, Levin HS, Eisenberg HM. Mild head injury classification. Neurosurgery. 1990;27:422–8. doi: 10.1097/00006123-199009000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Ratcliff JJ, Adeoye O, Lindsell CJ, Hart KW, Pancioli A, McMullan JT, et al. ED disposition of the Glasgow Coma Scale 13 to 15 traumatic brain injury patient: analysis of the Transforming Research and Clinical Knowledge in TBI study. Am J Emerg Med. 2014;32:844–50. doi: 10.1016/j.ajem.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vos PE, Battistin L, Birbamer G, Gerstenbrand F, Potapov A, Prevec T, et al. EFNS guideline on mild traumatic brain injury: report of an EFNS task force. Eur J Neurol. 2002;9:207–19. doi: 10.1046/j.1468-1331.2002.00407.x. [DOI] [PubMed] [Google Scholar]
- 10.Sifri ZC, Livingston DH, Lavery RF, Homnick AT, Mosenthal AC, Mohr AM, et al. Value of repeat cranial computed axial tomography scanning in patients with minimal head injury. Am J Surg. 2004;187:338–42. doi: 10.1016/j.amjsurg.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 11.Washington CW, Grubb RL., Jr Are routine repeat imaging and intensive care unit admission necessary in mild traumatic brain injury? J Neurosurg. 2012;116:549–57. doi: 10.3171/2011.11.JNS111092. [DOI] [PubMed] [Google Scholar]
- 12.Homnick A, Sifri Z, Yonclas P, Mohr A, Lingingston D. The temporal course of intracranial haemorrhage progression: how long is observation necessary? Injury. 2012;43:2122–5. doi: 10.1016/j.injury.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 13.Nishijima DK, Haukoos JS, Newgard CD, Staudenmayer K, White N, Slattery D, et al. Variability of ICU use in adult patients with minor traumatic intracranial hemorrhage. Ann Emerg Med. 2013;61:509–17:e4. doi: 10.1016/j.annemergmed.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kreitzer N, Lyons MS, Hart K, Lindsell CJ, Chung S, Yick A, et al. Repeat neuroimaging of mild traumatic brain-injured patients with acute traumatic intracranial hemorrhage: clinical outcomes and radiographic features. Acad Emerg Med. 2014;21:1083–91. doi: 10.1111/acem.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huynh T, Jacobs DG, Dix S, Sing RF, Miles WS, Thomason MH. Utility of neurosurgical consultation for mild traumatic brain injury. AmSurg. 2006;72:1162–5. [discussion 6–7] [PubMed] [Google Scholar]
- 16.Nishijima DK, Shahlaie K, Echeverri A, Holmes JF. A clinical decision rule to predict adult patients with traumatic intracranial haemorrhage who do not require intensive care unit admission. Injury. 2012;43:1827–32. doi: 10.1016/j.injury.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishijima DK, Sena MJ, Holmes JF. Identification of low-risk patients with traumatic brain injury and intracranial hemorrhage who do not need intensive care unit admission. J Trauma. 2011;70:E101–7. doi: 10.1097/TA.0b013e3181e88bcb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De’ath G, Fabricius KE. Classification and regression trees: a powerful yet simple technique for ecological data analysis. Ecology. 2000;81:3178–92. [Google Scholar]
- 19.von der Brelie C, Schneegans I, van den Boom L, Meier U, Hedderich J, Lemcke J. Impaired coagulation is a risk factor for clinical and radiologic deterioration in patients with traumatic brain injury and isolated traumatic subarachnoid hemorrhage. J Trauma Acute Care Surg. 2015;79:295–300. doi: 10.1097/TA.0000000000000722. [DOI] [PubMed] [Google Scholar]
- 20.Sharifuddin A, Adnan J, Ghani AR, Abdullah JM. The role ofrepeat head computed tomography in the management of mild traumatic brain injury patients with a positive initial head CT. Med J Malaysia. 2012;67:305–8. [PubMed] [Google Scholar]
- 21.Fabbri A, Servadei F, Marchesini G, Bronzoni C, Montesi D, Arietta L. Antiplatelet therapy and the outcome of subjects with intracranial injury: the Italian SIMEU study. Crit Care. 2013;17:R53. doi: 10.1186/cc12575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishijima DK, Sena M, Galante JM, Shahlaie K, London J, Melnikow J, et al. Derivation of a clinical decision instrument to identify adult patients with mild traumatic intracranial hemorrhage at low risk for requiring ICU admission. Ann Emerg Med. 2014;63:448–56:e2. doi: 10.1016/j.annemergmed.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. R Core Team. 2014 [Google Scholar]
- 24.Mark Stevenson with contributions from Telmo Nunes CH, Jonathon Marshall JS, Ron Thornton, Jeno Reiczigel, Jim, Robison-Cox PS, Peter Solymos, Kazuki Yoshida and, of SFeTftA, 0.9-62. EDRpv.
- 25.Schuster R, Waxman K. Is repeated head computed tomography necessary for traumatic intracranial hemorrhage? Am Surg. 2005;71:701–1. [PubMed] [Google Scholar]
- 26.Velmahos GC, Gervasini A, Petrovick L, Dorer DJ, Doran ME, Spaniolas K, et al. Routine repeat head CT for minimal head injury is unnecessary. J Trauma. 2006;60:494–9. doi: 10.1097/01.ta.0000203546.14824.0d. [discussion 9–501] [DOI] [PubMed] [Google Scholar]
- 27.Sifri ZC, Homnick AT, Vaynman A, Lavery R, Liao W, Mohr A, et al. Aprospective evaluation of the value of repeat cranial computed tomography in patients with minimal head injury and an intracranial bleed. J Trauma. 2006;61:862–7. doi: 10.1097/01.ta.0000224225.54982.90. [DOI] [PubMed] [Google Scholar]
- 28.Brown CV, Zada G, Salim A, Inaba K, Kasotakis G, Hadjizacharia P, et al. Indications for routine repeat head computed tomography (CT) stratified by severity of traumatic brain injury. J Trauma. 2007;62:1339–44. doi: 10.1097/TA.0b013e318054e25a. [discussion 44–5] [DOI] [PubMed] [Google Scholar]
- 29.Jagoda AS, Bazarian JJ, Bruns JJ, Jr, Cantrill SV, Gean AD, Howard PK, et al. Clinical policy: neuroimaging and decisionmaking in adult mild traumatic brain injury in the acute setting. Ann Emerg Med. 2008;52:714–48. doi: 10.1016/j.annemergmed.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 30.Phelan HA, Richter AA, Scott WW, Pruitt JH, Madden CJ, Rickert KL, et al. Does isolated traumatic subarachnoid hemorrhage merit a lower intensity level of observation than other traumatic brain injury? J Neurotrauma. 2014;31:1733–6. doi: 10.1089/neu.2014.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shukla D, Devi BI. Mild traumatic brain injuries in adults. J Neurosci Rural Pract. 2010;1:82–8. doi: 10.4103/0976-3147.71723. [DOI] [PMC free article] [PubMed] [Google Scholar]


