Abstract
Background
Conventional ECMO is cumbersome and is associated with high morbidity and mortality. We are developing the Paracorporeal Ambulatory Assist Lung (PAAL), designed to ambulate lung failure patients during bridge to transplant or recovery. In this study, we investigated the in-vitro and acute in-vivo performance of the PAAL.
Methods
The PAAL features a 1.75 inch diameter cylindrical HFM bundle of stacked sheets, with a surface area of 0.65 m2 integrated with a centrifugal pump. The PAAL was tested on the bench for hydrodynamic performance, gas exchange and hemolysis. The PAAL was then tested in 40–60 kg adult sheep (n=4) for 6h. The animals were cannulated with an Avalon Elite 27 Fr. DLC inserted through the right external jugular into the SVC, RA and IVC.
Results
The PAAL pumped over 250 mmHg at 3.5 L/min at a rotation speed of 2100 RPM. Oxygenation performance met the target of 180 ml/min at 3.5 L/min of blood flow in vitro, resulting in a gas exchange efficiency of 278 ml/min/m2. The normalized index of hemolysis (NIH) for the PAAL and cannula was 0.054 g/100L (n=2) at 3.5 L/min, as compared to 0.020 g/100L (n=2) for control (DLC cannula and a Centrimag pump). Plasma-free hemoglobin (pfHb) was below 20 mg/dL for all animals. Blood left the device 100% oxygenated in vivo and oxygenation reached 181 ml/min at 3.8 L/min.
Conclusions
The PAAL met in vitro and acute in vivo performance targets. 5 day chronic sheep studies are planned in the near term.
Introduction
Chronic lung disease still remains a major clinical problem which has the potential to be addressed through an artificial lung device. Much like the present artificial heart devices used as a bridge to transplant (BTT) or bridge to recovery (BTR)1, an implanted artificial lung device would simplify ECMO technology and provide relief to patients serving as a bridge2. Current intervention techniques include mechanical ventilation (MV) and ECMO. Prolonged MV injures the patient in the form of barotrauma and volutrauma, and results in poor post-transplant outcomes3,4. Conventional ECMO is cumbersome and expensive in addition to being associated with high morbidity and mortality5,6,7. Additionally, patients are confined in MV and ECMO, which exacerbates morbidity through progressive deconditioning8,9. Recently clinical implementation of the Maquet Cardiohelp10,11 and novel cannula such as the Avalon Elite® (Maquet Cardiovascular LLC, Wayne, NJ) dual lumen cannula have simplified ambulation on ECMO12. Ambulation using such systems improves patient outcomes as this allows patients to walk, eat and exercise during therapy – reducing muscle deconditioning11,12,13,14. Yet, the newer generation of ECMO systems remain bulky and cumbersome. An artificial lung device designed to compact and integrate several ECMO circuit components, have high gas exchange efficiency and be durable would fulfill the role of a BTT or BTR in patients in patients suffering prolonged reversible respiratory failure8,12,15.
We are developing a highly integrated blood pump and oxygenator as a wearable artificial lung or respiratory assist device. The Pittsburgh Ambulatory Assist Lung (PAAL) integrates an efficient hollow fiber membrane (HFM) bundle for gas exchange (278 ml/min/m2) directly with an efficient centrifugal pump and is intended to be a truly wearable device that allows for patient ambulation. The PAAL requirements include a relatively small overall form factor and long term (1–3 month) durability. The small form factor is achieved by minimizing the size of the HFM bundle, which typically represents the largest component of the pump oxygenator system. Our unique HFM bundle design leverages form factor to increase gas exchange efficiency, which allows minimizing device size22. With this HFM bundle we achieve over 180 ml/min oxygenation at 3.5L/min, with a 0.65 m2 surface area (half the surface area of the HLS 5.0 Cardiohelp16). In this study, we integrated our HFM bundle design directly with a centrifugal pump into a single housing. In-vitro gas exchange, hemolysis and pumping studies were conducted to demonstrate the device meets our design specifications. We then proceeded to 6-hour acute sheep studies to verify that the in-vivo performance met specifications as well.
Methods
PAAL Device Design
The integrated PAAL device is intended for ambulatory respiratory support applications and is designed to be wearable. The PAAL features a high efficiency small sized stacked type HFM bundle we previously developed17, directly integrated with a centrifugal pump. The fiber bundle is manufactured using commercially available Membrana® PMP 90/200 type hollow fiber sheets (44 fibers/inch) (Membrana GmbH, Wuppertal, Germany) and is potted round to eliminate corners. Bundle manufacturing further described elsewhere23. The device components are designed in SolidWorks (Dassault Systèmes, Waltham, MA) and CNC-machined from clear polymethyl methacrylate. The pump impeller has embedded magnets which couple to rotating magnets on an external motor driver to maintain a hermetic seal. The impeller has ceramic pivots which are held within ultra-high molecular weight polyethylene (UHMWPE). All device surfaces are polished to a mirror finish. The PAAL prototype and schematic are shown in Figure 1. Blood enters the voluted centrifugal pump and is channeled into the fiber bundle oxygenating while flowing across the fibers. The PAAL overall dimensions are 5 inch × 4.8 inch × 4.6 inch and the prototype weighs 4lb. Device weight can be significantly reduced (50%–80%) if injection molded at the product development stage.
Figure 1.
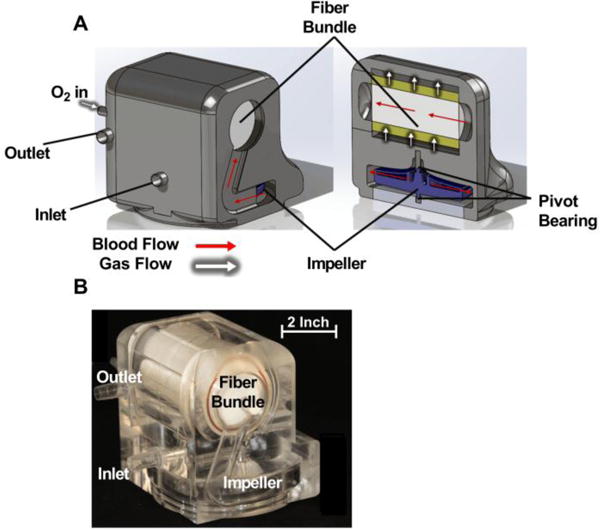
Cross section drawing of the PAAL device (A); and machined prototype (B)
In-Vitro Testing
Standard pump testing was conducted in a solution of 8.5g/L low viscosity carboxymethylcellulose sodium salt (Sigma Aldrich, St. Louis, MO) to match blood viscosity. The PAAL was placed in a flow loop consisting of an 800 mL venous reservoir (Medtronic, Minneapolis, MN) kept at 37C using a water bath connected to a PolyScience 210 heater (PolyScience Inc., Niles, IL). A hoffman’s clamp on the outlet tubing was used to adjust afterload on the device. Honeywell 143 PC03D pressure transducers (Honeywell, Morris Plains, NJ) were placed before and after the PAAL device to measure pressure generated while simultaneously measuring flowrate using an ultrasound flow probe (Transonic Systems Inc., Ithaca, NY). Measurements were made at 1500, 1700, 1900 and 2100 RPMs to generate the H-Q curve at flowrates between 0 and 4 L/min.
Pressure drop of the 27 Fr. Avalon Elite® DLC (Maquet Cardiovascular LLC, Wayne, NJ) was measured in the same loop setup in which flow was driven with a Biomedicus BP 80-X pump (Medtronic, Minneapolis, MN) and pressure was measured across the cannula. Measurements were made at flowrates between 0 and 3.5 L/min.
Blood testing followed published standards18,19,20 using locally collected slaughterhouse porcine or bovine blood. Oxygenation was characterized in a single pass loop system in which blood was conditioned to have an oxygen saturation of 65% ±5% and a pCO2 of 45 mmHg ± 5 mmHg. Blood flowrates tested were varied between 1 and 4 L/min at a constant 7 L/min gas flowrate. CO2 removal performance was characterized in a continuous flow loop in which blood was conditioned to have a pCO2 of 45 mmHg ± 5 mmHg. Blood flowrate was constant at 3.5 L/min and gas flowrates tested were varied between 3 L/min and 18 L/min. Each point was repeated once.
Hemolysis was characterized in a continuous flow loop system using an 800 mL compliant blood reservoir (Medtronic, Minneapolis, MN). The test loop comprised of the PAAL and a 27 Fr. Avalon Elite® DLC (Maquet Cardiovascular LLC, Wayne, NJ). The control loop comprised of a Centrimag pump (Thoratec, Pleasanton, CA) the 27 Fr. DLC. Both loops were run simultaneously at 3.5 L/min for a duration of 6 hours. Plasma free hemoglobin versus time was measured and a normalized index of hemolysis (NIH) was calculated to represent the level of hemolysis in each loop. Each experiment was repeated once. A detailed description of the in-vitro methods are published elsewhere21,22.
In-Vivo Testing
Four acute (6 hour) acute studies were conducted at the McGowan Institute’s Center for Preclinical Studies (CPCS) on adult Dorsett sheep (40 – 60 kg) to assess in vivo hemodynamics, gas exchange and biocompatibility of the PAAL. All animals received humane care in accordance with the Guide for Care and Use of Laboratory Animals (NIH publication 86–23, revised 1996). The surgical protocol and animal care were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh.
Prior to surgery, anesthesia was induced with atropine (0.05 mg/kg) followed by an intramuscular injection of Ketamine (22mg/kg). Surgical plane anesthesia was maintained following intubation of a cuffed Magill type ET tube with isoflurane inhalation (1.0–3.5%). A Swan Ganz catheter was placed into the PA via left external jugular after which a heparin bolus (100IU/kg) was administered just prior to cannulation in Acute 1. This original lower dose was chosen based on a human clinical practice guideline. With experience we determined that a longer circulation time and higher dose were going to be optimal to prevent thrombus formation in our model. Thus, a longer circulation time (10 minutes) was allowed in Acute 2. A larger heparin bolus (150 IU/kg) was administered during Acute 3 and 4, with the longer circulation time prior to cannulation. These modifications to the heparin protocol maintained ACT over two times baseline prior to cannulation until initiation of extracorporeal support and resulted in a mature protocol that had no thrombus formation seen. A heparin drip was then initiated to target ACTs between 1.5 to 2 times baseline. The animal was then cannulated with a 27 Fr. Avalon Elite Dual Lumen Cannula through the right external jugular traversing the SVC and IVC through a cut down. The cannula was placed under fluoroscopy, and checked under fluoroscopy after suturing the cut down site.
A pre-primed device (1U/ml heparin in normal saline) was connected to the cannula. Mechanical ventilation and inspired oxygen were adjusted to maintain normal blood-gas tensions entering the device. A blend of 95% O2 and 5% CO2 flowed through the device to maintain a normal arterial pCO2. Flowrates were then varied (1–4 L/min) over the 6 hours. At each operating point, hematocrit, PfHb, arterial and device blood gases, and hemodynamics were recorded. Each point was tested three times. Hemodynamics at the start and end of the study were statistically compared using a two-sample t-test. A gross examination of the device after the study was done to look for possible areas of thrombus. A gross examination of the cannula site and vital organs was also done during a necropsy.
Results
The PAAL met all in-vitro performance targets. The PAAL pumped over 250 mmHg at 3.5 L/min at a rotation speed of 2100 RPM, as shown in Figure 2A. The PAAL oxygenates 180 ml/min at 3.5 L/min, shown in Figure 2B with up to 150 ml/min CO2 removal. CO2 removal can be controlled by adjusting sweep gas flowrate as shown in Figure 3. A low NIH of 0.054 ± 0.005 g/100L was achieved for the PAAL including the Avalon DLC at 3.5 L/min as seen in Figure 4. The control condition NIH representing the loop and cannula was 0.02 ±0.007 g/100L.
Figure 2.
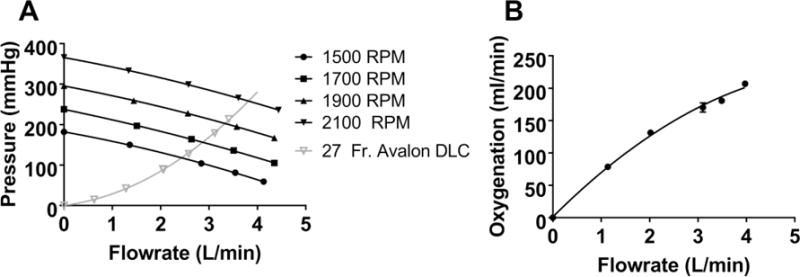
In-vitro pump function (A) and oxygenation (B) of the PAAL device
Figure 3.
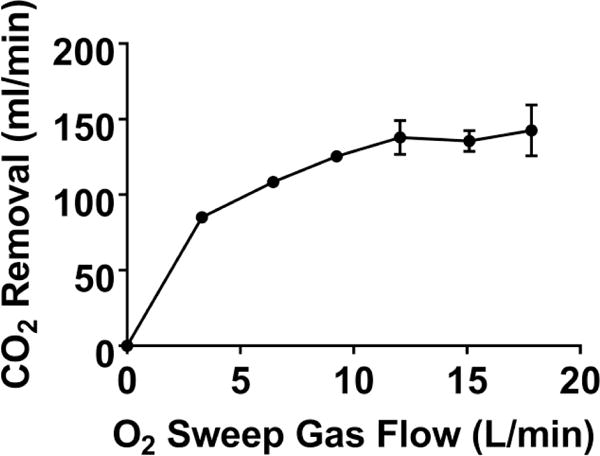
In-vitro CO2 removal of the PAAL device
Figure 4.
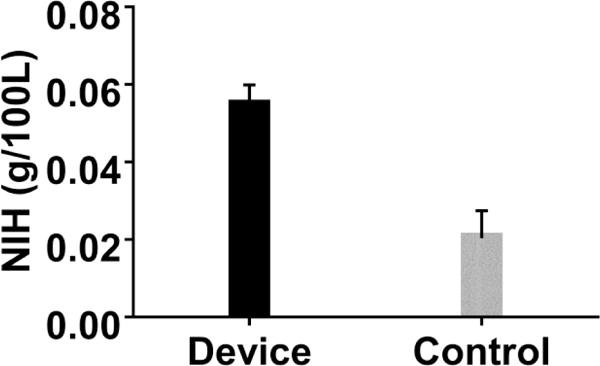
In-vitro hemolysis in the PAAL device
In-vivo, the PAAL pumped as much as 3.8 L/min oxygenating up to 181±15.9 ml/min as shown in Figure 5. At all flowrates, blood left the PAAL 100% saturated. Hemolysis in-vivo for all the animals was low (pfHb < 20 mg/dL) as shown in Figure 6. In Acute 1, a small pfHb spike was seen at 3.5 hours, after which the hemolysis stabilized. In Acutes 2–4, pfHb at the end of the study was at most within 3mg/dL of the animal’s baseline. MAP dropped (p<0.05) from 79 mmHg to 40 mmHg in Acute 1; hemodynamics were stable for Acute 2–4 as shown in Table 1. CVP was increased (p<0.05) by saline bolus for Acute 1 and 4 to prevent suction on the cannula. All flow channels including the pivot bearings in devices tested were free of thrombus formation. Acute 1–2 had bundle small thrombi form, particularly at the inlet to the fiber bundle and the exit port of the device. Acutes 3–4 were free of thrombus formation. The necropsy did not show device related damage to the heart, lungs, kidney or liver.
Figure 5.
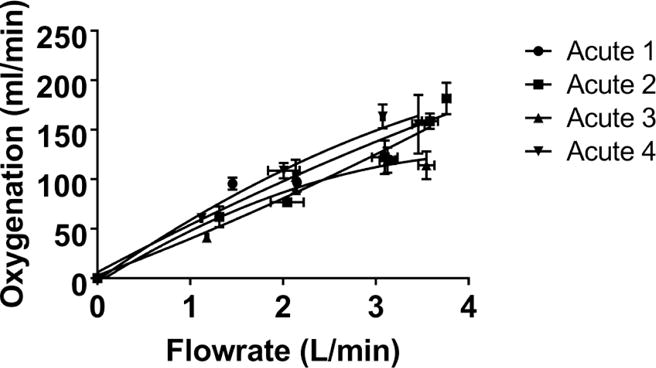
In-vivo gas exchange in the PAAL device
Figure 6.
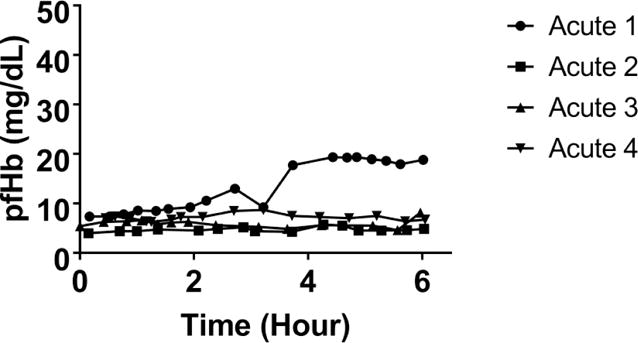
In-vivo hemolysis in the PAAL device
Table 1.
Hemodynamics during the in-vivo study
| MAP (mmHg) | PAP (mmHg) | CVP (mmHg) | HR (BPM) | |||||
|---|---|---|---|---|---|---|---|---|
| Sheep # | Baseline | End | Baseline | End | Baseline | End | Baseline | End |
| 1 | 79 ± 16 | 40 ± 2.0* | 10.0 ± 5.0 | 11 ± 0.8 | 10.0 ± 1.9 | 14 ± 0.9* | 82 ± 2.4 | 65 ± 14 |
| 2 | 79 ± 11 | 74 ± 5 | 29 ± 4.7 | 25 ± 1.9 | 7.9 ± 2.3 | 4.9 ± 0.9 | 64 ± 4.0 | 110 ± 23* |
| 3 | 88 ± 35 | 73 ± 12 | 32 ± 3.4 | 20.0 ± 2.0* | 6.9 ± 1.4 | 4.5 ± 1.8 | 93 ± 14 | 94 ± 6.6 |
| 4 | 110 ± 10 | 100 ± 13 | 19 ± 3 | 22 ± 0.4 | 2.9 ± 2 | 8.0 ± 1* | 120 ± 2.4 | 120 ± 4.5 |
p < 0.05
Discussion
The only viable treatment options for lung failure patients at the end stage is MV and ECMO. These treatments limit patient mobility, driving up already high morbidity and mortality in these patients11,12,13,14,15. Contemporary clinical literature has shown ambulation improves outcomes in lung failure patients19,20 Given this, we are developing the Pittsburgh Ambulatory Assist Lung or PAAL, a truly wearable, compact artificial lung that allows for patient ambulation during BTR or BTT. In this study, we integrated a HFM bundle with a high efficiency of 278 ml/min/m2, much higher than standard blood oxygenators used for ECMO today (160–170ml/min/m2) into a wearable device.21 The PAAL met our design targets and performed successfully in 6h sheep studies.
Our in-vivo study found that performance was consistent with our in-vitro experiments. In-vitro the PAAL produced sufficient oxygenation (180 ml/min) as well as CO2 removal (150 ml/min) at 3.5 L/min while still maintaining a low level of hemolysis (NIH 0.054 g/100L) compared to clinically accepted oxygenators23. Of the measured in-vitro hemolysis, only a portion (60%) is due the device, the rest is attributed to the cannula. We found that the in-vivo oxygenation reached our target at 3.8 L/min, however hemoglobin saturation was 100% at all our test conditions for all inlet saturations (52%–76%), thus underestimated the full potential of the device. In-vivo hemolysis was low in all animals (< 20 mg/dL) validating the low benchtop hemolysis. The flow channels of all devices including pivot bearings were free of thrombus formation. Time course of administering the heparin bolus was not optimized in Acute 1 and 2 due to which ACT did not rise fast enough prior to cannulation (Acute 1) and did not maintain at 2 times baseline (Acute 2). This led to device change out was required in Acute 1, and some small thrombi were seen in Acute 2. Keeping this experience in mind, timing of the initial heparin bolus and drip were improved in Acute 3 and 4 after which bundles were free of thrombus formation. The hypotension in Acute 1 was likely due to an inflammatory response from device implant. Additionally the device change out could have contributed to the inflammatory response as well. Device flow or oxygenation was not compromised despite the drop in MAP.
There are other wearable artificial lung devices under research development for treating patients with lung failure24,25. The ambulatory pump lung (APL) device features a fully magnetically levitated centrifugal pump integrated into a 0.8m2 surface area annular fiber bundle30. The compliant thoracic artificial lung (cTAL) device relies upon native right ventricle function as a pump, and has a 2.4m2 surface area rectangular fiber bundle31. Clinically, the Maquet Cardiohelp is gaining popularity. The Small Adult Cardiohelp (HLS 5.0) features a Rotaflow pump integrated with a Quadrox oxygenator surface area 1.3 m2 into a portable however not wearable (10kg) device21. In comparison to these devices, the PAAL is a wearable device that delivers the same respiratory support at 80% of the surface area of the APL, 27% of the surface area of the cTAL and 50% of the surface area of the Cardiohelp. This creates a more compact artificial lung but also potentially reduces the adverse blood – material interactions associated with a larger blood contacting area. As part of the PAAL project we are also developing novel thromboresistant coatings26. Additionally, dead flow zones which can occur in square cross section oxygenators such as the Quadrox are eliminated in the PAAL as our fiber bundle has a circular cross section.
In conclusion, we have developed a compact integrated wearable artificial lung capable of providing at least 180 ml/min oxygenation and 150 ml/min CO2 removal at low levels of hemolysis. This has been shown through in-vitro and acute in-vivo studies. Future work involves CFD optimization of the device and 5-day chronic studies planned in the near term.
Acknowledgments
This study was supported by NIH grant number RO1 HL117637, the Commonwealth of PA and the McGowan Institute for Regenerative Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
Authors do not have any financial disclosures related to the work presented in this manuscript.
References
- 1.Kirklin JK, Naftel DC, Pagani FD, et al. Seventh INTERMACS annual report: 15,000 patients and counting. The Journal of Heart and Lung Transplantation. 2015;34:1495–1504. doi: 10.1016/j.healun.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Lick SD, Zwischenberger JB. Artificial lung: bench toward bedside. ASAIO journal. 2004;50:2–5. doi: 10.1097/01.mat.0000107282.22793.49. [DOI] [PubMed] [Google Scholar]
- 3.Villar J, Blanco J, Añón JM, et al. The ALIEN study: incidence and outcome of acute respiratory distress syndrome in the era of lung protective ventilation. Intensive Care Med. 2011;37:1932–1941. doi: 10.1007/s00134-011-2380-4. [DOI] [PubMed] [Google Scholar]
- 4.Nieman GF, Gatto LA, Bates JHT, Habashi NM. Mechanical ventilation as a therapeutic tool to reduce ards incidence. Chest. 2015 doi: 10.1378/chest.15-0990. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.MacLaren G, Combes A, Bartlett RH. Contemporary extracorporeal membrane oxygenation for adult respiratory failure: life support in the new era. Intensive Care Med. 2011;38:210–220. doi: 10.1007/s00134-011-2439-2. [DOI] [PubMed] [Google Scholar]
- 6.Garcia JP, Iacono A, Kon ZN, Griffith BP. Ambulatory extracorporeal membrane oxygenation: a new approach for bridge-to-lung transplantation. J Thorac Cardiovasc Surg. 2010;139:137–139. doi: 10.1016/j.jtcvs.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 7.Strueber M. Artificial Lungs: Are We There yet? Thoracic Surgery Clinics. 2015;25:107–113. doi: 10.1016/j.thorsurg.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 8.Biscotti M, Sonett J, Bacchetta M. ECMO as Bridge to Lung Transplant. Thoracic Surgery Clinics. 2015;25:17–25. doi: 10.1016/j.thorsurg.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 9.Maury G, Langer D, Verleden G, et al. Skeletal Muscle Force and Functional Exercise Tolerance Before and After Lung Transplantation: A Cohort Study. American Journal of Transplantation. 2008;8:1275–1281. doi: 10.1111/j.1600-6143.2008.02209.x. [DOI] [PubMed] [Google Scholar]
- 10.Haneya A, Philipp A, Foltan M, et al. First experience with the new portable extracorporeal membrane oxygenation system Cardiohelp for severe respiratory failure in adults. Perfusion. 2012;27:150–155. doi: 10.1177/0267659111432330. [DOI] [PubMed] [Google Scholar]
- 11.Palanzo D, Qiu F, Baer L, Clark JB, Myers JL, Ündar A. Evolution of the Extracorporeal Life Support Circuitry. Artificial Organs. 2010;34:869–873. doi: 10.1111/j.1525-1594.2010.01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang D, Zhou X, Liu X, Sidor B, Lynch J, Zwischenberger JB. Wang-Zwische Double Lumen Cannula—Toward a Percutaneous and Ambulatory Paracorporeal Artificial Lung. ASAIO Journal. 2008;54:606–611. doi: 10.1097/MAT.0b013e31818c69ab. [DOI] [PubMed] [Google Scholar]
- 13.Perme CS, Southard RE, Joyce DL, Noon GP, Loebe M. Early Mobilization of LVAD Recipients Who Require Prolonged Mechanical Ventilation. Tex Heart Inst J. 2006;33:130–133. [PMC free article] [PubMed] [Google Scholar]
- 14.Pruijsten R, van Thiel R, Hool S, Saeijs M, Verbiest M, Miranda DR. Mobilization of patients on venovenous extracorporeal membrane oxygenation support using an ECMO helmet. Intensive Care Med. 2014;40:1595–1597. doi: 10.1007/s00134-014-3410-9. [DOI] [PubMed] [Google Scholar]
- 15.Nolan H, Wang D, Zwischenberger JB. Artificial lung basics. Organogenesis. 2011;7:23–27. doi: 10.4161/org.7.1.14025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maquet Cardiohelp Technical Data Sheet:
- 17.Madhani SP, Frankowski B. ASAIO 60th Annual Conference. Washington, D.C: 2014. Development of a high efficiency Passive exchange Paracorporeal Ambulatory Assist Lung (P-PAAL) Through Bundle Geometry Variation; p. 101. [Google Scholar]
- 18.ISO 7199:2009 Cardiovascular implants and artificial organs – Blood-gas exchangers (oxygenators):, 2009
- 19.ASTM F1841-97 Standard Practice for Assessment of Hemolysis in Continuous Flow Blood Pumps:, 2013
- 20.Koller T, Hawrylenko A. Contribution to the in vitro testing of pumps for extracorporeal circulation. J Thorac Cardiovasc Surg. 1967;54:22–29. [PubMed] [Google Scholar]
- 21.Svitek RG, Frankowski BJ, Federspiel WJ. Evaluation of a Pumping Assist Lung That Uses a Rotating Fiber Bundle. ASAIO J. 2005;51:773–780. doi: 10.1097/01.mat.0000178970.00971.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang J, Taskin ME, Koert A, et al. Computational Design and In Vitro Characterization of an Integrated Maglev Pump-Oxygenator. Artificial Organs. 2009;33:805–817. doi: 10.1111/j.1525-1594.2009.00807.x. [DOI] [PubMed] [Google Scholar]
- 23.Kawahito S, Maeda T, Yoshikawa M, et al. Blood trauma induced by clinically accepted oxygenators. ASAIO journal. 2001;47:492–495. doi: 10.1097/00002480-200109000-00019. [DOI] [PubMed] [Google Scholar]
- 24.Zhang T, Wei X, Bianchi G, et al. A novel wearable pump-lung device: In vitro and acute in vivo study. The Journal of Heart and Lung Transplantation. 2012;31:101–105. doi: 10.1016/j.healun.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schewe RE, Khanafer KM, Arab A, Mitchell JA, Skoog DJ. Cook KE Design and In Vitro Assessment of an Improved, Low-Resistance, Compliant Thoracic Artificial Lung. ASAIO J. 2012;58:583–589. doi: 10.1097/MAT.0b013e31826dcd23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ye S-H, Arazawa DT, Zhu Y, et al. Hollow Fiber Membrane Modification with Functional Zwitterionic Macromolecules for Improved Thromboresistance in Artificial Lungs. Langmuir. 2015;31:2463–2471. doi: 10.1021/la504907m. [DOI] [PMC free article] [PubMed] [Google Scholar]


