Abstract
Objectives
The primary aim of this study was to assess subcortical auditory processing in individuals with chronic symptoms following mild traumatic brain injury (mTBI) by measuring auditory brainstem responses (ABRs) to standard click and complex speech stimuli. Consistent with reports in the literature of auditory problems following mTBI (despite normal hearing thresholds), it was hypothesized that individuals with mTBI would have evidence of impaired neural encoding in the auditory brainstem compared to non-injured controls, as evidenced by delayed latencies and reduced amplitudes of ABR components. We further hypothesized that the speech-evoked ABR would be more sensitive than the click-ABR to group differences due to its complex nature, particularly when recorded in a background noise condition.
Design
Click- and speech-ABRs were collected in 32 individuals diagnosed with mTBI in the past 3–18 months. All mTBI participants were experiencing on-going injury symptoms for which they were seeking rehabilitation through a brain injury rehabilitation management program. The same data were collected in a group of 32 age- and gender-matched controls with no history of head injury. ABRs were recorded in both left and right ears for all participants in all conditions. Speech-ABRs were collected in both quiet and in a background of continuous 20-talker babble ipsilateral noise. Peak latencies and amplitudes were compared between groups, and across subgroups of mTBI participants categorized by their behavioral auditory test performance.
Results
Click-ABR results were not significantly different between the mTBI and control groups. However, when comparing the control group to only those mTBI subjects with measurably decreased performance on auditory behavioral tests, small differences emerged including delayed latencies for waves I, III, and V. Similarly, few significant group differences were observed for peak amplitudes and latencies of the speech-ABR when comparing at the whole group level but were again observed between controls and those mTBI subjects with abnormal behavioral auditory test performance. These differences were seen for the onset portions of the speech-ABR waveforms in quiet, and were close to significant for the onset wave. Across groups, quiet vs. noise comparisons were significant for most speech-ABR measures but the noise condition did not reveal more group differences than speech-ABR in quiet, likely due to variability and overall small amplitudes in this condition for both groups.
Conclusions
The outcomes of this study indicate that subcortical neural encoding of auditory information is affected in a significant portion of individuals with long-term problems following mTBI. These subcortical differences appear to relate to performance on tests of auditory processing and perception, even in the absence of significant hearing loss on the audiogram. While confounds of age and slight differences in audiometric thresholds can’t be ruled out, these preliminary results are consistent with the idea that mTBI can result in neuronal changes within the subcortical auditory pathway that appear to relate to functional auditory outcomes. Although further research is needed, clinical audiological evaluation of individuals with ongoing post-mTBI symptoms is warranted for identification of individuals who may benefit from auditory rehabilitation as part of their overall treatment plan.
Keywords: mild traumatic brain injury, central auditory processing, auditory brainstem response, audiology, concussion, auditory dysfunction
Introduction
Traumatic brain injury (TBI) can result in damage throughout the central nervous system, including sensory and cognitive processing pathways. Mild traumatic brain injury (mTBI), often interchangeably referred to as concussion, accounts for nearly 90% of these injuries but presents increased challenges in diagnosis and treatment. Given that more than 1.5 million TBIs occur in the United States each year (National Center for Injury Prevention and Control 2003; Cassidy et al. 2004), this represents a substantial number of individuals. Growing evidence and reports from sports and military populations suggests that long-term effects of mTBI or concussion are significant, the term “mild” referring to the initial physical injury not the severity of the longer term consequences. While many recover from mTBI within the days and weeks following injury, perhaps 15%–50% of mTBI patients experience persistent post-concussion symptoms beyond the usual 4-week period (Rimel et al. 1981; Bohnen et al. 1993; Ingebrigtsen et al. 1998; Ponsford et al. 2000; Wood 2004; Vanderploeg et al. 2007; Fourtassi et al. 2011). It is now generally accepted that mTBI is more serious than previously thought and that even without sustained loss of consciousness, nerve fibers/axons throughout the brain are susceptible to widespread damage in diffuse axonal injury (DAI), particularly when the brain is exposed to rotational forces (Inglese et al. 2005; Biasca & Maxwell 2007; Browne et al. 2011).
In the auditory system, damage from TBI could include injury to the auditory periphery by direct impact to the temporal bone housing the outer, middle and inner ear or various structures within the central auditory system through focal or diffuse lesions. The auditory brainstem nuclei may be particularly susceptible to the effects of rotational and shearing forces and resulting DAI (Gennarelli & Graham 1998), while the primary auditory cortex may be vulnerable to impact against bony ridges of the sphenoid and temporal bones (Gutierrez-Cadavid 2005). Brain structures in the vulnerable frontal and temporal lobes involved in cognition are also likely to be affected by TBI and have top-down influences on the processing of auditory information.
Excluding blast-related injuries and temporal bone fractures, peripheral hearing may be relatively unaffected or only transiently affected by TBI, especially in mild compared to more moderate or severe injuries (Barber 1969; Munjal et al. 2010a). Despite this, disproportionate rates of auditory problems such as difficulty listening in background noise, inability to remember and follow oral instructions, and difficulty understanding rapid or degraded speech following have been reported following TBI (Jury & Flynn 2001; Lew et al. 2007a; Lew et al. 2007b). Existing research suggests that between 16% to >50%, of individuals who sustain TBI have evidence of central auditory dysfunction (Cockrell & Gregory 1992; Bergemalm & Borg 2001; Bergemalm 2003; Bergemalm & Lyxell 2005; Flood et al. 2005). It is likely that much of this auditory dysfunction results from DAI within the central auditory pathway.
Determining where and how severely mTBI affects neural processing within the central auditory system and/or higher level structures that affect auditory processing would provide insight into both diagnosis and prediction of outcomes in individuals with mTBI, for whom standard clinical neuroimaging tests including head computed tomography (CT) and magnetic resonance (MR) imaging scans are typically normal. Auditory evoked potentials (AEPs) offer a potentially sensitive tool to assess neuronal encoding of fine timing of acoustic information at multiple levels within this pathway, including subcortical and cortical structures. The long term goals of this research are to determine the sensitivity of AEPs representing various levels of processing within the central auditory system to mTBI and their relationship to post-injury auditory deficits. While it is hoped that this knowledge will lead to improved clinical assessment and individualized intervention and monitoring of recovery for those with mTBI, this initial stage is designed to establish whether some proportion of individuals with mTBI have evidence of deficits in neural processing within the central auditory nervous system. The current study specifically examines neural processing at the subcortical level in a group of individuals who are symptomatic following mTBI, meaning they are not among those who recover quickly and spontaneously but have long-term consequences.
At the subcortical level, the auditory brainstem response (ABR) is a highly sensitive measure of synchronous neuronal response to an acoustic stimulus. As the accuracy of sound encoding at this sensory, precognitive level influences the input to higher-level structures in the auditory processing pathway, it is important to determine whether mTBI-related damage may disrupt neuronal function at this level. The majority of studies evaluating auditory brainstem function after TBI have used the standard ABR evoked by a click stimulus (click-ABR). Results of such studies have been mixed, but abnormal click-ABR responses in individuals have been most commonly reported in the acute phase after injury and as severity of TBI increases (Greenberg et al. 1977; Hall et al. 1983; Ottaviani et al. 1986; Fligor et al. 2002; Munjal et al. 2010b). In milder forms of TBI, significant changes in ABR latencies have not always been observed (Schoenhuber et al. 1988; Nolle et al. 2004). There are some studies that have demonstrated prolonged inter-peak latencies in individuals with mTBI (Rowe & Carlson 1980; Noseworthy et al. 1981; Schoenhuber & Gentilini 1986; Soustiel et al. 1995). However, even those studies showing significant click-ABR latency differences found little evidence of correlation with post-concussion functional outcomes (Schoenhuber & Gentilini 1986; Soustiel et al. 1995).
Evoked potentials from the brainstem can also be recorded by complex stimuli, such as a speech syllable, which may provide a more sensitive indicator of neural encoding differences in individuals with mTBI. The neural response to such complex sounds consists of both sustained and transient features, which encode both transient and periodic features of a sound stimulus. The complex ABR provides a more behaviorally relevant stimulus, in this case speech, and it has been demonstrated that the response to a complex sound provides additional information about brainstem processing not predictable from the response to the simpler click stimulus (Song et al. 2006; Aiken & Picton 2008; Johnson et al. 2008). The ABR evoked by speech (speech-ABR) is a precise temporal representation of the events in a consonant-vowel stimulus, with transient components encoding syllable onset and offset, and a sustained portion of the response encoding the periodicity of the fundamental frequency and vowel formants. In clinical populations, significant differences in speech-ABRs have been shown between children with and without auditory learning disabilities (Wible et al. 2004; Banai et al. 2005; Wible et al. 2005) and between groups of older and younger adults (Vander Werff & Burns 2011; Anderson et al. 2012). The response has also been shown to have some relationship with speech processing in the presence of background noise (Anderson et al. 2011; Parbery-Clark et al. 2011). The speech-ABR, therefore may prove useful as an objective indicator of impaired neural encoding of important time-varying speech features in the subcortical central auditory system in individuals following mTBI.
In the current study, both click- and speech-evoked ABRs were recorded in a population of individuals with mTBI experiencing ongoing post-concussion problems 3–18 months post-injury. That is, the measures were taken at a non-acute stage and with knowledge of whether the individual was experiencing long-term consequences of concussion. Subjects were part of a larger study examining auditory behavioral performance, cognitive performance, and AEP recordings at brainstem, cortical and cognitive levels. The goals of this particular study were to determine whether ABR responses to clicks and complex speech stimuli were significantly different in mTBI subjects compared to controls, and whether there was a relationship between ABR results and auditory behavioral test outcomes in this population. If ABR results are sensitive to mTBI and/or mTBI-related auditory deficits, they may prove a useful inclusion to multi-disciplinary clinical assessment in this population.
Methods
Participants
The experimental group consisted of 32 individuals (22 female), aged 19–61 years (mean 42.3 ± 13.1) with confirmed mTBI using diagnostic criteria consistent with those established by the American Congress of Rehabilitation Medicine (ACRM, 1993). Participants were recruited from a concussion management and rehabilitation program where they were being evaluated and/or treated for ongoing post-concussion problems. No participants were recruited who were within an initial acute or semi-acute stage (<3 months) following injury. To be enrolled, subjects were required to be 18–60 years of age and within 3–18 months post-injury with no other significant past head injuries requiring medical treatment. Penetrating trauma, blast, and crush injuries were excluded, along with temporal bone fracture or direct injury to the outer ear. Other exclusionary criteria included a pre-injury history of significant neurologic disorders (e.g. multiple sclerosis, seizure disorders, Parkinson’s disease), mental illness or psychological disorders (e.g. schizophrenia, bipolar disorder), or diagnosed learning disability or attention deficit disorders. All subjects were native speakers of English. In order to reduce the influence of peripheral hearing loss on auditory outcomes, all subjects were required to have no more than a mild hearing loss as described in the following section.
A control group of age- and gender-matched participants with no history of concussion/TBI was also recruited. This group consisted of 32 adults (22 female), aged 18–60 years (mean 41.2 ± 12.7). Participants in the control group were required to meet all exclusion criteria listed above. All subjects completed a series of questionnaires including case history information and symptoms questionnaires and participated in a battery of audiological, neuropsychological, and electrophysiological tests. In this paper, the brainstem electrophysiological measures and their comparison to the audiological outcomes are discussed.
Audiometric and auditory behavioral test measures
A comprehensive audiometric evaluation was conducted on all subjects including pure tone air and bone conduction thresholds, tympanometry, acoustic reflex thresholds, and distortion product otoacoustic emissions (DPOAEs). Any participants with hearing losses greater than 40 dB hearing level (HL; ANSI, 2010) as defined by the pure-tone average of 1.0, 2.0 and 4.0 kHz, or evidence of middle ear pathology by tympanometry or air-bone differences, were excluded from further participation in order to limit the influence of peripheral hearing loss on brainstem electrophysiological measures. As shown in Figure 1, mean audiograms were similar between groups. T-tests revealed no significant differences for individual frequencies in the left or right ears, with the exception of 1000 Hz in the right ear, where the mean threshold was 11.7 dB HL for the mTBI group and 7.7 dB HL for the control group (p = 0.038).
Figure 1.
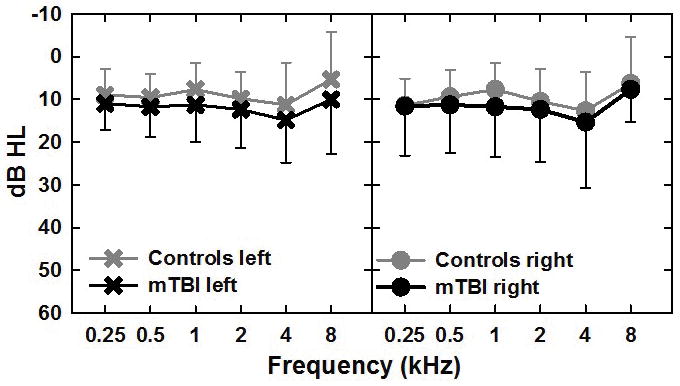
Mean pure-tone air-conduction audiometric thresholds in dB HL for the left and right ears for the control (gray symbols) and mTBI (black symbols) groups from 250 to 8000 Hz. Error bars are standard deviation and are shown in the up and down direction for the control and mTBI groups respectively for clarity.
Those who passed the initial audiological testing then completed a series of behavioral auditory tests. All tests were administered in a double-walled sound-treated booth using CD recordings via the GSI-61 audiometer and ER-3A insert phones. Calibration was performed prior to each test using the recorded calibration tones on each CD and all tests were presented at 50 dB sensation level (SL) re: the .5, 1.0 and 2.0 kHz average or at 60 dB HL as indicated in the test instructions. The battery of tests included monaural word recognition in quiet (WR Quiet) and binaural word recognition in a background of noise (WR Bin +10). Separate 50-word NU-6 lists (Tillman & Carhart 1966) were used for each condition and ear for these word recognition tests. The binaural word recognition in noise test was performed at a constant +10 signal-to-noise ratio (SNR) using 20-talker babble pre-recorded on the second track of the same CD from Auditec of St. Louis. The babble used in this test was the same babble used for the background noise condition for the speech-ABR testing.
The remaining battery of auditory behavioral tests included the Words in Noise test (WIN, Wilson & Burks 2005; Wilson & McArdle 2007), time-compressed speech recognition (NU-6 words with carrier phrases, 65% compression), the Gaps in Noise test (GIN, Musiek et al. 2005), and the Staggered Spondaic Word test (Katz & Smith 1991). All tests were performed and scored according to the provided standard instructions for each test.
It is important to note that this battery of tests is not inclusive of all tests recommended for clinical assessment and diagnosis of central auditory processing disorders (CAPD; American Academy of Audiology 2010), nor is the diagnosis of CAPD proposed for individuals in this study. The battery was also not specifically focused on measures known to be sensitive to brainstem lesions. As part of the larger study that included several hours and multiple sessions of testing AEPs at brainstem, cortical and cognitive levels, a battery of cognitive/neuropsychological tests, the auditory behavioral test battery was chosen to concentrate mainly on measures of speech in noise and temporal resolution tests, along with the dichotic listening to correlate with functional outcomes as well as AEP measures at multiple levels.
Electrophysiological test parameters
Click-and speech-evoked auditory brainstem responses (ABRs) were collected and analyzed separately for left and right ears of all subjects using the Bio-logic Navigator Pro System (Bio-logic Systems Corp., Natus Medical Inc., Mundelein, IL). All ABRs were recorded using a 2-channel montage, with electrodes at Cz (non-inverting), mastoids (inverting), and forehead (ground). Contact impedances were all less than 5 kΩ and within 1.5 kΩ of each other. Stimuli were presented through unshielded insert earphones (ER-3A, Etymotic Research, Elk Grove Village, IL). Click-ABRs were recorded in response to a 100-μs click presented at a rate of 11.1/sec at 80 dB nHL (103 dB ppeSPL). Responses were band pass filtered from 30–3000 Hz, using 256 sampling points over a 16-ms time window. Individual traces exceeding 15 μV were rejected online. Two repetitions of 1500 artifact-free sweeps were collected for each ear.
Speech-ABRs were recorded in responses to a 40-ms synthesized syllable/da/utilized in previous studies (Vander Werff & Burns 2011) and obtained from the Auditory Neuroscience Lab of Nina Kraus and colleagues at Northwestern University. Stimuli were presented using the custom stimulus option in the standard Bio-logic AEP software (version 6.1.0) at a rate of 11.1/sec. Stimulus levels were 80 dB SPL as measured using a 2cc DB-0138 coupler, Bruel & Kjaer Type 2203 sound level meter, and 1-inch microphone. Alternating polarity was used to minimize stimulus artifact. Responses were band pass filtered online from 100–2000 Hz, using 512 digital sampling points over a 74.67 ms epoch. Two repetitions of 2000 artifact-free sweeps were recorded for each ear. Speech-ABRs were collected under two conditions in each ear: quiet and in a background of speech babble. The level of the 20-talker babble was 70 dB SPL to maintain a +10 dB SNR.
Subjects were seated in a comfortable, reclining chair in a double-walled sound-treated booth with 1/3 octave band ambient noise floor measurements were lower than maximum permissible levels for testing with or without headphones (ANSI S3.1–1999, R 2008). Lights were dimmed and subjects were encouraged to relax with their eyes closed and sleep if possible. No formal assessment of subject state was conducted.
Analyses
Click-ABR waveforms for each ear were analyzed by averaging the two replications and visually identifying peaks I, III, and V. Speech-ABR waveforms were similarly analyzed by averaging the two repetitions for each ear and visually identifying peaks V, A, C, D, E, F, and O, using nomenclature previously established for the speech-ABR (Russo, N. et al. 2004; Johnson et al. 2005). The transient response was considered to consist of the onset (waves V and A) and the offset (wave O) responses to the syllable, while the sustained response was considered to consist of peaks D, E, and F reflecting the timing of the fundamental and formant frequencies of the vowel. Peak C may represent a transition between the consonant burst and vowel, and was marked in those subjects for whom it could be reliably identified but was not analyzed statistically due to the relatively small number of subjects for whom it was present.
Peaks were visually determined within latency windows based on the literature and our previous paper (Vander Werff & Burns 2011) as well as ongoing data collection for the current study. Peaks were judged as absent if they were not replicable between traces or not reliably above the noise floor for the individual’s average waveform. In addition, waveforms for individual conditions judged to be contaminated by significant artifact were eliminated from analyses. This artifact was primarily observed in the sustained portion of the speech-ABR as large peaks unlikely to represent the true neural response and thought to be related to post-auricular muscle artifact. Data were not usable from 5 right and 7 left ears for controls in quiet, and 5 right and 8 left ears from controls in noise. For the mTBI group 4 right and 4 left ears were excluded from quiet condition analysis, and 5 right and 5 left ears in the noise condition. The excluded ears were not always from the same subjects in the quiet and noise conditions.
Components of the speech-ABR were further analyzed using custom MATLAB (version R2013a, MathWorks, Inc., Natick, MA) routines developed by Erika Skoe and Trent Nicol at Northwestern University (Brainstem Toolbox 2010). Peak latencies and amplitudes were automatically adjusted from those picked visually in the software (within ± 2 sampling points) to obtain the absolute minimum or maximum values.
Statistical analyses
Mixed model analysis of variance (ANOVA) was used to test for significant group differences (control vs. mTBI) with repeated factors of ear (right and left) for click-ABR peak latencies and amplitudes and with an additional repeated factor of condition (quiet and noise, nested within each ear) for speech-ABR peak latencies and amplitudes. Point-by-point t-tests comparisons were calculated to evaluate significant differences across waveforms and groups. T-tests were also conducted within each ear and condition (e.g. right quiet, left quiet, right noise, left noise) to evaluate for significant differences in latency and amplitude between subgroups of individuals with normal or abnormal auditory behavioral test performance. All statistical tests were considered to be significant at p < .05. It should be noted that the multiple time point t-tests and those of the subgroups by condition were not corrected for multiple comparisons, which increases the possibility of type I errors. The results should therefore be interpreted with appropriate caution.
Results
Click-ABR
Grand mean click-ABRs for the left and right ears for the control group compared to those of all mTBI subjects are shown in the left panels of Figure 2. There were no significant differences between grand mean group waveforms at each time point found by between-group independent t-test comparisons of amplitude, meaning the mTBI and control groups had similar click-ABR average morphology.
Figure 2.
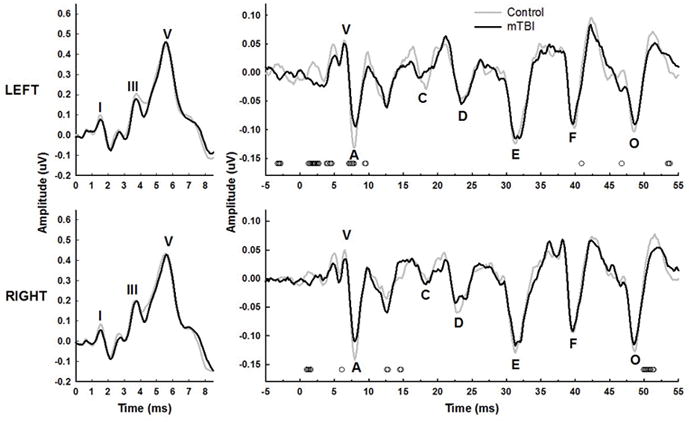
Grand mean click- (left panels) and speech-evoked (right panels) ABRs in the quiet condition for the control (gray lines) and mTBI (black lines) groups. Waveforms recorded from left ears are shown in the top panels, and right ears in the bottom panels. General location of individual peaks in the group mean waveforms are marked in each panel. Small open symbols at the bottom of the panels represent significant p-values (p<0.05) for between group independent t-tests of amplitude at each time point. There were no significant t-tests within the time window shown for the click-ABRs for either ear.
Absolute peak amplitudes were picked for each individual waveform and averaged, as shown in Table 1, and absolute and interpeak latencies are shown in Table 2. Statistics were conducted on these means of individually picked peaks, rather than on the peaks in the grand mean waveform. Using two-way mixed model ANOVA (group x ear, results shown in Tables 1 and 2), there were no significant group differences for the absolute latencies or amplitudes for waves I, III, or V between the control and mTBI groups or by left vs. right ears overall. There were also no significant differences in interpeak latencies by group or ear, although there was a significant ear x group interaction for the I–III (F1,53 = 6.42, p = 0.014) and I–V intervals (F1,56 = 4.74, p = 0.034). For the I–III interval, the mTBI group had slightly shorter I–III intervals, more so for the right ear than the left. I–V intervals for the right ear intervals were the same between groups, while the mTBI group had slightly shorter I–V intervals than the control group in the left ear. These results appear to be due to slightly delayed wave I latencies in the mTBI group, although this difference in wave I latency did not reach statistical significance. Overall, the click-ABR showed little evidence of brainstem level processing differences of click stimuli between controls and individuals with mTBI at the whole group level.
Table 1.
Mean (s.d.) click- and speech-ABR amplitude (μV) values
| Left | Right | RMANOVA results (p-values) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Controls | All mTBI | Controls | All mTBI | Group | Ear | Cond. | Interactions | |||
| Amplitude Click-ABR | I | 0.10 (0.19) | 0.15 (0.26) | 0.10 (0.16) | 0.18 (0.25) | 0.224 | 0.705 | -- | NS | |
| III | 0.24 (0.20) | 0.26 (0.33) | 0.28 (0.21) | 0.32 (0.22) | 0.617 | 0.095 | -- | NS | ||
| V | 0.42 (0.24) | 0.50 (0.30) | 0.42 (0.21) | 0.50 (0.25) | 0.021 | 0.967 | -- | NS | ||
|
| ||||||||||
| Amplitude Speech-ABR Quiet | V | 0.09 (0.05) | 0.09 (0.05) | 0.08 (0.04) | 0.07 (0.05) | 0.057 | 0.624 | <0.001 | NS | |
| A | −0.16 (0.05) | −0.14 (0.06) | −0.17 (0.05) | −0.15 (0.07) | 0.322 | 0.212 | <0.001 | NS | ||
| D | −0.09 (0.08) | −0.10 (0.09) | −0.10 (0.06) | −0.09 (0.07) | 0.206 | 0.839 | <0.001 | 0.051 | G x E x C | |
| E | −0.16 (0.09) | −0.18 (0.07) | −0.04 (0.04) | −0.06 (0.05) | 0.923 | 0.900 | <0.001 | NS | ||
| F | −0.16 (0.09) | −0.14 (0.07) | −0.16 (0.09) | −0.14 (0.09) | 0.453 | 0.745 | 0.933 | NS | ||
| O | −0.16 (0.09) | −0.14 (0.07) | −0.16 (0.06) | −0.16 (0.07) | 0.877 | 0.906 | 0.023 | 0.034 | E x C | |
| V-A amp | 0.25 (0.09) | 0.24 (0.10) | 0.25 (0.07) | 0.24 (0.10) | 0.829 | 0.237 | <0.001 | NS | ||
| V-A area | 0.16 (0.07) | 0.15 (0.07) | 0.16 (0.06) | 0.13 (0.06) | 0.468 | 0.766 | <0.001 | 0.054 | G x C | |
|
| ||||||||||
| Amplitude Speech-ABR Noise | V | 0.04 (0.04) | 0.05 (0.05) | 0.04 (0.03) | 0.06 (0.05) | |||||
| A | −0.12 (0.05) | −0.11 (0.06) | −0.13 (0.04) | −0.12 (0.08) | ||||||
| D | −0.04 (0.04) | −0.06 (0.05) | −0.04 (0.05) | −0.07 (0.05) | ||||||
| E | −0.12 (0.09) | −0.14 (0.07) | −0.13 (0.09) | −0.12 (0.07) | ||||||
| F | −0.14 (0.09) | −0.17 (0.09) | −0.15 (0.09) | −0.14 (0.09) | ||||||
| O | −0.13 (0.07) | −0.15 (0.08) | −0.13 (0.06) | −0.14 (0.07) | ||||||
| V-A amp | 0.15 (0.07) | 0.18 (0.08) | 0.18 (0.06) | 0.19 (0.09) | ||||||
| V-A area | 0.10 (0.06) | 0.12 (0.07) | 0.12 (0.05) | 0.13 (0.08) | ||||||
NS = no significant interactions present; G = group, E = ear, C = condition (quiet vs.
Table 2.
Mean (s.d.) click- and speech-ABR peak latency (μV) values
| Left | Right | RMANOVA results (p-values) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Controls | All mTBI | Controls | All mTBI | Group | Ear | Cond. | Interactions | |||
| Latency Click-ABR | I | 1.57 (0.13) | 1.67 (0.19) | 1.58 (0.13) | 1.63 (0.18) | 0.120 | 0.515 | -- | NS | |
| III | 3.77 (0.20) | 3.79 (0.22) | 3.72 (0.23) | 3.79 (0.21) | 0.499 | 0.215 | -- | NS | ||
| V | 5.80 (0.22) | 5.87 (0.29) | 5.78 (0.23) | 5.88 (0.29) | 0.175 | 0.558 | -- | NS | ||
| I–III | 2.13 (0.21) | 2.11 (0.18) | 2.20 (0.18) | 2.15 (0.21) | 0.395 | 0.594 | -- | 0.014 | G x E | |
| III–V | 2.08 (0.17) | 2.07 (0.20) | 2.05 (0.18) | 2.07 (0.20) | 0.757 | 0.443 | -- | NS | ||
| I–V | 4.21 (0.22) | 4.19 (0.29) | 4.24 (0.21) | 4.24 (0.29) | 0.998 | 0.954 | -- | 0.034 | G x E | |
|
| ||||||||||
| Latency Speech-ABR Quiet | V | 6.70 (0.34) | 6.82 (0.51) | 6.68 (0.32) | 7.00 (0.64) | 0.160 | 0.008 | <0.001 | 0.006 | G x E |
| A | 7.87 (0.42) | 7.99 (0.48) | 7.91 (0.32) | 8.09 (0.70) | 0.181 | 0.002 | <0.001 | 0.039 | G x E | |
| D | 23.30 (0.76) | 23.61 (0.84) | 23.36 (1.15) | 23.89 (1.33) | 0.189 | 0.243 | 0.011 | NS | ||
| E | 31.58 (0.66) | 31.56 (0.70) | 31.45 (0.81) | 31.80 (0.88) | 0.368 | 0.377 | 0.585 | NS | ||
| F | 39.80 (0.72) | 39.80 (0.73) | 39.62 (0.50) | 39.91 (0.80) | 0.323 | 0.941 | 0.050 | NS | ||
| O | 48.43 (0.57) | 48.63 (0.67) | 48.55 (0.42) | 48.71 (0.75) | 0.241 | 0.195 | 0.006 | NS | ||
| V-A lat | 1.18 (0.30) | 1.17 (0.29) | 1.24 (0.22) | 1.09 (0.25) | 1.000 | 0.680 | 0.382 | NS | ||
| V-A slope | −0.22 (0.09) | −0.21 (0.10) | −0.21 (0.07) | −0.23 (0.12) | 0.511 | 0.289 | <0.001 | NS | ||
|
| ||||||||||
| Latency Speech-ABR Noise | V | 6.86 (0.31) | 6.95 (0.60) | 6.84 (0.32) | 7.11 (0.64) | |||||
| A | 7.98 (0.28) | 8.17 (0.57) | 8.03 (0.30) | 8.36 (0.74) | ||||||
| D | 23.59 (1.12) | 24.39 (1.55) | 23.11 (0.44) | 23.90 (1.31) | ||||||
| E | 31.32 (0.65) | 31.58 (0.79) | 31.37 (0.57) | 31.62 (0.90) | ||||||
| F | 39.75 (0.59) | 39.90 (0.92) | 39.78 (0.60) | 39.96 (0.81) | ||||||
| O | 48.53 (0.70) | 48.71 (0.65) | 48.74 (0.53) | 48.77 (0.58) | ||||||
| V-A lat | 1.11 (0.30) | 1.21 (0.29) | 1.19 (0.26) | 1.25 (0.30) | ||||||
| V-A slope | −0.14 (0.05) | −0.15 (0.07) | −0.15 (0.04) | −0.17 (0.08) | ||||||
NS = no significant interactions present; G = group, E = ear, C = condition (quiet vs.
Speech-ABR in Quiet and Noise
Grand mean speech-ABRs recorded in quiet for the two groups are shown in the right panels of Figure 2 for the left and right ears separately. A trend for smaller transient peaks at the onset waves V and A can be observed for the mTBI group for both ears, as well as some decrease in the size of wave O. Point-by-point independent t-tests revealed some significant group differences across the waveforms, as shown by the small open circles at the bottom of each panel, primarily for the onset and offset time periods. Figure 3 shows the grand mean speech-ABR waveforms for the two groups recorded in the presence of multi-talker babble noise. Overall, amplitudes for both groups and both ears were reduced compared to the quiet waveforms shown in Figure 3. A smaller but still visible reduction in the size of the onset complex for the mTBI group compared to controls can still be observed for both ears. A few points along the waveforms, primarily at wave A for the left and right ears, were found to significantly differ between groups in the point-by-point t-test comparisons.
Figure 3.
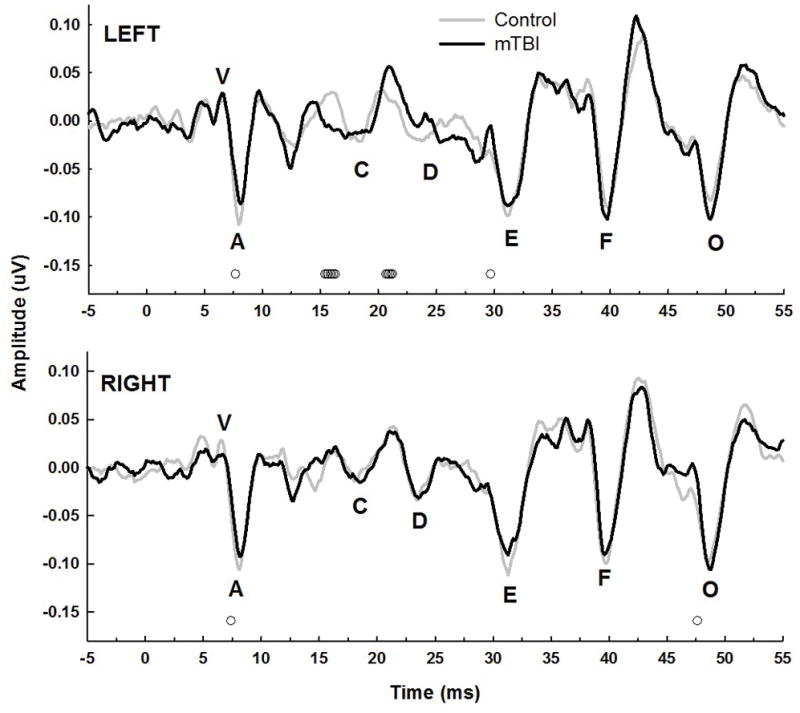
Speech-evoked ABRs in the noise condition for the control and mTBI groups. Symbols, lines, and scaling are the same as in the right panels of Figure 2.
Mean amplitudes and latencies for the speech-ABR peaks are shown in Tables 1 and 2 respectively, along with the results of the three-way (group x ear x condition) mixed model ANOVA analyses conducted for each. P-values for each main effect and significant interactions are shown in the rightmost columns of each table. As shown in Table 1, amplitude values were not significantly different overall by group or by ear for any of the absolute peak amplitudes or for the V-A amplitude difference or V-A area measurement. The effect of condition, quiet vs. noise, was significant for all but wave F (F1,43 = 0.01, p = 0.933), meaning that the amplitudes were significantly smaller in noise compared quiet across all subjects in both groups. The ear x condition interaction was significant for wave O overall. The condition difference was seen more in the right ear than in the left across groups for this offset wave.
The three-way interaction approached significance for wave D (F1,43 = 4.03, p = 0.051), with the smallest average amplitude (−0.03 μV) seen for the controls in the right noise condition compared to any other combination of ear and condition in either group. The group x condition interaction also approached significance for V-A area (F1,41 = 3.93, p = 0.054) with quiet areas greater than noise for the control group (areas = .16, .12, .16, .10 for right quiet and noise and left quiet and noise areas respectively) but not the mTBI group (.14, .14, .16, .13). Overall, these results indicate that on the whole group level, amplitude trends between groups seen in the grand mean waveform data are generally not robust enough to translate into significant differences in marked peak amplitude between control and concussion participants by this analysis.
Latency analyses were conducted the same way as described for amplitude and are shown in Table 2. As with peak amplitude, there were no significant main effects of group in terms of absolute peak latency or the V-A latency difference or slope. There were significant latency differences between ears for peaks V (F1,42 = 7.78, p = 0.008) and A (F1,42 = 11.02, p = .002), as well as significant group x ear interactions for both peaks (F1,42 = 8.29 and 4.52; p = .006 and 0.039 for V and A respectively). In both cases, the right ear latencies were longer than the left ear latencies for peaks V and A in the mTBI group, while for the controls right ear latency was longer than left for wave A, but the opposite was true for wave V . The effect of condition was not as consistent for latency as it was for amplitude, as it did not reach significance for the V-A latency difference or wave E, and was right at the significance level for wave F. There were no other significant interactions.
In summary, absolute peak amplitude and latency did not differ significantly between the controls and the mTBI participants on the whole group level for any peaks. There were few significant ear differences (latency of waves V and A) across the entire pool of subjects. The most robust effects were that peaks were generally smaller and more delayed in noise compared to quiet conditions. However, overall, the quiet vs. noise differences were not group-specific. A few exceptions were seen, such as the longer right ear latencies for the concussion subjects compared to controls.
Relationships between ABR and auditory test performance
Because of inherent variability across individuals with head injury involving factors including, but certainly not limited to, type and mechanism of injury, place and force of impact, loss of consciousness, and other important but less quantifiable factors, patterns of findings at the whole group level are often difficult to observe. In characterizing neural processing of acoustic information at the brainstem level, it may be important to consider whether, on an individual basis, there is evidence of functional auditory problems. As an initial probe of this question, click- and speech-evoked ABR characteristics were compared to performance classifications based on the battery of auditory behavioral tests completed as part of the larger study.
Individual performance on each of the auditory behavioral tests was categorized as abnormal or normal based on available test norms and/or published data. Performance was considered abnormal for a particular test if scores for one or both ears (when tested individually) fell outside of the specified cutoff from test manuals and published normative studies for normal performance. Cumulatively, the mTBI group performed abnormally on 56% of the auditory behavioral tests, with one or more abnormal test results obtained from 22 of the 32 individuals. Comparatively, 19% of all of the tests were abnormal for controls, with only 6 individuals showing abnormal performance on any tests. In most cases the few control subjects who were classified as abnormal were just outside of the normal range for the individual test. The highest percentage of abnormal test results in the mTBI group were obtained for the SSW (50%), WIN (38%), and the GIN (22%) tests, with 19% abnormal on both WR Bin+10 and TC speech. All mTBI participants performed normal on WR Quiet.
Correlations between ABR variables and performance on auditory behavioral tests were examined for the mTBI group overall. This resulted in an extensive number of comparisons given the number of conditions and outcome variables, therefore results are not reported here. The majority of significant correlations were found between the right ear speech-ABR in quiet and noise and behavioral test scores. Figure 4 shows some examples of variables which were significantly correlated between ABR and behavioral performance.
Figure 4.
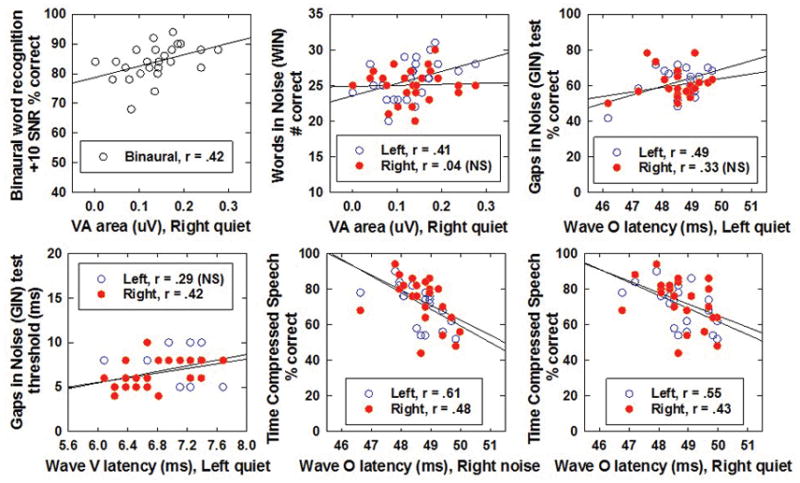
Correlations between speech-ABR variables and auditory behavioral performance on selected tests. Data are shown for the left (open) and right (filled) ears of the speech-ABR in all panels except the top left (averaged across ears), which is a binaural word recognition score in noise. Note that the speech-ABR variable (X-axis) and the behavioral test scores (Y-axis) change in each panel.
In order to better understand the overall relationship between ABR results and auditory test performance, the mTBI group was divided into two subgroups based on normal or abnormal performance on the auditory behavioral tests, as shown in Table 3. Group A consisted of those who had mostly normal auditory test performance, with no abnormal or only one abnormal auditory test result (n=19). Those who performed abnormally on 2 or more of the auditory behavioral tests were considered Group B (n = 9). Despite abnormal performance on most of the auditory behavioral tests, 4 of the mTBI subjects (labelled as subgroup IE for insufficient effort in Table 3) were excluded from further analysis. Their exclusion was due to abnormal performance on multiple cognitive tests and a test of effort, the Medical Symptom Validity Test (Green 2004), completed as part of the neuropsychological test battery – meaning it could not be determined if their behavioral test performance was abnormal due to low effort or auditory dysfunction. It is important to note that these 4 subjects performed abnormally, with very poor scores, on almost all auditory behavioral tests and if their low effort were unknown could be inappropriately classified as having auditory processing problems.
Table 3.
| Subject | Sub-group | Age (yrs) | Gender | Right PTA | Left PTA | Post-injury (mos) | Type/Cause of injury | Area of head |
|---|---|---|---|---|---|---|---|---|
| MIT02 | A | 24 | M | 1.7 | −3.3 | 4.1 | MVAa/direct impact | Left side |
| MIT05 | A | 29 | F | 11.7 | 16.7 | 12.3 | MVA/Fall from height | Forehead, Left side |
| MIT06 | A | 35 | F | 6.7 | 10.0 | 9.0 | MVA/direct impact | Forehead |
| MIT11 | A | 28 | F | 10.0 | 11.7 | 12.8 | MVA/direct impact | Forehead, Left side |
| MIT12 | A | 49 | F | 15.0 | 16.7 | 10.0 | Fall from height | Back |
| MIT13 | A | 31 | F | 13.3 | 8.3 | 7.8 | MVA/direct impact | Left side |
| MIT14 | A | 49 | F | 11.7 | 13.3 | 4.1 | Sportb/fall | No direct blow to head |
| MIT16 | A | 36 | F | 10.0 | 8.3 | 18.4 | Blow to head/fall | Face |
| MIT19 | A | 20 | F | 6.7 | 5.0 | 6.6 | MVA/accel & decelc | No direct blow to head |
| MIT20 | A | 60 | M | 10.0 | 15.0 | 4.2 | MVA/hit by car | Unknown |
| MIT23 | A | 41 | M | 8.3 | 8.3 | 6.3 | Other | Left side, Back |
| MIT24 | A | 48 | M | 11.7 | 10.0 | 4.2 | Fall ground level | Back |
| MIT29 | A | 48 | F | 10.0 | 5.0 | 8.7 | MVA | Forehead, Left side, Back, Face |
| MIT30 | A | 46 | F | 6.7 | 10.0 | 8.4 | Fall ground level | Left side, Back |
| MIT31 | A | 53 | F | 10.0 | 13.3 | 4.5 | Fall | Back |
| MIT32 | A | 18 | F | 8.3 | 10.0 | 4.8 | Blow to head | Right side |
| MIT34 | A | 21 | F | 3.3 | 5.0 | 3.6 | Sport | Right side |
| MIT36 | A | 19 | F | 10.0 | 15.0 | 2.9 | Sport | Top |
| MIT37 | A | 49 | M | 8.3 | 11.7 | 3.8 | MVA | Back |
| 37.0 ± 13.2 | 14F, 5M | 9.1 ± 3.2 | 10.0 ± 4.9 | 7.2 ± 4.0 | ||||
| MIT07 | B | 41 | F | 16.7 | 18.3 | 4.5 | MVA/direct impact | Right side, Back |
| MIT09 | B | 49 | M | 5.0 | 5.0 | 5.6 | Assault | Left side |
| MIT21 | B | 53 | F | 35.0 | 31.7 | 16.5 | Blow to head | Right side |
| MIT22 | B | 56 | M | 11.7 | 13.3 | 4.4 | Blow to head | Right side, Back |
| MIT26 | B | 60 | F | 16.7 | 6.7 | 7.1 | Fall from height | Left side, Back |
| MIT27 | B | 52 | F | 10.0 | 10.0 | 3.7 | Assault | Right side, Left side |
| MIT28 | B | 51 | F | 10.0 | 6.7 | 5.1 | Assault | Forehead |
| MIT33 | B | 35 | M | 11.7 | 11.7 | 3.1 | MVA/blow to head | Right side |
| MIT35 | B | 58 | F | 13.3 | 10.0 | 5.1 | Fall ground level | Right side |
| 50.6 ± 8.0 | 6 F, 3 M | 14.4 ± 8.5 | 12.6 ± 8.2 | 5.7 ± 3.5 | ||||
| MIT01 | IE | 39 | M | 15.1 | 30.0 | 5.5 | MVA/direct impact | Left side, Top |
| MIT04 | IE | 58 | M | 21.0 | 18.3 | 4.0 | MVA/accel & decel | No direct blow to head |
| MIT10 | IE | 40 | F | 15.1 | 3.3 | 3.1 | Fall ground level | Back |
| MIT25 | IE | 59 | F | 22.5 | 21.7 | 6.5 | Blow to head | Back |
| 49.0 ± 11.0 | 2 F, 2M | 18.4 ± 3.9 | 18.3 ± 11.1 | 4.8 ± 1.5 |
MVA = motor vehicle accident; includes motor vehicle and motorcycle accidents and pedestrians hit by vehicles
includes traditional sports, equestrian, recreational activities
acceleration/deceleration injuries including whiplash
Click-evoked ABR comparisons between grand mean waveforms for the control group and subgroups A and B are shown in the top panels of Figure 5 for the left and right ears. Although overall morphologies are still similar, the solid lines of subgroup B appear to be slightly shifted compared to controls or group A for waves I and V particularly. Point-by-point t-tests, however showed no significant differences between controls and either subgroup at any time point within the window shown. The onset and offset portions of speech-ABR waveforms for the left and right ears in quiet and in noise for the three subgroups are shown in the bottom two rows of panels in Figure 5. Again, some differences in waveforms appear for some of the onset and offset portions of the waveform, more visible for the responses recorded in quiet. There were significant point-by-point differences between the control grand mean waveform and the group B (abnormal performance) waveform at several time points for both quiet and noise (open circles), while there were only a few significantly different time points in the onset portion of the left quiet waveform between controls and group A (open triangles).
Figure 5.
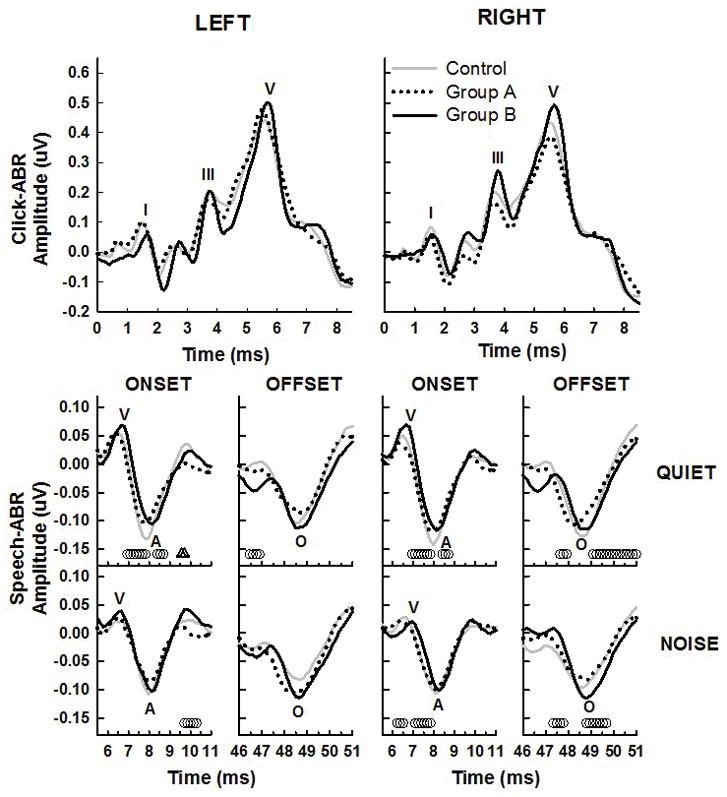
Grand mean waveforms for the control group (gray lines) compared to the two subgroups of mTBI participants. Individuals in group A, who had normal behavioral auditory test performance, are shown with dotted lines and those in group B, with abnormal auditory test performance, are shown with solid black lines. The top panels show the click-ABR and the bottom two rows of panels are the speech-ABR in quiet and in noise. Left ear responses are in the left columns and right ear in the right columns. Partial speech-ABR waveforms are shown to highlight the onset (waves V and A) and offset portions (wave O) of the waveforms only for clarity. Small open symbols at the bottom of the panels represent significant p-values (p<0.05) for t-tests of amplitude at each time point between controls and each subgroup, with the triangles representing group A and the circles representing group B.
Table 4 shows the mean and standard deviations of latency and amplitude values of peaks for the subgroups for click- and speech-ABR. Univariate independent two-tail t-tests were performed to compare amplitude and latency values between controls and each of the two subgroups of mTBI participants, those with normal behavioral audiological outcomes (group A) and those with abnormal audiological outcomes (group B). Separate t-tests were performed for right ear in quiet, right ear in noise, left ear in quiet, and left ear in noise.
Table 4.
Mean (s.d.) speech-ABR amplitude (μV) and latency (ms) values for mTBI subgroups
| Left | Right | ||||
|---|---|---|---|---|---|
| Group A | Group B | Group A | Group B | ||
| Amplitude Click-ABR | I | 0.19 (0.33) | 0.10 (0.10) | 0.20 (0.32) | 0.14 (0.13) |
| III | 0.33 (0.33) | 0.27 (0.14) | 0.35 (0.26) | 0.32 (0.17) | |
| V | 0.55 (0.35) | 0.51 (0.21) | 0.55 (0.30) | 0.48 (0.19) | |
|
| |||||
| Amplitude Speech-ABR Quiet | V | 0.10 (0.05) | 0.09 (0.06) | 0.09 (0.06) | 0.05 (0.02) |
| A | −0.15 (0.07) | −0.14 (0.03) | −0.16 (0.07) | −0.14 (0.03) | |
| D | −0.11 (0.10) | −0.10 (0.05) | −0.08 (0.08) | −0.10 (0.06) | |
| E | −0.18 (0.08) | −0.19 (0.06) | −0.05 (0.05) | −0.09 (0.04) | |
| F | −0.14 (0.08) | −0.15 (0.07) | −0.15 (0.09) | −0.15 (0.06) | |
| O | −0.13 (0.05) | −0.15 (0.10) | −0.17 (0.08) | −0.15 (0.07) | |
| V-A amp | 0.25 (0.12) | 0.23 (0.07) | 0.26 (0.10) | 0.19 (0.06) | |
| V-A area | 0.16 (0.08) | 0.15 (0.06) | 0.15 (0.05) | 0.10 (0.04) | |
|
| |||||
| Amplitude Speech-ABR Noise | V | 0.07 (0.05) | 0.03 (0.03) | 0.07 (0.05) | 0.04 (0.05) |
| A | −0.12 (0.07) | −0.12 (0.05) | −0.12 (0.08) | −0.11 (0.06) | |
| D | −0.05 (0.05) | −0.09 (0.04) | −0.07 (0.05) | −0.09 (0.05) | |
| E | −0.15 (0.08) | −0.13 (0.04) | −0.12 (0.08) | −0.13 (0.06) | |
| F | −0.17 (0.10) | −0.13 (0.06) | −0.14 (0.10) | −0.14 (0.09) | |
| O | −0.17 (0.09) | −0.12 (0.07) | −0.14 (0.07) | −0.13 (0.07) | |
| V-A amp | 0.20 (0.08) | 0.15 (0.06) | 0.21 (0.08) | 0.17 (0.07) | |
| V-A area | 0.14 (0.08) | 0.10 (0.03) | 0.14 (0.08) | 0.13 (0.05) | |
|
| |||||
| Left | Right | ||||
| Group A | Group B | Group A | Group B | ||
|
| |||||
| Latency Click-ABR | I | 1.64 (0.22) | 1.70 (0.16) | 1.59 (0.18) | 1.68 (0.19) |
| III | 3.73 (0.22) | 3.83 (0.19) | 3.71 (0.20) | 3.87 (0.18) | |
| V | 5.82 (0.33) | 5.90 (0.21) | 5.84 (0.34) | 5.89 (0.20) | |
|
| |||||
| Latency Speech-ABR Quiet | V | 6.76 (0.54) | 6.81 (0.44) | 6.99 (0.78) | 6.93 (0.24) |
| A | 7.93 (0.52) | 7.99 (0.39) | 8.13 (0.85) | 7.97 (0.28) | |
| D | 23.55 (0.82) | 23.72 (0.99) | 23.90 (1.50) | 23.49 (1.04) | |
| E | 31.46 (0.84) | 31.60 (0.40) | 31.69 (0.98) | 32.05 (0.60) | |
| F | 39.82 (0.74) | 39.91 (0.57) | 39.85 (0.91) | 40.06 (0.59) | |
| O | 48.53 (0.65) | 48.72 (0.75) | 48.55 (0.75) | 49.10 (0.65) | |
| V-A lat | 1.17 (0.32) | 1.19 (0.29) | 1.14 (0.27) | 1.04 (0.21) | |
| V-A slope | −0.23 (0.12) | −0.19 (0.04) | −0.26 (0.12) | −0.19 (0.04) | |
|
| |||||
| Latency Speech-ABR Noise | V | 6.95 (0.67) | 6.76 (0.23) | 7.16 (0.74) | 7.06 (0.36) |
| A | 8.20 (0.59) | 7.89 (0.33) | 8.37 (0.87) | 8.37 (0.45) | |
| D | 24.42 (1.49) | 23.83 (1.42) | 23.95 (1.43) | 23.41 (0.70) | |
| E | 31.49 (0.73) | 31.45 (0.78) | 31.54 (0.99) | 31.62 (0.58) | |
| F | 39.91 (0.98) | 40.16 (0.64) | 39.97 (0.94) | 39.93 (0.57) | |
| O | 48.61 (0.68) | 48.95 (0.69) | 48.64 (0.63) | 49.12 (0.41) | |
| V-A lat | 1.25 (0.32) | 1.12 (0.26) | 1.21 (0.31) | 1.31 (0.32) | |
| V-A slope | −0.16 (0.07) | −0.14 (0.06) | −0.19 (0.09) | −0.14 (0.06) | |
Group A = normal auditory behavioral test performance, Group B = abnormal auditory behavioral test performance
For the click-ABR, subgroup A (with normal auditory test performance) had significantly larger wave V amplitudes than controls (.55 μV vs. .43 μV; t96 = −2.373, p = .020), which was the only significant latency or amplitude difference between these two groups. However, group B with abnormal auditory performance had significantly delayed wave I (1.70 vs. 1.50 ms; t77 = −2.304, p = .024), wave III (3.86 vs. 3.74 ms; t71 = −2.041, p = .045), and wave V (5.91 vs. 5.78 ms; t76 = −2.155, p = .033) latencies compared to controls. These results suggest that although there were no significant differences in click ABR latencies at the whole group level between the mTBI group and controls, those with mTBI who also exhibited decreased auditory behavioral test performance had evidence of significantly delayed click-ABR latencies compared to controls. Between groups A and B, only the latency of wave III differed significantly (t52 = −2.419, p = .019).
Speech-ABR peak amplitudes were not significantly different between the controls and group A for any of the peaks, except for the amplitude of wave V in the left noise condition, where group A (with normal auditory performance) had a significantly larger mean amplitude in noise (0.06 μV) than did the controls (0.4 μV) (t39 = −2.33, p= 0.027). All other amplitude comparisons between controls and group A were non-significant. Comparisons between amplitudes between controls and group B, however, showed significantly larger amplitudes for controls for waves V (t33 = 3.00, p = 0.008), D (t29 = 2.60, p = 0.025), the V-A amplitude difference (t33 = 2.56, p = 0.024), and the V-A area (t33 = 3.02, p = 0.009). The wave A amplitude also approached significant difference between groups (t33 = −2.01, p = 0.062). All of these were significant for the right quiet condition, with the exception of wave D which was for the left noise condition. None of the amplitude comparisons between the two subgroups reached significance.
Similar comparisons performed on peak latencies showed no significant differences between the control group and group A for any of the peak latency variables. The concussion participants who had abnormal auditory performance, however, had significantly longer latencies than controls for waves V (t33 = −2.47, p = 0.026), E (t32 = −2.30, p = 0.036) and the V-A latency difference (t33 = 2.28, p = 0.042), and approached significance for wave O (t33 = −2.22, p = 0.054). All of these differences were found for the right ear in quiet only. Again, none of the latency comparisons between the two subgroups for speech-ABR reached significance.
Differences in demographic variables by subgroups were assessed using independent t-tests, with adjustments for unequal variance where appropriate as indicated by Levene’s test. The overall control and mTBI groups were matched by age and gender, and as described, the PTA did not differ between groups. However, the mean age of group B with abnormal behavioral results and differences in brainstem processing was 50.6 ± 8.0 years and range of 32–60 years. Compared to group A with a mean age of 37.0 ± 13.2 years and range of 18–60. The mean age of the control group was 41.2 ± 12.7 years, with a range of 18–60. The age differences between group B and group A (t24 = −3.32, p = 0.003) and group B and controls (t22 = −2.47, p = 0.022) were significant, but there was no significant age difference between group A and controls (t49 = 1.20, p = 0.236). In Figure 6, an example of the relationship between age and wave O latency of the speech-ABR is shown. This figure demonstrates that while there was a relationship between increasing age and longer wave O latencies in controls (p < 0.005), it was not significant for the mTBI subjects (p =0.21). This suggests that both age and mTBI have an effect on some ABR variables, and this potential interaction between age and mTBI that should be studied further in a larger study.
Figure 6.
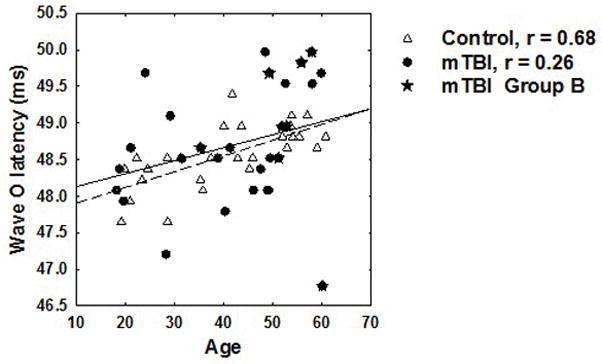
Relationship between age and the latency of wave O (offset) of the speech-evoked ABR. Open triangles represent control subjects and the regression is shown as a dashed line. The solid line represents the regression for the mTBI subjects represented by filled symbols. The star symbols indicate the subjects in Group B with abnormal auditory performance. Correlation coefficients for each group are shown in the legend.
Neither the left (t26 = 0.65, p = −1.079) nor the right (t9 = −1.844, p = 0.098) PTA differed significantly between the two mTBI subgroups. There were no significant differences in PTA between the control group and group A for either ear, but there was a significant difference in the right PTA (t39 = −2.29, p = .027) between the control group (9.2 ± 5.5 dB HL) and group B (14.6 dB ± 8.5 dB HL). Time between injury and testing was similar between subgroups, with subgroup B tested at 6.1 ± 4.0 months and group A at 7.2 ± 4.0 months, which was not a significant difference (t26 = 0.65, p = 0.521).
To summarize, individually picked peak amplitudes and latencies showed no significant differences at the whole group level. There were some significant correlations between ABR and auditory test performance variables in the mTBI group and importantly, when comparing controls to those with abnormal audiological test performance, click-ABRs showed delayed latencies and speech-ABRs showed both reduced amplitudes and delayed latencies for some peaks. These differences for the speech-ABR were seen primarily in the right ear in the quiet condition. The mTBI subjects with normal audiological performance on the other hand had no significant reduction in amplitude or reduced latency compared to controls, and in fact had larger amplitudes than controls in some conditions (click-ABR wave V amplitude; speech-ABR wave V amplitude for left ear in noise). Differences in age and PTA, with group B being older and having poorer overall PTA than group A may contribute to these results.
Discussion
The objective of the current study was to compare and characterize neural encoding of subcortical auditory processing in individuals with a recent history of mTBI and ongoing post-concussion symptoms compared to non-injured controls. All of the mTBI participants had at maximum a mild hearing loss, with most presenting clinically normal hearing sensitivity, yet the majority reported auditory problems. It is well known that auditory information processing at both cortical and subcortical levels is critical to speech understanding. The structures in the auditory brainstem provide precise encoding of fine spectrotemporal features of sound which allow us to identify the features of individual consonant and vowel sounds and patterns in ongoing speech. If axonal injury within the brainstem structures occurs in mTBI, it could result in a degraded representation of this acoustic information being passed on to higher levels of processing in the cortex.
At the overall group level, there were no differences in click-ABR observed between mTBI and control subjects. Click-ABR abnormalities have not been consistently observed in previous TBI literature, except in more severe injuries. In a recent study, Gallun et al (2012) found that click-ABRs did not differ significantly between a group of 29 normal controls and a group of blast-exposed subjects, 19 out of 55 of whom were diagnosed with mTBI (the remaining had a diagnosis of no TBI). In that previous study, there were no group differences in terms of peak amplitudes or latencies even though the blast-exposed subjects, similar to the mTBI subjects in the current study, had a higher rate of abnormal performance on behavioral tests of central auditory processing. In another study, Nolle et al (2004) did not find group differences in ABR, but did report differences in otoacoustic emission suppression and stapedial reflex thresholds in a group of 31 patients with post-concussion syndrome (4–17 months post-injury), possibly indicating major sites-of-lesion in the brainstem and auditory midbrain. Other published studies have shown some evidence that even mTBI can result in auditory dysfunction at the brainstem level as evidenced by delayed peak latencies for the click-ABR (Rowe & Carlson 1980; Noseworthy et al. 1981; Schoenhuber & Gentilini 1986; Soustiel et al. 1995). However, only a few of these studies (Schoenhuber & Gentilini 1986; Soustiel et al. 1995) have indicated any correlation between ABR results and functional outcomes such as long-lasting post-concussion symptoms or the scores on neuropsychological tests. Our results provide some link between click-ABR and functional outcomes, indicating that only when comparing the control group to those mTBI subjects with measurably decreased performance on auditory behavioral tests did small differences in the click-ABR emerge, including delayed latencies for waves I, III, and V.
No known published studies have examined the speech-evoked ABR in the mTBI population, although it has been used to study other populations with auditory processing dysfunction. Because difficulty listening in background noise is one of the biggest complaints, reported by 50% of the mTBI subjects as frequently a problem and 38% as sometimes a problem, we hypothesized that recording the speech-ABR in background noise would potentially be sensitive to group differences in neural processing. Noise condition was significant for most speech-ABR absolute amplitudes and latencies; however, contrary to our hypothesis, the noise condition did not reveal more group differences than recording the speech-ABR in quiet. This is likely due to the smaller amplitude and higher variability in the quiet waveforms of the mTBI group even in the quiet condition. The changes in variables between the quiet and noise conditions were therefore not as great.
Although some trends were observed in the grand mean waveforms, as with the click-ABR, few significant group differences were observed for peak amplitudes and latencies of the speech-ABR when comparing the control group to all mTBI subjects. However, there were relationships between ABR variables and outcomes on speech tests in the mTBI group, and when divided into subgroups, some significant differences were observed between controls and those mTBI subjects with abnormal behavioral auditory test performance. These differences were primarily observed for the onset portions of the waveforms in quiet, and were close to significant for the onset wave. While not reported here, we did not find any group differences or relationship to behavioral measures in sustained measures such as the overall RMS energy or energy at the fundamental or higher formant frequencies. That is, differences were observed for the transient portions rather than the sustained portions of the speech-ABR. This may reflect a decrease in synchronous activity of neurons in the brainstem to the fast components of speech. It would be valuable to further examine the sustained responses in comparison to more specific tests of temporal processing such as pitch or frequency pattern testing in a larger study.
Overall, there were not many significant differences in ABRs between left vs. right stimulation in this study. However, when the subgroups were examined, most of the significant differences between the poor auditory behavioral test performers in the mTBI group compared to controls were seen in the right quiet condition. A right ear advantage for some portions of the speech-ABR has been reported by Hornickel et al. (2009), but not consistently observed for all components (Ahadi et al. 2014) or in all studies (Vander Werff & Burns 2011). Although injury variables certainly complicate whether ear asymmetries may exist in mTBI, it is interesting that that observed differences were most apparent in the right ear.
The outcomes of this study indicate that subcortical sensory processing may be affected by mTBI on a long-term basis for some, but not all, individuals with chronic post-concussion problems. These subcortical neural processing differences appear to relate to performance on tests of auditory processing and perception, even in the absence of significant hearing loss on the audiogram. This finding is in agreement with other studies that have suggested possible central auditory processing deficits despite normal audiometric results in blast-exposed Veteran populations (Fausti et al. 2009; Folmer et al. 2011; Gallun et al. 2012) and sports concussion populations (Turgeon et al. 2011), although these previous papers have not shown brainstem level differences between mTBI and control populations. We also attempted to restrict the time frame between injury and testing which often varies between days and years, and to limit our population to only those who reported ongoing post-concussion problems after 3 months and up to 18 months following injury. Therefore any observed differences were not acute or transient changes, nor were they several years post-injury. This is also a civilian population without additional complication of blast injury and noise trauma, which may represent different or more complex mechanisms of injury.
It is difficult to rule out some other confounding variables that could account for these results, including demographic and injury factors. The individuals in the mTBI subgroup with abnormal auditory performance and some reduced amplitudes and delayed latencies in click- and speech-evoked ABR were significantly older than the other mTBI subgroup and the controls. While there is evidence of aging related changes in the speech-ABR in older adults (Vander Werff & Burns 2011; Anderson et al. 2012), these previous studies have only included younger (<30 year old) and older (>60 year old) adults. The subjects in the current study largely fell between these ages, yet there was some evidence of an age-effect at least in controls that was not the same in the mTBI group (at least for the offset latency of speech-ABR). There was also a small (5 dB) but significant difference in the right PTA between the control group and mTBI group B. Although it can’t be ruled out, it seems unlikely that relatively small differences in pure-tone thresholds between groups would account for the observed supra-threshold ABR results. However, the combination of age, PTA, and injury variables could influence the findings in this study. Due to the small number of subjects in the subgroups, more complex ANOVA testing with covariates was not carried out. It will be critical, however, to follow up with larger studies examining the interaction and controlling for the influence of these variables.
Injury variables may also contribute to the small but significant differences observed between subgroups as well. Time between injury and testing was similar between subgroups, with subgroup B tested at 6.1 ± 4.1 months and group A at 7.2 ± 4.0 months, which was not a significant difference (t26 = 0.65, p = 0.521). However, it was not possible to control other injury factors such as the direction, location, and nature of the impact. As shown in Table 3, the majority of the participants in the current study suffered a direct blow to the head (75%) or acceleration/deceleration injuries (19%), mostly from motor vehicle accidents (38%) or falls (22%). DAI has been reported as a consistent and predominant feature in transportation and sports-related injuries (Meythaler et al. 2001). The amount and direction of rotational or linear acceleration forces each individual received may relate to the likelihood of axonal injury and brainstem level pathology. In a mTBI model in swine, Browne et al. (2011) have shown that axonal pathology was significantly greater for injuries with axial rotation compared to coronal rotation of the head. In both planes of injury, axonal pathology was found in the frontal lobe, cerebellum, the midbrain, and the brainstem, but was far greater for the brains of animals injured in the axial plane especially in the occipital lobe, cerebellum, and brainstem. In humans, rather than in animal models, most injuries are likely a combination of these forces causing both focal and diffuse injuries (Gennarelli et al. 1998).
Just as a small but significant minority of those with mTBI experience long term but life-altering problems, some but not all of these individuals may have particular problems processing auditory information. Given the millions of mTBIs seen in military, sports, accidents, and other injuries, this preliminary evidence suggests that auditory evaluations may be an important part of multi-disciplinary assessment in this population. Although for the relatively small samples in the subgroups in the current study these contributing variables, particularly the confound of age difference between groups, can’t be excluded from contributing to the differences in brainstem processing of auditory information, it is interesting that a subset of individuals with mTBI had both reduced performance on auditory behavioral tests and evidence of delayed latencies and reduced amplitudes for portions of the click- and especially the speech-evoked ABR. Further research controlling for these factors may help illuminate whether different age-groups or injury types are more susceptible to auditory brainstem dysfunction. The inclusion of a test of effort to provide an indication whether behavioral test results can be interpreted as reliable was another important component of this study and the classification of subgroups. Further results of the auditory and cognitive behavioral test performance as well as cortical event-related potential from the current study will be presented in upcoming publications to provide a broader picture of the possible effects of mTBI on the central auditory system and auditory processing.
Conclusions and clinical implications
Audiological evaluation of individuals with mTBI is recommended and warranted, in both civilian and military populations, as a significant portion of individuals may experience auditory processing difficulty that can be identified and remediated. The heterogeneity of TBI presents a significant problem for all fields of research and clinical practice, and the results of this study are in line with the idea that individuals with mTBI cannot be treated as a homogenous clinical population. Although further research is needed to better understand the relationship between auditory processing and overall mTBI outcomes, it has been shown in other populations that both subcortical and cortical processing can be affected by remediation including auditory training (Tremblay, K. et al. 2001; Tremblay, K. L. & Kraus 2002; Hayes et al. 2003; Russo, N. M. et al. 2005; Murphy et al. 2011). As axonal damage in mTBI is typically not visible on conventional imaging it is possible that both behavioral and objective audiological assessment may also help identify more subtle pathology in both acute and chronic post-injury stages as part of a multi-disciplinary team approach. Such information and collaboration could lead to better individualized treatment plans for those with long-term problems associated with mTBI.
Acknowledgments
Source of Funding: Funded by: NIH/National Institute on Deafness and Other Communication Disorders, R03 DC010246
References
- Ahadi M, Pourbakht A, Jafari AH, et al. Effects of stimulus presentation mode and subcortical laterality in speech-evoked auditory brainstem responses. Int J Audiol. 2014;53:243–249. doi: 10.3109/14992027.2013.866281. [DOI] [PubMed] [Google Scholar]
- Aiken SJ, Picton TW. Human cortical responses to the speech envelope. Ear Hear. 2008;29:139–157. doi: 10.1097/aud.0b013e31816453dc. [DOI] [PubMed] [Google Scholar]
- American Academy of Audiology. Clinical Practice Guidelines. 2010. Diagnosis, treatment and management of children and adults with central auditory processing disorder. [Google Scholar]
- American National Standards Institute. American National Standard Maximum Permissible Ambient Noise Levels for Audiometric Test Rooms. 2008. (ANSI S3.1–1999, R2008) [Google Scholar]
- Anderson S, Parbery-Clark A, White-Schwoch T, et al. Aging affects neural precision of speech encoding. J Neurosci. 2012;32:14156–14164. doi: 10.1523/JNEUROSCI.2176-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S, Parbery-Clark A, Yi HG, et al. A Neural Basis of Speech-in-Noise Perception in Older Adults. Ear Hear. 2011 doi: 10.1097/AUD.0b013e31822229d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banai K, Nicol T, Zecker SG, et al. Brainstem timing: implications for cortical processing and literacy. Journal of Neuroscience. 2005;25:9850–9857. doi: 10.1523/JNEUROSCI.2373-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber HO. Head injury audiological and vestibular findings. Annals of Otology, Rhinology and Laryngology. 1969;78:239–252. doi: 10.1177/000348946907800204. [DOI] [PubMed] [Google Scholar]
- Bergemalm PO. Progressive hearing loss after closed head injury: a predictable outcome? Acta Otolaryngol. 2003;123:836–845. doi: 10.1080/00016480310002474. [DOI] [PubMed] [Google Scholar]
- Bergemalm PO, Borg E. Long-term objective and subjective audiologic consequences of closed head injury. Acta Otolaryngol. 2001;121:724–734. doi: 10.1080/00016480152583674. [DOI] [PubMed] [Google Scholar]
- Bergemalm PO, Lyxell B. Appearances are deceptive? Long-term cognitive and central auditory sequelae from closed head injury. Int J Audiol. 2005;44:39–49. doi: 10.1080/14992020400022546. [DOI] [PubMed] [Google Scholar]
- Biasca N, Maxwell WL. Minor traumatic brain injury in sports: a review in order to prevent neurological sequelae. Prog Brain Res. 2007;161:263–291. doi: 10.1016/S0079-6123(06)61019-4. [DOI] [PubMed] [Google Scholar]
- Bohnen N, Jolles J, Verhey FR. Persistent neuropsychological deficits in cervical whiplash patients without direct headstrike. Acta Neurol Belg. 1993;93:23–31. [PubMed] [Google Scholar]
- Browne KD, Chen XH, Meaney DF, et al. Mild traumatic brain injury and diffuse axonal injury in swine. J Neurotrauma. 2011;28:1747–1755. doi: 10.1089/neu.2011.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy JD, Carroll LJ, Peloso PM, et al. Incidence, risk factors and prevention of mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med. 2004:28–60. doi: 10.1080/16501960410023732. [DOI] [PubMed] [Google Scholar]
- Cockrell JL, Gregory SA. Audiological deficits in brain-injured children and adolescents. Brain Inj. 1992;6:261–266. doi: 10.3109/02699059209029667. [DOI] [PubMed] [Google Scholar]
- Fausti SA, Wilmington DJ, Gallun FJ, et al. Auditory and vestibular dysfunction associated with blast-related traumatic brain injury. J Rehabil Res Dev. 2009;46:797–810. doi: 10.1682/jrrd.2008.09.0118. [DOI] [PubMed] [Google Scholar]
- Fligor BJ, Cox LC, Nesathurai S. Subjective hearing loss and history of traumatic brain injury exhibits abnormal brainstem auditory evoked response: a case report. Arch Phys Med Rehabil. 2002;83:141–143. doi: 10.1053/apmr.2002.26813. [DOI] [PubMed] [Google Scholar]
- Flood GM, Dumas HM, Haley SM. Central auditory processing and social functioning following brain injury in children. Brain Inj. 2005;19:1019–1026. doi: 10.1080/02699050500110223. [DOI] [PubMed] [Google Scholar]
- Folmer RL, Billings CJ, Diedesch-Rouse AC, et al. Electrophysiological assessments of cognition and sensory processing in TBI: Applications for diagnosis, prognosis and rehabilitation. Int J Psychophysiol. 2011 doi: 10.1016/j.ijpsycho.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Fourtassi M, Hajjioui A, Ouahabi AE, et al. Long term outcome following mild traumatic brain injury in Moroccan patients. Clin Neurol Neurosurg. 2011;113:716–720. doi: 10.1016/j.clineuro.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Gallun FJ, Diedesch AC, Kubli LR, et al. Performance on tests of central auditory processing by individuals exposed to high-intensity blasts. J Rehabil Res Dev. 2012;49:1005–1025. doi: 10.1682/jrrd.2012.03.0038. [DOI] [PubMed] [Google Scholar]
- Gennarelli TA, Graham DI. Neuropathology of the Head Injuries. Semin Clin Neuropsychiatry. 1998;3:160–175. [PubMed] [Google Scholar]
- Gennarelli TA, Thibault LE, Graham DI. Diffuse Axonal Injury: An Important Form of Traumatic Brain Damage. The Neuroscientist. 1998;4:202–215. [Google Scholar]
- Green P. Green’s Medical Symptom Validity Test (MSVT). User’s manual. Edmonton, Canada: Green’s Publishing; 2004. [Google Scholar]
- Greenberg RP, Becker DP, Miller JD, et al. Evaluation of brain function in severe human head trauma with multimodality evoked potentials. Part 2: Localization of brain dysfunction and correlation with posttraumatic neurological conditions. J Neurosurg. 1977;47:163–177. doi: 10.3171/jns.1977.47.2.0163. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Cadavid JE. Imaging of head trauma. In: Latchow RE, Kucharczyk J, Moselely ME, editors. Imaging of the Nervous System. Philadelphia: Elsevier Mosby; 2005. pp. 869–904. [Google Scholar]
- Hall JW, 3rd, Huangfu M, Gennarelli TA, et al. Auditory evoked responses, impedance measures, and diagnostic speech audiometry in severe head injury. Otolaryngol Head Neck Surg. 1983;91:50–60. doi: 10.1177/019459988309100110. [DOI] [PubMed] [Google Scholar]
- Hayes EA, Warrier CM, Nicol TG, et al. Neural plasticity following auditory training in children with learning problems. Clin Neurophysiol. 2003;114:673–684. doi: 10.1016/s1388-2457(02)00414-5. [DOI] [PubMed] [Google Scholar]
- Hornickel J, Skoe E, Kraus N. Subcortical laterality of speech encoding. Audiol Neurootol. 2009;14:198–207. doi: 10.1159/000188533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingebrigtsen T, Waterloo K, Marup-Jensen S, et al. Quantification of post-concussion symptoms 3 months after minor head injury in 100 consecutive patients. J Neurol. 1998;245:609–612. doi: 10.1007/s004150050254. [DOI] [PubMed] [Google Scholar]
- Inglese M, Makani S, Johnson G, et al. Diffuse axonal injury in mild traumatic brain injury: a diffusion tensor imaging study. Journal of Neurosurgery. 2005;103:298–303. doi: 10.3171/jns.2005.103.2.0298. [DOI] [PubMed] [Google Scholar]
- Johnson KL, Nicol T, Zecker SG, et al. Developmental plasticity in the human auditory brainstem. J Neurosci. 2008;28:4000–4007. doi: 10.1523/JNEUROSCI.0012-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KL, Nicol TG, Kraus N. Brain stem response to speech: a biological marker of auditory processing. Ear Hear. 2005;26:424–434. doi: 10.1097/01.aud.0000179687.71662.6e. [DOI] [PubMed] [Google Scholar]
- Jury MA, Flynn MC. Auditory and vestibular sequelae to traumatic brain injury: a pilot study. N Z Med J. 2001;114:286–288. [PubMed] [Google Scholar]
- Katz J, Smith PS. The Staggered Spondaic Word Test. A ten-minute look at the central nervous system through the ears. Annals of the New York Academy of Sciences. 1991;620:233–251. doi: 10.1111/j.1749-6632.1991.tb51587.x. [DOI] [PubMed] [Google Scholar]
- Lew HL, Jerger JF, Guillory SB, et al. Auditory dysfunction in traumatic brain injury. Journal of Rehabilitation Research and Development. 2007a;44:921–928. doi: 10.1682/jrrd.2007.09.0140. [DOI] [PubMed] [Google Scholar]
- Lew HL, Thomander D, Chew KT, et al. Review of sports-related concussion: Potential for application in military settings. J Rehabil Res Dev. 2007b;44:963–974. doi: 10.1682/jrrd.2006.12.0169. [DOI] [PubMed] [Google Scholar]
- Meythaler JM, Peduzzi JD, Eleftheriou E, et al. Current concepts: diffuse axonal injury-associated traumatic brain injury. Arch Phys Med Rehabil. 2001;82:1461–1471. doi: 10.1053/apmr.2001.25137. [DOI] [PubMed] [Google Scholar]
- Munjal SK, Panda NK, Pathak A. Audiological deficits after closed head injury. J Trauma. 2010a;68:13–18. doi: 10.1097/TA.0b013e3181c9f274. discussion 18. [DOI] [PubMed] [Google Scholar]
- Munjal SK, Panda NK, Pathak A. Dynamics of hearing status in closed head injury. J Neurotrauma. 2010b;27:309–316. doi: 10.1089/neu.2009.0957. [DOI] [PubMed] [Google Scholar]
- Murphy CF, Fillippini R, Palma D, et al. Auditory training and cognitive functioning in adult with traumatic brain injury. Clinics (Sao Paulo) 2011;66:713–715. doi: 10.1590/S1807-59322011000400030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musiek FE, Shinn JB, Jirsa R, et al. GIN (Gaps-In-Noise) test performance in subjects with confirmed central auditory nervous system involvement. Ear and Hearing. 2005;26:608–618. doi: 10.1097/01.aud.0000188069.80699.41. [DOI] [PubMed] [Google Scholar]
- National Center for Injury Prevention and Control. Report to Congress on Mild Traumatic Brain Injury in the United States: Steps to Prevent a Serious Public Health Problem. Atlanta, GA: Centers for Disease Control and Prevention; 2003. [Google Scholar]
- Nolle C, Todt I, Seidl RO, et al. Pathophysiological changes of the central auditory pathway after blunt trauma of the head. J Neurotrauma. 2004;21:251–258. doi: 10.1089/089771504322972040. [DOI] [PubMed] [Google Scholar]
- Noseworthy JH, Miller J, Murray TJ, et al. Auditory brainstem responses in postconcussion syndrome. Arch Neurol. 1981;38:275–278. doi: 10.1001/archneur.1981.00510050041004. [DOI] [PubMed] [Google Scholar]
- Ottaviani F, Almadori G, Calderazzo AB, et al. Auditory brain-stem (ABRs) and middle latency auditory responses (MLRs) in the prognosis of severely head-injured patients. Electroencephalogr Clin Neurophysiol. 1986;65:196–202. doi: 10.1016/0168-5597(86)90054-7. [DOI] [PubMed] [Google Scholar]
- Parbery-Clark A, Marmel F, Bair J, et al. What subcortical-cortical relationships tell us about processing speech in noise. Eur J Neurosci. 2011;33:549–557. doi: 10.1111/j.1460-9568.2010.07546.x. [DOI] [PubMed] [Google Scholar]
- Ponsford J, Willmott C, Rothwell A, et al. Factors influencing outcome following mild traumatic brain injury in adults. J Int Neuropsychol Soc. 2000;6:568–579. doi: 10.1017/s1355617700655066. [DOI] [PubMed] [Google Scholar]
- Rimel RW, Giordani B, Barth JT, et al. Disability caused by minor head injury. Neurosurgery. 1981;9:221–228. [PubMed] [Google Scholar]
- Rowe MJ, 3rd, Carlson C. Brainstem auditory evoked potentials in postconcussion dizziness. Arch Neurol. 1980;37:679–683. doi: 10.1001/archneur.1980.00500600027002. [DOI] [PubMed] [Google Scholar]
- Russo N, Nicol T, Musacchia G, et al. Brainstem responses to speech syllables. Clin Neurophysiol. 2004;115:2021–2030. doi: 10.1016/j.clinph.2004.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo NM, Nicol TG, Zecker SG, et al. Auditory training improves neural timing in the human brainstem. Behavioural Brain Research. 2005;156:95–103. doi: 10.1016/j.bbr.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Schoenhuber R, Gentilini M. Auditory brain stem responses in the prognosis of late postconcussional symptoms and neuropsychological dysfunction after minor head injury. Neurosurgery. 1986;19:532–534. doi: 10.1227/00006123-198610000-00006. [DOI] [PubMed] [Google Scholar]
- Schoenhuber R, Gentilini M, Orlando A. Prognostic value of auditory brain-stem responses for late postconcussion symptoms following minor head injury. J Neurosurg. 1988;68:742–744. doi: 10.3171/jns.1988.68.5.0742. [DOI] [PubMed] [Google Scholar]
- Song JH, Banai K, Russo NM, et al. On the relationship between speech- and nonspeech-evoked auditory brainstem responses. Audiol Neurootol. 2006;11:233–241. doi: 10.1159/000093058. [DOI] [PubMed] [Google Scholar]
- Soustiel JF, Hafner H, Chistyakov AV, et al. Trigeminal and auditory evoked responses in minor head injuries and post-concussion syndrome. Brain Inj. 1995;9:805–813. doi: 10.3109/02699059509008236. [DOI] [PubMed] [Google Scholar]
- Tillman T, Carhart R. An expanded test for speech discrimination utilizing CNC monosyllabic words (Northwestern University Auditory Test No. 6), Technical Report No. SAM-TR-66–55. USAF School of Aerospace Medicine, Brooks Air Force Base; Texas: 1966. [DOI] [PubMed] [Google Scholar]
- Tremblay K, Kraus N, McGee T, et al. Central auditory plasticity: changes in the N1-P2 complex after speech-sound training. Ear Hear. 2001;22:79–90. doi: 10.1097/00003446-200104000-00001. [DOI] [PubMed] [Google Scholar]
- Tremblay KL, Kraus N. Auditory training induces asymmetrical changes in cortical neural activity. J Speech Lang Hear Res. 2002;45:564–572. doi: 10.1044/1092-4388(2002/045). [DOI] [PubMed] [Google Scholar]
- Turgeon C, Champoux F, Lepore F, et al. Auditory processing after sport-related concussions. Ear Hear. 2011;32:667–670. doi: 10.1097/AUD.0b013e31821209d6. [DOI] [PubMed] [Google Scholar]
- Vander Werff KR, Burns KS. Brain stem responses to speech in younger and older adults. Ear and Hearing. 2011;32:168–180. doi: 10.1097/AUD.0b013e3181f534b5. [DOI] [PubMed] [Google Scholar]
- Vanderploeg RD, Curtiss G, Luis CA, et al. Long-term morbidities following self-reported mild traumatic brain injury. J Clin Exp Neuropsychol. 2007;29:585–598. doi: 10.1080/13803390600826587. [DOI] [PubMed] [Google Scholar]
- Wible B, Nicol T, Kraus N. Atypical brainstem representation of onset and formant structure of speech sounds in children with language-based learning problems. Biol Psychol. 2004;67:299–317. doi: 10.1016/j.biopsycho.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Wible B, Nicol T, Kraus N. Correlation between brainstem and cortical auditory processes in normal and language-impaired children. Brain. 2005;128:417–423. doi: 10.1093/brain/awh367. [DOI] [PubMed] [Google Scholar]
- Wilson RH, Burks CA. Use of 35 words for evaluation of hearing loss in signal-to-babble ratio: A clinic protocol. J Rehabil Res Dev. 2005;42:839–852. doi: 10.1682/jrrd.2005.01.0009. [DOI] [PubMed] [Google Scholar]
- Wilson RH, McArdle R. Intra- and inter-session test, retest reliability of the Words-in-Noise (WIN) test. J Am Acad Audiol. 2007;18:813–825. doi: 10.3766/jaaa.18.10.2. [DOI] [PubMed] [Google Scholar]
- Wood RL. Understanding the ‘miserable minority’: a diasthesis-stress paradigm for post-concussional syndrome. Brain Inj. 2004;18:1135–1153. doi: 10.1080/02699050410001675906. [DOI] [PubMed] [Google Scholar]


