Abstract
Objective
This report describes results of a series of experiments where we use the neural response telemetry (NRT) system of the Nucleus cochlear implant (CI) to measure the response of the peripheral auditory system to acoustic stimulation in Nucleus Hybrid CI users. Our objectives were to determine whether we could separate responses from hair cells and neurons, and to evaluate the stability of these measures over time.
Design
Forty-four CI users participated. They all had residual acoustic hearing and used a Nucleus Hybrid S8, S12, or L24 CI or the standard lateral wall CI422 implant. The NRT system of the CI was used to trigger an acoustic stimulus (500 Hz tone burst or click), which was presented at a low stimulation rate (10, 15 or 50 per second) to the implanted ear via an insert earphone and to record the cochlear microphonic (CM), the auditory nerve neurophonic (ANN) and the compound action potential (CAP) from an apical intracochlear electrode. To record acoustically evoked responses, a longer time window than is available with the commercial NRT software is required. This limitation was circumvented by making multiple recordings for each stimulus using different time delays between the onset of stimulation and the onset of averaging. These recordings were then concatenated offline. Matched recordings elicited using positive and negative polarity stimuli were added off line to emphasize neural potentials (SUM) and subtracted off line to emphasize potentials primarily generated by cochlear hair cells (DIF). These assumptions regarding the origin of the SUM and DIF components were tested by comparing the magnitude of these derived responses recorded using various stimulation rates. Magnitudes of the SUM and DIF components were compared to each other and to behavioral thresholds.
Results
SUM and DIF components were identified for most subjects, consistent with both hair cell and neural responses to acoustic stimulation. For a subset of the study participants, the DIF components grew as stimulus level was increased, but little or no SUM components were identified. Latency of the CAPs in response to click stimuli was long relative to reports in the literature of recordings obtained using extracochlear electrodes. This difference in response latency and general morphology of the CAPs recorded was likely due to differences across subjects in hearing loss configuration. Use of high stimulation rates tended to decrease SUM and CAP components more than DIF components. We suggest this effect reflects neural adaptation. In some individuals, repeated measures were made over intervals as long as 9 months. Changes over time in DIF, SUM and CAP thresholds mirrored changes in audiometric threshold for the subjects who experienced loss of acoustic hearing in the implanted ear.
Conclusions
The Nucleus NRT software can be used to record peripheral responses to acoustic stimulation at threshold and supra-threshold levels, providing a window into the status of the auditory hair cells and the primary afferent nerve fibers. These acoustically evoked responses are sensitive to changes in hearing status and consequently could be useful in characterizing the specific pathophysiology of the hearing loss experienced by this population of CI users.
Keywords: Neural Response Telemetry, Cochlear Microphonic, Compound Action Potential, Auditory Nerve Neurophonic, Hybrid Cochlear Implant
INTRODUCTION
The past decade has seen a significant relaxation of cochlear implant (CI) candidacy criteria. Recent modifications to surgical techniques and to the design of the implanted electrode array have allowed individuals with good low frequency hearing but significant high frequency hearing loss to consider cochlear implantation. These individuals are likely to have surviving hair cells and auditory nerve fibers near the apex of the cochlea, but extensive loss of hair cells (and possibly neural degeneration) near the base. Hearing aids often provide limited benefit for individuals with this configuration of hearing loss and “Hybrid” cochlear implants were developed with this population in mind. The original Hybrid CIs featured electrode arrays that were shorter than the electrode arrays used with traditional CIs and often had fewer electrode contacts. The original Hybrid CI system included a speech processor with both acoustic and electrical “components”. The acoustic component functioned like a traditional hearing aid, providing amplification for low frequency sounds. High frequency information was transmitted electrically via intracochlear electrodes surgically implanted into the basal turn of the cochlea (Gantz & Turner, 2003). Many of those devices are still in use today.
In 2014, Cochlear Corporation obtained FDA approval for the Nucleus Hybrid CI. This device features an L24 electrode array that is 16 mm long and has 22 electrode contacts. The electrode array is thin, flexible, and designed to lie along the lateral wall of the scala tympani. Before the Nucleus Hybrid CI was FDA approved, many patients opted to be implanted with an investigational version of the Hybrid device, also manufactured by Cochlear Corporation. In this report we refer to those investigational devices as Hybrid S8 and S12 CIs. The Hybrid S8 CI has 6 electrode contacts mounted on a silastic carrier that is 10 mm in length. The Hybrid S12 CI is also 10 mm long but with 10 intracochlear electrodes. Successful preservation of acoustic hearing has been possible with all three versions of the Nucleus Hybrid CI, though results of clinical trials have only been published for two versions [Hybrid S8: Gantz et al, 2009; 2016; Hybrid L24: Roland et al, 2015]. In-house unpublished data also indicates successful hearing preservation for Hybrid S12 CI users who have been seen in our clinic.
The Nucleus 422 electrode array is 20 mm long and holds 22 intracochlear electrode contacts. Like the Hybrid L24 implant, the electrode contacts are small and the silastic carrier is straight, thin and flexible. The Nucleus 422 CI was designed for use by patients who may have little or no residual acoustic hearing but who wished to preserve cochlear structures in the implanted ear. Many clinics report hearing preservation is also possible with this cochlear implant system (Jurawitz et al, 2014; Skarzynski et al, 2014; van Abel et al, 2015).
A number of studies have been published describing the benefits that preserving acoustic hearing provides to the Hybrid CI user. Specifically, they show improved performance on measures of speech perception in background noise and music perception when listeners use a combination of acoustic and electric stimulation versus acoustic or electric stimulation alone (Turner et al., 2004; Gfeller et al 2006; Gantz et al, 2009, 2016; Lenarz et al 2009; Brockmeier et al, 2010; Roland et al, 2015). Success with a Hybrid CI depends, however, on how much acoustic hearing can be preserved. The data suggest that most Hybrid CI users experience a relatively mild hearing loss of approximately 12 dB in the implanted ear immediately after surgery (Gantz et al, 2009, 2016; Gifford et al, 2008; Podkarbi-Fayette et al, 2010). This loss is likely a direct result of insertion trauma. However, in some cases, significant hearing loss can occur after several months of successful CI use. Kopelovich et al (2015) followed 82 Hybrid S8 CI users and compiled pure tone averages (PTAs) over several time points from before surgery to approximately one year after surgery. PTAs were calculated as an average of unaided audiometric thresholds obtained at 125, 250, 500, 750, and 1000 Hz. Study participants exhibited a permanent loss of approximately 15 dB at the time of activation. Sixty five CI users (80%) showed no additional loss of acoustic hearing after 4 months of implant use. The remaining 17 study participants (20%) experienced an additional delayed hearing loss that averaged approximately 24 dB.
The cause of this delayed onset hearing loss is not known. Kopelovich et al (2014) examined patient files and showed that age, male gender and a history of noise exposure were all correlated with increased susceptibility to delayed onset hearing loss in the implanted ear but based on available evidence, several different etiologies are possible. Tanaka et al (2014) reported results from implanted guinea pigs suggesting that mechanisms of delayed hearing loss may be related to changes in the stria vascularis and/or new bone growth. Additional work by Reiss et al (2015) showed a relationship between strial capillary density near the implanted electrode insertion and hearing loss. They suggested that excitotoxicity and/or inflammation could also cause damage to hair cell-neuron synapses remote from the electrode location. Kopelovich et al (2015) reported deleterious effects of excessively high electrical stimulation levels on neural structures of rat cochlea – including blebbing of afferent nerve fibers and reduction of fibers innervating hair cells. These changes were observed despite a finding of minimal change in the number of surviving inner and outer hair cells. Some studies have shown relatively poor correlations of hearing loss with hair cell and/or spiral ganglion cell loss in guinea pigs (Tanaka et al 2014; O’Leary et al 2013). Other studies have shown that electrode insertion trauma can result in programmed cell death in cochlear structures (Eshraghi et al., 2013, 2015). Finally, post-implant fibrosis due to electrode insertion may have an effect on cochlear mechanical responses to sound (Choi and Oghalai 2005). Quesnel et al (2015) performed a temporal bone study on a single Hybrid S8 CI user from the University of Iowa cohort who died of unrelated causes after experiencing total loss of his acoustic hearing 3 months after implant activation. They observed extensive cochlear scarring in the basal turn and hypothesized that the effect of the fibrous tissue on cochlear mechanics could be a possible mechanism of hearing loss in this case. Histology showed no difference in hair cell or spiral ganglion cell counts compared to the contralateral ear.
These findings suggest there may be several possible mechanisms for the delayed onset hearing loss that some patients experience after receiving a Hybrid CI. Having a better understanding of the changes that take place in individual CI users could be one way to distinguish among the various etiologies of hearing loss. For instance measures that separate the response of hair cells from that of neurons could be helpful in separating etiologies resulting from changes in synaptic function versus etiologies associated with changes in mechanical transduction or hair cell loss. We reasoned that we might be able to use electrophysiologic recording techniques to separate neural responses from cochlear hair cell responses. Measurements from an intracochlear electrode can record the compound action potential (CAP), the phase-locked response of the auditory nerve (auditory nerve neurophonic; ANN) as well as responses from hair cells including the cochlear microphonic (CM) and/or summating potential (SP). This study describes our initial attempts to make such measures in a group of Hybrid CI users with residual acoustic hearing.
Several research groups have used electrocochleography (ECoG), with an electrode placed on or near the round window, to record responses from the cochlea to the presentation of an acoustic stimulus in cochlear implant patients (Choudhury et al, 2012; Harris et al, 2011; Mandala et al 2012; Adunka et al 2006; Calloway et al, 2014). Formeister et al (2014) and Fitzpatrick et al. (2014) demonstrated that the average amplitude of the CM response across several stimulus frequencies shows a strong correlation to postoperative speech perception in adult and pediatric recipients of standard cochlear implants. Their results suggest that acoustic responses, particularly response amplitude at relatively high levels, may provide a measure of overall cochlear health that affects neuronal responses to electrical stimulation. More recently, Campbell et al (2014; 2015) reported using the telemetry system of the Nucleus cochlear implant to record responses to acoustic stimulation. They adapted the neural response telemetry (NRT) software to allow them to record a longer time window than is possible with the standard NRT software. These investigators were able to record both the CM and the ANN from an intracochlear electrode. They reported data from five individuals tested while they were in the operating room at the time of surgery, demonstrating the feasibility of such measures.
In this study we focus on using the telemetry system of the Nucleus CI in a manner that required minimal adaptations to the software to record responses to acoustic stimulation from hybrid CI users postoperatively. Standard clinical software (Custom Sound EP) was used to control the telemetry system of the Hybrid CI. This allowed us to record responses to acoustic stimulation from an intracochlear electrode. We subtracted responses recorded using opposite polarity stimuli off line to emphasize the contribution of the hair cells in the cochlea and added these two responses to minimize the CM and emphasize the response of the auditory nerve (Aran and Charlet de Sauvage, 1976; Lichtenham et al. 2013). Although this technique does not result in a pure separation (Forgues et al. 2014), we test the partial division by comparing responses obtained at two stimulation rates, under the assumption that neural contributions would be susceptible to adaptation at the higher rate, but hair cell contributions would not. We also use the intracochlear electrode to measure the CAP evoked using clicks. We hypothesized that the physiological measures would be positively correlated with behavioral measures of auditory threshold. Some subjects were tested only once. Others, including several individuals who experienced delayed onset hearing loss, were tested multiple times. Repeated measures on individuals with stable hearing thresholds provided a means for exploring stability of the physiological measures. Repeated measures on individuals with hearing loss provided a means for exploring the sensitivity of the physiological measures to a change in auditory status. Although recordings at the time of surgery are important, we reasoned that postoperative recordings of acoustically evoked auditory potentials could provide a method to monitor physiological activity over time, detect changes in function associated with increased hearing thresholds and, in turn, help us define the underlying pathophysiology of the hearing loss.
METHODS
Custom Sound EP software is routinely used to control the NRT system and to record electrically evoked compound action potentials (ECAPs). In this study we used the Custom Sound EP software to record responses from an intracochlear electrode, but the stimulus was presented acoustically rather than electrically. This required a modification of the clinical software so that an external trigger could be generated and used to synchronize the neural recordings with the presentation of externally generated acoustic clicks or tone bursts.
Participants
Forty-four individuals participated in this study. They all received their CIs at the University of Iowa Hospitals and Clinics (UIHC) between 2005 and 2015 and returned to UIHC frequently for programming and follow-up testing. Demographic information about the individual study participants is provided in Table 1. Study participants used one of several different cochlear implants. Four used the Hybrid S8 (Subject ID=A; insertion=10 mm); 5 used the Hybrid S12 (ID=T; insertion=10 mm); 28 used the Hybrid L24 (ID=L, CL; insertion=17.5-18 mm); 7 used the Nucleus CI 422 (ID=S; insertion=20-25 mm). While there was considerable variability across subjects in the configuration of the audiograms, prior to surgery, most had high frequency hearing loss and some degree of hearing at frequencies below 1000 Hz. The average post-implant audiogram for the group of 44 study participants is shown in Figure 1.
Table 1.
| Subject | Implant | DOB | Activation | Age (at activation) | Test Dates (months post activation) |
|---|---|---|---|---|---|
| A12 | S8 | 6/28/1953 | 1/10/2007 | 53 | 103 |
| A2 | S8 | 6/18/1945 | 7/11/2005 | 60 | 120 |
| A4 | S8 | 8/29/1955 | 10/6/2005 | 50 | 108 |
| A8 | S8 | 9/7/1950 | 6/12/2006 | 55 | 108 |
| CL5 | L24 | 4/7/2000 | 5/22/2012 | 12 | 35 |
| CL6 | L24 | 9/13/1999 | 4/12/2013 | 13 | 18, 24 |
| CL7L | L24 | 6/1/1997 | 7/9/2013 | 16 | 24 |
| L15R | L24 | 12/18/1953 | 3/6/2013 | 59 | 24 |
| L17L | L24 | 8/7/1958 | 12/12/2013 | 55 | 12 |
| L18R | L24 | 9/19/1977 | 4/7/2014 | 36 | 9, 12 |
| L19L | L24 | 9/18/1964 | 7/3/2014 | 49 | 3, 6, 11 |
| L19R | L24 | 10/22/1957 | 6/4/2014 | 56 | 7 |
| L2 | L24 | 12/6/1947 | 3/31/2010 | 62 | 61 |
| L20R | L24 | 3/2/1969 | 8/11/2014 | 45 | 3, 6 |
| L21L | L24 | 5/7/1956 | 2/13/2014 | 57 | 12 |
| L22R | L24 | 8/8/1965 | 8/25/2014 | 49 | 3, 6, 12, 12.1 |
| L23R | L24 | 3/1/1961 | 9/22/2014 | 53 | 1, 3 |
| L24R | L24 | 1/7/1969 | 5/29/2014 | 45 | 14 |
| L25L | L24 | 8/27/1996 | 9/26/2014 | 18 | 3, 6, 12 |
| L26L | L24 | 6/6/1981 | 12/5/2014 | 33 | 0.5, 1, 3 |
| L27R | L24 | 4/13/1990 | 12/17/2014 | 24 | 1 |
| L28L | L24 | 11/11/1960 | 1/5/2015 | 54 | 0.5, 1, 3, 5 |
| L29R | L24 | 7/21/1957 | 4/21/2015 | 57 | 0.5, 1, 2 |
| L30L | L24 | 1/19/1951 | 4/22/2015 | 64 | 0.5 |
| L31L | L24 | 9/26/1957 | 4/23/2015 | 57 | 0.5, 1, 3 |
| L32L | L24 | 11/11/1993 | 6/25/2015 | 21 | 0.5, 1 |
| L32R | L24 | 5/20/1964 | 4/24/2015 | 50 | 0.5, 3 |
| L33L | L24 | 3/9/1953 | 6/12/2015 | 62 | 1, 2, 9 wks, 3 |
| L3R | L24 | 5/24/1969 | 6/2/2010 | 41 | 60 |
| L4R | L24 | 12/27/1955 | 6/6/2010 | 54 | 60 |
| L6R | L24 | 8/19/1947 | 9/29/2011 | 64 | 36 |
| L9R | L24 | 9/5/1949 | 2/22/2012 | 62 | 36 |
| S24R | 422 | 2/20/1936 | 10/27/2014 | 78 | 5 |
| S27R | 422 | 11/10/1940 | 12/5/2013 | 73 | 11 |
| S31R | 422 | 8/3/1964 | 1/7/2013 | 48 | 26 |
| S35L | 422 | 4/13/1942 | 10/24/2014 | 72 | 7 |
| S36R | 422 | 10/13/1941 | 3/27/2015 | 73 | 3 |
| S38R | 422 | 11/4/1934 | 10/17/2013 | 78 | 21 |
| S5L | 422 | 11/14/1948 | 1/17/2013 | 64 | 24 |
| T10L | S12 | 10/28/1969 | 1/10/2011 | 41 | 48 |
| T12L | S12 | 8/23/1954 | 12/19/2013 | 59 | 12 |
| T13L | S12 | 11/2/1980 | 1/2/2014 | 33 | 11 |
| T14R | S12 | 2/5/1950 | 1/15/2015 | 64 | 0.5, 1, 3, 6 |
| T6 | S12 | 5/9/1946 | 4/28/2009 | 62 | 72 |
Figure 1.
Average audiometric threshold plotted as a function of frequency for all 44 study participants. Error bars indicate ± 1 standard deviation. Thresholds used in the calculation are from the implanted ear and were measured at or near the time the electrophysiological recordings were made.
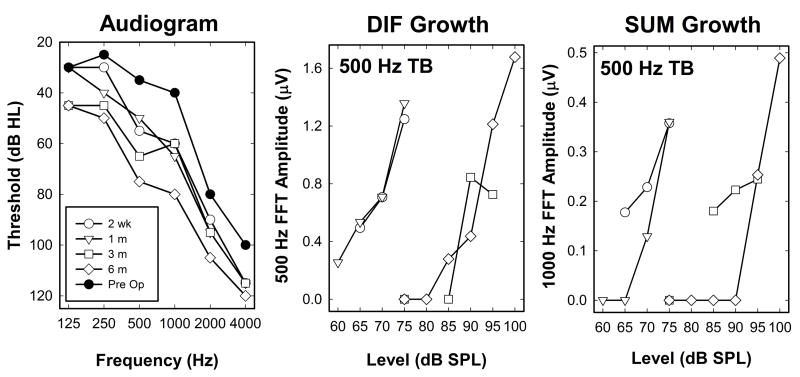
We began using the NRT system to record peripheral physiological responses to acoustic stimulation in 2014. Twenty nine of our study participants received their CI before that point in time. These individuals typically used one of the devices with a short electrode array (S8, S12 or L24) and most participated in only a single recording session. Fifteen individuals who received their CI after 2014 were tested repeatedly during the first year following surgery, typically at 2 weeks, 1 month, 3 months, 6 months, and 12 months post activation. Five of those 15 study participants (L22R, L25L, L29R, L33L, T14R) experienced some loss of acoustic hearing during the first year of CI use and two of them were seen more frequently for evoked potential and audiometric testing.
Stimulation procedures
Intracochlear recordings were measured using a series of clicks and 500-Hz tone bursts routed through an audiometer and presented to the implanted ear via an insert earphone. The 500-Hz tone bursts were generated digitally using LabVIEW at a sampling rate of 44100 samples/s. Each tone burst was 12 ms in duration and was shaped with a rectangular gating function to maximize the chance we would record a compound action potential. Two stimulation rates were used (10 and 50 per second). The external trigger pulse generated by Custom Sound EP software was used to synchronize the click stimulus (a 100 μs pulse). These were presented at a rate of 15 per second.
For both tone burst and click stimuli, neural responses recorded to opposite polarity signals were stored separately. The stimulation levels ranged from below the subject’s detection threshold to levels that approached their maximum comfortable level or to 110 dB SPL, whichever was lower. Step-size varied but typically was 5 or 10 dB. Time constraints limited the number of stimulation levels used for some subjects. Levels were calibrated by placing the insert earphone into a 2 cc cavity with a calibrated microphone placed at the position approximating that of the tympanic membrane.
Recording Procedures
The most apical electrode in the intracochlear array was selected as the recording electrode under the assumption that the electrode is located closest to the region of preserved acoustic hearing and therefore will be most likely to record an acoustic response if one is generated. Another advantage is that the recording is presumably dominated by activity nearer the characteristic frequency region of the toneburst stimuli than a more basal or promontory electrode location. However, for individuals with steeply sloping high frequency hearing loss, the most basal regions should have minimal contributions regardless of electrode location. In this study, the exact position of that electrode in the cochlea varied depending on the specific implant type the study participant used, because the array lengths/insertion depths differ (see Table 1). Moreover, even for individuals with the same implant type, electrode position varied depending upon cochlear anatomy and on the actual insertion depth achieved. Because responses recorded from the most apical electrode in the intracochlear array tended to have the largest amplitudes (Campbell et al., 2015), achieving the best signal-to-noise ratio was given preference over cross-subject consistency. Also the within-subject nature of the analyses meant that the position of the recording electrode would likely have a minimal effect on the comparisons of the three physiological responses that were recorded. When comparing behavioral to physiological responses, the cross-subject variability in recording electrode location is potentially more relevant, and is addressed in the analysis.
Electrically evoked CAPs are typically recorded using a two-pulse, forward masking paradigm. The Custom Sound EP software is designed such that a “masker” and “probe” pulse are presented sequentially, separated by a brief masker probe interval (MPI) that can be varied by the experimenter. The voltage at the recording electrode is sampled shortly after the probe pulse is presented. Because our goal was to record neural responses evoked by presentation of an acoustic stimulus, the masker and probe levels were set to 0 CL. Therefore, the electrical pulses were not audible and were used only to control the timing of the recordings relative to the onset of the acoustic stimulus. Because sampling begins after the probe is presented and because the masker and probe pulses were set to zero, electrical stimulus artifact issues were minimal. The “Artefact Cancellation Technique” that allows users to determine how they want to deal with stimulus artifact when they record ECAPs was set to “None” and the “Artefact Reduction” option was set to “Off”.
The maximum recording window available with Custom Sound EP is 3.2 ms. This time window works well for recording short latency electrically evoked responses, but we wished to extend that window to capture the longer latency response evoked using a long duration acoustic tone burst and clicks. To work around this limitation in the software, we recorded several responses to each stimulus while systematically varying the length of the masker (trigger/stimulus onset) to probe (recording onset) interval. The responses in each time window were then concatenated off line, creating an effectively longer time interval for the recordings. The schematic in Figure 2 illustrates this technique. The trigger pulse (Figure 2A) initiates a 500-Hz tone burst (Figure 2B) that is synchronized with the timing of the masker pulse (shown in Figure 2C and 2D). The experimenter can select the length of the MPI, the duration of the probe pulse and the length of the “recording delay”, all of which determine the time at which sampling is initiated. In this study, the recording delay was fixed at 898 μs and the duration of the probe pulse was fixed at 25 μs. Figure 2C illustrates a short MPI, and Figure 2D illustrates a longer MPI. The exact time that the probe is presented is determined by the length of the MPI. The time at which sampling is initiated at the recording electrode site is determined by a combination of the length of the MPI, the duration of the probe pulse, and the length of the recording delay (See Figure 2C). In this study, each concatenated response was the sum of 7 (0.1 to 15.1 ms for toneburst) or 8 (1 to 18.5 ms for clicks) recordings each measured using a different MPI. The recording delays and MPIs were chosen to capture the entire time period of the physiological response. In reporting results we corrected the latency to account for the acoustic delay introduced by the insert earphone.
Figure 2.
Schematic diagram illustrating the method used for data collection. See text for details.
After completing all recordings at each sound level, a recording was also collected for which the acoustic stimulus was presented at the highest level used for testing but the insert phone was removed from the ear canal. These recordings are absent of the physiological responses of interest and were used to characterize stimulus artifact or artifact that resulting from switching on the internal amplifier that may be synchronized to the acoustic stimulus. This recording condition was collected for each stimulus type but at only one MPI (i.e. one recording window) and one stimulus rate.
The sampling rate used to record the physiological responses was set via Custom Sound EP to 20 kHz, and the gain of the recording amplifier was set to 50 dB. Each recording consisted of an average of 200-400 sweeps at each MPI; the recording used to measure stimulus/internal amplifier switching artifact consisted of an average of 1000 sweeps. During data collection, study participants were seated in a comfortable chair and were allowed to read, sleep or watch captioned movies. They were given breaks as necessary and were encouraged to let the experimenter know if any sound was too loud or otherwise uncomfortable.
Data Analysis
Raw waveforms were exported from Custom Sound EP and analyzed using a custom MATLAB script. An example of the analysis technique for a response collected using a 500-Hz tone burst is shown in Figure 3. The same technique was used for the click-evoked recordings. Figure 3A shows raw waveforms recorded for a single polarity stimulus at 7 different delays. Some artifact is evident at the beginning of each trace. It is clear, however, that there are sinusoidal variations in the recordings that persist across the 20-ms time window. To remove the recording artifact, the waveform recorded with the insert earphone removed was subtracted from each of the raw waveforms. The result of that subtraction is shown in Figure 3B. Note that although the recording window was 3.2 ms, the spacing of MPIs was selected in order to create some overlap (approximately 0.7 ms) in the individual recordings. The recordings were shifted up or down by adding or subtracting a fixed voltage amount until the overlapping segments were aligned (see Figure 3C). The shifted waveforms were concatenated and fit with a regression line. The slope of the regression line was used to “baseline correct” the tilt seen in the concatenated waveform in Figure 3C. The final waveform used for analysis is shown in Figure 3D. For each stimulus, this process was repeated twice: once using positive-leading and once using negative-leading stimuli.
Figure 3.
Panel A shows 7 responses. Each recording is 3.2 ms in duration but started at a different time interval relative to the onset of the acoustic stimulus. Panel B plots the same series of responses after subtraction of the “no stimulus” condition. Panel C shows the waveform that resulted from concatenation of all seven recordings. Panel D shows the same waveform after the final correction for baseline drift/tilt was applied. The time scale on the abscissa is the same for all four tracings and represents time after onset of the 500-Hz tone burst.
Tone-burst recordings
Measures of ECoG have typically used averaging of alternating polarity stimuli to cancel stimulus artifact and cochlear microphonic. Use of alternating polarity stimuli helps to isolate the neural response. Recent data from Forgues et al (2014) in gerbils has demonstrated that since CM recordings from hair cells are not necessarily linear, particularly at high stimulus levels relative to CM threshold, the addition of alternating polarity responses can contain contributions both from the hair cells and from the auditory nerve. In the following sections we address this issue but for convenience we have chosen to refer to the summed responses as simply SUM and the difference responses as DIF. SUM and DIF components were quantified using a custom MATLAB program that performed a Fast Fourier Transform (FFT) of both the SUM and DIF waveforms. The magnitudes of the 500 Hz, 1000 Hz, and 1500 Hz components of the FFT were noted. Higher harmonics were not evident in the recordings that we have obtained to date.
Click recordings
Recordings obtained using condensation and rarefaction clicks were added together in the analysis, and this SUM response was analyzed in the time domain by measuring the amplitude and latency of the negative and positive peaks.
Human Subjects
This study was approved by the University of Iowa Institutional Review Board. Experimental protocols, potential benefits and risks were explained to each participant prior to data collection. All participants signed an informed consent document.
RESULTS
Responses to clicks and tone bursts
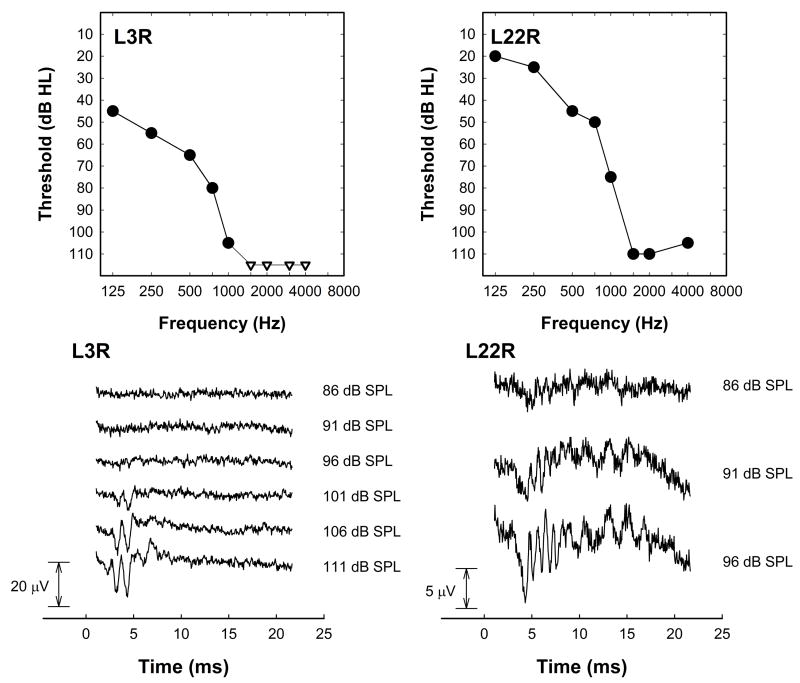
Figure 4 shows a series of responses from the right ear of two individual study participants to an acoustic click presented at several stimulation levels. The audiogram for each subject shows the extent of acoustic hearing that this individual has in the test (e.g. implanted) ear at the time of recording. Each waveform shown in Figure 4 is the sum of the responses to rarefaction and condensation clicks. The level of the acoustic click used to evoke these responses is also shown on the figure. While using the sum of two polarities tends to minimize the CM in response to a click stimulus, we cannot rule out hair cell contributions to these recordings, either summating potential and/or CM distortion.
Figure 4.
Responses to click stimuli presented at several different levels are shown for two subjects (L3R and L22R). Audiograms for both subjects measured on the day these recordings were obtained are also shown. Note the different amplitude scales used for these two study participants.
For the subject L3R, the SUM response consists of a two negative peaks. The first negative peak has a latency of approximately 3 ms. That latency is somewhat long compared to N1 peak latencies in the literature that are recorded from normal hearing listeners but is fairly typical of the recordings we have obtained from other Hybrid CI users with residual acoustic hearing. Given the extent of high frequency hearing loss, the prolonged N1 latency likely reflects the more apical site of stimulation and consequent traveling wave delay compared to responses typically recorded from individuals with less extensive amounts of hearing loss, where an earlier latency response may arise from stimulation in the more basal “tail” of the traveling wave.
In some individuals we have observed ringing in the response to a click stimulus as illustrated for subject L22R shown on the right side of Figure 4. The response that is recorded shows multiple peaks, the period of which is typically within the range of 1-2 ms. This individual has significantly better hearing at low frequencies than the subject with less ringing, but to date we have not observed this pattern consistently. That is, we have not been able to relate either the extent or configuration of hearing loss with the degree of periodicity in the recorded waveforms evoked using click stimuli.
Figure 5 shows responses evoked from an individual study participant using a 500-Hz tone burst presented at a rate of 10 per second. Figure 5A shows this subject’s audiogram for the implanted ear. Figure 5B shows two recordings. One was obtained using a positive-leading 500 Hz toneburst. The other was obtained using a negative-leading tone burst. The physiological responses are periodic but there is also evidence of distortion in these waveforms. The phase inversion that is apparent in this response is consistent with a recording that is dominated by the response of the auditory hair cells (i.e. the CM). The FFT amplitude to each polarity stimulus is plotted as a function of frequency in Figure 1C. As expected there is a strong component at the fundamental frequency but also a strong component evident at the second harmonic frequency, 1000 Hz. The presence of the second harmonic suggests that there is a significant neural component and that the CM may obscure any neural response (CAP or ANN) in the time waveforms. There is also a third harmonic evident in this example at 1500 Hz. It was typical for us to observe a clear second harmonic in this population, but third harmonics were often absent or below the noise floor in our recordings.
Figure 5.
An example of responses recorded using a 500-Hz tone burst. Panel A shows the audiogram measured at the time of evoked potential testing. Panel B shows the response to positive leading and negative leading tone bursts plotted as a function of time after stimulus onset. Panel C shows the results of fast Fourier transform analysis of the two time waveforms shown in panel B. The amplitude scale in this and subsequent figures indicates the amplitude of each frequency component of the transform (in μV) calculated using 376 time samples recorded at a rate of 20,000 samples/s. Panel D shows the sum of the positive and negative -leading traces (labeled SUM) which tends to enhance the neural response as well as the difference between positive and negative leading responses (labeled DIF) which tends to enhance the hair cell response. Panel E shows the results of a fast Fourier transform of the time waveforms shown in Panel D.
We expect that the neural response will be more asymmetric than the CM and the results will be an enhanced 2nd harmonic. By adding the two opposite polarity responses together, we minimize the CM (at least the fundamental and odd harmonics) and the response that remains is likely to reflect primarily the response of the auditory nerve (i.e. the ANN). By subtracting the two waveforms, we minimize the neural response and enhance the contribution of the CM. The result of these two manipulations is shown in Figure 5D. Figure 5E shows the magnitude of the FFT for both the DIF and SUM waveforms. The gray dashed trace in Figure 5D labeled DIF should contain relatively little neural component. In theory, it will also contain primarily the fundamental frequency component (i.e. 500 Hz) of the ANN but this contribution is expected to be smaller than the 500 Hz component of the CM.
A number of other observations support this conclusion:
The DIF waveform has a shorter latency than the SUM waveform. This could be the result of a number of factors including site of origin of CM and ANN but is consistent with pre-synaptic origin.
The SUM waveform has clear periodicity at the 2nd harmonic (i.e. 1000 Hz; Fig 5E). Neural firing that is phase-locked to the periodic stimulus and the asymmetry of the neural waveforms would contribute to this frequency component. The SUM also shows a negative peak in the time waveform near the response onset that is most likely the compound action potential.
Examination of the time waveform in Figure 5D labeled DIF that shows little evidence of an onset response. The FFT of the difference waveform (Fig. 5E) has a peak at 500 Hz, consistent with CM. Therefore, we presume that this relatively large sinusoidal response is dominated by hair cell potentials. While we have observed variations in the amplitude of the DIF over time (larger responses at onset), as in this example, the waveforms do not generally show a clear negative peak as in the SUM response, typical of the compound action potential.
These observations are all consistent with a hypothesis that the CM is dominant in the DIF waveforms and the ANN is dominant in the SUM response. This occurs because the neural component tends to have a more asymmetric response than the CM, resulting in the dominance of the ANN in the SUM response.
Figures 6 and 7 show how changes in stimulus intensity impact these responses. In both figures, panel A plots the audiogram for the implanted ear of the study participant (dB HL). Panels B and D show a series of SUM and DIF waveforms recorded using a 500 Hz tone burst presented at several different stimulus levels. The stimulation levels used for each subject varied and are indicated on the graphs. Panel C displays FFT magnitude at 500 Hz for the DIF (gray) and at 1000 Hz for the SUM (black). The vertical line in Panel C designates the audiometric threshold converted to dB SPL for a 500 Hz tone in the implanted ear.
Figure 6.
An example of responses showing growth of both DIF and SUM components with level are shown. Panel A shows the audiogram from the test ear measured at the time of recording. Panels B and D show the SUM and DIF waveforms plotted as a function of time after stimulus onset for the three stimulation levels indicated at the right. Panel C shows the results of an FFT analysis of the SUM and DIF waveforms shown in panels B and D.
Figure 7.
An example of responses from a subject who had a clear Diff component but no measurable SUM response. Organization of the figure is identical to Figure 6.
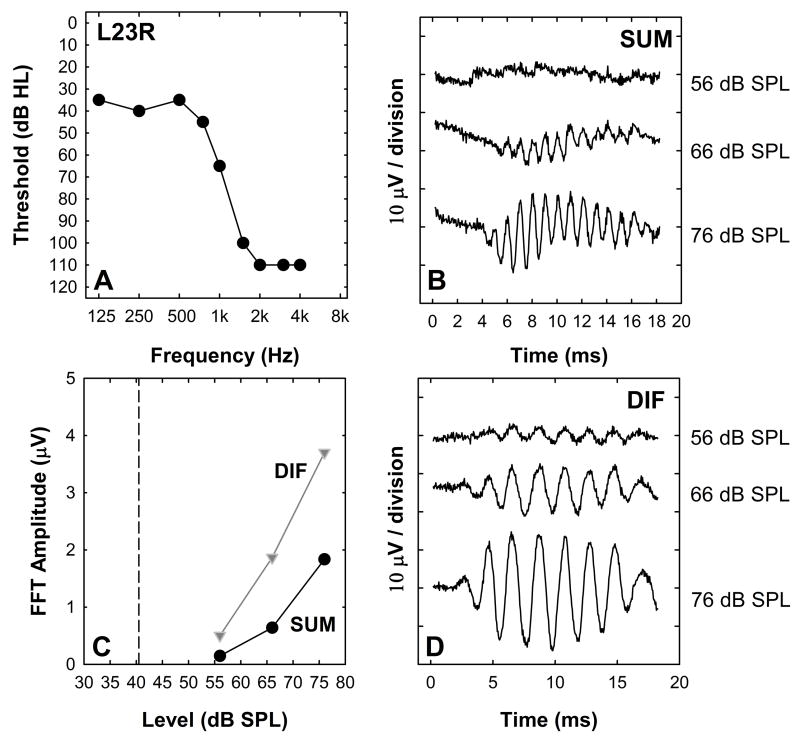
For the subject whose data are shown in Figure 6, the DIF responses are relatively large and consist of a 500 Hz component while the SUM amplitude is smaller and the energy is primarily at 1000 Hz. The waveforms in B and D show growth of both the SUM and DIF components as stimulation level was increased. The FFT amplitude measures in 5C are typical in that these intracochlear responses become measureable and begin to grow in amplitude once the stimulation level is above detection threshold. The majority of Hybrid CI users (73%) show this same pattern of results. The DIF FFT amplitude growth functions typically are steeper and the response amplitudes are larger than the SUM FFT growth functions.
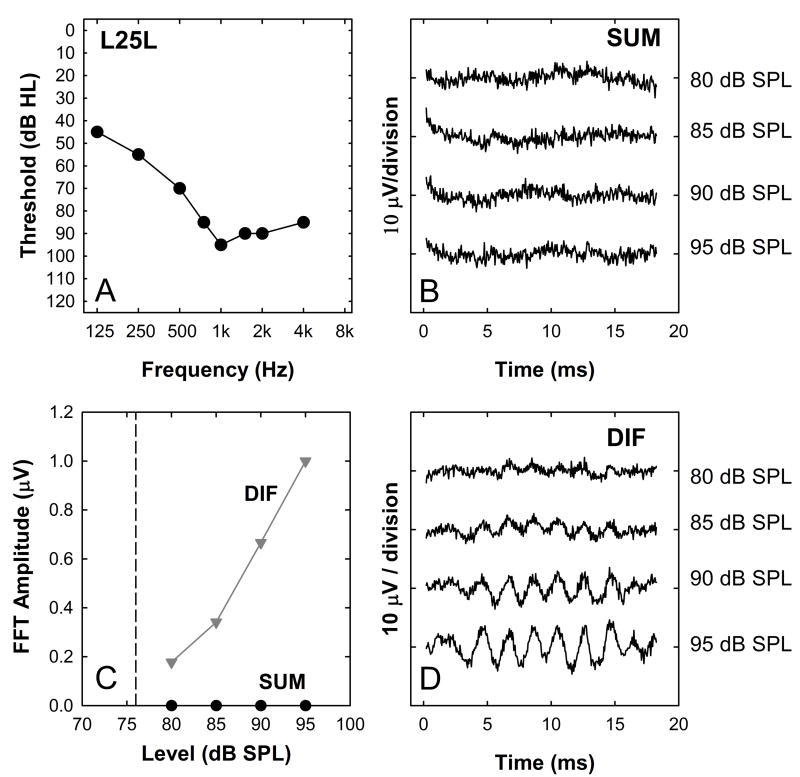
For 20% of study participants a different trend is observed. This is illustrated in Figure 7. The DIF exhibits growth with increasing stimulus level and the threshold is above the 500 Hz audiometric threshold, but there is no evidence of a SUM response at any stimulation level. The magnitude of the 1000 Hz component of the FFT computed using the SUM is shown in Panel C.
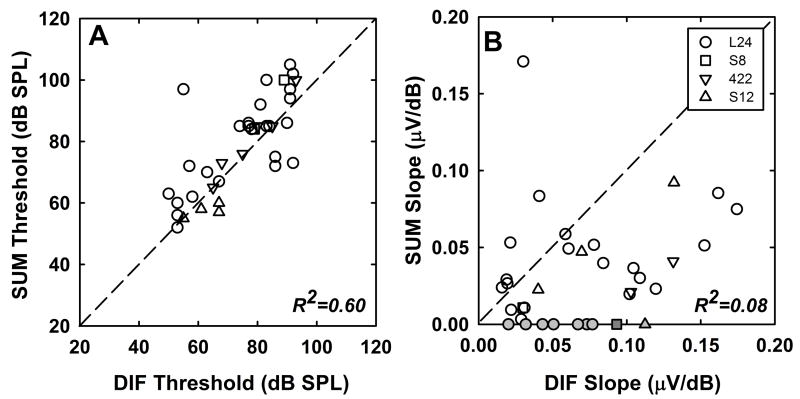
Figure 8 compares DIF and SUM thresholds (A) and the rates of growth (B) with increasing stimulation level. As in the previous figures, when we analyze the SUM, we report threshold of the 1000-Hz component of the FFT and when we analyze the DIF, we report threshold of the 500-Hz component of the FFT. In all cases, the acoustic stimulus was a 500 Hz toneburst. Threshold of the SUM tends to be higher than threshold of the DIF (Figure 8A). The correlation between these two threshold measures, however, is significant (r2=0.60, p < 0.01). The SUM tends to grow more slowly than the DIF with increasing stimulus level, however, there was not a significant correlation between slope of the SUM growth function and slope of the DIF growth function (r2 = 0.08, p = 0.14; Figure 8B). The data points in gray are used to illustrate the results obtained from individuals that showed no measurable neural response to the acoustic stimulus (slope=0). Results from those individuals were not included in the regression calculation, but the distribution of these data points along the x-axis shows that growth of the DIF spanned a large range, i.e., they were not only small amplitude responses. We assume that these data showing zero slope do not indicate complete absence of a neural response. These individuals do hear the stimulus and it is likely that they have neural potentials that are lower than our measurement noise floor. We also note that for all but one subject who showed no SUM response we were able to record a compound action potential in response to electrical stimulation using standard NRT procedures. (Due to time constraints, we were not able to attempt an ECAP recording for that remaining participant)
Figure 8.
Scatterplots illustrate the relationship between the DIF and SUM components across the population of subjects tested. Panel A plots threshold of the SUM component as a function of DIF threshold. Panel B shows the relationship between the slope of the SUM and DIF growth functions. Thresholds were determined using linear regression of the amplitude growth data. Different symbols are used to represent the device type used by each study participant. Gray symbols represent cases where no ANN responses were observed. Linear regression analysis was used to calculate the R2 values shown. The data shown in gray were not included in that analysis. The dashed line has a slope of one.
Positive correlations between SUM and DIF thresholds and slopes were expected since neural responses are dependent upon cochlear input. However, our data used to explore rate adaptation (described below) suggests that the separation is incomplete; therefore, the correlation may also partially reflect that the same source contributes to both. With this caveat, we note that the threshold data show a clear correlation while amplitude growth does not. This suggests that there may be relative differences in neural and hair cell response amplitudes among individuals at suprathreshold levels, which might provide information about differences in auditory status.
Relationship to behavioral threshold
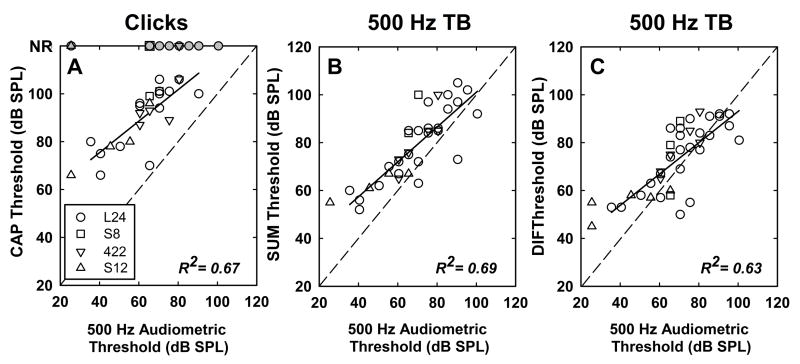
The scatterplots in Figure 9 show the relationship between the 500 Hz audiometric threshold (on the abscissa) and threshold of the click evoked CAP, the SUM and the DIF (on the ordinate). Note that click, SUM and DIF could not be obtained for all subjects. Thus, the number of data points used for correlation analyses are different in all three scatterplots (n = 25 for clicks, 41 for DIF, and 37 for SUM). Correlations among all three of these physiological measures and audiometric threshold are similar and all are statistically significant (r2 = 0.67, 0.69, and 0.63 respectively; p < 0.01 in all cases). Clearly the click thresholds tend to be higher, likely due to the broad spectrum that may not efficiently stimulate the region of low-frequency hearing. Both SUM and DIF thresholds show strong correlations to behavioral thresholds and in some cases show thresholds lower than behavioral threshold. This is more common with the DIF component and could indicate that there is a hair-cell component that does not effectively stimulate the spiral ganglion cells. We note however that behavioral thresholds are determined using typical clinical procedures and likely have ±5 dB test-retest reliability.
Figure 9.
Scatterplots illustrate the relationship between physiologic thresholds and behavioral thresholds. Panel A shows CAP thresholds measured using click stimuli plotted as a function of the subject’s 500-Hz audiometric threshold. Panels B and C show the SUM and DIF thresholds respectively plotted as a function of the 500-Hz audiometric threshold. Data for the four different implant types are plotted using different symbols. Grey symbols are used to indicate instances where no physiologic response was detected. The dashed line has a slope of one. Results of linear regression analysis are shown using a solid line and did not include data from subjects with no measurable CAP. R2 values are indicated on each graph.
In each plot, the data for different implant types are shown with different symbols as indicated in the legend. Because longer arrays such as the L24 or 422 are inserted deeper into the cochlear, the recording electrode (most apical) is closer to the presumed site of response generation. Therefore, the physiological amplitudes may be affected by array type; whereas, behavioral thresholds would not be. Physiological thresholds in those individuals are similar to those observed with shorter electrode arrays, indicating that array length was not a primary factor in the unexplained variability in the correlations between physiological and behavioral measures. Further, we calculated the correlations for L24 subjects alone and found no difference in the R2 value.
Comparison of the three scatterplots shows that the threshold of the CM component tends to be most similar to audiometric threshold. The threshold of the ANN and the CAP are elevated relative to the audiometric threshold. These differences in absolute thresholds (behavioral – DIF threshold vs. behavioral – SUM threshold) may reflect differences in location of the recording electrode relative to the presumed generator, i.e., the electrode array is closer to the hair cell than the spiral ganglion cell.
Stimulus rate effects
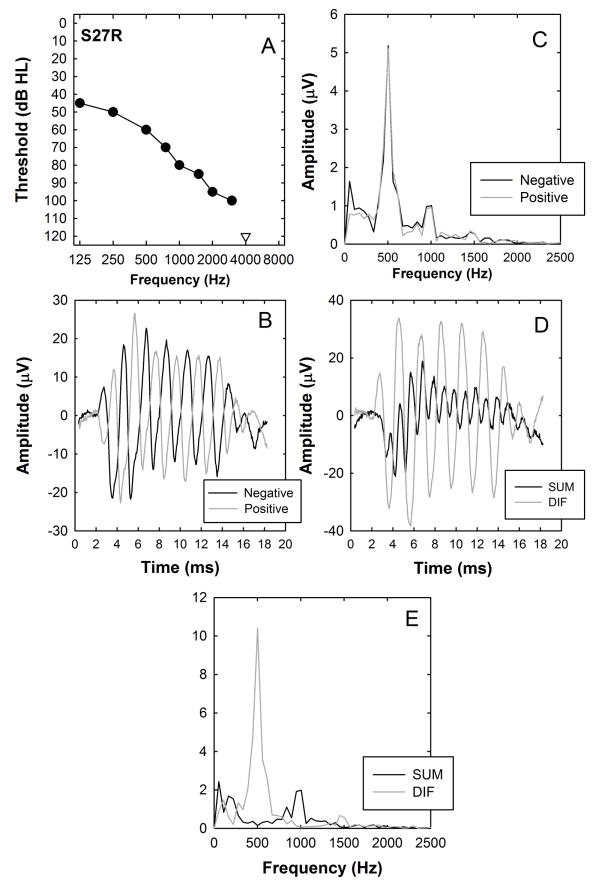
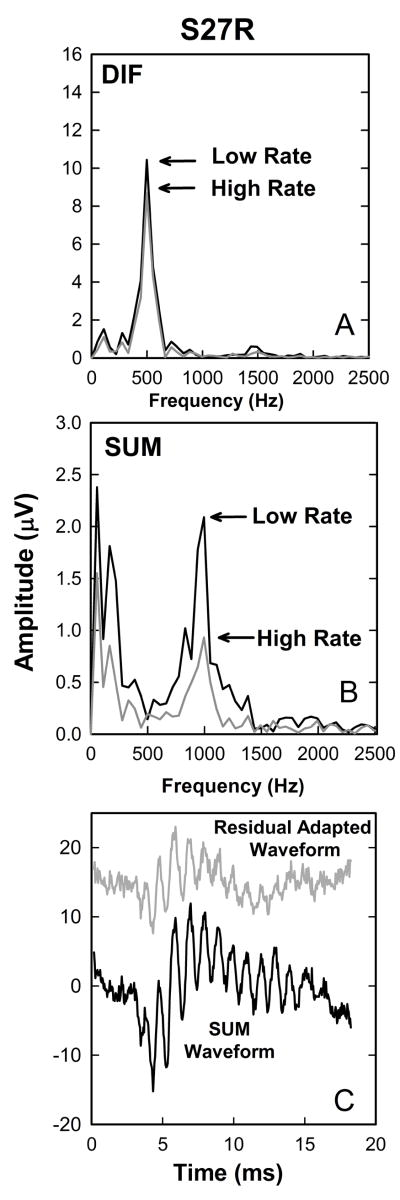
Animal recordings have demonstrated that simply summing the positive and negative responses can minimize CM in the recordings but does not completely isolate neural responses (Forgues et al, 2014). Another way to distinguish neural and hair cell responses would be in terms of their adaptation properties (Snyder and Schreiner, 1984; Mouney et al., 1978). We recorded responses to tone bursts in each individual at two stimulation rates (10 and 50 per second). Our hypothesis was that increasing the stimulation rate should cause adaptation, likely due to synaptic depletion at the hair-cell neural interface or due to neural refractory properties. If that is the case, the neural responses should be affected by the increase in stimulation rate but the response from the hair cell, because they are presynaptic, should not. Figs 10A and B illustrate the trends seen in this study. FFT analysis of the DIF waveforms (Fig. 10A) reveals a strong 500 Hz component that decreases minimally when the stimulation rate increased from 10 to 50 tone bursts per second. The same change in rate has a stronger effect on the SUM component which has a clear peak at 1000 Hz (10B).
Figure 10.
Additional recordings obtained from the same subject (S27R) whose data are plotted in Figure 5. Panels A and B show the results of fast Fourier transform analysis of the DIF and SUM waveforms recorded using both a low (10/s) and high (50/s) stimulation rate. Panel C shows the SUM waveforms in black and the residual adapted SUM waveform (see text) in grey.
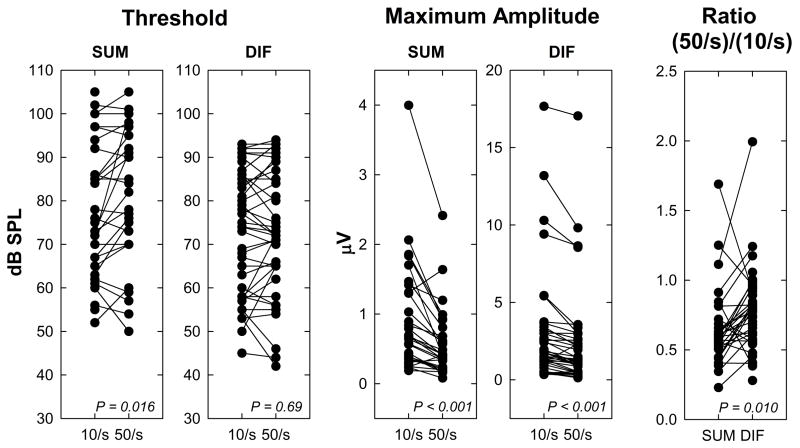
Figure 11 shows a series of plots that compare response threshold and maximum amplitude of evoked responses recorded using 500 Hz tone bursts presented at high (50 stimuli/s) and low (10 stimuli/s) stimulation rates. As predicted, the effect of increasing the stimulation rate was greater for the SUM components than the DIF components. For example, paired t-tests revealed no significant difference in DIF threshold at the low and high stimulation rates (t=0.39, df=42, p=0.69). However, increasing the stimulation rate resulted in a significant increase in SUM threshold (mean values, two-tailed test, t=2.54, df=29 p=0.016). The center graphs in Figure 10 show the effect of increasing stimulation rate on the maximum amplitude both in the FFT at 500 Hz for the DIF response and 1000 Hz for the SUM response. For both responses, the lower stimulation rate (10 Hz) resulted in larger response amplitudes (SUM: t=4.71, df=31, p<0.001; DIF: t=4.79, df =45, p<0.001), suggesting that the subtracted response still has some neural component. Note that the stimulation level at which maximum amplitude is measured differs across individuals but for each individual the comparison is made at the same level for high and low rates. Finally, in order to assess the differential effects of adaptation in SUM and DIF we compute a ratio of the FFT amplitude measured using the 50 Hz stimulation rate to the FFT amplitude measured using the 10 Hz rate for each measure (DIF vs SUM). While there are exceptions in individuals, most show greater adaptation (smaller ratios) for the SUM than for the DIF (t=2.71, df=30, p=0.01). These results are consistent with our general hypothesis that adding responses recorded using opposite polarity stimuli results in an evoked response that is dominated by postsynaptic, auditory nerve activity while subtracting the two recordings results in a response that is dominated by presynaptic, hair cell activity.
Figure 11.
The effect of changing stimulation rate for individual subjects is shown. Threshold (dB SPL) and maximum amplitude (μV) of the SUM and DIF components are plotted for the two stimulation rates (10/s and 50/s). Maximum amplitude of the 500-Hz component of the FFT presented at the highest stimulation level used is shown for the DIF responses. Maximum amplitude of the 1000-Hz component of the FFT presented at the same level is shown for the SUM responses. Data from individual subjects are connected by a line to show trends. In order to compare the changes in response amplitude due to adaptation for the DIF and SUM recordings, the ratio of maximum amplitude for the fast rate relative to the slow rate is also shown in the panel on the right.
In an attempt to extract a waveform that represents a “true” (or at least more pure) neural response, the “adapted” waveforms recorded at a higher stimulation rate (50/s) were subtracted from those recorded using a low stimulation rate (10/s). In equation form:
We refer to this derived waveform as the “residual adapted” response. Considering the components assumed to be present within each waveform, the equation can be rewritten as:
Contributions from the cochlear hair cells in the residual adapted waveform are assumed to be minimal because increasing the stimulation rate causes adaptation of the neurons but should not affect the hair cell response. The hair cell contributions will theoretically be identical in the original waveforms, and will be completely subtracted out.
We performed this manipulation on responses to each stimulus polarity and added the two “residual adapted” responses. When plotted in Fig 10C along with the original SUM waveform, the “residual adapted” waveforms are smaller but have similar latency and morphology characteristics. This suggests, again, that the SUM is dominated by the response of the nerve rather than the hair cell. The “residual adapted” response is smaller than the SUM likely because the neural response is not completely adapted by the high-rate stimulus presentation and lend support to the thesis that while we may not be able to completely separate the response of the hair cell and the auditory nerve, these manipulations can, at the very least, emphasize the relative contributions from these two generator sites.
Changes over time
We made repeated measures of both behavioral thresholds and physiological responses on 15 of the 44 subjects. We observed less than 10 dB change in behavioral threshold over 20 intervals in those 15 subjects. Average magnitude of that change was 4 dB (SD=3.48). For those individuals, the average magnitude of the threshold changes for DIF growth functions was 5.1 dB (SD= 4.99) and for SUM growth functions was 3.9 dB (SD=2.7). The changes in electrophysiological thresholds were small and approximately the same range as changes in behavioral threshold.
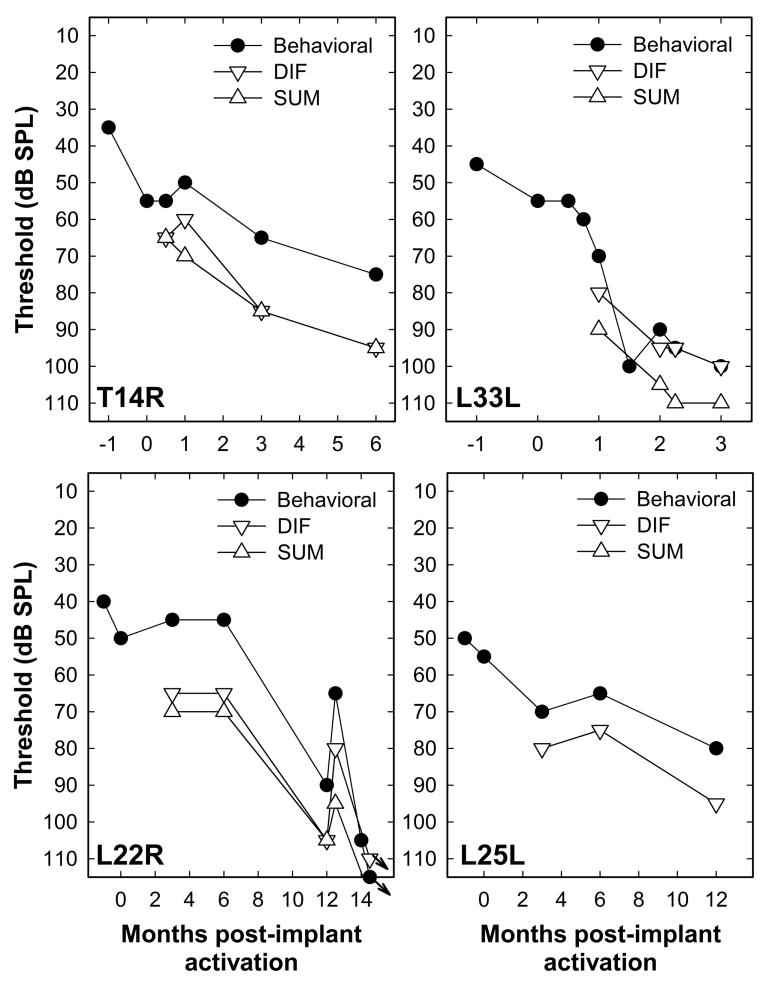
Five subjects experienced changes in behavioral audiometric thresholds greater than 10 dB during the time period over which we monitored their hearing. That gave us the unique opportunity to monitor changes in physiological potentials over time and to compare them with behavioral measures of change in their hearing status. Figure 12 shows data from one such individual (Subject T14R – S12 Hybrid user). The graph on the left (Figure 12A) shows a series of audiograms. One was recorded prior to surgery. This subject experienced a loss of about 20 dB at initial stimulation two weeks after surgery. This loss was likely due to insertion trauma and was stable for the first month after surgery. Hearing then declined at two months and again at 6 months post operatively. Delayed, progressive hearing loss is a trend that has been previously reported for some Hybrid CI users (Kopelovich et al, 2015). The two graphs on the right show results of physiological testing conducted at each of these four postoperative visits (12B: DIF; 12C: SUM). In both graphs, the responses recorded at 2 weeks and 1 month have larger amplitudes than responses recorded at 3 and 6 months. Both the SUM and DIF responses show growth with stimulus level, but the responses obtained after the 2-month drop in hearing exhibit a shift in threshold similar to that observed in the audiogram at 500 Hz.
Figure 12.
These plots show an example of a series of recordings obtained from an individual study participant (T14R) who experienced a loss of hearing several months after implantation. The panel on the left shows audiometric thresholds plotted as a function of stimulus frequency both preoperatively and at several times after implantation. Initially there some hearing loss but by the 3 and 6 month visits the extent of the hearing loss was more severe. The plots on the right show growth functions constructed using the DIF and SUM responses at each of the four postoperative appointments.
Figure 13 shows data from four of the five individuals who experienced thresholds shifts (we were not able to test for physiological responses on one individual). The filled symbols indicate audiometric thresholds at 500 Hz converted to dB SPL. Also plotted are thresholds for the SUM (up triangles) and DIF (down triangles) components. In each case, a change in the behavioral detection threshold was accompanied by similar changes in both DIF and SUM responses. One study participant (L22R) was treated with steroids at 12 months post implant. At this point, some recovery of both behavioral and physiologic thresholds occurred. However, further deterioration in hearing was noted at the 14 month postoperative visit. Another round of steroids was prescribed, however, this time there was no recovery of function. The pattern of responses in these individuals provides further evidence that the acoustic responses measured from the intracochlear electrode are sensitive to relatively small changes in hearing status.
Figure 13.
Changes in the DIF and SUM responses over time post implant are shown for four subjects who experienced a loss of acoustic hearing at 500 Hz following the initial activation of their CI. In each case, the stimulus was a 500 Hz toneburst presented via an insert earphone at a rate of 10 tone bursts per second. Also shown are changes in the subject’s 500 Hz audiometric threshold.
DISCUSSION
The results of this study demonstrate that it is possible to record intracochlear responses to acoustic stimuli using NRT software. Several previous studies have measured similar responses intraoperatively, either with a round window electrode or using the NRT system (McClellan et al., 2014; Calloway et al., 2014; Campbell et al, 2014, 2015). This work expands on those findings by showing postoperative longitudinal data for a larger cohort of CI users, all of whom have preserved acoustic hearing. We show a strong and statistically significant correlation between these measures of peripheral neural response to acoustic stimulation and behavioral thresholds. Additionally, four individuals who lost some degree of acoustic hearing during the time period over which recordings were monitored showed a parallel change in the acoustically evoked potentials recorded from the most apical electrode in the implanted electrode array. CI users with stable acoustic behavioral hearing similarly showed stability in acoustic physiological responses. We would argue this result suggests potential clinical utility of these measures and that they may complement traditional audiometric thresholds. Given that many patients also experience a drop in hearing prior to implant activation, the intracochlear recordings may provide further insight to the mechanisms associated with that initial loss.
Unfortunately, the method we use is time consuming, and limits the potential clinical utility of these measures. Multiple recordings need to be collected and combined to fully capture acoustically evoked responses. In this study, we obtained 7 or 8 different responses, each starting at a different time after the probe (See Figure 3). This window could likely be reduced for shorter duration stimuli and could provide adequate sampling to assess the neurophonic and CM components. However, a recent study indicated success with recording similar responses utilizing an adaptation of the Custom Sound EP software (Campbell et al, 2014, 2015) that allowed for a 10-ms recording window rather than the commercially available 3.2-ms window. This modification would greatly facilitate efficient data collection and make it possible for these measures to move more quickly from the laboratory into the clinic.
Another important issue that will require more attention in future studies is the extent to which we can use post-implant measures recorded using acoustic stimulation to separate hair cell responses from neural responses. The technique we use of summing or subtracting responses collected using stimuli that are out of phase with each other to cancel or enhance the neural components has been used previously (Aran and Charlet de Sauvage, 1976; Campbell et al., 2014). A basic assumption of this technique is that the cochlear microphonic is linear, i.e., sinusoidal in response to a sinusoidal stimulus. However, Forgues et al (2014) recently used round window recordings from normally hearing gerbils to demonstrate important limitations of that method. They noted that the major source of distortion was neural only for low frequency stimuli presented at relatively low intensities. These observations suggest that hair cell transduction rather than differences in basilar membrane motion was likely responsible for the distortion observed at higher stimulation levels. They further noted that hair cells began distorting not at a particular stimulus level but rather at a fixed level above a response threshold.
There are clearly a number of assumptions in comparing human impaired ears with normal gerbil responses but on the basis of the animal data, one might have concerns that a significant part of the summed response responses could be the result of hair cell distortion. We would argue, however, that the stimulus frequency used in the recordings reported was 500 Hz, which is in the frequency range where cochlear microphonic could show linear responses in normal hearing subjects. Further, while many of the recordings reported here required the use of relatively high stimulation levels, since the test subjects had significant amounts of hearing loss, many responses were measured at levels less than 40 dB relative to threshold of the DIF response. Thus, if hair cell distortion is limited at levels close to threshold, we may expect significant cancellation of the cochlear microphonic responses in the SUM waveforms.
Forgues et al (2014) describe clear differences in harmonic distortion produced by hair cells compared to neural responses. Theoretically, those analyses could help to separate CM and ANN responses. In our recordings we could observe 2nd harmonic response in most subjects but not 3rd harmonic (1500 Hz component). Since 2nd harmonic amplitudes were, in many cases, close to the noise floor of recordings (for example, see Figure 5), it is not clear whether higher harmonics were absent or simply below the noise floor of the recording available through the telemetry system.
Since further analysis of harmonic structure was not possible we compared data for low and high stimulus rates to assess relative effects of adaptation on the DIF and SUM components. On average, both components show some degree of adaptation at the high stimulus rate. The fact that DIF responses show some adaptation suggests that this response is not purely from hair cells and that there is some significant neural component in those recordings. Nevertheless, the data in Figures 10 and 11 suggest a clear difference in adaptation for the two measures, consistent with a hypothesis that there is generally a greater neural component in the SUM response providing further support that responses recorded at levels close to threshold have more neural contributions. Observations on individual subjects may prove to be more informative in that in some cases there is little or no difference in the DIF response for different rates while the SUM response shows a clear decrease. In those cases we may conclude that the DIF traces are largely hair cell responses.
The recordings illustrated in the case in Figure 11C show further support that SUM recordings are dominated by a neural component. In that work we used the two different stimulus rates to derive a “residual adapted response” that represents the activity that is affected by the change in stimulus presentation rate, presumably due to neural adaptation effects. The fact that the SUM waveform shows a similar latency and morphology but a larger amplitude is consistent with a hypothesis that the SUM is dominated by neural phase-locked responses.
In the recordings reported here we have focused on 500-Hz tone burst and broadband click stimuli. In addition, all recordings have been made using the most apical electrode of the implant array. We chose these parameters under the assumption that 500 Hz would show a strong neurophonic component of the responses and the thresholds at 500 Hz would be variable across individuals with the Hybrid implant. The most apical electrode was chosen to presumably be closer to the source of the responses and to maximize signal-to-noise ratios. Previous intraoperative recordings have demonstrated increased response amplitudes when a more apical recording electrode is used in cochlear implant recipients (Calloway et al 2014, Campbell et al 2015). The use of other frequencies and variations in response with different recording electrodes may provide a more complete picture of the relative status of the cochlea and the auditory nerve and, in turn, may lead to methods by which better separation of hair cell and neural components of the response could be possible. In addition, the use of different recording electrodes potentially will reflect variations in the neural status along the cochlear partition. Unfortunately, time limitations due to the recording technique did not allow for more extensive measures.
The group of subjects that showed no SUM response to acoustic stimulation is particularly intriguing. One might hypothesize that these subjects have some type of neuropathy, affecting either synaptic or neural responses while sparing their auditory hair cells. We would presume, if that were the case, that we might also see differences in performance in speech perception tests. This study did not include measures of speech perception or performance with the CI but examination of their clinical records does not reveal any striking differences between the group of subjects who had measurable SUM responses and those that did not. The fact that no SUM was evident could, of course, be due to a signal-to-noise issue, i.e. the SUM responses were generally smaller than DIF in these recordings. The fact that slope of the growth functions for DIF component varied greatly across the group of nine subjects that showed no SUM growth (see Figure 8) also suggests that at least in some users noise floor could be an issue, i.e., SUM responses may be below noise floor and consequently not measurable. In addition, one might expect that if there is no measureable neural component then the DIF component should show little or no adaptation , i.e., if there were truly no neural component evident, then adaption should be minimal. This was, in fact, the case for 5 of the 9 subjects that showed no SUM growth, i.e., there was no change in the DIF maximum amplitude for high and low rates. Other subjects in this group showed more typical decreases at high stimulus rates. All of these are topics that require further research to adequately address. We also note that of the 9 subjects, we were able to record an ECAP for 8 of them, which demonstrates a neural response was recordable. (We were not able to attempt an ECAP recording for the 9th subject due to time limitations.)
Another reason one might be interested in measuring the response of the auditory periphery to acoustic stimulation in Hybrid CI recipients is that it may provide a novel way of exploring acoustic-electric interactions. Hybrid CI users listen to combined acoustic and electrical stimulation in the same ear. One major clinical issue that remains somewhat controversial is related to how the speech processor should be programmed in order to deliver an appropriate balance of acoustic and electrical stimulation. Certainly the low frequencies will be processed via the hearing aid and high frequencies will be processed by the implant, but there is not currently a clear consensus regarding how the frequency boundary between acoustic and electric stimulation should be set (Karsten et al., 2013). Cochlear’s recommendation is to stimulate acoustically for audiometric thresholds less than 90 dB HL out to 2200 Hz, and set the lower frequency boundary of electric stimulation corresponding to where audiometric threshold is greater than 70 dB HL (Cochlear Ltd, 2015). There is likely some interaction of both acoustic and electrical stimulations that contribute to a patient’s performance. Prior animal studies have shown evidence of suppression of electrically evoked compound action potentials in the presence of acoustic noise (Nourski et al, 2005), and there is some psychophysical evidence of acoustic electric interaction in Hybrid CI users in that some showed elevated acoustic thresholds in the presence of an electric masker and vice-versa (Lin et al, 2011). Assessment of possible interaction at the peripheral level may impact cochlear implant programming strategies, especially the frequency boundary of acoustic and electrical stimulation. We have previously tried to suppress ECAPs by measuring them in the presence of acoustic broadband and narrowband noise in our hearing preservation patients but have not been successful to date. In addition, we have tried a modification of the current experimental approach where instead of presenting zero current and a tone burst, we presented current to evoke an ECAP. We did this with and without concurrent presentation of the tone burst, and did not see an effect of acoustic stimulation on electrical responses. It’s unclear whether the lack of measured acoustic electric interaction is due to limitations in experimental methods. It may also be that there is truly a lack of interaction at the periphery.
Finally, CAPs were recorded in response to clicks from 23 of the 44 subjects tested. For other subjects, responses could not be obtained at the highest level tested, or recordings were not attempted due to patient inability to hear the click stimulus. For the 23 subjects who had CAPs, growth functions were measured with as many levels as possible. The average click response latency amongst all the responses was 4.16 ms (SD = 1.40 ms). This average is based on the minimum latency observed at each subject’s appointment, which presumably reflects the response evoked at the highest stimulation level used at the appointment. If there was more than one appointment for the subject, then the latest appointment was used. ECoG latencies recorded from normal hearing listeners generally decrease with increasing level and asymptote at 1.5-2 ms (Portmann, 1977). When evoked using click stimuli, ECoG responses – like the ABR – primarily reflect neural activity from the base of the cochlea (Teas et al., 1962). However, the individuals who participated in this study all had significant high frequency hearing loss (See Figure 1). The delayed response we observed may reflect the fact that cochlear damage is extensive near the base and, as a result, the CAPs we recorded may have been dominated by neurons located closer to the apex of the cochlea. Data obtained using toneburst stimuli to record the ABR suggest that the difference in latency for a 500 Hz and a 4000 Hz stimulus is approximately 3-4 ms and is largely the result of delays introduced by the travelling wave (Gorga et al., 1988). ECoG data in subjects with high frequency hearing loss can show a similar latency shift (particularly at low levels where one might expect more sensitive low frequencies would dominate the response (Portmann, 1977). The latency values that we report are here are consistent with a hypothesis that responses in these subjects may be dominated by contributions for more apical regions than ECOG in normal hearing individuals.
In addition to variations in response latencies across participants, we also noted variations in waveform morphology. Some responses exhibited a typical negative peak followed by a positive peak. There were quite a few responses, however, that exhibited multiple negative peaks, as if there was some ringing in the waveform. There was no obvious reason for the multiple peaks. We theorized that a steeply sloping hearing loss would cause the ringing to occur (due to sharp filtering of the stimulus); however, there did not appear to be a relationship between audiometric configuration and presence of ringing. This point deserves further attention.
CONCLUSIONS
The NRT system of the Nucleus CI can be used to record responses to acoustic stimulation from an intracochlear electrode. These recordings are noninvasive, provide an objective assessment of acoustic threshold, can be used to study neural response to suprathreshold stimuli and are repeatable over time. Importantly, they also show sensitivity to changes in the hearing. We argue that it may be possible to use these recordings to partially separate hair cell responses from neural responses and as such, these acoustic recordings measured from an intracochlear electrode could prove to be particularly useful in characterizing the etiology of hearing loss as well as the cause of changes in hearing sensitivity over time for Hybrid CI users with preserved residual acoustic hearing.
Acknowledgments
Sources of funding: This study was funded by grants from the NIH/NIDCD (P50 DC000242; R01 DC012082).
Footnotes
Conflicts of interest: None of the authors report having a conflict of interest.
References
- Adunka O, Roush P, Grose J, et al. Monitoring of cochlear function during cochlear implantation. Laryngoscope. 2006;116:1017–1020. doi: 10.1097/01.mlg.0000217224.94804.bb. [DOI] [PubMed] [Google Scholar]
- Aran J-M, Charlet de Sauvage R. Clinical value of cochlear microphonic recordings. In: Ruben RJ, Elberling C, Salomon G, editors. Electrocochleography. University Park Press; 1976. pp. 55–65. [Google Scholar]
- Brockmeier SJ, Peterreins M, Lorens A, et al. Music perception in electric acoustic stimulation users as assessed by the Mu.S.I.C. Test. Adv Otorhinolaryngol. 2010;67:70–80. doi: 10.1159/000262598. [DOI] [PubMed] [Google Scholar]
- Calloway NH, Fitzpatrick DC, Campbell AP, et al. Intracochlear electrocochleography during cochlear implantation. Otol Neurotol. 2014;35:1451–1457. doi: 10.1097/MAO.0000000000000451. [DOI] [PubMed] [Google Scholar]
- Campbell L, Kaicer A, Briggs R, et al. Cochlear response telemetry: Intracochlear electrocochleography via cochlear implant neural response telemetry pilot study results. Otol Neurotol. 2014;36:399–405. doi: 10.1097/MAO.0000000000000678. [DOI] [PubMed] [Google Scholar]
- Campbell L, Kaicer A, Briggs R, et al. Cochlear response telemetry: Real-time monitoring of intraoperative electrocochleography; Poster presentation at the Conference on Implantable Auditory Prostheses; Lake Tahoe, CA. 2015. [Google Scholar]
- Cochlear Ltd. CochlearTM Nucleus® HybridTM Hearing Solution: Desk Reference Guide. 2015 Retrieved from: http://www.cochlear.com/wps/wcm/connect/1c88513a-7e5b-4cea-b6bf-cf3a3ff797c7/FUN1975+ISS3+JAN15+Hybrid+Reference+Guide.pdf?MOD=AJPERES&CONVERT_TO=url&CACHEID=1c88513a-7e5b-4cea-b6bf-cf3a3ff797c7.
- Choi C, Oghalai JS. Predicting the effect of post-implant cochlear fibrosis on residual hearing. Hear Res. 2005;205:193–200. doi: 10.1016/j.heares.2005.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury B, Fitzpatrick DC, Buchman CA, et al. Intraoperative round window recordings to acoustic stimuli from cochlear implant patients. Otol Neurotol. 2012;33:1507–1515. doi: 10.1097/MAO.0b013e31826dbc80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshraghi AA, Gupta C, Van De Water TR, et al. Molecular mechanisms involved in cochlear implantation trauma and the protection of hearing and auditory sensory cells by inhibition of c-Jun-N-terminal kinase signaling. Laryngoscope. 2013;123:S1–S14. doi: 10.1002/lary.23902. [DOI] [PubMed] [Google Scholar]
- Eshraghi AA, Lang DM, Roell J, et al. Mechanisms of programmed cell death signaling in hair cells and support cells post-electrode insertion trauma. Acta Oto-Laryngologica. 2015;135(4):328–334. doi: 10.3109/00016489.2015.1012276. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick DC, Campbell AP, Choudhury B, Dillon MT, Forgues M, Buchman CA, Adunka OF. Round window electrocochleography just prior to cochlear implantation: Relationship to word recognition outcomes in adults. Otol Neurotol. 2014;35(1):64–71. doi: 10.1097/MAO.0000000000000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forgues M, Koehm HA, Dunnon AK, et al. Distinguishing hair cell from neural potentials recorded at the round window. J Neurophysiol. 2014;111:580–593. doi: 10.1152/jn.00446.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formeister EJ, McClellan JH, Merwin WH, et al. Intraoperative round window electrocochleography and speech perception outcomes in pediatric cochlear implant recipients. Ear Hear. 2015;36:249–260. doi: 10.1097/AUD.0000000000000106. [DOI] [PubMed] [Google Scholar]
- Gantz BJ, Turner CW. Combining acoustic and electric hearing. Laryngoscope. 2003;113:1726–1730. doi: 10.1097/00005537-200310000-00012. [DOI] [PubMed] [Google Scholar]
- Gantz BJ, Hansen MR, Turner CW, et al. Hybrid 10 clinical trial. Audiol Neurotol. 2009;14(suppl 1):32–38. doi: 10.1159/000206493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantz BJ, Dunn C, Oleson J, Hansen M, Parkinson A, Turner C. Multicenter clinical trial of the Nucleus Hybrid S8 cochlear implant: Final outcomes. The Laryngoscope. 2016;126:962–973. doi: 10.1002/lary.25572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gfeller KE, Olszewski C, Turner C, et al. Music perception with cochlear implants and residual hearing. Audiol Neurotol. 2006;11(suppl 1):12–15. doi: 10.1159/000095608. [DOI] [PubMed] [Google Scholar]
- Gifford RH, Dorman MF, Spahr AJ, et al. Hearing preservation surgery: Psychophysical estimates of cochlear damage in recipients of a short electrode array. J Acoust Soc Am. 2008;124(4):2164–2173. doi: 10.1121/1.2967842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorga MP, Kaminski JR, Beauchaine KA, et al. Auditory brainstem responses to tone bursts in normally hearing subjects. J Speech Hear Res. 1988;31(1):87–97. doi: 10.1044/jshr.3101.87. [DOI] [PubMed] [Google Scholar]
- Harris R, Cruise A, Gibson W, et al. Preliminary results and technique for electrophysiological intra-operative monitoring of residual hearing during cochlear implantation. Cochlear Implants Int. 2011;12:209–215. doi: 10.1179/146701011X12950038111657. [DOI] [PubMed] [Google Scholar]
- Jurawitz M, Buchner A, Harpel T, et al. Hearing preservation outcomes with different cochlear implant electrodes: Nucleus Hybrid™-L24 and Nucleus Freedom™ CI422. Audiol Neurotol. 2014;19:293–309. doi: 10.1159/000360601. [DOI] [PubMed] [Google Scholar]
- Karsten SA, Turner CW, Brown CJ, et al. Optimizing the combination of acoustic and electric hearing in the implanted ear. Ear Hear. 2013;34(2):142–150. doi: 10.1097/AUD.0b013e318269ce87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopelovich JC, Reiss LAJ, Oleson JJ, et al. Risk factors for loss of ipsilateral residual hearing after hybrid cochlear implantation. Otol Neurotol. 2014;35:1403–1408. doi: 10.1097/MAO.0000000000000389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopelovich JC, Reiss LAJ, Etler CP, et al. Hearing loss after activation of hearing preservation cochlear implants might be related to afferent cochlear innervation injury. Otol Neurotol. 2015a;36:1035–1044. doi: 10.1097/MAO.0000000000000754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenarz T, Stover T, Buechner A, et al. Hearing conservation surgery using the Hybrid-L electrode. Audiol Neurotol. 2009;14(suppl 1):22–31. doi: 10.1159/000206492. [DOI] [PubMed] [Google Scholar]
- Lichtenham JT, Cooper NP, Guinan JJ., Jr A new auditory threshold estimation technique for low frequencies: Proof of concept. Ear Hear. 2013;34(1):42–51. doi: 10.1097/AUD.0b013e31825f9bd3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P, Turner CW, Gantz BJ, et al. Ipsilateral masking between acoustic and electric stimulation. J Acoust Soc Am. 2011;130(2):858–865. doi: 10.1121/1.3605294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandala M, Colletti L, Tonoli G, et al. Electrocochleography during cochlear implantation for hearing preservation. Otol Neurotol. 2012;146(5):774–781. doi: 10.1177/0194599811435895. [DOI] [PubMed] [Google Scholar]
- McClellan JJ, Formeister EJ, Merwin WH, III, et al. Round window electrocochleography and speech perception outcomes in adult cochlear implant subjects: Comparison with audiometric and biographical information. Otol Neurotol. 2014;35:e245–e252. doi: 10.1097/MAO.0000000000000557. [DOI] [PubMed] [Google Scholar]
- Mouney DF, Berline CI, Cullen JK, et al. Changes in human eighth nerve action potential as a function of stimulation rate. Arch Otolaryngol. 1978;104:551–554. doi: 10.1001/archotol.1978.00790100005001. [DOI] [PubMed] [Google Scholar]
- Nourski KV, Abbas PJ, Miller CA, et al. Effects of acoustic noise on the auditory nerve compound action potentials evoked by electric pulse trains. Hear Res. 2004;202:141–153. doi: 10.1016/j.heares.2004.10.001. [DOI] [PubMed] [Google Scholar]
- O’Leary SJ, Monksfield P, Kel G, et al. Relations between cochlear histopathology and hearing loss in experimental cochlear implantation. Hear Res. 2013;298:27–35. doi: 10.1016/j.heares.2013.01.012. [DOI] [PubMed] [Google Scholar]
- Portmann M. Electrocochleography. J Laryngol Otol. 1977;91:655–678. doi: 10.1017/s0022215100084218. [DOI] [PubMed] [Google Scholar]
- Podskarbi-Fayette R, Pilka A, Skarzynski H. Electric stimulation complements functional residual hearing in partial deafness. Acta Otolaryngol. 2010;130(8):888–896. doi: 10.3109/00016480903567189. [DOI] [PubMed] [Google Scholar]
- Quesnel AM, Nakajima HH, Rosowski JJ, et al. Delayed loss of hearing after preservation cochlear implantation: Human temporal bone pathology and implications for etiology. Hear Res. 2015 doi: 10.1016/j.heares.2015.08.018. http://dx.doi.org/10.1016/j.heares.2015.08.018. epub ahead of print. [DOI] [PMC free article] [PubMed]
- Reiss LA, Stark G, Nguyen-Huynh AT, et al. Morphological correlates of hearing loss after cochlear implantation and electro-acoustic stimulation in a hearing-impaired guinea pig model. Hear Res. 2015;327:163–174. doi: 10.1016/j.heares.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland JT, Gantz BJ, Waltzman SB, et al. United States multicenter clinical trial of the Cochlear Nucleus Hybrid implant system. Laryngoscope. 2015;126(1):175–181. doi: 10.1002/lary.25451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarzynski H, Lorens A, Matusiak M, et al. Cochlear implantation with the nucleus slim straight electrode in subjects with residual low-frequency hearing. Ear Hear. 2014;35:e33–e43. doi: 10.1097/01.aud.0000444781.15858.f1. [DOI] [PubMed] [Google Scholar]
- Snyder RL, Schreiner CE. The auditory neurophonic: Basic properties. Hear Res. 1984;15:261–280. doi: 10.1016/0378-5955(84)90033-9. [DOI] [PubMed] [Google Scholar]
- Tanaka C, Nguyen-Huynh A, Loera K, et al. Factors associated with hearing loss in a normal-hearing guinea pig model of hybrid cochlear implants. Hear Res. 2014;316:82–93. doi: 10.1016/j.heares.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teas DC, Eldredge DH, Davis H. Cochlear responses to acoustic transients: An interpretation of whole-nerve action potentials. J Acoust Soc Am. 1962;34:1438–1458. [Google Scholar]
- Turner CW, Gantz BJ, Vidal C, et al. Speech recognition in noise for cochlear implant listeners: Benefits of residual acoustic hearing. J Acoust Soc Am. 2004;115(4):1729–1735. doi: 10.1121/1.1687425. [DOI] [PubMed] [Google Scholar]
- Van Abel KM, Dunn CC, Sladen DP, et al. Hearing preservation among patients undergoing cochlear implantation. Otol Neurotol. 2015;36(3):416–421. doi: 10.1097/MAO.0000000000000703. [DOI] [PMC free article] [PubMed] [Google Scholar]