Abstract
Binge alcohol drinking has emerged as a typical phenomenon in young people. This pattern of drinking, repeatedly leading to extremely high blood and brain alcohol levels and intoxication is associated with severe risks of neurodegeneration and cognitive damage. Mechanisms involved in excitotoxicity and neuroinflammation are pivotal elements in alcohol-induced neurotoxicity. Evidence has demonstrated that PPARγ receptor activation shows anti-inflammatory and neuroprotective properties. Here we examine whether treatment with the PPARγ agonist pioglitazone is beneficial in counteracting neurodegeneration, neuroinflammation and cognitive damage produced by binge alcohol intoxication. Adult Wistar rats were subjected to a 4-day binge intoxication procedure, which is commonly used to model excessive alcohol consumption in humans. Across the 4-day period, pioglitazone (0, 30, 60 mg/kg) was administered orally twice daily at 12-h intervals. Degenerative cells were detected by fluoro-jade B (FJ-B) immunostaining in brain regions where expression of pro-inflammatory cytokines was also determined. The effects of pioglitazone on cognitive function were assessed in an operant reversal learning task and the Morris water maze task. Binge alcohol exposure produced selective neuronal degeneration in the hippocampal dentate gyrus and the adjacent entorhinal cortex. Pioglitazone reduced FJ-B positive cells in both regions and prevented alcohol-induced expression of pro-inflammatory cytokines. Pioglitazone also rescued alcohol-impaired reversal learning in the operant task and spatial learning deficits in the Morris water maze. These findings demonstrate that activation of PPARγ protects against neuronal and cognitive degeneration elicited by binge alcohol exposure. The protective effect of PPARγ agonist appears to be linked to inhibition of pro-inflammatory cytokines.
Keywords: pioglitazone, PPARγ, neurodegeneration, alcohol, cognitive flexibility, cytokines
1. INTRODUCTION
Chronic alcohol intoxication resulting from binge drinking results in significant activation of neurodegenerative processes (Vetreno and Crews, 2015; Vetreno et al, 2016). The alcoholic brain shows reduction in brain volume and weight, enlargement of ventricles and gray as well as white matter shrinkage in cortical and subcortical structures (Pfefferbaum et al, 1992; Vetreno et al, 2015). Alcohol-induced neuropathological alterations in brain structure and function are often correlated with impairments in cognitive processes (Bowden and McCarter, 1993; White, 2003). In laboratory animals, it is well established that large doses of alcohol (11–15 g/kg/day) administered over a short period of time (4 days) reliably produce neurotoxicity in various corticolimbic areas including the hippocampal dentate gyrus (DG) and the entorhinal cortex [EC, (Collins et al, 1996; Crews et al, 2004; Obernier et al, 2002a)]. The binge model of human alcoholic neurodegeneration, which mimics a single cycle of binge intoxication in human alcoholics (Braconi et al, 2010), is further validated by the fact that during alcohol intoxication animals reach sustained, high blood alcohol levels (BALs), commonly observed among alcoholics. Furthermore, neuronal deficits in animals treated with this binge alcohol model are associated with significant cognitive dysfunctions, such as learning and memory impairment as well as behavioral deficits including maladaptive perseverant behavior (Cippitelli et al, 2010a; Cippitelli et al, 2010b; Obernier et al, 2002b).
It has been proposed that alcohol-induced brain damage may result from imbalance in expression and activation of transcription factors that regulate anti-inflammatory /pro-survival versus pro-inflammatory/pro-death processes (Crews and Nixon, 2009). Indeed, both in vitro and in vivo evidence have shown that alcohol shifts this balance toward neuroinflammation by decreasing cAMP responsive element-binding protein (CREB)-mediated signaling (Zou and Crews, 2006) or increasing toll-like receptor (TLR) 4 and downstream nuclear factor κB (NF-κB) signaling (Alfonso-Loeches et al, 2010; Crews et al, 2006; Davis and Syapin, 2004; Fernandez-Lizarbe et al, 2013).
Peroxisome proliferator-activated receptors (PPARs) are ligand-activated transcription factors of the nuclear hormone receptor superfamily. They are involved in the transcriptional control of genes regulating various physiological processes such as lipid-homeostasis, glucose metabolism, inflammation, and cellular differentiation and proliferation (Desvergne and Wahli, 1999). Agonists of the isoform PPARγ such as pioglitazone or rosiglitazone are commonly used medications in the treatment of type II diabetes. Beside their effect on metabolic disorders, PPARγ agonists also modulate inflammatory responses, including immune activity in the central nervous system (Kapadia et al, 2008). This is consistent with the observation that PPARγ was found in several brain regions both in neuronal and non-neuronal cells (Moreno et al, 2004) although a recent study indicates that PPARγ is constitutively expressed at higher levels in neurons than non-neuronal cells (Warden et al, 2016). Thus, PPARγ may be an important therapeutic target for neurodegenerative diseases. Accordingly, neuroprotective potential of PPARγ agonists has been demonstrated in animal models of acute CNS insults [i.e., spinal cord injury (Park et al, 2007)] and models of chronic CNS injuries including Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, and multiple sclerosis (Diab et al, 2002; Kapadia et al, 2008; Kaundal and Sharma, 2010; Schintu et al, 2009). Efficacy of PPARγ agonists was also shown in a mouse model of fetal alcohol spectrum disorders (Drew et al, 2015; Kane et al, 2011).
Immune or brain pro-inflammatory signaling is not only relevant for neurodegeneration. Recent evidence supports the influence of the neuroimmune system on learning and memory and neuroplasticity (Williamson and Bilbo, 2013). Thus, actions of alcohol on neuroimmune function may be important for the development of aspects of alcohol dependence such as escalation of consumption, craving, tolerance, and withdrawal (Crews et al, 2011; Robinson et al, 2014). PPARγ agonists have been recently shown to modulate excessive alcohol consumption, alcohol withdrawal symptoms and stress but not cue-induced reinstatement of alcohol seeking in rats (Stopponi et al, 2013; Stopponi et al, 2011), thus providing promising preclinical evidence for novel and effective alcohol addiction medications.
Stemming from the initial work, which supports a role of pioglitazone in reducing alcohol addiction and its emerging protective effects in various neurological dysfunctions, here we examine whether treatment with pioglitazone is beneficial in attenuating neurodegeneration elicited by excessive alcohol exposure. First we characterized fluorojade B (FJ-B) immunohistochemistry in the DG and the EC, two brain regions known to be sensitive to alcohol-induced neurotoxicity. Subsequently, using operant reversal learning and spatial orientation strategy-shift tasks, we monitored the effect of PPARγ activation on re-learning and cognitive flexibility. Finally, we examined the ability of pioglitazone to modulate expression of pro-inflammatory cytokines as a possible mechanism for its neuroprotective actions following binge alcohol exposure.
2. MATERIALS AND METHODS
2.1 Animals
Male Wistar rats (Charles River, Calco, Italy), weighing 300 to 350 g (8–10 weeks old) at the beginning of the experiments, were pair-housed with free access to tap water and food pellets (4RF18, Settimo Milanese, Italy) except where specified. The animals were maintained in a temperature- and humidity-controlled vivarium on a reverse 12-hour light/dark cycle (lights off at 8:30 AM). Behavioral experiments were conducted during the dark phase of the cycle. Animals were handled three times before each experiment. All procedures followed the EU Directive for Care and Use of Laboratory Animals and were approved by the Ethical Committee of the University of Camerino, Italy.
2.2 Drugs
Pioglitazone was prepared from the pharmaceutical form Actos (30 mg tablets, Takeda). Tablets were suspended in distilled water to reach dosages of 30 mg/kg and 60 mg/kg. Pioglitazone was administered orally (by gavage) in a 1 ml/kg volume. Alcohol solution (20% vol/vol) was prepared by diluting 95% alcohol with water and made available orally.
2.3 Binge alcohol treatment
All rats used in the present study (N=157) were subjected to a 4-day binge intoxication (or to a control) procedure in which alcohol (20% vol/vol) was administered orally every 8 hours to reach doses of 11–15 g/kg/day as previously described (Cippitelli et al, 2014; Majchrowicz, 1975). Alcohol was administered in a vehicle made up with water, 6% sucrose and 14.7% milk powder (Mellin, Milan, Italy). Alcohol treated animals were given a priming dose of 5 g/kg of body weight. Additional alcohol was administered every 8 hours for 4 consecutive days at 8:00 AM, 4:00 PM, and 12:00 AM based on the animals’ estimated BAL, as determined using a six-point intoxication scale (Majchrowicz, 1975). Control (CON) rats received equal volumes of the vehicle. Four batches of rats were used. One batch (N=29) was employed for histochemical analysis of FJ-B. These rats were assigned to two groups of 17 alcohol exposed, in turn divided into three groups of 5–6 receiving vehicle, pioglitazone (PIO) 30 mg/kg, or PIO 60 mg/kg twice daily at 12-h intervals across the 4-day binge period (7:00 AM and 11:00 PM), and 12 control exposed divided into three groups of 4 rats receiving vehicle, PIO 30 mg/kg, or PIO 60 mg/kg. A second group (N=30; 8 non-alcohol exposed vehicle treated, 8 non-alcohol exposed treated with PIO 60 mg/kg, 7 alcohol exposed vehicle treated and 7 alcohol exposed receiving PIO 60 mg/kg) was employed for gene expression analysis. The third (N=53) and the fourth (N=43) batch of rats were divided into 4 groups as described above and used to examine operant reversal learning and to determine BALs and reversal learning in the Morris Water Maze (MWM), respectively (Supplemental Table 1).
2.4 BALs
Twenty-four blood samples (150–200 μl) consisting of 6 non-alcohol exposed vehicle treated, 6 non-alcohol exposed treated with PIO 60 mg/kg, 6 alcohol exposed vehicle treated and 6 alcohol exposed receiving PIO 60 mg/kg were taken from the rat tail vein 60 min after the last administration of the 4-day binge alcohol procedure (after the 8:00 AM gavage). Samples were kept on ice and then immediately centrifuged (10 min, 1400 g). Alcohol content was then assayed from 5 μl plasma aliquots using an Analox instrument (Lunenburg, MA). Single point calibrations from 25–400 mg% were done for each set of samples with reagents provided by Analox (Gilpin et al, 2009; Richardson et al, 2008). Instrumentation background determined from non-alcohol treated samples was subtracted to calculate BAL of alcohol-treated animals.
2.5 FJ-B staining
FJ-B was purchased from Histochem, Inc., (Jefferson, AR) and used as a marker of degenerating neurons (Schmued and Hopkins, 2000). Three hours after the last alcohol gavage, rats were perfused with 4% paraformaldehyde under isoflurane anesthesia. Horizontal 20-μm cryosections were obtained, allowing visualization in the same section of both ventral hippocampi containing the DG and the EC. Sections were mounted directly on gelatin-coated slides and stained for FJ-B according to the manufacturer’s protocol. Dry slides were cleared in xylene and cover-slipped with Cytoseal (Richard-Allan Scientific, Kalamazoo, MI). For cell density analysis an Olympus microscope (Olympus Corporation, Japan) equipped with a Fitch filter was used. Six horizontal sections containing the bilateral hippocampi and the respective EC regions were analyzed for degenerating cells between 5.6 to 6.6 mm ventral from bregma. Cell counting was conducted as previously described (Cippitelli et al, 2010a; Cippitelli et al, 2014; Cippitelli et al, 2010b). Degenerating granule cells of the entire DG were measured using the program Image J (Schneider et al, 2012). Results for EC degeneration are depicted as counts per square mm by dividing the total number of degenerating cells found in 48 examined microscope fields, equivalent to 16.8 square millimeters (single field area was 0.35 square millimeters × 4 fields per side × 2 sides per section × 6 sections per animal, for a total of 16.8 square millimeters) with a 20× microscope objective. Data for EC and DG degenerating cells are presented as number per square millimeter.
2.6 RNA Isolation, cDNA Synthesis and Real Time Polymerase Chain Reaction
Rats were decapitated three hours after the last alcohol gavage, the same time point used to harvest samples for histochemical analysis of FJ-B. Brains were quickly removed, areas of interest were dissected and snap frozen in −40°C isopentane, and stored at −80°C until use. RNA isolation and cDNA synthesis was executed as previously described (Drew et al, 2015). Briefly, tissue was homogenized using a BBX24B Bullet Blender Blue homogenizer with 0.5mm RNase-free beads for approximately 6 minutes at speed 8 (Next Advance, Averill Park, NY). RNA was isolated from tissue homogenate using the RNeasy Lipid Tissue Mini Kit and optional on-column DNA digestion using the supplementary RNase-free DNase set according to the manufacturer’s instructions (Qiagen, Valencia, CA). RNA concentration and integrity were evaluated using an Agilent 2100 Bioanalyzer with its associated RNA 6000 Nano kit (Agilent Technologies, Santa Clara, CA). The iScript™ cDNA synthesis kit was used to prepare cDNA as described by the manufacturer (Bio-Rad, Hercules, CA). Quantitative real-time polymerase chain reaction (qRTPCR) was performed using TaqMan® primers (assays # Rn00580432m1 for IL-1β, Rn01410330m1 for IL-6, Rn00667869 m1 for β-actin; ThermoFisher Scientific, Waltham, MA) and SsoAdvanced™ Universal Probes Supermix (Bio-Rad). Data were calculated as the mean ΔCt relative to the housekeeping gene β-actin. The ΔΔCt method was employed to generate fold expression variance of ethanol and drug treated groups compared to control.
2.7 Operant reversal learning task
This procedure was conducted in operant conditioning chambers (Med Associates, Inc., St. Albans, VT) enclosed in lit, sound-attenuating and ventilated cubicles and equipped with two retractable levers located in the front panel, laterally (on the right and left side) to a food pellet magazine. The pellet dispenser was positioned behind the front panel of the chambers. Chambers were also equipped with visual stimuli located above the levers (right and left cue lights) and near the top of the chamber on the back panel (house light). A microcomputer controlled the delivery of the reinforcer, presentation of visual stimuli and recording of the behavioral data. Operant training and testing methods used are described elsewhere (Abdul-Monim et al, 2003).
Training
Rats maintained at food restriction regimen (16–18 g per day) were initially trained for 4 days to press the lever for delivery of one 45-mg food pellet (Test Diet, 5-TUM, Richmond, IN) in 20-min daily sessions under a fixed ratio 1 (FR-1) reinforcement schedule using two active levers. Subsequently, rats were trained to press either the right or the left lever for food delivery under FR-1 for an additional 4 days. The active lever was varied from day to day to avoid lever bias. Rats were then trained to respond for food in presence and absence of a visual cue for 20 days. In the former case reward contingencies were right lever-right cue (RR) and left-lever left cue (LL), in the latter case, reward contingencies were right lever-left cue (RL) or left lever-right cue (LR). Thus, half of animals that were initially rewarded following RR or LL schedule for 10 days (varied day to day) were switched to RL or LR for other 10 days and the other half did the opposite. The experimental session began with the house light being illuminated. After 3 s, the levers, together with the stimulus cue, were presented. Following a lever press, the levers were retracted and the house light was extinguished for a 3-s time out period. A correct response on the active lever resulted in delivery of a food pellet and an incorrect response resulted in no food delivery. The house light was then turned on again and the cycle repeated. The experimental session was terminated following 128 lever presses (the total number including active and inactive lever presses) or 40 min (cut-off). At the end of this training period all rats were subjected to the 4-day binge alcohol or control treatment in which rats were pre-treated with PIO 60 mg/kg dose or its vehicle as described above.
Testing
Following 5-day recovery from alcohol intoxication the operant training on all reward contingencies was briefly recalled (2 sessions for each randomly scheduled reward contingency). On testing day, rats were first given access to the same reward contingency of the training session used on the previous day for a maximum period of 5 min or 25 lever presses (including both active and inactive lever presses, initial task). Then, after 2 min time-out signalled by the house light being turned off in which animals remained in the operant chamber, the reward contingency was reversed so that animals rewarded in the presence of the visual cue (RR or LL) were rewarded for responses made on the lever not signalled by the cue (LR or RL, respectively) and vice versa (reversal task). Similar to the initial task, the reversal task was carried out for a maximum period of 5 minutes or following a total number of 25 lever presses including correct and incorrect responses.
2.8 MWM task
Spatial acquisition and cognitive flexibility were assessed following methods described previously (Cippitelli et al, 2010a; Obernier et al, 2002b; Vorhees and Williams, 2006). The apparatus consisted of a circular pool (55 cm diameter) and a squared platform (16 cm2) submerged below the water surface. Behavior was recorded and analyzed using a computerized video-tracking system (Ethovision 4, Noldus Information Technology, Wageningen, The Netherlands). The pool was placed in a room with distal cues represented by objects present in the text room (i.e., doors, black rectangle drawn in a wall).
Reference memory
Beginning 5 days after the final gavage treatment, in which rats were pretreated with PIO 60 mg/kg or its vehicle and treated with alcohol or control, acquisition of reference memory was assessed on four daily trials over 6 days. Four random entry points were used. If a rat did not escape to the platform within 60 seconds, it was guided to the platform and allowed to remain on the platform for 10 seconds. The rat was then removed and placed into a holding cage for 50 seconds before the next trial, so the total intertrial interval was 60 seconds.
The platform remained in the same location throughout the 6 days of acquisition training. Results are means of four daily trials for latency to reach the submerged platform. Swimming speed was an index of locomotor activity.
Learning of a new platform position
Following reference memory testing, which provided 6 days of acquisition training and was 11 days after the last alcohol dose, the platform was moved diagonally across from its initial position, and the new learning was assessed. Each animal performed four trials separated by 60-second intertrial intervals, starting from four random entry points. The rest of the procedure was as described above. Time swum to reach the new platform location, distance to reach the new location, entries into the quadrant of the previous platform location as well as time swum in the previous platform location indicated learning of the new condition. For each re-learning variable, differences between trials (“savings”) served as an index of re-learning. Swimming speed was an index of locomotor activity.
2.9 Statistical analysis
Histological data from alcohol-induced neurodegeneration were analyzed separately for DG and EC using the non-parametric multiple independent samples Kruskal-Wallis test in which FJ-B positive cells/mm2 was the dependent variable and treatment groups were the independent variable. This overall analysis was followed by group comparisons using the Mann–Whitney U test. The rate of correct responses during operant training was analyzed by two-way mixed model ANOVA with reward contingency (RR-LL vs RL-LR or treatment group) used as between-subject and “day” as within-subject factor. Operant reversal learning data of both initial and reversal tasks were analyzed by a two-way ANOVA using the percent of correct responses as the dependent variable and “alcohol effect” and “pioglitazone treatment” as the two independent variables. The same approach was used for speed navigation and analysis of savings (i.e., trial 1- trial 2) for the new learning variables (MWM task). These variables were also analyzed to examine the effects of drug treatments across trials during reversal learning by means of a two-way mixed model ANOVA. Memory acquisition and speed navigation in the MWM was analyzed using three-way ANOVA, where the within-subject variable was “day” and the between-subject variables were “alcohol effect” and “pioglitazone treatment”. For all statistical analyses, differences between groups were considered significant if p<0.05. When a statistically significant threshold was reached, ANOVA was followed by Newman-Keuls. For gene expression analysis, interactions were tested with two-way ANOVA and Tukey’s multiple comparisons test. Grubbs test (alpha = 0.05) of expression data identified one outlier in the non-alcohol exposed PIO treated and alcohol exposed PIO treated groups.
3. RESULTS
3.1 Pioglitazone treatment prevents alcohol-induced neurodegeneration in the hippocampal DG and the EC
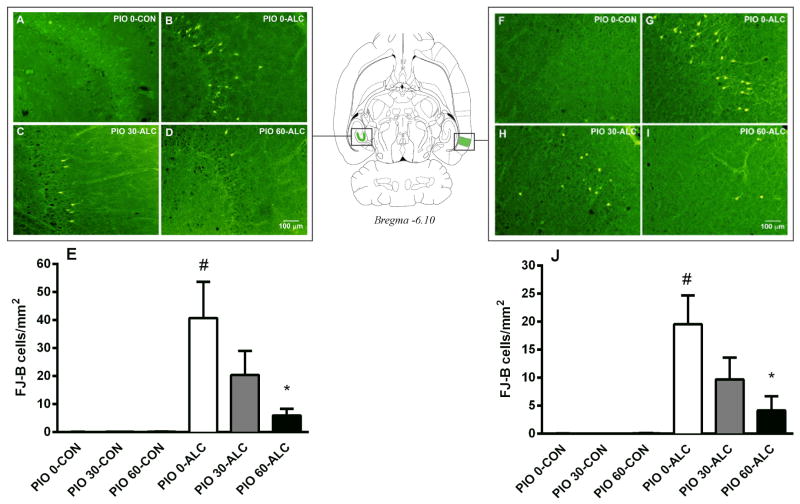
Because very low numbers of FJ-B positive cells were present in the groups that were not alcohol exposed, these groups had low variance compared with groups that did receive alcohol. The analysis was therefore conducted using non-parametric approaches (see Statistical Analysis). These analyses demonstrated an overall significant effect of alcohol treatment in inducing neuronal cell death in the DG (p<0.05) as well as the EC (p<0.05). Specifically, the most intense damage was found in the granule cells of the DG and the layer III pyramidal cells of the lateral EC (Supplemental Figure S1). In the DG, neurotoxicity was reduced by daily treatment with pioglitazone. Pairwise comparisons showed that alcohol exposed animals treated with the 60 mg/kg dose of pioglitazone had significantly reduced levels of neurodegeneration as compared to rats treated with only alcohol (p<0.05, Figure 1A–E). Similar to the DG findings, treatment with 60 mg/kg of pioglitazone prevented alcohol-induced neurotoxicity in the EC (p<0.05, Figure 1F–J). Pioglitazone 30 mg/kg did not block neurodegeneration in the DG or EC, but a nonsignificant statistically reduction was observed. In areas other than the hippocampal DG granule cells and the EC layer III pyramidal cells, FJ-B positive cells were too few to be reliably counted. Thus, although alcohol-induced neurotoxicity was also observed in brain regions such as the piriform cortex, neurodegeneration was not quantified.
Figure 1.
Binge alcohol-induced neurotoxicity in the dentate gyrus (DG) and the entorhinal cortex (EC), and its prevention by pioglitazone (0, 30, 60 mg/kg). Sections were stained by Fluoro-Jade B (FJ-B) to visualize neurodegeneration. Panels (A), (B), (C), and (D) show representative sections (−6.1 mm from bregma, 20× magnification) visualizing labeled DG neurons in animals non-alcohol exposed treated with vehicle (PIO 0-CON), alcohol exposed treated with vehicle (PIO 0-ALC), pioglitazone 30 (PIO 30-ALC) and 60 mg/kg (PIO 60-ALC), respectively. Panels (F), (G), (H), (I) show representative sections (−6.1 mm from bregma, 20× magnification) visualizing labeled EC neurons in PIO 0-CON, PIO 0-ALC, PIO 30-ALC and PIO 60-ALC treated rats, respectively. Panels (E) and (J) show quantification of the histological data demonstrating alcohol-induced neurodegeneration and its prevention by pioglitazone in the DG and the EC, respectively. Data are the mean number of FJ-B positive cells/mm2 ± SEM (N=4–6 per group). #p<0.05 vs. PIO 0-CON group; *p<0.05 vs. PIO 0-ALC group. For detailed statistics, see “Results”.
3.2 Binge treatment leads to high, sustained BALs that are not influenced by pioglitazone
BALs were 393.1 ± 24.2 and 401.5 ± 20.0 in alcohol treated animals without or with pioglitazone treatment, respectively.
3.3 Neuroprotection by pioglitazone is associated with inhibition of alcohol-induced increase in cytokine expression in hippocampus and EC
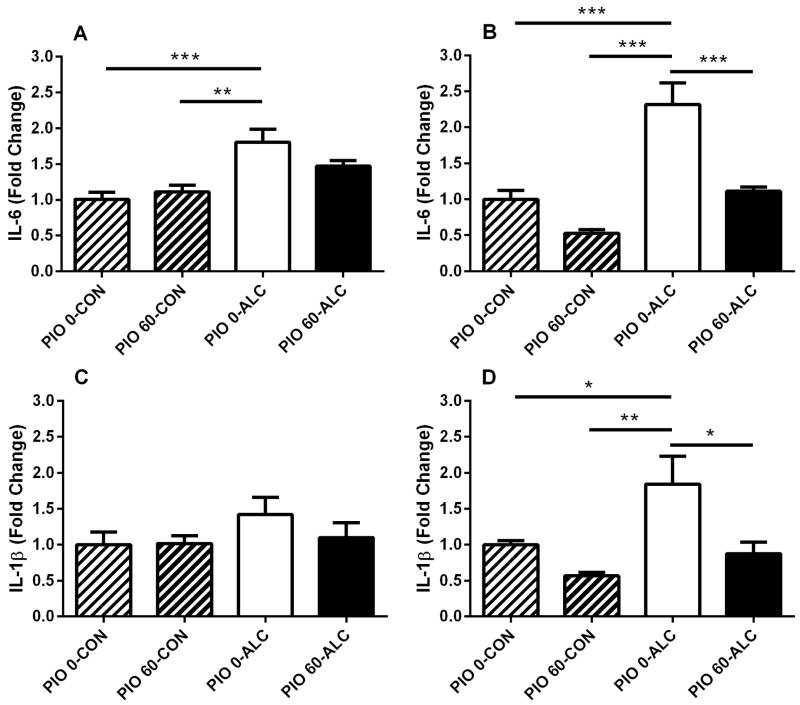
Analysis of gene expression revealed 1.8-fold induction of interleukin 6 (IL-6) mRNA in the hippocampus of rats treated with alcohol [main alcohol effect: (F(1,24)=21.2, p<0.001)]. This effect was not accompanied by a main pioglitazone effect (F(1,24)=0.8, NS) or interaction between alcohol and pioglitazone (F(1,24)=3.1, NS). Administration of pioglitazone attenuated alcohol induction of IL-6 expression in the hippocampus (Figure 2A). Analysis of gene expression also revealed robust 2.5 fold induction of IL-6 mRNA in the EC of rats treated with alcohol (F(1,26)=36.1, p<0.001). Administration of pioglitazone blocked alcohol induction of IL-6 expression in the EC to levels observed in vehicle treated controls or treatment with pioglitazone alone [(main pioglitazone effect (F(1,26)=28.1, p<0.001); interaction “pioglitazone × alcohol” (F(1,26)=5.4, p<0.05), Figure 2B].
Figure 2.
Effect of alcohol (ALC) and pioglitazone (PIO) on expression of interleukin 6 (IL-6) and interleukin 1β (IL-1β) genes in the hippocampus (A and C) and entorhinal cortex (EC, B and D). Pioglitazone (60 mg/kg) blocked alcohol-induced neuroinflammation in the hippocampus and EC. Brain structures were dissected, RNA prepared, cDNA synthesized, and mRNA levels evaluated by real-time polymerase chain reaction. β-actin expression served as normalization control. Results are expressed as fold change relative to vehicle treated controls (PIO 0-CON). Values are mean ± SEM. N=6–8 per treatment group. *p<0.05, **p<0.01, ***p<0.001. For detailed statistics see “Results”.
Interleukin 1 beta (IL-1β) mRNA expression in the hippocampus was not significantly altered by treatment with alcohol or pioglitazone, or co-administration [(F(1,24)=1.7, NS), (F(1,24)=0.6, NS) and (F(1,24)=0.8, NS), respectively]. However, there was a trend to increased IL-1β expression with alcohol treatment, and suppression of the alcohol effect by pioglitazone (Figure 2C). In contrast, IL-1β mRNA expression in the EC was increased 1.8-fold by alcohol treatment (F(1,24)=7.5, p<0.05) and blocked by co-administration of pioglitazone (F(1,24)=11.1, p<0.01), but no interaction between the alcohol and pioglitazone was detected (F(1,24)=1.7, NS). Pioglitazone blocked alcohol induction of IL-1β expression to the level in vehicle treated control or treatment with pioglitazone alone (Figure 2D).
3.4 Pioglitazone prevents alcohol-impaired reversal learning ability in the operant task
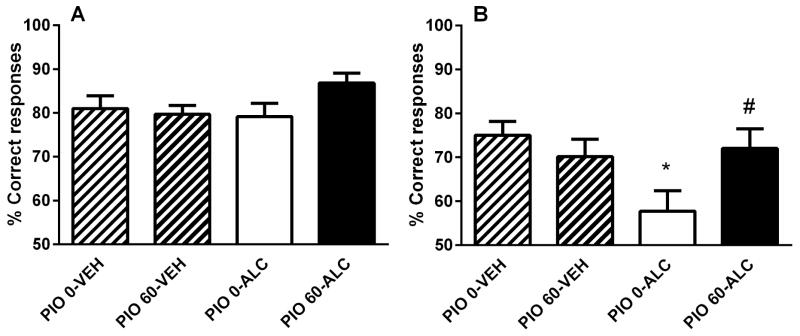
The sequence of the reward contingencies did not alter the correct performance of the task during training [(F(1,49)=0.0, NS) and (F(1,49)=0.1, NS), Supplemental Figure S2A and S2B, respectively]. Post-intoxication training was not altered either by alcohol, pioglitazone or the combined treatment [(F(3,49)=0.6, NS), Supplemental Figure S3]. Also, these manipulations did not affect the performance in the initial task [(F(1,49)=3.1, NS), Figure 3A]. In contrast, overall performance in the reversal task was significantly modified. ANOVA showed “alcohol effect” × “pioglitazone treatment” interaction (F(1,49)=5.1, p<0.05) with pairwise comparisons showing reduced ability of rats treated with alcohol only to perform the correct response (p<0.05) while pioglitazone reversed the cognitive deficit induced by alcohol intoxication (p=0.05, Figure 3B).
Figure 3.
Alcohol-induced impairment of operant reversal learning and its prevention by pioglitazone. (A) Alcohol, pioglitazone (60 mg/kg) or the combined treatment did not influence operant learning of the initial task. (B) Performance in the reversal task was reduced in alcohol exposed while pioglitazone (60 mg/kg) prevented alcohol-induced impairment of reversal learning. Data are expressed as mean ±SEM percent (%) of correct responses (N=53, 12–14 per group). #p<0.05 vs control (CON) group receiving vehicle (PIO 0-CON); *p<0.05 vs alcohol group receiving vehicle (PIO 0-ALC). For detailed statistics, see “Results”.
3.5 Pioglitazone improves acquisition of spatial memory and prevents alcohol-induced deficits in spatial learning in the MWM
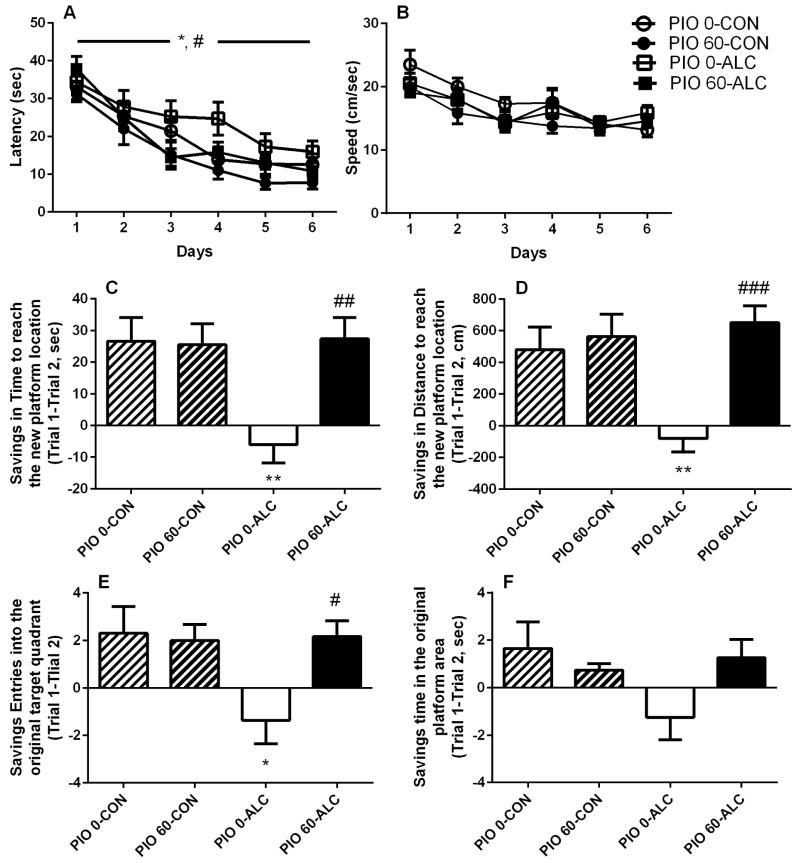
Patterns of acquisition of spatial memory in the MWM were modified by the treatments with both alcohol and pioglitazone (60 mg/kg). ANOVA revealed a main effect of alcohol intoxication (F(1,39)=5.0, p<0.05) accompanied by a main effect of pioglitazone (F(1,39)=6.0, p<0.05) indicating that alcohol impaired reference memory abilities while pioglitazone improved them. Interestingly, the pioglitazone-induced enhancement of spatial memory acquisition occurred regardless of the contingent alcohol treatment, as demonstrated by the absence of “alcohol effect” × “pioglitazone effect” interaction. However, navigation latency to reach the hidden platform decreased over the 6 days of training [main time effect: (F(5,195)=37.6, p<0.001)] and did so in all groups, as shown by a lack of “alcohol effect” × “pioglitazone effect” × “day” interaction [(F(5,195)=0.5, NS), Figure 4A]. Swim speed (Figure 4B), a measure of locomotor activity, was not altered by alcohol treatment (main effect: F(1,39)=0.0, NS) or pioglitazone (main effect: F(1,39)=2.3, NS). Speed decreased across the 6 training days (F(5,195)=16.4, p<0.001) in all treatment groups examined (F(5,195)=1.3, NS).
Figure 4.
Reference memory and spatial learning of a new condition in the Morris water maze. (A) Acquisition of spatial memory is improved by pioglitazone and impaired by alcohol intoxication. Data are shown as mean ±SEM latency (sec) of four daily trials across 6 days (N=43, 10–12 per group). *p<0.05 difference between alcohol exposed and controls; #p<0.05 difference between pioglitazone treated and vehicle treated groups. (B) Motor performance measured as mean of swim speed ±SEM (cm/sec) was not affected by alcohol exposure or following pretreatment with pioglitazone. Alcohol exposure induced impairment of spatial learning in the Morris water maze which was prevented by chronic treatment with pioglitazone 60 mg/kg. Performance for the new learning is described as savings between trial 1 and trial 2 (±SEM, N=43, 10–12 per group) in (C) time swum to reach the new platform location, (D) distance swum to reach the new platform placement, (E) number of entries into the previously trained quadrant, (F) navigation time into the original platform quadrant. *p<0.05, **p<0.01 alcohol vs non-exposed; #p<0.05, ## p<0.01, ###p<0.001 PIO 60-ALC vs PIO 0-ALC. For detailed statistics, see “Results”.
Following 6 days of acquisition trials, animals were tested for a new spatial learning task, in which the platform was moved to the opposite quadrant of the pool. Learning of the new platform location differed between groups for distance traveled to reach the new platform location (F(3,39)=3.3, p<0.05) and time swum to reach this new location (F(3,39)=4.2, p<0.05) while the time course of this learning was different in all variables examined [“group” × “trial” interaction for distance traveled to reach the new platform location (F(9,117)=3.3, p<0.01), time to reach this new location (F(9,117)=2.5, p<0.05), entries into the original training quadrant (F(9,117)=2.8, p<0.01) and time swum in the original platform quadrant [(F(9,117)=2.2, p<0.05), Supplemental Figure S4].
The differential course of re-learning reflected differences in savings (improvement of performance). When analyzing savings between trial 1 and trial 2, when most of the learning normally occurs, savings for time spent swimming to reach the new platform location (Figure 4C) differed among treatments. ANOVA showed a main effect of alcohol (F(1,39)=5.2, p<0.05) accompanied by a main effect of pioglitazone (F(1,39)=5.8, p<0.05) and interaction of the two factors (F(1,39)=6.6, p<0.05) with post hoc comparisons indicating decreased savings of alcohol exposed rats as compared to non-exposed controls (p<0.01), an effect prevented by pioglitazone (p<0.01 vs alcohol exposed group). A similar pattern was found for total distance swum to reach the new location (Figure 4D; main effect of alcohol: (F(1,39)=3.9, p=0.05); main effect of pioglitazone: (F(1,39)=11.6, p<0.01); interaction: (F(1,39)=7.3, p<0.05) with post hoc analysis showing alcohol-induced decrease in this variable (p<0.01) reversed by pioglitazone (p<0.001 vs alcohol exposed group). Savings for entries into the previously trained quadrant (Figure 4E) also differed [“alcohol” × “pioglitazone” interaction (F(1,39)=4.8, p<0.05)] with post hoc analysis showing difference between alcohol exposed and control exposed rats (p<0.05) as well as prevention by pioglitazone (p<0.05). Significant treatment interaction was also observed for time spent swimming in the original platform quadrant (F(1,39)=4.1, p<0.05, Figure 4F). Conversely, swim speed did not differ between the groups during the new learning task [main alcohol effect: (F(1,39)=0.2, NS); main pioglitazone effect: (F(1,39)=0.0, NS); interaction (F(1,39)=0.0, NS)].
4. DISCUSSION
We show here that binge-like alcohol exposure produces neuronal death in the EC and hippocampal DG and that this is associated with alcohol-induced immune responses. Pioglitazone prevents neurodegeneration and neuroinflammation. In parallel, pioglitazone prevents alcohol-impaired re-learning performance both in an operant task and in a spatial navigation task.
Extensive neurodegeneration was indicated by FJ-B staining in the DG of the hippocampus and in the adjacent EC. Sparse degenerative neurons were detected in other areas of the brain but their number was low compared to the DG and the EC. These findings are consistent with previous observations indicating that binge alcohol intoxication caused significant neuronal loss, particularly in these regions (Cippitelli et al, 2010a; Collins et al, 1996; Crews et al, 2004; Obernier et al, 2002a). We also found that the PPARγ agonist pioglitazone prevented in a dose dependent manner neurotoxic consequences of the binge alcohol treatment by suppressing the number of FJ-B positive cells in both the hippocampal DG and the EC. The dose dependence of the effect suggests that, in order to evaluate the potential clinical implications, the plasma drug levels at which this response is achieved should be compared to the levels that are achieved in humans with FDA-approved doses. In agreement with these results, previous studies have shown that activation of PPARγ exerts a marked protective response in various models of neurodegenerative deseases (Diab et al, 2002; Kapadia et al, 2008; Kaundal et al, 2010). This effect appears to be linked to the ability of PPARγ to attenuate neuronal inflammation and to reduce activation of inflammatory cytokines (Kapadia et al, 2008; Yi et al, 2008). Interestingly, the PPARα agonist oleoylethanolamide protects against alcohol-induced inflammatory signaling and apoptosis in the cerebral cortex of adolescent rats with binge drinking (Anton et al, 2016). Here, for the first time, we provided evidence that PPARγ activation is also neuroprotective against binge alcohol drinking, possibly through inhibition of the neuroimmune response (Drew et al, 2015; Kane et al, 2013; Kane et al, 2014; Kane et al, 2011).
To confirm this hypothesis, we examined IL-1β and IL-6 mRNA expression levels. These cytokines are involved in neuroinflammation initiated by excessive alcohol use (Alfonso-Loeches et al, 2010; Crews et al, 2009; Drew et al, 2015; Kane et al, 2013; Kane et al, 2014). IL-1β and IL-6 are well characterized as pro-inflammatory molecules. In addition, it should be noted that IL-6 is suggested to possess anti-inflammtory activities under some circumstances (Schaper and Rose-John, 2015). We found that both the DG and the EC are sensitive to neuroinflammatory processes as dynamic changes were observed in the expression of these inflammatory cytokines in response to alcohol treatment. However, these alcohol-induced changes were more prominent in the EC. There was robust alcohol-induced upregulation of IL-6 and IL-1β expression in the EC that was abolished by concurrent administration of pioglitazone. This suggests, on the one hand, these cytokines may contribute to alcohol-induced neurodegeneration and, on the other, the effect of pioglitazone may be related to its ability to blunt alcohol-induced activation of these immune response mediators.
The intimate mechanisms linking PPARγ function to immune system regulation has not been fully determined yet. However, it is known that PPARγ agonists suppress the production of pro-inflammatory molecules including cytokines and chemokines by CNS glial cells. PPARγ agonists are believed to principally suppress inflammation by repressing the transcription of genes encoding these pro-inflammatory molecules. This is believed to occur through a mechanism referred to as receptor-dependent trans-repression in which PPARγ physically interacts with transcription factors, blocking the activation of immune responsive genes (Daynes and Jones, 2002). PPARγ has been shown to act through receptor-dependent trans-repression to suppress the activity of a variety of transcription factors including NF-κB (Li et al, 2000). PPARγ can also alter transcription through receptor-independent mechanisms, including suppressing specific steps in NF-κB signaling pathways (Rossi et al, 2000; Straus et al, 2000). Interestingly, alcohol has been demonstrated to activate NF-κB (Blanco and Guerri, 2007; Ward et al, 1996). PPARγ can be expressed by glia in vivo, particularly under neuroinflammatory conditions (Diab et al, 2002; Warden et al, 2016). Thus, PPARγ suppression of immune activity by glia may indirectly protect neurons since pro-inflammatory molecules can be toxic to neurons. In addition, PPARγ agonists may directly alter the viability of neurons. Neurons have previously been demonstrated to express PPARγ suggesting that pioglitazone may regulate neuron viability directly through receptor-dependent mechanisms (Inestrosa et al, 2005). PPARγ agonists have been demonstrated to protect neurons from toxic insults by maintaining mitochondrial function and limiting reactive oxygen species formation in these cells (Fuenzalida et al, 2007). It will be important in future studies to further define the mechanisms by which PPARγ agonists protect hippocampal and EC neurons from the toxic effects of alcohol.
The EC and the hippocampus are closely interconnected regions and the circuitry from the EC to the hippocampal formation is considered to be critical for memory formation and spatial learning (Aggleton et al, 2000). Lesion studies have shown that damage of the hippocampal area mostly produces spatial reference deficits (Jarrard, 1993; Morris et al, 1982) whereas damage of the EC may also compromise reversal learning abilities (Eijkenboom et al, 2000; Hagan et al, 1992). However, cognitive impairment resulting from binge alcohol exposure includes reversal learning and object recognition deficits rather than deficits in acquisition of spatial reference memory in navigation or exploration tasks in rats (Cippitelli et al, 2010a; Cippitelli et al, 2010b; Obernier et al, 2002b). In these studies, re-learning deficits observed in binge alcohol treated animals have been closely associated with different navigation or exploratory patterns reflecting increased perseverative behavior as well as decreased cognitive flexibility. Here we tested the possibility that the observed neuroprotective role of pioglitazone was associated with prevention of alcohol-induced deficits in learning abilities. This hypothesis was verified by employing two behavioral strategy-shift tasks, both requiring intact cognitive ability and flexibility, though one devoid of a spatial component (i.e., operant reversal learning task) and the other heavily based on spatial orientation (i.e., learning of a new task in the MWM). In the non-spatial operant reversal learning task, we found a selective though weak deficit in cued reversal learning following alcohol treatment, which was reversed by concurrent administration of pioglitazone. These data confirm previous evidence that excessive alcohol treatment causes learning disabilities in non-spatial tasks (Cippitelli et al, 2010b; Garcia-Moreno and Cimadevilla, 2012; Takahashi et al, 2015) and demonstrate that pioglitazone ameliorates re-learning performance in a new cue-reward contingency. The learning of a new condition in the MWM is very sensitive to hippocampal as well as EC damage and, therefore, cognitive consequences of alcohol exposure were assumed to result in a more pronounced impairment. As expected, variables describing navigation strategies of alcohol treated rats were significantly changed from the control group. Since velocity of swimming was similar between groups, these data indicate alcohol-induced damage of new learning performance and reduced cognitive flexibility to engage the correct navigation strategy to reach the new platform location, similar to what has been described previously (Cippitelli et al, 2010a; Obernier et al, 2002b). Pioglitazone successfully prevented this impairment. However, surprisingly, alcohol-induced cognitive impairment was not restricted to the re-learning performance, as a deficit in the acquisition of reference memory was also detected following binge alcohol treatment in the present study. This finding suggests that, under certain experimental conditions (i.e., different use of navigation cues or different experimental design), alcohol can produce damage to the hippocampal-mediated spatial reference memory. This evidence reconciles with previous observations obtained from lesion studies and supports the hypothesis that alcohol-induced impairments in spatial learning and memory parallel those induced by lesions (Matthews and Silvers, 2004). Even more surprisingly, we also observed that pioglitazone treatment was able to improve spatial reference memory for a fixed platform location compared to non-pioglitazone treated groups. Similarly, PPARγ agonist treatment was previously associated with improvements of hippocampal-dependent memory in mouse models for Alzheimer’s disease, indicating that PPARγ agonists may act as cognitive enhancers (Nenov et al, 2014). In addition, PPARγ activation has been shown to modulate alcohol intake and preference in two-bottle choice models of consumption but not in a limited access binge drinking model (Blednov et al, 2015). Investigation of potential anxiolytic or antidepressant activity of pioglitazone (Domi et al, 2016; Kemp et al, 2014) may suggest additional mechanisms of cognitive protection by pioglitazone following alcohol intoxication. Further study of the therapeutic potential of approved PPARγ agonists, including pioglitazone, in alcohol-induced neuropathology, and cognitive impairments, as well as alcohol consumption, may provide new strategies for intervention in alcohol use disorders.
In the present study, molecular events were assessed acutely and behavioral effects were assessed at later time points. As reported by Crews and Nixon (2009), neurodegeneration peaks shortly after the last dose of alcohol in this model, the time point that we used to measure neurodegeneration. We have previously demonstrated that spatial memory remains impaired 10 weeks in this alcohol model (Cippitelli et al, 2010b) and cognitive function was tested in the present study in a two week period following alcohol treatment. Alcohol treatment also inhibits neurogenesis, contributing to neuron loss and perhaps cognitive impairment (Nixon, 2006; Nixon and Crews, 2004). Given our finding that pioglitazone protects against alcohol-induced cognitive impairment, it is interesting that PPARγ activation has been shown to protect neurogenesis and cognitive function in disease models (Ormerod et al, 2013). The protective effects of pioglitazone appear to be long lasting as suggested by our data demonstrating pioglitazone protection against cognitive impairments two weeks after completion of alcohol and pioglitazone treatment.
5. CONCLUSIONS
Here we show a clear protective effect by the PPARγ agonist pioglitazone against neuronal damage caused by binge alcohol intoxication in brain regions underlying learning and memory processes. Consistently, pioglitazone confers a wide spectrum of protection against alcohol-induced impairment of spatial and non-spatial learning abilities, and improves spatial reference memory. We also show that neuroinflammation importantly contributes to alcohol-induced neurotoxicity as dynamic changes in the expression of pro-inflammatory markers were detected following alcohol treatment. These changes were blocked by pioglitazone suggesting that anti-inflammatory mechanisms may be responsible for the protective effects of PPARγ agonists on alcohol-induced neuronal and cognitive damages. The present findings, together with our previous evidence describing the ability of PPARγ agonists to reduce excessive alcohol intake and vulnerability to relapse into alcohol seeking (Stopponi et al, 2011), provide a strong rationale for consideration of pioglitazone or its congeners as effective treatments for alcohol use disorders.
Supplementary Material
Highlights.
The PPARγ agonist pioglitazone reduces alcohol-induced neurodegeneration
Pioglitazone prevents alcohol-induced expression of pro-inflammatory cytokines
Pioglitazone prevents alcohol-impaired reversal learning ability in an operant task
Pioglitazone improves reference memory in the Morris water maze task
Pioglitazone prevents alcohol-induced deficits in spatial learning
Acknowledgments
This work is dedicated to the memory of Dr. Robert L. Eskay (LCTS/NIAAA, Bethesda, MD). We are thankful to Rina Righi and Mariangela Fiorelli for animal care and Marino Cucculelli and Alfredo Fiorelli for technical support. This work was supported by the fellowship for research projects provided by the Italian Society of Pharmacology (SIF) and the support of Merck Sharp & Dohme (MSD) Italy to Esi Domi and by the U.S. National Institutes of Health AA017447 (to MR), AA18839 (to PD), AA19108 (to PD), AA018834 (to CK), AA023723 (to CK), and NIGMS IdeA award P30GM110702 (to CK).
Footnotes
FINANCIAL DISCLOSURES
Dr. Demopulos is the Chairman and CEO of Omeros Corporation and Dr. Gaitanaris is Chief Scientific Officer of Omeros Corporation. Omeros exclusively controls the intellectual property rights directed to the use of PPARγ receptor agonists for the treatment of addiction and addictive behaviors obtained from the University of Camerino and Dr. Ciccocioppo. Dr. Ciccocioppo is the inventor of a number of patent applications, which have been assigned to Omeros, relating to the therapeutic use of PPARγ agonists in addiction. He is entitled to receive payments and royalties from Omeros under such licensing arrangement. The other authors have no conflict of interest.
Author Contributions
A.C., R.C. designed research; A.C., E.D., M.U., J.C.D., H.L. performed research; G.D., G.G., P.D.D., C.J.M.K. provided essential tools; A.C., C.J.M.K. analyzed data; A.C., P.D.D., C.J.M.K., R.C. wrote the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdul-Monim Z, Reynolds GP, Neill JC. The atypical antipsychotic ziprasidone, but not haloperidol, improves phencyclidine-induced cognitive deficits in a reversal learning task in the rat. Journal of psychopharmacology. 2003;17(1):57–65. doi: 10.1177/0269881103017001700. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Vann SD, Oswald CJ, Good M. Identifying cortical inputs to the rat hippocampus that subserve allocentric spatial processes: a simple problem with a complex answer. Hippocampus. 2000;10(4):466–474. doi: 10.1002/1098-1063(2000)10:4<466::AID-HIPO13>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Alfonso-Loeches S, Pascual-Lucas M, Blanco AM, Sanchez-Vera I, Guerri C. Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30(24):8285–8295. doi: 10.1523/JNEUROSCI.0976-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton M, Alen F, Gomez de Heras R, Serrano A, Pavon FJ, Leza JC, et al. Oleoylethanolamide prevents neuroimmune HMGB1/TLR4/NF-kB danger signaling in rat frontal cortex and depressive-like behavior induced by ethanol binge administration. Addiction biology. 2016 doi: 10.1111/adb.12365. [DOI] [PubMed] [Google Scholar]
- Blanco AM, Guerri C. Ethanol intake enhances inflammatory mediators in brain: role of glial cells and TLR4/IL-1RI receptors. Frontiers in bioscience : a journal and virtual library. 2007;12:2616–2630. doi: 10.2741/2259. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Benavidez JM, Black M, Ferguson LB, Schoenhard GL, Goate AM, et al. Peroxisome proliferator-activated receptors alpha and gamma are linked with alcohol consumption in mice and withdrawal and dependence in humans. Alcoholism, clinical and experimental research. 2015;39(1):136–145. doi: 10.1111/acer.12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden SC, McCarter RJ. Spatial memory in alcohol-dependent subjects: using a pushbutton maze to test the principle of equiavailability. Brain and cognition. 1993;22(1):51–62. doi: 10.1006/brcg.1993.1024. [DOI] [PubMed] [Google Scholar]
- Braconi S, Sidhpura N, Aujla H, Martin-Fardon R, Weiss F, Ciccocioppo R. Revisiting intragastric ethanol intubation as a dependence induction method for studies of ethanol reward and motivation in rats. Alcoholism, clinical and experimental research. 2010;34(3):538–544. doi: 10.1111/j.1530-0277.2009.01119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cippitelli A, Damadzic R, Frankola K, Goldstein A, Thorsell A, Singley E, et al. Alcohol-induced neurodegeneration, suppression of transforming growth factor-beta, and cognitive impairment in rats: prevention by group II metabotropic glutamate receptor activation. Biological psychiatry. 2010a;67(9):823–830. doi: 10.1016/j.biopsych.2009.12.018. [DOI] [PubMed] [Google Scholar]
- Cippitelli A, Damadzic R, Hamelink C, Brunnquell M, Thorsell A, Heilig M, et al. Binge-like ethanol consumption increases corticosterone levels and neurodegneration whereas occupancy of type II glucocorticoid receptors with mifepristone is neuroprotective. Addiction biology. 2014;19(1):27–36. doi: 10.1111/j.1369-1600.2012.00451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cippitelli A, Zook M, Bell L, Damadzic R, Eskay RL, Schwandt M, et al. Reversibility of object recognition but not spatial memory impairment following binge-like alcohol exposure in rats. Neurobiology of learning and memory. 2010b;94(4):538–546. doi: 10.1016/j.nlm.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins MA, Corso TD, Neafsey EJ. Neuronal degeneration in rat cerebrocortical and olfactory regions during subchronic “binge” intoxication with ethanol: possible explanation for olfactory deficits in alcoholics. Alcoholism, clinical and experimental research. 1996;20(2):284–292. doi: 10.1111/j.1530-0277.1996.tb01641.x. [DOI] [PubMed] [Google Scholar]
- Crews F, Nixon K, Kim D, Joseph J, Shukitt-Hale B, Qin L, et al. BHT blocks NFkappaB activation and ethanol-induced brain damage. Alcoholism, clinical and experimental research. 2006;30(11):1938–1949. doi: 10.1111/j.1530-0277.2006.00239.x. [DOI] [PubMed] [Google Scholar]
- Crews FT, Collins MA, Dlugos C, Littleton J, Wilkins L, Neafsey EJ, et al. Alcoholinduced neurodegeneration: when, where and why? Alcoholism, clinical and experimental research. 2004;28(2):350–364. doi: 10.1097/01.alc.0000113416.65546.01. [DOI] [PubMed] [Google Scholar]
- Crews FT, Nixon K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol and alcoholism. 2009;44(2):115–127. doi: 10.1093/alcalc/agn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Zou J, Qin L. Induction of innate immune genes in brain create the neurobiology of addiction. Brain, behavior, and immunity. 2011;25(Suppl 1):S4–S12. doi: 10.1016/j.bbi.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RL, Syapin PJ. Ethanol increases nuclear factor-kappa B activity in human astroglial cells. Neuroscience letters. 2004;371(2–3):128–132. doi: 10.1016/j.neulet.2004.08.051. [DOI] [PubMed] [Google Scholar]
- Daynes RA, Jones DC. Emerging roles of PPARs in inflammation and immunity. Nature reviews Immunology. 2002;2(10):748–759. doi: 10.1038/nri912. [DOI] [PubMed] [Google Scholar]
- Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocrine reviews. 1999;20(5):649–688. doi: 10.1210/edrv.20.5.0380. [DOI] [PubMed] [Google Scholar]
- Diab A, Deng C, Smith JD, Hussain RZ, Phanavanh B, Lovett-Racke AE, et al. Peroxisome proliferator-activated receptor-gamma agonist 15-deoxy-Delta(12,14)-prostaglandin J(2) ameliorates experimental autoimmune encephalomyelitis. Journal of immunology. 2002;168(5):2508–2515. doi: 10.4049/jimmunol.168.5.2508. [DOI] [PubMed] [Google Scholar]
- Domi E, Uhrig S, Soverchia L, Spanagel R, Hansson AC, Barbier E, et al. Genetic Deletion of Neuronal PPARgamma Enhances the Emotional Response to Acute Stress and Exacerbates Anxiety: An Effect Reversed by Rescue of Amygdala PPARgamma Function. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2016;36(50):12611–12623. doi: 10.1523/JNEUROSCI.4127-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew PD, Johnson JW, Douglas JC, Phelan KD, Kane CJ. Pioglitazone blocks ethanol induction of microglial activation and immune responses in the hippocampus, cerebellum, and cerebral cortex in a mouse model of fetal alcohol spectrum disorders. Alcoholism, clinical and experimental research. 2015;39(3):445–454. doi: 10.1111/acer.12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eijkenboom M, Blokland A, van der Staay FJ. Modelling cognitive dysfunctions with bilateral injections of ibotenic acid into the rat entorhinal cortex. Neuroscience. 2000;101(1):27–39. doi: 10.1016/s0306-4522(00)00342-0. [DOI] [PubMed] [Google Scholar]
- Fernandez-Lizarbe S, Montesinos J, Guerri C. Ethanol induces TLR4/TLR2 association, triggering an inflammatory response in microglial cells. Journal of neurochemistry. 2013;126(2):261–273. doi: 10.1111/jnc.12276. [DOI] [PubMed] [Google Scholar]
- Fuenzalida K, Quintanilla R, Ramos P, Piderit D, Fuentealba RA, Martinez G, et al. Peroxisome proliferator-activated receptor gamma up-regulates the Bcl-2 anti-apoptotic protein in neurons and induces mitochondrial stabilization and protection against oxidative stress and apoptosis. The Journal of biological chemistry. 2007;282(51):37006–37015. doi: 10.1074/jbc.M700447200. [DOI] [PubMed] [Google Scholar]
- Garcia-Moreno LM, Cimadevilla JM. Acute and chronic ethanol intake: effects on spatial and non-spatial memory in rats. Alcohol. 2012;46(8):757–762. doi: 10.1016/j.alcohol.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Smith AD, Cole M, Weiss F, Koob GF, Richardson HN. Operant behavior and alcohol levels in blood and brain of alcohol-dependent rats. Alcoholism, clinical and experimental research. 2009;33(12):2113–2123. doi: 10.1111/j.1530-0277.2009.01051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan JJ, Verheijck EE, Spigt MH, Ruigt GS. Behavioural and electrophysiological studies of entorhinal cortex lesions in the rat. Physiology & behavior. 1992;51(2):255–266. doi: 10.1016/0031-9384(92)90139-s. [DOI] [PubMed] [Google Scholar]
- Inestrosa NC, Godoy JA, Quintanilla RA, Koenig CS, Bronfman M. Peroxisome proliferator-activated receptor gamma is expressed in hippocampal neurons and its activation prevents beta-amyloid neurodegeneration: role of Wnt signaling. Experimental cell research. 2005;304(1):91–104. doi: 10.1016/j.yexcr.2004.09.032. [DOI] [PubMed] [Google Scholar]
- Jarrard LE. On the role of the hippocampus in learning and memory in the rat. Behavioral and neural biology. 1993;60(1):9–26. doi: 10.1016/0163-1047(93)90664-4. [DOI] [PubMed] [Google Scholar]
- Kane CJ, Phelan KD, Douglas JC, Wagoner G, Johnson JW, Xu J, et al. Effects of ethanol on immune response in the brain: region-specific changes in aged mice. Journal of neuroinflammation. 2013;10:66. doi: 10.1186/1742-2094-10-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane CJ, Phelan KD, Douglas JC, Wagoner G, Johnson JW, Xu J, et al. Effects of ethanol on immune response in the brain: region-specific changes in adolescent versus adult mice. Alcoholism, clinical and experimental research. 2014;38(2):384–391. doi: 10.1111/acer.12244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane CJ, Phelan KD, Han L, Smith RR, Xie J, Douglas JC, et al. Protection of neurons and microglia against ethanol in a mouse model of fetal alcohol spectrum disorders by peroxisome proliferator-activated receptor-gamma agonists. Brain, behavior, and immunity. 2011;25(Suppl 1):S137–145. doi: 10.1016/j.bbi.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapadia R, Yi JH, Vemuganti R. Mechanisms of anti-inflammatory and neuroprotective actions of PPAR-gamma agonists. Frontiers in bioscience : a journal and virtual library. 2008;13:1813–1826. doi: 10.2741/2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaundal RK, Sharma SS. Peroxisome proliferator-activated receptor gamma agonists as neuroprotective agents. Drug news & perspectives. 2010;23(4):241–256. doi: 10.1358/dnp.2010.23.4.1437710. [DOI] [PubMed] [Google Scholar]
- Kemp DE, Schinagle M, Gao K, Conroy C, Ganocy SJ, Ismail-Beigi F, et al. PPARgamma agonism as a modulator of mood: proof-of-concept for pioglitazone in bipolar depression. CNS drugs. 2014;28(6):571–581. doi: 10.1007/s40263-014-0158-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Pascual G, Glass CK. Peroxisome proliferator-activated receptor gamma-dependent repression of the inducible nitric oxide synthase gene. Molecular and cellular biology. 2000;20(13):4699–4707. doi: 10.1128/mcb.20.13.4699-4707.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majchrowicz E. Induction of physical dependence upon ethanol and the associated behavioral changes in rats. Psychopharmacologia. 1975;43(3):245–254. doi: 10.1007/BF00429258. [DOI] [PubMed] [Google Scholar]
- Matthews DB, Silvers JR. The use of acute ethanol administration as a tool to investigate multiple memory systems. Neurobiology of learning and memory. 2004;82(3):299–308. doi: 10.1016/j.nlm.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Moreno S, Farioli-Vecchioli S, Ceru MP. Immunolocalization of peroxisome proliferatoractivated receptors and retinoid X receptors in the adult rat CNS. Neuroscience. 2004;123(1):131–145. doi: 10.1016/j.neuroscience.2003.08.064. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297(5868):681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Nenov MN, Laezza F, Haidacher SJ, Zhao Y, Sadygov RG, Starkey JM, et al. Cognitive enhancing treatment with a PPARgamma agonist normalizes dentate granule cell presynaptic function in Tg2576 APP mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34(3):1028–1036. doi: 10.1523/JNEUROSCI.3413-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon K. Alcohol and adult neurogenesis: roles in neurodegeneration and recovery in chronic alcoholism. Hippocampus. 2006;16(3):287–295. doi: 10.1002/hipo.20162. [DOI] [PubMed] [Google Scholar]
- Nixon K, Crews FT. Temporally specific burst in cell proliferation increases hippocampal neurogenesis in protracted abstinence from alcohol. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24(43):9714–9722. doi: 10.1523/JNEUROSCI.3063-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obernier JA, Bouldin TW, Crews FT. Binge ethanol exposure in adult rats causes necrotic cell death. Alcoholism, clinical and experimental research. 2002a;26(4):547–557. [PubMed] [Google Scholar]
- Obernier JA, White AM, Swartzwelder HS, Crews FT. Cognitive deficits and CNS damage after a 4-day binge ethanol exposure in rats. Pharmacology, biochemistry, and behavior. 2002b;72(3):521–532. doi: 10.1016/s0091-3057(02)00715-3. [DOI] [PubMed] [Google Scholar]
- Ormerod BK, Hanft SJ, Asokan A, Haditsch U, Lee SW, Palmer TD. PPARgamma activation prevents impairments in spatial memory and neurogenesis following transient illness. Brain, behavior, and immunity. 2013;29:28–38. doi: 10.1016/j.bbi.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SW, Yi JH, Miranpuri G, Satriotomo I, Bowen K, Resnick DK, et al. Thiazolidinedione class of peroxisome proliferator-activated receptor gamma agonists prevents neuronal damage, motor dysfunction, myelin loss, neuropathic pain, and inflammation after spinal cord injury in adult rats. The Journal of pharmacology and experimental therapeutics. 2007;320(3):1002–1012. doi: 10.1124/jpet.106.113472. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Lim KO, Zipursky RB, Mathalon DH, Rosenbloom MJ, Lane B, et al. Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: a quantitative MRI study. Alcoholism, clinical and experimental research. 1992;16(6):1078–1089. doi: 10.1111/j.1530-0277.1992.tb00702.x. [DOI] [PubMed] [Google Scholar]
- Richardson HN, Lee SY, O’Dell LE, Koob GF, Rivier CL. Alcohol self-administration acutely stimulates the hypothalamic-pituitary-adrenal axis, but alcohol dependence leads to a dampened neuroendocrine state. The European journal of neuroscience. 2008;28(8):1641–1653. doi: 10.1111/j.1460-9568.2008.06455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson G, Most D, Ferguson LB, Mayfield J, Harris RA, Blednov YA. Neuroimmune pathways in alcohol consumption: evidence from behavioral and genetic studies in rodents and humans. International review of neurobiology. 2014;118:13–39. doi: 10.1016/B978-0-12-801284-0.00002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi A, Kapahi P, Natoli G, Takahashi T, Chen Y, Karin M, et al. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IkappaB kinase. Nature. 2000;403(6765):103–108. doi: 10.1038/47520. [DOI] [PubMed] [Google Scholar]
- Schaper F, Rose-John S. Interleukin-6: Biology, signaling and strategies of blockade. Cytokine & growth factor reviews. 2015;26(5):475–487. doi: 10.1016/j.cytogfr.2015.07.004. [DOI] [PubMed] [Google Scholar]
- Schintu N, Frau L, Ibba M, Caboni P, Garau A, Carboni E, et al. PPAR-gamma-mediated neuroprotection in a chronic mouse model of Parkinson’s disease. The European journal of neuroscience. 2009;29(5):954–963. doi: 10.1111/j.1460-9568.2009.06657.x. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Hopkins KJ. Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain research. 2000;874(2):123–130. doi: 10.1016/s0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nature methods. 2012;9(7):671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopponi S, de Guglielmo G, Somaini L, Cippitelli A, Cannella N, Kallupi M, et al. Activation of PPARgamma by pioglitazone potentiates the effects of naltrexone on alcohol drinking and relapse in msP rats. Alcoholism, clinical and experimental research. 2013;37(8):1351–1360. doi: 10.1111/acer.12091. [DOI] [PubMed] [Google Scholar]
- Stopponi S, Somaini L, Cippitelli A, Cannella N, Braconi S, Kallupi M, et al. Activation of nuclear PPARgamma receptors by the antidiabetic agent pioglitazone suppresses alcohol drinking and relapse to alcohol seeking. Biological psychiatry. 2011;69(7):642–649. doi: 10.1016/j.biopsych.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Straus DS, Pascual G, Li M, Welch JS, Ricote M, Hsiang CH, et al. 15-deoxy-delta 12,14-prostaglandin J2 inhibits multiple steps in the NF-kappa B signaling pathway. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(9):4844–4849. doi: 10.1073/pnas.97.9.4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi TT, Vendruscolo LF, Takahashi RN. Binge-like ingestion of a combination of an energy drink and alcohol leads to cognitive deficits and motivational changes. Pharmacology, biochemistry, and behavior. 2015;136:82–86. doi: 10.1016/j.pbb.2015.07.007. [DOI] [PubMed] [Google Scholar]
- Vetreno RP, Crews FT. Binge ethanol exposure during adolescence leads to a persistent loss of neurogenesis in the dorsal and ventral hippocampus that is associated with impaired adult cognitive functioning. Frontiers in neuroscience. 2015;9:35. doi: 10.3389/fnins.2015.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetreno RP, Yaxley R, Paniagua B, Johnson GA, Crews FT. Adult rat cortical thickness changes across age and following adolescent intermittent ethanol treatment. Addiction biology. 2016 doi: 10.1111/adb.12364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nature protocols. 2006;1(2):848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward RJ, Zhang Y, Crichton RR, Piret B, Piette J, de Witte P. Identification of the nuclear transcription factor NFkappaB in rat after in vivo ethanol administration. FEBS letters. 1996;389(2):119–122. doi: 10.1016/0014-5793(96)00545-5. [DOI] [PubMed] [Google Scholar]
- Warden A, Truitt J, Merriman M, Ponomareva O, Jameson K, Ferguson LB, et al. Localization of PPAR isotypes in the adult mouse and human brain. Scientific reports. 2016;6:27618. doi: 10.1038/srep27618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AM. What happened? Alcohol, memory blackouts, and the brain. Alcohol research & health : the journal of the National Institute on Alcohol Abuse and Alcoholism. 2003;27(2):186–196. [PMC free article] [PubMed] [Google Scholar]
- Williamson LL, Bilbo SD. Chemokines and the hippocampus: a new perspective on hippocampal plasticity and vulnerability. Brain, behavior, and immunity. 2013;30:186–194. doi: 10.1016/j.bbi.2013.01.077. [DOI] [PubMed] [Google Scholar]
- Yi JH, Park SW, Brooks N, Lang BT, Vemuganti R. PPARgamma agonist rosiglitazone is neuroprotective after traumatic brain injury via anti-inflammatory and anti-oxidative mechanisms. Brain research. 2008;1244:164–172. doi: 10.1016/j.brainres.2008.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Crews F. CREB and NF-kappaB transcription factors regulate sensitivity to excitotoxic and oxidative stress induced neuronal cell death. Cellular and molecular neurobiology. 2006;26(4–6):385–405. doi: 10.1007/s10571-006-9045-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.