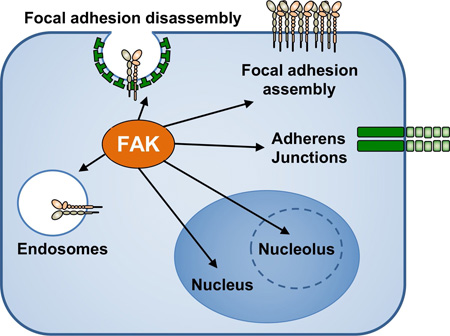
Abstract
Focal adhesion kinase (FAK) is a cytoplasmic protein-tyrosine kinase first identified at extracellular matrix and integrin receptor cell adhesion sites and is a key regulator of cell movement. FAK is activated by a variety of stimuli. Herein, we discuss advances in conformational-associated FAK activation and dimerization mechanisms. Additionally, new roles have emerged for FAK signaling at cell adhesions, adherens junctions, endosomes, and the nucleus. In light of these new findings, we review how FAK activation at these sites is connected to the regulation of integrin recycling-activation, vascular permeability, cell survival, and transcriptional regulation, respectively. Studies uncovering FAK signaling connections in unexpected places within cells have yielded important new regulatory insights in cell biology.
Graphical abstract
Introduction
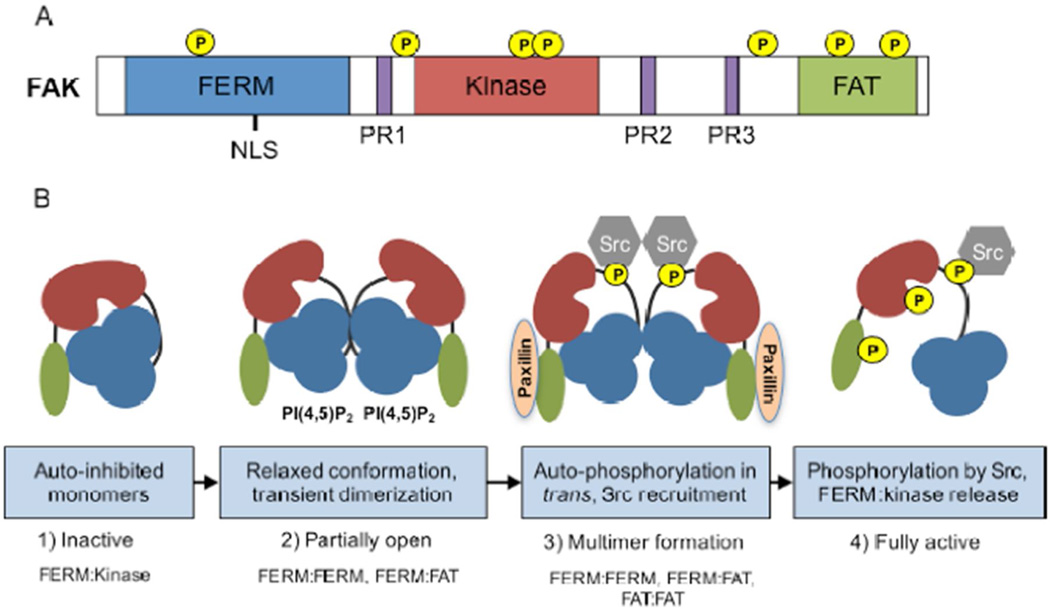
FAK is an important contributor to growth factor receptor and integrin signals governing fundamental processes in normal and cancer cells. FAK is comprised of an N-terminal FERM (band 4.1-ezrin-radixin-moesin) domain, a central kinase domain, and three proline-rich regions that are binding sites for Src-homology 3 (SH3) domain-containing proteins (Figure 1A). FAK contains multiple tyrosine phosphorylation sites that serve as binding sites for SH2 domain-containing proteins, serine phosphorylation sites that may alter FAK conformational dynamics, and a C-terminal FAT (focal adhesion-targeting) domain that indirectly links FAK to integrins.
Figure 1.
Schematic of FAK domains and mechanisms leading to activation. A) FAK contains an amino-terminal protein band 4.1-ezrin-radixin-moesin (FERM) homology domain (blue), a central kinase domain (red), and a carboxy-terminal focal adhesion targeting (FAT) domain (green). These domains are connected by linker regions containing proline-rich regions (PR1–3, purple). Location of phosphorylation sites and a nuclear localization sequence (NLS) is shown. B) Proposed mechanism of FAK activation. 1) Inactive FAK exists as an auto-inhibited monomer, a conformation maintained by interactions between the FERM (blue) and kinase (red) domains. 2) FERM binding to phosphatidylinositol-4,5-biphosphate [PI(4,5)P2] induces a relaxed conformation, FAK clustering, and transient FERM:FERM and FAT:FAT dimerization. 3) Transient FERM:FERM and FAT:FAT dimerization allows FAK autophosphorylation at Y397 in trans (yellow). A FERM:FAT (FAT shown in green) interaction can be reinforced by paxillin at focal adhesions. Y397 FAK phosphorylation creates a binding site for the SH2 domain of Src. 4) Src further phosphorylates FAK at multiple sites inducing full FAK activation by a conformational shift that includes FERM:kinase release.
During development, FAK is expressed and activated during gastrulation. Deletion of FAK or disruption of FAK activity in mice result in embryonic lethality with vascular, cell proliferation, and cell survival defects [1]. Whereas, conditional inactivation of FAK expression or activity in tissues of adult mice result is not lethal [2,3], expression of the FAK-related Pyk2 protein complicates the interpretation of causal relationships of conditional FAK knockouts [4,5] as there are overlapping functions of FAK and Pyk2 in some signaling pathways [6–8].
Importantly, FAK activity has been linked to some cancers, and small molecule inhibitors of FAK activity are being tested as anti-tumor therapies in early phase human clinical trials [1–3,9]. Over the last five years, expansion of genetic tumor profiling has revealed both FAK DNA amplification (PTK2 gene at 18q24.3) and elevated FAK mRNA levels in several cancers, including breast and ovarian carcinomas [3]. DNA sequencing has revealed several small nucleotide polymorphisms that result in amino acid substitutions within FAK, but mutational activation or inactivation of FAK is infrequent. In this review, we discuss the regulation of FAK activation at various sub-cellular locations that will provide researchers with insights into the effects of blocking FAK function.
Current Models of FAK Activation
In simple terms, FAK activity is defined as the ability of this protein-tyrosine kinase to transfer phosphate from ATP onto site-specific tyrosine residues in substrate proteins such as paxillin, p130Cas, Src, cortactin, β-catenin, VE-cadherin, and α-actinin as a means to alter protein function [3]. In addition, FAK forms phosphorylation-independent complexes with a number of different proteins as a scaffold, as the FAK FERM domain can enhance cell survival in a kinase-independent manner [10,11]. Research efforts to understand and distinguish FAK kinase-dependent and -independent signaling functions are ongoing [12,13]. We will discuss recent insights into the molecular mechanisms regulating FAK conformational activation and FAK auto-phosphorylation at Y397 that have been obtained from crystal structure, super-resolution microscopy, biosensor, biochemical, and cell biological analyses.
FAK at Focal Adhesions
Integrin-mediated signaling is one of the strongest activators of FAK. Integrin receptor binding to extracellular matrix proteins results in the formation of adhesomes where FAK localizes to a membrane-proximal signaling layer containing integrin cytoplasmic tails and paxillin [14,15]. FAK does not bind directly to integrin tails and is recruited rapidly in part through complexes formed by integrin tails, kindlin-2, and paxillin [16,17]. FAK binding to paxillin facilitates recruitment to adhesions but this is not sufficient as the loss of other FAK FAT domain binding partners, such as Rgnef, prevent FAK recruitment to adhesions [18]. The partners and sequence of events for FAK recruitment to adhesions remains unclear as FAK can precede paxillin localization at adhesions [19], FAK can function upstream of Src phosphorylation events [20], and loss of Rgnef delays integrin-mediated FAK activation [18]. Notably, FAK expression is not essential for adhesion formation [21] and blocking FAK signaling does not alter adhesion protein composition [22]. However, the dynamics of adhesion formation versus adhesion disassembly in migrating cells is associated with temporal differences in FAK and/or Src activation [23].
Evidence supports the notion that FAK exists as monomers in an auto-inhibited state prior to adhesion recruitment [24] (Figure 1B). This inhibitory conformation is maintained by interactions between the FERM and kinase domains that block FAK autophosphorylation at Y397 [25]. Point mutations within the FERM F2 lobe (Y180A/M183A) or within the kinase domain (F596D) release inhibitory FERM-kinase domain interactions and promote elevated FAK tyrosine phosphorylation. Another important region in FERM-mediated regulation of FAK activity is a solvent-facing “patch” of basic residues (KAKTLR) within the FERM F2 lobe. These residues can bind to phosphatidylinositol-4,5-bisphosphate [PI(4,5)P2] and induce a “partially open” conformation [26] but not necessarily release of FERM-kinase domain auto-inhibitory interactions. Instead, FAK binding to PI(4,5)P2 induces FAK clustering and a shift in equilibrium towards dimerization. FAK dimers featuring a FERM:FERM interaction are observed in crystal structures, and transient FAK dimerization is a proposed mechanism of FAK activation [27]. In support of this hypothesis, a point mutation within the FERM F3 lobe (W266A) reduces FAK activity through reduction of FAK Y397 phosphorylation in trans [27].
It is possible that an active FAK-Src complex may form multimers at adhesion sites via stabilization of FAK dimers. In addition to FERM:FERM interactions, FAK dimerization also involves binding between the FERM and C-terminal FAT domain [27] which may be reinforced by paxillin binding to the FAT domain (Figure 1B). Additionally, conformational changes within the FAT domain influence the formation of FAT:FAT dimers [28]. The FAT domain forms a four-helix barrel bundle [29], and nuclear magnetic resonance studies revealed an “opening” or flexibility of the first helix of the FAT four-helix bundle [30]. Notably, pH change over the range of 5.5−7.5 affects the conformational dynamics of the FAT domain and leads to differential phosphorylation of FAK Y1007 and FAK Y925 by Src [31]. Although the role of FAK Y1007 remains unknown, FAK Y925 phosphorylation creates a binding site for the SH2 domain of Grb2, linking FAK to the activation of the ERK/MAP kinase signaling cascade [3]. The overall contribution of FERM:FERM, FERM:FAT, and FAT:FAT binding interactions to sustained FAK activity remain unknown.
In general, FAK phosphorylation at Y397 creates a binding site for the SH2 domain of Src, and the stability of a FAK-Src complex is enhanced by Src SH3 domain binding to the first proline-rich motif in a region following the FERM domain. Within this complex, Src phosphorylates FAK at Y576 and Y577, and FAK phosphorylates Src at Y416, sites located within the activation loop of each kinase domain. Src binding to and phosphorylation of FAK promotes an open FAK conformation capable of functioning as both a scaffold and a kinase. Other important factors leading to release of FERM domain restraint on FAK kinase activity are the binding of other proteins to the FERM domain [32], pH-dependent changes leading to the protonation of FERM H48 [33,34], and the phosphorylation of FAK FERM Y194 by growth factor receptor tyrosine kinases [35].
The majority of studies evaluating the role of FAK signaling at adhesions have focused on mechanisms associated with cell motility [36–38] and cross-talk to other signaling pathways [39]. Integrins also serve as important receptors for mechanosensing [40]. Force fluctuations within adhesions can drive FAK activation and increase paxillin phosphorylation leading to a stronger cytoskeletal linkage, vinculin recruitment to adhesions, and focal adhesion maturation [41,42]. Force has been proposed to induce the opening of the FERM-kinase interface and to increase FAK Y397 phosphorylation [43]. Downstream signaling connections of force-mediated FAK activation can induce mitotic spindle reorganization [44], trigger mechanosensitive cell proliferation [45], and can increase inflammatory cytokine production associated with fibrosis [46]. As tumor tissues can exhibit stiffness and pharmacological FAK inhibition can reverse fibrosis in models of pancreatic cancer [47,48], it will be interesting to determine if this reversal is an effect of FAK inhibition in tumor, stromal, or multiple cell types.
FAK at Endosomes
Integrins are constitutively endocytosed and undergo endosomal sorting that determines degradation or receptor recycling [49]. Polarized integrin recycling to the leading edge of migrating cells is a fundamental process required for adhesion formation and efficient cell movement [50]. FAK interactions with the GTPase dynamin are also linked to the early steps of integrin endocytosis during adhesion turnover [51,52]. Interestingly, Y397-phosphorylated FAK co-localizes with β1 integrins, talin, and Rab GTPases on early endosomes [53]. In vitro, FAK binds to purified endosomes from FAK−/− cells via the FERM domain and this is associated with FAK activation [53]. The protein- or lipid-binding target of the FAK FERM domain on endosomes remains unknown.
Although intrinsic FAK activity is not required for endosomal targeting [53], the maintenance of active endosomal integrins is dependent on FAK activity [54]. On endosomes, FAK phosphorylates and activates type I phosphatidylinositol phosphate kinase, that facilitates PIP2 lipid generation and talin binding to endosomes. As talin maintains conformational integrin activation by binding to integrin cytoplasmic tails [55], FAK activity confers “conformational memory” that allows active integrins to be recycled and delivered to the leading edge of migrating cells [54]. Thus, FAK signaling can affect cell migration in locations other than focal adhesions.
FAK at Adherens Junctions
Adherens junctions (AJs) are protein complexes that form at endothelial or epithelial cell-cell contacts, and are comprised of transmembrane cadherins that form extracellular homo-dimers and maintain cell junctional integrity [56]. Although cadherins, catenins, and α-actinin are primary constituents of AJs, a number of focal adhesion proteins are also recruited to AJs such as Src, FAK, vinculin, and the Rac GTPase in response to different cell stimuli [57]. The activation of Src and FAK at both adhesions and AJs likely facilitates crosstalk between integrin and cadherin receptors.
Using real-time confocal imaging of confluent endothelial cells expressing a green fluorescent fusion protein (GFP) fused to FAK, vascular endothelial growth factor (VEGF) stimulation resulted in the rapid redistribution of cytosolic GFP-FAK to AJs within 30 to 60 seconds [58]. VEGF-stimulated recruitment of FAK to AJs promoted FAK association with vascular endothelial cadherin (VE-Cadherin), and pharmacological or genetic blockage of FAK kinase activity prevented VEGF-stimulated vascular permeability in vitro and in mice [58]. Endothelial-specific and conditional expression of kinase-inactive FAK (K454R) in adult mice reduced VE-cadherin Y658 and β-catenin Y142 phosphorylation in response to circulating VEGF. This genetic inhibition of FAK in endothelial cells prevented tumor cell extravasation and metastasis by enhancing vascular barrier function [59]. As pharmacological FAK inhibition can also alter immune cell infiltration into tumors [48,60], it will be interesting to determine if this is mediated in part by alterations in vascular permeability.
On a molecular level, FAK and VE-cadherin association is mediated by direct binding of the FERM domain to the VE-cadherin cytoplasmic domain [58]. Binding is dependent on a FAK “open” conformational state and enhanced by FERM domain Y180A/M183A point mutations. Elucidating the molecular and physical stimuli that can trigger conformational changes in FAK is crucial for understanding the processes that control FAK recruitment to both AJs and adhesions.
FAK in the Nucleus
FAK nuclear recruitment can occur in response to chemical or genetic stress and FERM F2 lobe residues (KAKTLR) function as a nuclear localization sequence in addition to facilitating PIP2 lipid binding [10]. Nuclear FAK enhances p53 tumor suppressor proteosomal degradation as a mechanism promoting cell survival in response to stress [5,61]. Although genetic or pharmacological FAK inhibition is associated with increased FAK nuclear accumulation, activated FAK also localizes to the nucleolus, a sub-nuclear compartment that has been proposed to act as a “stress sensor” as well as a site for ribosomal RNA biogenesis [62,63]. In the nucleolus, FAK binds and protects nucleostemin, a cancer stem cell marker, from stress-induced degradation. As tumor aggressiveness is associated with increased nucleoli number and size [63], further investigation into roles of nucleolar FAK may reveal new connections promoting tumor cell survival.
FAK nuclear localization is associated with cell transformation as increased active nuclear FAK is present in squamous carcinoma cells compared to normal keratinocytes [60]. While canonical signaling cascades downstream of membrane-associated FAK signals can induce gene expression changes [3], nuclear FAK influences gene expression through interaction with an expanding set of nuclear binding partners [60,64–66]. The majority of these interactions are mediated by FERM domain binding. However, the FAT domain also play a role as it forms a complex with the MEF2 transcription factor in cardiomyocytes to upregulate transcriptional activity in response to mechanical stress [66]. As the regulation of p21 cyclin dependent kinase inhibitor gene expression by FAK can occur via kinase-dependent [67] or kinase-independent [10] linkages, unraveling the complexity of FAK nuclear signaling will require the development of new genetic models incorporating point mutations that can inactivate FAK nuclear localization and/or activity.
Conclusions and Future Perspectives
FAK research has focused primarily on the role of FAK at focal adhesions, but newly identified roles for FAK at adherens junctions, the nucleus, and endosomes point to a more versatile role for FAK in cells. In fact, referring to “focal adhesion kinase” by its alternative nomenclature, “PTK2,” may be a more accurate reflection of multiple roles for FAK in cells. As a multi-domain protein that changes conformations upon activation, FAK can act as an assembly platform for protein complexes or as a bridge between proteins. FAK also functions as a protein-tyrosine kinase, phosphorylating different targets throughout the cell. Through these dual kinase-dependent and kinase-independent scaffolding roles, FAK lies at the intersection between many signaling pathways that control adhesion, migration, proliferation, and survival.
Subcellular FAK recruitment may act as an additional layer of signaling specificity, but this also yields additional questions. For instance, do different FAK effector proteins exist at different locations? Although some focal adhesion-associated proteins such as paxillin can shuttle to the nucleus [68], it is unclear whether nuclear paxillin involves an association with FAK. Moreover, as distinct subcellular localizations allow FAK to interact with different binding partners, it will be important to identify common or divergent signaling pathways associated with FAK recruitment to distinct sites or “unexpected places” within cells.
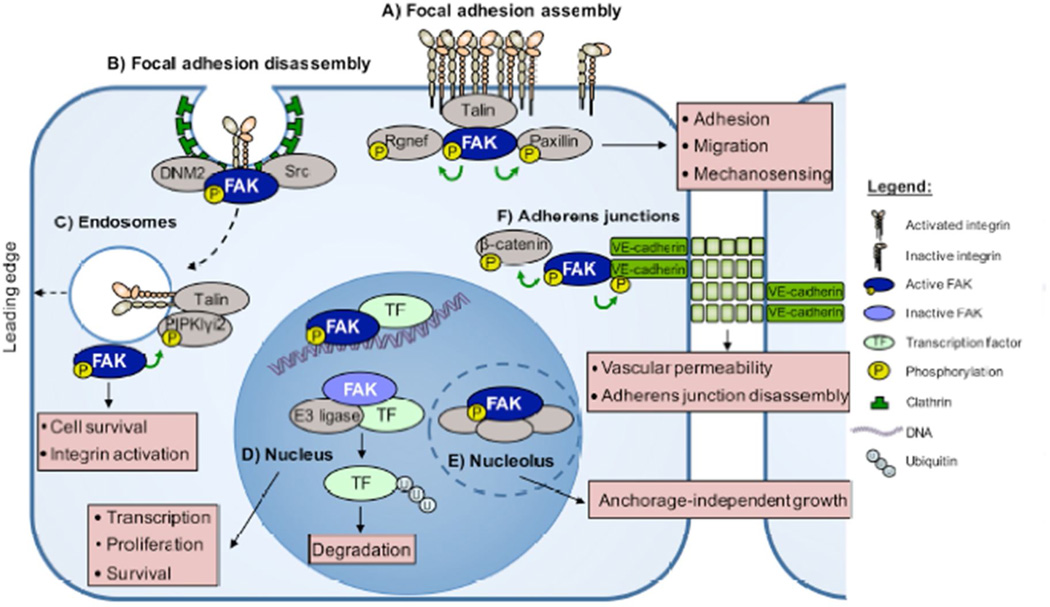
Figure 2. FAK functions throughout the cell.
A) Proteins such as Rgnef, talin, and paxillin contribute to FAK localization at sites of integrin clustering. Activated FAK phosphorylates Rgnef and paxillin, promoting the assembly of focal adhesions. B) FAK binds dynamin-2 (DNM2), triggering focal adhesion disassembly and integrin endocytosis. FAK at adhesions regulates cell adhesion, migration, and mechanosensing. C) FAK binds to and is activated at endosomes. FAK phosphorylation of PIPKIγi2 results in talin recruitment, which in turn maintains the active conformation of endosome-associated integrins. FAK-endosome signaling facilitates the polarized reassembly of activated integrins at the leading edge of migrating cells. FAK at endosomes facilitates integrin activation and cell survival. D) In the nucleus, activated FAK binds to transcription factors (TFs) to modulate gene expression. Inactive FAK in the nucleus functions as a scaffold to facilitate transcription factor turnover via enhanced ubiquitination by complexing with different E3 ligases. E) In the nucleolus, activated FAK complexes with proteins important in promoting stem cell phenotypes. FAK in the nucleus and nucleolus alters transcription, survival, and anchorage-independent cell growth. F) Active FAK localizes to adherens junctions (AJs) and directly binds VE-cadherin in response to vascular endothelial growth factor stimulation of endothelial cells. FAK phosphorylates β-catenin and VE-cadherin, triggering AJ disassembly. Right, depiction of figure elements.
Highlights.
FAK undergoes conformational activation and dimerization.
FAK activation can occur at adhesions, on endosomes, and in the nucleus.
FAK localizes to adherens junctions and can promote their disassembly.
Nuclear FAK can alter targets through kinase-dependent and –independent mechanisms.
The gene name for FAK (PTK2) reflects a more versatile role in cells.
Acknowledgments
E. Kleinschmidt and D. Schlaepfer declare no conflicts of interest. Supported by grants CA180769 and CA102310 from the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yoon H, Dehart JP, Murphy JM, Lim ST. Understanding the roles of FAK in cancer: inhibitors, genetic models, and new insights. J Histochem Cytochem. 2015;63:114–128. doi: 10.1369/0022155414561498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roy-Luzarraga M, Hodivala-Dilke K. Molecular Pathways: Endothelial Cell FAK-A Target for Cancer Treatment. Clin Cancer Res. 2016;22:3718–3724. doi: 10.1158/1078-0432.CCR-14-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sulzmaier FJ, Jean C, Schlaepfer DD. FAK in cancer: mechanistic findings and clinical applications. Nat Rev Cancer. 2014;14:598–610. doi: 10.1038/nrc3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weis SM, Lim ST, Lutu-Fuga KM, Barnes LA, Chen XL, Gothert JR, Shen TL, Guan JL, Schlaepfer DD, Cheresh DA. Compensatory role for Pyk2 during angiogenesis in adult mice lacking endothelial cell FAK. J Cell Biol. 2008;181:43–50. doi: 10.1083/jcb.200710038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim ST, Miller NL, Nam JO, Chen XL, Lim Y, Schlaepfer DD. Pyk2 inhibition of p53 as an adaptive and intrinsic mechanism facilitating cell proliferation and survival. J Biol Chem. 2010;285:1743–1753. doi: 10.1074/jbc.M109.064212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan H, Guan JL. Compensatory function of Pyk2 protein in the promotion of focal adhesion kinase (FAK)-null mammary cancer stem cell tumorigenicity and metastatic activity. J Biol Chem. 2011;286:18573–18582. doi: 10.1074/jbc.M110.200717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao C, Chen G, Kuan SF, Zhang DH, Schlaepfer DD, Hu J. FAK/PYK2 promotes the Wnt/beta-catenin pathway and intestinal tumorigenesis by phosphorylating GSK3beta. Elife. 2015;4 doi: 10.7554/eLife.10072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verma N, Keinan O, Selitrennik M, Karn T, Filipits M, Lev S. PYK2 sustains endosomal-derived receptor signalling and enhances epithelial-to-mesenchymal transition. Nat Commun. 2015;6:6064. doi: 10.1038/ncomms7064. [DOI] [PubMed] [Google Scholar]
- 9.Lee BY, Timpson P, Horvath LG, Daly RJ. FAK signaling in human cancer as a target for therapeutics. Pharmacol Ther. 2015;146:132–149. doi: 10.1016/j.pharmthera.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Lim ST, Chen XL, Lim Y, Hanson DA, Vo TT, Howerton K, Larocque N, Fisher SJ, Schlaepfer DD, Ilic D. Nuclear FAK promotes cell proliferation and survival through FERM-enhanced p53 degradation. Mol Cell. 2008;29:9–22. doi: 10.1016/j.molcel.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frame MC, Patel H, Serrels B, Lietha D, Eck MJ. The FERM domain: organizing the structure and function of FAK. Nat Rev Mol Cell Biol. 2010;11:802–814. doi: 10.1038/nrm2996. [DOI] [PubMed] [Google Scholar]
- 12.Lim ST, Chen XL, Tomar A, Miller NL, Yoo J, Schlaepfer DD. Knock-in mutation reveals an essential role for focal adhesion kinase activity in blood vessel morphogenesis and cell motility-polarity but not cell proliferation. J Biol Chem. 2010;285:21526–21536. doi: 10.1074/jbc.M110.129999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao X, Peng X, Sun S, Park AY, Guan JL. Role of kinase-independent and - dependent functions of FAK in endothelial cell survival and barrier function during embryonic development. J Cell Biol. 2010;189:955–965. doi: 10.1083/jcb.200912094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanchanawong P, Shtengel G, Pasapera AM, Ramko EB, Davidson MW, Hess HF, Waterman CM. Nanoscale architecture of integrin-based cell adhesions. Nature. 2010;468:580–584. doi: 10.1038/nature09621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Horton ER, Byron A, Askari JA, Ng DH, Millon-Fremillon A, Robertson J, Koper EJ, Paul NR, Warwood S, Knight D, et al. Definition of a consensus integrin adhesome and its dynamics during adhesion complex assembly and disassembly. Nat Cell Biol. 2015;17:1577–1587. doi: 10.1038/ncb3257. [see comments with reference 22]
- 16.Bachir AI, Zareno J, Moissoglu K, Plow EF, Gratton E, Horwitz AR. Integrin-associated complexes form hierarchically with variable stoichiometry in nascent adhesions. Curr Biol. 2014;24:1845–1853. doi: 10.1016/j.cub.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Theodosiou M, Widmaier M, Bottcher RT, Rognoni E, Veelders M, Bharadwaj M, Lambacher A, Austen K, Muller DJ, Zent R, et al. Kindlin-2 cooperates with talin to activate integrins and induces cell spreading by directly binding paxillin. Elife. 2016;5 doi: 10.7554/eLife.10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller NL, Lawson C, Kleinschmidt EG, Tancioni I, Uryu S, Schlaepfer DD. A non-canonical role for Rgnef in promoting integrin-stimulated focal adhesion kinase activation. J Cell Sci. 2013;126:5074–5085. doi: 10.1242/jcs.135509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu YL, Lu S, Szeto KW, Sun J, Wang Y, Lasheras JC, Chien S. FAK and paxillin dynamics at focal adhesions in the protrusions of migrating cells. Sci Rep. 2014;4:6024. doi: 10.1038/srep06024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qu H, Tu Y, Guan JL, Xiao G, Wu C. Kindlin-2 tyrosine phosphorylation and interaction with Src serve as a regulatable switch in the integrin outside-in signaling circuit. J Biol Chem. 2014;289:31001–31013. doi: 10.1074/jbc.M114.580811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schlaepfer DD, Hauck CR, Sieg DJ. Signaling through focal adhesion kinase. Progress in Biophysics & Molecular Biology. 1999;71:435–478. doi: 10.1016/s0079-6107(98)00052-2. [DOI] [PubMed] [Google Scholar]
- 22. Horton ER, Humphries JD, Stutchbury B, Jacquemet G, Ballestrem C, Barry ST, Humphries MJ. Modulation of FAK and Src adhesion signaling occurs independently of adhesion complex composition. J Cell Biol. 2016;212:349–364. doi: 10.1083/jcb.201508080. Using specific kinase inhibitors, these comprehensive studies [15] show that integrin adhesion complex composition are not altered by FAK or Src inhibition. However, kinase inhibition significantly diminishes phosphotyrosine signal propagation through adhesions, reducing cell migration and proliferation.
- 23.Wu Y, Zhang K, Seong J, Fan J, Chien S, Wang Y, Lu S. In-situ coupling between kinase activities and protein dynamics within single focal adhesions. Sci Rep. 2016;6:29377. doi: 10.1038/srep29377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walkiewicz KW, Girault JA, Arold ST. How to awaken your nanomachines: Site-specific activation of focal adhesion kinases through ligand interactions. Prog Biophys Mol Biol. 2015;119:60–71. doi: 10.1016/j.pbiomolbio.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Lietha D, Cai X, Ceccarelli DF, Li Y, Schaller MD, Eck MJ. Structural basis for the autoinhibition of focal adhesion kinase. Cell. 2007;129:1177–1187. doi: 10.1016/j.cell.2007.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goni GM, Epifano C, Boskovic J, Camacho-Artacho M, Zhou J, Bronowska A, Martin MT, Eck MJ, Kremer L, Grater F, et al. Phosphatidylinositol 4,5-bisphosphate triggers activation of focal adhesion kinase by inducing clustering and conformational changes. Proc Natl Acad Sci U S A. 2014;111:E3177–E3186. doi: 10.1073/pnas.1317022111. Here the authors detail a sequential mechanism(s) that link integrin engagement with FAK activation. Binding of the FAK FERM domain to PI(4,5)P2 triggers FAK clustering and relaxes the “closed” FAK conformation, allowing FAK autophosphorylation at Y397.
- 27. Brami-Cherrier K, Gervasi N, Arsenieva D, Walkiewicz K, Boutterin MC, Ortega A, Leonard PG, Seantier B, Gasmi L, Bouceba T, et al. FAK dimerization controls its kinase-dependent functions at focal adhesions. EMBO J. 2014 doi: 10.1002/embj.201386399. This paper establishes that FAK autophosphorylation at Y397 requires transient FAK FERM:FERM homodimerization. The dimers are strengthened by interaction of the FAT domain and paxillin, suggesting that site-specific ligands may enhance localized FAK activation.
- 28. Kadare G, Gervasi N, Brami-Cherrier K, Blockus H, El Messari S, Arold ST, Girault JA. Conformational dynamics of the focal adhesion targeting domain control specific functions of focal adhesion kinase in cells. J Biol Chem. 2015;290:478–491. doi: 10.1074/jbc.M114.593632. This paper demonstrates that the ability of the FAK FAT domain to shift between an “open” and “closed” conformation is required for proper FAK focal adhesion targeting and turnover, without affecting FAK activation.
- 29.Hayashi I, Vuori K, Liddington RC. The focal adhesion targeting (FAT) region of focal adhesion kinase is a four-helix bundle that binds paxillin. Nat. Struct. Biol. 2002;9:101–106. doi: 10.1038/nsb755. [DOI] [PubMed] [Google Scholar]
- 30.Dixon RD, Chen Y, Ding F, Khare SD, Prutzman KC, Schaller MD, Campbell SL, Dokholyan NV. New insights into FAK signaling and localization based on detection of a FAT domain folding intermediate. Structure. 2004;12:2161–2171. doi: 10.1016/j.str.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 31.Cable J, Prutzman K, Gunawardena HP, Schaller MD, Chen X, Campbell SL. In vitro phosphorylation of the focal adhesion targeting domain of focal adhesion kinase by Src kinase. Biochemistry. 2012;51:2213–2223. doi: 10.1021/bi300123a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jung O, Choi S, Jang SB, Lee SA, Lim ST, Choi YJ, Kim HJ, Kim DH, Kwak TK, Kim H, et al. Tetraspan TM4SF5-dependent direct activation of FAK and metastatic potential of hepatocarcinoma cells. J Cell Sci. 2012;125:5960–5973. doi: 10.1242/jcs.100586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi CH, Webb BA, Chimenti MS, Jacobson MP, Barber DL. pH sensing by FAK-His58 regulates focal adhesion remodeling. J Cell Biol. 2013;202:849–859. doi: 10.1083/jcb.201302131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ritt M, Guan JL, Sivaramakrishnan S. Visualizing and manipulating focal adhesion kinase regulation in live cells. J Biol Chem. 2013;288:8875–8886. doi: 10.1074/jbc.M112.421164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen TH, Chan PC, Chen CL, Chen HC. Phosphorylation of focal adhesion kinase on tyrosine 194 by Met leads to its activation through relief of autoinhibition. Oncogene. 2011;30:153–166. doi: 10.1038/onc.2010.398. [DOI] [PubMed] [Google Scholar]
- 36.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 37.Lawson C, Lim ST, Uryu S, Chen XL, Calderwood DA, Schlaepfer DD. FAK promotes recruitment of talin to nascent adhesions to control cell motility. J Cell Biol. 2012;196:223–232. doi: 10.1083/jcb.201108078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swaminathan V, Fischer RS, Waterman CM. The FAK-Arp2/3 interaction promotes leading edge advance and haptosensing by coupling nascent adhesions to lamellipodia actin. Mol Biol Cell. 2016;27:1085–1100. doi: 10.1091/mbc.E15-08-0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim NG, Gumbiner BM. Adhesion to fibronectin regulates Hippo signaling via the FAK-Src-PI3K pathway. J Cell Biol. 2015;210:503–515. doi: 10.1083/jcb.201501025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Przybyla L, Muncie JM, Weaver VM. Mechanical Control of Epithelial-to-Mesenchymal Transitions in Development and Cancer. Annu Rev Cell Dev Biol. 2016;32:527–554. doi: 10.1146/annurev-cellbio-111315-125150. [DOI] [PubMed] [Google Scholar]
- 41.Pasapera AM, Schneider IC, Rericha E, Schlaepfer DD, Waterman CM. Myosin II activity regulates vinculin recruitment to focal adhesions through FAK-mediated paxillin phosphorylation. J Cell Biol. 2010;188:877–890. doi: 10.1083/jcb.200906012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Plotnikov SV, Pasapera AM, Sabass B, Waterman CM. Force fluctuations within focal adhesions mediate ECM-rigidity sensing to guide directed cell migration. Cell. 2012;151:1513–1527. doi: 10.1016/j.cell.2012.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou J, Aponte-Santamaria C, Sturm S, Bullerjahn JT, Bronowska A, Grater F. Mechanism of Focal Adhesion Kinase Mechanosensing. PLoS Comput Biol. 2015;11:e1004593. doi: 10.1371/journal.pcbi.1004593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petridou NI, Skourides PA. FAK transduces extracellular forces that orient the mitotic spindle and control tissue morphogenesis. Nat Commun. 2014;5:5240. doi: 10.1038/ncomms6240. [DOI] [PubMed] [Google Scholar]
- 45. Bae YH, Mui KL, Hsu BY, Liu SL, Cretu A, Razinia Z, Xu T, Pure E, Assoian RK. A FAK-Cas-Rac-lamellipodin signaling module transduces extracellular matrix stiffness into mechanosensitive cell cycling. Sci Signal. 2014;7:ra57. doi: 10.1126/scisignal.2004838. This study details how FAK can translate mechanical stiffness into modified cell behavior. High matrix stiffness induces FAK/p130Cas phosphorylation, which in turn activates the GTPase Rac, resulting in increased intracellular stiffness and expression of cyclin D1.
- 46.Wong VW, Rustad KC, Akaishi S, Sorkin M, Glotzbach JP, Januszyk M, Nelson ER, Levi K, Paterno J, Vial IN, et al. Focal adhesion kinase links mechanical force to skin fibrosis via inflammatory signaling. Nat Med. 2011;18:148–152. doi: 10.1038/nm.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laklai H, Miroshnikova YA, Pickup MW, Collisson EA, Kim GE, Barrett AS, Hill RC, Lakins JN, Schlaepfer DD, Mouw JK, et al. Genotype tunes pancreatic ductal adenocarcinoma tissue tension to induce matricellular fibrosis and tumor progression. Nat Med. 2016;22:497–505. doi: 10.1038/nm.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jiang H, Hegde S, Knolhoff BL, Zhu Y, Herndon JM, Meyer MA, Nywening TM, Hawkins WG, Shapiro IM, Weaver DT, et al. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat Med. 2016;22:851–860. doi: 10.1038/nm.4123. This study found that administration of the FAK inhibitor VS-4718 in a mouse model of pancreatic ductal adenocarcinoma altered the tumor microenvironment by reducing fibrosis and increasing infiltration of cytotoxic T lymphocytes. The addition of VS-4718 to combinatorial gemcitabine + anti-PD1/anti-CTLA4 therapy significantly improved median survival time in mice.
- 49.De Franceschi N, Hamidi H, Alanko J, Sahgal P, Ivaska J. Integrin traffic - the update. J Cell Sci. 2015;128:839–852. doi: 10.1242/jcs.161653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paul NR, Jacquemet G, Caswell PT. Endocytic Trafficking of Integrins in Cell Migration. Curr Biol. 2015;25:R1092–R1105. doi: 10.1016/j.cub.2015.09.049. [DOI] [PubMed] [Google Scholar]
- 51.Ezratty EJ, Partridge MA, Gundersen GG. Microtubule-induced focal adhesion disassembly is mediated by dynamin and focal adhesion kinase. Nat Cell Biol. 2005;7:581–590. doi: 10.1038/ncb1262. [DOI] [PubMed] [Google Scholar]
- 52.Wang Y, Cao H, Chen J, McNiven MA. A direct interaction between the large GTPase dynamin-2 and FAK regulates focal adhesion dynamics in response to active Src. Mol Biol Cell. 2011;22:1529–1538. doi: 10.1091/mbc.E10-09-0785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Alanko J, Mai A, Jacquemet G, Schauer K, Kaukonen R, Saari M, Goud B, Ivaska J. Integrin endosomal signalling suppresses anoikis. Nat Cell Biol. 2015;17:1412–1421. doi: 10.1038/ncb3250. This study demonstrates that FAK can be recruited to and activated at integrin-containing endosomes, and that endocytosis is necessary for full ECM-induced FAK signaling. Blocking endocytosis rendered cells sensitive to anoikis in a FAK-dependent manner.
- 54. Nader GP, Ezratty EJ, Gundersen GG. FAK, talin and PIPKIgamma regulate endocytosed integrin activation to polarize focal adhesion assembly. Nat Cell Biol. 2016 doi: 10.1038/ncb3333. This study found that FAK was necessary for polarized focal adhesion reassembly in migrating cells. Specifically FAK, talin, and PIPKIγ2 associate with integrins in endosomes, maintaining an active integrin conformation.
- 55.Ginsberg MH. Integrin activation. BMB Rep. 2014;47:655–659. doi: 10.5483/BMBRep.2014.47.12.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cavallaro U, Dejana E. Adhesion molecule signalling: not always a sticky business. Nat.. Rev. Mol. Cell. Biol. 2011;12:189–197. doi: 10.1038/nrm3068. [DOI] [PubMed] [Google Scholar]
- 57.Mui KL, Chen CS, Assoian RK. The mechanical regulation of integrin-cadherin crosstalk organizes cells, signaling and forces. J Cell Sci. 2016;129:1093–1100. doi: 10.1242/jcs.183699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen XL, Nam JO, Jean C, Lawson C, Walsh CT, Goka E, Lim ST, Tomar A, Tancioni I, Uryu S, et al. VEGF-induced vascular permeability is mediated by FAK. Dev Cell. 2012;22:146–157. doi: 10.1016/j.devcel.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jean C, Chen XL, Nam JO, Tancioni I, Uryu S, Lawson C, Ward KK, Walsh CT, Miller NL, Ghassemian M, et al. Inhibition of endothelial FAK activity prevents tumor metastasis by enhancing barrier function. J Cell Biol. 2014;204:247–263. doi: 10.1083/jcb.201307067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Serrels A, Lund T, Serrels B, Byron A, McPherson RC, von Kriegsheim A, Gomez-Cuadrado L, Canel M, Muir M, Ring JE, et al. Nuclear FAK controls chemokine transcription, Tregs, and evasion of anti-tumor immunity. Cell. 2015;163:160–173. doi: 10.1016/j.cell.2015.09.001. This paper finds that nuclear FAK binds to transcription factors that regulate expression of cytokines, which support an immunosuppressive environment in squamous cell carcinoma. Interestingly, this role for FAK is dependent upon its kinase activity.
- 61.Golubovskaya VM, Finch R, Cance WG. Direct Interaction of the N-terminal domain of focal adhesion kinase with the N-terminal transactivation domain of p53. J. Biol. Chem. 2005;280:25008–25021. doi: 10.1074/jbc.M414172200. [DOI] [PubMed] [Google Scholar]
- 62. Tancioni I, Miller NL, Uryu S, Lawson C, Jean C, Chen XL, Kleinschmidt EG, Schlaepfer DD. FAK activity protects nucleostemin in facilitating breast cancer spheroid and tumor growth. Breast Cancer Res. 2015;17:47. doi: 10.1186/s13058-015-0551-x. This study was the first to demonstrate a role for FAK in the nucleolus. FAK protected nucleostemin from proteasomal degradation, and both FAK activity and nucleostemin were required for anchorage-independent breast cancer spheroid growth.
- 63.Hein N, Hannan KM, George AJ, Sanij E, Hannan RD. The nucleolus: an emerging target for cancer therapy. Trends in Molecular Medicine. 2013;19:643–654. doi: 10.1016/j.molmed.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 64.Luo SW, Zhang C, Zhang B, Kim CH, Qiu YZ, Du QS, Mei L, Xiong WC. Regulation of heterochromatin remodelling and myogenin expression during muscle differentiation by FAK interaction with MBD2. EMBO J. 2009;28:2568–2582. doi: 10.1038/emboj.2009.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lim ST, Miller NL, Chen XL, Tancioni I, Walsh CT, Lawson C, Uryu S, Weis SM, Cheresh DA, Schlaepfer DD. Nuclear-localized focal adhesion kinase regulates inflammatory VCAM-1 expression. J Cell Biol. 2012;197:907–919. doi: 10.1083/jcb.201109067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cardoso AC, Pereira AH, Ambrosio AL, Consonni SR, Rocha de Oliveira R, Bajgelman MC, Dias SM, Franchini KG. FAK Forms a Complex with MEF2 to Couple Biomechanical Signaling to Transcription in Cardiomyocytes. Structure. 2016;24:1301–1310. doi: 10.1016/j.str.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 67.Nakazawa N, Sathe AR, Shivashankar GV, Sheetz MP. Matrix mechanics controls FHL2 movement to the nucleus to activate p21 expression. Proc Natl Acad Sci U S A. 2016 doi: 10.1073/pnas.1608210113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sathe AR, Shivashankar GV, Sheetz MP. Nuclear transport of paxillin depends on focal adhesion dynamics and FAT domains. J Cell Sci. 2016;129:1981–1988. doi: 10.1242/jcs.172643. [DOI] [PMC free article] [PubMed] [Google Scholar]