Abstract
Compelling evidence suggests that physical activity is an effective intervention for cancer survivors, including for those undergoing active cancer treatments. However, to date most evidence has emerged from interventions that have promoted moderate to vigorous physical activity. In this conceptual review, we argue that attention should be given to the entire continuum of physical activity from reducing sedentary behavior to increasing higher levels of physical activity when possible. In addition, considerable evidence in the cancer literature supports the value of mindfulness-based interventions as a means of helping patients and survivors cope with the variety of threats that accompany this disease. Based on the success of these two areas of research, we argue for conceptualizing and promoting physical activity as Mindfulness-Based Movement, using Polyvagal Theory as a theoretical framework to understand the role and value of Mindfulness-Based Movement as a potential intervention for cancer care and control.
Keywords: mindfulness, cancer survivorship, physical activity, sedentary behavior, polyvagal theory
Exercise is Medicine is a global health initiative managed by the American College of Sports Medicine, which encourages primary care physicians and other health care providers to include exercise in the treatment plan for patients. Although there is strong empirical support for the health benefits of exercise, rates of recidivism are high, and the thought of exercise does not resonate well with many patients who are frequently overweight, burdened by physical symptoms, and often have limitations in physical capacities. Also, exercise does not negate the ill effects of excessive sedentary behavior. In this article, we focus on cancer, introducing the concept of Mindfulness-Based Movement (MBM), a novel intervention that cuts across the continuum of physical activity.
There are 3 fundamental tenets that serve as the scaffolding for this intervention. First, MBM is conceptually rooted in polyvagal theory.1 Second, it is important to move and to move often, no matter how simple or limited the movement may be. Third, all movement—simple or complex, low demand or high demand—deserves participants’ full attention. This orientation creates opportunities for embodied and relational experience; movement becomes a state of being rather than doing.2 And fourth, as symptoms and conditions permit, patients should experiment with and have goals for both decreasing sedentary behavior and increasing moderate to vigorous levels of physical activity.
As a brief overview for the reader, MBM represents an intervention for promoting a broad range of physical activity behaviors both during and following cancer treatment. It acknowledges the substantial health benefits that can be derived from both reducing sedentary behavior and increasing moderate to vigorous forms of physical activity. MBM is delivered as a group-mediated intervention, fostering positive emotions as well as the relational and playful nature of various forms of movement. Consistent with the view of Porges,3 playful oriented forms of physical activity that involve engagement with others constitute a neural exercise, helping patients transition from aroused motoric states associated with physical movements to calm states. Polyvagal theory, which serves as the foundation for MBM, posits that recruiting and exercising the social engagement system is the “go-to” default activity that humans use to modulate stress. This capacity resides within the social engagement system because positive face-to face interactions activate neural pathways through the vagus nerve that downregulate the sympathetic activation associated with both exercise and stress.
From the perspective of polyvagal theory, MBM functions as a neural exercise in which physiological state is manipulated by exercise, social engagement, and mindful focused attention. During calm states in which the social engagement system is functioning, the social cues from voice and face maintain a physiology of safety characterized by a strong vagal influence to the heart that supports health, growth, and restoration. Exercise requires an increase in cardiac output through both a removal of vagal inhibition and an increase in sympathetic influences on the heart. The time course of alternating physical activity and social engagement behaviors provides opportunities to exercise the neural regulation of the autonomic nervous system. Through repeated withdrawal of vagal inhibition to support movement and the recovery of vagal inhibition, the autonomic nervous system develops and promotes a more efficient shift from a physiological state of arousal to calm. Opportunities to enhance neural regulation of the autonomic nervous system are becoming relevant in the treatment of cancer, with recent findings linking autonomic nervous system function to outcomes in prostate and breast cancer.4,5
The article is divided into 4 sections. First, we briefly overview the field of physical activity in the context of cancer care and control. Second, we examine the concept of mindfulness as applied to cancer, emphasizing how it relates to embodiment and human relationships. Third, we discuss in detail the value of MBM, laying the groundwork for the fourth and final section, which details MBM from the perspective of polyvagal theory.
The Role of Physical Activity in Cancer Care and Control
Physical activity has received increasing attention in the context of cancer care and control. A key feature of MBM is that it encompasses the entire continuum of physical activity. The rationale for this decision will be developed and reinforced throughout the article. Briefly, as shown in Figure 1, movement is inherent in a range of activities from postural shifts, to activities of daily living, and in leisure pursuits that involve light to vigorous exercise. Of particular importance is both reducing time spent in sedentary behavior to the left and increasing time in light- and higher-intensity activities in the middle and to the right of the continuum.
Figure 1.

Physical activity continuum.
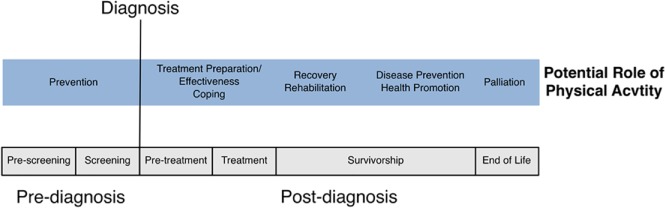
Some of the first studies to examine physical activity with cancer patients were performed in the 1980s by McVicar, Winningham, and colleagues.6-8 A total of 45 breast cancer patients receiving adjuvant chemotherapy were randomized to 1 of 3 conditions: (a) 10 weeks of high-intensity aerobic exercise, (b) stretching/flexibility, or (c) a control group. The exercise intervention was found to be safe and resulted in positive changes in aerobic capacity, body composition, and self-reported nausea. Since then, there has been a significant increase in physical activity research from prediagnosis to palliative care. The physical exercise across the cancer experience (PEACE) framework is a conceptual model that is useful in identifying specific time points, prediagnosis and postdiagnosis or during and following treatment, when physical activity may be of most benefit (Figure 2).9,10 In the current article, we will use the term survivors to refer to persons in long-term care toward the right of the cancer continuum, whereas patients are those in active treatments closer to diagnosis.
Figure 2.
Framework PEACE (adapted from Courneya and Friedenreich.9(p243) Copyright 2007 Elsevier Inc. Adapted with permission.)
Courneya has argued that, for cancer research involving physical activity to flourish, investigators need to specify how physical activity may be linked to cancer variables.11 Cancer variables can be either disease or treatment related. How these variables interact with one another and with various types of movement across the physical activity continuum (Figure 1) will shape each individual’s response and ultimately health outcomes. There are 4 main types of relationships in this framework: cancer variables can be (a) outcomes of physical activity such as mortality, (b) moderators of health outcomes associated with physical activity, (c) determinants of adherence to physical activity, and (d) moderators of the known determinants of physical activity. It is important to recognize that whereas moderate to vigorous physical activity is important, reduction of sedentary behavior is an independent predictor of adverse health outcomes.12-14 Furthermore, for some patients or at a particular stage of cancer, the only reasonable goal may be reducing sedentary behavior.
To date, physical activity research on cancer has focused primarily on exercise for survivors of breast, prostate, and colorectal cancer. More recently, research has expanded to include a variety of disease subtypes and has also included patients who are undergoing treatment.15,16 For example, a question of interest is whether physical activity can reduce the adverse effects of primary cancer therapies because, for certain cancers, these therapies are known to be cardiotoxic.17,18 There is also a growing understanding of the negative effects of high levels of sedentary behavior, independent of time spent in higher-intensity activities, on morbidity and mortality. However, it is not yet clear how much or what type of physical activity is necessary in these circumstances and whether the effects are uniform or moderated by factors such as demographics, medical history, or genetic profiles.
A systematic review and meta-analysis of 82 randomized controlled trials of physical activity interventions both during and following treatment, including 66 high-quality studies, found that exercise resulted in large effects on muscular strength, moderate effects on fatigue and breast cancer–specific concerns, and small to moderate effects on physical activity level, cardiovascular fitness, and quality of life during treatment. Overall physical activity was found to be safe, feasible, and efficacious for patients undergoing a variety of treatment modalities.16 Prostate cancer patients who receive treatment with androgen deprivation therapy are prone to experience a loss of muscle mass and increases in adipose tissue deposition, potentially leading to functional decline, disability, and loss of independence.19-21 Evidence suggests that engaging in physical activity from the onset of androgen deprivation therapy may offer one way of offsetting these musculoskeletal changes and protecting against functional decline.22,23 Recent studies have also examined the safety, feasibility, and preliminary efficacy of exercise for patients diagnosed and being treated for acute myelogenous leukemia (AML)24,25—a very sick population that experiences significant declines in physical function and quality of life, with a high rate of mortality. On the other end of the physical activity continuum, the impact of sedentary behavior on cancer survivorship is receiving growing attention. It is estimated that cancer survivors spend two-thirds of their time in sedentary behaviors.26 Sedentary behaviors have been shown to be associated with increased risk of mortality in postdiagnosis breast cancer survivors27 and with increased all-cause mortality in colorectal cancer survivors.28 A harmonized analysis with data from 3 large breast cancer cohorts found that there was a 22% (hazard ratio = 1.22; 95% CI = 1.05, 1.42) increased risk of breast cancer mortality for women considered to be sedentary, even when controlling for the effects of comorbidities.27 The high levels of sedentary behavior among cancer survivors and a lack of data on the biological mechanisms through which increased sedentary behavior affects health makes this a significant topic for future scientific inquiry.29
Despite the merits of physical activity for cancer patients and survivors, adherence to guidelines has been disappointing.30,31 Of note, breast cancer survivors have been found to engage in higher levels of moderate to vigorous physical activity compared with healthy women, yet they also have much higher levels of sedentary behavior,32 a finding that supports the focus both for reducing sedentary behavior and increasing moderate to vigorous physical activity.33 In addition, for cancer patients undergoing active therapies, focusing on sedentary behavior allows for greater flexibility in patient-centered goals and increases the likelihood that patients will remain active beyond the supervised care setting. High competing care demands, symptoms, and functional decline are all barriers to cancer patients being physically active. If we further burden patients with a single-minded approach of increasing moderate to vigorous physical activity, we may well be setting patients up for failure.
Mindfulness and Mindfulness-Based Interventions for Cancer
Over the past 20 years, there has been a growing body of literature supporting the therapeutic value of mindfulness-based interventions (MBIs) in the treatment of cancer patients.34-37 In brief, meta-analytic studies of MBIs and cancer have reported moderate effect sizes on anxiety and somewhat smaller but significant effect sizes on depression.37-39 Positive effects have also been reported for enhanced well-being40,41 and improved quality of life42; there is some evidence that MBIs have favorable effects on biomarkers of health and aging, including more favorable slopes for daily cortisol levels43 and improved telomere length.44 In a recent article from the Annals of the New York Academy of Sciences, Carlson45 stated that support for the efficacy of MBIs in cancer is unequivocal.
As we will demonstrate throughout this article, a working definition of the mind is critical to the concept of mindfulness and to the structure of MBM. As defined by Siegel, “The human mind is a relational and embodied process that regulates the flow of energy and information.”46(p52) That is, one can view the mind as a process that establishes a network to support the flow of energy and information between the body, brain, and relationships—see Figure 3. As emphasized in our own work, the relational nature of the mind enables important connections not only with other people, but with the surrounding environment as well.47 The embodied nature of the mind underscores the fact that the body plays a central role in how people regulate energy and information flow that is then processed by the brain. A well-known example is the effect of different types of breathing techniques on anxiety.48 Further evidence comes from the role that intestinal microbes play in shaping both mood and behavior.49 It is also important to emphasize that the embodied and relational mind are interdependent; synergy between the two is essential to human thriving, a proposition that is central to polyvagal theory.1 Specifically, in early infancy, a secure attachment relationship between mother and child affects the flow of information to skeletal muscles and to the heart in a manner that downregulates states of defense by increasing vagal tone and promoting feelings of safety and human connection—the body has direct effects on shaping the brain but, in this case, in collaboration with interpersonal connection.
Figure 3.
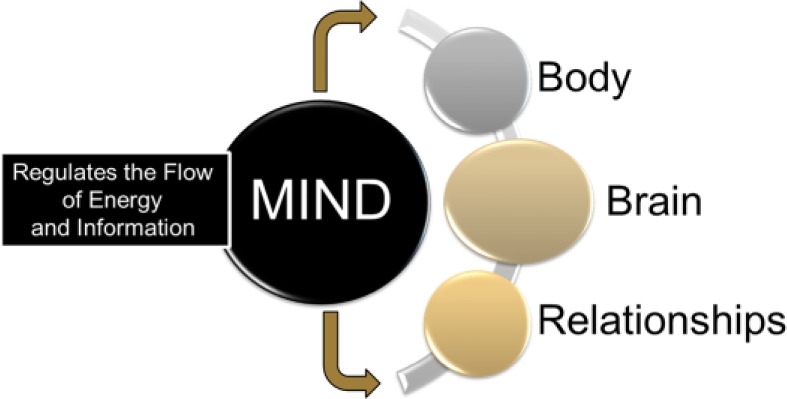
The mind: an embodied and relational process.
So what is mindfulness? In 2007, Brown et al50(p212) published a seminal article arguing that, as a psychological construct, mindfulness is “receptive attention to and conscious awareness of present events and experience,” a definition that reflected early ideas articulated by Kabat-Zinn51(p2) who in 1990 suggested that “simply put, mindfulness is moment-to-moment awareness. It is cultivated by purposefully paying attention to things we ordinarily never give a moment’s thought to.” Brown et al50 pointed out that awareness involves the conscious registration of events and experience—that it is our most proximal contact with reality. Particularly important to understanding the boundaries of this construct is the following passage from their article:
Commonly sensory objects are held in focal awareness only briefly, if at all, before some cognitive and emotional reaction to them is made. These rapid perceptual reactions have several characteristics of relevance to subjective experience and functioning: First, they are often of a discriminative nature, in which a primary appraisal of the object is made as, most basically, “good,” “bad,” or “neutral,” usually in reference to the self. Second, they are usually conditioned by past experience of the sensory object or other objects of sufficient similarity to evoke an association in memory. Third, perceptual experience is easily assimilated or, through further cognitive operations upon the object made to assimilate into existing cognitive schemas. The consequence of such processing is that concepts, labels, and judgments are often imposed, often automatically, on everything that is encountered.50(p212)
Prior to this publication and subsequent to it, a number of authors expanded on the definition of mindfulness that was offered by Kabat-Zinn in 1990.51 In fact, in 1994, Kabat-Zinn2(p4) himself stated that mindfulness involved “paying attention in a particular way; that is, on purpose, in the present moment, and nonjudgmentally.” Note that in this version of the definition, the term nonjudgmental enters the picture. Brown et al50 would argue that although being nonjudgmental may be an important quality of being able to achieve mindful awareness, from a scientific and theoretical perspective, it is not an attribute of the construct itself. This is a critical distinction because the skills and strategies necessary to achieving mindful states of awareness are central to the design of behavioral interventions that have the goal of promoting mindful states of being. However, to reiterate, these skills and strategies are not what it means to be mindful; rather, they are important elements of mindful training programs.
Interestingly, within the field of neuroscience, there has been an explosion of interest in mindfulness.52,53 Important to our discussion is the position espoused by Siegel54 that the mindful brain is an embodied, nonconceptual state of consciousness that has a strong relational orientation. In fact, the conceptual self, which is largely a product of the left hemisphere, can be a barrier to achieving mindful awareness55 because as emphasized by Brown et al,50 the conceptual self filters and spins the raw experience of awareness for its own self-interest or protection. Porges1 also argues, as have others, that the brain has an evolutionary bias to protect humans from harm; thus, it expends a great deal of time as a watchtower for threat. However, according to Porges, polyvagal theory provides an understanding of the cues of safety that are embedded in positive social engagement behaviors, including prosodic voice, endearing facial expressions, and welcoming gestures that are capable of reflexively turning off vigilance and enable an individual to feel safe. Fredrickson et al56 posit that this evolutionary bias of the brain can be countered by the intentional experience of positive emotion, moving one toward brain states that promote openness, curiosity, and creativity—the broaden and build theory of positive emotion.
The theories posited by Porges1 and Fredrickson et al56 are particularly relevant to the design of MBIs from 2 perspectives. First, both emphasize the critical role of relationships to human thriving. In fact, the fundamental tenet of polyvagal theory is that the brain is a social organ that does not require conscious awareness to respond appropriately and reciprocally to cues of social engagement. And second, as shown by Fredrickson and colleagues, repeated conscious awareness of positive affect from relational experience enhances vagal tone and is a powerful enabling force in promoting positive well-being57 and the experience of embodied, nonconceptual, self-awareness.58
Despite the value of being mindful in daily life,50 even under the best of circumstances, mindfulness can be difficult to achieve given our production-oriented society and its ever increasing dependence on technology. For cancer patients, whose lives become fragmented by the disease and its treatment, the norm is to become preoccupied with the threat of physical symptoms and existential concerns, to get stuck in destructive modes of thinking and feeling about the conceptual self. As described by Carlson,45 cancer results in most people experiencing significant fear, anxiety, and depression—life is no longer predictable or controllable. These emotional experiences are associated with an activation of the sympathetic nervous system, producing physiological states that support defense and are incompatible with mindfulness. Moreover, current research suggests chronic sympathetic activation without being constrained and regulated by vagal circuit is linked to poor outcomes following cancer diagnoses.4,5 However, it is also important to recognize that despite the challenges of achieving mindful awareness in the face of cancer, most people do experience such states. This can occur when listening to a piece of music, gazing upon a scene in nature, or thinking about a loved one. The goal in MBIs is to help people access these states more readily and frequently.
Humans are relational beings and will engage readily in social interactions when they feel safe from harm.1 A diagnosis of cancer can disrupt the balance in people’s lives and can serve to disconnect patients from their own bodies. Ongoing treatments can lead to physical deconditioning that places patients at risk of losing their independence.59 The ability to function independently is critical to feeling autonomous, and cancer can easily morph into a traumatic experience. The question is how can people who are dealing with a diagnosis and treatment for cancer be supported in returning to a more self-determined life? What does MBM offer beyond the current available options for lifestyle interventions in these populations?
Benefits of a MBM Intervention for Cancer
To date, most research on MBIs in cancer has been modeled after Mindfulness-Based Stress Reduction (MBSR) developed by Kabat-Zinn51; however, a program known as Mindfulness-Based Cancer Recovery (MBCR) includes material specific to coping with cancer, fear of recurrence, management of physical symptoms, and key principles related to cognitive coping strategies adapted from cognitive behavioral therapy.43,44 These modifications to MBSR are important because promoting mindful states of being requires intervening on factors that derail cancer patients and survivors from achieving such states. In this section, we discuss 3 distinct structural/conceptual innovations of a MBM intervention.
Whereas physical activity has favorable effects on the heart and improves metabolic, autonomic, inflammatory, and immune function, the ability of physical activity to rebuild the physical self for cancer patients and its potential to reestablish functioning in both social and physical roles are clear priorities as patient-centered outcomes. Additionally, cancer treatment has deleterious effects on the brain; physical activity may prove to be a viable antidote to declining brain health and associated cognitive decline.60,61 Whereas previous MBIs utilize gentle yoga and/or walking meditation, there is not an explicit focus on the benefits of physical activity per se. Of interest are reports that cancer patients express an interest in alternative therapies such as yoga and Tai Chi. As Porges1 has reported, when the autonomic nervous system is out of balance, people have a tendency to unconsciously self-select engaging in such activities in an attempt to counter their discomfort. Hence, there is a strong conceptual rationale for the integration of mindfulness-based skills with exercise therapy.
Other innovations of MBM include the following: (a) promoting movement across a broad range of activities, (b) fostering the mindset that physical activity can be used to cultivate a sense of “being” rather than “doing,”2 and (c) using the power of a group-mediated approach to treatment. Let us first consider promoting a range of activities as opposed to an exclusive focus on moderate to vigorous physical activity. The advantages of such a perspective are considerable because sedentary behavior is an independent risk factor for compromised health and functional fitness beyond the effects of moderate to vigorous physical activity.62-65 Also, we have found that sedentary behavior interventions are well tolerated and valued by sedentary populations.33 Ultimately, what we seek to achieve with MBM is acceptance of the highs and lows of life—a central tenet of mindfulness51—and developing an understanding of and awareness for the role that even simple movements play in our physical and emotional lives. A greater awareness of moment-to-moment movement is a cornerstone of MBM and of mindful awareness.2,51
Second, MBM focuses on using movement as a time of “being” rather than “doing.”51 Too often, exercise is approached as an additional chore or commitment to add to daily life—something else “to do.” This orientation can be daunting for people with a chronic disease who are already overwhelmed by treatment regimens. In addition, this mindset risks fragmenting from movement the embodied and relational value of such experience. This is unfortunate because both theory66 and recent research67 underscore the important role that value has in the promotion and maintenance of health behavior. In contrast, MBM promotes active living as an opportunity to consciously connect with the body—the embodied mind—in moment-to-moment movements throughout the day. MBM is also used to promote the relational mind both with others and in conjunction with the physical environment. Part of this experience is rediscovering the value inherent in movement and how it affects our lives. Positive emotion promotes an increase in vagal tone,56 enhances resilience, and helps people navigate painful life experience,68 which are important steps in coping with loss.
And third, MBM is based on a model of group-mediated lifestyle behavior change that we developed and tested over the past 20 years on a variety of aging patient populations.69-73 Originally, we successfully used the group as a means of increasing motivation and developing self-regulatory skills; however, in our more recent trials, we have expanded the scope of treatment to focus more on the relational value of the group experience.74 By creating and fostering a safe and supportive environment for participants, MBM provides an opportunity for participants to develop a heartfelt connection with one another. A critical part of this process is the shared challenge that patients face with the cancer experience and, in particular, the feeling of common humanity. As Neff and colleagues have shown,75,76 a sense of common humanity—seeing one’s experience as shared by others—is a core feature of developing self-compassion, which is central to psychological well-being. In fact, we recently showed that a group-mediated exercise intervention for patients with peripheral artery disease substantially increased patients’ social resources as compared with those in a health education control group.77 We have also recently applied this group-based approach to promoting adoption and adherence to physical activity and dietary behavior in prostate cancer patients undergoing androgen deprivation therapy.78
MBM and Cancer: A Polyvagal Perspective
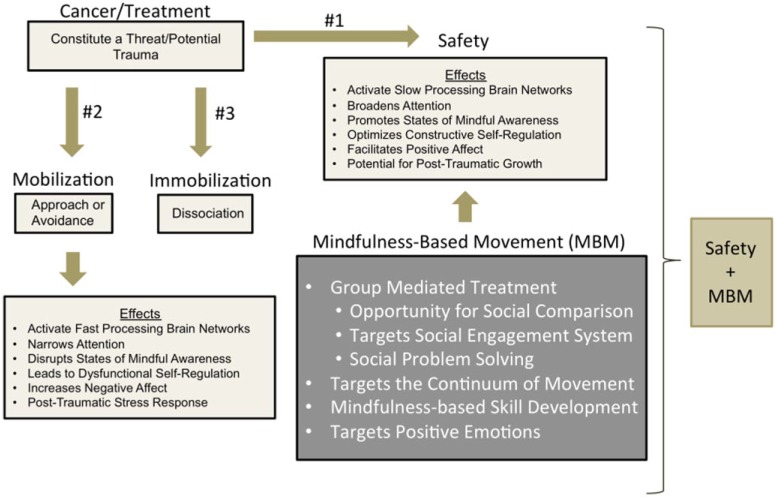
As noted in the introduction to this article, the conceptual model (Figure 4) for MBM has its foundation in polyvagal theory.1 A central tenet of this theory, rooted in evolution, is that the most basic human need or motive is safety. From a developmental perspective, it is no accident that safety is first secured through a successful infant-mother attachment relationship, making the social engagement system described in polyvagal theory a key feature of managing threat. The social engagement system involves the regulation of the muscles of the face and head and the heart through motor fibers of 5 cranial nerves (ie, special visceral efferent pathways) that originate in the brainstem. This system enables emotional communication through facial expressions and prosodic vocalizations, enhances listening to voices, and calms the physiological and behavioral state by increasing the vagal influence to the heart through a branch of the vagus originating in the nucleus ambiguus. Functionally, the social engagement system can be collectively described as the ventral vagal complex. In fact, in the absence of safety, the second most basic human need is to reduce threat; however, the removal of threat is not sufficient to trigger the neural circuits that support connectedness and health, growth, and restoration.
Figure 4.
The polyvagal perspective of mindfulness-based movement.
The relevance of polyvagal theory in the management of cancer is that, as mentioned earlier, the disease and its treatment constitute a traumatic experience—whether real or imagined, cancer is often life-threatening. However, even if one’s life is not threatened, the cancer experience strikes at the very fabric of patients’ day-to-day lives. For example, in prostate and breast cancer, the effects of surgeries and radiation therapy can be devastating to the self-image and sexuality of survivors. The ability to interact in intimate and meaningful ways with partners and significant others as well as to perform social roles can be compromised and valued leisure activities may be lost.
When threatening events occur, particularly severe threat, polyvagal theory79 proposes that 1 of 3 phylogenetically ordered networks is innervated. The networks range from newer, highly developed responses to primitive, survival-mode reactions. The latter occur only when more highly developed networks are rendered ineffective. First, it is possible that people find safety through attuned social interaction and that the threat is neutralized or becomes manageable. According to polyvagal theory, interpersonal attunement involves a heartfelt connection with another human being that is manifested in the behavioral features of the social engagement system and an increase in cardiac vagal tone mediated through the ventral vagal complex. This hypothesis is supported in the findings of a recent study focused on autonomic flexibility, where it was shown that vagal tone was associated with the degree to which people felt connected and socially engaged over a 9-week period.80 Individuals with higher initial levels of vagal tone experienced greater self-reported social connectivity and social engagement. Interestingly, those people who also reported an increase in their social connection and engagement also had greater increases in vagal tone, independent of levels at baseline. The work of Cozolino81 has also explored the neurobiology of attachment and how it has shaped the social and cultural evolution of humans and their communities. It is this experience of interpersonal attachment that provides theoretical support for the group-mediated approach of MBM. A second possible reaction is activation of the sympathetic nervous system and the hypothalamic pituitary adrenal (HPA) axis, preparing the individual for mobilization. However, there are actually 2 directions mobilization can take: (a) it can result in direct action to neutralize the threat or (b) it may invoke psychological defenses such as avoidance. Whereas mobilization through direct action is functional in the face of acute stressors, chronic activation of the HPA axis leads to systemwide dysregulation and is known to have adverse effects on the hippocampus.82 Additionally, although avoiding threat may seem adaptive at a conscious level, avoidance results in threat having a chronic unconscious presence that disrupts adaptive neural circuitry adversely, affecting both physical and psychological health. Finally, a third possible direction is immobilization triggered by activation of vagal motor fibers originating in the dorsal motor nucleus of the vagus. Fatigue and depression are often symptoms of an immobilization response to trauma. In this instance, we hypothesize that reducing sedentary behavior through the promotion of movement across the day stimulates dopamine and other neuromodulators that serve as antidotes to immobilization. Furthermore, as we shall argue further on in our discussion, there is additional potency to a reduction in sedentary behavior when movement is mindfulness based.
A key feature of threat is that it mobilizes neural networks that are quick to respond—a fast circuit83—which bypass brain structures involved in conscious cognitive deliberation. This reduces the utility of conscious models of behavior change such as Social Cognitive Theory84 until such time that the threat has passed or is neutralized. This is precisely why mindfulness-based training is central to MBM. Specifically, centering-based practices such as mindfulness-based stress reduction85 stimulate the ventral vagal pathways to the heart86,87 and encourage the activation of the slow brain circuit.83 This slow circuit potentiates processes such as conscious self-regulation of behavior, self-reflection, and the opportunity for posttraumatic growth that has been a reported consequence of cancer and other chronic diseases.88,89
What do we actually mean by mindful movement? And, why is movement, particularly mindful movement, a viable intervention from the perspective of polyvagal theory? First, it is important to note that humans can be characterized as having the capacity for 2 distinct forms of movement: (a) movement of the body in space, activities that are supported by the spinal nerves, and (b) movement of the muscles of the face and middle ear controlled by the cranial nerves. As a central feature of polyvagal theory,1 movement of the muscles of the face and middle ear facilitate reciprocal social interactions and the experience of attachment—interpersonal attunement. Indeed, as mentioned previously, research by Fredrickson and colleagues56,57 has shown that social connection facilitates positive affect, which increases the calming effect of the vagus on the heart. Additionally, however, being attuned with another person is an experience in mindful awareness, as is attunement with our bodies as we move, or attunement with physical environments in which our lives are embedded. In essence, our hypothesis is that mindful awareness is mediated by regulating the ventral vagus as one totally embraces the present moment through movement in space. There is a coherent integration between the spinal and cranial nerves that support human movement. Within this context MBM is an active “neural exercise” that promotes resilience and improves the efficiency and effectiveness of engaging and disengaging the calming ventral vagal pathways to adjust the cardiac output necessary for movement. MBM promotes the experience of a heartfelt connection between participants by (a) providing a safe and supportive environment where (b) cancer survivors can share their experiences with others who can relate to and empathize with one another, and by (c) providing a specific set of tools and techniques for breaking up periods of immobilization, thereby engaging in opportunities to move within a social and physical environment. Finally, MBM aims to teach participants how to self-regulate their own behavior by first developing an awareness of their experience and then applying the skills learned though the MBM program. As we noted in the previous section of this article, too often, physical activity and engagement with life is experienced as a series of exercises—things “to do” on the treadmill of living productively—rather than an experience of “being” a part of life, fully engaged and playfully connected in an embodied and relational manner in moment-to-moment existence.
Summary
In summary, there are significant health benefits to a lifestyle that includes physical activity, especially for people living with cancer. However, cancer patients and survivors face challenges in either returning to activity or beginning an active lifestyle following the disease and its treatment. As we have argued in our presentation of MBM, there is an advantage in promoting activity across the continuum of PA, teaching patients and survivors to appreciate the continuous flow of movement across the day and being mindfully aware as they do so. Polyvagal theory offers a sound conceptual perspective for delivering MBM. The theory posits that for people to engage in approach behaviors that help foster social engagement and human development, they first need to feel safe. The theory also posits that when the social engagement system with the ventral vagal complex is optimally functioning, the older components of the autonomic nervous system (ie, sympathetic nervous system, dorsal vagal complex) support health, growth, and restoration. In contrast, when the social engagement system is not optimally functioning, the older components assume control, with the main goal of activating cognitive and behavioral defensive strategies. Consistent with this model, recent research illustrates that the older components of the autonomic nervous system are involved in the initiation and proliferation of prostate cancer,4 whereas greater ventral vagal regulation (indexed by heart rate variability) increased postdiagnosis survival duration in metastatic pancreatic cancer.5 The structure of social groups and communities can be one way of providing this safety and support, helping people identify opportunities for positive affective experience and reinforce relationships through movement and social connection—the core of MBM. Finally, we believe that MBM provides a vehicle to experience the embodied and relational nature of the mind, reconnecting people with the meaning and value inherent in being active rather than viewing physical activity as just another thing to do in the context of the challenges posed by cancer and its treatment.
Footnotes
Authors’ Note: Interested readers are welcome to contact the first or senior authors of the study to receive an overview of the proposed MBM intervention and for any further discussion.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded, in part, by a grant from National Institutes of Health/National Heart, Lung and Blood Institute, R18 HL076441, awarded to Dr Rejeski and by a National Institutes for Aging Grant, P30 AG021332. Dr Lucas’s work on this article was also supported by a Cancer Control Traineeship, National Cancer Institute/National Institute of Health (NCI/NIH; R25CA122061).
References
- 1. Porges SW. The polyvagal perspective. Biol Psychol. 2007;74:116-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kabat-Zinn J. Wherever You Go There You Are: Mindfulness Meditation in Everyday Life. New York, NY: Hyperion; 1994. [Google Scholar]
- 3. Porges S. Play as neural exercise: insights from the polyvagal theory. In: Pearce-McCall D, ed. The Power of Play for Mind Brain Health. Mindgains.org, GAINS; 2015:3-7. [Google Scholar]
- 4. Magnon C, Hall SJ, Lin J, et al. Autonomic nerve development contributes to prostate cancer progression. Science. 2013;341:1236361. [DOI] [PubMed] [Google Scholar]
- 5. Couck MD, Marechal R, Moorthamers S, Laethem JL, Gidron Y. Vagal nerve activity predicts overall survival in metastatic pancreatic cancer, mediated by inflammation. Cancer Epidemiol. 2018;40:47-51. [DOI] [PubMed] [Google Scholar]
- 6. MacVicar MG, Winningham ML, Nickel JL. Effects of aerobic interval training on cancer patients’ functional capacity. Nurs Res. 1989;38:348-351. [PubMed] [Google Scholar]
- 7. Winningham ML, MacVicar MG. The effect of aerobic exercise on patient reports of nausea. Oncol Nurs Forum. 1988;15:447-450. [PubMed] [Google Scholar]
- 8. Winningham ML, MacVicar MG, Bondoc M, Anderson JI, Minton JP. Effect of aerobic exercise on body weight and composition in patients with breast cancer on adjuvant chemotherapy. Oncol Nurs Forum. 1989;16:683-689. [PubMed] [Google Scholar]
- 9. Courneya KS, Friedenreich CM. Physical activity and cancer control. Semin Oncol Nurs. 2007;23:242-252. [DOI] [PubMed] [Google Scholar]
- 10. Courneya KS, Friedenreich CM. Framework PEACE: an organizational model for examining physical exercise across the cancer experience. Ann Behav Med. 2001;23:263-272. [DOI] [PubMed] [Google Scholar]
- 11. Courneya KS. Physical activity and cancer survivorship: a simple framework for a complex field. Exerc Sport Sci Rev. 2014;42:102-109. [DOI] [PubMed] [Google Scholar]
- 12. Healy GN, Dunstan DW, Salmon J, Shaw JE, Zimmet PZ, Owen N. Television time and continuous metabolic risk in physically active adults. Med Sci Sports Exerc. 2008;40:639-645. [DOI] [PubMed] [Google Scholar]
- 13. Seguin R, Lamonte M, Tinker L, et al. Sedentary behavior and physical function decline in older women: findings from the women’s health initiative. J Aging Res. 2012;2012:271589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lynch BM. Sedentary behavior and cancer: a systematic review of the literature and proposed biological mechanisms. Cancer Epidemiol Biomarkers Prev. 2010;19:2691-2709. [DOI] [PubMed] [Google Scholar]
- 15. Schmitz KH, Holtzman J, Courneya KS, Masse LC, Duval S, Kane R. Controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2005;14:1588-1595. [DOI] [PubMed] [Google Scholar]
- 16. Speck RM, Courneya KS, Masse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010;4:87-100. [DOI] [PubMed] [Google Scholar]
- 17. Jones LW, Haykowsky MJ, Swartz JJ, Douglas PS, Mackey JR. Early breast cancer therapy and cardiovascular injury. J Am Coll Cardiol. 2007;50:1435-1441. [DOI] [PubMed] [Google Scholar]
- 18. Jones LW, Eves ND, Haykowsky M, Freedland SJ, Mackey JR. Exercise intolerance in cancer and the role of exercise therapy to reverse dysfunction. Lancet Oncol. 2009;10:598-605. [DOI] [PubMed] [Google Scholar]
- 19. Boxer RS, Kenny AM, Dowsett R, Taxel P. The effect of 6 months of androgen deprivation therapy on muscle and fat mass in older men with localized prostate cancer. Aging Male. 2005;8:207-212. [DOI] [PubMed] [Google Scholar]
- 20. Bylow K, Mohile SG, Stadler WM, Dale W. Does androgen-deprivation therapy accelerate the development of frailty in older men with prostate cancer? A conceptual review. Cancer. 2007;110:2604-2613. [DOI] [PubMed] [Google Scholar]
- 21. Bylow K, Dale W, Mustian K, et al. Falls and physical performance deficits in older patients with prostate cancer undergoing androgen deprivation therapy. Urology. 2008;72:422-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Segal RJ, Reid RD, Courneya KS, et al. Resistance exercise in men receiving androgen deprivation therapy for prostate cancer. J Clin Oncol. 2003;21:1653-1659. [DOI] [PubMed] [Google Scholar]
- 23. Bourke L, Smith D, Steed L, et al. Exercise for men with prostate cancer: a systematic review and meta-analysis. Eur Urol. 2018;69:693-703. [DOI] [PubMed] [Google Scholar]
- 24. Klepin HD, Danhauer SC, Tooze JA, Stott K. Exercise for older adult inpatients with acute myelogenous leukemia: a pilot study. J Geriatr Oncol. 2011;2:11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alibhai SMH, O’Neill S, Fisher-Schlombs K, et al. A clinical trial of supervised exercise for adult inpatients with acute myeloid leukemia (AML) undergoing induction chemotherapy. Leuk Res. 2012;36:1255-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lynch BM, Dunstan DW, Healy GN, Winkler E, Eakin E, Owen N. Objectively measured physical activity and sedentary time of breast cancer survivors, and associations with adiposity: findings from NHANES (2003-2006). Cancer Causes Control. 2010;21:283-288. [DOI] [PubMed] [Google Scholar]
- 27. Nelson SH, Marinac CR, Patterson RE, et al. Impact of very low physical activity, BMI, and comorbidities on mortality among breast cancer survivors. Breast Cancer Res Treat. 2018;155:551-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Campbell PT, Patel AV, Newton CC, Jacobs EJ, Gapstur SM. Associations of recreational physical activity and leisure time spent sitting with colorectal cancer survival. J Clin Oncol. 2013;31:876-885. [DOI] [PubMed] [Google Scholar]
- 29. Lynch BM, Dunstan DW, Vallance JK, Owen N. Don’t take cancer sitting down. Cancer. 2013;119:1928-1935. [DOI] [PubMed] [Google Scholar]
- 30. Bellizzi KM, Rowland JH, Jeffery DD, McNeel T. Health behaviors of cancer survivors: examining opportunities for cancer control intervention. J Clin Oncol. 2005;23:8884-8893. [DOI] [PubMed] [Google Scholar]
- 31. Mowls DS, Brame LS, Martinez SA, Beebe LA. Lifestyle behaviors among US cancer survivors. J Cancer Surviv. 2018;10:692-698. [DOI] [PubMed] [Google Scholar]
- 32. Phillips SM, Dodd KW, Steeves J, McClain J, Alfano CM, McAuley E. Physical activity and sedentary behavior in breast cancer survivors: new insight into activity patterns and potential intervention targets. Gynecol Oncol. 2015;138:398-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nicklas BJ, Gaukstern JE, Beavers KM, Newman JC, Leng X, Rejeski WJ. Self-monitoring of spontaneous physical activity and sedentary behavior to prevent weight regain in older adults. Obesity (Silver Spring). 2014;22:1406-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Smith JE, Richardson J, Hoffman C, Pilkington K. Mindfulness-based stress reduction as supportive therapy in cancer care: systematic review. J Adv Nurs. 2005;52:315-327. [DOI] [PubMed] [Google Scholar]
- 35. Baer RA, Smith GT, Allen KB. Assessment of mindfulness by self-report: The Kentucky inventory of mindfulness skills. Assessment. 2004;11:191-206. [DOI] [PubMed] [Google Scholar]
- 36. Shennan C, Payne S, Fenlon D. What is the evidence for the use of mindfulness-based interventions in cancer care? A review. Psychooncology. 2011;20:681-697. [DOI] [PubMed] [Google Scholar]
- 37. Zainal NZ, Booth S, Huppert FA. The efficacy of mindfulness-based stress reduction on mental health of breast cancer patients: a meta-analysis. Psychooncology. 2013;22:1457-1465. [DOI] [PubMed] [Google Scholar]
- 38. Cramer H, Lauche R, Paul A, Dobos G. Mindfulness-based stress reduction for breast cancer-a systematic review and meta-analysis. Curr Oncol. 2012;19:e343-e352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Piet J, Wurtzen H, Zachariae R. The effect of mindfulness-based therapy on symptoms of anxiety and depression in adult cancer patients and survivors: a systematic review and meta-analysis. J Consult Clin Psychol. 2012;80:1007-1020. [DOI] [PubMed] [Google Scholar]
- 40. Hoffman CJ, Ersser SJ, Hopkinson JB, Nicholls PG, Harrington JE, Thomas PW. Effectiveness of mindfulness-based stress reduction in mood, breast- and endocrine-related quality of life, and well-being in stage 0 to III breast cancer: a randomized, controlled trial. J Clin Oncol. 2012;30:1335-1342. [DOI] [PubMed] [Google Scholar]
- 41. Branstrom R, Kvillemo P, Moskowitz JT. A randomized study of the effects of mindfulness training on psychological well-being and symptoms of stress in patients treated for cancer at 6-month follow-up. Int J Behav Med. 2012;19:535-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McNeely ML, Campbell KL, Rowe BH, Klassen TP, Mackey JR, Courneya KS. Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. Can Med Assoc J. 2006;175:34-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Carlson LE, Doll R, Stephen J, et al. Randomized controlled trial of mindfulness-based cancer recovery versus supportive expressive group therapy for distressed survivors of breast cancer. J Clin Oncol. 2013;31:3119-3126. [DOI] [PubMed] [Google Scholar]
- 44. Carlson LE, Beattie TL, Giese-Davis J, et al. Mindfulness-based cancer recovery and supportive-expressive therapy maintain telomere length relative to controls in distressed breast cancer survivors. Cancer. 2015;121:476-484. [DOI] [PubMed] [Google Scholar]
- 45. Carlson LE. Mindfulness-based interventions for coping with cancer. Ann N Y Acad Sci. 2018;1373:5-12. [DOI] [PubMed] [Google Scholar]
- 46. Siegel D. Mindsight. New York, NY: Bantam Books; 2010. [Google Scholar]
- 47. Rejeski WJ, Gauvin L. The embodied and relational nature of the mind: implications for clinical interventions in aging individuals and populations. Clin Interv Aging. 2013;8:657-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chen YF, Huang XY, Chien CH, Cheng JF. The effectiveness of diaphragmatic breathing relaxation training for reducing anxiety. Perspect Psychiatr Care. 2017;53:329–336. [DOI] [PubMed] [Google Scholar]
- 49. Friedrich MJ. Unraveling the influence of gut microbes on the mind. JAMA. 2015;313:1699-1701. [DOI] [PubMed] [Google Scholar]
- 50. Brown KW, Ryan RA, Creswell JD. Mindfulness: theoretical foundations and evidence for its salutary effects. Psychol Inq. 2007;18:211-237. [Google Scholar]
- 51. Kabat-Zinn J. Full Catastrophe Living. New York, NY: Dell Publishing; 1990. [Google Scholar]
- 52. Dahl CJ, Lutz A, Davidson RJ. Reconstructing and deconstructing the self: cognitive mechanisms in meditation practice. Trends Cogn Sci. 2015;19:515-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Marchand WR. Neural mechanisms of mindfulness and meditation: evidence from neuroimaging studies. World J Radiol. 2014;6:471-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Siegel D. The Mindful Brain. New York, NY: W W Norton; 2007. [Google Scholar]
- 55. McGilchrist I. The Master and His Emissary: The Divided Brain and the Making of the Western World. New Haven, CT: Yale University Press; 2009. [Google Scholar]
- 56. Fredrickson BL. The role of positive emotions in positive psychology: the broaden-and-build theory of positive emotions. Am Psychol. 2001;56:218-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kok BE, Fredrickson BL. Upward spirals of the heart: autonomic flexibility, as indexed by vagal tone, reciprocally and prospectively predicts positive emotions and social connectedness. Biol Psychol. 2010;85:432-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fogel A. The Psychophysiology of Self-awareness. New York, NY: W W Norton; 2009. [Google Scholar]
- 59. Hewitt M, Rowland JH, Yancik R. Cancer survivors in the United States: age, health, and disability. J Gerontol A Biol Sci Med Sci. 2003;58:82-91. [DOI] [PubMed] [Google Scholar]
- 60. Li C, Zhou C, Li R. Can exercise ameliorate aromatase inhibitor-induced cognitive decline in breast cancer patients? Mol Neurobiol. 2018;53:4238-4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cormie P, Nowak AK, Chambers SK, Galvao DA, Newton RU. The potential role of exercise in neuro-oncology. Front Oncol. 2015;5:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Healy GN, Matthews CE, Dunstan DW, Winkler EA, Owen N. Sedentary time and cardio-metabolic biomarkers in US adults: NHANES 2003-06. Eur Heart J. 2011;32:590-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Edwardson CL, Gorely T, Davies MJ, et al. Association of sedentary behaviour with metabolic syndrome: a meta-analysis. PLoS One. 2012;7:e34916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Santos DA, Silva AM, Baptista F, et al. Sedentary behavior and physical activity are independently related to functional fitness in older adults. Exp Gerontol. 2012;47:908-912. [DOI] [PubMed] [Google Scholar]
- 65. Wilmot EG, Edwardson CL, Achana FA, et al. Sedentary time in adults and the association with diabetes, cardiovascular disease and death: systematic review and meta-analysis. Diabetologia. 2012;55:2895-2905. [DOI] [PubMed] [Google Scholar]
- 66. Rothman AJ. Toward a theory-based analysis of behavioral maintenance. Health Psychol. 2000;19(1, suppl):64-69. [DOI] [PubMed] [Google Scholar]
- 67. Falk EB, O’Donnell MB, Cascio CN, et al. Self-affirmation alters the brain’s response to health messages and subsequent behavior change. Proc Natl Acad Sci U S A. 2015;112:1977-1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tudage MM FB, Barrett LF. Psychological resilence and positive emotional granularity: examining the benefits of positive emotions on coping and health. J Pers Assess. 2004;72:1161-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Brawley LR, Rejeski WJ, Lutes L. A group-mediated cognitive-behavioral intervention for increasing adherence to physical activity in older adults. J Appl Biobehav Res. 2000;5:47-65. [Google Scholar]
- 70. Rejeski WJ, Brawley LR, Ambrosius WT, et al. Older adults with chronic disease: benefits of group-mediated counseling in the promotion of physically active lifestyles. Health Psychol. 2003;22:414-423. [DOI] [PubMed] [Google Scholar]
- 71. Rejeski WJ, Brubaker PH, Goff DC, et al. Translating weight loss and physical activity programs into the community to preserve mobility in older, obese adults in poor cardiovascular health. Arch Intern Med. 2011;171:880-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. McDermott MM, Domanchuk K, Liu K, et al. The Group Oriented Arterial Leg Study (GOALS) to improve walking performance in patients with peripheral arterial disease. Contemp Clin Trials. 2012;33:1311-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Rejeski WJ, Marsh AP, Chmelo E, et al. The Lifestyle Interventions and Independence for Elders Pilot (LIFE-P): 2-year follow-up. J Gerontol A Biol Sci Med Sci. 2009;64:462-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Marsh AP, Janssen JA, Ambrosius WT, et al. The Cooperative Lifestyle Intervention Program-II (CLIP-II): design and methods. Contemp Clin Trials. 2013;36:382-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Neff K. Self-compassion: an alternative conceptualization of a healthy attitude toward oneself. Self Identity. 2003;2:85-101. [Google Scholar]
- 76. Neff KD, Kirkpatrick KL, Rude SS. Self-compassion and adaptive psychological functioning. J Res Pers. 2007;41:139-154. [Google Scholar]
- 77. Rejeski WJ, Spring B, Domanchuk K, et al. A group-mediated, home-based physical activity intervention for patients with peripheral artery disease: effects on social and psychological function. J Transl Med. 2014;12:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Focht BC, Lucas AR, Grainger E, Simpson C, Thomas-Ahner JM, Clinton SK. The Individualized Diet and Exercise Adherence Pilot Trial (IDEA-P) in prostate cancer patients undergoing androgen deprivation therapy: study protocol for a randomized controlled trial. Trials. 2014;15:354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Byrne EA, Fleg JL, Vaitkevicius PV, Wright J, Porges SW. Role of aerobic capacity and body mass index in the age-associated decline in heart rate variability. J Appl Physiol. 1996;81:743-750. [DOI] [PubMed] [Google Scholar]
- 80. Kok BE, Fredrickson BL. Upward spirals of the heart: autonomic flexibility, as indexed by vagal tone, reciprocally and prospectively predicts positive emotions and social connectedness. Biol Psychol. 2010;85:432-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Cozolino L. The Neuroscience of Psychotherapy: Healing the Social Brain (Norton Series on Interpersonal Neurobiology). New York, NY: W W Norton; 2010. [Google Scholar]
- 82. Sapolsky RM, Krey LC, McEwen BS. Prolonged glucocorticoid exposure reduces hippocampal neuron number: implications for aging. J Neurosci. 1985;5:1222-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ledoux JE. Emotion, memory and the brain. Sci Am. 1994;270(6):50-57. [DOI] [PubMed] [Google Scholar]
- 84. Bandura A. Self-efficacy mechanism in physiological activation and health-promoting behavior. In: Madden J. ed. Neurobiology of Learning, Emotion, and Affect. New York, NY: Raven; 1991:229-269. [Google Scholar]
- 85. Carlson LE. Mindfulness-based interventions for physical conditions: a narrative review evaluating levels of evidence. ISRN Psychiatry. 2012;2012:651583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Tang YY, Ma Y, Fan Y, et al. Central and autonomic nervous system interaction is altered by short-term meditation. Proc Natl Acad Sci U S A. 2009;106:8865-8870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wu SD, Lo PC. Inward-attention meditation increases parasympathetic activity: a study based on heart rate variability. Biomed Res. 2008;29:245-250. [DOI] [PubMed] [Google Scholar]
- 88. Morris BA, Shakespeare-Finch J, Scott JL. Posttraumatic growth after cancer: the importance of health-related benefits and newfound compassion for others. Support Care Cancer. 2012;20:749-756. [DOI] [PubMed] [Google Scholar]
- 89. Danhauer SC, Russell G, Case LD, et al. Trajectories of posttraumatic growth and associated characteristics in women with breast cancer. Ann Behav Med. 2015;49:650-659. [DOI] [PMC free article] [PubMed] [Google Scholar]