Abstract
Objective
To assess whether a shared decision-making intervention decreases the quantity of oxycodone tablets prescribed after cesarean delivery.
Technique
A tablet computer-based decision aid formed the basis of a shared decision-making session to guide opioid prescribing after cesarean delivery. Women first received information on typical trajectories of pain resolution and expected opioid use after cesarean delivery, and then chose the number of tablets of oxycodone 5mg they would be prescribed, up to the institutional standard prescription of 40 tablets.
Experience
From April 11, 2016, to June 10, 2016, 105 women were screened, 75 were eligible, and 51 consented to participate; one patient was excluded after enrollment due to prolonged hospitalization. The median number of tablets (oxycodone 5 mg) women chose for their prescription was 20.0 [interquartile range of 15.0, 25.0], which was less than the standard 40-tablet prescription (p<0.001).
Conclusion
A shared decision-making approach to opioid prescribing after cesarean delivery was associated with approximately a 50% decrease in the number of opioids prescribed postoperatively in this cohort compared with our institutional standard prescription. This approach is a promising strategy to reduce the amount of leftover opioid medication after treatment of acute postcesarean pain.
Clinical Trial Registration
NCT02770612 at clinicaltrials.gov
Introduction
Cesarean delivery is the most common inpatient surgical procedure in the United States, and prescription opioids are one of the mainstays of pain management after discharge [1]. Survey data suggest that the amount of prescription opioid dispensed after cesarean delivery frequently exceeds what women use by a significant margin, leading to large amounts of leftover opioid medication [2]. Leftover opioids from legitimate prescriptions represent a primary source of misused or diverted opioids [3–5]. Strategies to align the number of prescription opioids dispensed with the amount used for acute indications are needed in order to reduce the quantity of leftover opioids introduced into communities.
Over the past two decades, shared decision-making has been demonstrated to improve outcomes and patient satisfaction in a variety of clinical settings [6–9]. We sought to assess the effects of an interactive shared decision-making session, based on a decision aid delivered by a tablet computer, on women’s choice regarding the number of oxycodone 5 mg tablets they would be prescribed at discharge after cesarean delivery. We hypothesized that this intervention would decrease the amount of opioids prescribed, as compared to our institution’s standard, while maintaining effective post-cesarean delivery pain management.
Technique
After approval from the Partners Institutional Review Board, we reviewed medical charts of women undergoing cesarean delivery daily (on the day prior to hospital discharge) to identify eligible patients. Women with a history of chronic pain or chronic opioid use, including methadone or buprenorphine were not eligible for participation. Additional exclusion criteria were age <18 years, non-English speaking, post-operative hospitalization >7 days, use of oral opioids other than oxycodone post-operatively, and contraindications to acetaminophen or non-steroidal anti-inflammatory drugs.
After providing written informed consent, women participated in an approximately 10 minute shared decision-making session in which a clinician (obstetrician (MP) or anesthesiologist (EMH)) reviewed information verbally while the participants viewed a tablet computer-based decision aid [Appendix 1, available online at http://links.lww.com/xxx]. The clinicians who facilitated the sessions followed a script and observed each other to ensure consistency in presentation. The decision aid was developed by the study investigators based on the standards of the International Patient Decision Aid Standards Collaboration [10] and included (1) information on anticipated patterns of pain in the first 2 weeks after cesarean delivery; (2) expected outpatient opioid use after cesarean delivery [2]; (3) risks and benefits of opioid and non-opioid analgesics; and (4) information on opioid disposal and access to refills if needed. At the conclusion of the session, participants chose the number of tablets (oxycodone 5 mg) they would be prescribed upon discharge, from 0 to 40 tablets; 40 tablets was the standard number of tablets prescribed by obstetric providers at our institution at the time of the study. The number of oxycodone tablets prescribed was confirmed by chart review. Two weeks after enrollment, telephone follow-up was performed by one of two investigators (MP and EMH).
As obtaining additional opioid after discharge requires returning to the hospital or clinic to obtain a new prescription, our primary safety concern was ensuring that participants were provided adequate pain control. We planned an a priori interim analysis with follow-up data on 25 patients to assess the need for refills, with a plan to modify the study procedure if more than 50% of patients required an additional prescription after discharge. At the interim analysis, 4 (16%) of patients required an additional prescription, and no changes were made to the study methodology.
Demographic information and medical history were abstracted from the medical record. Study data were collected and managed using REDCap, which is a secure, web-based application designed to support data capture for research studies [11].
Data were analyzed in Stata 14. We used summary statistics to describe study participants and performed a one-sided, one-sample Wilcoxon test comparing the median [interquartile range, IQR] number of oxycodone tablets chosen and prescribed to the institutional standard of 40 tablets. The sample size of 50 women was based on 90% power to show a reduction in the mean oxycodone prescription from 40 to 35, assuming a standard deviation (SD) of 12 and alpha=0.05. The study was registered with Clinicaltrials.gov, NCT02770612.
Experience
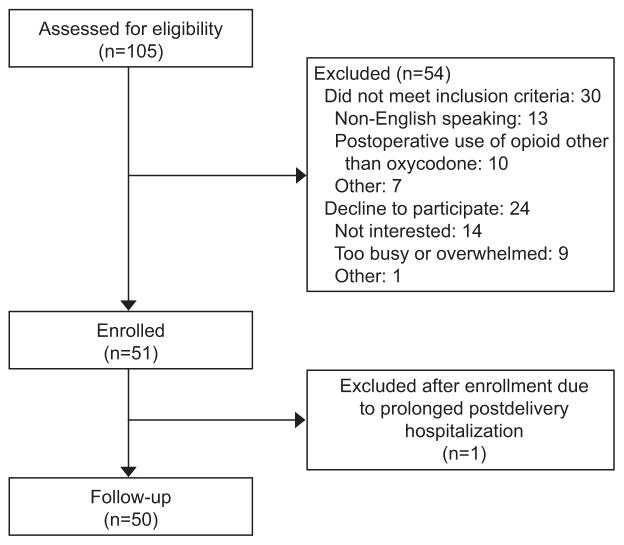
From April 11, 2016 to June 10, 2016, we assessed 105 women for eligibility: 30 (28.6%) were ineligible for the study and 24 (22.9%) declined participation (Figure 1). One woman was initially enrolled but was subsequently excluded because she had a prolonged postdelivery hospitalization. Of the remaining 50 women enrolled, none were lost to follow-up. The median maternal age of participants was 33 years, and consistent with the patient population at our institution, the majority were white, privately insured, and healthy (Table 1). Seventy percent of participants underwent a primary cesarean delivery, and median [IQR] postoperative length of stay was 4.0 [3.8, 4.4] days.
Figure 1.
Study flowchart
Table 1.
Baseline characteristics of participants
| Patient Demographics | Median [IQR], or n (%) |
|---|---|
| Age, median [IQR] years | 33.0 [29.0, 37.0] |
| Nulliparous, n(%) | 26 (52.0%) |
| Race/Ethnicity, n(%) | |
| White | 27 (54.0) |
| Black | 0 (0) |
| Hispanic | 8 (16.0) |
| Asian | 14 (28.0) |
| Other | 1 (2.0) |
| Insurance, n(%) | |
| Private | 36 (72.0) |
| Medicaid | 14 (28.0) |
| Current non-opioid drug abuse, n(%) | 1 (2.0) |
| Current alcohol/tobacco use, n(%) | 2 (4.0) |
| Cesarean Delivery Characteristics | |
| Type, n(%) | |
| Primary cesarean delivery | 35 (70.0) |
| Repeat cesarean delivery | 15 (30.0) |
| Planned, n(%) | |
| Planned cesarean delivery | 19 (38.0) |
| Unplanned cesarean delivery | 31 (62.0) |
| Anesthetic mode, n(%) | |
| Spinal/combined spinal-epidural | 19 (38.0) |
| Epidural | 29 (58.0) |
| General | 2 (4.0) |
| Use of neuraxial morphine, n(%) | 46 (92.0) |
| Post-operative length of stay, median [IQR] days | 4.0 [3.8, 4.4] |
| Post-operative Pain Characteristics | |
| Pain scores with activity, median [IQR] | |
| Post-op day 1 | 7.0 [5.0, 8.5] |
| Post-op day 2 | 5.0 [4.0, 6.0] |
| Post-op day 3 | 4.0 [3.0, 5.0] |
IQR = interquartile range
The median [IQR] number of oxycodone 5mg tablets selected was 20.0 [15.0, 25.0] oxycodone 5 mg tablets, which was lower than the 40 tablets usually prescribed (p<0.001) (Table 2). For 6 patients, the number of tablets prescribed was different from the number chosen by the patients, such that the overall median [IQR] number of tablets dispensed was 20.0 [20.0, 30.0] (p<0.001 compared to 40). Whether this was a deliberate choice based on a clinical judgment or simply accidental non-compliance with the study protocol is not known. The median [IQR] oxycodone used was 15.5 [8.0, 25.0] tablets over the first two weeks after discharge, and the median [IQR] number of leftover oxycodone tablets was 4.0 [0.0, 8.0].
Table 2.
Oxycodone utilization after discharge and satisfaction with pain regimen
| Outcomes | Median [IQR] or n (%) |
|---|---|
| Number of oxycodone tablets chosen* | 20.0 [15.0, 25.0] |
| Number of oxycodone tablets used | 15.5 [8.0, 25.0] |
| Number of oxycodone tablets remaining | 4.0 [0.0, 8.0] |
| Need for oxycodone refills | 4 (8.0) |
| Satisfied with outpatient pain management | 26 (52.0) |
| Very satisfied with outpatient pain management | 19 (38.0) |
IQR = interquartile range
For 6 patients, the number of tablets prescribed was different from the number chosen such that the median [IQR] number of tablets dispensed was 20.0 [20.0, 30.0]
Overall, 26 (52.0%) women were satisfied and 19 (38.0%) women were very satisfied with pain management. Forty-three (86.0%) women found the intervention valuable to their post-operative care. These women were asked about which elements of the intervention they found helpful (response choices were not mutually exclusive); 20 (46.5%) cited the education regarding expectations for their outpatient course and 19 (44.2%) cited the education surrounding the risks and benefits of oxycodone.
Four women (8.0%) required refills of oxycodone; their initial choice for oxycodone prescription was 5, 30, 30, and 40 tablets. Three of these women had complications that likely resulted in greater than usual postoperative pain and one patient had continued post-operative pain for which the initial prescription was inadequate. These patients were prescribed between 20 and 30 additional tablets of oxycodone.
Among the 32 participants with unused oxycodone tablets who did not receive a refill, and were not using oxycodone at the time of follow-up, plans for disposal included: flushing the pills [11 (34.3%)], returning pills to a disposal station [10 (31.3%)], and no plan [11 (34.3%)].
Additional analyses of oxycodone tablets chosen, used, and remaining, stratified by type of cesarean delivery (primary versus repeat) and timing (planned versus unplanned) are shown in Appendix 2, available online at http://links.lww.com/xxx.
Discussion
The use of a shared decision-making intervention to optimize post-cesarean delivery pain management was associated with a reduction in the number of prescribed oxycodone tablets by approximately half among patients who participated in the study, compared to our institution’s standard. The refill rate was low, and 90% of participants reported being satisfied or very satisfied with their outpatient pain management. Use of this approach to guide opioid prescribing after cesarean delivery represents a novel strategy to reduce the amount of leftover opioid available for misuse and diversion without compromising the effective treatment of pain.
Our study’s strengths include the application of a multifaceted shared decision-making intervention to acute pain management after cesarean delivery, the most commonly performed inpatient surgical procedure in the United States [1]. In addition to decreasing the median number of oxycodone tablets prescribed, two-thirds of patients had plans to dispose of unused opioids. Of note, flushing opioids was included as a potential disposal strategy, as this is endorsed by the U.S. Food and Drug Administration as reasonable in order to permanently remove potentially harmful medications from the community. However, the U.S. Environmental Protection Agency recommends the disposal of medications by other methods, due to potential concerns for the safety of the water supply [12–13].
Our pilot study is subject to limitations inherent in its design. We cannot isolate the influence of each component within our intervention, including the relative impact of the contents of the decision aid, the time spent with an obstetrician or anesthesiologist, or the patient engagement in the decision on their satisfaction. Immediately after the session, 79% of patients noted either improved expectations regarding their outpatient post-operative course or education about pharmacologic options for pain management, suggesting that perhaps education was a key factor driving the choice for a lower number of oxycodone tablets. Future studies testing the individual components of the shared decision-making intervention and incorporating measures of decision quality are needed to establish which components are most effective. Also, while the frequency of refill was low and satisfaction scores high, future randomized evaluations with a control group receiving routine care will be necessary to fully define the impact of this approach on these measures. Also of note, one-third of eligible patients declined participation. Nonparticipation may influence generalizability of our findings; since we did not have IRB-approval to conduct chart reviews once women declined participation, we cannot comment on the characteristics of this population or their motivations for declining beyond what they reported. Additional studies are needed to establish the feasibility of these approaches in clinical settings with variable resources and diverse patient populations to determine generalizability and identify strategies to maximize participation. However, if the consent rate and proportion of patients excluded is generalizable, this intervention would be expected to result in a 25% overall reduction in the number of opioid tablets dispensed after cesarean delivery.
In conclusion, our study demonstrates that shared decision-making is a promising strategy to align opioid prescribing with patient needs after cesarean delivery, and thus may reduce the number of unused opioid tablets in the community while still ensuring adequate pain control and patient satisfaction.
Supplementary Material
Acknowledgments
Brian T. Bateman is supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the NIH (Bethesda, Maryland, United States) under Award Number K08HD075831.
Footnotes
Financial Disclosure
Brian T. Bateman is an investigator on grants to his institution from Lilly, Pfizer, Baxalta, GSK, and Pacira. Malavika Prabhu is an investigator on a grant to her institution from Pacira. The other authors did not report any potential conflicts of interest.
Each author has indicated that he or she has met the journal’s requirements for authorship.
Presented at the Society for Maternal-Fetal Medicine, Las Vegas, Nevada January 28, 2017 and the Society for Obstetric Anesthesia and Perinatology Annual Meeting, Seattle, Washington May 10–14, 2017.
Contributor Information
Malavika Prabhu, Division of Maternal-Fetal Medicine, Department of Obstetrics and Gynecology, Massachusetts General Hospital, Boston, MA.
Emily McQuaid-Hanson, Division of Obstetric Anesthesia, Department of Anesthesia, Critical Care, and Pain Medicine, Massachusetts General Hospital, Boston, MA.
Stephanie Hopp, Division of Obstetric Anesthesia, Department of Anesthesia, Critical Care, and Pain Medicine, Massachusetts General Hospital, Boston, MA. Current affiliation: Alabama College of Osteopathic Medicine.
Sara M. Burns, Department of Anesthesia, Critical Care, and Pain Medicine, Massachusetts General Hospital, Boston, MA.
Lisa R. Leffert, Division of Obstetric Anesthesia, Department of Anesthesia, Critical Care, and Pain Medicine, Massachusetts General Hospital, Boston, MA.
Ruth Landau, Division of Obstetric Anesthesia, Center for Precision Medicine in Anesthesiology, Department of Anesthesiology, Columbia University Medical Center, New York City, NY.
Julie C. Lauffenburger, Division of Pharmacoepidemiology and Pharmacoeconomics and Center for Healthcare Delivery Sciences, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA.
Niteesh K. Choudhry, Division of Pharmacoepidemiology and Pharmacoeconomics and Center for Healthcare Delivery Sciences, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA.
Anjali Kaimal, Division of Maternal-Fetal Medicine, Department of Obstetrics and Gynecology, Massachusetts General Hospital, Boston, MA.
Brian T. Bateman, Division of Pharmacoepidemiology and Pharmacoeconomics, Department of Medicine, Brigham and Women’s Hospital, and The Department of Anesthesiology, Perioperative and Pain Medicine, Brigham and Women’s Hospital, Boston, MA.
References
- 1.Hamilton BE, Martin JA, Osterman MJ, Curtin SC, Matthews TJ. Births: Final data for 2014. Natl Vital Stat Rep. 2015;64:1. [PubMed] [Google Scholar]
- 2.Bateman BT, Huybrechts KF, Booth J, Briggs H, Flood P, Bauer M, Landau R. Opioid Use Following Discharge After Cesarean Delivery. Pharmacoepidemiology and drug safety; Abstracts of the 32nd International Conference on Pharmacoepidemiology & Therapeutic Risk Management; August 25–28, 2016; Dublin, Ireland: The Convention Centre Dublin; 2016. pp. 3–680. [DOI] [Google Scholar]
- 3.Inciardi JA, Surratt HL, Cicero TJ, Beard RA. Prescription opioid abuse and diversion in an urban community: the result of an ultrarapid assessment. Pain Med. 2009;10:537–48. doi: 10.1111/j.1526-4637.2009.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCabe SE, West BT, Teter CJ, Boyd CJ. Medical and nonmedical use of prescription opioids among high school seniors in the United States. Arch Pediatr Adolesc Med. 2012;166:797–802. doi: 10.1001/archpediatrics.2012.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kennedy-Hendricks A, Gielen A, McDonald E, McGinty EE, Shields W, Barry CL. Medication sharing, storage, and disposal practices for opioid medications among US adults. JAMA Intern Med. 2016;176:1027. doi: 10.1001/jamainternmed.2016.2543. [DOI] [PubMed] [Google Scholar]
- 6.Barry MJ, Edgman-Levitan S. Shared decision making--pinnacle of patient-centered care. N Engl J Med. 2012;366:780–1. doi: 10.1056/NEJMp1109283. [DOI] [PubMed] [Google Scholar]
- 7.Clever SL, Ford DE, Rubenstein LV, Rost KM, Meredith LS, Sherbourne CD, et al. Primary care patients’ involvement in decision-making is associated with improvement in depression. Med care. 2006;44:398–405. doi: 10.1097/01.mlr.0000208117.15531.da. [DOI] [PubMed] [Google Scholar]
- 8.Loh A, Leonhart R, Wills CE, Simon D, Harter M. The impact of patient participation on adherence and clinical outcome in primary care of depression. Patient Educ Couns. 2007;65:69–78. doi: 10.1016/j.pec.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Loh A, Simon D, Wills CE, Kriston L, Biebling W, Harter M. The effects of a shared decision-making intervention in primary care of depression: a cluster randomized controlled trial. Patient Educ Couns. 2007;67:324–32. doi: 10.1016/j.pec.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 10.International Patient Decision Aid Standards Collaboration. [Accessed January 10, 2016];Criteria for judging the quality of patient decision aids. http://www.ipdas.ohri.ca/IPDAS_checklist.pdf.
- 11.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Food and Drug Administration. [Accessed 19 March 2017];Disposal of Unused Medicines: What You Should Know. https://www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicineSafely/EnsuringSafeUseofMedicine/SafeDisposalofMedicines/ucm186187.htm#Flush_List.
- 13.Environmental Protection Agency. [Accessed 19 March 2017];How to Dispose of Drugs Safely. https://www.epa.gov/sites/production/files/2015-06/documents/how-to-dispose-medicines.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.