Abstract
Although numerous studies have implicated Akt and Src kinases in vascular endothelial growth factor (VEGF) and Angiopoietin-1 (Ang-1)-induced endothelial-barrier regulation, a link between these two pathways has never been demonstrated. We determined the long-term effects of Akt inhibition on Src activity and vice versa, and in turn, on the human microvascular endothelial cell (HMEC) barrier integrity at the basal level, and in response to growth factors. Our data showed that Akt1 gene knockdown increases gap formation in HMEC monolayer at the basal level. Pharmacological inhibition of Akt, but not Src resulted in exacerbated VEGF-induced vascular leakage and impaired Ang-1-induced HMEC-barrier protection in-vitro at 24 hours. Whereas inhibition of Akt had no effect on VEGF-induced HMEC gap formation in the short term, inhibition of Src blunted this process. In contrast, inhibition of Akt disrupted the VEGF and Ang-1 stabilized barrier integrity in the long-term while inhibition of Src did not. Interestingly, both long-term Akt inhibition and Akt1 gene knockdown in HMECs resulted in increased Tyr416 phosphorylation of Src. Treatment of HMECs with transforming growth factor-β1 (TGFβ1) that inhibited Akt Ser473 phosphorylation in the long-term, activated Src through increased Tyr416 phosphorylation and decreased HMEC-barrier resistance. The effect of TGFβ1 on endothelial-barrier breakdown was blunted in Akt1 deficient HMEC monolayers, where endothelial-barrier resistance was already impaired compared to the control. To our knowledge, this is the first report demonstrating a direct cross-talk between Akt and Src in endothelial-barrier regulation.
Keywords: Akt, Src, vascular permeability, endothelial-barrier, VE-cadherin
Introduction
Vascular permeability is a complex yet a highly coordinated process that not only regulates vesicular trafficking but also integrates complex junction rearrangements, and refined cytoskeletal dynamics (Goddard and Iruela-Arispe 2013). The endothelium plays a key role in regulating vascular integrity. Recent studies suggest that impairment of endothelial function, as observed in the presence of cardiovascular risk factors, is not only a marker but also contributes to the pathogenesis of cardiovascular diseases (Landmesser, Hornig et al. 2004). Thus, improving endothelial function is an important therapeutic target for reducing vascular diseases (Bonetti, Lerman et al. 2003, Melo, Gnecchi et al. 2004). It is evident that Akt1 is highly involved in the vascular endothelial growth factor (VEGF)-mediated vascular permeability as the phosphorylation of Akt1 increases considerably in VEGF-stimulated endothelial cells (Chen, Somanath et al. 2005). However, since Akt1 is activated by not only vascular permeability-inducing agents such as VEGF and tumor necrosis factor-α (Fairaq, Goc et al. 2015, Gao, Artham et al. 2016) but also by agents that promote barrier integrity such as angiopoietin-1 (Ang-1), roundabout guidance receptor-4 and sphingosine-1-phosphate, the precise role of Akt1 in regulating vascular permeability was not clear until recently (Daly, Wong et al. 2004, De Palma, Meacci et al. 2006, Somanath, Kandel et al. 2007). Studies from our laboratory have demonstrated that Akt1 is important for vascular maturation and that suppression of Akt1 activity leads to increased vascular permeability (Chen, Somanath et al. 2005). There are also reports demonstrating the role of Akt1-FoxO signaling in the transcriptional up-regulation of claudin-5 as a result of vascular endothelial-cadherin (VE-cadherin) clustering at the endothelial junctions (Taddei, Giampietro et al. 2008) and in response to long-term treatments with VEGF and Ang-1 (Gao, Artham et al. 2016). Apart from Akt, Src family of non-receptor tyrosine kinases is well known for its contributions to the VEGF-induced vascular leakage (Ha, Bennett et al. 2008). Pathological levels of VEGF result in endothelial barrier breakdown via Src-mediated degradation of VE-cadherin (Gavard, Patel et al. 2008, Azzi, Hebda et al. 2013).
Although a lot has been reported about the involvement of Akt and Src in the modulation of growth factor-mediated endothelial-barrier integrity and vascular permeability, it is not clear until today whether there is any cross-talk between Akt and Src in mediating endothelial-barrier function. Given the importance of Akt and Src in endothelial function, survival, and barrier regulation, we wanted to study the effect of Akt inhibition on Src activity and vice versa, in turn, regulating the endothelial-barrier. Our results indicated that whereas Src, but not Akt is necessary for the early VEGF-induced HMEC-barrier breakdown, in the later stages, Akt, but not Src is responsible for the long-term HMEC-barrier stability offered by VEGF and Ang-1. Interestingly, whereas long-term pharmacological inhibition of Akt and ShRNA-mediated Akt1 gene knockdown in HMECs resulted in increased activating Src Tyr416 phosphorylation and increased HMEC-barrier breakdown, long-term treatment with transforming growth factor-β1 (TGFβ1) resulted in decreased Akt1 Ser473 phosphorylation, increased Src Tyr416 phosphorylation and increased HMEC-barrier breakdown. Effect of TGFβ1 on HMEC-barrier was blunted in Akt1 deficient HMEC monolayers apparently due to the already reduced Akt activity and increased Src activity in Akt1 deficient HMECs. Together, we show for the first time that Akt and Src maintain a reciprocal phosphorylation/activity pattern in HMECs and thus provide novel insights into the cross-talk between Akt and Src, and their interplay in the regulation of endothelial-barrier function.
Materials and Methods
Cell culture and preparation of ShAkt1 stable cell lines
Telomerase-immortalized HMECs (CRL-4025; ATCC, Manassas, VA) were maintained in EBM-2 with a Growth factor-2 Bullet Kit (Lonza; Walkersville, MD). Cultures were maintained in a humidified 5% CO2 incubator at 37 °C and routinely passaged when 80– 90% confluent.
Stable ShControl, ShAkt1 (sequence: ACGCTTAACCTTTCCGCTG) HMECs were generated using SMART vector 2.0 lentivirus particles (109 pfu) (Thermo Scientific, Waltham, MA). Lentiviral particles mixed in 1ml Hyclone SFM4Transfx-293 (Fisher, Hanover Park, IL) were added along with 1 μl Polybrene (10 mg/ml, American bioanalytical, Natick, MA). Three days later, transfection efficiency was tested through Turbo-GFP expression and subjected to 4 μg/ml Puromycin (Life Technologies, Grand Island, NY) selection until all the cells expressed GFP.
Measurement of endothelial-barrier resistance
Endothelial-barrier integrity was measured as the electrical resistance of the HMEC monolayer using ‘electric cell-substrate impedance sensing’ (ECIS) equipment (Applied Biophysics, NY) as described previously (Goc, Al-Azayzih et al. 2013, Gao, Al-Azayzih et al. 2015). To synchronize the HMECs before treatment, cells were cultured in serum-free EBM-2 for 5 hours, followed by treatment with either 20 ng/ml VEGF or 50 ng/ml Ang-1. Endothelial-barrier resistance was measured at multiple frequency modes for 24 hours. Growth factors for stimulation such as VEGF, Ang-1 and TGFβ1 were obtained from R&D Systems (Minneapolis, MN).
Immunofluorescence staining
Immunofluorescence staining of HMEC monolayers was performed using chamber slides (Fisher, Hanover Park, IL). Cells were cultured to monolayer, treated with either vector or 20 ng/ml VEGF or 50 ng/ml Ang-1 and co-treated with either 10 μM Triciribine (TCBN) or 1 μM pp-2 for 24 hours and washed twice with ice-cold PBS, fixed using 2% paraformaldehyde for 30 min, permeabilized with 0.1% Triton X-100 for 15 min, and blocked with 2% BSA in sterile PBS for 1 hour. Cell monolayers were then incubated with antibodies against VE-cadherin (1:100, Rabbit antibodies, Cell Signaling, Danvers, MA) at 4°C overnight. Immunofluorescence was revealed using goat anti-rabbit AlexaFlour-488 secondary antibodies (1:2000, Life Technologies, Grand Island, NY). Cells were mounted on to a glass slide using DAPI containing mounting medium (Vector Laboratories, PA). Images were captured using a confocal microscope equipped with argon and helium/neon lasers (LSM510, Zeiss, Germany). Controls were performed by omitting primary antibodies. All controls gave negative results with no detectable non-specific labeling.
Western blot analysis
Cell lysates were prepared using complete lysis buffer (EMD Millipore, San Diego, CA) with protease and phosphatase inhibitor cocktails (Roche Diagnostics, Indianapolis, IN). Protein quantification was performed using DC protein assay (Bio-Rad, Hercules, CA. Western blot analysis was performed as described previously (Goc, Al-Azayzih et al. 2013, Gao, Al-Azayzih et al. 2015). Antibodies used include Src Tyr416, total-Src, Akt Ser473, total-Akt (Cell Signaling, Danvers, MA) and anti- β-actin (Sigma, St. Louis, MO). Densitometry was done using NIH Image J software.
Statistical Analysis
All the data are presented as Mean ± SD and were calculated from multiple independent experiments performed in quadruplicates. For normalized data analysis, data was confirmed that normality assumption was satisfied and analyzed using paired sample t-test (dependent t-test) and/or further confirmed with non-parametric test Wilcoxon signed rank test. For all other analysis, Student’s two-tailed t-test or ANOVA test were used to determine significant differences between treatment and control values using the GraphPad Prism 4.03 and SPSS 17.0 software.
Results
Long-term Akt inhibition disrupts, but Src inhibition protects the endothelial-barrier
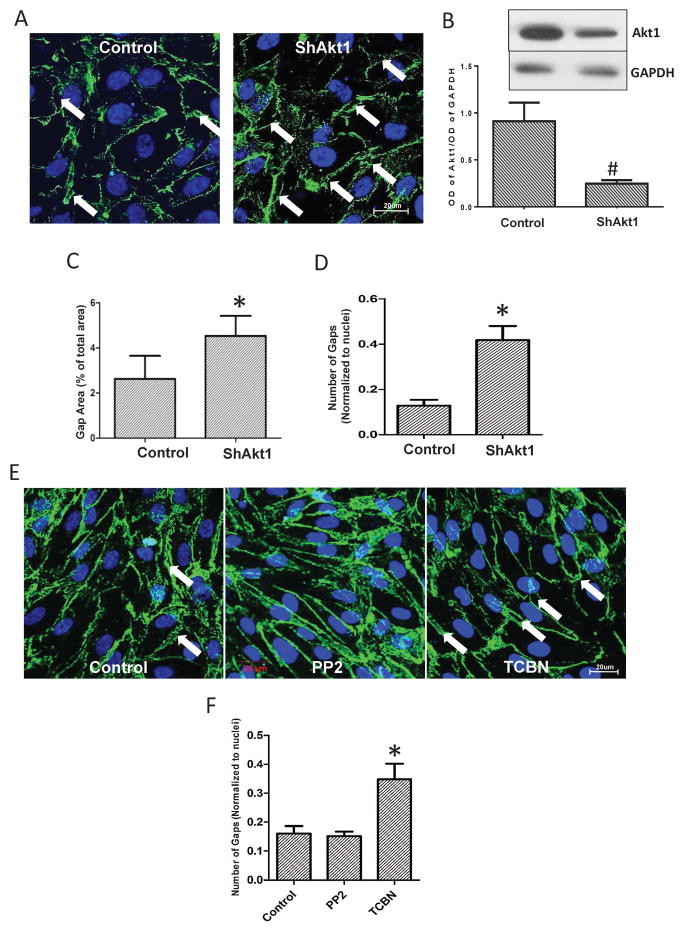
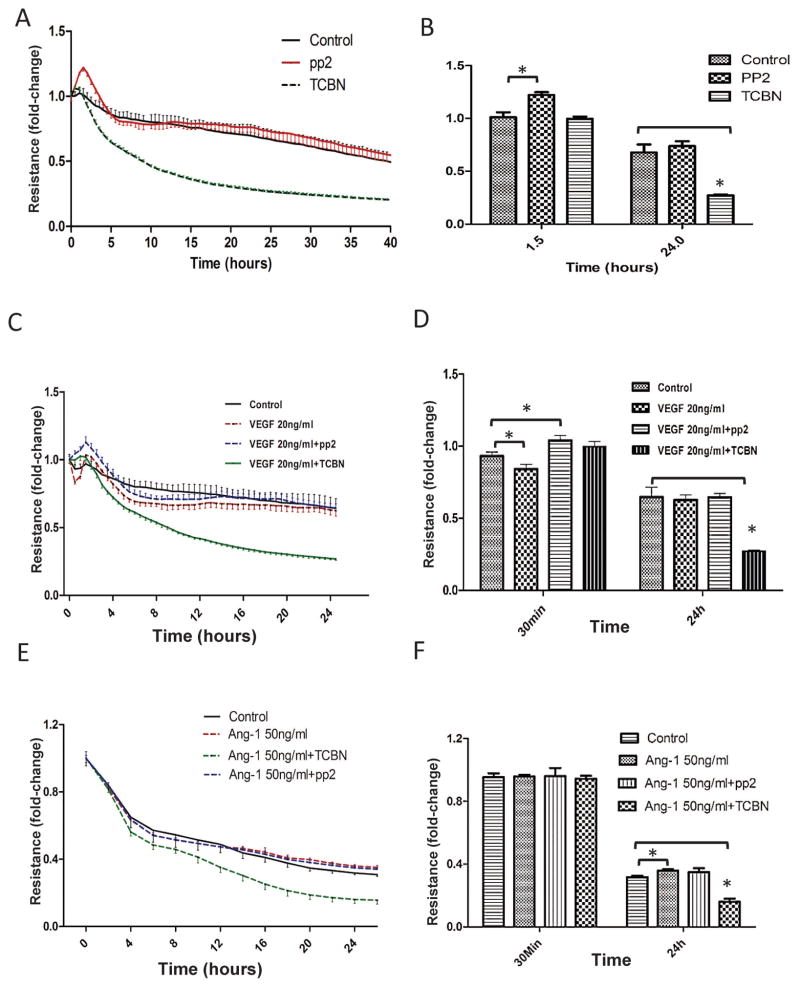
Whereas the role of Src in VE-cadherin internalization is well known, our immunocytochemistry analysis indicated that ShRNA-mediated Akt1 gene knockdown in HMEC monolayers results in increased gap formation, compared to ShConrol HMEC monolayers (Figure 1A–D). Whereas no significant difference was observed in the number of gaps in Src inhibitor (pp2) treated HMEC monolayers, treatment with Akt inhibitor TCBN resulted in increased number of gaps in the HMEC monolayers, compared to control (DMSO treated) monolayers (Figure 1E–F). A similar result on the Src and Akt inhibitor was also observed in another analysis on endothelial-barrier resistance as measured using the ECIS approach. Whereas Src inhibition, but not Akt inhibition promoted HMEC-barrier resistance at 90 minutes post treatment, Akt inhibition, but not Src inhibition reduced HMEC-barrier resistance in the long-term (24 hours) (Figure 2A–B). To explore the differential effects of Akt and Src on barrier permeability, HMEC monolayers were treated with either 20 ng/ml VEGF (Figure 2C–D) or 50 ng/ml Ang-1 (Figure 2E–F) and co-treated with either 10 μM TCBN, a pharmacological inhibitor of Akt or 1 μM pp2, a selective Src inhibitor. Barrier resistance was measured in real-time using ECIS assay for 24 hours. Inhibition of Akt, but not Src resulted in exacerbated VEGF-induced vascular leakage and reduced Ang-1 induced barrier protection in-vitro at 24 hours suggesting the vascular protective role of Akt in the long term.
Figure 1. Long-term Akt inhibition disrupts, but Src inhibition protects the endothelial-barrier.
(A) Representative confocal images showing immunofluorescence staining of VE-cadherin on HMEC monolayers transfected with either scrambled ShRNA or ShRNA targeting Akt1. (B) Representative Western blot images and band densitometry quantification of stable ShControl and ShAkt1 HMEC lysates showing reduced Akt1 expression in ShAkt1 HMEC compared to ShControl HMEC (n=3). (C) Quantification of the gap area in control and Akt1 knockdown HMEC monolayers normalized to the total area (n=4). (D) Quantification of the number of gaps in control and Akt1 knockdown HMEC monolayers normalized to the number of nuclei per field (n=4). (E) Representative confocal images showing VE-cadherin staining on the vehicle (DMSO), Src inhibitor (PP2) and Akt inhibitor (TCBN) treated HMEC monolayers 24 hours after treatment. (F) Quantification of the number of gaps in the vehicle (DMSO), Src inhibitor (PP2) and Akt inhibitor (TCBN) treated HMEC monolayers 24 hours after treatment (n=4). Data are represented as mean ± SD. *P<0.01, #P<0.05, scale bar 20 μm.
Figure 2. Akt inhibition promotes, but Src inhibition reduces endothelial-barrier leakage.
(A) Real-time changes in the barrier resistance of control, 1 μM pp2 and 10 μM TCBN treated HMEC monolayers as measured using ECIS equipment. (B) Quantification of the changes in barrier resistance between control, 1 μM pp2 and 10 μM TCBN treated HMEC monolayers at 90 minutes and 24 hours after plating an equal number of cells in array wells (n=3). (C) Real-time changes in the barrier resistance of control and 20 ng/ml VEGF-treated HMEC monolayers co-treated with either TCBN or pp2 as measured using ECIS equipment. (D) Quantification of the changes in barrier resistance between control and 20 ng/ml VEGF-treated HMEC monolayers at 30 minutes and 24 hours after plating an equal number of cells in array wells (n=3). (E) Real-time changes in the barrier resistance of control and 50 ng/ml Ang-1 treated HMEC monolayers co-treated with either TCBN or pp2 as measured using ECIS equipment. (F) Quantification of changes in the barrier resistance between control and 50 ng/ml treated HMEC monolayers at 30 minutes and 24 hours after plating an equal number of cells in array wells (n=3). Data are represented as mean ± SD. *P<0.01.
Inhibition of Akt, but not Src results in HMEC monolayer gap formation in the long-term
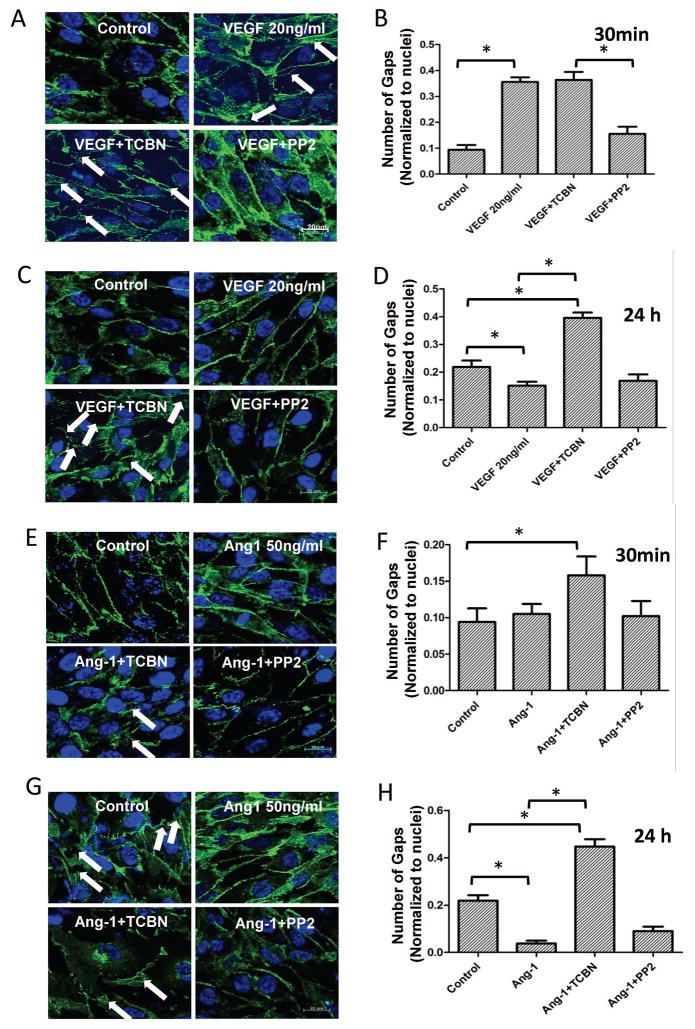
HMEC monolayers were treated with either the vehicle (DMSO), 20 ng/ml VEGF or 50 ng/ml Ang-1, and co-treated with either 10 μM TCBN or 1 μM pp2 for 30 minutes and 24 hours. Immunofluorescence staining was performed using VE-Cadherin antibodies to study the effect of inhibitors on the number of “gaps” formed in the monolayers. As expected, VEGF increased the number of gaps in 30 minutes compared to that of control. While inhibition of Akt did not affect it further, inhibition of Src reduced the VEGF-induced gap formation (Figure 3A–B). In contrast, at 24 hours, VEGF stabilized the HMEC-barrier integrity, which was disrupted by Akt inhibition (Figure 3C–D). Inhibition of Src had no effect on VEGF-induced stabilization of barrier integrity in the long term. Ang-1-induced long-term HMEC-barrier protection was disrupted by Akt inhibition while Src inhibition had no significant effect (Figure 3E–F).
Figure 3. Inhibition of Akt, but not Src results in HMEC monolayer gap formation in the long-term.
(A–B) Representative confocal images and quantification of the gap number in VE-cadherin stained HMEC monolayers treated with 20 ng/ml VEGF in the presence and absence of Akt inhibitor TCBN or Src inhibitor pp2 for 30 minutes. (C–D) Representative confocal images and quantification of the gap number in VE-cadherin stained HMEC monolayers treated with 20 ng/ml VEGF in the presence and absence of Akt inhibitor TCBN or Src inhibitor pp2 for 24 hours. (E–F) Representative confocal images and quantification of the gap number in VE-cadherin stained HMEC monolayers treated with 50 ng/ml Ang-1 in the presence and absence of Akt inhibitor TCBN or Src inhibitor pp2 for 30 minutes. (G–H) Representative confocal images and quantification of the gap number in VE-cadherin stained HMEC monolayers treated with 50 ng/ml Ang-1 in the presence and absence of Akt inhibitor TCBN or Src inhibitor pp2 for 24 hours. Data are represented as mean ± SD. (n=4), *P<0.01, scale bar 20 μm.
Akt and Src maintain a reciprocal relationship in the phosphorylation/activity pattern
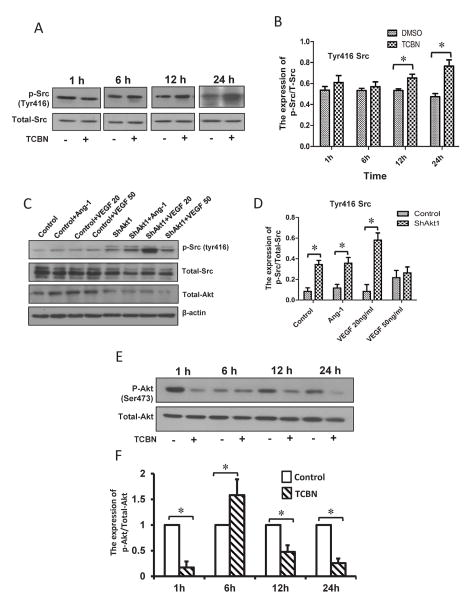
To elucidate the relationship between the expression and phosphorylation patterns of Akt and Src upon mutual inhibition and growth factor stimulation in the long-term, HMECs were treated in the presence or absence of TCBN for 1 to 24 hours and changes in phosphorylation of Tyr416 Src were studied using Western blot analysis. Inhibition of Akt promoted Tyr416 phosphorylation of Src starting from 12 hours after treatment with TCBN (Figure 4A–B). No changes in the Src Tyr416 phosphorylation were observed during the early stages of TCBN treatment. Alternately, HMECs were subjected to ShRNA-mediated Akt1 knockdown followed by treatment with either 20 ng/ml VEGF or 50 ng/ml Ang-1, and changes in Src Tyr416 phosphorylation were examined. Loss of Akt1 by itself induced an increase in Src Tyr416 phosphorylation, which was further increased in the presence of Ang-1 or 20 ng/ml VEGF (Figure 4C–D), but not 50 ng/ml VEGF. In order to directly compare the changes in Akt phosphorylation upon TCBN treatment and its correlation with changes in Src phosphorylation, we determined a time course effect of TCBN on Akt Ser473 phosphorylation. Our data indicates that significant increase in Src Tyr416 phosphorylation at 12 and 24 hours after TCBN treatment correlates with a significant decrease in Akt Ser473 phosphorylation (Figure 4E–F).
Figure 4. Akt and Src maintain a reciprocal relationship in the phosphorylation/activity pattern.
(A–B) Representative Western blot images of HMEC lysates treated with or without TCBN showing Src Tyr416 phosphorylation and total Src, and a corresponding bar graph showing changes in the Src Tyr416 phosphorylation normalized to total Src from 1–24 hours, respectively. (C–D) Representative Western blot images of HMEC lysates transfected with scrambled ShRNA or ShRNA targeting Akt1 followed by treatment with either 20 and 50 ng/ml VEGF or 50 ng/ml Ang-1 showing changes in the Src Tyr416 phosphorylation and total Src, and corresponding bar graph showing changes in the Src Tyr416 phosphorylation normalized to total Src at 24 hours, respectively. (E–F) Representative Western blot images of HMEC lysates treated with or without TCBN showing Akt Ser473 phosphorylation and total Akt, and a corresponding bar graph showing changes in the Akt Ser473 phosphorylation normalized to total Akt from 1–24 hours, respectively. Data are represented as mean ± SD. (n=4), *p<0.01.
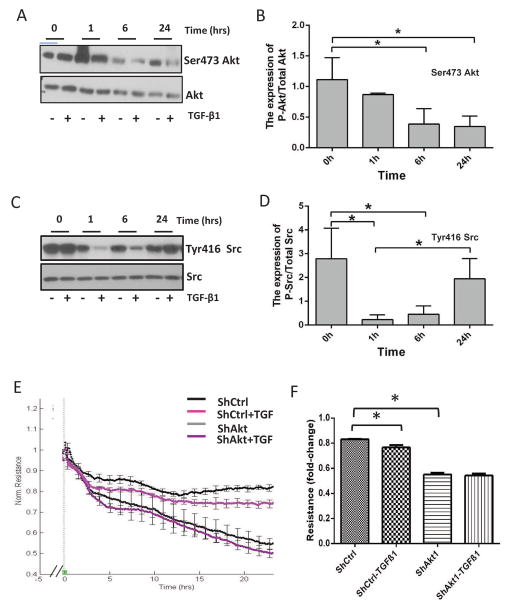
TGFβ1 inhibits Ser473 phosphorylation of Akt and enhances Tyr416 phosphorylation of Src to induce HMEC-barrier breakdown in a time-dependent manner
To investigate the cross-talk between the activity of Akt and Src upon stimulus from a growth factor known to induce endothelial injury in the long-term, HMECs were treated with 5 ng/ml TGFβ1 for 0–24 hours. Western blot analysis of the cell lysates collected at various time points following TGFβ1 treatment indicated a time-dependent reduction in Akt Ser473 phosphorylation (Figure 5A and 5B). In contrast, although TGFβ1 treatment inhibited Tyr416 Src phosphorylation early on (6 hours), prolonged stimulation resulted in increased Tyr416 Src phosphorylation (Figure 5C and 5D), showing a reciprocal relationship with Akt in the long-term. The barrier permeability of HMEC monolayers was increased with TGFβ1 treatment in a time-dependent manner, an effect that was blunted in already permeable Akt1 knockdown cells (Figure 5E–F), suggesting that TGFβ1 inhibits Akt and activates Src in the long-term to induce endothelial injury (Figure 6).
Figure 5. TGFβ1 inhibits Ser473 phosphorylation of Akt and enhances Tyr416 phosphorylation of Src to induce HMEC-barrier breakdown in a time-dependent manner.
(A–B) Representative Western blot images and corresponding bar graph of Akt Ser473 phosphorylation and total-Akt in HMEC lysates treated in the presence and absence of 5 ng/ml TGFβ1 for 1–24 hours, respectively. (C–D) Representative Western blot images and corresponding bar graph of Src Tyr416 phosphorylation and total-Src in HMEC lysates treated in the presence and absence of 5 ng/ml TGFβ1 for 1–24 hours, respectively. (E–F) Real-time changes and a bar graph showing the quantification of changes in the ShControl and ShAkt1 HMEC-barrier resistance upon treatment with either vehicle (PBS) or 5 ng/ml TGFβ1 as measured using ECIS equipment. Data are represented as mean ± SD. (n=3), *p<0.01.
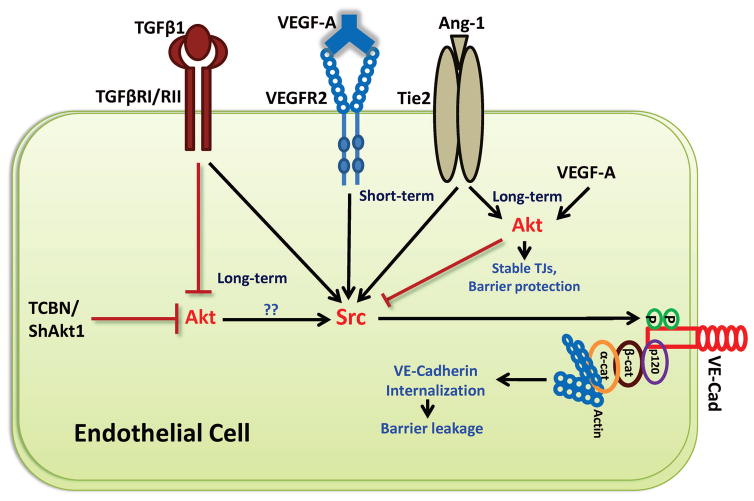
Figure 6. Schematic representation of cross-talk between Akt and Src pathways in the long-term endothelial barrier regulation in response to VEGF, Ang-1, and TGFβ.
Our study concludes that whereas long-term stimulation with VEGF and Ang-1 in HMEC increases Akt and normalizes Src activities leading to endothelial-barrier protection, treatment with TGFβ1 promotes endothelial-barrier breakdown in the long-term associated with inhibition of Akt and activation of Src activities. Direct inhibition of Akt activity through pharmacological (TCBN) and genetic (ShRNA for Akt1) approaches also lead to Src activation in the long-term indicating that Akt and Src are reciprocally regulated in the growth factor-induced long-term endothelial-barrier regulation.
Discussion
Vascular permeability is the hallmark of several diseases including cancer, acute kidney and lung injuries, cerebral edema, diabetes-related vascular complications, chronic inflammatory diseases, and cardiovascular diseases. (Leu, Berk et al. 2000, Azzi, Hebda et al. 2013, Moughon, He et al. 2015). The healthy and intact endothelium is essential for vascular homeostasis. Although numerous signaling pathways are involved in regulating the endothelial-barrier function, PI3K-Akt and Src pathways are of prime importance in regulating endothelial cell survival, proliferation, migration, endothelial-barrier function and gene expression (Coffer, Jin et al. 1998, Eliceiri, Paul et al. 1999, Minshall, Tiruppathi et al. 2002, Shiojima and Walsh 2002, Laird, Li et al. 2003, Yeatman 2004). A variety of stimuli including growth factors, cytokines, vascular permeability-inducing agents such as VEGF, and barrier protective agents such as Ang-1 activate Akt and Src and hence are greatly implicated in the regulation of vascular wall integrity (Eliceiri, Paul et al. 1999, Shiojima and Walsh 2002, Gavard and Gutkind 2006, Wallez, Cand et al. 2007, Aghajanian, Wittchen et al. 2008, Guignabert and Montani 2013). While there have been contradicting reports about the role of Akt in endothelial-barrier regulation, our recent study has demonstrated the integral role of Akt1 in the long-term protection of endothelial-barrier in response to VEGF and Ang-1 (Gao, Artham et al. 2016). The src family of non-receptor tyrosine kinases has been implicated in vascular permeability and injury (Paul, Zhang et al. 2001, He, Liu et al. 2015). Following ischemia and injury, phosphorylation of Src is highly upregulated causing activation of downstream signaling to induce barrier breakdown and vascular permeability (Esser, Lampugnani et al. 1998, Ha, Bennett et al. 2008). Src is activated in pathological conditions following elevated levels of VEGF and causes internalization of VE-cadherin, a key component of adherens junctions which is responsible for maintaining intact endothelium (Azzi, Hebda et al. 2013). Although a great amount of light has been shed on the independent roles of Akt and Src and their specific roles in the regulation of endothelial-barrier function and vascular permeability, a cross-talk between these two pathways in response to various stimuli, if there is one, has not been studied yet.
The objective of the current study was to investigate the changes in Ser473 Akt and Tyr416 Src-activating phosphorylations in response to growth factors that make or break endothelial-barrier junctions, determine the effect of direct activity modulation of Akt on Src and vice versa, and finally establish a cross-talk between Akt and Src in the modulation of endothelial-barrier function. Our results show that although VEGF-induced endothelial-barrier permeability is short-term and is reversed on due course of time, TCBN-mediated Akt inhibition blunts the reversal and diminishes the long-term barrier protective effects of VEGF and Ang-1. In contrast, pp2-mediated Src inhibition reversed VEGF-induced short-term vascular permeability but had no significant effect on the VEGF and Ang-1-mediated long-term barrier protection. Genetic loss of Akt1 achieved by ShRNA-mediated knockdown resulted in increased gap formation at the basal levels compromising the barrier integrity, thus confirming our previous observations that Akt1 loss results in increased vascular permeability in vivo (Chen, Somanath et al. 2005, Gao, Artham et al. 2016), and a report by Mukai et al (Mukai, Rikitake et al. 2006) demonstrating that endothelial-specific activation of Akt1 suppresses lesion formation and maintains integrity of vascular wall. As expected, VEGF increased the number of gaps in the short-term, an effect that was blunted by the inhibition of Src, but not Akt. However, in the long-term (24 hours), both VEGF and Ang-1 stabilized the endothelial-barrier, which was disrupted by inhibition of Akt but not Src, thus indicating that both Src and Akt play different roles in the short- and long-term endothelial-barrier regulation in response to VEGF and Ang-1.
Interestingly, inhibition of Akt although did not exhibit any significant change in the levels of Tyr416 Src phosphorylation in the short-term (0–6 hours), it resulted in increased levels of activating Tyr416 Src phosphorylation in the long-term (12–24 hours). The increase in Src Tyr416 phosphorylation was accompanied by a decrease in Akt Ser473 phosphorylation at 12 and 24 hours following TCBN treatment, indicating that long-term inactivation of Akt enhances Src activity, thus contributing to the long-term endothelial-barrier disruption following Akt inhibition. Similarly, a significant increase in Tyr416 Src phosphorylation was also observed in Akt1 deficient HMECs. Although Akt-mediated effects are specific to the tight-junction protein turnover, and not adherens junction modulation (Gao, Artham et al. 2016), activation of Src upon long-term inhibition of Akt would explain why we still see gap formations in VE-cadherin stained Akt1 deficient HMEC-monolayers.
To further confirm the Akt and Src cross-talk in the long-term endothelial-barrier regulation, we involved TGFβ, a cytokine known to induce endothelial and vascular injury in the long-term. Stimulation of HMECs with TGFβ1 although did not affect Ser473 Akt phosphorylation in the short-term, it resulted in reduced Ser473 Akt phosphorylation and hence its inhibition in the long-term. In contrast, although TGFβ1 inhibited Tyr416 Src phosphorylation in the short-term, it promoted Tyr416 Src phosphorylation in the long term, once again indicating a reciprocal regulation of Akt and Src activities in the long-term in HMECs. Furthermore, whereas TGFβ1 treatment resulted in HMEC-barrier disruption in the long-term, this effect was blunted in ShAkt1 HMEC monolayers, thus indicating that Akt inhibition is necessary for the TGFβ1-induced endothelial-barrier injury.
Although our data indicate a reciprocal regulation of Akt and Src pathways in the long-term endothelial barrier function, the underlying molecular mechanisms regulating this cross-talk need further extensive analysis. Nevertheless, since the growth factors such as VEGF, Ang-1, and TGFβ that modulate endothelial-barrier function and angiogenesis in various vascular beds in physiological, hypoxic as well as pathological conditions, the existence of Akt-Src cross-talk in these conditions in vivo is very likely. However, since Akt is a serine-threonine kinase and Src a tyrosine kinase, it is very clear that the effects are not direct. The fact that Akt-Src cross-talk occurs only in the long-term, the possibility of secondary events including paracrine effects, in this process, cannot be ruled out.
In conclusion, we report for the first time that Akt and Src maintain a reciprocal regulation of their activities in response to various growth factors in the regulation of long-term endothelial-barrier function (Figure 6). Whereas VEGF and Ang-1 activate Akt to return Src activity to the basal levels in the long-term thus protecting the endothelial-barrier, TGFβ stimulation inhibits Akt and activates Src in the long-term leading to endothelial-barrier injury. In support of this newly characterized Akt and Src cooperation by three different growth factors in the modulation of long-term endothelial-barrier function, treatment with Akt inhibitor TCBN that promotes endothelial-barrier breakdown in the long-term is also associated with increased Src activation. Based on these observations and support from our previous findings in this field (Chen, Somanath et al. 2005, Gao, Artham et al. 2016) we demonstrate the programmed orchestration of adherens-junction and tight-junction protein turnover by Src and Akt respectively, in the modulation of endothelial-barrier function.
Acknowledgments
Funds were provided by the National Institutes of Health grant (R01HL103952) to PRS. This work has been accomplished using the resources and facilities at the Charlie Norwood Medical Center in Augusta, GA. The funders had no role in the study design, data collection, analysis and decision to publish the data. The contents of the manuscript do not represent the views of Department of Veteran Affairs or the United States Government.
Footnotes
Conflict of Interest: The authors declare that no conflict of interest exists.
References
- 1.Aghajanian A, Wittchen ES, Allingham MJ, Garrett TA, Burridge K. Endothelial cell junctions and the regulation of vascular permeability and leukocyte transmigration. J Thromb Haemost. 2008;6(9):1453–1460. doi: 10.1111/j.1538-7836.2008.03087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azzi S, Hebda JK, Gavard J. Vascular permeability and drug delivery in cancers. Front Oncol. 2013;3:211. doi: 10.3389/fonc.2013.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23(2):168–175. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- 4.Chen J, Somanath PR, Razorenova O, Chen WS, Hay N, Bornstein P, Byzova TV. Akt1 regulates pathological angiogenesis, vascular maturation, and permeability in vivo. Nat Med. 2005;11(11):1188–1196. doi: 10.1038/nm1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coffer PJ, Jin J, Woodgett JR. Protein kinase B (c-Akt): a multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem J. 1998;335(Pt 1):1–13. doi: 10.1042/bj3350001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daly C, Wong V, Burova E, Wei Y, Zabski S, Griffiths J, Lai KM, Lin HC, Ioffe E, Yancopoulos GD, Rudge JS. Angiopoietin-1 modulates endothelial cell function and gene expression via the transcription factor FKHR (FOXO1) Genes Dev. 2004;18(9):1060–1071. doi: 10.1101/gad.1189704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Palma C, Meacci E, Perrotta C, Bruni P, Clementi E. Endothelial nitric oxide synthase activation by tumor necrosis factor alpha through neutral sphingomyelinase 2, sphingosine kinase 1, and sphingosine 1 phosphate receptors: a novel pathway relevant to the pathophysiology of endothelium. Arterioscler Thromb Vasc Biol. 2006;26(1):99–105. doi: 10.1161/01.ATV.0000194074.59584.42. [DOI] [PubMed] [Google Scholar]
- 8.Eliceiri BP, Paul R, Schwartzberg PL, Hood JD, Leng J, Cheresh DA. Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol Cell. 1999;4(6):915–924. doi: 10.1016/s1097-2765(00)80221-x. [DOI] [PubMed] [Google Scholar]
- 9.Esser S, Lampugnani MG, Corada M, Dejana E, Risau W. Vascular endothelial growth factor induces VE-cadherin tyrosine phosphorylation in endothelial cells. J Cell Sci. 1998;111(Pt 13):1853–1865. doi: 10.1242/jcs.111.13.1853. [DOI] [PubMed] [Google Scholar]
- 10.Fairaq A, Goc A, Artham S, Sabbineni H, Somanath PR. TNFalpha induces inflammatory stress response in microvascular endothelial cells via Akt- and P38 MAP kinase-mediated thrombospondin-1 expression. Mol Cell Biochem. 2015;406(1–2):227–236. doi: 10.1007/s11010-015-2440-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao F, Al-Azayzih A, Somanath PR. Discrete functions of GSK3alpha and GSK3beta isoforms in prostate tumor growth and micrometastasis. Oncotarget. 2015;6(8):5947–5962. doi: 10.18632/oncotarget.3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao F, Artham S, Sabbineni H, Al-Azayzih A, Peng XD, Hay N, Adams RH, Byzova TV, Somanath PR. Akt1 promotes stimuli-induced endothelial-barrier protection through FoxO-mediated tight-junction protein turnover. Cell Mol Life Sci. 2016 doi: 10.1007/s00018-016-2232-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gavard J, Gutkind JS. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol. 2006;8(11):1223–1234. doi: 10.1038/ncb1486. [DOI] [PubMed] [Google Scholar]
- 14.Gavard J, Patel V, Gutkind JS. Angiopoietin-1 prevents VEGF-induced endothelial permeability by sequestering Src through mDia. Dev Cell. 2008;14(1):25–36. doi: 10.1016/j.devcel.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 15.Goc A, Al-Azayzih A, Abdalla M, Al-Husein B, Kavuri S, Lee J, Moses K, Somanath PR. P21 activated kinase-1 (Pak1) promotes prostate tumor growth and microinvasion via inhibition of transforming growth factor beta expression and enhanced matrix metalloproteinase 9 secretion. J Biol Chem. 2013;288(5):3025–3035. doi: 10.1074/jbc.M112.424770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goddard LM, Iruela-Arispe ML. Cellular and molecular regulation of vascular permeability. Thromb Haemost. 2013;109(3):407–415. doi: 10.1160/TH12-09-0678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guignabert C, Montani D. Key roles of Src family tyrosine kinases in the integrity of the pulmonary vascular bed. Eur Respir J. 2013;41(1):3–4. doi: 10.1183/09031936.00091912. [DOI] [PubMed] [Google Scholar]
- 18.Ha CH, Bennett AM, Jin ZG. A novel role of vascular endothelial cadherin in modulating c-Src activation and downstream signaling of vascular endothelial growth factor. J Biol Chem. 2008;283(11):7261–7270. doi: 10.1074/jbc.M702881200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He YX, Liu J, Guo B, Wang YX, Pan X, Li D, Tang T, Chen Y, Peng S, Bian Z, Liang Z, Zhang BT, Lu A, Zhang G. Src inhibitor reduces permeability without disturbing vascularization and prevents bone destruction in steroid-associated osteonecrotic lesions in rabbits. Sci Rep. 2015;5:8856. doi: 10.1038/srep08856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laird AD, Li G, Moss KG, Blake RA, Broome MA, Cherrington JM, Mendel DB. Src family kinase activity is required for signal tranducer and activator of transcription 3 and focal adhesion kinase phosphorylation and vascular endothelial growth factor signaling in vivo and for anchorage-dependent and -independent growth of human tumor cells. Mol Cancer Ther. 2003;2(5):461–469. [PubMed] [Google Scholar]
- 21.Landmesser U, Hornig B, Drexler H. Endothelial function: a critical determinant in atherosclerosis? Circulation. 2004;109(21 Suppl 1):II27–33. doi: 10.1161/01.CIR.0000129501.88485.1f. [DOI] [PubMed] [Google Scholar]
- 22.Leu AJ, Berk DA, Lymboussaki A, Alitalo K, Jain RK. Absence of functional lymphatics within a murine sarcoma: a molecular and functional evaluation. Cancer Res. 2000;60(16):4324–4327. [PubMed] [Google Scholar]
- 23.Melo LG, Gnecchi M, Pachori AS, Kong D, Wang K, Liu X, Pratt RE, Dzau VJ. Endothelium-targeted gene and cell-based therapies for cardiovascular disease. Arterioscler Thromb Vasc Biol. 2004;24(10):1761–1774. doi: 10.1161/01.ATV.0000142363.15113.88. [DOI] [PubMed] [Google Scholar]
- 24.Minshall RD, Tiruppathi C, Vogel SM, Malik AB. Vesicle formation and trafficking in endothelial cells and regulation of endothelial barrier function. Histochem Cell Biol. 2002;117(2):105–112. doi: 10.1007/s00418-001-0367-x. [DOI] [PubMed] [Google Scholar]
- 25.Moughon DL, He H, Schokrpur S, Jiang ZK, Yaqoob M, David J, Lin C, Iruela-Arispe ML, Dorigo O, Wu L. Macrophage Blockade Using CSF1R Inhibitors Reverses the Vascular Leakage Underlying Malignant Ascites in Late-Stage Epithelial Ovarian Cancer. Cancer Res. 2015 doi: 10.1158/0008-5472.CAN-14-3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mukai Y, Rikitake Y, Shiojima I, Wolfrum S, Satoh M, Takeshita K, Hiroi Y, Salomone S, Kim HH, Benjamin LE, Walsh K, Liao JK. Decreased vascular lesion formation in mice with inducible endothelial-specific expression of protein kinase Akt. J Clin Invest. 2006;116(2):334–343. doi: 10.1172/JCI26223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paul R, Zhang ZG, Eliceiri BP, Jiang Q, Boccia AD, Zhang RL, Chopp M, Cheresh DA. Src deficiency or blockade of Src activity in mice provides cerebral protection following stroke. Nat Med. 2001;7(2):222–227. doi: 10.1038/84675. [DOI] [PubMed] [Google Scholar]
- 28.Shiojima I, Walsh K. Role of Akt signaling in vascular homeostasis and angiogenesis. Circ Res. 2002;90(12):1243–1250. doi: 10.1161/01.res.0000022200.71892.9f. [DOI] [PubMed] [Google Scholar]
- 29.Somanath PR, Kandel ES, Hay N, Byzova TV. Akt1 signaling regulates integrin activation, matrix recognition, and fibronectin assembly. J Biol Chem. 2007;282(31):22964–22976. doi: 10.1074/jbc.M700241200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taddei A, Giampietro C, Conti A, Orsenigo F, Breviario F, Pirazzoli V, Potente M, Daly C, Dimmeler S, Dejana E. Endothelial adherens junctions control tight junctions by VE-cadherin-mediated upregulation of claudin-5. Nat Cell Biol. 2008;10(8):923–934. doi: 10.1038/ncb1752. [DOI] [PubMed] [Google Scholar]
- 31.Wallez Y, Cand F, Cruzalegui F, Wernstedt C, Souchelnytskyi S, Vilgrain I, Huber P. Src kinase phosphorylates vascular endothelial-cadherin in response to vascular endothelial growth factor: identification of tyrosine 685 as the unique target site. Oncogene. 2007;26(7):1067–1077. doi: 10.1038/sj.onc.1209855. [DOI] [PubMed] [Google Scholar]
- 32.Yeatman TJ. A renaissance for SRC. Nat Rev Cancer. 2004;4(6):470–480. doi: 10.1038/nrc1366. [DOI] [PubMed] [Google Scholar]