Abstract
Recent studies of indigenous human populations at high altitude have provided proof-of-principle that genome scans of DNA polymorphism can be used to identify candidate loci for hypoxia adaptation. When integrated with experimental analyses of physiological phenotypes, genome-wide surveys of DNA polymorphism and tissue-specific transcriptional profiles can provide insights into actual mechanisms of adaptation. It has been suggested that adaptive phenotypic evolution is largely mediated by cis-regulatory changes in genes that are located at integrative control points in regulatory networks. This hypothesis can be tested by conducting transcriptomic analyses of hypoxic signaling pathways in conjunction with experimental measures of vascular oxygen supply and metabolic pathway flux. Such studies may reveal whether the architecture of gene regulatory networks can be used to predict which loci (and which types of loci) are likely to be “hot spots” for adaptive physiological evolution. Functional genomic studies of deer mice (Peromyscus maniculatus) demonstrate how the integrated analysis of variation in tissue-specific transcriptomes, whole-animal physiological performance, and various subordinate traits can yield insights into the mechanistic underpinnings of high-altitude adaptation.
Keywords: Functional genomics, Hypoxia, Peromyscus, Population genomics, Systems genetics, Transcriptomics
8.1 Introduction
There is currently a growing interest in conducting genome scans of DNA polymorphism to identify loci that have contributed to high-altitude adaptation in humans [1, 2, 5–7, 25, 45, 55, 82–85]. This population genomics approach is premised on the idea that the locus-specific effects of positive selection can be detected against the genome-wide backdrop of stochastic variation. Consider a genome-wide survey of DNA polymorphism in individuals sampled from a pair of high- and low-altitude populations. If a given trait is subject to directional selection only at high altitude, then the underlying loci are expected to undergo shifts in allele frequency in the highland population relative to the lowland population. In principle, it is possible to identify chromosomal regions that harbor such loci by exploiting theoretical predictions about the effects of positive selection on patterns of DNA polymorphism at linked neutral sites. However, the effects of selection on patterns of variation at or near causative loci depend strongly on the genetic architecture of the selected trait and numerous other factors [13, 28, 31, 32, 46, 60, 68, 73, 74].
The population genomics approach can be used as a means of “outlier detection” to nominate candidate loci as putative targets of positive selection, or it can be used to assess evidence for selection on previously identified candidate loci by assessing whether such loci emerge as outliers against a backdrop of genome-wide variation. Both approaches have been used successfully to identify loci involved in adaptation to high-altitude environments. In humans, for example, genome-wide surveys of DNA polymorphism in Tibetan highlanders revealed evidence for strong and recent positive selection on several genes that function as upstream regulators of the hypoxia inducible-factor (HIF) oxygen signaling pathway [5–7, 45, 55, 82–85]. The HIF family of transcription factors plays a key role in regulating oxygen homeostasis by coordinating the transcriptional response to hypoxia. One of the HIF genes that exhibited an especially clear signal of positive selection in the Tibetan population was the EPAS1 gene (endothelial PAS domain protein 1) (Fig. 8.1), also known as HIF2α, which encodes the oxygen-sensitive α subunit of the HIF-2 transcription factor. The product of EPAS1 is known to play an especially important role in regulating the erythropoietic response to hypoxia [47]. Another HIF-regulatory gene that exhibited strong evidence for selection in both Tibetan and Andean populations was EGLN1 (Egl Nine homolog 1) [1, 6, 7, 45, 55, 82, 84, 85], which encodes the prolyl hydroxlase isozyme (PHD2) that is responsible for hydroxylating the α subunit of the HIF1 transcription factor. Results of these studies provide proof-of-principle that the genome scan approach can successfully identify targets of recent positive selection, and the integration of such analyses with functional studies can provide additional insights into possible phenotypic targets of selection [34].
Fig. 8.1.
A genome-wide scan of allelic differentiation between population samples of Tibetans (resident at 3200–3500 m in Yunnan Province, China) and Han Chinese. The vertical axis of the graph shows the negative log of site-specific P-values for allele frequency differences between the Tibetan and Han Chinese population samples (low P-values denote allele frequency differences that are too large to explain by genetic drift). The horizontal axis of the graph shows the genomic positions of each assayed nucleotide site, arranged by chromosome number. The red line indicates the threshold for genome-wide statistical significance (P = 5 × 10−7). Values are shown after correction for background population stratification using an intragenomic control. Several noncoding variants flanking the EPAS1 gene are highly significant outliers. Reprinted from [5]
An example of how the population genomics approach can be combined with the functional analysis of individual candidate genes is provided by an integrative analysis of hemoglobin polymorphism in natural populations of North American deer mice (Peromyscus maniculatus). Multilocus surveys of nucleotide polymorphism in high- and low-altitude populations revealed evidence for a history of spatially varying selection at two α-globin gene duplicates and two β-globin gene duplicates [40, 62–66], and site-directed mutagenesis experiments involving recombinant hemoglobins quantified the additive and nonadditive effects of the causative mutations [41]. The population genetic evidence for spatially varying selection and the experimental measures of mutational effects corroborated previous research on wild-derived strains of deer mice, which had demonstrated that allelic variation in hemoglobin-oxygen affinity contributes to adaptive variation in whole-animal aerobic performance under hypoxia [11, 12, 59]. Similarly, genome-wide surveys of nucleotide variation in a number of Andean birds species have provided insights into the evolutionary forces that have shaped altitudinal patterns of hemoglobin polymorphism [17, 21, 42]. In each of these studies, population-genomic inferences about the adaptive significance of observed amino acid polymorphisms were tested by conducting functional analyses of native hemoglobin variants and engineered recombinant hemoglobin mutants that quantified the phenotypic effects of individual mutations.
In addition to identifying particular candidate loci for high-altitude adaptation, genome scans for signatures of positive selection can also be used to gain more general insights into the nature of adaptation to different environments. For example, in comparisons between high- and low-altitude populations of a given species, it is possible to assess whether certain classes of loci make disproportionate contributions to adaptive phenotypic evolution. We can assess whether genes that occupy particular positions in metabolic pathways or regulatory networks make disproportionate contributions to adaptation, and we can assess the relative importance of structural mutations (e.g., amino acid mutations that alter the catalytic efficiency of an enzyme) and regulatory mutations (e.g., cis- or trans-acting mutations that alter the expression of the enzyme-encoding gene).
8.2 The Relative Importance of Structural vs. Regulatory Changes in Physiological Adaptatation
It has been suggested that cis-acting regulatory mutations may make a disproportionate contribution to adaptive evolution because such changes generally have fewer pleiotropic effects relative to changes in coding sequence [10, 57, 58]. This is because cis-regulatory elements (e.g., promoters, enhancers, and 5′ and 3′ untranslated regions [UTRs]) are often functionally modular—distinct sequence motifs control discrete temporal phases and/or spatial patterns of gene expression [10, 56, 81]. Each cis-regulatory module represents a collection of transcription factor binding sites that encodes a particular transcriptional output, and mutational changes in a single module will typically alter a small part of the gene’s total transcriptional pattern. For example, a cis-regulatory mutation may affect transcription in one particular tissue or cell type, and will therefore have minimal pleiotropic effects on the regulatory network as a whole. By contrast, structural changes in the coding sequence of a given gene would be manifest in every tissue or cell type in which the affected protein is expressed. Likewise, mutations in the coding sequence of a transcription factor that affect DNA binding affinity could potentially affect the transcriptional control of myriad downstream regulatory targets. For these reasons, coding mutations are generally expected to have more far-reaching pleiotropic effects than cis-regulatory mutations, and may therefore have smaller net fitness benefits.
A number of recent studies have documented evolutionary changes in phenotype that were caused by mutations in modular cis-regulatory elements [26, 48, 75]. In humans, persistence of lactase expression into adulthood has evolved independently in several different ethnic groups, and in all cases the ontogenetic changes in the expression of the Lct gene were attributable to point mutations in cis-regulatory elements [75]. Another good example involves the evolution of reduced abdominal pigmentation in Drosophila santomea, which is caused by several distinct inactivating mutations in an abdomen-specific cis-regulatory element of the tan gene [26].
There are good reasons to expect that a disproportionate number of the mutations that contribute to phenotypic evolution are concentrated in the cis-regulatory regions of transcription factor genes that serve as central control points in regulatory networks [57, 58]. This expectation is based on the observation that pleiotropic effects are determined by how regulatory networks shape the phenotype. For example, among species in the genus Drosophila, evolutionary changes in larval trichome patterning are mediated by cis-regulatory substitutions in a transcription factor called shavenbaby/ovo (svb) [38, 71, 72]. The svb transcription factor integrates a vast array of cellular signals and produces an on/off transcriptional output that determines cellular differentiation into trichomes or naked cuticle. It appears that anatomically localized changes in trichome patterning can be achieved most efficiently through mutations in specific cis-regulatory enhancers of svb because such mutations have minimal pleiotropic effects. By contrast, coding mutations in svb would produce changes in trichome patterning in every spatial domain of the cuticle in which the protein is expressed, and changes in upstream regulators of svb would alter the development of other epidermal structures besides trichomes. Thus, genes located at integrative control points in a regulatory network can accumulate mutations with specific, minimally pleiotropic effects, and these mutations are predicted to be especially common in cis-regulatory regions of “control point” genes [57, 58].
In analogy with the role of svb in the development of trichome patterning in Drosophila embryos, the EPAS1 gene may occupy an analogous position in the regulatory network that governs the transcriptional response to hypoxia. It may be that the physiological response to hypoxia is most efficiently accomplished by modulating the transcription of EPAS1, which then causes a coordinated change in the expression of all downstream target genes.
Future research should reveal whether cis-regulatory mutations in EPAS1 and other upstream regulators of the HIF signaling pathway have made disproportionate contributions to hypoxia adaptation in other animal species that are native to high-altitude environments. Beyond the important goal of identifying convergent mechanisms of hypoxia adaptation in different species, such research could also contribute to the more general goal of discovering whether the architecture of gene regulatory networks can be used to predict which genes are likely to be “hot spots” for adaptive physiological evolution.
8.3 Integrating the Analysis of Coding Sequence Variation and Transcriptional Variation
Gene expression profiles represent an important source of phenotypic data at the molecular level, and detailed studies of transcriptional variation may help to identify mechanisms of genetic adaptation and/or physiological acclimatization [61, 67, 79]. Plasticity in most physiological traits is probably mediated to a large extent by environment-specific changes in the transcriptional activity of multiple underlying genes. As stated by Hochachka and Somero [24]: “The evolution of phenotypic plasticity requires development of a complex set of tightly integrated environmental sensing and gene regulation mechanisms that allow the organism to sense and then respond appropriately to an environmental change”.
In principle, genomic technologies that permit the simultaneous analysis of sequence variation and expression profiles for a set of genes in the same pathway can be used to identify both structural and regulatory mechanisms of adaptation. For example, RNA-seq technology can be used to characterize sequence polymorphism and transcript abundance in thousands of expressed mRNAs from specific tissue types [27, 43, 78]. The integrated analysis of DNA sequence variation, genome-wide variation in transcriptional profiles, and variation in organismal phenotypes in a linkage or association mapping population can yield important insights into how regulatory networks shape variation in complex traits [3, 36, 49]. This approach is most powerful when implemented in a common garden or reciprocal-transplant experimental design to quantify the environmental and genetic components of gene expression differences between populations.
To appreciate the types of evolutionary inferences that can be drawn from a typical RNA-seq analysis, consider a common garden experiment involving individuals sampled from a pair of high- and low-altitude populations. Using animals that have experienced uniform acclimation histories to control for environmentally induced variation in gene expression, tissue specific cDNA libraries are then constructed for transcriptome sequencing. For each protein-coding gene, it is possible to measure nucleotide differentiation between high- and low-altitude populations and the corresponding differentiation in expression levels that is attributable to additive genetic effects. If adaptive genetic differentiation in the expression of a given gene is attributable to changes in proximal cis-regulatory elements (i.e., mutations in the promoter region), then the gene in question may exhibit an elevated level of nucleotide differentiation due to divergent selection at the immediately adjacent noncoding sites. When adaptive differentiation in the expression of a given gene is attributable to trans-acting mutations, then we would not expect a correlated increase in nucleotide differentiation at or near the gene itself because flanking regulatory mutations would not have contributed to the selection response.
For the purpose of identifying candidate loci for environmental adaptation, one of the disadvantages of the RNA-seq approach is that the assayed variation is exclusively restricted to the transcripts of protein-coding genes. Thus, adaptive changes in noncoding DNA may go undetected if the causal mutations are distant from genic regions. Population genomic analyses of spatially varying selection in D. melanogaster provide suggestive evidence that noncoding DNA polymorphisms may make an unexpectedly large contribution to environmental adaptation. For example, in genomic comparisons between temperate and tropical Australian populations of D. melanogaster, chromosomal regions that exhibited the highest levels of differentiation contained an over-representation of unannotated noncoding sequence [30 ]. Secondly, RNA-seq analysis can only identify expression differences that are mediated by changes in the rate of transcription or mRNA stability—regulatory changes that are mediated by posttranslational modifications would remain undetected.
8.4 Dissecting the Genetic Architecture of Adaptive Regulatory Variation
It is possible to further assess the contributions of cis- and trans-acting regulatory mutations to variation in the expression of a given gene by quantifying the relative abundance of allele-specific transcripts (provided that the transcripts are distinguished by at least one diagnostic nucleotide change). In a diploid cell, trans-acting regulatory mutations affect the expression of both alleles of a given target gene, whereas cis-acting regulatory mutations exert effects that are allele-specific [19, 39, 80]. For this reason, it is possible to distinguish the effects of cis- and trans-acting regulatory mutations in modulating the expression of a given gene by comparing the relative expression of alternative alleles in heterozygotes. Assuming that the expression of each allele is independent of the other, a marked asymmetry in allele-specific transcript abundance is typically attributable to cis-regulatory changes because both alleles are transcribed in the same trans-regulatory cellular environment [19, 39]. RNA-seq allows for high-throughput assessment of allele-specific gene expression because it provides a means of quantifying transcript abundance on an allele-specific basis. Although analyses designed to characterize allele-specific expression patterns are most powerful when applied to controlled crosses between inbred strains [22, 29, 39, 70], powerful evolutionary inferences can still be made in RNA-seq analyses of samples from outbred, natural populations.
Ideally, inferences derived from genomic or transcriptomic analyses can help guide the design of detailed follow-up experiments to obtain insights into the mechanistic basis of adaptive trait variation [68, 69]. For example, at the biochemical level, variation in metabolic traits may stem from allelic variation in enzyme concentration, enzyme kinetics, or a combination of the two. The combined effects of enzyme concentration and enzyme kinetics are measured by the maximal velocity of the enzyme-catalyzed reaction, Vmax, which is defined as the product [E] × kcat, where [E] is the enzyme concentration and kcat is the kinetic constant. In RNA-seq analyses of samples from high- and low-altitude populations, metabolic genes that exhibit extreme differences in expression or allele frequency can be targeted for functional studies of enzyme kinetics. For example, cases where Vmax and gene expression vary in the same direction suggest that differences in Vmax may be attributable to differences in enzyme concentration (regulatory mutations). Conversely, cases in which differences in Vmax are not accompanied by changes in gene expression would implicate mutations that alter enzyme catalytic efficiency (structural mutations). Similarly, traditional expression QTL (eQTL) mapping approaches can be used to confirm inferences about the genetic architecture of the observed regulatory variation. Thus, results of RNA-seq analyses can generate novel hypotheses that can be experimentally tested.
8.5 Integrating Functional Genomics and Experimental Analyses of Whole-Animal Physiology
Integrating analyses of transcriptomic variation with measures of physiological traits can reveal how variation in regulatory networks gives rise to phenotypic variation at different hierarchical levels of organization. This is the goal of the “systems genetics” approach—to achieve a mechanistic understanding of the mapping function that relates genotype to phenotype by examining how genetic variation in intermediate molecular phenotypes such as transcript abundance is transduced into variation in higher-level traits [3, 18, 33, 36, 49, 54].
In analyses of tissue-specific transcriptome profiles, statistical associations between particular traits and levels of transcript abundance can reveal trait-specific modules of co-regulated genes. These transcriptional modules typically comprise sets of genes that interact in the same pathway or regulatory network. For this reason, correlations in transcript abundance among genes in the same module provide a means of constructing genetic interaction networks, and can suggest hypotheses about causal effects [49].
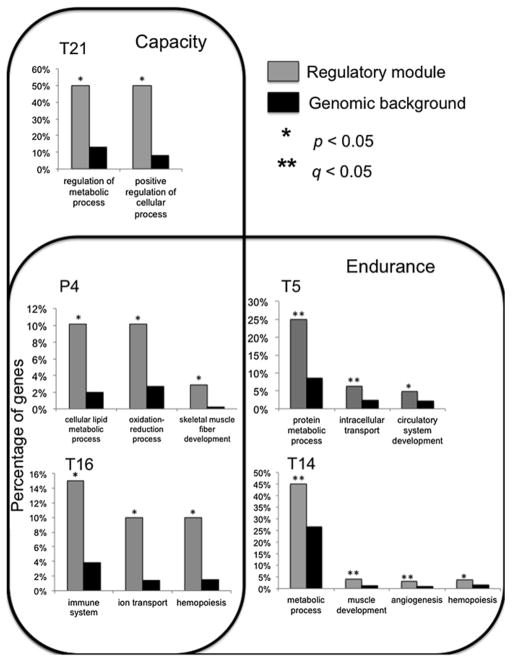
Integrated analyses of transcriptomic variation and physiological phenotypes have been successfully applied in studies of high-altitude adaptation in North American deer mice. Relative to mice that are native to lowland environments, high-altitude deer mice have evolved enhanced capacities for aerobic performance under hypoxia [14–16, 23, 35], and these population differences in whole-organism performance stem from genetically based physiological changes at several hierarchical levels of biological organization. For example, the elevated thermogenic capacity of high-altitude deer mice is associated with an increased capacity to oxidize lipids as a primary fuel source during aerobic thermogenesis [14]. This difference in lipid catabolic capacities is associated with differences in the activities of enzymes that influence flux through fatty acid oxidation and oxidative phosphorylation pathways, and with concerted changes in gene expression in these same pathways [14, 16]. For example, high-altitude mice that were acclimated to low-altitude conditions for 6 weeks exhibited wholesale upregulation of genes in the β-oxidation and oxidative phosphorylation pathways compared to their lowland counterparts that were acclimated to the same laboratory conditions [14]. Follow-up experiments on mice with different acclimation histories revealed that the regulatory changes associated with enhanced performance are highly plastic. Specifically, four of five transcriptional modules that were significantly associated with whole-organism thermogenic performance comprised a set of genes that exhibited significant effects of rearing environment (native elevation vs. common garden) that were independent of population-of-origin (highland vs. lowland), suggesting that a large fraction of the transcriptional variation is environmentally induced [16]. These environmentally sensitive transcriptional modules were enriched for genes involved in hematopoiesis, angiogenesis, muscle development, and immune response (Fig. 8.2). Interestingly, the single transcriptional module that exhibited a significant population-of-origin effect (expression differences persisted in the F1 generation) was enriched for genes involved in lipid oxidation, and this module was expressed at higher levels in high-altitude natives. Again, there was generally good correspondence between these transcriptional patterns and enzyme activities that serve as biomarkers for the flux capacity of the β-oxidation pathway and other core metabolic pathways, lending further support to the hypothesis that the enhanced aerobic performance of high-altitude deer mice under hypoxia is partly attributable to their enhanced capacities for lipid oxidation.
Fig. 8.2.
Functional enrichment of transcriptional modules that are associated with thermogenic performance in deer mice. Categorical enrichments are shown for five separate modules that are associated with thermogenic capacity (cold-induced VO2 max) (T21), thermogenic endurance (the length of time individuals can maintain >90 % of VO2 max (T5 and T14), or both measures of performance (P4 and T16). Modules T5, T14, T16, and T21 are comprised of genes that differ in expression across rearing environments independent of population of origin, whereas module P4 is comprised of genes differ in expression between highland and lowland mice across rearing environments. For each performance-associated transcriptional module, the proportional representation of genes in different gene ontology categories is compared between the transcriptional module (gray bars) and the genome as a whole (black bars). Asterisks denote gene ontology categories that are significantly enriched within the transcriptional module (*uncorrected p < 0.05, **FDR corrected q < 0.05). Reprinted from [16]
Taken together, these studies suggest that metabolic adaptation to high-altitude in deer mice involves the maintenance of a highly aerobic phenotype in the face of reduced oxygen availability, and this aerobic phenotype is achieved through both genetically based, constitutive differences in gene expression and transcriptional plasticity. Elite endurance athletes and highly aerobic nonhuman mammals are also characterized by an enhanced capacity for fatty acid oxidation under normoxic conditions [4, 8, 37]. Under conditions of chronic oxygen deprivation at high-altitude, a similar enhancement of fatty acid oxidation capacity could promote enhanced thermogenic performance, but it would require additional physiological changes to ensure adequate oxygen and fuel flux through oxidative pathways, suggesting that modifications of upstream steps in the oxygen-transport cascade may be necessary to support the increased lipid oxidation rate of high-altitude deer mice.
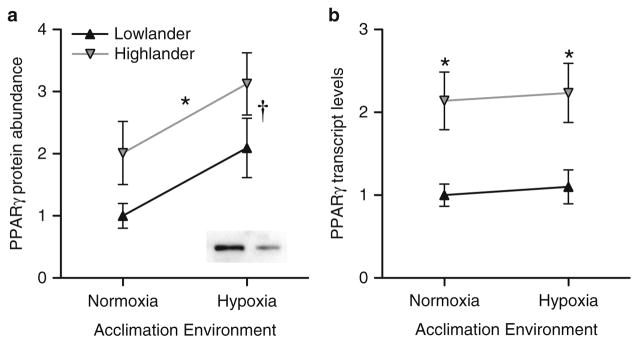
Indeed, the enhanced aerobic capacity of high-altitude deer mice under hypoxia is also partly attributable to increases in capillary density, oxidative fiber abundance, and oxidative enzyme activity in skeletal muscle [14, 16, 35]. These changes enhance the oxidative capacity and oxygen-diffusion capacity of working muscle, which could compensate for the diminished tissue oxygen supply under hypoxia [9, 51, 53, 77]. The transcriptional underpinnings of these phenotypic changes were identified in a common-garden experiment involving wild-caught high- and low- altitude mice as well as laboratory-reared F1 progeny of wild-caught mice [35, 52]. Expression analysis of a panel of genes that regulate angiogenesis and energy metabolism revealed that the increased capillarity and oxidative capacity of skeletal muscle in high-altitude mice was associated with an increased transcript abundance and protein abundance of peroxisome proliferator-activated receptor γ (PPARγ) (Fig. 8.3), a transcription factor that regulates mitochondrial biogenesis. PPARγ protein expression also increased during acclimation to chronic hypoxia [35]. Intriguingly, the underlying gene (pparg) exhibits a strong signature of recent positive selection in indigenous Tibetan and Mongolian highlanders [83].
Fig. 8.3.
Relative to low-altitude deer mice, high-altitude mice have higher expression of PPARγ protein (a) and mRNA (b) in the gastrocnemius muscle. Population-of-origin (i.e., high- or low-altitude) had significant main effects on PPARγ protein abundance and transcript abundance, but acclimation environment only had a significant main effect on protein abundance. There was no significant interaction between population-of-origin and acclimation treatment in either case [35]. The inset image in panel A shows representative immunoreactive bands for a high-altitude mouse (left) and low-altitude mouse (right) after acclimation to hypoxia. Modified from [35]
Expression patterns of the deer mouse pparg gene proved to be somewhat anomalous, however, as most other genes involved in angiogenesis were actually down-regulated during acclimation to hypoxia [35] and were either not differentially expressed between hypoxia-acclimated high- and low-altitude mice or were down-regulated in the high-altitude mice [52]. Transcriptomic analysis of gastrocnemius muscle revealed a small set of transcripts with genetically based expression differences between high- and low-altitude mice (i.e., nonplastic differences that persisted in the F1 progeny of mice with different populations-of-origin), and these transcripts clustered into two discrete modules. Some genes involved in regulating angiogenesis (Cadherin-7 and Notch-4) were significantly upregulated in high-altitude mice, but most genes within these modules were actually expressed at a lower level in high-altitude mice and expression levels were negatively correlated with measures of muscle capillarity and oxidative capacity. A possible explanation for this seemingly paradoxical result is that particular genes like Notch-4 may be responsible for maintaining the high muscle capillarity of high-altitude mice, and the associated increase in cellular oxygen tension simply dampens the stimulus for the expression of other genes that respond to oxygen limitation [35, 52]. As stated by Scott et al. (Ref. [52]: 1972): “A negative association between muscle phenotypes and expression could thus result for genes that do not cause the differences in muscle phenotype but are sensitive to its effects.” This highlights the difficulty of distinguishing the effects of genes that cause a given phenotype vs. effects that are simply consequences of the induced changes.
These integrative studies of deer mouse physiology revealed that genetically based regulatory changes and acclimation responses both contribute to improvement in whole-animal aerobic performance under hypoxia via changes in oxygen-transport capacity and oxygen utilization [14–16, 35, 52, 76]. Thus, variation in whole-organism aerobic performance seems to stem from changes at several hierarchical levels of biological organization, and changes at each level appear to stem from concerted expression changes in co-regulated sets of genes.
8.6 Future Outlook
Whole-genome and transcriptome sequencing will continue to play a central role in studies of hypoxia adaptation in humans and other animals. Analyses of genomic/ transcriptomic data and gene ontology databases can provide lists of candidate loci for hypoxia adaptation, but such analyses need to be integrated with experimental measures of whole-animal performance and other subordinate traits to obtain insights into actual mechanisms of physiological adaptation. Gene ontology analyses can be useful for generating hypotheses but, in the absence of experimental validation, in silico approaches lend themselves to overinterpretation and storytelling [44, 61]. Surveys of genomic/transcriptomic variation can suggest hypotheses about the adaptive significance of particular polymorphisms or expression changes, but functional experiments are required to test such hypotheses [17, 20, 50, 68].
In analyses of transcriptomic variation, it is also critical to use common-garden and/or reciprocal transplant experimental designs to disentangle genetic and environmental components of variation in gene expression, otherwise it is not possible to distinguish between evolved changes and plastic changes. Studies of high-altitude physiology in humans face obvious constraints with regard to experimental manipulations. However, recent work by Lorenzo et al. [34] provides an example of how reverse-genetics approaches and in vitro experiments can be used to follow up on results of population genomic studies to gain insights into mechanisms of hypoxia adaptation.
Acknowledgments
We thank C.M. Beall and G.R. Scott for providing figures, and we gratefully acknowledge funding support from the National Institutes of Health/National Heart, Lung, and Blood Institute (HL087216 [JFS]) and the National Science Foundation (IOS-1354390 [JFS], MCB-1517636 [JFS], IOS-1354934 [ZAC]), and IOS-1444161 [ZAC]).
Contributor Information
Jay F. Storz, School of Biological Sciences, University of Nebraska, Lincoln, NE, USA
Zachary A. Cheviron, Division of Biological Sciences, University of Montana, Missoula, MT, USA
References
- 1.Aggarwal S, Negi S, Jha P, Singh PK, Stobdan T, Pasha MAQ, Ghosh S, Agrawal A, Prasher B, Mukerji M Indian Genome Variation C. EGLN1 involvement in high-altitude adaptation revealed through genetic analysis of extreme constitution types defined in Ayurveda. Proc Natl Acad Sci U S A. 2010;107:18961–6. doi: 10.1073/pnas.1006108107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alkorta-Aranburu G, Beall CM, Witonsky DB, Gebremedhin A, Pritchard HK, Di Rienzo A. The genetic architecture of adaptations to high altitude in Ethiopia. PLoS Genet. 2012;8:e1003110. doi: 10.1371/journal.pgen.1003110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ayroles JF, Carbone MA, Stone EA, Jordan KW, Lyman RF, Magwire MM, Rollmann SM, Duncan LH, Lawrence F, Anholt RRH, Mackay TFC. Systems genetics of complex traits in Drosophila melanogaster. Nat Genet. 2009;41:299–307. doi: 10.1038/ng.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bangsbo J, Mohr M, Poulsen A, Perez-Gomez J, Krustrup P. Training and testing the elite athlete. J Exerc Sci Fit. 2006;4:1–14. [Google Scholar]
- 5.Beall CM, Cavalleri GL, Deng LB, Elston RC, Gao Y, Knight J, Li CH, Li JC, Liang Y, McCormack M, Montgomery HE, Pan H, Robbins PA, Shianna KV, Tam SC, Tsering N, Veeramah KR, Wang W, Wangdui PC, Weale ME, Xu YM, Xu Z, Yang L, Zaman MJ, Zeng CQ, Zhang L, Zhang XL, Zhaxi PC, Zheng YT. Natural selection on EPAS1 (HIF2 alpha) associated with low hemoglobin concentration in Tibetan highlanders. Proc Natl Acad Sci U S A. 2010;107:11459–64. doi: 10.1073/pnas.1002443107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bigham A, Bauchet M, Pinto D, Mao XY, Akey JM, Mei R, Scherer SW, Julian CG, Wilson MJ, Herraez DL, Brutsaert T, Parra EJ, Moore LG, Shriver MD. Identifying signatures of natural selection in Tibetan and Andean populations using dense genome scan data. PLoS Genet. 2010;6:e1001116. doi: 10.1371/journal.pgen.1001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bigham AW, Mao XY, Mei R, Brutsaert T, Wilson MJ, Julian CG, Parra EJ, Akey JM, Moore LG, Shriver MD. Identifying positive selection candidate loci for high-altitude adaptation in Andean populations. Hum Genomics. 2009;4:79–90. doi: 10.1186/1479-7364-4-2-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bjorntorp P. Importance of fat as a support nutrient for energy: metabolism of athletes. J Sport Sci. 1991;9:71–6. doi: 10.1080/02640419108729867. [DOI] [PubMed] [Google Scholar]
- 9.Cano I, Mickael M, Gomez-Cabrero D, Tegnér J, Roca J, Wagner PD. Importance of mitochondrial PO2 in maximal O2 transport and utilization: a theoretical analysis. Respir Physiol Neurobiol. 2013;189:477–83. doi: 10.1016/j.resp.2013.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carroll SB. Evo-devo and an expanding evolutionary synthesis: a genetic theory of morphological evolution. Cell. 2008;134:25–36. doi: 10.1016/j.cell.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 11.Chappell MA, Hayes JP, Snyder LRG. Hemoglobin polymorphisms in deer mice (Peromyscus maniculatus): physiology of beta-globin variants and alpha-globin recombinants. Evolution. 1988;42:681–8. doi: 10.1111/j.1558-5646.1988.tb02486.x. [DOI] [PubMed] [Google Scholar]
- 12.Chappell MA, Snyder LRG. Biochemical and physiological correlates of deer mouse α chain hemoglobin polymorphisms. Proc Natl Acad Sci U S A. 1984;81:5484–8. doi: 10.1073/pnas.81.17.5484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chevin LM, Hospital F. Selective sweep at a quantitative trait locus in the presence of background genetic variation. Genetics. 2008;180:1645–60. doi: 10.1534/genetics.108.093351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheviron ZA, Bachman GC, Connaty AD, McClelland GB, Storz JF. Regulatory changes contribute to the adaptive enhancement of thermogenic capacity in high-altitude deer mice. Proc Natl Acad Sci U S A. 2012;109:8635–40. doi: 10.1073/pnas.1120523109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheviron ZA, Bachman GC, Storz JF. Contributions of phenotypic plasticity to differences in thermogenic performance between highland and lowland deer mice. J Exp Biol. 2013;216:1160–6. doi: 10.1242/jeb.075598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheviron ZA, Connaty AD, McClelland GB, Storz JF. Functional genomics of adaptation to hypoxic cold-stress in high-altitude deer mice: transcriptomic plasticity and thermogenic performance. Evolution. 2014;68:48–62. doi: 10.1111/evo.12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheviron ZA, Natarajan C, Projecto-Garcia J, Eddy DK, Jones J, Carling MD, Witt CC, Moriyama H, Weber RE, Fago A, Storz JF. Integrating evolutionary and functional tests of adaptive hypotheses: a case study of altitudinal differentiation in hemoglobin function in an Andean sparrow. Zonotrichia capensis. Mol Biol Evol. 2014;31:2948–62. doi: 10.1093/molbev/msu234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Civelek M, Lusis AJ. Systems genetics approaches to understand complex traits. Nat Rev Genet. 2014;15:34–48. doi: 10.1038/nrg3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cowles C, Hirschhorn J, Altshuler D, Lander E. Detection of regulatory variation in mouse genes. Nat Genet. 2002;32:432–7. doi: 10.1038/ng992. [DOI] [PubMed] [Google Scholar]
- 20.Dean AM, Thornton JW. Mechanistic approaches to the study of evolution: the functional synthesis. Nat Rev Genet. 2007;8:675–88. doi: 10.1038/nrg2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galen SC, Natarajan C, Moriyama H, Weber RE, Fago A, Benham PM, Chavez AN, Cheviron ZA, Storz JF, Witt CC. Contribution of a mutational hotspot to hemoglobin adaptation in high-altitude Andean house wrens. Proc Natl Acad Sci U S A. 2015;112:13958–13963. doi: 10.1073/pnas.1507300112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Genissel A, McIntyre L, Wayne M, Nuzhdin S. Cis and trans regulatory effects contribute to natural variation in transcriptome of Drosophila melanogaster. Mol Biol Evol. 2008;25:101–10. doi: 10.1093/molbev/msm247. [DOI] [PubMed] [Google Scholar]
- 23.Hayes JP. Field and maximal metabolic rates of deer mice (Peromyscus maniculatus) at low and high altitudes. Physiol Zool. 1989;62:732–44. [Google Scholar]
- 24.Hochachka PW, Somero GN. Mechanism and process in physiological evolution. Oxford: Oxford University Press; 2002. Biochemical adaptation. [Google Scholar]
- 25.Huerta-Sanchez E, DeGiorgio M, Pagani L, Tarekegn A, Ekong R, Antao T, Cardona A, Montgomery HE, Cavalleri GL, Robbins PA, Weale ME, Bradman N, Bekele E, Kivisild T, Tyler-Smith C, Nielsen R. Genetic signatures reveal high-altitude adaptation in a set of Ethiopian populations. Mol Biol Evol. 2013;30:1877–88. doi: 10.1093/molbev/mst089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeong S, Rebeiz M, Andolfatto P, Werner T, True J, Carroll SB. The evolution of gene regulation underlies a morphological difference between two Drosophila sister species. Cell. 2008;132:783–93. doi: 10.1016/j.cell.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 27.Jones MR, Good JM. Targeted capture in evolutionary and ecological genomics. Mol Ecol. 2016;85:185–202. doi: 10.1111/mec.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelly JK. Geographical variation in selection, from phenotypes to molecules. Am Nat. 2006;167:481–95. doi: 10.1086/501167. [DOI] [PubMed] [Google Scholar]
- 29.Kiekens R, Vercauteren A, Moerkerke B, Goetghebeur E, van Den Daele H, Sterken R, Kuiper M, van Eeuwijk F, Vuylsteke M. Genome-wide screening for cis-regulatory variation using a classical diallel crossing scheme. Genome Res. 2006;34:3677–86. doi: 10.1093/nar/gkl510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kolaczkowski B, Kern AD, Holloway AK, Begun DJ. Genomic differentiation between temperate and tropical Australian populations of Drosophila melanogaster. Genetics. 2011;187:245–60. doi: 10.1534/genetics.110.123059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Latta RG. Differentiation of allelic frequencies at quantitative trait loci affecting locally adaptive traits. Am Nat. 1998;151:283–92. doi: 10.1086/286119. [DOI] [PubMed] [Google Scholar]
- 32.Le Corre V, Kremer A. Genetic variability at neutral markers, quantitative trait loci and trait in a subdivided population under selection. Genetics. 2003;164:1205–19. doi: 10.1093/genetics/164.3.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lehner B. Genotype to phenotype: lessons from model organisms for human genetics. Nat Rev Genet. 2013;14:168–78. doi: 10.1038/nrg3404. [DOI] [PubMed] [Google Scholar]
- 34.Lorenzo FR, Huff C, Myllymaki M, Olenchock B, Swierczek S, Tashi T, Gordeuk V, Wuren T, Ri-Li G, McClain DA, Khan TM, Koul PA, Guchhait P, Salama ME, Xing J, Semenza GL, Liberzon E, Wilson A, Simonson TS, Jorde LB, Kaelin WG, Jr, Koivunen P, Prchal JT. A genetic mechanism for Tibetan high-altitude adaptation. Nat Genet. 2014;46:951–6. doi: 10.1038/ng.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lui MA, Mahalingam S, Patel P, Connaty AD, Ivy CM, Cheviron ZA, Storz JF, McClelland GB, Scott GR. High-altitude ancestry and hypoxia acclimation have distinct effects on exercise capacity and muscle phenotype in deer mice. Am J Physiol Regul Integr Comp Physiol. 2015;308:R779–91. doi: 10.1152/ajpregu.00362.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mackay TFC, Stone EA, Ayroles JF. The genetics of quantitative traits: challenges and prospects. Nat Rev Genet. 2009;10:565–77. doi: 10.1038/nrg2612. [DOI] [PubMed] [Google Scholar]
- 37.McClelland G, Zwingelstein G, Taylor CR, Weber J-M. Increased capacity for circulatory fatty acid transport in a highly aerobic mammal. Am J Physiol. 1994;266(35):R1280–6. doi: 10.1152/ajpregu.1994.266.4.R1280. [DOI] [PubMed] [Google Scholar]
- 38.McGregor AP, Orgogozo V, Delon I, Zanet J, Srinivasan DG, Payre F, Stern DL. Morphological evolution through multiple cis-regulatory mutations at a single gene. Nature. 2007;448:587–90. doi: 10.1038/nature05988. [DOI] [PubMed] [Google Scholar]
- 39.McManus C, Coolon J, Duff M, Eiper-Mains J, Graveley B, Wittkopp P. Regulatory divergence in Drosophila revealed by mRNA-seq. Genome Res. 2010;20:816–25. doi: 10.1101/gr.102491.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Natarajan C, Hoffman FG, Lanier HC, Wolf CJ, Cheviron ZA, Spangler ML, Weber RE, Fago A, Storz JF. Intraspecific polymorphism, interspecific divergence, and the origins of function- altering mutations in deer mouse hemoglobin. Mol Biol Evol. 2015;32:978–97. doi: 10.1093/molbev/msu403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Natarajan C, Inoguchi N, Weber RE, Fago A, Moriyama H, Storz JF. Epistasis among adaptive mutations in deer mouse hemoglobin. Science. 2013;340:1324–7. doi: 10.1126/science.1236862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Natarajan C, Projecto-Garcia J, Moriyama H, Weber RE, Munoz-Fuentes V, Green AJ, Kopuchian C, Tubaro PL, Alza L, Bulgarella M, Smith MM, Wilson RE, Fago A, McCracken KG, Storz JF. Convergent evolution of hemoglobin function in high-altitude Andean waterfowl involves limited parallelism at the molecular sequence level. PLoS Genet. 2015;11:e1005681. doi: 10.1371/journal.pgen.1005681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ozsolak F, Milos P. RNA sequencing: advances, challenges and opportunities. Nat Rev Genet. 2011;12:87–98. doi: 10.1038/nrg2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pavlidis P, Jensen JD, Stephan W, Stamatakis A. A critical assessment of storytelling: gene ontology categories and the importance of validating genomic scans. Mol Biol Evol. 2012;29:3237–48. doi: 10.1093/molbev/mss136. [DOI] [PubMed] [Google Scholar]
- 45.Peng Y, Yang Z, Zhang H, Cui C, Qi X, Luo X, Tao X, Wu T, Ouzhuluobu B, Ciwangsangbu D, Chen H, Shi H, Su B. Genetic variations in Tibetan populations and high-altitude adaptation at the Himalayas. Mol Biol Evol. 2011;28:1075–81. doi: 10.1093/molbev/msq290. [DOI] [PubMed] [Google Scholar]
- 46.Przeworski M, Coop G, Wall JD. The signature of positive selection on standing genetic variation. Evolution. 2005;59:2312–23. [PubMed] [Google Scholar]
- 47.Rankin EB, Biju MP, Liu Q, Unger TL, Rha J, Johnson RS, Simon MC, Keith B, Haase VH. Hypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivo. J Clin Invest. 2007;117:1068–77. doi: 10.1172/JCI30117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rebeiz M, Pool JE, Kassner VA, Aquadro CF, Carroll SB. Stepwise modification of a modular enhancer underlies adaptation in a Drosophila population. Science. 2009;326:1663–7. doi: 10.1126/science.1178357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rockman MV. Reverse engineering the genotype-phenotype map with natural genetic variation. Nature. 2008;456:738–44. doi: 10.1038/nature07633. [DOI] [PubMed] [Google Scholar]
- 50.Runck AM, Weber RE, Fago A, Storz JF. Evolutionary and functional properties of a two- locus β-globin polymorphism in Indian house mice. Genetics. 2010;184:1121–31. doi: 10.1534/genetics.109.113506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scott GR. Elevated performance: the unique physiology of birds that fly at high altitudes. J Exp Biol. 2011;214:2455–62. doi: 10.1242/jeb.052548. [DOI] [PubMed] [Google Scholar]
- 52.Scott GR, Elogio TS, Lui MA, Storz JF, Cheviron ZA. Adaptive modifications of muscle phenotype in high-altitude deer mice are associated with evolved changes in gene regulation. Mol Biol Evol. 2015;32:1962–76. doi: 10.1093/molbev/msv076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scott GR, Milsom WK. Flying high: a theoretical analysis of the factors limiting exercise performance in birds at altitude. Respir Physiol Neurobiol. 2006;154:284–301. doi: 10.1016/j.resp.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 54.Sieberts SK, Schadt EE. Moving toward a system genetics view of disease. Mamm Genome. 2007;18:389–401. doi: 10.1007/s00335-007-9040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Simonson TS, Yang YZ, Huff CD, Yun HX, Qin G, Witherspoon DJ, Bai ZZ, Lorenzo FR, Xing JC, Jorde LB, Prchal JT, Ge RL. Genetic evidence for high-altitude adaptation in Tibet. Science. 2010;329:72–5. doi: 10.1126/science.1189406. [DOI] [PubMed] [Google Scholar]
- 56.Stern DL. Evolutionary developmental biology and the problem of variation. Evolution. 2000;54:1079–91. doi: 10.1111/j.0014-3820.2000.tb00544.x. [DOI] [PubMed] [Google Scholar]
- 57.Stern DL, Orgogozo V. Is genetic evolution predictable? Science. 2009;323:746–51. doi: 10.1126/science.1158997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stern DL, Orgogozo V. The loci of evolution: how predictable is genetic evolution? Evolution. 2008;62:2155–77. doi: 10.1111/j.1558-5646.2008.00450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Storz JF. Hemoglobin function and physiological adaptation to hypoxia in high-altitude mammals. J Mammal. 2007;88:24–31. [Google Scholar]
- 60.Storz JF. Using genome scans of DNA polymorphism to infer adaptive population divergence. Mol Ecol. 2005;14:671–88. doi: 10.1111/j.1365-294X.2005.02437.x. [DOI] [PubMed] [Google Scholar]
- 61.Storz JF, Bridgham JT, Kelly SA, Garland T. Genetic approaches in comparative and evolutionary physiology. Am J Physiol Regul Integr Comp Physiol. 2015;309:R197–214. doi: 10.1152/ajpregu.00100.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Storz JF, Kelly JK. Effects of spatially varying selection on nucleotide diversity and linkage disequilibrium: insights from deer mouse globin genes. Genetics. 2008;180:367–79. doi: 10.1534/genetics.108.088732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Storz JF, Natarajan C, Cheviron ZA, Hoffmann FG, Kelly JK. Altitudinal variation at duplicated β-globin genes in deer mice: effects of selection, recombination, and gene conversion. Genetics. 2012;190:203–16. doi: 10.1534/genetics.111.134494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Storz JF, Runck AM, Moriyama H, Weber RE, Fago A. Genetic differences in hemoglobin function between highland and lowland deer mice. J Exp Biol. 2010;213:2565–74. doi: 10.1242/jeb.042598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Storz JF, Runck AM, Sabatino SJ, Kelly JK, Ferrand N, Moriyama H, Weber RE, Fago A. Evolutionary and functional insights into the mechanism underlying high-altitude adaptation of deer mouse hemoglobin. Proc Natl Acad Sci U S A. 2009;106:14450–5. doi: 10.1073/pnas.0905224106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Storz JF, Sabatino SJ, Hoffmann FG, Gering EJ, Moriyama H, Ferrand N, Monteiro B, Nachman MW. The molecular basis of high-altitude adaptation in deer mice. PLoS Genet. 2007;3(e45):448–59. doi: 10.1371/journal.pgen.0030045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Storz JF, Scott GR, Cheviron ZA. Phenotypic plasticity and genetic adaptation to high-altitude hypoxia in vertebrates. J Exp Biol. 2010;213:4125–36. doi: 10.1242/jeb.048181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Storz JF, Wheat CW. Integrating evolutionary and functional approaches for inferring adaptation at specific loci. Evolution. 2010;64:2489–509. doi: 10.1111/j.1558-5646.2010.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Storz JF, Zera AJ. Experimental approaches to evaluate the contributions of candidate protein-coding mutations to phenotypic evolution. In: Orgogozo V, Rockman MV, editors. Molecular methods in evolutionary genetics. New York: Springer; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stupar R, Springer N. Cis-transcriptional variation in maize inbred lines b73 and m017 leads to additive expression patterns in the F1 hybrid. Genetics. 2006;173:2199–210. doi: 10.1534/genetics.106.060699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sucena E, Delon I, Jones I, Payre F, Stern DL. Regulatory evolution of shavenbaby/ovo underlies multiple cases of morphological parallelism. Nature. 2003;424:935–8. doi: 10.1038/nature01768. [DOI] [PubMed] [Google Scholar]
- 72.Sucena E, Stern DL. Divergence of larval morphology between Drosophila sechellia and its sibling species caused by cis-regulatory evolution of ovo/shaven-baby. Proc Natl Acad Sci U S A. 2000;97:4530–4. doi: 10.1073/pnas.97.9.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Teshima KM, Coop G, Przeworski M. How reliable are empirical genomic scans for selective sweeps? Genome Res. 2006;16:702–12. doi: 10.1101/gr.5105206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Teshima KM, Przeworski M. Directional positive selection on an allele of arbitrary dominance. Genetics. 2006;172:713–8. doi: 10.1534/genetics.105.044065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tishkoff SA, Reed FA, Ranciaro A, Voight BF, Babbitt CC, Silverman JS, Powell K, Mortensen HM, Hirbo JB, Osman M, Ibrahim M, Omar SA, Lema G, Nyambo TB, Ghori J, Bumpstead S, Pritchard JK, Wray GA, Deloukas P. Convergent adaptation of human lactase persistence in Africa and Europe. Nat Genet. 2007;39:31–40. doi: 10.1038/ng1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tufts DM, Revsbech I, Cheviron ZA, Weber RE, Fago A, Storz JF. Phenotypic plasticity in blood-oxygen transport in highland and lowland deer mice. J Exp Biol. 2013;216:1167–73. doi: 10.1242/jeb.079848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wagner PD. Determinants of maximal oxygen transport and utilization. Annu Rev Physiol. 1996;58:21–50. doi: 10.1146/annurev.ph.58.030196.000321. [DOI] [PubMed] [Google Scholar]
- 78.Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet. 2009;10:57–63. doi: 10.1038/nrg2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Whitehead A. Comparative genomics in ecological physiology: toward a more nuanced understanding of acclimation and adaptation. J Exp Biol. 2012;215:884–91. doi: 10.1242/jeb.058735. [DOI] [PubMed] [Google Scholar]
- 80.Wittkopp PJ, Haerum BK, Clark AG. Regulatory changes underlying expression differences within and between Drosophila species. Nat Genet. 2008;40:346–50. doi: 10.1038/ng.77. [DOI] [PubMed] [Google Scholar]
- 81.Wray GA. The evolutionary significance of cis-regulatory mutations. Nat Rev Genet. 2007;8:206–16. doi: 10.1038/nrg2063. [DOI] [PubMed] [Google Scholar]
- 82.Xiang K, Ouzhuluobo, Peng Y, Yang Z, Zhang X, Cui C, Zhang H, Li M, Zhang Y, Bianba G, Basang C, Wu T, Chen H, Shi H, Qi X, Su B. Identification of a Tibetan-specific mutation in the hypoxic gene EGLN1 and its contribution to high-altitude adaptation. Mol Biol Evol. 2013;30:1889–98. doi: 10.1093/molbev/mst090. [DOI] [PubMed] [Google Scholar]
- 83.Xing J, Wuren T, Simonsen TS, Watkins WS, Witherspoon DJ, Wu W, Qin G, Huff CD, Jorde LB, Ge RL. Genomic analysis of natural selection and phenotypic variation in high-altitude Mongolians. PLoS Genet. 2013;9:e1003634. doi: 10.1371/journal.pgen.1003634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xu S, Li SG, Yang YZ, Tan J, Lou H, Jin W, Yang L, Pan X, Wang J, Shen Y, Wu B, Wang H, Jin L. A genome-wide search for signals of high-altitude adaptation in Tibetans. Mol Biol Evol. 2011;28:1003–11. doi: 10.1093/molbev/msq277. [DOI] [PubMed] [Google Scholar]
- 85.Yi X, Liang Y, Huerta-Sanchez E, Jin X, Cuo ZXP, Pool JE, Xu X, Jiang H, Vinckenbosch N, Korneliussen TS, Zheng HC, Liu T, He WM, Li K, Luo RB, Nie XF, Wu HL, Zhao MR, Cao HZ, Zou J, Shan Y, Li SZ, Yang Q, Asan, Ni PX, Tian G, Xu JM, Liu XA, Jiang T, Wu RH, Zhou GY, Tang MF, Qin JJ, Wang T, Feng SJ, Li GH, Huasang, Luosang JB, Wang W, Chen F, Wang YD, Zheng XG, Li Z, Bianba ZM, Yang G, Wang XP, Tang SH, Gao GY, Chen Y, Luo Z, Gusang L, Cao Z, Zhang QH, Ouyang WH, Ren XL, Liang HQ, Zheng HS, Huang YB, Li JX, Bolund L, Kristiansen K, Li YR, Zhang Y, Zhang XQ, Li RQ, Li SG, Yang HM, Nielsen R, Wang J, Wang JA. Sequencing of 50 human exomes reveals adaptation to high altitude. Science. 2010;329:75–8. doi: 10.1126/science.1190371. [DOI] [PMC free article] [PubMed] [Google Scholar]