Abstract
Longevity varies among individuals, but how natural genetic variation contributes to variation in lifespan is poorly understood. Drosophila melanogaster presents an advantageous model system to explore the genetic underpinnings of longevity, since its generation time is brief and both the genetic background and rearing environment can be precisely controlled. The bellwether (blw) gene encodes the α subunit of mitochondrial ATP synthase. Since metabolic rate may influence lifespan, we investigated whether alternative haplotypes in the blw promoter affect lifespan when expressed in a co-isogenic background. We amplified 521 bp upstream promoter sequences containing alternative haplotypes and assessed promoter activity both in vitro and in vivo using a luciferase reporter system. The AG haplotype showed significantly greater expression of luciferase than the GT haplotype. We then overexpressed a blw cDNA construct driven by either the AG or GT haplotype promoter in transgenic flies and showed that the AG haplotype also results in greater blw cDNA expression and a significant decrease in lifespan relative to the GT promoter haplotype, in male flies only. Thus, our results show that naturally occurring regulatory variants of blw affect lifespan in a sex-specific manner.
Introduction
Lifespan is highly variable among individuals and is determined by the complex interplay between genetic and environmental factors1, 2. Evolutionary theories regarding genetic limitations on lifespan have proposed the persistence of deleterious alleles in the genome that are activated at later age after reproduction3–6, or antagonistic pleiotropy of alleles that are beneficial early in life and deleterious later on7, 8. Oxidative stress9–11, genomic instability12–15, telomere length16–18 and DNA repair mechanisms19–22 have been implicated as mechanisms that affect aging and longevity. However, little is known about the mechanisms by which naturally occurring allelic variants within a population affect variation in lifespan.
Oxidative stress occurs through the production of reactive oxygen species (ROS) as a byproduct of mitochondrial oxidative phosphorylation23, 24. Previously, single nucleotide polymorphisms (SNPs) in the promoter region of the Drosophila bellwether (blw) gene have been associated with differences in lifespan between control flies and long-lived lines of flies originally selected for delayed reproduction25, 26. This study showed that all four of the long-lived lines selected for postponed reproduction that were genotyped for the blw promoter were fixed for the GT haplotype, but this haplotype was lost or at very low frequency in the five controls that were genotyped for the blw promoter25. The blw gene encodes the α subunit of mitochondrial ATP synthase, suggesting that sequence variants in this gene could give rise to subtle differences in metabolic rate which could affect the production of ROS during the organism’s lifespan27. Here, we show that alternative haplotypes in the promoter region of blw result in different levels of gene expression and that introduction of a transgenic blw construct driven by these alternative promoters in a co-isogenic background causes a profound sex-specific effect on lifespan. These results provide a mechanistic link between lifespan and allelic variation in a central metabolic gene.
Results
RNAi-mediated inhibition of blw expression results in lethality
The Drosophila blw gene is located on chromosome 2 (Chr2R:22,799,099…22,802,180 [+]) and generates a single transcript composed of a 5′-UTR (105 bp), 4 exons (66 bp, 581 bp, 802 bp and 210 bp) and a 3′-UTR (488 bp) (Fig. 1)28. Prior to examining regulation of blw expression under alternative promoters, we assessed the effect of RNAi-mediated knockdown of blw expression by crossing flies homozygous for a UAS-blw-RNAi transgene to flies that drive GAL4 expression under ubiquitin or actin promoters. No embryos developed when blw was knocked down with the actin promoter. When blw was knocked-down using the ubiquitin promoter, flies reached the pupal stage but did not eclose from the pupal cases. These results demonstrate that blw is an essential gene for development and viability of D. melanogaster.
Figure 1.
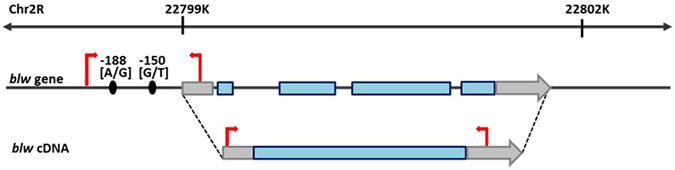
Diagram of the structure of the D. melanogaster blw gene. The blw gene is located on Chr2R:22,799,099…22,802,180 [+] and generates a single transcript composed of a 5′-UTR (gray box), four exons (blue boxes) and a 3′-UTR (gray arrow) (Flybase.org). The two SNPs are located 150 bp [G/T] and 188 bp [A/G] upstream of the blw transcriptional start site (gray box). The location of PCR primers used to generate the blw-promoter or blw-cDNA are indicated by red arrows.
Functional analysis of the blw promoter region
To examine whether alternative haplotypes in the blw promoter affect gene expression, we generated promoter constructs of different lengths containing the AG haplotype and tested their activity in an in vitro firefly luciferase reporter system. All blw promoter constructs encompass the 56 bp region containing the GAGA and Adf-1 elements essential for promoter activity29. All constructs were effective in driving luciferase expression (Fig. 2a) and therefore we selected the shortest, 521 bp promoter region for further studies.
Figure 2.
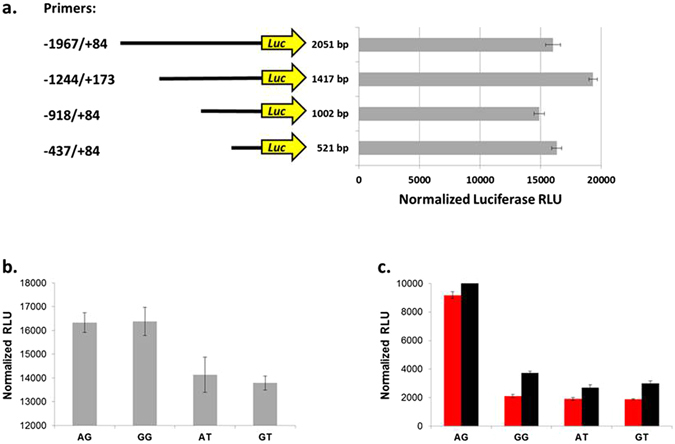
Relative strength of the putative promoter region of the blw gene. (a) Four different lengths of the blw-promoter region harboring the AG haplotype were cloned in the pGL3-basic vector and expressed in Drosophila S2 cells. Primer locations are indicated with respect to the transcriptional start site of the blw gene (see Fig. 1). Black lines represent the promoter length and the yellow arrow represents the luciferase reporter gene. (b) The 521 bp blw-promoter region harboring the AG, GG, AT and GT haplotypes were cloned in the pGL3-basic vector and expressed in Drosophila S2 cells. (c) The same promoters were expressed in flies using the Gal4-UAS binary expression system. For each experiment, protein lysates were extracted and luciferase expression was measured and normalized (relative light units; RLU). Gray bars represent the Drosophila S2 cells (Panels a and b). Female flies are represented by red bars and males by black bars (Panel c). Error bars are standard errors of the mean.
We cloned the four haplotypes of the 521 bp blw promoter region, −188A/−150G, −188A/−150T, −188G/−150G and −188G/−150T (designated AG, AT, GG and GT respectively), into the pGL3-basic-Luciferase vector and transfected Drosophila S2 cells. The AG and GG haplotypes showed substantially greater luciferase expression than the AT and GT haplotypes (P < 0.0001) (Fig. 2b).
Next, we asked whether the same difference would be replicated in vivo. We cloned the same promoters in a pattB-Gal4-hsp70 vector and drove luciferase expression in transgenic flies by crossing flies homozygous for the Gal4-blw-promoter with flies carrying a UAS-luciferase construct. We measured luciferase activity in protein lysates from the F1, sexes separately, to compare the strength of each promoter (Fig. 2c). Here, the AG haplotype showed approximately 3-fold greater luciferase expression than the other haplotypes in both sexes (P < 0.0001).
We compared our in vitro and in vivo observations. Analyses of variance revealed a significant difference among the haplotypes and in promoter strength between the −150G and −150T SNPs both in cell culture and in flies (P = 0.0008 (in vitro); P < 0.001 (in vivo) Fig. 2). In both cases, the −150G allele results in stronger promoter expression. In contrast, the −188[A/G] appears to have no effect when expressed in cell culture (P = 0.823). When expressed in flies, however, the −188A allele is significantly stronger than the −188G allele (P < 0.0001). These results reveal the effect of each individual SNP on the strength of the blw promoter. Based on our in vivo results, we focused further experiments on the effects of the AG and GT haplotypes on lifespan.
Overexpression of blw from the AG promoter shortens lifespan
We used the Gal4-UAS binary expression system30, 31 to investigate the effects of overexpression of a blw cDNA construct driven by promoters with either the AG or GT haplotype in a co-isogenic background. Quantitative real-time PCR showed that the promoter with the AG haplotype drives stronger blw cDNA expression than the GT haplotype (P = 0.01), in line with observations from our in vivo and in vitro luciferase reporter gene experiments (Fig. 3a). Flies in which blw cDNA expression is driven by the AG promoter, however, have a reduced median lifespan compared to flies in which blw cDNA expression is driven by the GT promoter (Fig. 3b). We fitted a mixed effects Cox model including the sex by haplotype term to the lifespan data. This analysis revealed a strong sex by haplotype effect (P < 6 × 10−5, Fig. 3b). Therefore we performed survival analyses for sexes separately. For each sex, we fitted another mixed effects Cox model, testing for significance of the differences of hazard between the two haplotypes and with controls. In males there was no difference between haplotype GT and control (HR = 0.99, P = 0.96), but the hazard for the GT haplotype was markedly higher than the AG haplotype (Fig. 3c, HR = 12.83, P = 2.47 × 10−32). By contrast, in females there was a significant effect between the AG and GT haplotypes with the controls (hazard ratio (HR) = 1.70 and P = 0.003 for AG versus control and HR = 1.69 and P = 0.004 for GT versus control) but no difference between the AG and GT haplotypes (HR = 0.99, P = 0.96; Fig. 3d).
Figure 3.
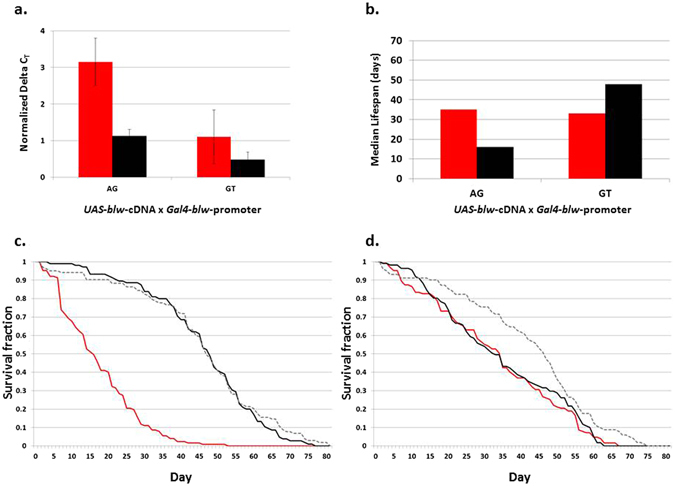
Effect of blw-cDNA overexpression on lifespan. (a) Normalized expression levels measured by quantitative RT-PCR of blw-cDNA when driven by the blw-AG and blw-GT promoters (red, females; black, males). (b) Median lifespan of flies that overexpress blw-cDNA from the blw-AG and blw-GT promoters (red, females; black, males). (c) Survival curves of male flies overexpressing blw-cDNA when driven by either the blw-AG (red) or blw-GT promoter (black). (d) Survival curves of female flies overexpressing blw-cDNA when driven by either the blw-AG (red) or blw-GT promoter (black). The control flies expressing the endogenous blw gene are shown by gray dotted lines.
Discussion
Sex differences in lifespan have been observed in model organisms including C. elegans, D. melanogaster, and Mus musculus 32, 33. Sexual dimorphism in genetic architecture34 is a common feature of quantitative traits and has been documented for morphological35–37, behavioral38, 39, physiological40, 41, and life history traits42, 43, including lifespan44, 45, in Drosophila. The genetic architecture of Drosophila lifespan in particular, is sexually dimorphic25, 45–50. Here, we investigated the effects of allelic variants in the promoter region of blw on lifespan and showed that overexpression of the AG haplotype shortens male lifespan compared to its GT counterpart. Our luciferase-reporter assays revealed a significant difference in promoter expression between the AG and GT haplotypes both in cell culture and in flies.
The blw gene encodes the ATP synthase α subunit, essential for oxidative phosphorylation27, and it is therefore not surprising that inhibition of blw expression by RNAi results in lethality. This observation is in line with previous studies on blw mutant alleles, which showed larval growth defects affecting all tissues, DNA endoreplication defects, and larval lethality with no homozygous animals reaching the pupal stage51. A previous study showed that a blw mutant, generated by insertion of a transposable element in the 5′ UTR (blw KG05893), reduced adipose tissue growth and triglyceride storage and increased ROS in third instar larval fat bodies52.
All four long-lived D. melanogaster lines (O-lines) selected for postponed reproduction that were genotyped for the blw promoter contain the GT haplotype, whereas this haplotype was lost or present at low frequency in all of the corresponding control base lines (B-lines)25. Assessment of feeding behavior, measured by a capillary feeding (CAFÉ) assay, showed that the B-lines consume more sucrose compared to the O-lines and that food consumption declines with age. The increased feeding behavior of the B-lines may correlate with increased metabolic rate and, therefore, shorter lifespan compared to the O-lines25. This corresponds with our results, since we observe a stronger promoter activity of the blw-AG haplotype (representative of the B-lines) resulting in higher expression levels of blw-cDNA, and a decreased male lifespan of the UAS-blw-cDNA x Gal4-blw-AG promoter lines.
Mechanisms of aging may involve metabolic regulation through the insulin signaling pathway, as evident from the effects of mutations in components of this pathway, including foxo, InR and chico 53–56. In addition, the major nutrient-signaling pathways, that depend on mTOR 57–59, Sir2 60, 61, and insulin-like 62, 63 genes, have been associated with extension of lifespan in flies subjected to dietary restriction. However, the benefits of dietary restriction on lifespan extension are eliminated by exposure to oligomycin, a specific inhibitor of mitochondrial ATP synthase64, implicating the electron transport chain.
Invadolysin, a lipid-droplet associated protein, interacts physically with three mitochondrial ATP synthase subunits: α (bellwether), β and δ52. Multiple proteomic screens have demonstrated that the ATP synthase subunits also interact with lipid droplets in Drosophila embryos, third instar larvae, and in human adipocytes65. Both invadolysin and blw mutants have defects in mitochondrial electron transport chain activity and thus produce high levels of ROS52. Furthermore, invadolysin mutants exhibit increased autophagy and decreased glycogen storage66. Together, these data suggest that blw plays a role in lifespan determination via its physical interaction with invadolysin.
Previous studies on aging in Drosophila have led to the discovery of additional genes that extend lifespan, including mth 67–69, Indy 70, 71, InR 55, 72, 73, chico 56, 74, 75, and SOD 76–79. Also, bride of sevenless (boss) null mutants have shortened lifespans, diminished locomotor performance and elevated ROS production80. In addition, boss mutant flies express higher levels of blw compared to control flies, further implicating a connection between decreased lifespan and increased metabolic rate, correlated with expression of blw.
It should be noted that our transgenic flies that overexpress blw from a cDNA construct still contain endogenous blw. The presence of the endogenous gene might amplify the deleterious effect of overexpression of blw under the AG promoter in males as it may allow overexpression of the transgene to surpass a critical threshold, which might not be reached in the absence of the endogenous gene.
It is tempting to speculate that greater expression of the blw ATP synthase α-subunit under the AG promoter may result in enhanced metabolic rate, generating more ROS, which results in shorter lifespan. In this scenario, the female sex environment would appear to be protective against the effects of the AG haplotype and metabolically generated oxidative stress. Although further experiments are necessary to consolidate or refute this hypothesis, our study demonstrates a link between allelic variation in the promoter of the blw gene and Drosophila lifespan.
Methods
In-vitro promoter luciferase assays
We used PCR to amplify four different lengths (521 bp, 1002 bp, 1417 bp and 2051 bp) of the promoter region, containing the AG haplotype, upstream of the blw coding region from genomic DNA using directional primers based on the Drosophila reference strain (line 2057) and cloned the amplicons into the Kpn1/Xho1 multiple cloning site of the pGL3-basic vector (Promega). We screened colonies by Kpn1/Xho1 double-digestion and Sanger sequencing to identify positive clones, and used site-directed mutagenesis to generate the other haplotypes using Pfu phusion HotStart Flex DNA polymerase (New England Biolabs). Following PCR-amplification the parental template was digested with Dpn1, and the DNA was transformed into JM109 competent cells (Promega). Clones were purified using the Qiagen MiniPrep kit (Qiagen) and validated by Sanger sequencing81.
Drosophila S2 cells were cultured at room temperature in Schneider’s Drosophila medium (Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum (Invitrogen) and 100 μg/ml of gentamicin (Gibco). Cells were counted 24 h prior to transfection using a Countess Automated Cell Counter (Invitrogen) and 1 million cells were transferred to the wells of a 6-well plate. Each blw promoter construct was co-transfected with a Renilla luciferase vector as an internal transfection control (pGL4.74[hRluc/TK] vector; Promega, E6921) using Cellfectin II reagent (Invitrogen). Transfections were performed in triplicate. After incubation for 72 h at 28 °C protein lysates were extracted and subjected to a Dual-Glo luciferase assay (Promega). Firefly and Renilla luciferase activity were measured with a GloMax luminometer. The firefly luciferase activity was normalized against the Renilla luciferase activity for each sample and data were analyzed using SAS software version 9.382. We performed an analysis of variance (ANOVA) for luciferase activity: Y = μ + H + ε, where Y is the observed value, μ is the mean, H is the promoter haplotype, and ε is the residual (error) variance. The normalized relative light units emitted by the assay revealed the strength of each promoter.
In-vivo promoter luciferase assays
We excised the 521 bp blw-promoter inserts from the pGL3-basic vector with Kpn1 and BglII and ligated them into the pattB-Gal4-synaptobrevin-hsp70 vector (Addgene; Plasmid #46107) after excision of the synaptobrevin promoter with BamH1 and EcoR1. Since the inserts and plasmids contained incompatible ends, cloning was achieved using the In-Fusion® HD Cloning Plus CE kit (Clontech) with the following primers to amplify the blw-promoter inserts: blw-InFusion-F, 5′-TTATGCTAGCGGATCTGGCGGCGTCCACATATA and blw-InFusion-R, 5′- CTTCATGTTGGAATTACTGTTCGCCGCAGAAGT. The PCR products were treated with Cloning Enhancer (Clontech) and subjected to InFusion cloning reactions with the linearized pattB-Gal4-hsp70 vector. We transformed the DNA constructs into Stellar competent cells (Clontech) and validated clones by Sanger sequencing81. Purified constructs were subjected to PhiC31 transformation83–86 in the Drosophila strain of genotype y w P[int, y +]; P[attP2, y +] where the attP2 landing site is located at 68A4 on the 3rd chromosome, by Model System Injections (Durham, NC). We identified positive transformants and, using balancer chromosomes and visible eye markers, created homozygous Gal4-attP2-blw-promoter flies. These were crossed to a homozygous UAS-luciferase reporter line. Protein lysates were extracted from sexes separately with 1X Luciferase Cell Culture Lysis Reagent (Promega) and quantified using the Bio-Rad DC Protein Assay kit II (Bio-Rad). The promoter activities were assessed with the Steady-Glo Luciferase Assay System (Promega) on a GloMax luminometer and data were analyzed using SAS software version 9.382. We performed an ANOVA for luciferase activity, separately for males and females with form: Y = μ + H + S + H × S + ε, where H and S are haplotype and sex, respectively, and ε is the residual (error) variance. The normalized relative light units emitted by the assay revealed the strength of each promoter.
Knockdown of blw using RNAi
A UAS-blw-RNAi line (ID = 34664) was obtained from the Vienna Drosophila Resource Center (VDRC)87. These flies were crossed to flies containing either an ubiquitin driver (Gal4-Ubi156) or an actin driver (Gal4-Actin) to disrupt blw expression. The progenitor VIE-260B genotype was also crossed to both drivers as a control.
Overexpression of blw cDNA
To overexpress blw in flies, we amplified blw cDNA from the Drosophila reference strain 2057 and cloned it into the pUAST-attb vector at the Not1/Xba1 restriction sites. The pUAST-attB-blw-cDNA purified construct was subjected to PhiC31 transformation83–86 to the Drosophila strain having the following genotype: y w P[int, y +]; P[attP2, y +] where the attP2 landing site is located at 68A4 on the 3rd chromosome (Model System Injections; Durham, NC). The injected G0 flies were crossed to a 2nd and 3rd chromosome balancer line (w 1118 iso CSB; CyO/Sp; TM3, Sb/H) and the G1 progeny were selected for the orange/red-eyed and Cy phenotype (positive transformants). w 1118 iso CSB is an isogenic X chromosome from the Canton S B (CSB) strain. Positive male transformants (G1) from G0 males were crossed to virgin females from a 3rd chromosome balancer line (w 1118 iso CSB; 2 iso CSB; TM3, Sb/H) and the resulting F1 flies (w 1118 iso CSB; 2 iso CSB/Cy; P[attP2, y + blw-cDNA w +]/Sb) were screened for red-eyed flies with the Cy and Sb phenotypes. 2 iso CSB is an isogenic 2nd chromosoems from the CSB strain Siblings were crossed to create homozygous flies of genotype w 1118 iso CSB; 2 iso CSB; P[attP2, y + blw-cDNA w +]). The homozygous flies were crossed to homozygous Gal4 driver lines (w 1118 iso CSB; 2 iso CSB; P[attP2, y + Gal4-blw-GT(or AG) w +]) and the lifespans of the resulting progeny were measured. As control for lifespan, we used F1 progeny from the cross between w 1118 iso CSB; 2 iso CSB; P[attP2, y +] and the Ubiquitin driver line, w 1118 iso CSB; 2 iso CSB;Ubi-Gal4[156].
Lifespan measurements
Flies were generated for each blw promoter haplotype under controlled adult density conditions, by allowing 6 males and 6 females to mate and lay eggs for one day in vials containing 10 ml cornmeal-molasses-agar medium (cornmeal, 65 g/L; molasses, 45 ml/L; yeast, 13 g/L) under a 12 h light-dark cycle. Offspring from these vials were collected at 1–3 days post-eclosion for lifespan assays. Lifespan was assessed for each haplotype using 48 replicate vials, each containing 3 males and 3 females on 5 ml culture medium. We transferred flies without anesthesia every 2–3 days to new vials containing 5 ml of fresh food. We removed dead flies upon observation and recorded deaths every 1–3 days until all individuals were deceased.
To assess statistical significance for differences in lifespan, we fitted a Cox mixed effects model, where the hazard function is determined by fixed effects for sex, haplotype and the interaction between sex and haplotype, and random effects replicate within haplotype and sex by replicate effects. The model was fitted using the ‘coxme’ library in R88. We further performed a stratified analysis in each sex separately. Assumption of the hazard proportionality was checked using the ‘cox.zph’ function in the ‘survival’ package in R and was found to be met for the models fitted.
Quantitative real time PCR
Total RNA was extracted from the progeny of the Gal4-blw-promoter x UAS-blw cDNA lines and the Gal4-ubiquitin x y,w, P[int, y +]; P[attP2, y +] control line. We synthesized cDNA from 120 ng of total RNA using the iScript cDNA synthesis kit (Bio-Rad) and performed quantitative RT-PCR using the Maxima SYBR Green/ROX qPCR master mix (Thermo Scientific) with the following primer pair specific to blw cDNA, 5′-ATGCAGACCGGTATCAAGG and 5′-GACGGTGGAACGCTTCTG. GAPDH was used as the internal control. The expression levels for blw-cDNA when driven by the blw-promoters were normalized against the control line to account for endogenous blw expression. The data were analyzed using the comparative CT (threshold cycle) method58. We performed an ANOVA for blw expression levels of form: Y = μ + H + S + H × S + ε, where H and S are line and sex, respectively, and ε is the residual (error) variance. ANOVAs were performed using SAS software version 9.382.
Data availability statement
All relevant data are contained within the manuscript. Additional raw data will be available upon request.
Acknowledgements
The pUAST-attb vector was kindly provided by Drs. Johannes Bischof and Konrad Basler from the University of Zurich. We would like to thank Aimee Durrett for assistance with the lifespan assays and Dr. Akihiko Yamamoto for help with the fly crosses. We thank Dr. Wen Huang for helpful discussions and performing the Cox mixed effects model analyses. This work was supported by NIH grants AG043490, AA016560 and GM059469 to TFCM and RRHA.
Author Contributions
M.A.C., T.F.C.M. and R.R.H.A. designed the experiments. J.F.G. and M.A.C. performed the experiments and compiled the data. M.A.C., T.F.C.M. and R.R.H.A. wrote the manuscript. T.F.C.M. and R.R.H.A. supervised the research.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bitto A, Wang AM, Bennett CF, Kaeberlein M. Biochemical genetic pathways that modulate aging in multiple species. Cold Spring Harb Perspect Med. 2015;5:pii: 1025114. doi: 10.1101/cshperspect.a025114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Briga M, Verhulst S. What can long-lived mutants tell us about mechanisms causing aging and lifespan variation in natural environments? Exp Gerontol. 2015;71:21–26. doi: 10.1016/j.exger.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Charlesworth B. Mutation-selection balance and the evolutionary advantage of sex and recombination. Genet Res. 1990;55:199–221. doi: 10.1017/S0016672300025532. [DOI] [PubMed] [Google Scholar]
- 4.Charlesworth, B. Evolution in age-structured populations. (Cambridge University Press, 1994).
- 5.Hamilton WD. The moulding of senescence by natural selection. J Theor Biol. 1966;12:12–45. doi: 10.1016/0022-5193(66)90184-6. [DOI] [PubMed] [Google Scholar]
- 6.Mueller LD. Evolution of accelerated senescence in laboratory populations of Drosophila. Proc Natl Acad Sci USA. 1987;84:1974–1977. doi: 10.1073/pnas.84.7.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charlesworth B. Evolutionary mechanisms of senescence. Genetica. 1993;91:11–19. doi: 10.1007/BF01435984. [DOI] [PubMed] [Google Scholar]
- 8.Sgro CM, Partridge L. A delayed wave of death from reproduction in Drosophila. Science. 1999;286:2521–2524. doi: 10.1126/science.286.5449.2521. [DOI] [PubMed] [Google Scholar]
- 9.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 10.Miquel J. An update on the oxygen stress-mitochondrial mutation theory of aging: genetic and evolutionary implications. Exp Gerontol. 1998;33:113–126. doi: 10.1016/S0531-5565(97)00060-0. [DOI] [PubMed] [Google Scholar]
- 11.Sies H. Oxidative stress: a concept in redox biology and medicine. Redox Biol. 2015;4:180–183. doi: 10.1016/j.redox.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bohr VA. Human premature aging syndromes and genomic instability. Mech Ageing Dev. 2002;123:987–993. doi: 10.1016/S0047-6374(02)00039-8. [DOI] [PubMed] [Google Scholar]
- 13.Busuttil RA, Dolle M, Campisi J, Vijga J. Genomic instability, aging, and cellular senescence. Ann N Y Acad Sci. 2004;1019:245–255. doi: 10.1196/annals.1297.041. [DOI] [PubMed] [Google Scholar]
- 14.Franceschi C, et al. Genomic instability and aging. Studies in centenarians (successful aging) and in patients with Down’s syndrome (accelerated aging) Ann N Y Acad Sci. 1992;663:4–16. doi: 10.1111/j.1749-6632.1992.tb38643.x. [DOI] [PubMed] [Google Scholar]
- 15.Moskalev AA, et al. The role of DNA damage and repair in aging through the prism of Koch-like criteria. Ageing Res Rev. 2013;12:661–684. doi: 10.1016/j.arr.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Anchelin M, Murcia L, Alcaraz-Perez F, Garcia-Navarro EM, Cayuela ML. Behaviour of telomere and telomerase during aging and regeneration in zebrafish. PLoS One. 2011;6:e16955. doi: 10.1371/journal.pone.0016955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kruk PA, Rampino NJ, Bohr VA. DNA damage and repair in telomeres: relation to aging. Proc Natl Acad Sci USA. 1995;92:258–262. doi: 10.1073/pnas.92.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanders JL, Newman AB. Telomere length in epidemiology: a biomarker of aging, age-related disease, both, or neither? Epidemiol Rev. 2013;35:112–131. doi: 10.1093/epirev/mxs008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ciccia A, Elledge SJ. The DNA damage response: making it safe to play with knives. Mol Cell. 2010;40:179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dolle ME, Vijg J. Genome dynamics in aging mice. Genome Res. 2002;12:1732–1738. doi: 10.1101/gr.125502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorbunova V, Seluanov A. DNA double strand break repair, aging and the chromatin connection. Mutat Res. 2016;788:2–6. doi: 10.1016/j.mrfmmm.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.White RR, Vijg J. Do DNA double-strand breaks drive aging? Mol Cell. 2016;63:729–738. doi: 10.1016/j.molcel.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kong Y, Trabucco SE, Zhang H. Oxidative stress, mitochondrial dysfunction and the mitochondria theory of aging. Interdiscip Top Gerontol. 2014;39:86–107. doi: 10.1159/000358901. [DOI] [PubMed] [Google Scholar]
- 24.Wang CH, Wu SB, Wu YT, Wei YH. Oxidative stress response elicited by mitochondrial dysfunction: implication in the pathophysiology of aging. Exp Biol Med (Maywood) 2013;238:450–460. doi: 10.1177/1535370213493069. [DOI] [PubMed] [Google Scholar]
- 25.Carnes MU, et al. The genomic basis of postponed senescence in Drosophila melanogaster. PLoS One. 2015;10:e0138569. doi: 10.1371/journal.pone.0138569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rose MR. Laboratory evolution of postponed senescence in Drosophila melanogaster. Evolution. 1984;38:1004–1010. doi: 10.1111/j.1558-5646.1984.tb00370.x. [DOI] [PubMed] [Google Scholar]
- 27.Jacobs H, Stratmann R, Lehner C. A screen for lethal mutations in the chromosomal region 59AB suggests that bellwether encodes the alpha subunit of the mitochondrial ATP synthase in Drosophila melanogaster. Mol Gen Genet. 1998;259:383–387. doi: 10.1007/s004380050826. [DOI] [PubMed] [Google Scholar]
- 28.Gramates LS, et al. FlyBase at 25: looking to the future. Nucleic Acids Res. 2017;45:D663–D671. doi: 10.1093/nar/gkw1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Talamillo A, et al. Expression of the Drosophila melanogaster ATP synthase alpha subunit gene is regulated by a transcriptional element containing GAF and Adf-1 binding sites. Eur J Biochem. 2004;271:4003–4013. doi: 10.1111/j.1432-1033.2004.04336.x. [DOI] [PubMed] [Google Scholar]
- 30.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 31.Fischer JA, Giniger E, Maniatis T, Ptashne M. GAL4 activates transcription in Drosophila. Nature. 1988;332:853–856. doi: 10.1038/332853a0. [DOI] [PubMed] [Google Scholar]
- 32.Maklakov AA, Lummaa V. Evolution of sex differences in lifespan and aging: causes and constraints. Bioessays. 2013;35:717–724. doi: 10.1002/bies.201300021. [DOI] [PubMed] [Google Scholar]
- 33.Tower J. Sex-specific regulation of aging and apoptosis. Mech Ageing Dev. 2006;127:705–718. doi: 10.1016/j.mad.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Williams TM, Carroll SB. Genetic and molecular insights into the development and evolution of sexual dimorphism. Nat Rev Genet. 2009;10:797–804. doi: 10.1038/nrg2687. [DOI] [PubMed] [Google Scholar]
- 35.Kopp A, Duncan I, Godt D, Carroll SB. Genetic control and evolution of sexually dimorphic characters in Drosophila. Nature. 2000;408:553–559. doi: 10.1038/35046017. [DOI] [PubMed] [Google Scholar]
- 36.Kopp A, Graze RM, Xu S, Carroll SB, Nuzhdin SV. Quantitative trait loci responsible for variation in sexually dimorphic traits in Drosophila melanogaster. Genetics. 2003;163:771–787. doi: 10.1093/genetics/163.2.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Testa ND, Dworkin I. The sex-limited effects of mutations in the EGFR and TGF-beta signaling pathways on shape and size sexual dimorphism and allometry in the Drosophila wing. Dev Genes Evol. 2016;226:159–171. doi: 10.1007/s00427-016-0534-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carbone MA, et al. Genetic architecture of natural variation in visual senescence in Drosophila. Proc Natl Acad Sci USA. 2016;113:E6620–E6629. doi: 10.1073/pnas.1613833113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swarup S, Huang W, Mackay TFC, Anholt RRH. Analysis of natural variation reveals neurogenetic networks for Drosophila olfactory behavior. Proc Natl Acad Sci USA. 2013;110:1017–1022. doi: 10.1073/pnas.1220168110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harbison ST, McCoy LJ, Mackay TFC. Genome-wide association study of sleep in Drosophila melanogaster. BMC Genomics. 2013;14:281. doi: 10.1186/1471-2164-14-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jordan KW, Morgan TJ, Mackay TFC. Quantitative trait loci for locomotor behavior in Drosophila melanogaster. Genetics. 2006;174:271–284. doi: 10.1534/genetics.106.058099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harbison ST, Chang S, Kamdar KP, Mackay TFC. Quantitative genomics of starvation stress resistance in Drosophila. Genome Biol. 2005;6:R36. doi: 10.1186/gb-2005-6-4-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ober U, et al. Accounting for genetic architecture improves sequence based genomic prediction for a Drosophila fitness trait. PLoS One. 2015;10:e0126880. doi: 10.1371/journal.pone.0126880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ivanov DK, et al. Longevity GWAS using the Drosophila Genetic Reference Panel. J Gerontol A Biol Sci Med Sci. 2015;70:1470–1478. doi: 10.1093/gerona/glv047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nuzhdin SV, Pasyukova EG, Dilda CL, Zeng ZB, Mackay TFC. Sex-specific quantitative trait loci affecting longevity in Drosophila melanogaster. Proc Natl Acad Sci USA. 1997;94:9734–9739. doi: 10.1073/pnas.94.18.9734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leips J, Mackay TFC. Quantitative trait loci for life span in Drosophila melanogaster: interactions with genetic background and larval density. Genetics. 2000;155:1773–1788. doi: 10.1093/genetics/155.4.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leips J, Mackay TFC. The complex genetic architecture of Drosophila life span. Exp Aging Res. 2002;28:361–390. doi: 10.1080/03610730290080399. [DOI] [PubMed] [Google Scholar]
- 48.Magwire MM, et al. Quantitative and molecular genetic analyses of mutations increasing Drosophila life span. PLoS Genet. 2010;6:e1001037. doi: 10.1371/journal.pgen.1001037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pasyukova EG, Vieira C, Mackay TFC. Deficiency mapping of quantitative trait loci affecting longevity in Drosophila melanogaster. Genetics. 2000;156:1129–1146. doi: 10.1093/genetics/156.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilson RH, Morgan TJ, Mackay TFC. High-resolution mapping of quantitative trait loci affecting increased life span in Drosophila melanogaster. Genetics. 2006;173:1455–1463. doi: 10.1534/genetics.105.055111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Galloni M, Edgar BA. Cell-autonomous and non-autonomous growth-defective mutants of Drosophila melanogaster. Development. 1999;126:2365–2375. doi: 10.1242/dev.126.11.2365. [DOI] [PubMed] [Google Scholar]
- 52.Di Cara F, Duca E, Dunbar DR, Cagney G, Heck MM. Invadolysin, a conserved lipid-droplet-associated metalloproteinase, is required for mitochondrial function in Drosophila. J Cell Sci. 2013;126:4769–4781. doi: 10.1242/jcs.133306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bauer JH, et al. Expression of dominant-negative Dmp53 in the adult fly brain inhibits insulin signaling. Proc Natl Acad Sci USA. 2007;104:13355–13360. doi: 10.1073/pnas.0706121104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pan H, Finkel T. Key proteins and pathways that regulate lifespan. J Biol Chem. 2017;292:6452–6460. doi: 10.1074/jbc.R116.771915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wessells RJ, Fitzgerald E, Cypser JR, Tatar M, Bodmer R. Insulin regulation of heart function in aging fruit flies. Nat Genet. 2004;36:1275–1281. doi: 10.1038/ng1476. [DOI] [PubMed] [Google Scholar]
- 56.Yamamoto R, Tatar M. Insulin receptor substrate chico acts with the transcription factor FOXO to extend Drosophila lifespan. Aging Cell. 2011;10:729–732. doi: 10.1111/j.1474-9726.2011.00716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kapahi P, et al. With TOR, less is more: a key role for the conserved nutrient-sensing TOR pathway in aging. Cell Metab. 2010;11:453–465. doi: 10.1016/j.cmet.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kapahi P, et al. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14:885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Katewa SD, Kapahi P. Role of TOR signaling in aging and related biological processes in Drosophila melanogaster. Exp Gerontol. 2011;46:382–390. doi: 10.1016/j.exger.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Parashar V, Rogina Bd. Sir2 mediates the increased spontaneous physical activity in flies on calorie restriction. Aging (Albany NY) 2009;1:529–541. doi: 10.18632/aging.100061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci USA. 2004;101:15998–16003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Britton JS, Lockwood WK, Li L, Cohen SM, Edgar BA. Drosophila’s insulin/PI3-kinase pathway coordinates cellular metabolism with nutritional conditions. Dev Cell. 2002;2:239–249. doi: 10.1016/S1534-5807(02)00117-X. [DOI] [PubMed] [Google Scholar]
- 63.Tatar M, Post S, Yu K. Nutrient control of Drosophila longevity. Trends Endocrinol Metab. 2014;25:509–517. doi: 10.1016/j.tem.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bahadorani S, Hur JH, Lo T, Jr., Vu K, Walker DW. Perturbation of mitochondrial complex V alters the response to dietary restriction in Drosophila. Aging Cell. 2010;9:100–103. doi: 10.1111/j.1474-9726.2009.00537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cobbe N, et al. The conserved metalloprotease invadolysin localizes to the surface of lipid droplets. J Cell Sci. 2009;122:3414–3423. doi: 10.1242/jcs.044610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chang, C. W. et al. A role for the metalloprotease invadolysin in insulin signaling and adipogenesis. Biol Chem398, 10.1515/hsz-2016–0226 (2016). [DOI] [PubMed]
- 67.Cvejic S, Zhu Z, Felice SJ, Berman Y, Huang XY. The endogenous ligand Stunted of the GPCR Methuselah extends lifespan in Drosophila. Nat Cell Biol. 2004;6:540–546. doi: 10.1038/ncb1133. [DOI] [PubMed] [Google Scholar]
- 68.Petrosyan A, Hsieh IH, Saberi K. Age-dependent stability of sensorimotor functions in the life-extended Drosophila mutant methuselah. Behav Genet. 2007;37:585–594. doi: 10.1007/s10519-007-9159-y. [DOI] [PubMed] [Google Scholar]
- 69.Schmidt PS, Duvernell DD, Eanes WF. Adaptive evolution of a candidate gene for aging in Drosophila. Proc Natl Acad Sci USA. 2000;97:10861–10865. doi: 10.1073/pnas.190338897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Knauf F, et al. The life-extending gene Indy encodes an exchanger for Krebs-cycle intermediates. Biochem J. 2006;397:25–29. doi: 10.1042/BJ20060409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Neretti N, et al. Long-lived Indy induces reduced mitochondrial ROS production and oxidative damage. Proc Natl Acad Sci USA. 2009;106:2277–2282. doi: 10.1073/pnas.0812484106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ismail MZ, et al. The Drosophila insulin receptor independently modulates lifespan and locomotor senescence. PLoS One. 2015;10:e0125312. doi: 10.1371/journal.pone.0125312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tatar M, et al. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science. 2001;292:107–110. doi: 10.1126/science.1057987. [DOI] [PubMed] [Google Scholar]
- 74.Clancy DJ, et al. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science. 2001;292:104–106. doi: 10.1126/science.1057991. [DOI] [PubMed] [Google Scholar]
- 75.Schriner SE, et al. Extension of Drosophila lifespan by cinnamon through a sex-specific dependence on the insulin receptor substrate chico. Exp Gerontol. 2014;60:220–230. doi: 10.1016/j.exger.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Curtis C, et al. Transcriptional profiling of MnSOD-mediated lifespan extension in Drosophila reveals a species-general network of aging and metabolic genes. Genome Biol. 2007;8:R262. doi: 10.1186/gb-2007-8-12-r262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kabil H, Partridge L, Harshman LG. Superoxide dismutase activities in long-lived Drosophila melanogaster females: chico1 genotypes and dietary dilution. Biogerontology. 2007;8:201–208. doi: 10.1007/s10522-006-9065-3. [DOI] [PubMed] [Google Scholar]
- 78.Kirby K, Hu J, Hilliker AJ, Phillips JP. RNA interference-mediated silencing of Sod2 in Drosophila leads to early adult-onset mortality and elevated endogenous oxidative stress. Proc Natl Acad Sci USA. 2002;99:16162–16167. doi: 10.1073/pnas.252342899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Parkes TL, et al. Extension of Drosophila lifespan by overexpression of human SOD1 in motor neurons. Nat Genet. 1998;19:171–174. doi: 10.1038/534. [DOI] [PubMed] [Google Scholar]
- 80.Kohyama-Koganeya A, Kurosawa M, Hirabayashi Y. Loss of BOSS causes shortened lifespan with mitochondrial dysfunction in Drosophila. PLoS One. 2017;12:e0169073. doi: 10.1371/journal.pone.0169073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.SAS 9.3 Software (SAS Institute Inc., Cary, NC, 2002–2010).
- 83.Bischof J, Maeda RK, Hediger M, Karch F, Basler K. An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc Natl Acad Sci USA. 2007;104:3312–3317. doi: 10.1073/pnas.0611511104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Groth AC, Calos MP. Phage integrases: biology and applications. J Mol Biol. 2004;335:667–678. doi: 10.1016/j.jmb.2003.09.082. [DOI] [PubMed] [Google Scholar]
- 85.Groth AC, Fish M, Nusse R, Calos MP. Construction of transgenic Drosophila by using the site-specific integrase from phage phiC31. Genetics. 2004;166:1775–1782. doi: 10.1534/genetics.166.4.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thorpe HM, Smith MC. In vitro site-specific integration of bacteriophage DNA catalyzed by a recombinase of the resolvase/invertase family. Proc Natl Acad Sci USA. 1998;95:5505–5510. doi: 10.1073/pnas.95.10.5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dietzl G, et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- 88.Dessau RB, Pipper CB. [“R”–project for statistical computing] Ugeskr Laeger. 2008;170:328–330. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are contained within the manuscript. Additional raw data will be available upon request.


