Abstract
We report a case of non-familial juvenile primary aldosteronism (PA). Super-selective adrenal venous sampling identified less aldosterone production in the right inferior adrenal segment than others. Bilateral adrenalectomy sparing the segment normalized blood pressure and improved PA. Both adrenals had similar histologies, consisting of a normal adrenal cortex and aldosterone synthase-positive hyperplasia/adenoma. An aldosterone-driving KCNJ5 mutation was detected in the lesions, but not in the histologically normal cortex. After taking into account that the two adrenal glands displayed a similar histological profile, as well as the fact that hyperplastic lesions in both glands exhibited a common KCNJ5 mutation, we conclude that the specific mutation may have occurred at an adrenal precursor mesodermal cell, at an early stage of development; its daughter cells were mixed with non-mutant cells and dispersed into both adrenal glands, resulting into a form of the condition known as genetic chimerism.
Keywords: Genetic chimerism, KCNJ5, Primary aldosteronism, Juvenile
1. Introduction
Primary aldosteronism (PA) is a condition where the adrenal glands autonomously produce excess aldosterone. In adults, PA is primarily caused by either an aldosterone-producing adenoma (APA) or idiopathic hyperaldosteronism (Funder et al., 2016). Somatic mutations in ion channel/pump genes, including KCNJ5 (potassium channel, inwardly rectifying subfamily J, member 5), have been identified in adult-onset APAs (Choi et al., 2011; Beuschlein et al., 2013; Scholl et al., 2013; Azizan et al., 2013). Such mutations lead to imbalanced ion exchange, which causes the depolarisation of the membrane potential; this in turn activates the voltage-dependent calcium channels, resulting in a massive influx of Ca2+. The activation of the calcium cascade leads to increased aldosterone production.
Juvenile PA is very rare and is mainly caused by three types of familial hyperaldosteronism (FH1-3) (Funder et al., 2016). FH1 is the result of the presence of a hybrid gene formed by an unequal cross-over between CYP11B1 and CYP11B2, where the CYP11B1 regulatory region directs CYP11B2 gene expression. CYP11B2 encodes aldosterone synthase, an enzyme that catalyses the last step of the aldosterone production pathway in the zona glomerulosa (ZG), under the control of the renin-angiotensin system, potassium, and the adrenocorticotropic hormone (ACTH). CYP11B1 encodes 11β-hydroxylase, which catalyses the last step of the cortisol production pathway in the zona fasciculata (ZF), under the control of ACTH (Hattangady et al., 2012). The presence of the hybrid gene leads to the ectopic, regulated by ACTH, expression of aldosterone synthase in ZF, resulting in aldosterone hyper-production. FH1 is treated with glucocorticoids, which suppress the secretion of ACTH. FH2 is diagnosed on the basis of family history of PA, while the related genes remain unknown. FH3 is caused by germ-line mutations in ion channel/pump genes including KCNJ5, and was reported by the same team that discovered the role of somatic KCNJ5 in adult-onset APAs (Choi et al., 2011).
M.Om and K.Ma in the Yokohama Rosai Hospital have developed a novel super-selective adrenal venous sampling (ssAVS, also called super-selective ACTH-stimulated adrenal venous sampling) method to explore additional surgical treatment options for PA (Nishikawa et al., 2010; Nishimoto et al., 2016; Nishikawa et al., 2009). In conventional adrenal venous sampling (cAVS), a catheter is inserted into both adrenal central veins, from which blood samples are collected. Thus, cAVS is only useful for judging the laterality of PA and not for identifying surgical options for bilateral PA. In ssAVS, blood samples are collected from small adrenal tributary veins (TV: smaller upstream branches of the adrenal central veins) using a specialized microcatheter (Gold Crest Micro Catheter KCV29S1S-OM, Koshin Medical Inc., Tokyo, Japan) (Nishikawa et al., 2010). Therefore, ssAVS enables the identification of specific adrenal segments not producing aldosterone autonomously within an affected adrenal, thereby allowing bilateral adrenal surgery to be performed on bilateral PA while sparing an unaffected adrenal segment(s). The first ssAVS was performed on August 2008. Between January 2014 to December 2015, in the Yokohama Rosai Hospital, cAVS was performed for 248 cases, and catheterization for both adrenal vein was accomplished in 246 patients (success rate: 99.2%, confirmed by PCC of adrenal venous samples (Nishikawa et al., 2011)). Among these cAVS-success patients, blood sample collection from more than 2 TVs of each of adrenal was accomplished in 242 patients (98.3%). Recently, the Tohoku University group followed our ssAVS protocol (they call the protocol segmental adrenal venous sampling) and showed similar success rate and availability with ours, suggesting that the protocol is potentially available in high volume referral centers (Satoh et al., 2015a,b; Satani et al., 2016; Satoh et al., 2015a,b; Morimoto et al., 2016).
Herein, we report a novel type of juvenile PA that is presumably caused by a somatic KCNJ5 mutation in both adrenals, which may have occurred in a prodromal cell of the adrenal cortex at an early stage of mesoderm development.
2. Subjects and methods
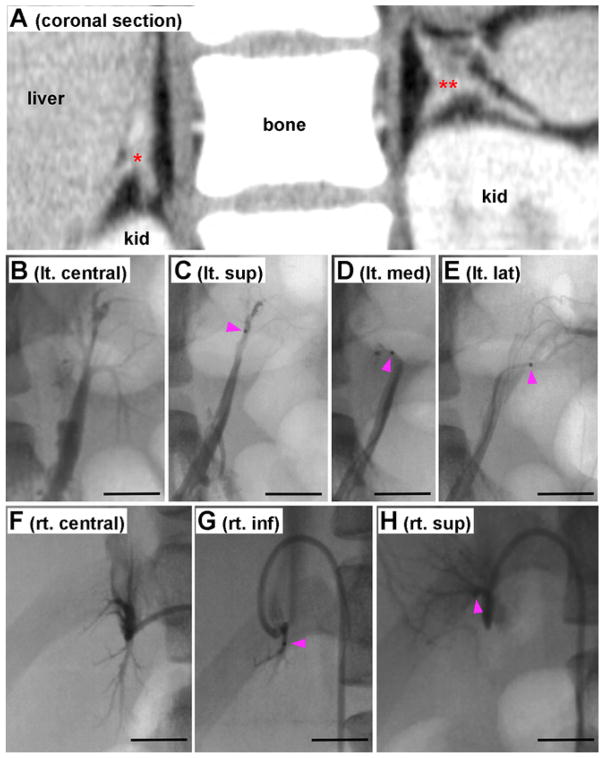
Detailed data on the clinical course of the case and the methods used are available in the Supplementary Data. Briefly, we report a 16-year-old woman with severe PA since the age of 8, diagnosed according to the existing guidelines (Funder et al., 2016; Nishikawa et al., 2011). Computed tomography revealed hyperplastic lesions in both adrenal glands (Fig. 1A). The administration of dexamethasone (a synthetic glucocorticoid used for the treatment of FH-1) did not alleviate her symptoms, while she had no familial history of PA; thereby all three types of familial hyperaldosteronism were excluded as possible causes of her PA. Results from both cAVS and ssAVS indicated that the inferior segment of the right adrenal gland produced less aldosterone than the other portions (Fig. 1B–H). She underwent bilateral adrenalectomy, sparing the right inferior segment; the operation significantly improved her PA symptoms, and normalized her blood pressure. Tissue and blood from the patient were used for histological and molecular analyses, after obtaining the patient’s written informed consent.
Fig. 1.
CT, cAVS, and ssAVS A: A frontal section image of computed tomography. Red * and ** indicate right and left adrenal glands, respectively. B: Conventional adrenal venous sampling (cAVS) from left adrenal vein. Superior, medial, and lateral tributary veins (TVs) were shown by infusion of contrast medium. C: Superselective AVS (ssAVS) from left superior TV. D: ssAVS from left median TV. E: ssAVS from left lateral TV. F: cAVS from right central adrenal vein. Superior and inferior TV were shown. G: ssAVS from inferior TV. H: ssAVS from superior TV. Bars indicate 1 cm. Pink arrowheads in panels C, D, E, G, and H indicate head of a microcatheter. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3. Results
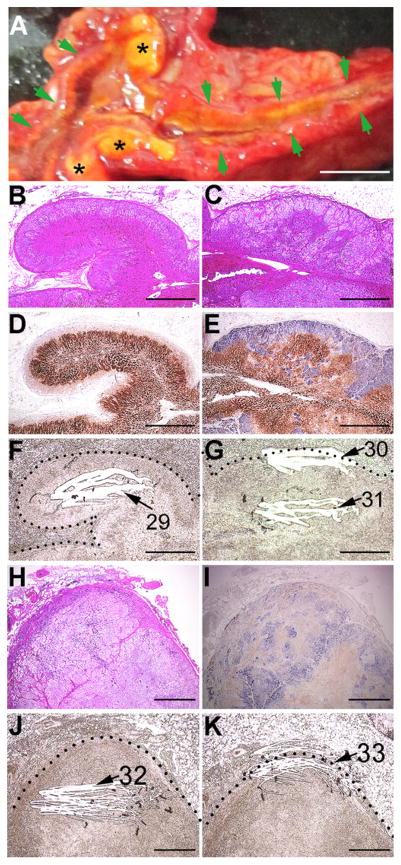
The surface of the extracted, fresh adrenal glands consisted of both mostly normal tissue and yellowish hyperplastic/adenomatous lesions, located in the outer portion of both adrenals (Fig. 2A). Samples from these putative aldosterone-producing lesions, left and right, were flash-frozen, and later used for DNA and RNA isolation (DNA/ RNA#76–77, Table 1).
Fig. 2.
Gross, histochemical, and immunohistochemical findings. A: Gross appearance of the left adrenal cut surface. Green arrows and black asterisks (*) indicate a normal adrenal gland portion and hyperplastic/adenomatous portions, respectively. B, C, and H: H&E staining. Enlarged images of the boxes in pages#1 and #4 of Supplementary Fig. 1. D, E, and I: Double immunostaining for CYP11B2 (blue) and CYP11B1 (brown). Enlarged images of the boxes in pages#1 and #4 of Supplementary Fig. 1. F, G, and J/K: Macro-dissected unstained adjacent FFPE sections of panels D, E, and I, respectively. Dotted lines indicate the adrenal capsule. White striations and corresponding numbers indicate scraped (macro-dissected) areas and DNA# in Table 1, respectively. The scale bar in panel A indicates 5 mm. Scale bars in panels B–K indicate 1 mm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 1.
NGS results of control and APL samples.
| Sample cohort | Sample portion | DNA/RNA# | High confidence somatic non-synonymous variants | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Gene | Location | Coding | Amino acid change | Variant allele frequency (%) | Frequency in matched controls | |||
| Flash-frozen | Control#1 (blood) | 78 | ||||||
| left APL | 77 | KCNJ5 | chr11:128781619 | c.451G > A | p.Gly151Arg | 7.8* | 0.0 | |
| right APL | 76 | KCNJ5 | chr11:128781619 | c.451G > A | p.Gly151Arg | 34.9 | 0.0 | |
| Left adrenal (FFPE) | Control#2 (ZF&ZR) | 29 | ||||||
| outer hyperplasia | 30 | KCNJ5 | chr11:128781619 | c.451G > A | p.Gly151Arg | 16.2 | 0.1 | |
| ATP1A1 | chr1:116930009 | c.283C > T | p.Gln95Ter | 76.3 | 0.1 | |||
| chr1:116932282 | c.976G > A | p.Gly326Ser | 19.1 | 0.1 | ||||
| inner hyperplasia | 31 | KCNJ5 | chr11:128781619 | c.451G > A | p.Gly151Arg | 24.3 | 0.1 | |
| inner APA | 32 | KCNJ5 | chr11:128781619 | c.451G > A | p.Gly151Arg | 9.4* | 0.1 | |
| ATP1A1 | chr1:116944213 | c.2887G > A | p.Ala963Thr | 20.3 | 0.0 | |||
| chr1:116944220 | c.2894C > T | p.Ala965Val | 20.9 | 0.0 | ||||
| outer APA | 33 | KCNJ5 | chr11:128781619 | c.451G > A | p.Gly151Arg | 9.9* | 0.1 | |
| ATP2B3 | chrX:152814194 | c.1220C > T | p.Thr407Met | 15.2 | 0.0 | |||
| Right adrenal (FFPE) | Control#3 (ZF&ZR) | 73 | ||||||
| hyperplasia | 75 | KCNJ5 | chr11:128781619 | c.451G > A | p.Gly151Arg | 42.9 | 0.0 | |
| APA | 74 | KCNJ5 | chr11:128781619 | c.451G > A | p.Gly151Arg | 35.1 | 0.0 | |
DNA and RNA were isolated from the flash-frozen and FFPE tissues of our case. RNA was used in CYP11B2-qPCR to confirm sample collection (see Supplemental Table 2). Libraries were prepared from DNA and used for NGS, the results of which are shown in Table 1 and Supplemental Table 1. The number of called variants prior to any filtering is shown in the column ‘# of variants called by the Variant Caller’. ‘The number of amino acid-changing variants within the called variants’ is in the column ‘# of amino acid-changing variants.’ of Supplemental Table 1. Among these amino acid-changing variants, high confidence (see filtering criteria in Supplemental Methods) somatic non-synonymous variants are summarized in the right-side columns of the table, in which the KCNJ5 (g.Gly151Arg) mutation with a variant allele frequency (%) less than 15 (*) is listed in the table since the mutation was commonly detected throughout bilateral aldosterone-producing lesions. The columns lists the gene symbol (gene), chromosome location in a human reference genome (i.e. hg19) (location), reference and variant alleles on each position (coding), amino acid changes, and % of variant allele frequency. The variant allele frequencies in the matched control tissues (Control#1–3) are shown for comparisons. KCNJ5 mutations are shown in bold characters. APL: aldosterone-producing lesions taken from the cut surface of extracted adrenal glands quickly after adrenalectomy. Ter: termination codon.
Histological analysis revealed that the normal tissue from the left adrenal gland displayed the three typical histological layers forming the adrenal cortex (zona glomeruloza, zona fasciculata, and zona reticularis) (Fig. 2B), whereas the hyperplastic lesions consisted of heterogeneous clear (lipid-rich) cells arranged in an irregular fashion (Fig. 2C). Double-immunostaining for aldosterone synthase (blue) and steroid 11β-hydroxylase (brown) was performed in both the normal tissue and the lesions. The ZG of the normal tissue lacked aldosterone synthase, suggesting that aldosterone production there was suppressed (possibly due to low circulating renin), while the ZF contained steroid 11β-hydroxylase, indicating that cortisol production at that zone remained unaffected (Fig. 2D). In the hyperplastic lesions, the immunostaining revealed a thick subcapsular area with high levels of aldosterone synthase, which expanded outward to form nodules and inward to invade a layer of cells positive for steroid 11β-hydroxylase (Fig. 2E). Unstained formalin-fixed, paraffin-embedded tissues (FFPE) were macro-dissected to collect control samples from the normal adrenal cortex (Control#2), as well as samples from the hyperplastic lesions (both the subcapsular area and the inner layer) for molecular analyses (DNA/RNA#29–31; Fig. 2F,G; Table 1).
In addition to these hyperplastic lesions, the left adrenal gland contained lesions resembling APAs, which consisted of heterogeneous tumour-like cells expressing either aldosterone synthase or steroid 11β-hydroxylase (Fig. 2H,I); these two types of cells were irregularly arranged, as is observed in APAs (Nishimoto et al., 2010). Inner and subcapsular portions of the APA-like hyperplasia were macro-dissected to isolate DNA and RNA (DNA/RNA#32–33, Fig. 2J, K).
The partially extracted right adrenal presented more or less the same histological profile as the left adrenal, suggesting that the two adrenal glands suffered from the same pathophysiology. After micro-dissection of the right adrenal cortex, samples from normal portions (Control#3) and hyperplastic lesions were extracted and used for DNA and RNA isolation (DNA/RNA#73–75, pages#6–7 in Supplementary Fig. 1).
Real-time quantitative-PCR analysis confirmed the elevation of CYP11B2 in the lesion samples (Supplementary Table 1). Afterwards, next generation sequencing (NGS) was conducted to detect reported APA-associated mutations as previously reported (Nishimoto et al., 2016; Nishimoto et al., 2015). NGS detected 114 variants before filtering (Supplementary Tables 1–2). The KCNJ5 p.Gly151Arg mutation, which is the most frequently (38–70%) detected mutation in APAs (Fernandes-Rosa et al., 2014; Kitamoto et al., 2016), was identified throughout the aldosterone-producing lesions (DNA#30–33, 74–77), although 3 lesions had a lower variant allele frequency (* in Table 1) than the one used by our filtration criteria (>15%, see Supplementary methods).
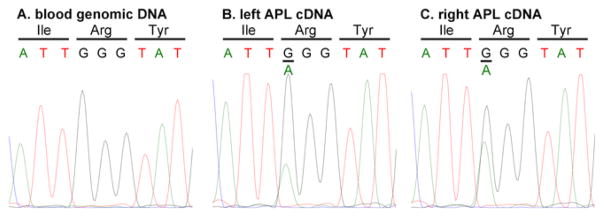
Sanger sequencing of cDNAs produced from RNA isolated from flash-frozen, putative aldosterone-producing lesions (RNA#76–77, Table 1) of both glands confirmed the expression of the KCNJ5 p.Gly151Arg mutated gene in the lesions (Fig. 3A–B). Determination of the sequence of genomic DNA isolated from the patient’s blood (Control#1, DNA#78, Table 1), again using the Sanger method, demonstrated that the KCNJ5 mutated gene was not present in blood cells (Fig. 3C).
Fig. 3.
Sanger sequencing. A–C: Sanger sequencing results from left aldosterone-producing lesion cDNA (cDNA#77), right aldosterone-producing lesion cDNA (cDNA#76), and blood genomic DNA (Control#1, DNA#78), respectively. A known heterozygous somatic mutation, p.Gly151Arg, was found in the genomic DNA sample from left and right aldosterone-producing lesion. This mutation was also detected in their corresponding DNA (DNA#77 and 76, respectively) by NGS at a variant allele frequency of 7.8% and 34.9%, respectively (Table 1).
4. Discussion
Both glands of the patient displayed hyperplasia, some areas of which resembled APA. The similarities in histology, as well as the identification of a common APA-associated mutation (KCNJ5 p.Gly151Arg) in the lesions from both glands, suggest that the abnormalities observed in the two glands share a common pathology. Although the KCNJ5 mutation could independently occurred in the different parts of the adrenals simultaneously, the mutation most likely occurred in a mesodermal cell in an early stage of development, and its daughter cells were mixed with non-mutant cells and distributed into both adrenal glands, a condition known as genetic chimerism.
Two models of adrenal zonation have been proposed; the model of centripetal migration from undifferentiated cells in the subcapsular region that initially differentiate into aldosterone-producing cells of the ZG which then migrate centripetally and undergo a lineage change into ZF and continue migrating to the zona reticularis where they eventually undergo apoptosis (Salmon and Zwemer, 1941). In the second zonal model states that each zone develops independently from zone-specific progenitor cells (Deane and Greep, 1946). Studies in mice using lineage tracing have shown that the adrenal cortex arises from cells of the intermediate mesoderm and after separation of individual adrenal and gonadal primordia from the shared adrenogonadal primordia; the mesenchymal cells migrate and encapsulate the foetal adrenal gland (Wood et al., 2013). From where, there is a centripetal migration of cells from the subcapsular region (zona glomerulosa) undergoing a phenotypic change to form the zona fasciculata (King et al., 2009). Using an aldosterone synthase-cre mice to knock out the Steroidogenic Factor 1 (SF-1, a master regulator of adrenal development) in zona glomerulosa demonstrated that the ZG no longer was able to contribute cells to the ZF, however, the ZF developed normally (Freedman et al., 2013). This indicated that some cells of the ZF correspond to a different lineage probably from the foetal adrenal that undergoes atrophy before birth.
The adrenals of the current case may have non-mutant and KC-NJ5-mutant progenitor cells in the adrenal capsule, which generate a normal adrenal cortex and hyperplasia, respectively. In fact, outer and inner tissues from hyperplasia in the left adrenal gland had similar KCNJ5 mutation frequencies (16.2% vs. 24.3% and 9.9% vs. 9.4%, respectively; Table 1) despite the inner hyperplastic portion containing more CYP11B1-positive cells than CYP11B2-positive cells (Fig. 2E,G). Overall, it is likely that the patient had a KCNJ5 genetic chimerism, and the mutant cells caused bilateral hyperplasia.
In addition to the KCNJ5 mutation, NGS identified high confidence unreported somatic non-synonymous variants of ATP1A1 and ATP2B3 in the left FFPE adrenal samples (Table 1). Although some variants may be caused by PCR amplification errors due to extensive amplification (30 cycles), others may be novo mutations associated with the KCNJ5 mutation and chronic aldosterone overproduction.
In the current case, although the ssAVS method is not universally approved, the ssAVS effectively found a less affected adrenal segment, which enabled surgical treatment sparing the segment. The spared adrenal have potential to exacerbate PA due to possible remaining KCNJ5 mutation. The current study revealed that the adrenal consisted of mutated and non-mutated adrenal, therefore, even if her PA recur in the future, she may have another surgical option such as removal of the remaining adrenal with auto-transplantation of unaffected adrenal. Further medical development on adrenal cortex is needed to explore curable options for severe “idiopathic hyperaldosteronism” including the current case.
In summary, we encountered a case of juvenile PA, in which bilateral adrenals had almost the same pathology consisting of a normal adrenal histology without genetic mutations and aldosterone-producing lesions harboring a KCNJ5 mutation. We propose this novel type of PA as ‘non-familial juvenile PA (NFJ-PA)’, which may be caused by a somatic KCNJ5 mutation arising in the early stage of mesodermal development. NFJ-PA may be improved by a combination treatment of ssAVS and bilateral adrenal surgery because the adrenals of NFJ-PA may have a non-mutated normal adrenal region(s).
Supplementary Material
Acknowledgments
Funding
This work was supported by the JSPS KAKENHI Grants (to KN [#15K10650] and KM [#26461387]); Yamaguchi Endocrine Research Foundation (to KN); Japanese Ministry of Health, Labour and Welfare (to TN); NIH grant HL27255 (to C.E.G-S), and Initiative for Rare and Undiagnosed Patients from AMED (to YS).
We thank Mr. Shinya Sasai in the Tachikawa Hospital, Tokyo, Japan for excellent technical assistance of immunostaining.
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.mce.2016.07.031.
Footnotes
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Azizan EA, Poulsen H, Tuluc P, Zhou J, Clausen MV, Lieb A, Maniero C, Garg S, Bochukova EG, Zhao W, Shaikh LH, Brighton CA, Teo AE, Davenport AP, Dekkers T, Tops B, Kusters B, Ceral J, Yeo GS, Neogi SG, McFarlane I, Rosenfeld N, Marass F, Hadfield J, Margas W, Chaggar K, Solar M, Deinum J, Dolphin AC, Farooqi IS, Striessnig J, Nissen P, Brown MJ. Somatic mutations in ATP1A1 and CACNA1D underlie a common subtype of adrenal hypertension. Nat Genet. 2013;45:1055–1060. doi: 10.1038/ng.2716. [DOI] [PubMed] [Google Scholar]
- Beuschlein F, Boulkroun S, Osswald A, Wieland T, Nielsen HN, Lichtenauer UD, Penton D, Schack VR, Amar L, Fischer E, Walther A, Tauber P, Schwarzmayr T, Diener S, Graf E, Allolio B, Samson-Couterie B, Benecke A, Quinkler M, Fallo F, Plouin PF, Mantero F, Meitinger T, Mulatero P, Jeunemaitre X, Warth R, Vilsen B, Zennaro MC, Strom TM, Reincke M. Somatic mutations in ATP1A1 and ATP2B3 lead to aldosterone-producing adenomas and secondary hypertension. Nat Genet. 2013;45:440–444. 444e1–2. doi: 10.1038/ng.2550. [DOI] [PubMed] [Google Scholar]
- Choi M, Scholl UI, Yue P, Bjorklund P, Zhao B, Nelson-Williams C, Ji W, Cho Y, Patel A, Men CJ, Lolis E, Wisgerhof MV, Geller DS, Mane S, Hellman P, Westin G, Akerstrom G, Wang W, Carling T, Lifton RP. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science. 2011;331:768–772. doi: 10.1126/science.1198785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane HW, Greep RO. A morphological and histochemical study of the rat’s adrenal cortex after hypoph ysectomy, with comments on the liver. Am J Anat. 1946;79:117–145. doi: 10.1002/aja.1000790104. [DOI] [PubMed] [Google Scholar]
- Fernandes-Rosa FL, Williams TA, Riester A, Steichen O, Beuschlein F, Boulkroun S, Strom TM, Monticone S, Amar L, Meatchi T, Mantero F, Cicala MV, Quinkler M, Fallo F, Allolio B, Bernini G, Maccario M, Giacchetti G, Jeunemaitre X, Mulatero P, Reincke M, Zennaro MC. Genetic spectrum and clinical correlates of somatic mutations in aldosterone-producing adenoma. Hypertension. 2014;64:354–361. doi: 10.1161/HYPERTENSIONAHA.114.03419. [DOI] [PubMed] [Google Scholar]
- Freedman BD, Kempna PB, Carlone DL, Shah MS, Guagliardo NA, Barrett PQ, Gomez-Sanchez CE, Majzoub JA, Breault DT. Adrenocortical zonation results from lineage conversion of differentiated zona glomerulosa cells. Dev cell. 2013;26:666–673. doi: 10.1016/j.devcel.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, Stowasser M, Young WF., Jr The management of primary aldosteronism: case detection, diagnosis, and treatment: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016:jc20154061. doi: 10.1210/jc.2015-4061. [DOI] [PubMed] [Google Scholar]
- Hattangady NG, Olala LO, Bollag WB, Rainey WE. Acute and chronic regulation of aldosterone production. Mol Cell Endocrinol. 2012;350:151–162. doi: 10.1016/j.mce.2011.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King P, Paul A, Laufer E. Shh signaling regulates adrenocortical development and identifies progenitors of steroidogenic lineages. Proc Natl Acad Sci U S A. 2009;106:21185–21190. doi: 10.1073/pnas.0909471106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamoto T, Suematsu S, Yamazaki Y, Nakamura Y, Sasano H, Matsuzawa Y, Saito J, Omura M, Nishikawa T. Clinical and steroidogenic characteristics of aldosterone-producing adenomas with ATPase or CACNA1D gene mutations. J Clin Endocrinol Metab. 2016;101:494–503. doi: 10.1210/jc.2015-3284. [DOI] [PubMed] [Google Scholar]
- Morimoto R, Satani N, Iwakura Y, Ono Y, Kudo M, Nezu M, Omata K, Tezuka Y, Seiji K, Ota H, Kawasaki Y, Ishidoya S, Nakamura Y, Arai Y, Takase K, Sasano H, Ito S, Satoh F. A case of bilateral aldosterone-producing adenomas differentiated by segmental adrenal venous sampling for bilateral adrenal sparing surgery. J Hum Hypertens. 2016;30:379–385. doi: 10.1038/jhh.2015.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa T, Matsuzawa Y, Saito J, Omura M. Super-selective ACTH-stimulated Adrenal Venous Sampling Can Simply Differentiate Bilateral Adrenal Hyperplasia from Bilateral Adenomas in Primary Aldosteronism. 35th INTERNATIONAL ALDOSTERONE CONFERRENCE; Washington, DC. 2009. [Google Scholar]
- Nishikawa T, Matsuzawa Y, Saito J, Omura M. Is it possible to extirpate cardiovascular events in primary aldosteronism after surgical treatment. Jpn Clin Med. 2010;1:21–23. doi: 10.4137/JCM.S6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa T, Omura M, Satoh F, Shibata H, Takahashi K, Tamura N, Tanabe A. Guidelines for the diagnosis and treatment of primary aldosteronism–the Japan Endocrine Society 2009. Endocr J. 2011;58:711–721. doi: 10.1507/endocrj.ej11-0133. [DOI] [PubMed] [Google Scholar]
- Nishimoto K, Nakagawa K, Li D, Kosaka T, Oya M, Mikami S, Shibata H, Itoh H, Mitani F, Yamazaki T, Ogishima T, Suematsu M, Mukai K. Adrenocortical zonation in humans under normal and pathological conditions. J Clin Endocrinol Metab. 2010;95:2296–2305. doi: 10.1210/jc.2009-2010. [DOI] [PubMed] [Google Scholar]
- Nishimoto K, Tomlins SA, Kuick R, Cani AK, Giordano TJ, Hovelson DH, Liu CJ, Sanjanwala AR, Edwards MA, Gomez-Sanchez CE, Nanba K, Rainey WE. Aldosterone-stimulating somatic gene mutations are common in normal adrenal glands. Proc Natl Acad Sci U S A. 2015;112:E4591–E4599. doi: 10.1073/pnas.1505529112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto K, Seki T, Kurihara I, Yokota K, Omura M, Nishikawa T, Shibata H, Kosaka T, Oya M, Suematsu M, Mukai K. Case report: nodule development from subcapsular aldosterone-producing cell clusters causes hyperaldosteronism. J Clin Endocrinol Metab. 2016;101:6–9. doi: 10.1210/jc.2015-3285. [DOI] [PubMed] [Google Scholar]
- Salmon TN, Zwemer RL. A study of the life history of cortico-adrenal gland cells of the rat by means of trypan blue injections. Anatomical Rec. 1941;80:421–429. [Google Scholar]
- Satani N, Ota H, Seiji K, Morimoto R, Kudo M, Iwakura Y, Ono Y, Nezu M, Omata K, Ito S, Satoh F, Takase K. Intra-adrenal aldosterone secretion: segmental adrenal venous sampling for localization. Radiology. 2016;278:265–274. doi: 10.1148/radiol.2015142159. [DOI] [PubMed] [Google Scholar]
- Satoh F, Morimoto R, Ono Y, Iwakura Y, Omata K, Kudo M, Satani N, Ota H, Seiji K, Takase K, Nakamura Y, Sasano H, Ito S. 8d.04: clinical benefits of administering super-selective segmental adrenal venous sampling and performing adrenal sparing surgery in the patients with primary aldosteronism. J Hypertens. 2015a;33(Suppl 1):e114. [Google Scholar]
- Satoh F, Morimoto R, Seiji K, Satani N, Ota H, Iwakura Y, Ono Y, Kudo M, Nezu M, Omata K, Tezuka Y, Kawasaki Y, Ishidoya S, Arai Y, Takase K, Nakamura Y, McNamara K, Sasano H, Ito S. Is there a role for segmental adrenal venous sampling and adrenal sparing surgery in patients with primary aldosteronism? Eur J Endocrinol. 2015b;173:465–477. doi: 10.1530/EJE-14-1161. [DOI] [PubMed] [Google Scholar]
- Scholl UI, Goh G, Stolting G, de Oliveira RC, Choi M, Overton JD, Fonseca AL, Korah R, Starker LF, Kunstman JW, Prasad ML, Hartung EA, Mauras N, Benson MR, Brady T, Shapiro JR, Loring E, Nelson-Williams C, Libutti SK, Mane S, Hellman P, Westin G, Akerstrom G, Bjorklund P, Carling T, Fahlke C, Hidalgo P, Lifton RP. Somatic and germline CACNA1D calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism. Nat Genet. 2013;45:1050–1054. doi: 10.1038/ng.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood MA, Acharya A, Finco I, Swonger JM, Elston MJ, Tallquist MD, Hammer GD. Fetal adrenal capsular cells serve as progenitor cells for steroidogenic and stromal adrenocortical cell lineages in M. musculus. Development. 2013;140:4522–4532. doi: 10.1242/dev.092775. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.