Abstract
Salivary glands are formed by branching morphogenesis with epithelial progenitors forming a network of ducts and acini (secretory cells). During this process, epithelial progenitors specialise into distal (tips of the gland) and proximal (the stalk region) identities that produce the acini and higher order ducts, respectively. Little is known about the factors that regulate progenitor expansion and specialisation in the different parts of the gland. Here, we show that Sox9 is involved in establishing the identity of the distal compartment before the initiation of branching morphogenesis. Sox9 is expressed throughout the gland at the initiation stage before becoming restricted to the distal epithelium from the bud stage and throughout branching morphogenesis. Deletion of Sox9 in the epithelium results in loss of the distal epithelial progenitors, a reduction in proliferation and a subsequent failure in branching. We demonstrate that Sox9 is positively regulated by mesenchymal Fgf10, a process that requires active Erk signalling. These results provide new insights into the factors required for the expansion of salivary gland epithelial progenitors, which can be useful for organ regeneration therapy.
KEY WORDS: Branching morphogenesis, Epithelial progenitors, Sox9, Fgf signalling, Salivary glands
Summary: Deletion of Sox9 in the salivary gland epithelium results in loss of the distal epithelial progenitors, a reduction in proliferation and a subsequent failure in branching in mice.
INTRODUCTION
To develop therapeutic strategies for organ regeneration, we first need to understand how progenitor cells contribute to organ formation. During development, organs such as lungs, lacrimal glands, pancreas and salivary glands undergo branching morphogenesis, a process that efficiently increases the surface area with a minimum increase of volume. Common to all branching epithelium, the embryonic salivary gland epithelium starts as a placode (also known as the prebud), which then elongates leading to the formation of a stalk attached to the bud (also known as the initial bud). The epithelium then undergoes sequential rounds of epithelial budding, clefting and epithelial outgrowth creating a highly branched network divided into ducts and endbuds (Affolter et al., 2003), these endbuds forming the secretory acini of the adult gland. Branching morphogenesis in many organs has been shown to require constant interactions between the epithelium, mesenchyme, blood vessels and nerves (Knosp et al., 2012). Salivary glands have long been used as a model to study branching morphogenesis because of their ease of ex vivo manipulation (Tucker, 2007). Among the three major types – submandibular (SMG) secreting seromucous saliva, sublingual (SL) secreting mucous saliva, and parotid (PG) secreting serous saliva – the SMG is the most commonly studied.
As the epithelium initiates and undergoes branching it becomes specialised into distinct epithelial compartments. In salivary glands, the earliest stage reported for this specialisation is after the initiation of branching at the pseudoglandular stage [embryonic day (E) 13.5] (Lombaert et al., 2011, 2013; Knox et al., 2010; Arnold et al., 2011). Based on the position of cells within the developing gland and the expression of progenitor markers, the epithelium is divided into proximal and distal progenitors. In salivary glands, the proximal progenitors, the cells located closer to the oral epithelium at the stalk region, express markers such as cytokeratin 5 (K5; also known as keratin 5, Krt5) and Sox2 [SRY (sex-determining region Y)-box 2] (Lombaert et al., 2011; Knox et al., 2010; Arnold et al., 2011). The distal progenitors, located at the end of the gland, express cytokeratin 14 (K14; also known as keratin 14, Krt14), Kit and Sox10 (Lombaert et al., 2013). Myb is also expressed by the distal epithelial progenitors, as shown at E17.5 when terminal differentiation starts to occur (Matsumoto et al., 2016). The location of epithelial progenitors at specific time points during development has been suggested to determine their progeny. When epithelial rudiments of E13.5 SMGs were cultured ex vivo with Fgf7 and Fgf1, the distal epithelial progenitors labelled after a day in culture contributed to the formation of acini (secretory cells producing saliva) and secondary- and tertiary-branched ducts. However, when labelled after 3 days in culture at a stage when pro-acinar differentiation had already initiated, their lineage was restricted to the acinar compartments. The more proximal progenitors, on the other hand, could only contribute to the formation of higher order branched ducts (Matsumoto et al., 2016). Lumen formation in the ducts is marked by F-actin deposition whereas acinar differentiation is marked by the expression of Mist1 (bHLHa15) (Walker et al., 2008; Aure et al., 2015). Interestingly, distal epithelial progenitors have been shown to be more proliferative than proximal progenitors (Steinberg et al., 2005; Matsumoto et al., 2016).
Although there is increasing information on the factors that regulate salivary gland branching morphogenesis, little is known about the signals that control the expansion of the different epithelial progenitors, or whether the distal epithelial progenitors alone are required for branching morphogenesis. Acetylcholine signalling through the parasympathetic ganglion was shown to promote the expansion of K5+ cells and their differentiation to the ductal K19 (Krt19)+ lineage by a process that required epidermal growth factor receptor (EGFR) signalling (Knox et al., 2010). On the other hand, epithelial Wnt and Fgf receptors in combination with Kit signalling were shown to promote the expansion of the distal Sox10+K14+ population (Lombaert et al., 2013; Matsumoto et al., 2016). Key pathway components for Fgf signalling in developing salivary glands are Fgf10 and its receptor Fgfr2, as mutations in either of these two genes lead to an arrest of salivary gland development at the placode stage (Jaskoll et al., 2005). Fgf10 is expressed in the neural crest-derived mesenchyme that surrounds the gland, with conditional knockout of Fgf10 in the neural crest mimicking the null phenotype (Teshima et al., 2016a), whereas Fgfr2 is expressed in the gland epithelium (Jaskoll et al., 2002). Similar to salivary glands, other branching organs were also arrested after knockout of Fgf10, including the lung and lacrimal glands, and the pancreas was hypoplastic (Ohuchi et al., 2000).
In the lungs, lacrimal glands and pancreas, Fgfr2 signalling has been shown to regulate the expression of Sox9, which appears to act as a distal epithelial marker (Abler et al., 2009; Chang et al., 2013; Chen et al., 2014; Seymour et al., 2012). Sox9 is a transcription factor that belongs to the highly conserved SOX family (subgroup E) characterised by the presence of the high mobility group DNA-binding domain of SRY (Pritchett et al., 2011). Initially Sox9 was identified as a gene linked to campomelic dysplasia, a syndrome that causes male-to-female sex reversal and skeletal defects (Wagner et al., 1994). Apart from its importance in gonadal formation and chondrogenesis, Sox9 is expressed in the epithelium of many developing branching organs, including lacrimal glands, lungs, pancreas and kidneys. Its requirement for their development varies as conditional Sox9 inactivation results either in complete agenesis, as in the case of the lacrimal glands (Chen et al., 2014), or in hypoplasticity, as in the case of the lungs and pancreas (Chang et al., 2013; Rockich et al., 2013; Seymour et al., 2007). Kidneys also rely on Sox9 expression for their development; however, the severity of the phenotype is variable and ranges from agenesis to hypoplasia (Reginensi et al., 2011). Despite these variabilities, in general epithelial Sox9 expression has been shown to promote progenitor cell expansion and extracellular matrix (ECM) deposition (Chang et al., 2013; Rockich et al., 2013; Chen et al., 2014). qPCR has shown that Sox9 is expressed in developing salivary glands, with a peak of expression at E15.5 (Lombaert and Hoffman, 2010). The role of Sox9 in salivary glands, however, has not been assessed.
Here, we investigated the importance of Sox9 in salivary gland development using the Sox9flox/flox; K14-Cre+ (Sox9CKO) mouse line, in which Sox9 is ablated in epithelial tissues from the initiation stage of salivary gland development. We find that Sox9 is highly expressed in the distal epithelial progenitors where it is required for their specification as a distal epithelial population and for subsequent branching morphogenesis. Sox9 expression is maintained by Fgf10 signalling by a process that requires active Erk signalling.
RESULTS
Sox9 is restricted to the distal epithelial compartment from the bud stage of development and is maintained in this region throughout development
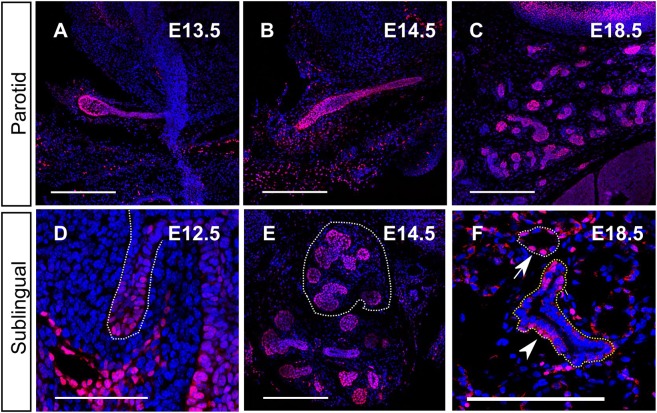
As a first approach to understanding the function of Sox9, we traced its protein distribution during SMG development. During all stages, Sox9, as expected for a transcription factor, was detected in the nucleus and was absent from the epithelium of Sox9CKO glands, indicating the high specificity of this antibody for Sox9 (Fig. 1; Fig. S1B). At gland initiation (E11.0-11.5), all the epithelial cells of the placode and the early invaginating bud were Sox9+ (Fig. 1A,B). However, at the bud stage (E12.5), high levels of Sox9 expression were only observed distally at the tip of the buds, with much lower expression proximally next to the oral surface (Fig. 1C). This pattern of epithelial Sox9 expression, with higher levels at the distal tips, was maintained throughout branching morphogenesis at E13.5 and E15.5 (Fig. 1D,E). As lumens started to form in the more distal ducts (as indicated by F-actin staining), Sox9 expression turned off (Fig. 1E). Interestingly, in the adult when differentiation had fully occurred, Sox9+ cells were still predominantly located in more distal structures, with large numbers of positive acinar cells, as shown by co-expression of Sox9 and Mist1 (Fig. 1F, insets). All Mist1+ cells were also Sox9+, indicating an important link between these two transcription factors in adult glands. Expression of Sox9 was also observed in the small distally located intercalated ducts (Fig. 1F, yellow outline), whereas fewer cells that stained less intensely were found in the bigger more proximal ducts (Fig. 1F, arrowheads). Interestingly, the intensity of Sox9 appeared to be lower in the acinar compartments than in the intercalated ducts in the adult (Fig. 1F), suggesting a change in role in fully differentiated glands. The PG (Fig. 2A-C) and SL (Fig. 2D-F) glands displayed a similar pattern of Sox9 expression compared with the SMG, suggesting that Sox9 plays a similar developmental role in all of the three major salivary glands (Fig. 2).
Fig. 1.
Sox9 is expressed throughout the development of the submandibular gland. (A-F) Sox9 immunofluorescence (red) at the placode [E11.0 (A), E11.5 (B)], initial bud [E12.5 (C)], pseudoglandular [E13.5 (D)], canalicular [E15.5 (E)] and adult (F) stages. DNA is shown in blue (DAPI), F-actin in yellow and Mist1 in green. Dotted lines in A-D delineate the salivary gland epithelium. Insets in F show magnifications of an acinus stained for Mist1 (green) and Sox9 (red). Arrowheads point to Sox9-positive cells within the striated duct. G, ganglion; MC, Meckel's cartilage; SL, sublingual gland; SMG, submandibular gland. Scale bars: 100 μm.
Fig. 2.
Sox9 expression in the parotid and sublingual gland is similar to the submandibular. (A-F) Sox9 immunofluorescence (red) in the parotid (A-C) and sublingual (D-F) glands at the bud stage (A,D) and at E14.5 (B,E) and E18.5 (C,F). Dotted white lines in D and E outline the sublingual glands. The white dotted line in F outlines an acinus and the yellow a duct. Arrow points to Sox9+ cells in the acinus and the arrowhead points to Sox9+ cells in the duct. DNA is shown in blue (DAPI). Scale bars: 250 μm (A-C,E,F); 50 μm (D).
Sox9+ epithelial cells are progenitors of the entire salivary gland epithelium
Our protein localisation analysis revealed early Sox9 expression in the placode epithelium. To investigate whether these early Sox9+ epithelial cells act as progenitors, we traced their progeny using Sox9-creERT2 mice crossed with Rosa-tdTomato mice. After tamoxifen administration at E10.5, the entire epithelium of the E14.5 submandibular gland was labelled in red including all the ductal and acinar structures (Fig. 3). In agreement with the early Sox9 expression in the ganglia (Fig. 1C), label was also detected in the ganglia cells found in close association with the submandibular gland epithelium (Fig. 3C). Earlier tamoxifen administration labelled the mesenchyme (date not shown) in agreement with Sox9 expression in the neural crest cells that form the salivary gland mesenchyme (Zhao et al., 1997).
Fig. 3.
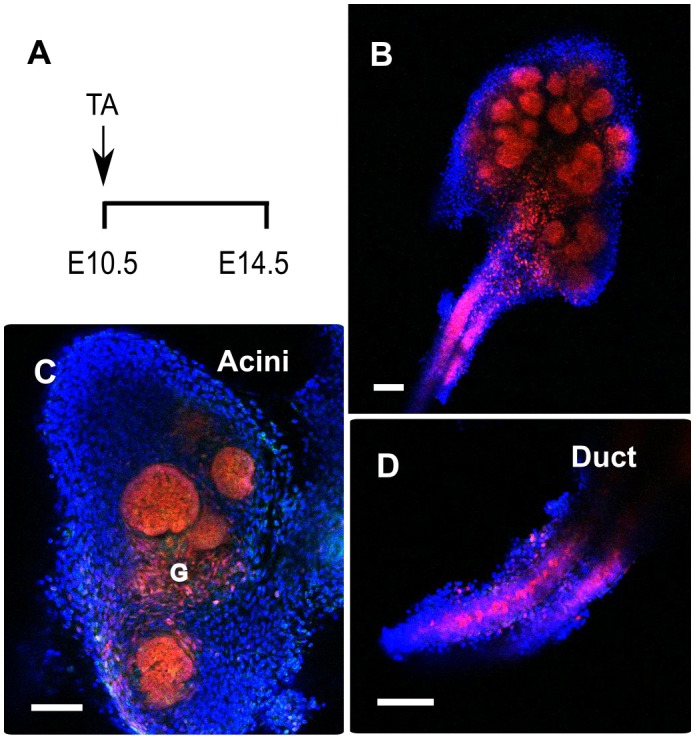
Sox9-positive cells are progenitors of the entire submandibular gland epithelium. (A) Experimental strategy used to follow the progeny of Sox9-expressing cells with the Sox9-creERT2; R26-tdTomato line. Tamoxifen (TA) was given at E10.5 and embryos were collected at E14.5. (B-D) Tomato-labelled cells (red) were detected in the whole submandibular gland at E14.5, (B) in the acini (C) and in the duct (D). G, ganglion. Scale bars: 100 μm.
Sox9 is required for the formation of distal epithelial progenitors and for branching morphogenesis
To assess the role of Sox9 during salivary gland development, we deleted Sox9 flox alleles in the oral epithelium using K14-Cre. The K14 promoter induces Cre recombination in almost all epithelial cells of the salivary gland from the initiation stage (Fig. S1A,B). Immunofluorescence for Sox9 confirmed almost complete loss of Sox9 in the salivary gland epithelium, although a very small number of cells remained positive for Sox9, both at the placode (Fig. S1A,B) and later at the bud stage (Fig. S1C,D). As expected, Sox9 was still expressed in the surrounding mesenchyme, including Meckel's cartilage and the ganglia (Fig. S1B,D).
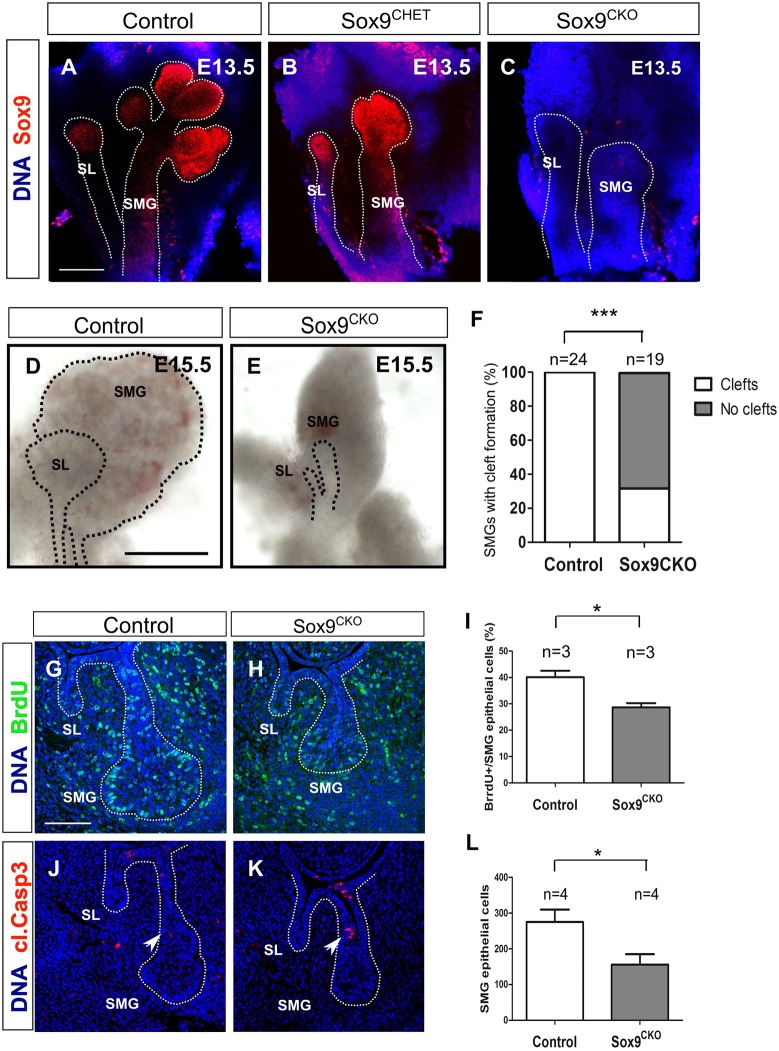
Although the initial thickening was normal, the bud was smaller at E12.5 (Fig. S1B,D). This defect was more marked as the gland continued to develop with Sox9CKO SMGs failing to branch (Fig. 4A-C). Development of the submandibular and sublingual glands arrested at the bud stage at time points when control glands had undergone extensive branching (Fig. 4D,E). A delay in branching was also evident in the heterozygous Sox9CHET mice (Sox9flox/+; K14-Cre+) (Fig. 4B). The mesenchymal capsule that develops around the epithelial tissue still formed in the Sox9CKO mutants (Fig. 4D,E), however, as has been observed in Fgf10 mutant mice (Wells et al., 2013). Similar to the SMG and SL, the PG was undetectable by E15.5 (Fig. 5) suggesting that Sox9 is required for the formation of all three major salivary glands.
Fig. 4.
Sox9 is required for branching morphogenesis. (A-C) Sox9 immunofluorescence (red) in control (A), Sox9CHET (B) and Sox9CKO (C) at the pseudoglandular stage (E13.5). (D,E) Submandibular and sublingual glands dissected from control (D) and Sox9CKO (E) mice at E15.5. (F) Quantification of cleft formation in the control and Sox9CKO submandibular glands at the pseudoglandular stage (E13.5). ‘n’ equals the number of submandibular glands. ***P<0.0001. (G,H) BrdU immunofluorescence (green) in control (G) and Sox9CKO (H) submandibular glands at the bud stage (E12.5). (I) Quantification of the percentage of epithelial BrdU+ cells in the control and Sox9CKO submandibular glands at the bud stage (E12.5). (J,K) Cleaved caspase 3 immunofluorescence (red) in control and Sox9CKO submandibular glands at the bud stage (E12.5). Arrowheads indicate apoptotic cells at the stalk region. (L) Quantification of epithelial cell number in control and Sox9CKO submandibular glands at the bud stage (E12.5). Dotted lines in A-E,G,H,J,K delineate the salivary gland epithelium. Error bars in I and L represent s.e.m.; *P<0.05. DNA is shown in blue (DAPI) for A-C and G,H,J,K. SL, sublingual gland; SMG, submandibular gland. Scale bars: 200 μm (A-C); 500 μm (D,E); 100 μm (G,H,J,K).
Fig. 5.
Sox9 is required for parotid gland development. (A-C) Sox9 immunofluorescence (red) in control (A), Sox9CHET (B) and Sox9CKO (C) parotid glands at E15.5. Arrowheads indicate the position of the parotid gland. DNA is shown in blue (DAPI). Scale bar: 250 μm (A-C).
As branching morphogenesis is a process that involves cleft formation and epithelial bud outgrowth through proliferation (Harunaga et al., 2011), we assessed cleft formation by morphological observation and laminin deposition (Fig. 4F; Fig. S2) and proliferation by detecting bromodeoxyuridine (BrdU) incorporation (Fig. 4G-I). Although some degree of variability was observed, approximately two-thirds of the Sox9CKO SMGs displayed no signs of cleft formation, i.e. no ingression of laminin into the bud (P<0.0001) (Fig. 4F; Fig. S2C) in contrast to controls, in which clefts were observed in every case (Fig. 4F; Fig. S2A). In the Sox9CKO SMGs in which a cleft formed, only a single cleft was observed, whereas in the wild type two or more clefts were evident (Fig. S2). In the lung, Sox9 ablation has been reported to cause aberrant laminin deposition on the basal surface of the epithelium. However, in our mutant salivary glands close examination of laminin in epithelial cells revealed no obvious deposition on the basal surface compared with controls (Fig. S2A′-C′), suggesting key differences between the lung and salivary glands.
When proliferation was assessed, the ratio of cells that incorporated BrdU as a proportion of the total number of cells in the epithelium was reduced by approximately 25% in the Sox9CKO SMGs compared with the control glands at the bud stage (P<0.05) (Fig. 4G-I). The total number of epithelial cells was also reduced, by approximately 50%, in the Sox9CKO SMGs compared with that of controls at this stage (P<0.05) (Fig. 4L).
Having established that proliferation levels were significantly reduced in the mutant, we then investigated cell death in the glands. Apart from a few activated caspase 3+ cells at the site of ductal formation in both control and mutant glands (Fig. 4J,K, arrowheads) (Teshima et al., 2016b), no aberrant activation was detected in the distal compartment, suggesting that loss of Sox9 does not lead to death of the epithelial cells of the gland.
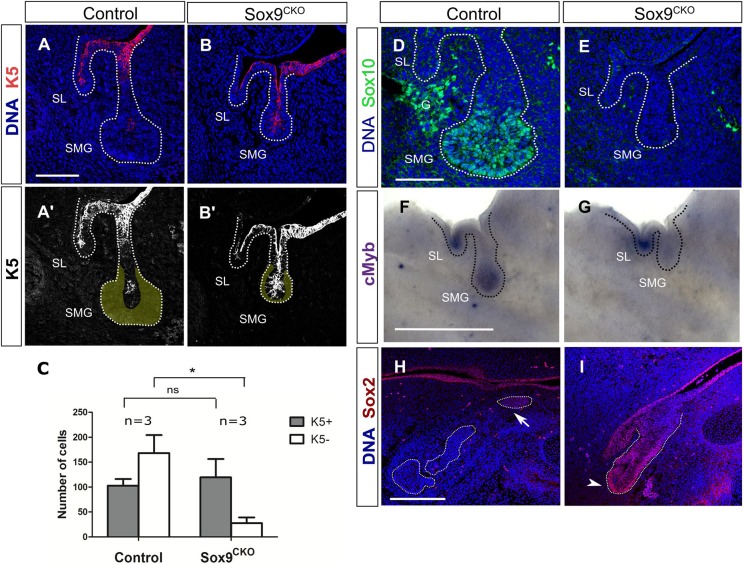
During branching morphogenesis (E13.5), epithelial progenitors express different markers depending on their location along the distal-proximal axis of the developing salivary gland. These distinct populations contribute to the formation of different epithelial structures (Matsumoto et al., 2016). Given that Sox9 is differentially expressed from E12.5 onwards, we assessed the expression of proximal (K5) and distal (Sox10, Myb) markers before the initiation of branching morphogenesis, and found that the epithelial cells could also be divided into two different populations at the bud stage, with proximal cells located at the stalk expressing K5 (Fig. 6A) and distal cells located at the tip of the endbud expressing Sox10 and cMyb (Fig. 6D,F). The early specification of the initial bud into distinct identities can be highlighted by dissecting the gland into distal and proximal compartments. The distal endbud goes on to branch in isolation, whereas the proximal stalk region fails to branch and has more limited growth (Fig. S3A-E). These data suggest that branching can initiate and progress independently of the proximal epithelium.
Fig. 6.
Sox9 is required for the specification of distal epithelial progenitors. (A-B′) Immunofluorescence for cytokeratin 5 (K5) (red) in control (A,A′) and Sox9CKO (B,B′) submandibular glands at the bud stage (E12.5). Yellow area in A′,B′ represents the K5− distal epithelial cells. (C) Total number of K5+ and K5− epithelial cells in control and Sox9CKO submandibular glands at the bud stage (E12.5). *P<0.01; ns, not significant. Error bars represent s.e.m. (D,E) Immunofluorescence for Sox10 (green) in control (D) and Sox9CKO (E) submandibular glands at the bud stage (E12.5). (F,G) In situ hybridisation for Myb in control (F) and Sox9CKO (G) submandibular glands at the bud stage (E12.5). (H,I) Immunofluorescence for Sox2 in control (H) and Sox9CKO (I) submandibular glands at the pseudoglandular stage (E13.5). Dotted lines (A-B',D-I) delineate the salivary gland epithelium. Arrow and arrowhead indicate the proximal and distal progenitors, respectively. DNA is shown in blue (DAPI) in A,B,D,E,H,I. G, ganglion; SL, sublingual gland; SMG, submandibular gland. Scale bars: 100 μm (A-B',H,I); 50 μm (D,E); 500 μm (F,G).
To understand the role of Sox9 in distal cell fate, we investigated the expression of Sox10 and Myb in Sox9CKO SMGs. Sox10 has been shown to be positively regulated by Sox9 in the lacrimal glands (Chen et al., 2014), whereas Myb has been shown to inhibit acinar differentiation in SMGs (Matsumoto et al., 2016). Both Sox10 and Myb were at low or undetectable levels in the Sox9CKO SMGs (Fig. 6D-G) indicating loss of this progenitor population in the absence of Sox9. To examine the identity of the epithelial progenitors in the Sox9CKO SMGs, we investigated the expression of the proximal marker K5 (Fig. 6A,B). When the total number of cells was compared, the number of cells with a proximal identity remained the same, but the number of K5− cells dramatically dropped (Fig. 6C). This suggests that, contrary to the distal progenitors, the proximal progenitors do not require Sox9 expression for their formation. To follow the proximal precursors at a later stage we then investigated the expression of another proximal marker, Sox2, at E13.5. Sox2 is normally expressed in the proximal epithelial progenitors in the ductal region of E13.5 control SMGs (Fig. 6H, arrow) (Lombaert et al., 2011). However, expression in the absence of Sox9 was found throughout the epithelium including the tip of the truncated Sox9CKO endbud (Fig. 6I, arrowhead) suggesting that normal differentiation can proceed in the absence of Sox9 in the remaining proximal progenitors. Altogether, these data illustrate the differential requirement of Sox9 for the formation of the distal progenitors as opposed to the proximal progenitors.
Conserved dependence of type II collagen on Sox9 expression in salivary gland epithelium
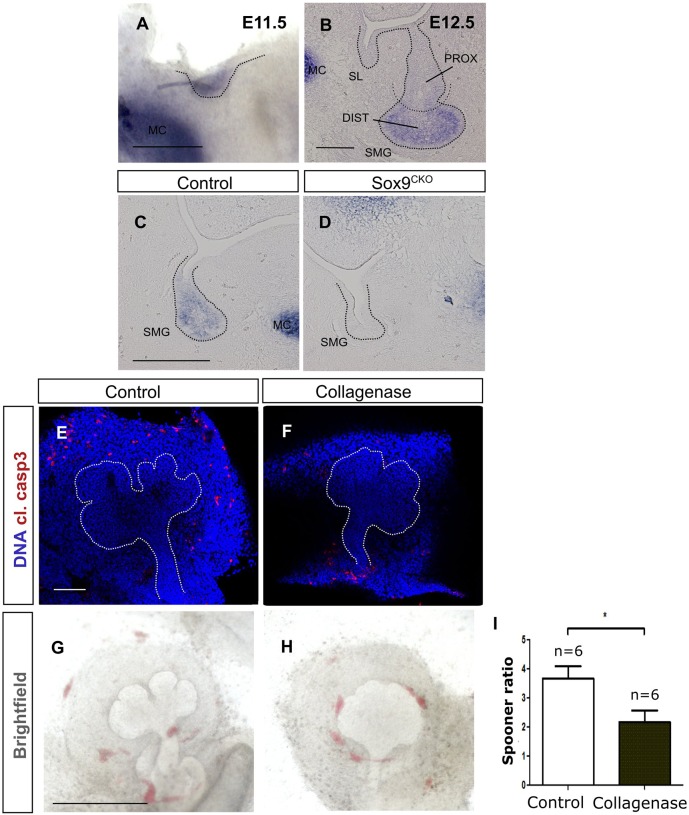
In the mesenchyme, Sox9 is part of a hierarchy of genes that control cartilage development (Bell et al., 1997). Some aspects of this pathway also appear to be conserved in epithelial tissues, for example with type II collagen being expressed in lung and lacrimal gland epithelium (Rockich et al., 2013; Chen et al., 2014). We therefore aimed to test whether type II collagen was also expressed in salivary gland epithelium. Col2a1 was observed in the salivary gland epithelium from E11.5, overlapping with Sox9 expression (Fig. 7A, compare with Fig. 1B). As with Sox9, Col2a1 was later restricted to the distal precursors (Fig. 7B). To test whether Col2a1 expression was dependent on Sox9, we assessed expression in our conditional mutants (Fig. 7C,D). In the absence of Sox9, Col2a1 expression was lost in the gland, suggesting a conserved relationship between these genes in both mesenchyme and epithelium (Fig. 7D).
Fig. 7.
Type II collagen (Col2a1) is expressed in the distal progenitors and acts downstream of Sox9 possibly by contributing to branching. (A,B) In situ hybridisation for Col2a1 at the placode (A) and bud stage (B). (C,D) In situ hybridisation for Col2a1 at the bud stage (E12.5) in control (C) and Sox9CKO (D) submandibular glands. (E,F) Immunofluorescence for cleaved caspase 3 (red) in control (E) and collagenase-treated (F) submandibular gland explants. DNA is shown in blue (DAPI). (G,H) Brightfield images of control (G) and collagenase-treated (H) submandibular gland explants. (I) Spooner ratio of the number of buds produced in the control and collagenase-treated submandibular gland explants. *P<0.05. Dotted lines (A-H) delineate the salivary gland epithelium. Error bars represent s.e.m. DIST, distal; MC, Meckel's cartilage; PROX, proximal; SL, sublingual gland; SMG, submandibular gland. Scale bars: 250 μm (A); 50 μm (B); 100 μm (C-F); 500 μm (G,H).
To investigate whether the reduction of Col2a1 expression could contribute to the branching defect observed in the Sox9CKO mice, submandibular glands were treated ex vivo with collagenase for 2 days (Fig. 7E-H). Collagenase treatment did not increase apoptosis in the epithelium, indicating no or low cytotoxic effects at this concentration (Fig. 7E,F). However, in agreement with previous observations from later stages (Nakanishi et al., 1986), collagenase treatment resulted in a reduction in branch formation (Fig. 7G-I). Thus, disruption of type II collagen in Sox9CKO salivary glands might contribute to the defect in epithelial branching morphogenesis.
Fgf10 maintains Sox9 expression through the Erk pathway during SMG development
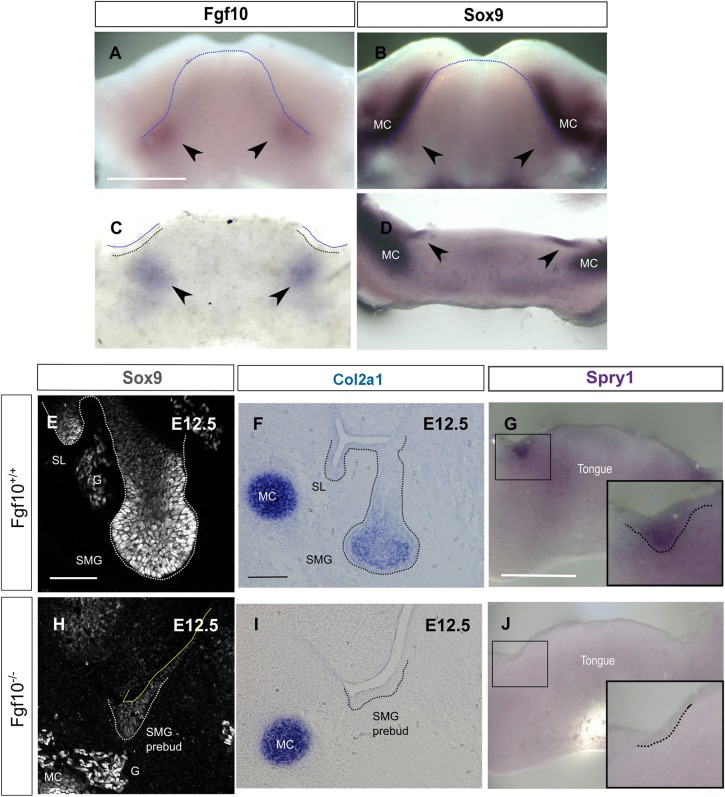
As Fgf signalling positively regulates Sox9 in other developing branching organs (Seymour et al., 2012; Chang et al., 2013; Chen et al., 2014), we hypothesised that Fgf10 might play a similar role in the SMGs. In keeping with this, qPCR analysis has previously shown upregulation of Sox9 after addition of Fgf7 or Fgf10 to wild-type epithelial rudiments of SMG at the pseudoglandular stage (Lombaert and Hoffman, 2010). We first examined the expression of Fgf10 and Sox9 at the SMG initiation stage by in situ hybridisation (Fig. 8A-D). As previously described, Fgf10 was expressed in the mesenchyme surrounding the site of placode formation (Fig. 8A,C) (Wells et al., 2013) whereas Sox9, as we have shown, was specifically expressed at the site of the epithelial thickening (Fig. 8B,D). Given the similar temporal and spatial localisation of Fgf10 and Sox9, we were interested to see whether this pattern correlates with a positive regulation. Thus, we examined the expression of Sox9 in Fgf10 null mice (Fig. 8E,H). Fgf10 null mice fail to develop a bud and their development is arrested at the placode stage (Jaskoll et al., 2005). Sox9 was highly expressed in the bud of the Fgf10+/+ SMGs but it was severely reduced in the developmentally arrested placodes of the E12.5 Fgf10 null SMGs. However, the mesenchymal expression of Sox9 in Meckel's cartilage and in the ganglion remained at the same levels (Fig. 8E,H), suggesting Sox9 in these tissues is not regulated by Fgf10. In addition, in keeping with the close relationship between Sox9 and type II collagen, expression of Col2a1 in the gland tissue was severely reduced at E12.5, with no effect on Col2a1 expression in the adjacent Meckel's cartilage (Fig. 8F,I). Loss of Sox9 in the epithelium correlated with a reduction in the expression of Spry1, a readout of Erk signalling, suggesting that activation of Sox9 by Fgf10 acts through the Erk pathway during these initial stages of SMG development (Fig. 8G,J).
Fig. 8.
Fgf10 maintains Sox9 expression during the initial stages of salivary gland development. (A-D) In situ hybridisation for Fgf10 (A,C) and Sox9 (B,D) on E11.0 mandibles (A,B) and frontal mandibular slices (C,D). Arrowheads indicate the site of expression in submandibular glands. (E,H) Immunofluorescence for Sox9 in Fgf10+/+ and Fgf10−/− submandibular glands at E12.5. (F-J) In situ hybridisation for Col2a1 (F,I) and Spry1 (G,J) in Fgf10+/+ (F,G) and Fgf10−/− (I,J) submandibular glands. Dotted lines delineate the tongue (A,B), the placode of the salivary glands (C,G,J) or the salivary gland epithelium (E,F,H,I). Boxes (G,J) indicate the placode of the developing submandibular glands, as magnified in insets. G, ganglion; MC, Meckel's cartilage; SL, sublingual gland; SMG, submandibular gland. Scale bars: 500 μm (A-D,G,J); 50 μm (E,H); 100 μm (F,I).
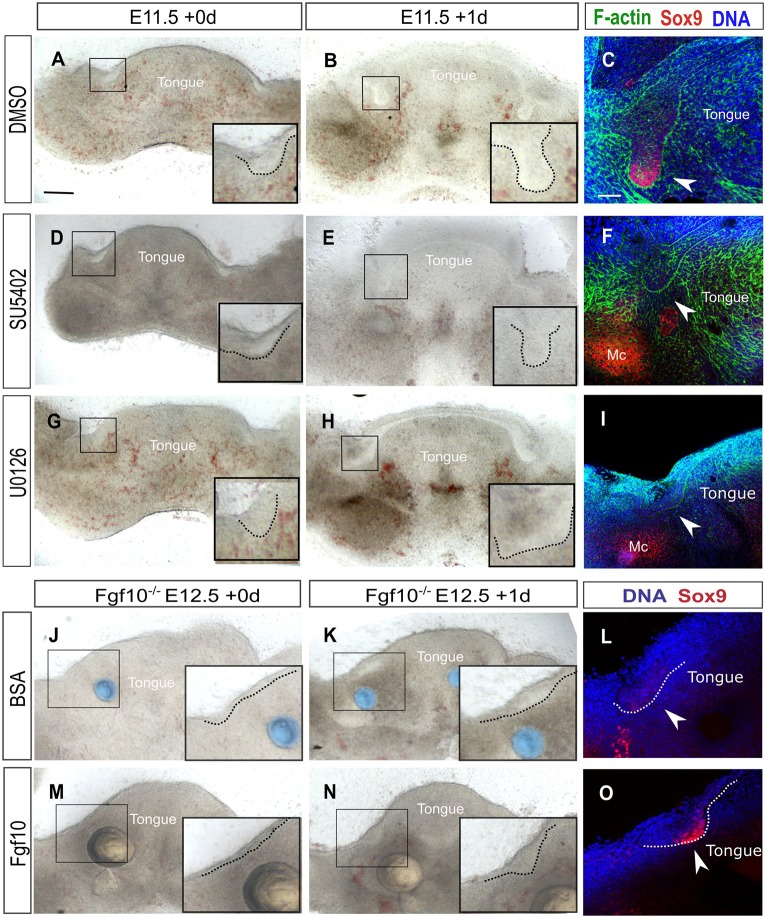
To study this positive regulation of Sox9 by Fgf10 further, we moved to an explant culture system. Mandibles were sliced frontally and slices with SMGs were cultured for 24 h (Fig. 9). In control cultures, the salivary gland tissue developed from a thickening to a bud and exhibited high levels of Sox9 (Fig. 9A-C). In contrast, slices cultured with SU5402, an inhibitor of the Fgf receptor signalling pathway, failed to develop a fully formed bud and Sox9 levels were undetectable (Fig. 9D-F), mimicking the Fgf10 knockout phenotype. In contrast, Sox9 levels were maintained at high levels in the cultures in the absence of the inhibitor (Fig. 9C).
Fig. 9.
Fgf receptor signalling maintains Sox9 expression through the Erk pathway. (A,B,D,E,G,H) Brightfield images of wild-type mandibular slice cultures treated with DMSO (A,B), the Fgf receptor inhibitor SU5402 (D,E) or the Erk inhibitor U0126 (G,H). (C,F,I) Immunofluorescence for Sox9 (red) and F-actin (green) in DMSO- (C), SU5402- (F) and U0126- (I) treated mandibular slice cultures. (J,K,M,N) Brightfield images of Fgf10−/− mandibular slice cultures treated with BSA-treated beads (blue) (J,K) or Fgf10-treated beads (pale yellow) (M,N). (L,O) Immunofluorescence for Sox9 in Fgf10−/− mandibles treated with BSA-treated beads (L) or Fgf10-treated beads (O). DNA is shown in blue (DAPI) in C,F,I,L,O. Boxes indicate the placode of the developing submandibular glands. Insets show higher magnifications of the boxed areas. Dotted lines outline the epithelium of the placodes. Arrowheads indicate the submandibular glands. Mc, Meckel's cartilage. Scale bars: 200 μm (C,F,I,L,O); 500 µm (A,B,D,E,G,H,J,K,M,N).
Fgf receptors signal through several transduction pathways the most common of which is the RAS-Erk pathway (Thisse and Thisse, 2005). To investigate which pathway controls Sox9 expression downstream of Fgf receptors, we inhibited the Erk pathway using the MAPK inhibitor U0126. Mandible slices were treated at E11.0 for 1 day with U0126 and DMSO-treated cultures were used as a control (Fig. 9G-I). Similar to the SU5402 treatment, the epithelium of the U0126-treated explants failed to form a fully developed bud (Fig. 9H) and to maintain Sox9 expression (Fig. 9I) suggesting that Fgf receptor signalling positively regulates Sox9 through the Erk pathway.
As Fgf10 is required to maintain Sox9, we went on to investigate whether exogenous Fgf10 treatment could restore Sox9 expression in Fgf10 null SMG epithelium (Fig. 9J-O). As an Fgf10 source we used heparin-coated beads treated with Fgf10 to provide a localised supply of the protein (Fig. 9M,N); bovine serum albumin (BSA)-treated beads were used as a control (Fig. 9J,K). Beads were placed on E12.5 Fgf10 null mandible slices in culture and the expression of Sox9 was assessed (Fig. 9L,O). The level of Sox9 expression was rescued in the Fgf10-treated slices compared with controls (Fig. 9L,O), further supporting the suggestion that Sox9 is positively regulated by Fgf10 in salivary glands.
Sox9 ablation does not lead to downregulation of Etv5
In the lacrimal glands, pancreas and kidney, Sox9 is involved in a positive-feedback loop with Fgf10 for further upregulation of Fgf signalling (Chen et al., 2014; Seymour et al., 2012; Reginensi et al., 2011), with Etv5 expression, a downstream target of the Fgf receptor pathway, reduced in the Sox9 mutant. To test whether Sox9 plays a similar role in salivary glands, we performed in situ hybridisation for Etv5 (Fig. S4A,B) and also for Fgf10 (Fig. S4C,D) on Sox9 mutant glands. In contrast to the development of other branching organs, no detectable difference was found between the mutants and control for both Etv5 (Fig. S4A,B) and Fgf10 (Fig. S4C,D), indicating that Sox9 does not act in a positive-feedback loop with Fgf signalling in salivary glands. Salivary glands, therefore, appear to have distinct differences in Fgf signalling compared with other branching organs.
DISCUSSION
Sox9 is a transcription factor involved in the development of many branching organs including pancreas, lacrimal glands, lungs and kidneys. Although salivary glands are also branching organs, the role of Sox9 during their development has not previously been addressed. Here, we have shown that Sox9 is expressed throughout the development of salivary glands from the salivary gland initiation stage to the fully differentiated adult salivary gland. These early Sox9+ epithelial cells are the progenitors of the entire salivary gland epithelium. In order to assess Sox9 function, we used the K14 promoter to specifically ablate epithelial Sox9 expression from the developing salivary glands. We demonstrated that Sox9 is required for salivary gland morphogenesis by promoting the formation of the distal epithelial progenitor population, the presence of which is essential for subsequent branching. Abnormal branching and gland formation was observed in all three major Sox9CKO glands, the submandibular, sublingual and parotid. Sox9 is therefore required for the development of all three major salivary glands, irrespective of whether the gland is mucous or serous.
Sox9 is required for the formation of distal epithelial progenitors and branching morphogenesis
Branching morphogenesis is a dynamic process that involves repetitive rounds of epithelial budding, clefting and epithelial outgrowth. This requires the coordination of different mechanisms, which includes ECM deposition, cell migration and epithelial proliferation (Harunaga et al., 2011). We have shown here that the mechanism of branch formation can be driven by the distal part of the epithelium alone (endbud) without the need of the proximal (stalk) epithelium. The branching defect observed in the Sox9CKO salivary glands is related to a failure in the specification of the distal epithelial population. Despite subtle differences in clefting, which could be attributed to differences in the number of Sox9+ cells that remained after recombination, all the Sox9CKO SMGs examined were arrested at the bud stage with an absence of the distal markers Myb and Sox10. Interestingly, this phenotype is specific to the salivary glands as Sox9 ablation in other branching organs leads either to complete agenesis (lacrimal glands) (Chen et al., 2014) or to reduced branching (lungs, pancreas) (Chang et al., 2013; Rockich et al., 2013; Seymour et al., 2007), suggesting that the requirement for Sox9 during development is specific to the branching organ. Despite the tissue-specific requirement for Sox9, we have shown that in salivary glands Sox9 can regulate a similar subset of genes important for branching. This includes Sox10 and Col2a1, which are also downregulated in the Sox9CKO lacrimal glands and lungs (Chen et al., 2014; Rockich et al., 2013).
Loss of type II collagen expression could contribute to the arrest in branch formation observed in the Sox9CKO SMGs as reduction of collagens with collagenase treatment in culture led to a loss of branching. In keeping with this, inhibition of collagenases has been shown to stimulate branching morphogenesis (Nakanishi et al., 1986). Our paper therefore provides a link between Sox9, distal progenitor formation and branching morphogenesis.
Fgf10 signalling positively regulates Sox9 expression through the Erk pathway
Sox9 has a distinct proximo-distal expression pattern from early bud stages; however, at the placode stage it is expressed throughout the epithelium. This change in expression might be driven by the changing pattern of Fgf10 expression, which becomes more focused around the distal part of the gland as it develops. In the Fgf10 null salivary gland, expression of Sox9 was lost at the late placode stage. In culture, Fgf7 has been shown to be able to strongly increase the expression levels of Sox9 (Lombaert and Hoffman, 2010), but in vivo Fgf10 appears to be the dominant Fgf for Sox9 expression. The Fgf10 null, however, had a more severe phenotype than the conditional Sox9 mutant with an arrest at the placode stage. Although some of the phenotype in the Fgf10 null might be generated by loss of Sox9, other genes are also likely to be affected. For example, inhibition of Fgf receptor signalling influences the activity of Wnt and Bmp signalling (Patel et al., 2011; Knosp et al., 2015; Hoffman et al., 2002).
Fgf10 heterozygous mice are viable but have been shown to have smaller salivary glands (May et al., 2015). Interestingly, at E13.5 the Sox9CHET glands were smaller than the control littermates and had reduced numbers of branches, it would therefore be interesting to study whether the glands stay small or are rescued later in development.
In our culture experiments, we were able to rescue the expression of Sox9 in Fgf10 null glands by addition of Fgf10 protein, implying that Sox9 is regulated by Fgf10 acting through the Erk pathway. Although loss of Sox9 has been associated with a subsequent loss of Fgf signalling in many branching organs, we saw no such reduction in the salivary glands (Chen et al., 2014; Seymour et al., 2012; Reginensi et al., 2011). This implies that a positive-feedback loop between Sox9 and Fgf10 is not a universal part of branching morphogenesis. In the lungs, although Etv5 is downregulated, Fgf10 itself appeared to be upregulated (Chang et al., 2013). Again, we found no change in Fgf10, confirming that Sox9 does not appear to be able to influence Fgf signalling in salivary glands. Interestingly, although inhibition of Fgf10 and Erk signalling led to a loss of Sox9 in the gland epithelium, no change in Sox9 expression was observed in the neighbouring developing cartilage, showing that although some aspects of the cartilage pathway are preserved in the glands (Sox9 induction of type II collagen), the specific involvement of Erk signalling is unique to the glands.
The current results lead us to introduce a working model in which mesenchymal Fgf10 via the Fgf and Erk pathway, activates Sox9 expression in the epithelium. Sox9 promotes the formation and proliferation of distal epithelial progenitors, and in the absence of this population the gland is unable to undergo branching morphogenesis (Fig. 10). These results provide insights into the mechanisms of progenitor cell function underlying normal salivary gland morphogenesis and could prove useful in designing methods for regeneration of branching organs.
Fig. 10.
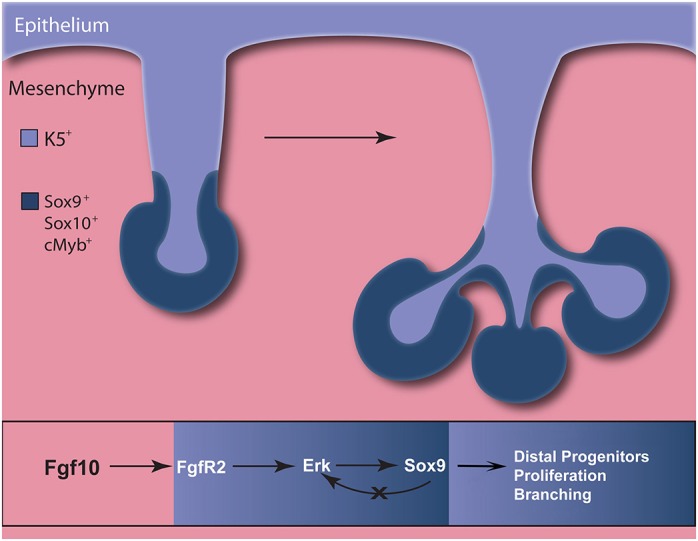
Model of Fgf10 and Sox9 function during salivary gland budding and branching morphogenesis. Sox9 is required for branching initiation by promoting the formation of distal epithelial progenitors and their proliferation. Mesenchymal Fgf10 maintains epithelial Sox9 expression during salivary gland development by activating the Erk pathway through Fgfr2.
MATERIALS AND METHODS
Mouse strains and lineage tracing
Sox9 floxed, Fgf10 null and K14-cre, Sox9-creERT2 and Rosa-tdTomato mice have been previously described (Kist et al., 2002; Min et al., 1998; Vasioukhin et al., 1999; Soeda et al., 2010; Madisen et al., 2010). For the lineage-tracing experiments, 75 mg tamoxifen/kg body weight was administered interperitoneally into E10.5 pregnant mice. The day of the vaginal plug was estimated as day 0.5 of embryonic development. All procedures and culling methods were compliant with UK Home Office regulations and with the approval of the King's College London Biological Safety committee.
Histology, immunofluorescence and in situ hybridisation
Tissue was embedded in paraffin as previously described (May et al., 2015). Immunofluorescence was performed either on paraffin-embedded tissue or on whole-mount dissected embryonic salivary glands and explant cultures (Gaete et al., 2015). Primary antibodies and dilutions were used as follows: anti-Sox9 1:300 (AB5535, Millipore); anti-BrdU 1:500 (ab6326, Abcam), anti-Sox2 1:200 (#2748, Cell Signaling Technology); anti-Mist1 1:50 (sc-98771, Santa Cruz Biotechnology), for which signal was amplified with the TSA kit (PerkinElmer); anti-laminin 1:300 (L9393, Sigma); anti-K5 1:300 (119-13621, Cambridge Bioscience); anti-Sox10 1:100 (sc-365692, Santa Cruz Biotechnology) using the TSA kit; anti-cleaved caspase 3 1:200 (#9661, Cell Signaling Technology). In situ hybridisation was performed as previously described (Gaete et al., 2015). Plasmids for probe generation have been described previously: Spry1 (Minowada et al., 1999), Fgf10 (Bellusci et al., 1997), Myb (Matalová et al., 2011), Etv5 (Hippenmeyer et al., 2002) and Col2a1 (Ng et al., 1997).
Proliferation and cell quantification analysis
For proliferation analysis, 20 mg BrdU per kg of pregnant mouse were injected intraperitoneally 30 min before harvesting. Tissue was then embedded in paraffin and processed for immunofluorescence. For BrdU immunofluorescence, samples were treated for 30 min with 2 M HCl at 40°C prior to the addition of primary antibody. The mean cell proliferation index (BrdU+/epithelial cells) for each gland was determined by analysing three different sections. For the cell quantification of epithelial progenitors, the section passing through the middle of the gland was quantified. Cells were quantified manually using the cell counter plug-in of Fiji/ImageJ (Schindelin et al., 2012). Results were plotted and statistically analysed using GraphPad Prism software. Data were analysed using a one-way ANOVA test apart from the cleft formation graph, which was analysed using the Chi-squared test. For all the quantification experiments, at least three independent biological replicates were used. Significance was taken as P<0.05 (*), P<0.01 (**) or P<0.001 (***).
Explant culture
Mandibular slice cultures were performed as previously described (Wells et al., 2013; Li et al., 2016). For the bead experiment, two types of beads were used to help distinguish between the control and treated conditions. For the Fgf10-treated explants, heparin beads (Sigma, 100-200 mesh) were incubated overnight at 4°C with 100 μg/ml Fgf10 (R&D Systems). For the control, Affi-Gel blue beads (Bio-Rad,153-7302) were treated with 0.5% BSA. For inhibiting Fgf receptor signalling or the Erk pathway, explant cultures were treated with 2.5 μM SU5402 (Merck) or 5 μM U0126 (Cell Signaling Technology), respectively, made up in DMSO. Control cultures were treated with equivalent concentrations of DMSO (0.25% DMSO for the SU5402 and 0.5% DMSO for the U0126 experiment). For the collagenase treatment, whole E12.5 submandibular glands were dissected and treated for 2 days with 1 μg/ml collagenase, Type II (Thermo Fisher Scientific) and HBSS-treated glands were used as a control. Spooner ratios were calculated as the number of buds at the end of culture divided by the number of buds at the start of culture.
Acknowledgements
We thank Dr Karine Rizzoti for kindly providing Sox9-CreERT2 embryos, from a line that originated from the lab of Prof. Haruhiko Akiyama. The Sox9 floxed mice were obtained from MRC-Harwell, which distributes these mice on behalf of the European Mouse Mutant Archive (https://www.infrafrontier.eu/). MRC-Harwell is also a member of the International Mouse Phenotyping Consortium (IMPC; www.mousephenotype.org). We thank T. von Kunowsky for help with graphics.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: L.C., M.G., A.S.T.; Validation: L.C.; Formal analysis: L.C.; Investigation: L.C., M.G.; Resources: A.S.T.; Writing - original draft: L.C., A.S.T.; Writing - review & editing: L.C., M.G., A.S.T.; Visualization: L.C., M.G.; Supervision: M.G., A.S.T.; Project administration: A.S.T.; Funding acquisition: A.S.T.
Funding
This work was funded by the Anatomical Society (PhD studentship for L.C. awarded to A.S.T.), the Wellcome Trust [102889/Z/13/Z to A.S.T.] and the Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT) [11140303 to M.G.]. Deposited in PMC for immediate release.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.146019.supplemental
References
- Abler L. L., Mansour S. L. and Sun X. (2009). Conditional gene inactivation reveals roles for Fgf10 and Fgfr2 in establishing a normal pattern of epithelial branching in the mouse lung. Dev. Dyn. 238, 1999-2013. 10.1002/dvdy.22032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Affolter M., Bellusci S., Itoh N., Shilo B., Thiery J.-P. and Werb Z. (2003). Tube or not tube: remodeling epithelial tissues by branching morphogenesis. Dev. Cell 4, 11-18. 10.1016/S1534-5807(02)00410-0 [DOI] [PubMed] [Google Scholar]
- Arnold K., Sarkar A., Yram M. A., Polo J. M., Bronson R., Sengupta S., Seandel M., Geijsen N. and Hochedlinger K. (2011). Sox2(+) adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell 9, 317-329. 10.1016/j.stem.2011.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aure M. H., Konieczny S. F. and Ovitt C. E. (2015). Salivary gland homeostasis is maintained through acinar cell self-duplication. Dev. Cell 33, 231-237. 10.1016/j.devcel.2015.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell D. M., Leung K. K. H., Wheatley S. C., Ng L. J., Zhou S., Ling K. W., Sham M. H., Koopman P., Tam P. P. and Cheah K. S. (1997). SOX9 directly regulates the type-II collagen gene. Nat. Genet. 16, 174-178. 10.1038/ng0697-174 [DOI] [PubMed] [Google Scholar]
- Bellusci S., Grindley J., Emoto H., Itoh N. and Hogan B. L. (1997). Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development 124, 4867-4878. [DOI] [PubMed] [Google Scholar]
- Chang D. R., Martinez Alanis D., Miller R. K., Ji H., Akiyama H., McCrea P. D. and Chen J. (2013). Lung epithelial branching program antagonizes alveolar differentiation. Proc. Natl. Acad. Sci. USA 110, 18042-18051. 10.1073/pnas.1311760110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Huang J., Liu Y., Dattilo L. K., Huh S.-H., Ornitz D. and Beebe D. C. (2014). FGF signaling activates a Sox9-Sox10 pathway for the formation and branching morphogenesis of mouse ocular glands. Development 141, 2691-2701. 10.1242/dev.108944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaete M., Fons J. M., Popa E. M., Chatzeli L. and Tucker A. S. (2015). Epithelial topography for repetitive tooth formation. Biol. Open 4, 1625-1634. 10.1242/bio.013672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harunaga J., Hsu J. C. and Yamada K. M. (2011). Dynamics of salivary gland morphogenesis. J. Dent. Res. 90, 1070-1077. 10.1177/0022034511405330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippenmeyer S., Shneider N. A., Birchmeier C., Burden S. J., Jessell T. M. and Arber S. (2002). A role for neuregulin1 signaling in muscle spindle differentiation. Neuron 36, 1035-1049. 10.1016/S0896-6273(02)01101-7 [DOI] [PubMed] [Google Scholar]
- Hoffman M. P., Kidder B. L., Steinberg Z. L., Lakhani S., Ho S., Kleinman H. K. and Larsen M. (2002). Gene expression profiles of mouse submandibular gland development: FGFR1 regulates branching morphogenesis in vitro through BMP- and FGF-dependent mechanisms. Development 129, 5767-5778. 10.1242/dev.00172 [DOI] [PubMed] [Google Scholar]
- Jaskoll T., Zhou Y. M., Chai Y., Makarenkova H. P., Collinson J. M., West J. D., Hajihosseini M. K., Lee J. and Melnick M. (2002). Embryonic submandibular gland morphogenesis: stage-specific protein localization of FGFs, BMPs, Pax6 and Pax9 in normal mice and abnormal SMG phenotypes in FgfR2-IIIc(+/Delta), BMP7(−/−) and Pax6(−/−) mice. Cells Tissues Organs 170, 83-98. 10.1159/000046183 [DOI] [PubMed] [Google Scholar]
- Jaskoll T., Abichaker G., Witcher D., Sala F. G., Bellusci S., Hajihosseini M. K. and Melnick M. (2005). FGF10/FGFR2b signaling plays essential roles during in vivo embryonic submandibular salivary gland morphogenesis. BMC Dev. Biol. 5, 11 10.1186/1471-213X-5-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kist R., Schrewe H., Balling R. and Scherer G. (2002). Conditional inactivation of Sox9: a mouse model for campomelic dysplasia. Genesis 32, 121-123. 10.1002/gene.10050 [DOI] [PubMed] [Google Scholar]
- Knosp W. M., Knox S. M. and Hoffman M. P. (2012). Salivary gland organogenesis. Wiley Interdiscip. Rev. Dev. Biol. 1, 69-82. 10.1002/wdev.4 [DOI] [PubMed] [Google Scholar]
- Knosp W. M., Knox S. M., Lombaert I. M. A., Haddox C. L., Patel V. N. and Hoffman M. P. (2015). Submandibular parasympathetic gangliogenesis requires sprouty-dependent Wnt signals from epithelial progenitors. Dev. Cell 32, 667-677. 10.1016/j.devcel.2015.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox S. M., Lombaert I. M. A., Reed X., Vitale-Cross L., Gutkind J. S. and Hoffman M. P. (2010). Parasympathetic innervation maintains epithelial progenitor cells during salivary organogenesis. Science 329, 1645-1647. 10.1126/science.1192046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Chatzeli L., Panousopoulou E., Tucker A. S. and Green J. B. (2016). Epithelial stratification and placode invagination are separable functions in early morphogenesis of the molar tooth. Development 143, 670-681. 10.1242/dev.130187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombaert I. M. A. and Hoffman M. P. (2010). Epithelial stem/progenitor cells in the embryonic mouse submandibular gland. Front. Oral Biol. 14, 90-106. 10.1159/000313709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombaert I. M. A., Knox S. M. and Hoffman M. P. (2011). Salivary gland progenitor cell biology provides a rationale for therapeutic salivary gland regeneration. Oral Dis. 17, 445-449. 10.1111/j.1601-0825.2010.01783.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombaert I. M. A., Abrams S. R., Li L., Eswarakumar V. P., Sethi A. J., Witt R. L. and Hoffman M. P. (2013). Combined KIT and FGFR2b signaling regulates epithelial progenitor expansion during organogenesis. Stem Cell Reports 1, 604-619. 10.1016/j.stemcr.2013.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L., Zwingman T. A., Sunkin S. M., Oh S. W., Zariwala H. A., Gu H., Ng L. L., Palmiter R. D., Hawrylycz M. J., Jones A. R. et al. (2010). A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133-140. 10.1038/nn.2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matalová E., Buchtová M., Tucker A. S., Bender T. P., Janečková E., Lungová V., Balková S. and Smarda J. (2011). Expression and characterization of c-Myb in prenatal odontogenesis. Dev. Growth Differ. 53, 793-803. 10.1111/j.1440-169X.2011.01287.x [DOI] [PubMed] [Google Scholar]
- Matsumoto S., Kurimoto T., Taketo M. M., Fujii S. and Kikuchi A. (2016). The WNT/MYB pathway suppresses KIT expression to control the timing of salivary proacinar differentiation and duct formation. Development 143, 2311-2324. 10.1242/dev.134486 [DOI] [PubMed] [Google Scholar]
- May A. J., Chatzeli L., Proctor G. B. and Tucker A. S. (2015). Salivary gland dysplasia in Fgf10 heterozygous mice: a new mouse model of Xerostomia. Curr. Mol. Med. 15, 674-682. 10.2174/1566524015666150831141307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min H., Danilenko D. M., Scully S. A., Bolon B., Ring B. D., Tarpley J. E., DeRose M. and Simonet W. S. (1998). Fgf-10 is required for both limb and lung development and exhibits striking functional similarity to Drosophila branchless. Genes Dev. 12, 3156-3161. 10.1101/gad.12.20.3156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minowada G., Jarvis L. A., Chi C. L., Neubüser A., Sun X., Hacohen N., Krasnow M. A. and Martin G. R. (1999). Vertebrate Sprouty genes are induced by FGF signaling and can cause chondrodysplasia when overexpressed. Development 126, 4465-4475. [DOI] [PubMed] [Google Scholar]
- Nakanishi Y., Sugiura F., Kishi J.-I. and Hayakawa T. (1986). Collagenase inhibitor stimulates cleft formation during early morphogenesis of mouse salivary gland. Dev. Biol. 113, 201-206. 10.1016/0012-1606(86)90122-3 [DOI] [PubMed] [Google Scholar]
- Ng L.-J., Wheatley S., Muscat G. E. O., Conway-Campbell J., Bowles J., Wright E., Bell D. M., Tam P. P. L., Cheah K. S. E. and Koopman P. (1997). Sox9 binds DNA, Activates transcription and coexpresses with type II collagen during chondrogenesis in the mouse. Dev. Biol. 183, 108-121. 10.1006/dbio.1996.8487 [DOI] [PubMed] [Google Scholar]
- Ohuchi H., Hori Y., Yamasaki M., Harada H., Sekine K., Kato S. and Itoh N. (2000). FGF10 acts as a major ligand for FGF receptor 2 IIIb in mouse multi-organ development. Biochem. Biophys. Res. Commun. 277, 643-649. 10.1006/bbrc.2000.3721 [DOI] [PubMed] [Google Scholar]
- Patel N., Sharpe P. T. and Miletich I. (2011). Coordination of epithelial branching and salivary gland lumen formation by Wnt and FGF signals. Dev. Biol. 358, 156-167. 10.1016/j.ydbio.2011.07.023 [DOI] [PubMed] [Google Scholar]
- Pritchett J., Athwal V., Roberts N., Hanley N. A. and Hanley K. P. (2011). Understanding the role of SOX9 in acquired diseases: lessons from development. Trends Mol. Med. 17, 166-174. 10.1016/j.molmed.2010.12.001 [DOI] [PubMed] [Google Scholar]
- Reginensi A., Clarkson M., Neirijnck Y., Lu B., Ohyama T., Groves A. K., Sock E., Wegner M., Costantini F., Chaboissier M.-C. et al. (2011). SOX9 controls epithelial branching by activating RET effector genes during kidney development. Hum. Mol. Genet. 20, 1143-1153. 10.1093/hmg/ddq558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockich B. E., Hrycaj S. M., Shih H. P., Nagy M. S., Ferguson M. A. H., Kopp J. L., Sander M., Wellik D. M. and Spence J. R. (2013). Sox9 plays multiple roles in the lung epithelium during branching morphogenesis. Proc. Natl. Acad. Sci. USA 110, E4456-E4464. 10.1073/pnas.1311847110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B. et al. (2012). Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676-682. 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour P. A., Freude K. K., Tran M. N., Mayes E. E., Jensen J., Kist R., Scherer G. and Sander M. (2007). SOX9 is required for maintenance of the pancreatic progenitor cell pool. Proc. Natl. Acad. Sci. USA 104, 1865-1870. 10.1073/pnas.0609217104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour P. A., Shih H. P., Patel N. A., Freude K. K., Xie R., Lim C. J. and Sander M. (2012). A Sox9/Fgf feed-forward loop maintains pancreatic organ identity. Development 139, 3363-3372. 10.1242/dev.078733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeda T., Deng J. M., de Crombrugghe B., Behringer R. R., Nakamura T. and Akiyama H. (2010). Sox9-expressing precursors are the cellular origin of the cruciate ligament of the knee joint and the limb tendons. Genesis 48, 635-644. 10.1002/dvg.20667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg Z., Myers C., Heim V. M., Lathrop C. A., Rebustini I. T., Stewart J. S., Larsen M. and Hoffman M. P. (2005). FGFR2b signaling regulates ex vivo submandibular gland epithelial cell proliferation and branching morphogenesis. Development 132, 1223-1234. 10.1242/dev.01690 [DOI] [PubMed] [Google Scholar]
- Teshima T. H., Lourenço S. V. and Tucker A. S. (2016a). Multiple cranial organ defects after conditionally knocking out Fgf10 in the neural crest. Front. Physiol. 7, 488 10.3389/fphys.2016.00488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teshima T. H., Wells K. L., Lourenço S. V. and Tucker A. S. (2016b). Apoptosis in early salivary gland duct morphogenesis and lumen formation. J. Dent. Res. 95, 277-283. 10.1177/0022034515619581 [DOI] [PubMed] [Google Scholar]
- Thisse B. and Thisse C. (2005). Functions and regulations of fibroblast growth factor signaling during embryonic development. Dev. Biol. 287, 390-402. 10.1016/j.ydbio.2005.09.011 [DOI] [PubMed] [Google Scholar]
- Tucker A. S. (2007). Salivary gland development. Semin. Cell Dev. Biol. 18, 237-244. 10.1016/j.semcdb.2007.01.006 [DOI] [PubMed] [Google Scholar]
- Vasioukhin V., Degenstein L., Wise B. and Fuchs E. (1999). The magical touch: genome targeting in epidermal stem cells induced by tamoxifen application to mouse skin. Proc. Natl. Acad. Sci. USA 96, 8551-8556. 10.1073/pnas.96.15.8551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner T., Wirth J., Meyer J., Zabel B., Held M., Zimmer J., Pasantes J., Bricarelli F. D., Keutel J., Hustert E. et al. (1994). Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell 79, 1111-1120. 10.1016/0092-8674(94)90041-8 [DOI] [PubMed] [Google Scholar]
- Walker J. L., Menko A. S., Khalil S., Rebustini I., Hoffman M. P., Kreidberg J. A. and Kukuruzinska M. A. (2008). Diverse roles of E-cadherin in the morphogenesis of the submandibular gland: insights into the formation of acinar and ductal structures. Dev. Dyn. 237, 3128-3141. 10.1002/dvdy.21717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells K. L., Gaete M., Matalova E., Deutsch D., Rice D. and Tucker A. S. (2013). Dynamic relationship of the epithelium and mesenchyme during salivary gland initiation: the role of Fgf10. Biol. Open 2, 981-989. 10.1242/bio.20135306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q., Eberspaecher H., Lefebvre V. and de Crombrugghe B. (1997). Parallel expression of Sox9 and Col2a1 in cells undergoing chondrogenesis. Dev. Dyn. 209, 377-386. [DOI] [PubMed] [Google Scholar]