Abstract
Proper oocyte development is crucial for female fertility and requires timely and accurate control of gene expression. K (lysine) acetyltransferase 8 (KAT8), an important component of the X chromosome dosage compensation system in Drosophila, regulates gene activity by acetylating histone H4 preferentially at lysine 16. To explore the function of KAT8 during mouse oocyte development, we crossed Kat8flox/flox mice with Gdf9-Cre mice to specifically delete Kat8 in oocytes. Oocyte Kat8 deletion resulted in female infertility, with follicle development failure in the secondary and preantral follicle stages. RNA-seq analysis revealed that Kat8 deficiency in oocytes results in significant downregulation of antioxidant genes, with a consequent increase in reactive oxygen species. Intraperitoneal injection of the antioxidant N-acetylcysteine rescued defective follicle and oocyte development resulting from Kat8 deficiency. Chromatin immunoprecipitation assays indicated that KAT8 regulates antioxidant gene expression by direct binding to promoter regions. Taken together, our findings demonstrate that KAT8 is essential for female fertility by regulating antioxidant gene expression and identify KAT8 as the first histone acetyltransferase with an essential function in oogenesis.
KEY WORDS: Kat8, H4K16ac, Oocyte, Follicle, Sterility, ROS
Highlighted Article: KAT8 represses ROS levels in oocytes by promoting the expression of antioxidant genes, while genetic deletion of KAT8 leads to female infertility.
INTRODUCTION
Oogenesis plays a key role in female fertility as it determines the production of healthy eggs (Rodrigues et al., 2008). Oocyte growth, which is accompanied by follicle development, is an important part of oogenesis. In mice, oocyte growth begins from 5 days postpartum and continues to the full-grown germinal vesicle (GV) stage (Lucifero et al., 2007). During this period, the oocyte grows rapidly in size and is transcriptionally active (Jagarlamudi and Rajkovic, 2012), which is essential for the oocyte to achieve further development and final maturation (van den Hurk and Zhao, 2005).
To ensure the normal growth of oocytes, excessive apoptosis must be avoided by promoting the expression of anti-apoptotic genes. Several factors can induce oocyte apoptosis in mammals, such as increased levels of reactive oxygen species (ROS) or destruction of oocyte-granulosa cell communication (Tiwari et al., 2015). However, how anti-apoptotic genes are regulated in response to these apoptosis inducers is still not fully understood.
In oocytes, transcriptional activity is associated with histone modifications, including acetylation (Gu et al., 2010). The level of histone acetylation is controlled by the opposing activity of histone acetyltransferases (HATs) and histone deacetylases (HDACs) (Ma et al., 2012). HATs, which comprise the MYST, Gcn5/PCAF and p300/CBP subfamilies (Gu et al., 2010), add acetyl groups to histone lysines, which weakens the electrostatic affinity between histones and DNA, resulting in an open chromatin structure that promotes transcription factor binding and thus gene activity. By contrast, HDACs mainly repress transcription by removing acetyl groups from histone lysine residues, which results in a more compact chromatin structure (Shahbazian and Grunstein, 2007). Among the multiple acetylatable lysines (K), the deacetylation of H4 at lysine 16 has been found to be essential for mouse oocyte maturation. Depletion of the HDACs HDAC2 or SIRT6 in oocytes within primary or antral follicles results in increased H4K16ac levels in metaphase II (MII) oocytes (Han et al., 2015; Ma and Schultz, 2013), decreases the number of antral follicles (Ma and Schultz, 2013) and causes defects in spindle assembly and chromosome segregation in MII oocytes (Han et al., 2015; Ma and Schultz, 2013). Although a high level of H4K16ac is observed in GV stage oocyte (Pan et al., 2012), whether it is necessary for oogenesis is unknown.
K (lysine) acetyltransferase 8 (KAT8, also known as MOF or MYST1) is a highly conserved MYST family member specifically responsible for H4K16 acetylation (Gupta et al., 2013). KAT8 contains a chromodomain for DNA binding and a C-terminal HAT domain for histone acetylation (Su et al., 2016). It is expressed in most tissues, including the ovary (Thomas et al., 2007), and affects gene expression in multiple biological processes. Drosophila MOF is essential for dosage compensation in males by inducing a 2-fold transcriptional upregulation of X-linked genes (Akhtar and Becker, 2000). In mouse embryonic stem cells (ESCs), Kat8 is necessary for the maintenance of self-renewal and pluripotency by promoting the expression of key transcription factors (Li et al., 2012). Although oocyte development is associated with dramatic changes in gene expression, whether KAT8 functions in this process is unknown.
Here, we specifically deleted Kat8 in mouse oocytes. The conditional knockout females were sterile, with defective oocyte and follicle development. Our findings identify KAT8 as the first HAT with an essential role in mouse follicle development and female fertility.
RESULTS
Kat8 expression in oocytes and granulosa cells
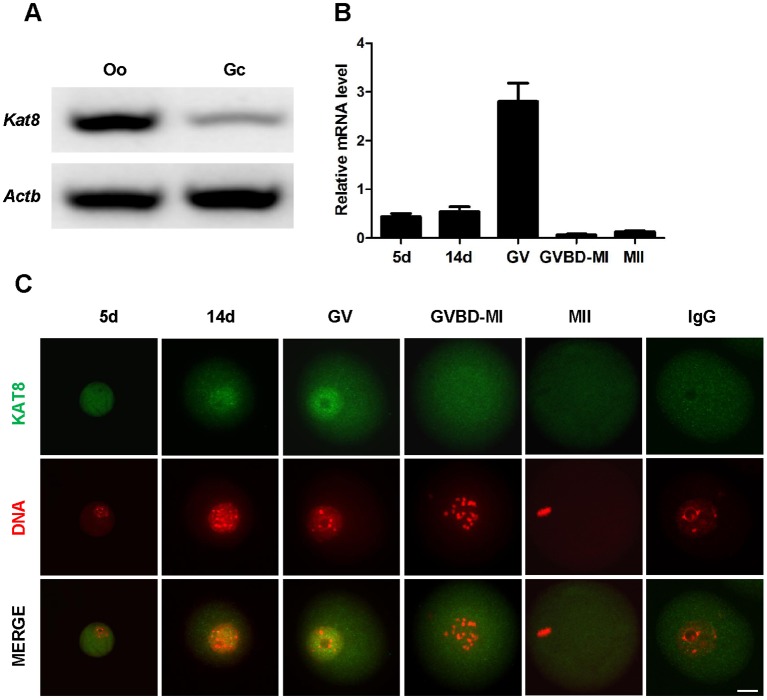
To explore the role of Kat8 during follicle development, we first examined gene expression in ovarian cells of wild-type (WT) mice. Analysis of Kat8 mRNA levels in oocytes and granulosa cells from antral follicles by RT-PCR revealed that both cell types express Kat8 (Fig. 1A), with much higher expression levels in oocytes than in granulosa cells. We next analyzed Kat8 expression during oocyte development and detected a dramatic increase (∼5-fold) between 14 days and full-grown GV stage oocytes, followed by a sharp decrease (2.3% to GV stage) in germinal vesicle breakdown (GVBD)-metaphase I (MI) stage oocytes (Fig. 1B). Immunostaining revealed that KAT8 protein is mainly located in the nucleus throughout the growth phase, but upon GVBD the staining intensity decreased and the signal became uniformly dispersed throughout the oocyte (Fig. 1C).
Fig. 1.
Kat8 expression in oocytes and granulosa cells. (A) RT-PCR analysis of Kat8 mRNA levels in oocytes (Oo) and granulosa cells (Gc) from antral follicles of WT mice. (B) Kat8 expression during oocyte development. Kat8 mRNA levels in oocytes at different growth phases were determined by real-time PCR, and normalized to Actb. 5 d, 5 days postpartum; 14 d, 14 days postpartum; GV, full-grown germinal vesicle; GVBD, germinal vesicle breakdown; MI, metaphase I; MII, metaphase II. Data are mean±s.e.m. from three independent experiments. (C) KAT8 immunostaining of oocytes at different growth phases. IgG was used as a negative control. Scale bar: 20 μm. Images (A,C) are representative of three independent experiments.
Given that Kat8 is essential for histone modification, we also analyzed H4K16 acetylation levels, the major histone target of KAT8, in oocytes at different developmental stages. Immunocytochemical assays showed that, consistent with the KAT8 localization profile, nuclear staining of H4K16ac increased from 14 days to GV stage and decreased significantly upon GVBD (Fig. S1).
Specific Kat8 deletion in oocytes leads to abnormal oogenesis and female sterility
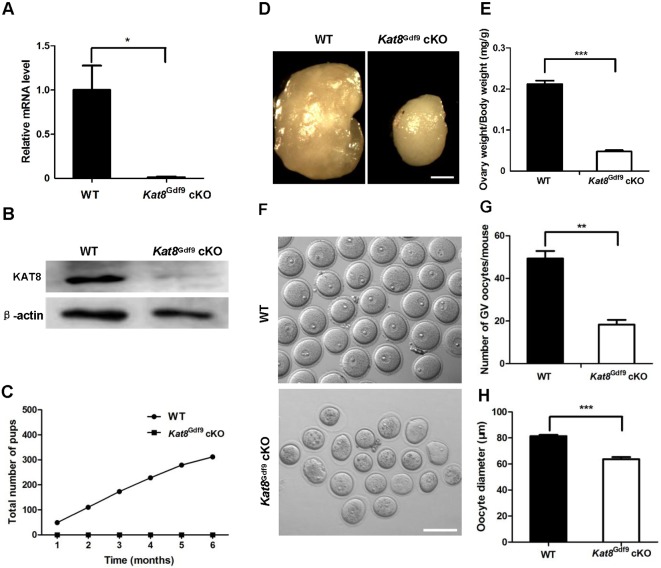
To study the role of Kat8 during oocyte development, we generated mice in which Kat8 was specifically disrupted in oocytes. We crossed female Kat8flox/flox mice, which have loxP sites flanking exons 1 to 3 of Kat8 (Gupta et al., 2008; Kumar et al., 2011), with Gdf9-Cre males. Expression of Cre recombinase begins in mouse oocytes at 3 days (Lan et al., 2004). Real-time PCR and western blot confirmed that oocytes from Gdf9-Cre; Kat8flox/flox (referred to as Kat8Gdf9 cKO) mice had negligible expression of Kat8 mRNA and protein (Fig. 2A,B), confirming efficient deletion of Kat8. Additionally, Kat8 deletion resulted in a decrease in H4K16ac without affecting acetylation at other detected histone residues in oocytes (Fig. S2).
Fig. 2.
Kat8Gdf9 cKO mice display abnormal oogenesis and sterility. (A) Real-time PCR analysis of Kat8 mRNA levels in oocytes from 2-week-old WT and Kat8Gdf9 cKO mice. Kat8 mRNA levels in WT oocytes were arbitrarily set to 1, and values were normalized and plotted against Actb. (B) Western blot analysis of KAT8 in oocytes from 2-week-old mice. Per sample, ∼200 oocytes were used; β-actin served as a protein loading control. Images shown are representative of three independent experiments. (C) Fertility test. The cumulative number of pups obtained from matings of eight WT and eight Kat8Gdf9 cKO females to WT males over a 6 month period. (D) Representative images of ovaries from 8-week-old females. Scale bar: 0.5 mm. (E) Ratio of ovary weight to body weight in 8-week-old mice. (F) Representative images of GV stage oocytes from 8-week-old mice. Scale bar: 200 μm. (G) Mean number of GV stage oocytes obtained per mouse after priming with PMSG. (H) Mean diameter of GV stage oocytes. (A,E,G,H) Data are mean±s.e.m. from three independent experiments. *P<0.05, **P<0.01, ***P<0.001, t-test.
To determine their fertility, Kat8Gdf9 cKO females and WT controls were bred to WT males for 6 months. A total of 312 pups were produced from eight WT females, whereas no offspring were born to the eight Kat8Gdf9 cKO females (Fig. 2C). Compared with WT mice, 8-week-old Kat8Gdf9 cKO females had substantially smaller ovaries (Fig. 2D) and a 77.4% lower ovary to body weight ratio (Fig. 2E). Following pregnant mare serum gonadotropin (PMSG) priming, ∼50 full-grown GV stage oocytes were recovered from each WT female, but only ∼20 oocytes from each Kat8Gdf9 cKO mouse (Fig. 2G). Notably, Kat8Gdf9 cKO oocytes were much smaller than WT oocytes (mean diameter 63.7 μm versus 81.4 μm, respectively; Fig. 2H), and exhibited abnormal morphology, including cytoplasmic granulation, shrinkage and an irregular shape (Fig. 2F). Together, these results indicate that Kat8 is essential for fertility and oogenesis.
Kat8Gdf9 cKO females display defects in follicle development and increased oocyte apoptosis
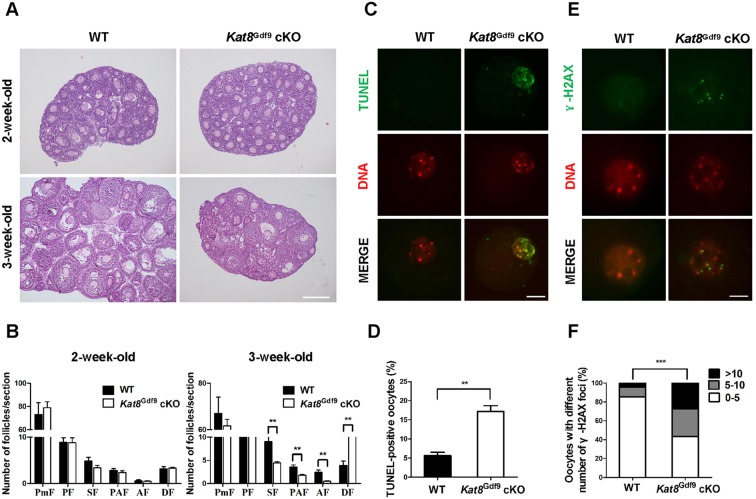
To investigate how Kat8 deletion disrupts oogenesis, we carefully analyzed follicle development in Kat8Gdf9 cKO females. Ovaries from 2-week-old Kat8Gdf9 cKO mice were indistinguishable from WT ovaries in morphology and contained comparable numbers of follicles at different developmental stages (Fig. 3A,B). However, Kat8Gdf9 cKO ovaries from 3-week-old females contained 50.4%, 49.6% and 78.7% fewer secondary, preantral and antral follicles, respectively, than WT ovaries, but 2-fold more atretic follicles (Fig. 3A,B).
Fig. 3.
Kat8 deletion in oocytes causes follicle loss and oocyte defects. (A) Histological sections of ovaries from 2- and 3-week-old females stained with Hematoxylin and periodic acid-Schiff (PAS) reagent. (B) Quantitative analysis of follicles at different stages in ovary sections from 2- and 3-week-old WT and Kat8Gdf9 cKO females. PmF, primordial follicles; PF, primary follicles; SF, secondary follicles; PAF, preantral follicles; AF, antral follicles; DF, degenerating follicles. (C) Detection of apoptosis by TUNEL assay in oocytes from 3-week-old mice. (D) Percentage of TUNEL-positive oocytes. (B,D) Data are mean±s.e.m. from three independent experiments. **P<0.01, t-test. (E) Visualization of DSBs in oocyte nuclei from 3-week-old mice by γ-H2AX staining. (F) Proportions of WT and Kat8Gdf9 cKO oocytes with various numbers of γ-H2AX foci per nucleus. ***P<0.001, Chi-square test. Images (A,C,E) are representative of three independent experiments. Scale bars: 200 μm in A; 20 μm in C; 10 μm in E.
The defective oogenesis and follicle development in Kat8Gdf9 cKO females strongly implied that Kat8-deficient oocytes undergo apoptosis. To confirm this, we performed terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assays in oocytes isolated from 3-week-old females (oocyte diameter of 50-70 μm, mainly from secondary and preantral follicles) (Monti and Redi, 2009). Indeed, Kat8Gdf9 cKO oocytes exhibited a significantly higher incidence of apoptosis than WT oocytes (∼17.2% versus ∼5.7%; Fig. 3C,D). As DNA damage is a major trigger of cell apoptosis (Ma et al., 2013), we measured DNA double-strand breaks (DSBs) in oocytes by immunostaining for phosphorylated histone H2AX (γ-H2AX), a commonly used surrogate marker for DSBs. The ratio of oocytes with more than five γ-H2AX foci (Fig. 3E,F) was significantly increased in Kat8Gdf9 cKO (∼56.8%) compared with WT (∼14.6%) oocytes. TUNEL assay and γ-H2AX immunostaining performed on ovary sections confirmed that oocytes undergoing apoptosis or exhibiting severe DNA damage were localized in secondary and preantral follicles, but not in primordial or primary follicles (Fig. S3). Additionally, we found that Kat8 deletion in oocytes did not affect the proliferation of granulosa cells, as confirmed by bromodeoxyuridine (BrdU) incorporation and PCNA immunostaining (Fig. S4A,B). Also, expression levels of the oocyte factors Gdf9 and Bmp15 (McNatty et al., 2005) did not differ significantly between WT and Kat8Gdf9 cKO oocytes (Fig. S4C). Taken together, these results indicate that Kat8 is essential for follicle development by maintaining oocyte survival.
Kat8Gdf9 cKO oocytes exhibit decreased antioxidant gene expression and excess ROS levels
To explore the mechanism of Kat8-dependent oogenesis and folliculogenesis, we performed whole-transcriptome sequencing (RNA-seq) on oocytes (50-70 μm diameter) from 2-week-old mice. Accuracy of the sequencing data was validated by real-time PCR of mRNA levels for randomly selected genes. Consistent expression trends from both real-time PCR and RNA-seq for all eight selected test genes confirmed reliability of the sequencing data (Fig. S5A). Data analysis identified 858 significant differentially expressed genes, 500 of which were upregulated and 358 downregulated in Kat8Gdf9 cKO oocytes (Tables S3 and S4, Fig. S5B).
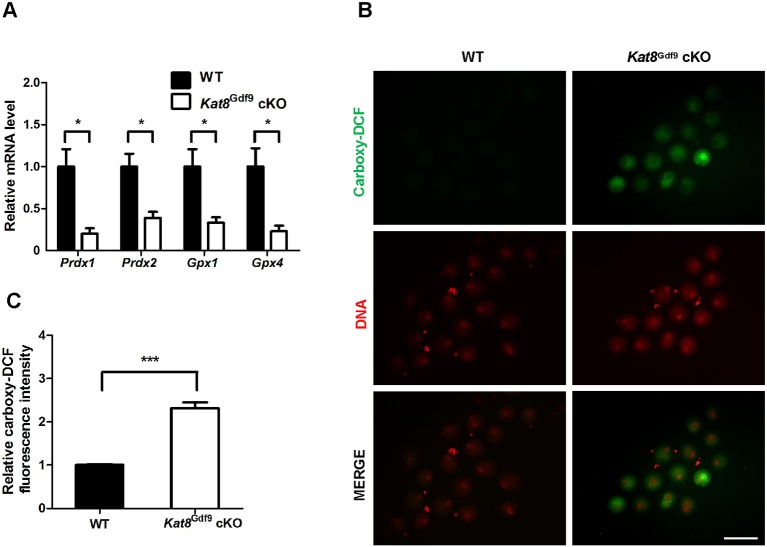
Gene Ontology (GO) enrichment analysis of the differentially expressed genes identified the term ʻoxidation-reduction process' among the most enriched downregulated biological processes (Fig. S5C). Continuous scavenging of ROS by antioxidant enzymes represents one cellular mechanism of defense against oxidative damage, which may compromise DNA integrity and cause oocyte apoptosis (Devine et al., 2012). Among the differently expressed genes after Kat8 deletion, the antioxidant genes peroxiredoxin 1 and 2 (Prdx1, Prdx2) and glutathione peroxidase 1 and 4 (Gpx1, Gpx4) were significantly downregulated in Kat8Gdf9 cKO oocytes (Table S4), which was confirmed by real-time PCR (Fig. 4A). As these genes are responsible for reducing endogenous oxidative stress (Brigelius-Flohé and Maiorino, 2013; Rhee, 2016), we evaluated oocyte ROS levels by measuring the fluorescence of 5(6)-carboxy-2′7′-dichlorofluorescein (carboxy-DCF), a fluorescent dye oxidized by ROS. Oocytes lacking Kat8 exhibited a greater than 2-fold increase in ROS compared with WT oocytes (Fig. 4B,C). These results demonstrate that Kat8Gdf9 cKO oocytes display decreased antioxidant gene expression and a corresponding increase in ROS levels.
Fig. 4.
Antioxidant gene expression decreases and ROS levels increase in Kat8Gdf9 cKO oocytes. (A) Relative mRNA levels of the antioxidant genes Prdx1, Prdx2, Gpx1 and Gpx4 in oocytes from 2-week-old WT and Kat8Gdf9 cKO females as detected by real-time PCR. The mRNA levels in WT oocytes were arbitrarily set to 1, and values were normalized to Actb. (B) Oocytes isolated from 2-week-old mice were treated with 5(6)-carboxy-2′7′-dichlorodihydrofluorescein diacetate (carboxy-H2DCFDA) for ROS detection. Images shown are representative of three independent experiments. Scale bar: 100 μm. (C) ROS levels in B were quantified by measuring carboxy-DCF fluorescence intensity. (A,C) Data are mean±s.e.m. from three independent experiments. *P<0.05, ***P<0.001, t-test.
N-acetylcysteine treatment rescues the Kat8 null phenotype
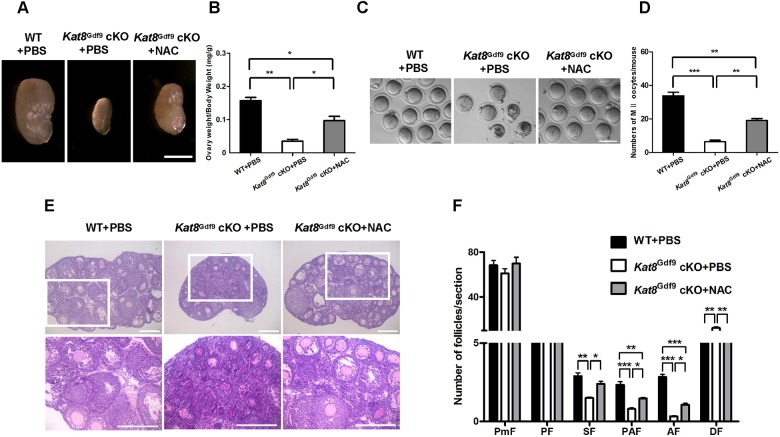
To further confirm that excessive ROS levels resulting from decreased expression of antioxidant genes caused the defective follicle development and increased oocyte apoptosis in Kat8Gdf9 cKO females, we performed a rescue experiment by supplying an exogenous antioxidant. N-acetylcysteine (NAC) is a precursor of intracellular cysteine and reduced glutathione and a proven potent antioxidant in various tissue types (Danilovic et al., 2011; Inci et al., 2007; Usta et al., 2008). Following intraperitoneal injection of 150 mg/kg NAC daily for 7 days, folliculogenesis in WT mice did not differ detectably from that of mice injected with PBS only (WT+PBS; data not shown). By contrast, Kat8Gdf9 cKO mice injected with NAC (Kat8Gdf9 cKO+NAC) had larger ovaries than PBS-injected Kat8Gdf9 cKO mice (Kat8Gdf9 cKO+PBS) (Fig. 5A,B). In addition, more MII oocytes were recovered from Kat8Gdf9 cKO+NAC mice than from Kat8Gdf9 cKO+PBS mice following gonadotropin injection (Fig. 5C,D). Histological analysis revealed significantly improved follicular development and survival in Kat8Gdf9 cKO mice following NAC injection. Compared with Kat8Gdf9 cKO+PBS ovaries, Kat8Gdf9 cKO+NAC ovaries exhibited a 60.0%, 83.8% and 224.2% increase in the number of secondary, preantral and antral follicles, respectively. Moreover, the number of atretic follicles in Kat8Gdf9 cKO+NAC groups decreased by 54.7% compared with that in Kat8Gdf9 cKO+PBS females (Fig. 5E,F).
Fig. 5.
NAC treatment partially rescues folliculogenesis in Kat8Gdf9 cKO females. (A) Ovaries from 3-week-old PBS- or NAC-treated mice. (B) Ratio of ovary weight to body weight in 3-week-old mice. (C) MII oocytes from 3-week-old mice. (D) Mean number of MII oocytes collected from each female following superovulation with PMSG and HCG. (E) Ovary sections from 3-week-old mice stained with Hematoxylin and PAS. Boxed regions are magnified beneath. (F) Quantitative analysis of follicles at different stages in ovary sections from PBS- or NAC-treated mice. (B,D,F) Data are mean±s.e.m. from three independent experiments. *P<0.05, **P<0.01, ***P<0.001, one-way ANOVA. Images (A,C,E) are representative of three independent experiments. Scale bars: 0.5 mm in A; 100 μm in C; 200 μm in E.
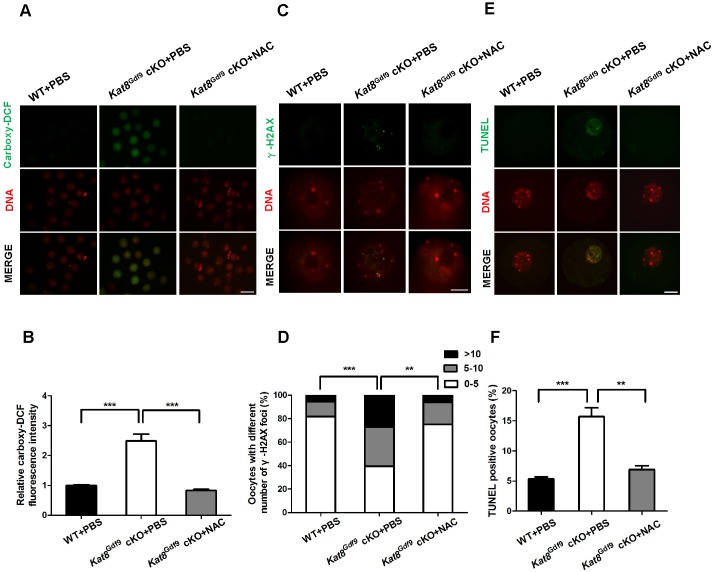
To confirm these rescue effects, we measured ROS levels, DSBs and apoptosis in Kat8Gdf9 cKO oocytes after NAC treatment. Fluorescence intensity analysis showed that ROS levels in Kat8Gdf9 cKO+NAC oocytes decreased by 67.1% compared with Kat8Gdf9 cKO+PBS oocytes (Fig. 6A,B). Furthermore, Kat8Gdf9 cKO+NAC oocytes exhibited significantly lower levels of DSBs and contained a significantly smaller proportion of TUNEL-positive oocytes, with 58.7% and 56.1% reductions, respectively, compared with Kat8Gdf9 cKO+PBS oocytes (Fig. 6C-F). These results further confirm that excessive ROS levels are the direct cause of the defective follicle development and increased oocyte apoptosis in Kat8Gdf9 cKO females.
Fig. 6.
Antioxidant treatment decreases ROS levels, DSBs and apoptosis in Kat8Gdf9 cKO oocytes. (A) Oocytes isolated from 3-week-old PBS- or NAC-injected mice were treated with carboxy-H2DCFDA for ROS detection. (B) ROS levels were quantified by measuring carboxy-H2DCFDA fluorescence intensity. (C) DSBs were detected by γ-H2AX staining in oocyte nuclei from 3-week-old PBS- or NAC-treated mice. (D) Proportions of oocytes containing various numbers of γ-H2AX foci. (E) Detection of apoptosis by TUNEL assay in oocytes from 3-week-old PBS- or NAC-treated mice by TUNEL assay. (F) Percentage of TUNEL-positive oocytes in 3-week-old PBS- or NAC-treated mice. Data (B,F) are mean±s.e.m. from three independent experiments. **P<0.01, ***P<0.001, one-way ANOVA (B,F) or Chi-square test (D). Images (A,C,E) are representative of three independent experiments. Scale bars: 100 μm in A; 10 μm in C; 20 μm in E.
KAT8 directly regulates antioxidant gene expression in oocytes
To determine whether endogenous KAT8 directly regulates antioxidant gene expression, we performed chromatin immunoprecipitation (ChIP) analysis on oocytes from 2-week-old mice. We detected a greater than 4-fold enrichment of KAT8 at the Prdx1, Gpx1 and Gpx4 promoters. Given that KAT8 is essential for H4K16ac, we additionally performed H4K16ac ChIP and detected a greater than 9-fold enrichment at the Gpx1 and Gpx4 promoters (Fig. 7), which implies that KAT8 can regulate the expression of these genes either through regulation of H4K16ac or by direct binding to the promoters. These data, together with the transcriptome analysis, indicate that KAT8 plays an essential and direct role in mediating antioxidant gene transcription during folliculogenesis.
Fig. 7.
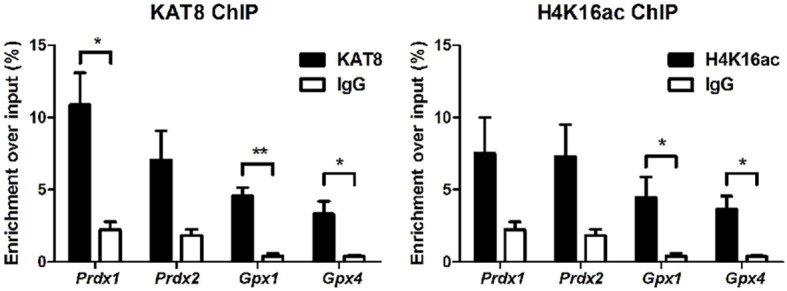
KAT8 and H4K16ac levels at antioxidant gene promoters in oocytes. ChIP was performed using KAT8 or H4K16ac antibodies in oocytes from 2-week-old WT mice. Promoter regions of antioxidant genes were analyzed by real-time PCR. Data are expressed as the enrichment over the input. IgG was used as a negative control. *P<0.05, **P<0.01, t-test. Data are mean±s.e.m. from three independent experiments.
Kat8 deletion results in abnormal heterochromatin configurations in oocytes
To examine the effect of Kat8 deletion on heterochromatin, we immunostained for heterochromatin-associated protein HP1β (also known as CBX1) and for H3K9 trimethylation (H3K9me3) in Kat8Gdf9 cKO oocytes. The majority (more than 80%) of heterochromatin regions in WT oocytes exhibited a normal oval or round shape, whereas more than 55% of heterochromatin regions in Kat8Gdf9 cKO oocytes were irregularly shaped (Fig. S6), indicating that Kat8 is essential for maintaining normal chromatin configurations in oocytes.
Granulosa cell-specific deletion of Kat8 does not affect follicle development or female fertility
To identify the potential function of Kat8 in granulosa cells, we generated mice in which Kat8 was specifically deleted in granulosa cells by crossing female Kat8flox/flox mice with Amhr2-Cre males. Amhr2-Cre; Kat8flox/flox (referred to as Kat8Amhr2 cKO) females exhibited negligible KAT8 protein levels in granulosa cells (Fig. S7A). However, Kat8Amhr2 cKO females exhibited normal fertility and follicle development, similar to WT mice (Fig. S7B-D). These findings indicate that Kat8 is dispensable in granulosa cells for female fertility.
DISCUSSION
Histone acetylation is important for the regulation of gene transcription and in many other cellular processes (Shahbazian and Grunstein, 2007). Here, we report that oocyte-specific deletion of Kat8, which encodes a HAT that controls the majority of H4K16 acetylation, results in a dramatic reduction in the number of secondary, preantral and antral follicles. Kat8-deficient oocytes have increased basal levels of DSBs and undergo apoptosis due to accumulation of ROS.
In oocytes, dramatic changes in gene expression occur during the transition from secondary follicle to small antral follicle (Pan et al., 2005), but the mechanisms regulating this transition are largely unknown. Here, we found that Kat8 deletion in oocytes leads to abnormal gene expression (Fig. S5, Tables S3 and S4) and significantly reduces the number of secondary, preantral and antral follicles (Fig. 3A,B). These results confirm that Kat8 is important for regulating gene expression in oocytes. Following Kat8 deletion in oocytes, follicle development was accompanied by morphological abnormalities and increased oocyte apoptosis (Fig. 3C,D). Many factors, including ROS, have been reported to induce oocyte apoptosis (Tiwari et al., 2015). Indeed, increased ROS levels were observed in Kat8Gdf9 cKO oocytes (Fig. 4B,C). Moreover, the ratio of apoptotic oocytes and the number of degenerating follicles per section were restored to near normal levels after NAC treatment for 7 days (Fig. 5F, Fig. 6E,F). These results not only confirm accumulation of ROS as the main cause for follicle degeneration in Kat8Gdf9 cKO mice, but also indicate that KAT8 protects the normal development of oocytes and follicles by indirectly controlling ROS levels.
Increased ROS levels can cause abnormal cellular redox circuits and damage cellular macromolecules, such as DNA (Kim et al., 2012; Roberts et al., 2009). We detected increased levels of DSBs in Kat8-deficient oocytes. Our observation that treatment of Kat8Gdf9 cKO oocytes with NAC, an antioxidant drug that can reduce ROS levels, significantly decreased DSB accumulation (Fig. 6A-D) indicates that ROS accumulation is the main cause of DNA damage in Kat8Gdf9 cKO oocytes. Although reports have shown that KAT8 can directly contribute to DSB repair (Gupta et al., 2014; Sharma et al., 2010), data from our rescue experiment suggest that, in oocytes, KAT8 primarily prevents DSB generation by limiting ROS levels.
In most cells, including oocytes, ROS are removed by a series of antioxidant enzymes (Devine et al., 2012). RNA-seq and real-time PCR showed that in Kat8Gdf9 cKO oocytes the antioxidant genes Prdx1, Prdx2, Gpx1 and Gpx4, which encode components of ROS-scavenging systems (Brigelius-Flohé and Maiorino, 2013; Rhee, 2016), were significantly downregulated (Table S4, Fig. 4A). ChIP assays further established that KAT8 directly binds the promoters of Prdx1, Gpx1 and Gpx4. Unexpectedly, although H4K16ac, the histone target of KAT8, is important for gene expression (Dion et al., 2005), ChIP results identified H4K16ac only at the Gpx1 and Gpx4 promoters. This indicates that KAT8 can directly regulate gene expression independently of H4K16 acetylation in mouse oocytes, which was also suggested by outcomes of a recent study in mouse ESCs (Taylor et al., 2013).
It should be noted that Kat8Gdf9 cKO mice are sterile, whereas mice lacking Gpx4 display embryonic lethality (Yant et al., 2003) and mice deficient for Prdx1, Prdx2 or Gpx1 are viable and fertile (Ho et al., 1997; Lee et al., 2003; Neumann et al., 2003). An explanation for this discrepancy is that the expression and/or functional loss may be compensated for by other Gpx and Prdx family members. For example, in the spleen of Prdx1 knockout mice, Gpx1 expression increases more than 2-fold (Rani et al., 2012). Silencing of Prdx2 alone in hepatocellular carcinoma cells has no effect on cell survival, whereas simultaneous silencing of Prdx1 and Prdx2 promotes cell death (Hou et al., 2014). These studies indicate synergetic effects on cells of Gpx and Prdx factors, and suggest that the simultaneous reduction of Prdx1, Prdx2, Gpx1 and Gpx4 could be the reason for excessive apoptosis in Kat8Gdf9 cKO oocytes.
Accumulation of ROS is a major cause of the defective oogenesis and follicle development in Kat8Gdf9 cKO mice. However, treatment of Kat8Gdf9 cKO mice with NAC did not entirely restore ovary size and oocyte and follicle numbers (Fig. 5). One explanation might be that oocyte apoptosis and follicle degeneration occurred before NAC administration/function. Other possibilities remain. For example, besides antioxidant genes, several oocyte-specific genes such as Oosp1 and Omt2b (West et al., 1996; Yan et al., 2001) were also downregulated in Kat8Gdf9 cKO oocytes (Fig. S5A, Table S4). Whether the misregulation of these genes contributes to the disrupted follicle development needs to be tested.
Our results show that H4K16ac levels increase during mouse oocyte development from 5 days to the GV stage, when transcription is becoming active (Lucifero et al., 2007), and then significantly decreases upon GVBD (Fig. S1), when the oocytes become transcriptionally silent and chromatin condensation starts (De La Fuente and Eppig, 2001; Kageyama et al., 2007). Mouse oocytes lacking H4K16ac due to Kat8 deletion displayed abnormal gene expression (Fig. S5, Tables S3 and S4) and increased apoptosis before reaching the full-grown GV stage (Fig. 3C,D). Interestingly, increasing H4K16ac by repressing Hdac2 or Sirt6 expression results in a decreased number of antral follicles (Ma and Schultz, 2013), abnormal spindle assembly, and defective chromosome segregation in MII cells (Han et al., 2015; Ma and Schultz, 2013). These findings show that the temporal regulation of H4K16ac levels is crucial for oogenesis, with high levels required for gene expression and normal oocyte growth, and low levels essential for progression through meiosis II.
Although oocyte-granulosa cell communication is important for follicle development by regulating granulosa cell proliferation (Su et al., 2009), Gdf9 and Bmp15, which encode two important oocyte-derived factors responsible for granulosa cell proliferation (McNatty et al., 2005), were expressed at comparable levels in WT and Kat8Gdf9 cKO oocytes (Fig. S4C). Consistent with this, BrdU incorporation and PCNA immunostaining showed that the proliferation of granulosa cells in Kat8Gdf9 cKO ovaries remained normal (Fig. S4A,B). These results suggest that the oocyte-granulosa cell communication responsible for granulosa cell proliferation is not affected by Kat8 deletion in oocytes.
In conclusion, our data show that the H4K16-specific HAT KAT8 promotes the expression of antioxidant genes in developing oocytes directly or by H4K16ac. These antioxidant proteins reduce ROS levels to prevent the generation of DSBs and to guarantee normal oocyte and follicle development. Our study demonstrates, for the first time, that KAT8 is essential for female fertility, and suggests that histone modifications, at least H4K16ac, should be considered in genetic counseling and when exploring the molecular basis of human ovarian folliculogenesis failure.
MATERIALS AND METHODS
Mice
Kat8flox/flox mice, which have loxP sites flanking exons 1 to 3 of Kat8, were described previously (Gupta et al., 2008; Kumar et al., 2011), Gdf9-Cre transgenic mice were purchased from The Jackson Laboratory and Amhr2-Cre knock-in mice were kindly donated by Dr. R. Behringer (Jamin et al., 2002). All the mice were maintained on a mixed C57BL/6 and SV129 genetic background. For fertility tests, 8- to 10-week-old WT, Kat8Gdf9 cKO and Kat8Amhr2 cKO females were separately housed with WT males for 6 months. The number of offspring from each pregnant female was recorded after birth. All animal experiments were performed according to the guidelines and procedures approved by the Institutional Animal Care Committee of the University of Science and Technology of China.
Oocyte and granulosa cell collection
Female mice (7-8 weeks old) were intraperitoneally injected with 5 IU PMSG (Ningbo Sansheng Pharmaceutical Corporation, Zhejiang, China). Forty-eight hours post injection, ovaries were collected and placed in M2 medium (Sigma, M7167) supplemented with 200 μM IBMX (Sigma, I7108) at 37°C to prevent GVBD. Full-grown GV oocytes and granulosa cells were released by puncturing antral follicles with a fine needle under the visual field of a dissecting microscope. GVBD-MI oocytes (absence of both GV and polar body) (Kim et al., 2015) were obtained after moving GV oocytes to IBMX-free M16 medium (Sigma, M7292) and culturing for 2-4 h (Ma and Viveiros, 2014). For MII oocyte collection, mice were injected with PMSG as above followed by intraperitoneal injection with 5 IU human chorionic gonadotropin (HCG; Ningbo Sansheng Pharmaceutical Corporation) 48 h later. MII oocytes were collected from the oviducts 16 h post HCG injection. Meiotically incompetent oocytes (20-30 μm diameter from 5-day mice, and 50-70 μm diameter from 2- and 3-week-old mice) were obtained as described previously (Svoboda et al., 2001).
RNA extraction, cDNA synthesis, RT-PCR and real-time PCR
Total RNA was extracted from oocytes of 2-week-old mice using the RNAprep Pure Micro Kit (DP420, Tiangen, Beijing, China), and cDNA synthesis was performed using the PrimeScript Reagent Kit (Takara, RR047A) according to the manufacturer's instructions. For RT-PCR, PCR reactions (30 μl) included: 3 μl 10×Easy Taq DNA Polymerase Buffer (AP111, Trans, Beijing, China), 2 μl 2.5 mM dNTPs (Trans, AD101), 1 μl 10 μM forward and reverse PCR primers, 2 μl cDNA and 2.5 U Easy Taq DNA polymerase (Trans, AP111). The PCR program consisted of 32 cycles of denaturing at 95°C for 20 s, annealing at 59°C for 30 s and extension at 72°C for 30 s. Real-time PCR was performed as previously described (Xu et al., 2011) and data were normalized against Actb. Quantification of the fold change in gene expression was determined by the comparative CT method. PCR primers are listed in Table S1.
Immunofluorescence
Oocytes were fixed in 4% paraformaldehyde (PFA) for 20 min at room temperature, followed by permeabilization in PBS containing 0.2% Triton X-100 for 15 min. After blocking with PBS containing 5% normal donkey serum (Jackson ImmunoResearch, 017-000-121) for 30 min, oocytes were incubated with primary antibodies at 37°C for 2 h, followed by incubation with secondary antibodies for 1 h at room temperature. After staining with Hoechst 33342 (Invitrogen, H21492), oocytes were transferred to slides and mounted using Vectashield (Vector Laboratories, H-1000). Fluorescence was detected on an Eclipse 80i microscope (Nikon) equipped with a digital camera (Hamamatsu, C4742-80). Antibodies are listed in Table S2.
Western blot analysis
Oocyte and granulosa cell lysates were prepared in lysis buffer (100 mM Tris-HCl pH 7.4, 3% SDS, 10 mM DTT, 17.3% glycerin, 0.15% Bromophenol Blue) supplemented with cOmplete Protease Inhibitor Cocktail (Roche, 04693116001). Western blot was performed as described previously (Wang et al., 2014) using the antibodies listed in Table S2.
Histological analysis
Ovaries were fixed in 4% PFA for 12-24 h before paraffin embedding, and 5 μm paraffin sections were attached to microscope slides for PAS-Hematoxylin or Hematoxylin staining. Samples were analyzed using an Eclipse 80i microscope equipped with a DS-Ri1 digital camera (Nikon).
TUNEL assay
TUNEL assay was performed in oocytes and ovary paraffin sections according to the instructions provided with the In Situ Cell Death Detection Kit (Roche, 11684795910). Fluorescence was detected using the Nikon Eclipse 80i microscope and Hamamatsu digital camera (C4742-80).
BrdU incorporation
Two-week-old mice were intraperitoneally injected with BrdU (100 mg/kg; Sigma, B9285) and were sacrificed 2 h post injection. Ovary sections were immunostained using an anti-BrdU antibody and analyzed using the Eclipse 80i microscope and DS-Ri1 camera. Antibodies used are listed in Table S2.
PCNA immunostaining
Ovary sections from 2-week-old mice were immunostained using anti-PCNA antibody (Table S2) and analyzed using the Eclipse 80i microscope and DS-Ri1 camera.
RNA-seq analysis
RNA-seq was conducted by Shanghai Biotechnology Corporation. Briefly, each sample, which contained ∼300 oocytes collected from eight 2-week-old WT and Kat8Gdf9 cKO mice, was lysed directly in 3.5 μl RNase-free water supplemented with 0.2% Triton X-100 and 7 U RiboLock RNase Inhibitor (Thermo, EO0381) and immediately stored at −80°C until use. RNA was amplified using the SMARTer Ultra Low Input RNA Kit (Clontech, 634823), the resulting cDNA fragmented using a Covaris S2 ultrasonicator, and paired-end libraries constructed using the Ovation Ultralow DR Multiplex System 1-96 (NuGEN Technologies, 0329-96). The libraries were sequenced on an Illumina HiSeq 2500 instrument (SY-401-2501), generating 54-63 million pairs of reads per sample. Each pair of reads represents a cDNA fragment from the library. The reads were mapped to the mouse reference genome (mm10) using TopHat (version 2.0.9) (Trapnell et al., 2009). Differential gene expression between WT and Kat8Gdf9 cKO oocytes was determined using Cufflinks (version 2.1.1) (Zhang et al., 2015). The threshold for significance was a false discovery rate ≤0.05 and a fold expression change ≥2. GO analysis was performed using Ingenuity Pathway Analysis (IPA) software. Processed RNA-seq data are presented in Tables S3 and S4 and the full data set has been deposited in the GEO database (accession GSE97026).
Oxidative stress assessment
ROS levels were assessed using carboxy-H2DCFDA (s0033, Beyotime, Jiangsu, China). Briefly, oocytes from 2- or 3-week-old mice were incubated in M2 medium containing 10 μM carboxy-H2DCFDA at 37°C for 20 min, followed by staining with Hoechst 33342. Fluorescence was detected on a Nikon TE2000E microscope equipped with a Nikon DS-Qi1Mc digital camera. Fluorescence intensity was quantified using ImageJ software (National Institutes of Health).
ChIP and real-time PCR
ChIP was performed using the Pierce Agarose ChIP Kit (Thermo, 26156) according to the manufacturer's instructions. In brief, ∼3000 oocytes from 2-week-old mice were cross-linked in 4% formaldehyde at room temperature for 15 min. Fixation was stopped by addition of 10×Glycine Solution. Oocytes were then incubated at room temperature for 5 min, washed in ice-cold PBS containing 1% Halt Cocktail, and immediately stored at −80°C before lysis. Following enzyme-mediated nuclear lysis, samples were sonicated for 1 min to completely fragment the chromatin. Promoter region primers spanning −700 to +300 bp relative to the gene transcription start sites were used, and gene enrichment was quantified by real-time PCR. PCR primers and antibodies used are listed in Tables S1 and S2.
N-acetylcysteine treatment
Two-week-old mice were injected intraperitoneally with NAC (150 mg/kg; Beyotime, s0077) or PBS once per day for 7 days. The NAC dose of 150 mg/kg was based on the studies by Mahmoodi et al. (2015). Ovaries were then collected for histological analysis and oocytes isolated for ROS detection, γ-H2AX staining and TUNEL assay.
Statistical analysis
Statistical comparison was performed using Student's unpaired two-tailed t-test, one-way ANOVA (Tukey's multiple comparison test) or Chi-square test. All experiments were performed at least three times and results are presented as mean±s.e.m. Statistical significance was set at P<0.05.
Acknowledgements
We are very thankful to Dr R. Behringer for donation of the Amhr2-Cre mice and S. Eckardt for help with manuscript modification.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: S.Y., Q.S.; Methodology: X.J., S.Y., Q.G., Y.Z.; Software: Y.Z.; Validation: S.Y., S.F.; Investigation: S.Y., Q.G.; Resources: H.J., T.K.P., H.-Y.F.; Writing - original draft: X.J., S.Y.; Writing - review & editing: X.J., F.W., T.K., N.J., M.K., A.A., P.X., T.K.P., Q.S.; Supervision: Q.S.; Funding acquisition: X.J., Q.S. F.W., T.K.P.
Funding
This work was supported by the National Health and Family Planning Commission of the People's Republic of China (2016YFC1000600), Ministry of Science and Technology of the People's Republic of China (2013CB945502), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB19000000), the National Natural Science Foundation of China (31501199, 31671557, 31501203 and 31371519), the National Natural Science Foundation of China - Israel Science Foundation (31461143013-1183/14), Major Program of Development Foundation of Hefei Institutes of Physical Science, Chinese Academy of Sciences (2014FXZY003), the Fundamental Research Funds for the Central Universities (WK2340000069), and the National Institutes of Health (RO1 CA129537, RO1 GM109768). Deposited in PMC for release after 12 months.
Data availability
RNA-seq data have been deposited at Gene Expression Omnibus under accession number GSE97026.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.149518.supplemental
References
- Akhtar A. and Becker P. B. (2000). Activation of transcription through histone H4 acetylation by MOF, an acetyltransferase essential for dosage compensation in Drosophila. Mol. Cell 5, 367-375. 10.1016/S1097-2765(00)80431-1 [DOI] [PubMed] [Google Scholar]
- Brigelius-Flohé R. and Maiorino M. (2013). Glutathione peroxidases. Biochim. Biophys. Acta 1830, 3289-3303. 10.1016/j.bbagen.2012.11.020 [DOI] [PubMed] [Google Scholar]
- Danilovic A., Lucon A. M., Srougi M., Shimizu M. H. M., Ianhez L. E., Nahas W. C. and Seguro A. C. (2011). Protective effect of N-acetylcysteine on early outcomes of deceased renal transplantation. Transplant. Proc. 43, 1443-1449. 10.1016/j.transproceed.2011.02.020 [DOI] [PubMed] [Google Scholar]
- De La Fuente R. and Eppig J. J. (2001). Transcriptional activity of the mouse oocyte genome: companion granulosa cells modulate transcription and chromatin remodeling. Dev. Biol. 229, 224-236. 10.1006/dbio.2000.9947 [DOI] [PubMed] [Google Scholar]
- Devine P. J., Perreault S. D. and Luderer U. (2012). Roles of reactive oxygen species and antioxidants in ovarian toxicity. Biol. Reprod. 86, 27 10.1095/biolreprod.111.095224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dion M. F., Altschuler S. J., Wu L. F. and Rando O. J. (2005). Genomic characterization reveals a simple histone H4 acetylation code. Proc. Natl. Acad. Sci. USA 102, 5501-5506. 10.1073/pnas.0500136102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu L., Wang Q. and Sun Q.-Y. (2010). Histone modifications during mammalian oocyte maturation: dynamics, regulation and functions. Cell Cycle 9, 1942-1950. 10.4161/cc.9.10.11599 [DOI] [PubMed] [Google Scholar]
- Gupta A., Guerin-Peyrou T. G., Sharma G. G., Park C., Agarwal M., Ganju R. K., Pandita S., Choi K., Sukumar S., Pandita R. K. et al. (2008). The mammalian ortholog of Drosophila MOF that acetylates histone H4 lysine 16 is essential for embryogenesis and oncogenesis. Mol. Cell. Biol. 28, 397-409. 10.1128/MCB.01045-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A., Hunt C. R., Pandita R. K., Pae J., Komal K., Singh M., Shay J. W., Kumar R., Ariizumi K., Horikoshi N. et al. (2013). T-cell-specific deletion of Mof blocks their differentiation and results in genomic instability in mice. Mutagenesis 28, 263-270. 10.1093/mutage/ges080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A., Hunt C. R., Hegde M. L., Chakraborty S., Chakraborty S., Udayakumar D., Horikoshi N., Singh M., Ramnarain D. B., Hittelman W. N. et al. (2014). MOF phosphorylation by ATM regulates 53BP1-mediated double-strand break repair pathway choice. Cell Rep. 8, 177-189. 10.1016/j.celrep.2014.05.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L., Ge J., Zhang L., Ma R., Hou X., Li B., Moley K. and Wang Q. (2015). Sirt6 depletion causes spindle defects and chromosome misalignment during meiosis of mouse oocyte. Sci. Rep. 5, 15366 10.1038/srep15366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho Y.-S., Magnenat J.-L., Bronson R. T., Cao J., Gargano M., Sugawara M. and Funk C. D. (1997). Mice deficient in cellular glutathione peroxidase develop normally and show no increased sensitivity to hyperoxia. J. Biol. Chem. 272, 16644-16651. 10.1074/jbc.272.26.16644 [DOI] [PubMed] [Google Scholar]
- Hou J.-K., Huang Y., He W., Yan Z.-W., Fan L., Liu M.-H., Xiao W.-L., Sun H.-D. and Chen G.-Q. (2014). Adenanthin targets peroxiredoxin I/II to kill hepatocellular carcinoma cells. Cell Death Dis. 5, e1400 10.1038/cddis.2014.345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inci I., Zhai W., Arni S., Hillinger S., Vogt P. and Weder W. (2007). N-acetylcysteine attenuates lung ischemia-reperfusion injury after lung transplantation. Ann. Thorac. Surg. 84, 240-246. 10.1016/j.athoracsur.2007.03.082 [DOI] [PubMed] [Google Scholar]
- Jagarlamudi K. and Rajkovic A. (2012). Oogenesis: transcriptional regulators and mouse models. Mol. Cell. Endocrinol. 356, 31-39. 10.1016/j.mce.2011.07.049 [DOI] [PubMed] [Google Scholar]
- Jamin S. P., Arango N. A., Mishina Y., Hanks M. C. and Behringer R. R. (2002). Requirement of Bmpr1a for Müllerian duct regression during male sexual development. Nat. Genet. 32, 408-410. 10.1038/ng1003 [DOI] [PubMed] [Google Scholar]
- Kageyama S.-I., Liu H., Kaneko N., Ooga M., Nagata M. and Aoki F. (2007). Alterations in epigenetic modifications during oocyte growth in mice. Reproduction 133, 85-94. 10.1530/REP-06-0025 [DOI] [PubMed] [Google Scholar]
- Kim S. B., Pandita R. K., Eskiocak U., Ly P., Kaisani A., Kumar R., Cornelius C., Wright W. E., Pandita T. K. and Shay J. W. (2012). Targeting of Nrf2 induces DNA damage signaling and protects colonic epithelial cells from ionizing radiation. Proc. Natl. Acad. Sci. USA 109, E2949-E2955. 10.1073/pnas.1207718109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Singh A. K., Takata Y., Lin K., Shen J., Lu Y., Kerenyi M. A., Orkin S. H. and Chen T. (2015). LSD1 is essential for oocyte meiotic progression by regulating CDC25B expression in mice. Nat. Commun. 6, 10116 10.1038/ncomms10116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R., Hunt C. R., Gupta A., Nannepaga S., Pandita R. K., Shay J. W., Bachoo R., Ludwig T., Burns D. K. and Pandita T. K. (2011). Purkinje cell-specific males absent on the first (mMof) gene deletion results in an ataxia-telangiectasia-like neurological phenotype and backward walking in mice. Proc. Natl. Acad. Sci. USA 108, 3636-3641. 10.1073/pnas.1016524108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan Z.-J., Xu X. and Cooney A. J. (2004). Differential oocyte-specific expression of Cre recombinase activity in GDF-9-iCre, Zp3cre, and Msx2Cre transgenic mice. Biol. Reprod. 71, 1469-1474. 10.1095/biolreprod.104.031757 [DOI] [PubMed] [Google Scholar]
- Lee T.-H., Kim S. U., Yu S. L., Kim S. H., Park D. S., Moon H. B., Dho S. H., Kwon K. S., Kwon H. J., Han Y. H. et al. (2003). Peroxiredoxin II is essential for sustaining life span of erythrocytes in mice. Blood 101, 5033-5038. 10.1182/blood-2002-08-2548 [DOI] [PubMed] [Google Scholar]
- Li X., Li L., Pandey R., Byun J. S., Gardner K., Qin Z. and Dou Y. (2012). The histone acetyltransferase MOF is a key regulator of the embryonic stem cell core transcriptional network. Cell Stem Cell 11, 163-178. 10.1016/j.stem.2012.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucifero D., La Salle S., Bourc'his D., Martel J., Bestor T. H. and Trasler J. M. (2007). Coordinate regulation of DNA methyltransferase expression during oogenesis. BMC Dev. Biol. 7, 36 10.1186/1471-213X-7-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma P. and Schultz R. M. (2013). Histone deacetylase 2 (HDAC2) regulates chromosome segregation and kinetochore function via H4K16 deacetylation during oocyte maturation in mouse. PLoS Genet. 9, e1003377 10.1371/journal.pgen.1003377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W. and Viveiros M. M. (2014). Depletion of pericentrin in mouse oocytes disrupts microtubule organizing center function and meiotic spindle organization. Mol. Reprod. Dev. 81, 1019-1029. 10.1002/mrd.22422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma P., Pan H., Montgomery R. L., Olson E. N. and Schultz R. M. (2012). Compensatory functions of histone deacetylase 1 (HDAC1) and HDAC2 regulate transcription and apoptosis during mouse oocyte development. Proc. Natl. Acad. Sci. USA 109, E481-E489. 10.1073/pnas.1118403109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J.-Y., Ou-Yang Y.-C., Wang Z.-W., Wang Z., Jiang Z.-Z., Luo S.-M., Hou Y., Liu Z., Schatten H. and Sun Q.-Y. (2013). The effects of DNA double-strand breaks on mouse oocyte meiotic maturation. Cell Cycle 12, 1233-1241. 10.4161/cc.24311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoodi M., Soleimani Mehranjani M., Shariatzadeh S. M. A., Eimani H. and Shahverdi A. (2015). N-acetylcysteine improves function and follicular survival in mice ovarian grafts through inhibition of oxidative stress. Reprod. Biomed. Online 30, 101-110. 10.1016/j.rbmo.2014.09.013 [DOI] [PubMed] [Google Scholar]
- McNatty K. P., Juengel J. L., Reader K. L., Lun S., Myllymaa S., Lawrence S. B., Western A., Meerasahib M. F., Mottershead D. G., Groome N. P. et al. (2005). Bone morphogenetic protein 15 and growth differentiation factor 9 co-operate to regulate granulosa cell function in ruminants. Reproduction 129, 481-487. 10.1530/rep.1.00517 [DOI] [PubMed] [Google Scholar]
- Monti M. and Redi C. A. (2009). Oogenesis specific genes (Nobox, Oct4, Bmp15, Gdf9, Oogenesin1 and Oogenesin2) are differentially expressed during natural and gonadotropin-induced mouse follicular development. Mol. Reprod. Dev. 76, 994-1003. 10.1002/mrd.21059 [DOI] [PubMed] [Google Scholar]
- Neumann C. A., Krause D. S., Carman C. V., Das S., Dubey D. P., Abraham J. L., Bronson R. T., Fujiwara Y., Orkin S. H. and Van Etten R. A. (2003). Essential role for the peroxiredoxin Prdx1 in erythrocyte antioxidant defence and tumour suppression. Nature 424, 561-565. 10.1038/nature01819 [DOI] [PubMed] [Google Scholar]
- Pan H., O'Brien M. J., Wigglesworth K., Eppig J. J. and Schultz R. M. (2005). Transcript profiling during mouse oocyte development and the effect of gonadotropin priming and development in vitro. Dev. Biol. 286, 493-506. 10.1016/j.ydbio.2005.08.023 [DOI] [PubMed] [Google Scholar]
- Pan Z., Zhang J., Li Q., Li Y., Shi F., Xie Z. and Liu H. (2012). Current advances in epigenetic modification and alteration during mammalian ovarian folliculogenesis. J. Genet. Genomics 39, 111-123. 10.1016/j.jgg.2012.02.004 [DOI] [PubMed] [Google Scholar]
- Rani V., Neumann C. A., Shao C. and Tischfield J. A. (2012). Prdx1 deficiency in mice promotes tissue specific loss of heterozygosity mediated by deficiency in DNA repair and increased oxidative stress. Mutat. Res. 735, 39-45. 10.1016/j.mrfmmm.2012.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S. G. (2016). Overview on Peroxiredoxin. Mol. Cells 39, 1-5. 10.14348/molcells.2016.2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R. A., Laskin D. L., Smith C. V., Robertson F. M., Allen E. M. G., Doorn J. A. and Slikker W. (2009). Nitrative and oxidative stress in toxicology and disease. Toxicol. Sci. 112, 4-16. 10.1093/toxsci/kfp179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues P., Limback D., McGinnis L. K., Plancha C. E. and Albertini D. F. (2008). Oogenesis: prospects and challenges for the future. J. Cell. Physiol. 216, 355-365. 10.1002/jcp.21473 [DOI] [PubMed] [Google Scholar]
- Shahbazian M. D. and Grunstein M. (2007). Functions of site-specific histone acetylation and deacetylation. Annu. Rev. Biochem. 76, 75-100. 10.1146/annurev.biochem.76.052705.162114 [DOI] [PubMed] [Google Scholar]
- Sharma G. G., So S., Gupta A., Kumar R., Cayrou C., Avvakumov N., Bhadra U., Pandita R. K., Porteus M. H., Chen D. J. et al. (2010). MOF and histone H4 acetylation at lysine 16 are critical for DNA damage response and double-strand break repair. Mol. Cell. Biol. 30, 3582-3595. 10.1128/MCB.01476-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y.-Q., Sugiura K. and Eppig J. J. (2009). Mouse oocyte control of granulosa cell development and function: paracrine regulation of cumulus cell metabolism. Semin. Reprod. Med. 27, 32-42. 10.1055/s-0028-1108008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J., Wang F., Cai Y. and Jin J. (2016). The functional analysis of histone acetyltransferase MOF in tumorigenesis. Int. J. Mol. Sci. 17, 99 10.3390/ijms17010099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda P., Stein P. and Schultz R. M. (2001). RNAi in mouse oocytes and preimplantation embryos: effectiveness of hairpin dsRNA. Biochem. Biophys. Res. Commun. 287, 1099-1104. 10.1006/bbrc.2001.5707 [DOI] [PubMed] [Google Scholar]
- Taylor G. C. A., Eskeland R., Hekimoglu-Balkan B., Pradeepa M. M. and Bickmore W. A. (2013). H4K16 acetylation marks active genes and enhancers of embryonic stem cells, but does not alter chromatin compaction. Genome Res. 23, 2053-2065. 10.1101/gr.155028.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T., Loveland K. L. and Voss A. K. (2007). The genes coding for the MYST family histone acetyltransferases, Tip60 and Mof, are expressed at high levels during sperm development. Gene Expr. Patterns 7, 657-665. 10.1016/j.modgep.2007.03.005 [DOI] [PubMed] [Google Scholar]
- Tiwari M., Prasad S., Tripathi A., Pandey A. N., Ali I., Singh A. K., Shrivastav T. G. and Chaube S. K. (2015). Apoptosis in mammalian oocytes: a review. Apoptosis 20, 1019-1025. 10.1007/s10495-015-1136-y [DOI] [PubMed] [Google Scholar]
- Trapnell C., Pachter L. and Salzberg S. L. (2009). TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25, 1105-1111. 10.1093/bioinformatics/btp120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usta U., Inan M., Erbas H., Aydogdu N., Oz Puyan F. and Altaner S. (2008). Tissue damage in rat ovaries subjected to torsion and detorsion: effects of L-carnitine and N-acetyl cysteine. Pediatr. Surg. Int. 24, 567-573. 10.1007/s00383-008-2123-y [DOI] [PubMed] [Google Scholar]
- van den Hurk R. and Zhao J. (2005). Formation of mammalian oocytes and their growth, differentiation and maturation within ovarian follicles. Theriogenology 63, 1717-1751. 10.1016/j.theriogenology.2004.08.005 [DOI] [PubMed] [Google Scholar]
- Wang Z., Yin H., Zhang Y., Feng Y., Yan Z., Jiang X., Bukhari I., Iqbal F., Cooke H. J. and Shi Q. (2014). miR-214-mediated downregulation of RNF8 induces chromosomal instability in ovarian cancer cells. Cell Cycle 13, 3519-3528. 10.4161/15384101.2014.958413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West M. F., Verrotti A. C., Sallés F. J., Tsirka S. E. and Strickland S. (1996). Isolation and characterization of two novel, cytoplasmically polyadenylated, oocyte-specific, mouse maternal RNAs. Dev. Biol. 175, 132-141. 10.1006/dbio.1996.0101 [DOI] [PubMed] [Google Scholar]
- Xu B., Hua J., Zhang Y., Jiang X., Zhang H., Ma T., Zheng W., Sun R., Shen W., Sha J. et al. (2011). Proliferating cell nuclear antigen (PCNA) regulates primordial follicle assembly by promoting apoptosis of oocytes in fetal and neonatal mouse ovaries. PLoS ONE 6, e16046 10.1371/journal.pone.0016046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C., Pendola F. L., Jacob R., Lau A. L., Eppig J. J. and Matzuk M. M. (2001). Oosp1 encodes a novel mouse oocyte-secreted protein. Genesis 31, 105-110. 10.1002/gene.10010 [DOI] [PubMed] [Google Scholar]
- Yant L. J., Ran Q., Rao L., Van Remmen H., Shibatani T., Belter J. G., Motta L., Richardson A. and Prolla T. A. (2003). The selenoprotein GPX4 is essential for mouse development and protects from radiation and oxidative damage insults. Free Radic. Biol. Med. 34, 496-502. 10.1016/S0891-5849(02)01360-6 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Li W., Dou Y., Zhang J., Jiang G., Miao L., Han G., Liu Y., Li H. and Zhang Z. (2015). Transcript quantification by RNA-Seq reveals differentially expressed genes in the red and yellow fruits of Fragaria vesca. PLoS ONE 10, e0144356 10.1371/journal.pone.0144356 [DOI] [PMC free article] [PubMed] [Google Scholar]