Abstract
Meaningful behavior requires successful differentiation of events surfacing from one's mind from those arising from the external world. Such judgements may be especially demanding during pain because of the strong contribution from psychological factors to this experience. It is unknown how the subjective reality of pain (SRP) is constructed in the human brain, and neuronal mechanisms of the subjective reality are poorly understood in general. To address these questions, 14 suggestion-prone healthy subjects rated reality of pain that was induced either by laser pulses to the skin or by hypnotic suggestion during functional MRI. Both pain states were associated with activation of the brain's pain circuitry. During laser stimulation, the sensory parts of this circuitry were activated more strongly, and their activation strengths correlated positively with the SRP. During suggestion-induced pain, the reality estimates were lower and correlated positively with activation strengths in the rostral and perigenual anterior cingulate cortex and in the pericingulate regions of the medial prefrontal cortex; a similar trend was evident during laser-induced pain. These findings support the view that information about sensory-discriminative characteristics of pain contributes to the SRP. Differences in such information between physically and psychologically induced pain, however, could be quantitative rather than qualitative and therefore insufficient for judging the reality of pain without knowledge about the source of this information. The medial prefrontal cortex is a likely area to contribute to such source monitoring.
Keywords: functional MRI, suggestion, hypnosis, human, cortex
Events surfacing from one's mind are usually differentiated from those arising from the external world by the subjective experience of reality. Ability to differentiate real and imaginal percepts, however, can be distorted in patients suffering from various organic and functional brain disorders and occasionally even in healthy subjects (1–3).
Brain correlates of pain are affected by various psychological factors (4–7). According to recent brain imaging studies, pain-related brain areas can be activated without any physical stimulus, solely by cognitive cues (7–9). It remains unknown, however, how real the subject's experiences of such a psychologically induced pain are, and how the subjective reality of pain (SRP) is constructed in the human brain. Because the clarity of a percept likely affects the experience of its reality, and because both real and hallucinatory percepts may be associated with activation of sensory brain areas (10, 11), we hypothesized that a neuronal system subserving the SRP should involve components that process pain-related sensory-discriminative information, i.e., the primary and secondary somatosensory cortices and the posterior insula (12, 13). We also expected this system to include brain regions such as the perigenual anterior cingulate cortex (pACC) (14) that are able to support source monitoring, because a failure to differentiate between internal and external sources has been argued to be essential for hallucinations (1, 2).
To study brain activations related to physically and psychologically induced pain, we induced pain to the left hand of healthy volunteers either by laser pulses or by hypnotic suggestion. Brain circuitries related to SRP were addressed by correlating strengths of brain signals, measured with functional MRI (fMRI), with the estimates of SRP given on a visual analog scale ranging from imaginal to real physical pain.
Materials and Methods
Subjects. Fourteen healthy subjects (ages 20–36 years, mean 26 years; 11 females, 3 males; 13 right-handed, one ambidextrous) were prescreened from among 103 healthy adults. Subjects who scored ≥8 on the Stanford Hypnotic Susceptibility Scale Form C (15) and responded to pain suggestion were selected for the fMRI experiments. The study had the acceptance of the local ethics committee, and each of the 14 subjects gave a written informed consent before participation.
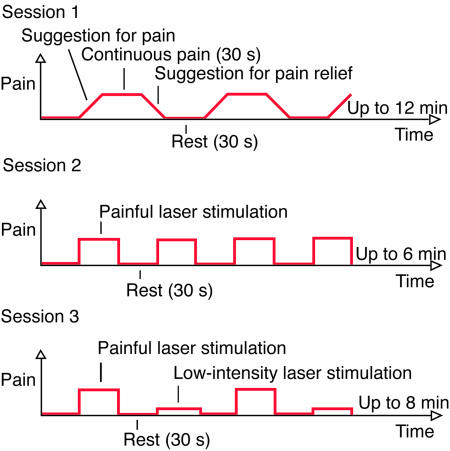
Experimental Setup. The subject's head was stabilized in the head coil by a vacuum pillow and a frontal bandage. The voice of the experimenter (S.N.) was conducted to the scanning room through headphones. Before the first imaging session, hypnosis was inducted by instructing sequential relaxation of body parts and by suggesting deepening relaxation during a number count from 1 to 10. The subjects were asked to report, by a small movement of the right foot, when the maximum tolerable pain was achieved, and when the pain was totally relieved. Then, the experience of pain was induced by verbal suggestion as follows, “Sensations in the back of your left hand start to become painful, more and more painful. The unpleasant experience of pain gets stronger and stronger, and, when it reaches the limit you can tolerate, it will not increase any further but will stay stable until I tell you that all pain will disappear.” Thirty seconds after the subject signaled maximal pain, a suggestion to relieve pain was given, “The pain goes further and further away, and soon you do not feel any pain at all. The pain is relieved, and your hand feels totally normal.” The periods including the suggestions and the experience of suggestion-induced pain alternated with 30-s rest periods until the end of the 12-min continuous scanning session (Fig. 1).
Fig. 1.
Imaging protocol. During each session, the experience of pain alternated with rest periods. During the first session, pain was induced by hypnotic suggestion, and during two other sessions by laser stimulation applied to the left hand. The subject stayed under hypnosis during the second session, whereas the third session was performed in the absence of hypnosis.
The second (6-min) session followed the first while the subject remained in hypnosis but did not receive any suggestions. Now rest periods of 30 s alternated with laser-pulse stimulation, delivered in a series of varying durations (mean 26 s, range 3–33 s, resulting in mean total amount of 89 pulses per subject, with an interstimulus interval of 0.6 s; the durations of the pulse series varied because of technical limitations of the stimulator). Laser stimuli (1 ms in duration, 2,000 nm in wavelength) were produced by a thulium–YAG stimulator (Baasel Lasertech, Starnberg, Germany), and the laser beam was conducted to the scanning room via an optic fiber. An assistant directed the laser beam to an area of ≈10 mm2 on the dorsal skin of the left hand. To avoid skin burns, the beam was moved after each pulse to a random direction on a skin area ≈5 cm in diameter. Stimulus intensity was on average 570 mJ (range 480–660 mJ), individually adjusted to equal 1.5–2 × the subjective pain threshold that was defined by increasing the stimulus intensity in 50-mJ steps.
In a separate 8-min session, performed in the absence of hypnosis, the painful laser stimulation was repeated, and every second stimulation block applied intensity of only 350 mJ (low-intensity laser stimulation), resulting in an experience of only mild pain or warmth (pulse series with mean duration of 28 s, range 6–33 s; on average 64 painful and 57 low-intensity pulses per subject with interstimulus interval of 0.6 s). To avoid memorizing the laser-induced pain during suggestions, sessions with laser stimulation always followed session with suggestions.
After the fMRI sessions, the subject filled out a questionnaire concerning the type and location of the pain and ratings of the pain intensity and unpleasantness on a visual analog scale (VAS). The subject also rated the reality of the experience on VAS ranging from “imaginal pain” to “real physical pain associated with injury or painful stimulation of the hand.”
Imaging. fMRI images were acquired by a Signa VH/i 3.0-T MRI scanner (General Electric) with a gradient-echo echo-planar imaging sequence (repetition time = 3.0 s, echo time = 32 ms, flip angle = 90°, field of view = 20 cm, 96 × 96 matrix, slice thickness 3 mm, no spacing between slices) to obtain a blood oxygenation level-dependent (BOLD) signal. Coverage of the whole brain required 37 oblique axial slices parallel to the paranasal sinuses. The first 4 volumes were discarded to allow stabilization of the T1 (longitudinal) relaxation effects.
High-resolution structural images were collected for each subject by the T1-weighted sequence 3D spoiled gradient-echo pulse sequence with repetition time = 8.4 ms, echo time = 1.8 ms, inversion time = 300 ms, flip angle 15°, and number of excitations = 2.
Data Analysis. Functional data were preprocessed and analyzed by statistical parametric mapping (spm2; www.fil.ion.ucl.ac.uk/spm) and matlab 6.1 (Mathworks, Natick, MA) softwares. Volumes for each subject were realigned to the first volume, spatially normalized to the average brain according to the Montreal Neurological Institute (MNI), resulting in voxel size of 2 × 2 × 2 mm3 (16), and spatially smoothed with an 8-mm (full-width at half-maximum) Gaussian kernel. High-pass filtering was applied to reduce the effect of slow signal drifts, and the serial correlation was compensated for by “prewhitening” the data with a first-order autoregressive model (17).
All functional series of a single subject were modeled with a common design matrix. Boxcar functions were created to model the periods of suggestion for pain, the subject's motor signals, the stable phase of suggestion-induced pain, the suggestion for pain relief, the painful laser stimulation, and the low-intensity laser stimulation. These functions were convolved with a hemodynamic response function, and the time derivative of this function was included in the model to compensate for small time shifts. The preprocessed data were fit to the model, and the resulting parameter estimates were compared voxel-wise between conditions to obtain individual contrast images (18).
For the random-effects group analyses, individual contrast images were fed into t tests (19). Although the subject was under hypnosis during the first painful laser stimulation and was not during the second, these sessions together were compared with the baseline and with the suggestion-induced pain. This pooling seemed feasible, because subjects reported similar pain during both sessions, and because the contrast between these two sessions showed no activation, suggesting close similarity of the patterns.
To study brain correlates of SRP, individual contrast strengths (pain vs. rest) were correlated with the subjective reality estimates across the subjects. The effect of perceived intensity and unpleasantness of pain on this correlation was tested by adding the intensity and unpleasantness estimates into the correlation analysis as confounding factors. Subjective estimates were tested for mutual correlation (Pearson).
Individual time courses were extracted from spherical (radius 5 mm) areas where the activation strengths (pain vs. rest) correlated with the SRP estimates. To study the functional connectivity of these areas, the time courses were used as regressors in the spm analysis (18, 20); laser stimulation, suggestions, and suggestion-induced pain were included as confounding factors. The resulting individual covariation images were fed into one sample t test.
In a priori brain regions, frequently reported to be associated with sensory-discriminative features of pain (i.e., the primary and secondary somatosensory cortices and the posterior insula) and with emotional aspects of pain [i.e., the caudal ACC (cACC) and the middle and anterior insula] (12, 13), clusters of at least 20 contiguous voxels (exceeding the threshold of P < 0.005; t > 3.0, Z > 2.6) were considered statistically significant. This threshold was applied also for other areas frequently associated with pain (the thalamus, several motor areas, and the dorsolateral prefrontal cortex; refs. 12 and 13) and for the pACC that has been suggested to be connected to the experience of reality of percepts (Talairach coordinates: x = 6, y = 48, and z = 0) (14). In searches from the whole brain, the threshold for activation was volume-corrected P < 0.05 at the cluster level, and only the most significant peaks from the interregional covariation analyses, clearly exceeding this threshold, are reported to avoid confounding by global signal changes.
For visualization of the BOLD responses, the individual subjects' BOLD signals' time courses were extracted from spherical areas (8 mm in diameter) centered according to the centers of mass of the clusters obtained at the group level. The resulting curves were averaged with respect to different conditions.
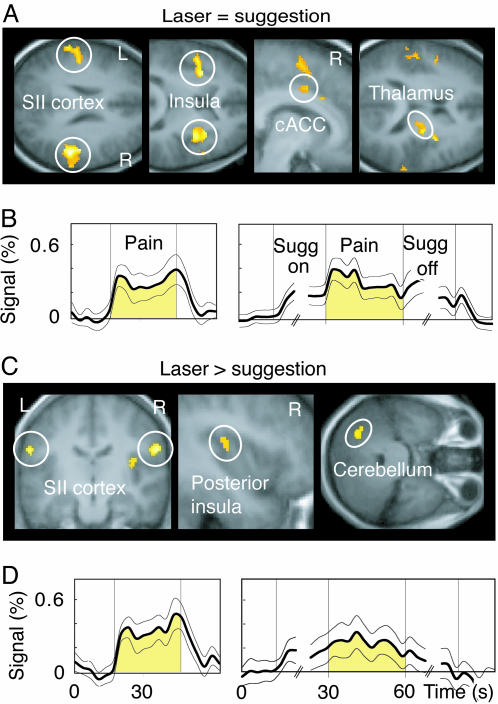
To visualize common activations for suggestion- and laser-induced pain (Fig. 2A), an inclusive mask image, corresponding to statistically significant activation during laser stimulation, was implemented in the second-level analysis of suggestion-induced pain.
Fig. 2.
Brain regions showing similarities and differences during laser- and suggestion-induced pain and time courses of the BOLD signal. (A) Brain regions activated during both suggestion- and laser-induced pain. A liberal threshold of P < 0.05 is used to illustrate the activation trend in cACC. (B) The (mean ± SEM across subjects) time courses of signal changes in the encircled brain areas of A during laser- (Left) and suggestion-induced pain (Right). Only the first three and the last three time points of the suggestion periods are illustrated, because time to pain appearance and to pain relief varied among subjects. Sugg on, suggestion for pain; Sugg off, suggestion for pain relief. (C) Areas where activation was stronger during laser- than suggestion-induced pain. (D) The signal time courses from the areas encircled in C.
The Montreal Neurological Institute (MNI) coordinates were converted to Talairach coordinates (21) by a matlab program (mnti2tal, author M. Brett, www.mrc-cbu.cam.ac.uk/Imaging/Common/mnispace.shtml). Structural images were spatially normalized into the T1 MNI template, and the average structural image across all subjects was calculated in spm2.
Results and Discussion
Subjective Evaluation of Laser- and Suggestion-Induced Pain. In the first session (see Fig. 1), subjects signaled, with a small foot movement, the maximum tolerable pain on average 29 ± 4 s (mean ± SEM reported throughout; range, 9–57 s) after the beginning of the suggestion for pain. Pain then remained stable in intensity until the beginning of the suggestion for pain relief, which was followed in 19 ± 3 s (range, 9–42 s) by the subjects' sign of absence of pain. Subjects described the suggestion-induced pain in various pain-related terms, most frequently as burning (8 of 14 subjects) or aching (7 subjects) in the left-hand dorsum; for details about subjective evaluations, see Table 2, which is published as supporting information on the PNAS web site.
Painful laser stimulation resulted in pain that included continuous burning and fluctuating pricking sensations. The location, intensity, and unpleasantness were similar for laser- and suggestion-induced pain: On the 0–100 Visual Analog Scale, the intensity was 65 ± 4 for laser-induced pain and 57 ± 5 for suggestion-induced pain (difference not significant, paired two-sample t test), and the corresponding estimates for unpleasantness were 58 ± 5 and 51 ± 6, respectively (difference not significant). However, all 14 subjects estimated the reality of pain to be higher during laser- than suggestion-induced pain (87 ± 3 vs. 62 ± 5; P < 0.001).
Activations During Laser- and Suggestion-Induced Pain. In rough agreement with a recent report (7), both laser- and suggestion-induced pain were associated with activation of the well known cerebral pain circuitry (Fig. 2 and Tables 3 and 4, which are published as supporting information on the PNAS web site). The primary somatosensory cortex was not activated during either condition, in line with about half of the prior pain imaging studies (13). At the group level, the activation strengths in the contralateral secondary somatosensory cortex (SII cortex) (x = 52, y = -30, and z = 27) correlated positively with the subjective intensities of suggestion-induced pain (R = 0.81, P < 0.001); a similar trend was evident during laser-induced pain (R = 0.44, P = 0.01). The activation pattern to painful laser stimulation was similar for contrasts against baseline and against low-intensity laser stimulation, thereby excluding the possibility that the activations would have been caused by nonpain-related physiological changes or would reflect stimulation-related artifacts. The small sample size prevented any detailed comparisons between activation patterns and the subjective quality of the suggestion-induced pain.
In contrast with an earlier study that included anticipation periods in the analysis (7), the present activations were associated with the stable phase of suggestion-induced pain, implying connection to the actual experience rather than anticipation of pain (22). This view is in line with prior findings of pain-related activation in the middle insula and cACC, similarly as in the present study, whereas anticipation-related activations were 2.7–3.4 cm more anterior (22).
Subjective pain intensities were similar during both laser and suggestion sessions, and they are thus unlikely explanations for the observed differences between activation patterns: the contralateral (right) posterior insula, the posterior superior SII cortex bilaterally, and the ipsilateral cerebellum were more strongly activated during laser- than suggestion-induced pain (P < 0.005, paired t test; Fig. 2; Table 5, which is published as supporting information on the PNAS web site). In contrast, the posterior cingulate cortex, adjacent to the cACC, was more strongly activated during suggestion- than laser-induced pain (P < 0.005, paired t test; Table 5). Suggestion-induced pain could therefore be related to more active processing of emotional aspects of pain, known to involve the cACC (4). On the other hand, the posterior insula and the SII cortex participate in the sensory-discriminative processing of pain (23, 24); such detailed analysis of pain intensity, quality, and location certainly contributes to the clarity of the percept and thereby most likely to SRP.
Brain Correlates of SRP. Supporting the connection between the sensory pain circuitry and SRP, we observed positive correlations between activation strengths within the sensory pain circuitry and SRP estimates of laser-induced pain (pooled data from Sessions 2 and 3; Table 1; Fig. 5, which is published as supporting information on the PNAS web site). Although none of the four correlations between activation strengths and SRPs (for the bilateral SII cortices and the bilateral posterior insula of the sensory pain circuitry) alone reached the statistical significance threshold for a priori areas (P < 0.005), the probability of these correlations occurring by chance in all these areas, under the assumption of independent activations, would be only 3 × 10-8.
Table 1. Correlation (R) between activation strengths and subjective reality of pain across subjects.
| Brain region | x | y | z | R | Peak Z | P | Volume, mm3 |
|---|---|---|---|---|---|---|---|
| Suggestion-induced | |||||||
| Bilateral rACC | 8 | 36 | 20 | 0.85 (0.64) | 3.9 (2.4) | 0.001* (0.01) | 910 (1600) |
| -8 | 32 | 19 | 0.83 (0.63) | 3.7 (2.3) | 0.001* (0.01) | 470 (380) | |
| Pericingulate cortex | -6 | 48 | 22 | 0.81 (0.51) | 3.5 (1.8) | 0.001* (0.03) | 1,170 (180) |
| Right pACC | 8 | 43 | 0 | 0.73 (0.74) | 3.4 (2.9) | <0.001 (0.002) | 300 (260) |
| Laser-induced | |||||||
| Left rACC | -8 | 36 | 20 | 0.69 (0.58) | 2.7 (2.1) | 0.003 (0.02) | 2,370 (2420)† |
| Left pACC | -4 | 48 | 4 | 0.48 (0.55) | 1.7 (2.5) | 0.04 (0.007) | 90 (260)† |
| Bilateral SII | 65 | -17 | 19 | - (0.41) | - (2.3) | - (0.01) | - (730)† |
| -56 | -20 | 19 | - (0.36) | - (1.8) | - (0.03) | - (90)† | |
| Bilateral posterior insula | 42 | -11 | 6 | - (0.45) | - (2.2) | - (0.01) | - (410)† |
| -44 | -13 | -8 | - (0.43) | - (2.3) | - (0.01) | - (530)† |
x, y, and z are Talairach coordinates, and Z is t value transformed into normal distribution. Volume consists of voxels where P < 0.005 (uncorrected) or P < 0.05 (†, uncorrected). All values within parentheses derive from multiple-regression analysis, where intensity and unpleasantness were included as confounding factors; the correlation between sensory pain-processing areas and subjective reality of pain was found only in this analysis. BA, Brodmann's area.
Corrected for multiple comparisons
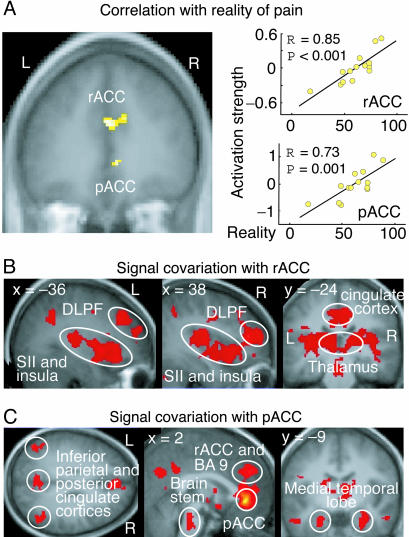
During suggestion-induced pain, the sensory pain-processing circuitry did not similarly correlate with the SRP estimates, whereas the pACC and the area extending from the rostral ACC (rACC) to the rostral pericingulate cortex (Fig. 3 and Table 1) clearly did. Although the subjective intensity and SRP were mutually correlated (R = 0.68, P = 0.01), a statistically significant correlation remained between activation strengths of these medial prefrontal areas and SRP when intensity and unpleasantness estimates were added to the analysis as confounding factors (Table 1; P < 0.05). A clear trend toward a similar correlation between activation strengths of medial prefrontal cortex (mPFC) and SRP was observed during laser-induced pain in the absence of hypnosis (Session 3, Table 1).
Fig. 3.
Medial prefrontal regions where activation strengths correlated with the SRP and brain regions where the fMRI signal's time behavior was similar to those of the medial prefrontal regions. (A) Areas rACC and pACC (Left) and the activation strengths as a function of subjective estimates of reality of pain. (B) Brain areas where the magnetic resonance signal covaried with that of rACC during sessions 1 and 3. (C) Brain areas where the magnetic resonance signal covaried with that of pACC during sessions 1 and 3. DLPF, dorsolateral prefrontal cortex; BA, Brodmann's area. For a complete list of covariation areas, see Table 6.
The correlation of pACC activation strengths with SRP resembles a prior finding during imaginal hearing (14). The pACC is frequently activated during emotional processing (25) and has been suggested to be related to source monitoring in subjects whose attention is affect-laden (14). In fact, emotional factors could play a prominent role in source monitoring; events often “feel” real or unreal rather than “are known” to be real or unreal.
We also found a strong correlation between the SRP estimates and activation strengths in the area extending from rACC to the rostral pericingulate cortex. Interestingly, the latter brain region is activated during self monitoring as well as evaluation of intentions of self and others (26), phenomena very close to source monitoring.
Covariation of mPFC fMRI Signals with Signals from Other Parts of the Brain. To shed more light on the role of these mPFC regions, we applied covariation analysis (20) to pinpoint brain areas where the BOLD signals would covary with those from pACC and rACC. Such a functional connectivity would suggest that the covariating areas either have common input or communicate with each other and thus could participate in the construction of the same experience.
During both Sessions 1 and 3, the mPFC covaried with a widespread neuronal network (Fig. 3; Table 6, which is published as supporting information on the PNAS web site), in line with the well established extensive anatomical connections of ACC (27). Because the covariation analysis included the entire Sessions 1 and 3, rest conditions included, the observed connectivity cannot be considered specific to pain. It is, however, interesting that the covariation areas of rACC included the pain circuitry, and the covariation areas of pACC included regions related to memory [the thalamus (28), the medial temporal lobe, the posterior cingulate, and the inferior parietal cortex], attention (the inferior parietal cortex), and imagery (the inferior parietal cortex) (29), i.e., factors suggested to contribute to the experience of reality (1, 2, 30–32).
Conclusion
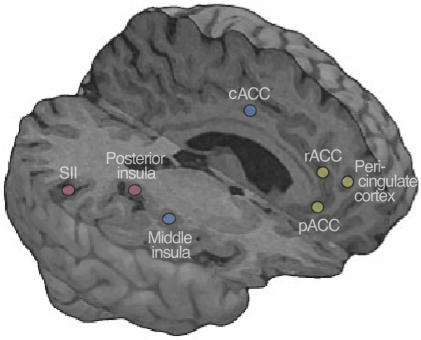
Although the applied covariation analysis cannot resolve causal relationships between brain function and subjective experience, nor between signals from different brain areas, our findings and psychological theories on the experience of reality (1, 2) converge to a neuronal system that could subserve the construction of the SRP, at least in suggestion-prone subjects. This network includes sensory pain circuitry where various sensory-discriminative aspects of pain may contribute to the clarity of percept, and thereby to the SRP, and mPFC that could monitor the source of this sensory-discriminative information. Fig. 4 gives a schematic summary of our findings and interpretations.
Fig. 4.
A schematic summary of the main findings and interpretations. The blue dots illustrate the cACC and the middle insula, i.e., areas related to emotional component of pain and activated similarly during both physically and psychologically induced pain. The red dots depict the SII cortex and the posterior insula that are related to the sensory component of pain and were activated more strongly during physically than psychologically induced pain. This difference, however, could reflect quantitative rather than qualitative differences in the processing of sensory information and therefore could be insufficient for judging the reality of pain without knowledge about the source of this information. On the basis of the known functional neuroanatomy and the observed correlation between the mPFC (yellow dots, rACC; pericingulate cortex; pACC) and SRP, we propose that the mPFC could be related to such source monitoring.
Supplementary Material
Acknowledgments
We thank S. Aulanko, N. Forss, R. Joensuu, V. Jousmäki, M. Kattelus, H. Renvall, M. Schürmann, and A. Tarkiainen for expert help and advice and M. Seppä for segmentation of the brain image in Fig. 4. This study was financially supported by the Academy of Finland, the Finnish Graduate School of Neuroscience, the Signe and Ane Gyllenberg Foundation, and the Louis Jeantet Foundation.
Abbreviations: SII cortex, secondary somatosensory cortex; ACC, anterior cingulate cortex; pACC, perigenual ACC; rACC, rostral ACC; cACC, caudal ACC; mPFC, medial prefrontal cortex; SRP, subjective reality of pain; fMRI, functional MRI.
References
- 1.Bentall, R. P. (1990) Psychol. Bull. 107, 82-95. [DOI] [PubMed] [Google Scholar]
- 2.David, A. S. (1999) Acta Psychiatr. Scand. Suppl. 395, 95-104. [DOI] [PubMed] [Google Scholar]
- 3.Bryant, R. A. & Mallard, D. (2003) Conscious. Cognit. 12, 219-230. [DOI] [PubMed] [Google Scholar]
- 4.Rainville, P., Duncan, G. H., Price, D. D., Carrier, B. & Bushnell, M. C. (1997) Science 277, 968-971. [DOI] [PubMed] [Google Scholar]
- 5.Petrovic, P. & Ingvar, M. (2002) Pain 95, 1-5. [DOI] [PubMed] [Google Scholar]
- 6.Wager, T. D., Rilling, J. K., Smith, E. E., Sokolik, A., Casey, K. L., Davidson, R. J., Kosslyn, S. M., Rose, R. M. & Cohen, J. D. (2004) Science 303, 1162-1167. [DOI] [PubMed] [Google Scholar]
- 7.Derbyshire, S. W., Whalley, M. G., Stenger, V. A. & Oakley, D. A. (2004) NeuroImage 23, 392-401. [DOI] [PubMed] [Google Scholar]
- 8.Eisenberger, N. I., Lieberman, M. D. & Williams, K. D. (2003) Science 302, 290-292. [DOI] [PubMed] [Google Scholar]
- 9.Singer, T., Seymour, B., O'Doherty, J., Kaube, H., Dolan, R. J. & Frith, C. D. (2004) Science 303, 1157-1162. [DOI] [PubMed] [Google Scholar]
- 10.Weiss, A. P. & Heckers, S. (1999) Psychiatry Res. 92, 61-74. [DOI] [PubMed] [Google Scholar]
- 11.Kosslyn, S. M., Thompson, W. L., Costantini-Ferrando, M. F., Alpert, N. M. & Spiegel, D. (2000) Am. J. Psychiatry 157, 1279-1284. [DOI] [PubMed] [Google Scholar]
- 12.Davis, K. D. (2000) in Pain Imaging, eds. Casey, K. L. & Bushnell, M. C. (IASP Press, Seattle), pp. 195-210.
- 13.Peyron, R., Laurent, B. & Garcia-Larrea, L. (2000) Neurophysiol. Clin. 30, 263-288. [DOI] [PubMed] [Google Scholar]
- 14.Szechtman, H., Woody, E., Bowers, K. S. & Nahmias, C. (1998) Proc. Natl. Acad. Sci. USA 95, 1956-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weitzenhoffer, A. M. & Hilgard, E. R. (1962) Stanford Hypnotic Susceptibility Scale: Form C (Consulting Psychologists, Palo Alto, CA).
- 16.Friston, K. J., Ashburner, J., Frith, C. D., Poline, J.-P., Heather, J. D. & Frackowiak, R. S. J. (1995a) Hum. Brain Mapp. 2, 165-189. [Google Scholar]
- 17.Bullmore, E., Brammer, M., Williams, S. C., Rabe-Hesketh, S., Janot, N., David, A., Mellers, J., Howard, R. & Sham, P. (1996) Magn. Reson. Med. 35, 261-277. [DOI] [PubMed] [Google Scholar]
- 18.Friston, K. J., Holmes, A. P., Worsley, K. J., Poline, J.-P., Frith, C. D. & Frackowiak, R. S. J. (1995b) Hum. Brain Mapp. 2, 189-210. [DOI] [PubMed] [Google Scholar]
- 19.Holmes, A. P. & Friston, K. J. (1998) NeuroImage, S754.
- 20.Friston, K. J. (1994) Hum. Brain Mapp. 2, 56-78. [Google Scholar]
- 21.Talairach, J. & Tournoux, P. (1988) A Co-Planar Stereotactic Atlas of the Human Brain (Thieme, Stuttgart).
- 22.Ploghaus, A., Tracey, I., Gati, J. S., Clare, S., Menon, R. S., Matthews, P. M. & Rawlins, J. N. (1999) Science 284, 1979-1981. [DOI] [PubMed] [Google Scholar]
- 23.Treede, R. D., Kenshalo, D. R., Gracely, R. H. & Jones, A. K. (1999) Pain 79, 105-111. [DOI] [PubMed] [Google Scholar]
- 24.Ostrowsky, K., Magnin, M., Ryvlin, P., Isnard, J., Guenot, M. & Mauguiere, F. (2002) Cereb. Cortex 12, 376-385. [DOI] [PubMed] [Google Scholar]
- 25.Phan, K. L., Wager, T., Taylor, S. F. & Liberzon, I. (2002) NeuroImage 16, 331-348. [DOI] [PubMed] [Google Scholar]
- 26.Gallagher, H. L. & Frith, C. D. (2003) Trends. Cognit. Sci. 7, 77-83. [DOI] [PubMed] [Google Scholar]
- 27.Öngür, D. & Price, J. L. (2000) Cereb. Cortex 10, 206-219. [DOI] [PubMed] [Google Scholar]
- 28.Lenz, F. A., Gracely, R. H., Romanoski, A. J., Hope, E. J., Rowland, L. H. & Dougherty, P. M. (1995) Nat. Med. 1, 910-913. [DOI] [PubMed] [Google Scholar]
- 29.Cabeza, R. & Nyberg, L. (2000) J. Cognit. Neurosci. 12, 1-47. [DOI] [PubMed] [Google Scholar]
- 30.Brebion, G., Amador, X., David, A., Malaspina, D., Sharif, Z. & Gorman, J. M. (2000) Psychiatry Res. 95, 119-131. [DOI] [PubMed] [Google Scholar]
- 31.Aleman, A., Bocker, K. B., Hijman, R., de Haan, E. H. & Kahn, R. S. (2003) Schizophr. Res. 64, 175-185. [DOI] [PubMed] [Google Scholar]
- 32.Barnes, J., Boubert, L., Harris, J., Lee, A. & David, A. S. (2003) Neuropsychologia 41, 565-574. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.