Abstract
Introduction
Although a role for alpha-2 adrenoceptors (alpha-2 ARs) in alcohol use disorder (AUD) and depression is suggested, very little information on a direct interaction between alcohol and these receptors is available.
Methods
In this study adult female Wistar and Wistar-Kyoto (WKY) rats, a putative animal model of depression, were exposed to alcohol vapor 3 h daily for 10 days (blood alcohol concentration ~150 mg%) followed by daily injection of 10 mg/kg of imipramine (IMP, a selective norepinephrine NE/serotonin reuptake inhibitor) or nomifensine (NOMI, a selective NE/dopamine reuptake inhibitor). On day 11 animals were tested for open field locomotor activity (OFLA) and forced swim test (FST) and were sacrificed 2 h later for measurement of alpha-2 ARs densities in the frontal cortex and hippocampus using [3H]RX 821002 as the specific ligand.
Results
Chronic alcohol treatment increased the immobility in the FST, without affecting OFLA in both Wistar and WKY rats, suggesting induction of depressive-like behavior in Wistar rats and an exacerbation of this behavior in WKY rats. Alcohol treatment also resulted in an increase in cortical but not hippocampal alpha-2 ARs densities in both Wistar and WKY rats. The behavioral effects of alcohol were completely blocked by IMP and NOMI and the neurochemical effects (increases in alpha-2 ARs) were significantly attenuated by both drugs in both strains.
Conclusions
The results suggest a role for cortical alpha-2 ARs in alcohol withdrawal-induced depression and that selective subtype antagonists of these receptors may be of adjunct therapeutic potential in AUD-depression co-morbidity.
Keywords: Alpha-2 adrenoceptors, depression, alcohol use disorder, alcohol withdrawal, Tricyclic antidepressants, Wistar-Kyoto (WKY) rats, Animal model
1. INTRODUCTION
A significant co-morbid expression of alcohol use disorders (AUD) and depression is evident in epidemiological studies (Boschloo et al., 2011; Dixit and Crum, 2000; Iovenio et al., 2011; Lai et al., 2015; Rodgers et al., 2000; Schuckit, 2006; Spak et al., 2000). Among the AUD treatment population, co-morbid depression can affect as much as 50% of people (Swendsen and Merikangas 2000). Similarly, depression treatment populations may have up to 40% life-time probability of developing AUD (Grant et al., 2011; Jane-Llopis, and Matytsina, 2006). Co-occurrence of AUD and depression results in greater disease burden than each disorder alone (Gadermann, et al., 2006). Such dual diagnosis is an important clinical assessment since treatment outcome for either condition, if considered separately, may not be fully adequate (Iovenio et al., 2011). Interestingly, pharmacological treatment of the depressive symptoms results in a better treatment outcome for AUD (Kessler et al., 1997; Schuckit et al., 1997). Likewise, treatment of primary AUD results in rapid reduction in depressive symptoms (Brown and Schuckit, 1988). Indeed, 80% of AUD patients with major depression no longer present depressive symptoms after 2 weeks of sobriety (Dackis et al., 1986). However, if unmanaged, depressive symptom especially during alcohol withdrawal can lead to relapse and increased alcohol intake (Dixit and Crum, 2000; Hodgins et al., 1995; Johanson and Fischman, 1989; Schulteis, et al., 1995).
A positive relationship between depressive symptoms and voluntary alcohol intake has also been observed in animal models. Thus, Wistar-Kyoto (WKY) rats, considered a putative and non-induced animal model of depression, voluntarily consume more alcohol than their control counterparts, Wistar rats or Sprague-Dawley rats (Jiao et al., 2006; Paré et al., 1999; Yaroslavsky and Tejani-Butt, 2010). Conversely, alcohol preferring (AA) rats may exhibit depressive-like characteristics following voluntary alcohol intake compared to alcohol non-preferring (ANA) rats (Viglinskaya et al. 1995). Although various theories have attempted to explain the association between AUD and depression, it appears that a number of factors, including genetic predisposition and alterations in neurochemical substrates, such as the noradrenergic system, may contribute to this co-morbidity (Balsamo et al., 2016; Bravo et al., 2017; Donadon and Osorio, 2016; Getachew et al., 2010; Jung et al., 2016; Kalejaye et al., 2013; Merikangas and Gelernter, 1990; Ovestreet et al., 2005; Rezvani et al., 2002, 2007; Rincon-Hoyos, et al., 2016).
Alpha adrenergic receptors (alpha ARs) are one of the major classes of G protein-coupled receptors for norepinephrine (NE) that are predominantly located pre-synaptically, but are also present post-synaptically in the central nervous system (Bylund, 1988; Bylund et al., 1995; Giovannitti et al., 2015; U’Prichard et al., 1979). There are two subtypes of alpha ARs (alpha 1 and alpha 2) (Bylund et al., 1995). In humans, three subtypes of alpha-2 ARs (alpha 2A, alpha 2B and alpha 2C) and in rats, four subtypes (alpha 2A, alpha 2B, alpha 2C and alpha 2D) have been identified. In the rat, alpha 2D is a species variation of human alpha 2A. These receptors play an important role in regulating the neuronal release of NE through presynaptic feedback inhibition and may be at least partially responsible for pathogenesis and symptomatic expression of depressive illness. Thus, a variety of antidepressants (e.g., desipramine) and other treatments of depression (e.g., electroconvulsive shock therapy) are associated with decreases in the density and sensitivity of central alpha-2 ARs (Barturen and Garcia-Sevilla, 1992; Cohen et al., 1980; Invernizzi and Garattini, 2004; Pilc and Vetulani, 1982; Smith et al., 1983; Tanaka and Telegdy, 2014). Interestingly, manipulations of the alpha-2 ARs may also affect alcohol intake. Hence, yohimbine, an alpha-2 AR antagonist can reinstate alcohol seeking after extinction (Funk et al., 2016; Marinelli et al., 2007). On the other hand, alpha-2A AR agonists that may decrease availability of NE reduce alcohol consumption (Fredriksson et al., 2015; Opitz, 1990; Rasmussen et al., 2014). However, very few studies have investigated direct effects of alcohol on apha-2 ARs, particularly in relation to depressive-like characteristics.
A major goal of this study was to investigate the effects of alcohol as well as two tricyclic antidepressants (imipramine, a selective norepinephrine/serotonin NE/5HT uptake inhibitor, and nomifensine, a selective NE/dopamine DA uptake inhibitor) on alpha-2 ARs in Wistar (control) and WKY rats. We hypothesized that alcohol withdrawal-induced depressive-like behavior will be associated with an increase in alpha-2 AR densities in two important brain regions, namely the frontal cortex and the hippocampus. Moreover, we anticipated that chronic treatment with antidepressants that affect the noradrenergic system would normalize the behavioral as well as the neurochemical effects of alcohol on alpha-2 ARs.
2. MATERIALS AND METHOD
2.1.1 Animals
Adult female WKY rats, approximately 4 months old (Harlan, IN, USA) were used throughout the study. We chose female rats because: 1.) there exists a higher incidence of depression in women, but there are sparse inclusion of female animals in such studies (Kessler et al., 1993; Nolen-Hoeksema, 1990; Weissman, et al., 1996); 2.) as in humans, there is also a higher incidence of depressive-like behavior in females compared to male WKY rats (Pare and Redei, 1993); 3.) both female rats (Carrier and Kabbaj, 2013; Kokras et al., 2015) and mice (Franceschelli et al., 2015) show enhanced response and greater sensitivity to antidepressant treatments compared to their male counterparts. Although menstrual cycle may have an important role in human behavioral outcome, it should be noted that the estrous cycle in rats is relatively very short (4 days, Spornitz et al. 1999), and that rats tend to synchronize their estrous cycle when housed in group or close proximity (Alekhina et al. 2015; McClintock, 1978) In addition, use of appropriate controls, as used in this study, can reduce the chance of false positive or negative results when using female rats.
Animals were housed in groups of three or four in standard polypropylene shoebox cages (42 × 20.5 × 20 cm) on hardwood chip bedding (alpha-dry) in a room designated for female rats. Throughout the experiment, animals had access to food (Harlan Tek Lab) and water ad libitum. The room was maintained at 24–26 °C at 51–66% relative humidity, on a 12-h reversed light/dark cycle (lights on at 1900 hr). All experiments were carried out in accordance with NIH guidelines and approved by the Institutional Animal Care and Use Committee.
In order to acclimate the subjects to the housing conditions, animals arrived at least one week prior to initiation of any experiment. During this period, they were gentled once daily in order to minimize any stress effects that might result from routine handling. All behavioral tests were carried out in the early portion of dark phase between 09:00 AM and 12:00 PM using a red light as source of illumination. The dark phase was chosen to coincide with the animals’ nocturnal nature (i.e., being active during the dark phase). Each experimental group consisted of 7–8 animals.
2.1.2 Vapor ethanol exposure and drug treatment
Inhalation chambers were used to expose the animals to ethanol (La Jolla Alcohol Research Inc., La Jolla, CA, USA). Briefly, 95% ethanol (EtOH) was pumped at regulated rate from 5-gallon reservoir via a peristaltic pump to a 5000 ml Erlenmeyer vacuum flask that was kept on a warming tray (52°C). EtOH was then volatilized and mixed with pressurized air. The flow of this mixture was controlled by a pressure gauge as it was delivered to individual chambers. The parameters used in EtOH vapor exposure were: air pressure ≈5 psi, airflow rate ≈ 15–20 liter/min and EtOH flow rate = 60 ml/hr. A control group received only air via a system that exactly mirrored the experimental condition. The animals were exposed to EtOH vapor for 3h daily, during which, a blood alcohol concentration (BAC) of approximately 150 mg% was maintained. The inhalation chamber has the advantage of easily achieving and maintaining the targeted BAC (Kliethermes et al., 2004). Moreover, the variability in the alcohol concentration between similarly controlled chambers is minimal (Lee et al., 2000; Getachew et al., 2008, 2010).
USP 200 proof EtOH was purchased from VWR Scientific Products (Bridgeport, New Jersey, USA) and was diluted down (95% v/v) with distilled water. Imipramine (IMP) HCl and nomefensine (NOMI) maleate were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA), were dissolved in saline and injected intraperitoneally (i.p.) (10 mg/kg) in a volume of 1 ml/kg, immediately after removal from the vapor chamber each day. Control animals were injected with the same volume of physiological saline. To verify the “depressogenic” effect of alcohol, a separate control group was exposed to air in an exactly same set up followed by saline injection only. The dosing of drugs was chosen based on works of others as well as ours that have been reported as optimally effective antidepressant doses (Tijani-Butt et al., 2003; Getachew et al., 2008).
2.1.3 Behavioral evaluations
2.1.3a Open Field Locomotor Activity (OFLA) Monitoring
On day 11, approximately 16 h after the last alcohol exposure, animals were tested in an open-field activity-monitoring cage (27 × 27 × 20.3 cm, Med Associates, Inc., St. Albans, VT) for 10 min, where ambulatory counts representing the number of infrared beam interruptions were recorded (Getachew et al., 2010).
2.1.3b Forced Swim Test (FST)
Immediately following the open field activity test, each animal was evaluated for its behavior (immobility) in the forced swim test (FST) (Getachew et al 2010). Briefly, each rat was placed in a round Pyrex cylinder pool measuring 17 cm in diameter and 60 cm in height for 5 min. The cylinder was filled with 30 cm water (25±1 °C) to ensure that animals could not touch the bottom of the container with their hind paws or their tails. The FST activity was video recorded for subsequent analysis. The rat was removed after 5 min, dried, and placed in its home cage. A time sampling scoring technique was used whereby the predominant behavior in each 5-s period of the 300-s test was recorded. Inactivity (immobility) and activity (swimming) were distinguished as mutually exclusive behavioral states. Swimming behavior was defined as movement (usually horizontal) throughout the cylinder. Immobility, reflective of helplessness, was defined when no additional activity was observed other than that required to keep the rat’s head above the water (Getachew et al., 2008, 2010).
2.1.3c Brain Collection
Animals were sacrificed by decapitation, approximately 2 h after the last behavioral test (i.e., FST). Brains were quickly removed, frozen on dry ice and stored at −80°C until dissection for receptor analysis (see below).
The flow chart below illustrates the timeline of the study:
Animal arrival ≫ 1week acclimation ≫ 10 days of alcohol vapor exposure (3h/day) followed immediately by drug injection ≫ behavioral tests on day 11 (10 min OFLA followed by 5 min FST) ≫ 2 h rest ≫ decapitation and brain collection.
2.1.4 Blood alcohol determination
To determine BAC at various time points, a separate group of Wistar and WKY were exposed to the chambers under identical conditions. Blood was sampled by tail bleed technique every three days immediately after the end of daily ethanol exposure. Briefly, tail blood (0.4ml) was collected in tubes coated with 0.2M ethylene-diamine-tetraacetic acid (EDTA) (Sigma-Aldrich Co., St. Louis, MO, USA) and centrifuged for 5 min at 1,500g at 4°C. The plasma was extracted and BAC was assayed by injecting 5μL plasma into GM7 Micro-Stat Analyzer (Analox Instruments Ltd., Lunenburg, MA, USA).
2.1.5 Brain dissection and Alpha-2 AR Binding
For sample collection, frozen brains were thawed on ice and two brain areas that are intimately associated with mood regulation and antidepressant effects of drugs, namely the frontal cortex and the hippocampus, were dissected alternating between strains and treatment groups as described previously (Tizabi et al. 1999, 2000; Getachew et al. 2010). The frontal cortex (bilateral) was delineated posteriorly by the genu of the corpus callosum and included the prefrontal cortex. The hippocampus included the entire hippocampal region from both hemispheres. Membrane preparations from these two regions were used for alpha-2 AR binding. For the assay we used the method described by O’Rourke et al (1994) with minor modifications. [3H]RX821002 [(1,4-[6,7-3H] benzodioxan-2-methoxy-2-yl)-2-imidazoline] (specific activity ~54 Ci/mmol) was purchased from Amersham (Buckinghamshire, UK). This compound is a 2-methoxy derivative of idazoxan, but has better selectivity and higher affinity for alpha-2 AR subtypes than idazoxan (Stillings et al., 1986). Thus, in binding and tissue studies it behaves as a selective antagonist of alpha-2 ARs, with at least 5 times greater affinity for these receptors than any other binding site, however, it does not have any specific selectivity for any alpha-2 AR subtype (Clarke and Harris 2002). Briefly, for receptor density measurements, duplicates of membrane preparations (200 μl) containing 100 μg of protein were incubated with 2.5 nM [3H]RX 821002. The buffer consisted of 0.3 mM MgCl2, 0.1 mM EGTA, 5 mM Tris-HCl, and 50 mM sodium phosphate, pH 7.4. Tissue homogenates were incubated for 2 h at room temperature Non-specific binding in all assays was determined in presence of 50 μM unlabeled (-)-NE hydrochloride (Sigma-Aldrich, St. Louis, MO, USA). Binding reactions were terminated by vacuum filtration through Whatman GF/C filters (Clifton, NJ, USA), which were mounted on a 48-sample manifold (Brandel cell Harvester; Biomedical Research and Development, Gaithersburg, MD) that was pre-wetted with 0.5% polyethylenimine to reduce nonspecific binding to the filter. The tubes and filters were then washed three times with 4 ml ice-cold 50 mM Tris-HCl buffer (pH. 8.0) and, the radioactivity on the filter was determined by liquid scintillation spectroscopy (Micro β-scintillation counter; PerkinElmer, Boston, MA). Specific binding was calculated as the difference between total and nonspecific binding. For saturation binding 8–12 concentrations of [3H]RX 821002 ranging from (0.01 nM to 12.5 nM) were used. The receptor affinity (Kd) values were calculated from nonlinear regression of bound versus free ligand concentrations using a statistical software program (Prism 7; GraphPad, San Diego, CA, USA). Protein concentrations were determined by the method of Bradford (1976) with bovine serum albumin as the standard.
2.1.6 Statistical Analysis
All data were analyzed using analysis of variance (ANOVA), followed by Student Newman-Keuls (SNK) post hoc test when significant main effects were indicated. Thus, if an overall effect of the treatments was noted in initial ANOVA analysis, this was followed by SNK post hoc test to determine which groups differed significantly from one another. All analyses were two-tailed and P<0.05 was considered significant. In addition, using SPSS, linear regression analysis was performed to verify if a correlation existed between immobility and alpha-2 binding.
3. RESULTS
3.1.1 BAC and the effects of alcohol withdrawal on OFLA and FST
Daily BAC fluctuated between 143–160 mg % (152 ± 6.1 mg % mean ± SEM) for all measurements in both strains. This BAC is equivalent to relatively high alcohol consumption in humans (Getachew et al 2008, 2010).
Alcohol exposure resulted in an increase in immobility in FST (Fig 1) without affecting the OFLA in both Wistar and WKY rats. Thus, there was approximately 94% increase (p<0.01) in immobility in FST following 10 days of alcohol exposure in Wistar rats (Fig 1A, columns 1 and 2) and approximately 55% increase in immobility in FST in WKY rats (p<0.01, Fig 1B, columns 1 and 2). Alcohol treatment did not affect the ambulatory counts in the open field in either strain (Figs 2A and 2B, columns 1 and 2). Thus, the findings that immobility score in FST is increased without changes in OFLA, indicates that the animals were not impaired in their locomotor activity. This further validates that the effects of the treatment on depressive characteristics (i.e., immobility) was not due to motor impairment.
Figure 1.
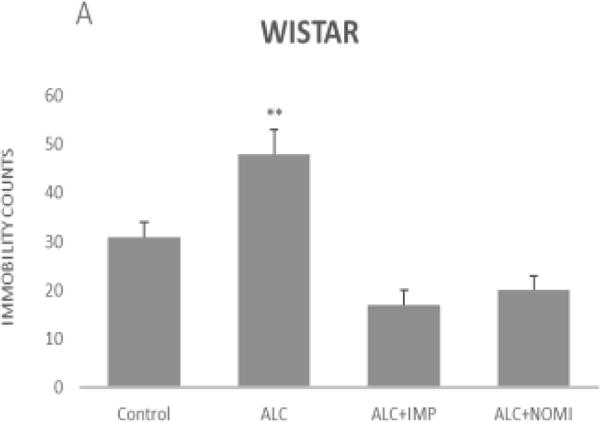
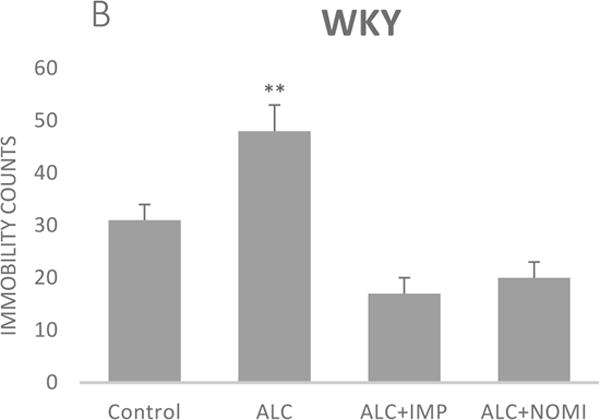
Effects of chronic alcohol, imipramine (IMP) and nomifensine (NOMI) on immobility scores in the forced swim test in female Wistar (A) and WKY (B) rats. Values are mean ± SEM (n=7–8). **p <0.01 compared to control.
Figure 2.
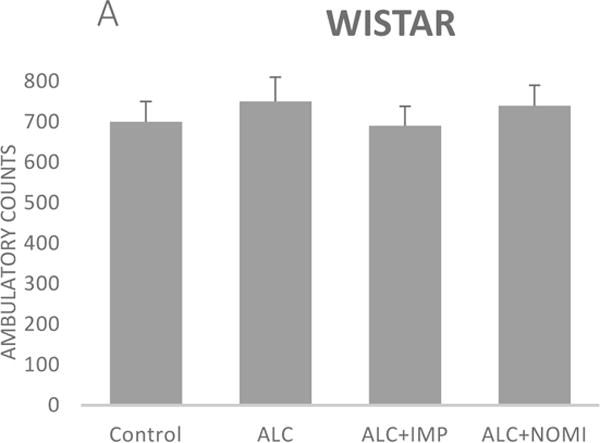
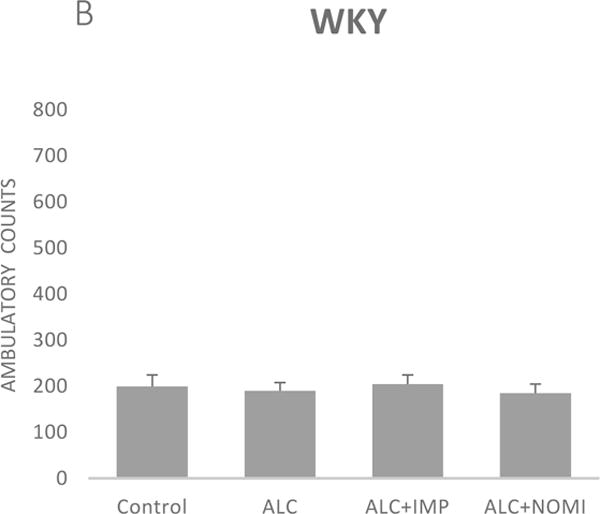
Effects of chronic alcohol, imipramine (IMP) and nomifensine (NOMI) on open field locomotor activity in female Wistar (A) and WKY (B) rats. Values are mean ± SEM (n=7–8).
3.1.2 Effects of IMP and NOMI on alcohol withdrawal-induced behavioral changes
Both IMP and NOMI treatment reversed alcohol withdrawal-induced increases in immobility in Wistar and WKY rats. Thus, following daily administration of IMP (10 mg/kg) and NOMI (10 mg/kg) the immobility scores were back to the original levels in Wistar rats and were further reduced in WKY rats to the basal levels that were observed in Wistar rats (Figs 1A and 1B, columns 3 and 4). Neither drug affected the OFLA in either strain (Figs 2A and 2B, columns 3 and 4).
3.1.3 Effects of alcohol withdrawal on Alpha-2 AR
The basal levels of alpha-2 ARs in the frontal cortex of WKY rats were approximately 50% higher than that of Wistar rats (Figs 3A and 3B, column 1). Alcohol exposure resulted in an increase in alpha-2 ARs in the frontal cortex of both Wistar and WKY rats. Thus, there was approximately 31% increase (p<0.01) in alpha-2 ARs following 10 days of alcohol exposure in Wistar rats (Fig 3A, columns 1 and 2) and approximately 32% increase in WKY rats (p<0.01, Fig 3B, columns 1 and 2).
Figure 3.
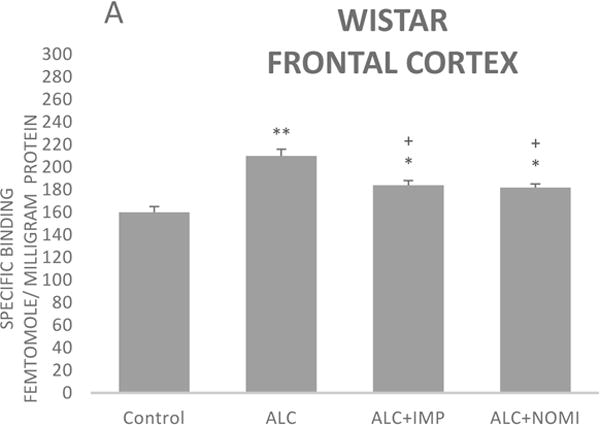
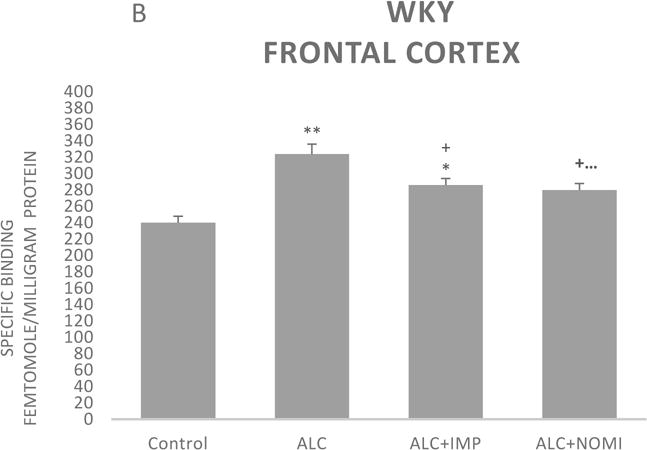
Effects of chronic alcohol, imipramine (IMP) and nomifensine (NOMI) on alpha-2 ARs density (fmole/mg Pr) in the frontal cortex of female Wistar (A) and WKY (B) rats. Values are mean ± SEM (n=7–8). *p<0.05, **p <0.01 compared to control. +p<0.05 compared to alcohol group.
Basal levels of alpha-2 ARs in the hippocampus were similar in Wistar and WKY rats (Figs 4A and 4B, column 1). Alcohol exposure did not affect alpha-2 ARs density in the hippocampus (Figs 4A and 4B, columns 1 and 2).
Figure 4.
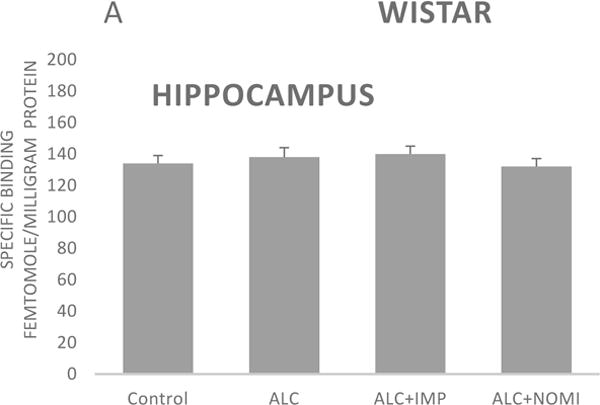
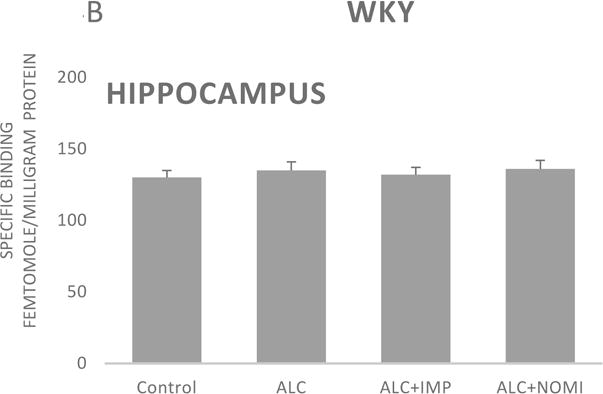
Effects of chronic alcohol, imipramine (IMP) and nomifensine (NOMI) on alpha-2 ARs density (fmole/mg Pr) in the hippocampus of female Wistar (A) and WKY (B) rats. Values are mean ± SEM (n=7–8).
3.1.4 Effects of IMP and NOMI on alcohol withdrawal-induced changes in Alpha-2 AR
Both IMP and NOMI treatment attenuated alcohol withdrawal-induced increases in the alpha-2 ARs density in the frontal cortex of both strains. Thus, daily administration of IMP (10 mg/kg) and NOMI (10 mg/kg), following alcohol exposure, reduced the density of alpha-2 ARs by approximately 50% in both Wistar (Fig 3A columns 3 and 4) and WKY rats (Fig 3B, columns 3 and 4).
There were no effects of any treatment on hippocampal alpha-2 ARs in either strain (Fig 4A and 4B, columns 3 and 4).
A correlation analysis of immobility scores and alpha-2 binding in the frontal cortex resulted in coefficient regression values (r = 8.1–8.4), suggesting a strong correlation between behavioral and neurochemical effects. However, it is important to note that correlation does not imply causation, but only an association.
3.1.5 Kd values
There were no changes in receptor affinities in either region with any treatment in either strain. The kd values were similar in both regions and fluctuated between 0.8 ± 0.09 and 1.0 ± 0.1 nM (mean ± SEM) for all measurements.
4. DISCUSSION
The results of this study confirm a “depressogenic” effect following withdrawal form high alcohol dose in Wistar rats and exacerbation of this behavior in WKY rats, a putative and non-induced animal model of depression (Getachew et al., 2010). Moreover, chronic treatment with the tricyclic antidepressants that are selective in inhibiting the uptake of NE/5HT (e.g., IMP) or NE/DA (e.g., NOMI) reversed these behavioral effects of alcohol in both strains, suggesting possible utility of such drugs in prevention of relapse. Previous studies in rodents have shown that both IMP and NOMI reduce FST immobility counts, reflective of the helplessness (Tejani-Butt et al., 2003; Yamada and Sugimoto 2001). The depressive-like characteristics of WKY rats were associated with an increase in alpha-2 ARs density in the frontal cortex. Alcohol exposure resulted in an increase in alpha-2 ARs in Wistar rats, and a further increase in these receptors in WKY rats. To our knowledge this is the first in-vivo study of alcohol effect on alpha-2 ARs density, which is in agreement with in-vitro studies where increases in these receptors were observed following alcohol exposure in NG108-15 cells (Hu et al 1993). The increases in alpha-2 ARs induced by chronic alcohol administration in our study were attenuated by chronic treatments with IMP and NOMI. Interestingly, Barturen and Garcia-Sevilla (1992) had proposed that sustained stimulation of alpha-2 ARs by endogenous norepinephrine, after inhibition of neuronal uptake, increases their disappearance rate (degradation), leading to a reduction in receptor number. Taken together, a role for alpha-2 ARs in alcohol withdrawal-induced depression and the effectiveness of tricyclic antidepressants is suggested.
The above contention is also supported by a number of studies. Thus, treatment of depression by tricyclic antidepressants (e.g., desipramine) or electroconvulsive shock therapy has been shown to be associated with decreases in the density and sensitivity of central alpha-2 ARs (Barturen and Garcia-Sevilla, 1992; Cohen et al., 1980; Invernizzi and Garattini, 2004; Nutt and Glue, 1991; Pilc and Vetulani, 1982; Smith et al., 1983; Tanaka and Telegdy 2014; Zhang et al.; 2009). These studies suggest that a super-sensitivity of the alpha-2 ARs might exist in patients with endogenous depression and that effective forms of therapy lead to a decrease in the number or down-regulation of central alpha-2 ARs (Cohen et al 1980, Nutt and Glue, 1991; Tanaka and Telegdy, 2014; Zhang et al., 2009). Moreover, pre-synaptic alpha-2 receptor antagonists such as mirtazapine can act as efficacious antidepressant (Airgas et al., 2002). Interestingly, WKY rats which manifest depressive-like characteristics also showed a higher alpha-2 ARs density in the frontal cortex compared to their control, Wistar rats (Fig 3 A, B). Indeed, it had been suggested that the antidepressant effect may be speeded up by blockade of the alpha-2 ARs and that alpha-2 ARs antagonist may improve the therapeutic effect of NE reuptake inhibitors (Invernizzi and Garattini, 2004). Although we did not detect any changes in alpha-2 ARs in the hippocampus, a functional role for these receptors in mood regulation and/or alcohol intake, in at least some sub-regions of the hippocampus, cannot be ruled out by our studies as NE may regulate hippocampal glutamatergic transmission via its interaction with alpha-2 ARs (Boehm, 1999). Moreover, it is of importance to note that other regions (e.g., amygdala and nucleus accumbens) may also play a role in mood regulation. Hence, deciphering the exact circuitry and precise cite of alpha-2 adrenoceptors in mood regulation not only in adult rats, but also in adolescence requires further investigation.
Manipulations of the alpha-2 ARs may also affect alcohol intake. Hence, yohimbine, which also contains alpha-2 ARs antagonism may reinstates alcohol seeking after extinction (Marinelli et al., 2007). On the other hand, alpha-2 AR agonists may reduce alcohol consumption or alcohol-withdrawal symptoms (Opitz, 1990; Muzyk et al., 2011; Rasmussen et al., 2014; Fredriksson et al., 2015; Giovanitti et al., 2015). However, it has also been reported that specific agonists or antagonists of alpha-2 ARs may produce only minor changes in the voluntary alcohol drinking in alcohol-preferring rats (Korpi 1990). Nonetheless, these findings may indicate limitation on use of alpha-2 ARs antagonists as adjunct therapy in alcohol-induced depression. However, careful consideration of the role of specific alpha-2 AR subtypes could overcome these limitations.
The radioligand used in our assay does not differentiate between various alpha-2 AR subtypes. However, it should be noted that in the frontal cortex of the rat more than 90% of the alpha-2 ARs are of 2A type (Wallace et al., 1994). Hence, it may be suggested that alcohol specifically interacts with this receptor subtype in the frontal cortex and that at least part of its depressogenic effects and the efficacy of the antidepressants in reversing alcohol effects may be mediated via these cortical receptors. It is also of relevance to note that yohimbine may interact with other adrenergic as well as non-adrenergic receptors and hence may not be an ideal tool to study alpha 2-adrenoceptor physiology and pharmacology (Haapalinna et al., 1997). A more recent study provides strong evidence that prazosin, which may be acting primarily as an antagonist at post-synaptic alpha-1 receptors can effectively block yohimbine-induced re-instatement (Funk et al., 2016). In addition, it was reported that prazosin prevents increased anxiety behavior that occurs in response to stress during alcohol deprivations (Rasmussen et al., 2016). Hence, further identification of the exact role of specific presynaptic alpha-2 ARs subtypes as well as postsynaptic alpha-1 ARs may lead to better intervention in AUD in general and depression-AUD co-morbidity in particular.
It should be recognized that our findings indicate only partial (approximately 50%) reversal of alcohol withdrawal-induced increases in alpha-2 ARs by IMP and NOMI, whereas the reversal of alcohol withdrawal-induced depressive like characteristics by these drugs were total. In fact, in WKY rats the two drugs reduced the immobility score in the FST to basal levels seen in Wistar rats. Thus, mechanisms other than alpha-2 ARs may be involved in the action of the antidepressants used in this study. Moreover, although the common factor between the two drugs that we used is their inhibition of NE uptake, contribution of 5HT and DA to the antidepressant effects of these drugs cannot be ruled out. It is also of relevance to note that tricyclic-induced antidepressants reduction of alpha-2 ARs may be due to alpha- 2A AR/arrestin recruitment, which can lead to receptor endocytosis and down-regulation of these receptors (Cottingham et al., 2015).
In summary, our findings provide evidence for involvement of cortical alpha-2 ARs in alcohol withdrawal-induced depression as well as exacerbation of the existing depressive behavior. Moreover, effectiveness of tricyclic antidepressants in inhibiting alcohol’s effects may be at least partially mediated through these receptors. Further elucidation of the roles of specific alpha-2 AR subtypes may lead to novel intervention in AUD-depression co-morbidity.
5. CONCLUSION
The results suggest a role for cortical alpha-2 ARs in alcohol withdrawal-induced depression and that selective subtype antagonists of these receptors may be at least of adjunct therapeutic potential in AUD-depression co-morbidity.
Highlights.
Alcohol withdrawal is associated with a depressive-like behavior in rats
Alcohol withdrawal is associated with an increase in cortical alpha2 adrenoceptors
Both imipramine and nomifensine block alcohol withdrawal depressive-like effects
Both imipramine and nomifensine attenuate alcohol withdrawal neurochemical effects
Selective alpha-2 adrenoceptors may have therapeutic potential in alcohol use disorder (AUD)-depression
Acknowledgments
The work was supported by National Institutes of Health and National Institute on Alcohol Abuse and Alcoholism NIH/NIAAA R03AA022479 and NIH/NIAAA P20 AA014643
Role of funding source
Nothing declared
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
All authors contributed significantly to the design, implementation, analysis, and reporting of this research. All authors have read and approved the final manuscript.
Conflict of interest statement
The authors have no conflict of interest to declare concerning the data presented in this report.
References
- Alekhina TA, Klochkov DV, Pal’chikova NA, Kuz’minova OI, Prokudina OI. Synchronization of the estrous cycle against the background of increased excitability in rats selected for catatonic type of reaction. Bull Exp Biol Med. 2015;159:73–6. doi: 10.1007/s10517-015-2893-x. [DOI] [PubMed] [Google Scholar]
- Artigas F, Nutt DJ, Shelton R. Mechanism of action of antidepressants Psychopharmacol. Bull. 2002;36:23–132. [PubMed] [Google Scholar]
- Balsamo DN, Douaihy A, Cornelius JR, Daley DC, Kirisci L, Hyman SM, Salloum IM. Differential impact of depressive and manic mood states on alcohol craving in comorbid bipolar alcoholism: Preliminary findings. Addict Disord Their Treat. 2016;15:107–110. doi: 10.1097/ADT.0000000000000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barturen F, Garcia-Sevilla JA. Long term treatment with desipramine increases the turnover of Alpha2-adrenoceptors in the rat brain. Mol Pharmacol. 1992;42:846–855. [PubMed] [Google Scholar]
- Boehm S. Presynaptic alpha2-adrenoceptors control excitatory, but not inhibitory, transmission at rat hippocampal synapses. J Physiol. 1999;519(Pt. 2):439–49. doi: 10.1111/j.1469-7793.1999.0439m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boschloo L, Vogelzangs N, Smit JH, van den Brink W, Vellman DJ, Beekman AT, Penninx BW. Comorbidity and risk indicators for alcohol use disorders among persons with anxiety and/or depressive disorders: findings from the Netherlands Study of Depression and Anxiety (NESDA) J Affect Disord. 2011;131:233–242. doi: 10.1016/j.jad.2010.12.014. [DOI] [PubMed] [Google Scholar]
- Bradford MM. Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown SA, Schuckit MA. Changes in depression among abstinent alcoholics. J Stud Alcohol. 1988;49:412–7. doi: 10.15288/jsa.1988.49.412. [DOI] [PubMed] [Google Scholar]
- Bravo AJ, Pearson MR, Henson JM. Drinking to cope with depressive symptoms and ruminative thinking: A multiple mediation model among college students. Subst Use Misuse. 2017;52:52–62. doi: 10.1080/10826084.2016.1214151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylund DB. Subtypes of alpha 2-adrenoceptors: Pharmacological and molecular biological evidence converge. Trends Pharmacol Sci. 1988;9:356–361. doi: 10.1016/0165-6147(88)90254-4. [DOI] [PubMed] [Google Scholar]
- Bylund DB. Pharmacological characteristics of alpha-2 adrenergic receptor subtypes. Ann N Y Acad Sci. 1995;763:1–7. doi: 10.1111/j.1749-6632.1995.tb32386.x. [DOI] [PubMed] [Google Scholar]
- Carrier N, Kabbaj M. Sex differences in the antidepressant-like effects of ketamine. Neuropharmacology. 2013;70:27–34. doi: 10.1016/j.neuropharm.2012.12.009. [DOI] [PubMed] [Google Scholar]
- Clarke RW, Harris J. RX 821002 as a tool for physiological investigation of alpha(2)-adrenoceptors. CNS Drug Rev. 2002;8:177–92. doi: 10.1111/j.1527-3458.2002.tb00222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RM, Campbell IC, Cohen MR, Torda T, Pickar D, Siever LJ, Murphy DL. Presynaptic noradrenergic regulation during depression and antidepressant drug treatment. Psychiatry Res. 1980;3:93–105. doi: 10.1016/0165-1781(80)90051-7. [DOI] [PubMed] [Google Scholar]
- Cottingham C, Ferryman CJ, Wang Q. α2 adrenergic receptor trafficking as a therapeutic target in antidepressant drug action. Prog Mol Biol Transl Sci. 2015;132:207–25. doi: 10.1016/bs.pmbts.2015.03.007. [DOI] [PubMed] [Google Scholar]
- Dackis CA, Gold MS, Pottash AL, Sweeney DR. Evaluating depression in alcoholics. Psychiatry Res. 1986;17:105–9. doi: 10.1016/0165-1781(86)90065-x. [DOI] [PubMed] [Google Scholar]
- Dixit AR, Crum RM. Prospective study of depression and the risk of heavy alcohol use in women. Am J Psychiatry. 2000;157:751–8. doi: 10.1176/appi.ajp.157.5.751. [DOI] [PubMed] [Google Scholar]
- Donadon MF, Osório FL. Personality traits and psychiatric comorbidities in alcohol dependence. Braz J Meb Biol Res. 2016;9:e5036. doi: 10.1590/1414-431X20155036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschelli A, Sens J, Herchick S, Thelen C, Pitychoutis PM. Sex differences in the rapid and the sustained antidepressant-like effects of ketamine in stress-naive and “epressed”mice exposed to chronic mild stress. Neuroscience. 2015;290:49–60. doi: 10.1016/j.neuroscience.2015.01.008. [DOI] [PubMed] [Google Scholar]
- Fredriksson I, Jayaram-Lindstrom N, Wirf M, Nylander E, Nystrom E, Jardemark K, Steensland P. Evaluation of guanfacine as a potential medication for alcohol use disorder in long-term drinking rats: Behavioral and electrophysiological findings. Neuropsychopharmacology. 2015;40:1130–1140. doi: 10.1038/npp.2014.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk D, Coen K, Tamadon S, Li Z, Loughlin A, Lê AD. Effects of prazosin and doxazosin on yohimbine-induced reinstatement of alcohol seeking in rats. Psychopharmacology (Berl) 2016;233:2197–207. doi: 10.1007/s00213-016-4273-2. [DOI] [PubMed] [Google Scholar]
- Gadermann AM, Alonso J, Vilagut G, Zaslavsky AM, Kessler RC. Comorbidity and disease burden in the National Comorbidity Survey Replication (NCS-R) Depress Anxiety. 2012;29:797–806. doi: 10.1002/da.21924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getachew B, Hauser SR, Taylor RE, Tizabi Y. Desipramine blocks alcohol induced anxiety- and depressive-like behaviors in two rat strains. Pharmacol Biochem Behav. 2008;91:97–103. doi: 10.1016/j.pbb.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getachew B, Hauser SR, Taylor RE, Tizabi Y. Alcohol-induced depressive-like behavior is associated with cortical norepinephrine reduction. Pharmacol Biochem Behav. 2010;96:395–401. doi: 10.1016/j.pbb.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannitti JA, Jr, Thoms SM, Crawford JJ. Alpha-2 adrenergic receptor agonists: A review of current clinical applications. Anesth Prog. 2015;62:31–38. doi: 10.2344/0003-3006-62.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, Pickering RP, Kaplan K. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004;61:807–816. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- Haapalinna A, Viitamaa T, MacDonald E, Savola JM, Tuomisto L, Virtanen R, Heinonen E. Evaluation of the effects of a specific alpha 2-adrenoceptor antagonist, atipamezole, on alpha 1- and alpha 2-adrenoceptor subtype binding, brain neurochemistry and behaviour in comparison with yohimbine. Naunyn-Schmiedeberg’s Arch Pharmacol. 1997;356:570–82. doi: 10.1007/pl00005092. [DOI] [PubMed] [Google Scholar]
- Hodgins DC, el-Guebaly N, Armstrong S. Prospective and retrospective reports of mood states before relapse to substance use. J Consult Clin Psychol. 1995;63:400–7. doi: 10.1037//0022-006x.63.3.400. [DOI] [PubMed] [Google Scholar]
- Hu G, Querimit LA, Downing LA, Charness ME. Ethanol differentially increases alpha 2-adrenergic and muscarinic acetylcholine receptor gene expression in NG108-15 cells. J Biol Chem. 1993;268:23441–23447. [PubMed] [Google Scholar]
- Invernizzi RW, Garattini S. Role of presynaptic alpha2-adrenoceptors in antidepressant action: recent findings from microdialysis studies. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:819–827. doi: 10.1016/j.pnpbp.2004.05.026. [DOI] [PubMed] [Google Scholar]
- Iovieno N, Tedeschini E, Bentley KH, Evins AE, Papakostas GI. Antidepressants for major depressive disorder and dysthymic disorder in patients with comorbid alcohol use disorders: A meta-analysis of placebo-controlled randomized trials. J Clin Psychiatry. 2011;72:1144–1151. doi: 10.4088/JCP.10m06217. [DOI] [PubMed] [Google Scholar]
- Jane-Llopis E, Matytsina I. Mental health and alcohol, drugs and tobacco: a review of the comorbidity between mental disorders and the use of alcohol, tobacco and illicit drugs. Drug Alcohol Rev. 2006;25:515–536. doi: 10.1080/09595230600944461. [DOI] [PubMed] [Google Scholar]
- Jiao X, Pare WP, Tejani-Butt SM. Alcohol consumption alters dopamine transporter sites in Wistar-Kyoto rat brain. Brain Res. 2006:1073–1074. doi: 10.1016/j.brainres.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Fischman MW. The pharmacology of cocaine related to its abuse. Pharmacol Rev. 1989;41:3–52. [PubMed] [Google Scholar]
- Jung J, Goldstein RB, Grant BF. Association of respondent psychiatric comorbidity with family history of comorbidity: Results from the National Epidemiologic Survey on Alcohol and Related Conditions-III. Compr Psychiatry. 2016;71:49–56. doi: 10.1016/j.comppsych.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalejaiye O, Bhatti BH, Taylor RE, Tizabi Y. Nicotine blocks the depressogenic effects of alcohol: Implications for drinking-smoking co-morbidity. J Drug Alcohol Res. 2013;2:235709. doi: 10.4303/jdar/235709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey I: Lifetime prevalence, chronicity, and recurrence. J Affect Disorders. 1993;29:85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Crum RM, Warner LA, Nelson CB, Schulenberg J, Anthony JC. Lifetime co-occurrence of DSM-IIIR alcohol abuse and dependence with other psychiatric disorders in the National Co-morbidity Survey. Arch Gen Psychiatry. 1997;154:313–321. doi: 10.1001/archpsyc.1997.01830160031005. [DOI] [PubMed] [Google Scholar]
- Kliethermes CL, Cronise K, Crabbe JC. Anxiety-like behavior in mice in two apparatuses during withdrawal from chronic ethanol vapor inhalation. Alcohol Clin Exp Res. 2004;28:1012–1019. doi: 10.1097/01.alc.0000131976.40428.8f. [DOI] [PubMed] [Google Scholar]
- Korpi ER. Effects of alpha 2-adrenergic drugs on the alcohol consumption of alcohol-preferring rats. Pharmacol Toxicol. 1990;66:283–6. doi: 10.1111/j.1600-0773.1990.tb00748.x. [DOI] [PubMed] [Google Scholar]
- Kokras N, Antoniou K, Mikail HG, Kafetzopoulos V, Papadopoulou-Daifoti Z, Dalla C. Forced swim test: What about females? Neuropharmacology. 2015;99:408–21. doi: 10.1016/j.neuropharm.2015.03.016. [DOI] [PubMed] [Google Scholar]
- Lai HM, Cleary M, Sitharthan T, Hun GE. Prevalence of comorbid substance use, anxiety and mood disorders in epidemiological surveys, 1990–2014: A systematic review and meta-analysis. Drug Alcohol Depend. 2015;154:1–13. doi: 10.1016/j.drugalcdep.2015.05.031. [DOI] [PubMed] [Google Scholar]
- Lee S, Schmidt D, Tilders F, Cole M, Smith A, Rivier C. Prolonged exposure to intermittent alcohol vapors blunts hypothalamic responsiveness to immune and non-immune signals. Alcohol Clin Exp Res. 2000;24:110–122. [PubMed] [Google Scholar]
- Marinelli PW, Funk D, Juzytsch W, Harding S, Rice KC, Shaham Y, Lê AD. The CRF1 receptor antagonist antalarmin attenuates yohimbine-induced increases in operant alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology (Berl) 2007;95:345–355. doi: 10.1007/s00213-007-0905-x. [DOI] [PubMed] [Google Scholar]
- McClintock M. Estrous synchrony and its mediation by Airborne chemical communication (Rattus norvegicus) Horm Behav. 1978;10:264–75. doi: 10.1016/0018-506x(78)90071-5. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Gelernter CS. Comorbidity for alcoholism and depression. Psychiatr Clin North Am. 1990;13:613–32. Review. [PubMed] [Google Scholar]
- Muzyk AJ, Fowler JA, Norwood DK, Chilipko A. Role of α2-agonists in the treatment of acute alcohol withdrawal. Ann Pharmacother. 2011;45:649–57. doi: 10.1345/aph.1P575. Review. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. Sex differences in depression. Stanford University Press; Stanford, CA: 1990. [Google Scholar]
- Nutt DJ, Glue P. Imipramine in panic disorder. 2. Effects on α2-adrenoceptor function. J Psychopharmacol. 1991;5:135–41. doi: 10.1177/026988119100500207. [DOI] [PubMed] [Google Scholar]
- Opitz K. The effect of clonidine and related substances on voluntary ethanol consumption in rats. Drug Alcohol Depend. 1990;25:43–48. doi: 10.1016/0376-8716(90)90139-6. [DOI] [PubMed] [Google Scholar]
- O’Rourke MF, Blaxall HS, Iversen LJ, Bylund DB. Characterization of [3H]RX821002 binding to alpha-2 adrenergic receptor subtypes. J Pharmacol Exp Ther. 1994;268:1362–1367. [PubMed] [Google Scholar]
- Overstreet DH, Friedman E, Mathé AA, Yadid G. The flinders sensitive line rat: A selectively bred putative animal model of depression. Neurosci Biobehav Rev. 2005;29:739–59. doi: 10.1016/j.neubiorev.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Paré WP, Redei E. Sex differences and stress response of WKY rats. Physiol Behav. 1993;54:1179–1185. doi: 10.1016/0031-9384(93)90345-g. [DOI] [PubMed] [Google Scholar]
- Paré AM, Paré WP, Kluczynski J. Negative affect and voluntary alcohol consumption in Wistar-Kyoto (WKY) and Sprague-Dawley rats. Physiol Behav. 1999;67:219–25. doi: 10.1016/s0031-9384(99)00054-2. [DOI] [PubMed] [Google Scholar]
- Porsolt D, Bertin A, Jalfre M. Behavioral despair in mice: A primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229:327–336. [PubMed] [Google Scholar]
- Pilc A, Vetulani J. Attenuation by chronic imipramine treatment of [3H] clonidine binding to cortical membranes and of clonidine-induced hypothermia: the influence of central chemosympathectomy. Brain Res. 1992;238:499–504. doi: 10.1016/0006-8993(82)90131-7. [DOI] [PubMed] [Google Scholar]
- Rasmussen DD, Alexander L, Malone J, Federoff D, Froehlich JC. The α2-adrenergic receptor agonist, clonidine, reduces alcohol drinking in alcohol preferring (P) rats. Alcohol. 2014;48:543–9. doi: 10.1016/j.alcohol.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezvani AH, Parsian A, Overstreet DH. The fawn-hooded (FH/Wjd) rat: A genetic animal model of comorbid depression and alcoholism. Psychiatr Genet. 2002;12:1–16. doi: 10.1097/00041444-200203000-00001. Review. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Overstreet DH, Cleves M, Parsian A. Further genetic characterization of the fawn-hooded (FH/Wjd) rat, an animal model of comorbid depression and alcoholism. Psychiatr Genet. 2007;17(2):77–83. doi: 10.1097/YPG.0b013e328012d7c3. [DOI] [PubMed] [Google Scholar]
- Rincon-Hoyos HG, Castillo A, Prada SI. Alcohol use disorders and psychiatric diseases in Colombia. Colomb Med (Cali) 2016;47:31–7. [PMC free article] [PubMed] [Google Scholar]
- Rodgers B, Korten AE, Jorm AF, Christensen H, Henderson S, Jacomb PA. Risk factors for depression and anxiety in abstainers, moderate drinkers and heavy drinkers. Addiction. 2000;95:1833–45. doi: 10.1046/j.1360-0443.2000.9512183312.x. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Tipp JE, Bergman M, Reich W, Hesselbrock VM, Smith TL. Comparison of induced and independent major depressive disorders in 2,945 alcoholics. Am J Psychiatry. 1997;54:948–957. doi: 10.1176/ajp.154.7.948. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Comorbidity between substance use disorders and psychiatric conditions. Addiction. 2006;101:76–88. doi: 10.1111/j.1360-0443.2006.01592.x. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Markou A, Cole M, Koob GF. Decreased brain reward produced by ethanol withdrawal. Proc Natl Acad Sci USA. 1995;92:5880–4. doi: 10.1073/pnas.92.13.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AP, et al. The effects and after effects of the α2-adrenoceptor antagonist idazoxan on mood, memory and attention in normal volunteers. J Psychopharmacol. 1992;6:376–381. doi: 10.1177/026988119200600306. [DOI] [PubMed] [Google Scholar]
- Spak L, Spak F, Allebeck P. Alcoholism and depression in a Swedish female population: Co-morbidity and risk factors. Acta Psychiatr Scand. 2000;102:44–51. doi: 10.1034/j.1600-0447.2000.102001044.x. [DOI] [PubMed] [Google Scholar]
- Spornitz UM, Socin CD, David AA. Estrous stage determination in rats by means of scanning electron microscopic images of uterine surface epithelium. Anat Rec. 1999;254:116–126. doi: 10.1002/(SICI)1097-0185(19990101)254:1<116::AID-AR15>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Stillings MR, England CD, Welbourn AP, Smith CF. Effect of methoxy substitution on the adrenergic activity of three structurally related alpha 2-adrenoreceptor antagonists. J Med Chem. 1986;29:1780–1783. doi: 10.1021/jm00159a037. [DOI] [PubMed] [Google Scholar]
- Swendsen JD, Merikangas KR. The comorbidity of depression and substance use disorders. Clin Psychol Rev. 2000;20:173–189. doi: 10.1016/s0272-7358(99)00026-4. [DOI] [PubMed] [Google Scholar]
- Syha K, Schraven E. Effects of a chronic administration of the new antidepressant pirlindole on biogenic amine receptors in rat prefrontal cortex and hippocampus. Comparison with desipramine, nomifensine and zimelidine. Arzneimittelforschung. 1990;40:843–7. [PubMed] [Google Scholar]
- Tanaka M, Telegdy G. Neurotransmissions of antidepressant-like effects of neuromedin U-23 in mice. Behav Brain Res. 2014;259:196–9. doi: 10.1016/j.bbr.2013.11.005. [DOI] [PubMed] [Google Scholar]
- Tejani-Butt S, Kluczynski J, Paré WP. Strain-dependent modification of behavior following antidepressant treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:7–14. doi: 10.1016/s0278-5846(02)00308-1. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Overstreet DH, Rezvani AH, Louis VA, Clark E, Jr, Janowsky DS, Kling MA. Antidepressant effects of nicotine in an animal model of depression. Psychopharmacology (Berl) 1999;142:193–9. doi: 10.1007/s002130050879. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Rezvani AH, Russell LT, Tyler KY, Overstreet DH. Depressive characteristics of FSL rats: involvement of central nicotinic receptors. Pharmacol Biochem Behav. 2000;66:73–7. doi: 10.1016/s0091-3057(00)00236-7. [DOI] [PubMed] [Google Scholar]
- U’Prichard DC, Bechtel WD, Rouot BM, Snyder SH. Multiple apparent alpha-noradrenergic receptor binding sites in rat brain: effect of 6-hydroxydopamine. Mol Pharmacol. 1979;16:47–60. [PubMed] [Google Scholar]
- Viglinskaya IV, Overstreet DH, Kashevskaya OP, Badishtov BA, Kampov-Polevoy AB, Seredenin SB, Halikas JA. To drink or not to drink: tests of anxiety and immobility in alcohol-preferring and alcohol-nonpreferring rat strains. Physiol Behav. 1995;57:937–41. doi: 10.1016/0031-9384(94)00368-f. [DOI] [PubMed] [Google Scholar]
- Wallace DR, Muskardin DT, Zahniser NR. Pharmacological characterization of [3H]idazoxan, [3H]RX821002 and p-[125I]iodoclonidine binding to alpha 2-adrenoceptors in rat cerebral cortical membranes. Eur J Pharmacol. 1994;258:67–76. doi: 10.1016/0014-2999(94)90058-2. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Bland RC, Canino GJ, Faravelli C, Greenwald S, Hwu HG, Joyce PR, Karam EG, Lee CK, Lellouch J, Lépine JP, Newman SC, Rubio-Stipec M, Wells JE, Wickramarante PJ, Wittchen H, Yeh EK. Cross-national epidemiology of major depression and bipolar disorder. JAMA. 1996;276:293–299. [PubMed] [Google Scholar]
- Yamada J, Sugimoto Y. Effects of 5-HT(2) receptor antagonists on the anti-immobility effects of imipramine in the forced swimming test with mice. Eur J Pharmacol. 2001;427:221–5. doi: 10.1016/s0014-2999(01)01240-7. [DOI] [PubMed] [Google Scholar]
- Yang Y, Wei Li W, Zhu B, Liu YB, Wang H, Wang Z. Sex differences in antidepressant-like effect of chronic repetitive transcranial magnetic stimulation in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:735–740. doi: 10.1016/j.pnpbp.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Yaroslavsky I, Tejani-Butt SM. Voluntary alcohol consumption alters stress-induced changes in dopamine-2 receptor binding in Wistar-Kyoto rat brain. Pharmacol Biochem Behav. 2010;94:471–476. doi: 10.1016/j.pbb.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HT, Whisler LR, Huang Y, Xiang Y, O’Donnell JM. Postsynaptic α-2 Adrenergic receptors are critical for the antidepressant-like effects of desipramine on behavior. Neuropsychopharmacology. 2009;34:1067–1077. doi: 10.1038/npp.2008.184. [DOI] [PMC free article] [PubMed] [Google Scholar]


