Abstract
Environmental stimuli that co-occur with tobacco use come to evoke drug-related conditioned responses (CRs) that appear involved in continued use of nicotine-containing products. In rats, nicotine can serve as a conditional stimulus (CS) for non-drug unconditioned stimuli (USs), prompting the question of whether the nicotine CS can compete with, or overshadow, a non-drug environmental stimulus for control of a CR. In Experiment 1, male Sprague-Dawley rats were assigned to a group [0, 0.01, 0.03, 0.045, or 0.06 mg nicotine (base)/kg/infusion]. During each session, there were 10 intravenous infusions followed by a 30-second houselight to form a compound CS. At light offset there was 4-second access to sucrose. For Experiment 2, groups were nicotine (0.03 mg/kg/infusion) + light compound paired, nicotine + light compound unpaired, nicotine paired and light unpaired, and nicotine unpaired and light paired. Paired stimuli were presented with sucrose similar to Experiment 1. Unpaired stimuli were temporally separated from sucrose. Following acquisition, tests of nicotine and light alone were conducted by intermixing non-reinforced trails into training sessions. Nicotine dose-dependently overshadowed the light CS as shown by reduced light control of conditioned responding with higher doses. The nicotine, light, and nicotine + light compound had to be paired with sucrose to evoke a CR. These results demonstrate nicotine overshadows an exteroceptive visual stimulus. Because exteroceptive stimuli are often the focus of cue-exposure therapy, such competition may help begin to explain the marginal effectiveness of these therapies.
Keywords: Addiction, nicotine, overshadowing, Pavlovian conditioning, smoking, treatment
INTRODUCTION
Possible treatment approaches for tobacco abuse currently range from nicotine replacement and other pharmacotherapeutics to a wide variety of behavioral interventions (Fiore et al. 2008). Among the behavioral interventions are individual, group and telephone counseling that may focus on topics such as problem solving, skills training or contingency management (Fiore et al. 2008). One behavioral method uses repeated cue exposure to extinguish (reduce) cue-induced reported craving and/or urges. In that therapy, nicotine is viewed as an unconditioned stimulus (US). That is, urges for tobacco are often elicited by other stimuli (e.g. smell of smoke, pack of cigarettes, etc.) that have come to be associated with the nervous system effects of nicotine (Pavlov 1927; Poulos, Hinson & Siegel 1981; Conklin & Tiffany 2001; Shiffman et al. 2002; Bevins & Palmatier 2004; Conklin 2006). According to conditioning theory, repeated exposure to these conditional stimuli (CSs) without the nicotine US should decrease the drug-related conditioned responses (CRs), thus decreasing the likelihood of relapse (see Pavlov 1927; Marlatt 1990). Behavioral tobacco cessation therapies utilize cue exposure to help reduce the occurrence of drug-related CRs in the presence of tobacco-related stimuli (see Conklin & Tiffany 2002; Conklin 2006). However, these therapies are only marginally effective (Niaura et al. 1999; Monti & MacKillop 2007), suggesting that other factors also contribute to tobacco abuse.
Along with its widespread US effects, nicotine has other stimulus effects that likely contribute to the tenacity of tobacco addiction (cf. Bevins 2009). Of particular interest in the present report is the recent research in rats showing that nicotine serves as a CS for other appetitive USs (e.g. Besheer et al. 2004; Murray & Bevins 2007a,b, 2009; see Discussion). That is, when nicotine is differentially paired with intermittent sucrose availability, the nicotine state comes to control anticipatory approach to the sucrose receptacle (i.e. goal-tracking CR; Farwell & Ayres 1979). Although there has only been a single study explicitly investigating the CS effects of nicotine in humans (Clements et al. 1996), this learning, and related associative processes, likely contributes to development of nicotine dependence as well as to high relapse rates (Alessi et al. 2002; Bevins & Palmatier 2004).
During tobacco use nicotine is experienced as a part of a multimodal compound stimulus composed of exteroceptive environmental stimuli, as well as the interoceptive stimulus effects of the drug. These stimuli may compete with each other for control of conditioned responding. The purpose of the present study is to begin to examine overshadowing of an exteroceptive stimulus by nicotine. Overshadowing is a form of cue competition in which two distinct stimuli are presented together as a compound stimulus controlling behavior (Pavlov 1927; Miles & Jenkins 1973). Following compound training, the stimulus elements of the compound are evaluated separately for control of responding (e.g. Järbe & Johansson 1984; Duncan 1986). The control each element has of responding presumably reflects the salience (perceptibility) of that element relative to the other element of the compound stimulus (Pavlov 1927; Mackintosh 1976). Further, compared with when a stimulus is trained alone, a reduction in responding evoked by that stimulus because of its compounding with the other has also been viewed as overshadowing (e.g. Matzel, Schachtman & Miller 1985; Mariathasan & Stolerman 1993; White & Stolerman 1996; Stout et al. 2003).
Because cue-exposure therapies target associations formed between exteroceptive stimuli and the effects of tobacco consumption (e.g. Brandon et al. 1995; Waters et al. 2004; Conklin 2006), it is of interest to determine whether the CS effects of nicotine can compete with an exteroceptive stimulus for control of an appetitive CR. Such competition could result in the exteroceptive stimuli controlling less of the CR than if there was no competition. Nicotine as part of the tobacco stimulus may therefore adversely impact the efficacy of therapy because the stimulus extinguished as part of the therapy is controlling a weaker CR than previously assumed.
There is some research on cue competition and overshadowing involving an interoceptive drug stimulus. For instance, early interoceptive effects of morphine administration can serve as a CS for later morphine-evoked effects (e.g. Sokolowska, Siegel & Kim 2002) and can overshadow an exteroceptive environmental CS (Kim, Siegel & Patenall 1999). In that experiment, rats were given IV morphine either gradually over 25–30 minutes or quickly over 14–17 seconds before a high dose of morphine was administered in a specific context. Both groups showed tolerance to the analgesic effects of the morphine with repeated exposure. However, rats that received the slow drug onset as a CS for a higher drug dose retained more of the tolerance CR than rats that received a fast drug onset as a CS when the external environment was switched at testing. These results were taken as evidence that the smaller drug effect presumably experienced with a long drug administration, when given before a larger drug onset, can overshadow an exteroceptive context for control of the CR.
To our knowledge the only two published reports on overshadowing with nicotine involve a two-lever operant drug discrimination task in which nicotine is an element of a compound stimulus composed of two drugs (Mariathasan & Stolerman 1993; White & Stolerman 1996). In those studies, rats were trained with a mixture of nicotine and midazolam as a discriminative stimulus. They found that control of nicotine-appropriate responding was overshadowed compared with rats trained with nicotine alone. However, there are no published reports examining nicotine overshadowing of an exteroceptive stimulus. Given the importance of associative learning processes involving exteroceptive cues in nicotine dependence and tobacco use (see Brandon et al. 1995; Conklin & Tiffany 2002; Bevins & Palmatier 2004), the current set of experiments examined competition between the nicotine CS and a visual CS for control of behavior.
GENERAL METHODS
Subjects
Eighty-three male Sprague-Dawley rats (345 ± 16 g at surgery) from Harlan (Indianapolis, IN, USA) were housed individually in clear 48.3 × 26.7 × 20.3 cm (l × w × h) polycarbonate cages lined with wood shavings. Water was continuously available in the home cage; access to chow (Harlan Teklad Rodent Diet) was restricted as described later. The colony was temperature and humidity controlled. Sessions were conducted during the light portion of a 12 hour light: dark cycle. Protocols were approved by the University of Nebraska-Lincoln Animal Care and Use Committee and followed the ‘Guide for the Care and Use of Laboratory Animals’ (National Research Council 1996).
Apparatus
Twenty conditioning chambers (ENV-008CT; Medical Associates, Inc., Georgia, VT, USA) measuring 30.5 × 24.1 × 21.0 cm (l × w × h) were used. Each chamber was enclosed in a light and sound attenuating cubicle with a fan providing airflow and masking noise. A houselight with two bulbs (28 V, 100 mA) was mounted on the back wall of the cubicle. It was centered side-to-side, 5 cm below the ceiling and 23.5 cm above the top of the conditioning chamber. Chamber sidewalls were aluminum; the ceiling, front, and back walls were clear polycarbonate. Chambers were equipped with a recessed receptacle (5.2 × 5.2 × 3.8 cm; l × w × d) into which a dipper raised 0.1-ml of 26% sucrose solution (w/v). An infrared emitter/detector unit, 1.2 cm into the receptacle and 3 cm from the floor, monitored head entries. Each chamber contained a spring leash attached to a balanced metal arm with a swivel. Tygon® tubing (AAQ04103; VWR, West Chester, PA) extended through the leash from a 5-ml syringe mounted on a syringe pump (Medical-Associates, PMH-100VS) located outside each cubical. The other end of the tubing was secured to the catheter (see Surgical Procedures). A personal computer with Medical Associates interface and software (Medical-PC for Windows, version IV) controlled infusions and sucrose deliveries and recorded dipper entries.
Drugs
(−)-Nicotine hydrogen tartrate from Sigma (St. Louis, MO, USA) was dissolved in 0.9% sterile saline and adjusted to a pH of 7.0 ± 0.2 using a dilute NaOH solution. Doses are reported in the base form. Infusions were given over 1 second at a volume of 35.7 μl.
Preliminary training
Rats were handled for at least 3 minutes per day for 3 days. Food was removed after handling on the last day. Dipper training began the following day in order to establish a high baseline of responding. Fifty-min sessions were conducted on 3 consecutive days with each session starting on a rat’s first dipper entry. The probability of receiving sucrose decreased from 0.167 to 0.05 per 60 seconds over the three sessions (approximately 2.5–0.75 sucrose deliveries per minute). After completing the first two sessions, rats received 20 g of food; following the third session, free access to food was returned.
Surgical procedures
Surgical implantation of catheters occurred within 2 days of the last preliminary training session. Rats were anesthetized with xylazine hydrochloride (20 mg/ml, 1 ml/kg, IP) and ketamine hydrochloride (100 mg/ml, 0.6 ml/kg, IP) purchased from Midwest Veterinary Supply (Des Moines, IA, USA). One end of a silastic catheter (CamCaths© IVSA28, Ely, Cambridgeshire, UK) was implanted into the external left jugular vein. The other end was positioned under the skin so that it exited just below the scapulae. The catheter was able to be accessed by a metal cannula to which the Tygon tubing was secured. Buprenorphine hydrochloride (0.1 mg/kg, SC) was given immediately following surgery. For the evening and full day following surgery, buprenorphine (0.5 mg/kg) was available in the drinking water to manage post-surgical pain. The catheter was flushed twice a day for the duration of the experiment with 0.2 ml of sterile saline mixed with heparin (30 Units/ml; Midwest Veterinary Supply) except for the first 5 post-surgical flushes in which 0.1 ml of sterile heparinized saline was mixed with streptokinase (c. 8000 Units/ml; Sigma). Rats were allowed 5–6 days of recovery in their home cage with free access to food before the start of the experiments. Catheter patency was assessed at predetermined points within the experiment with a 0.05 ml xylazine infusion. This concentration produces clear locomotor ataxia within 5 seconds (cf. Bevins 2005; Reichel et al. 2009). Only rats with patent catheters were included in analyses.
PROCEDURES
Experiment 1: Dose-dependence of nicotine overshadowing a light CS
Training
Rats were assigned to a solution (0, 0.01, 0.03, 0.045, or 0.06 mg/kg nicotine) irrespective of preliminary training performance (n = 9 for 0 mg/kg; n = 10 for all others). During 2-hour sessions, they received 10 infusions of their assigned solution. Each 1-second infusion was followed by the houselight, which remained on for 30 seconds. The offset of the houselight coincided with 4-second access to sucrose. Therefore, the 30-second nicotine/light presentation was conceptualized as the compound CS paired with the sucrose US; the 0 mg/ kg + light group served as a benchmark for the light CS. The average time to the first infusion was 11 minutes with a range of 8–14 minutes; the average time between infusions was also 11 minutes with a range of 8–14 minutes. Training continued for 10 sessions.
Testing
Following training, rats were tested to determine the relative control of conditioned responding by the nicotine and light elements of the compound CS. Each test was conducted across two sessions. In the test session, three test trials (i.e. no sucrose following presentation of the stimulus of interest) were intermixed among seven training trials. The order of testing each element (i.e. nicotine and light) was counterbalanced such that approximately half of the rats in a given group were tested with the light in the first session and with nicotine in the second session. Test order was reversed for the remaining rats. The first set of test sessions were followed by four more training sessions and a second set of test sessions.
Experiment 2: Assessing non-associative explanations for nicotine overshadowing
Training
This experiment was conducted to provide additional control groups for a single effective drug dose. Rats were assigned to a group (N−/L+, N+/L−, NL+, or NL−) irrespective of preliminary training performance (n = 8 for N−/L+ and NL−; n = 9 for N+/L− and NL+). For the group names, N denotes nicotine and L denotes light. The lowest dose of nicotine that evoked equivalent responding between nicotine and the light in Experiment 1 (0.03 mg/kg) was used in all groups. The/indicates a temporal separation of element presentations during training. The + indicates which stimulus or stimuli were paired with sucrose, and the − indicates the stimulus or stimuli were unpaired with sucrose. Groups with paired stimuli (i.e. N−/L+, N+/L−, NL+) were trained as described in Experiment 1. For groups whose training included unpaired stimulus presentations (i.e. N−/L+, N+/L−, NL−), there were at least 4 minutes between any two stimuli, including any CS and US. Groups that had a paired and unpaired stimulus (i.e. N−/L+, N+/L−) received 10 paired and 10 unpaired presentations. Each CS-US presentation was a mean of 11 minutes apart as in Experiment 1. The unpaired stimulus was presented at least 4 minutes from any paired CS-US presentation. Training continued for 10 sessions.
Testing
Testing and continued training was conducted as described in Experiment 1.
Dependent measures
Conditioned responding as measured by infrared beam breaks within the sucrose receptacle (i.e. dipper entries) during each CS presentation was converted to an elevation score by subtracting the number of dipper entries occurring in the 30 seconds before the CS from the number of dipper entries that occur during the 30-second CS period. A positive elevation score indicates more dipper entries during the CS relative to the same interval immediately before CS onset. The elevation score is a widely used measure of conditioned responding in Pavlovian conditioning literature that accounts for individual difference in baseline responding (e.g. Brooks & Bouton 1994; Simon & Setlow 2006; Murray et al. 2007) and has been used recently in a study examining IV nicotine as CS (Murray & Bevins 2009). In order to compare responding to the elements across the groups of Experiment 1, we converted the data from the element tests into a response ratio. That is, responding to each element was divided by the sum of responding on both elements. In Experiment 2, we analyzed elevation scores rather than response ratio. Hypothetically, there should be no responding for the elements in the NL− group, so normalization would create the appearance of effects where none existed.
Data analyses
Conditioned responding during acquisition, testing, and maintenance between tests was analyzed using two-way mixed analyses of variance with Dose or Group as the between-subjects factor and Session or Element as the within-subjects factor for each training and testing segment. Significant interactions were followed by pairwise comparisons using Fisher’s protected least significant difference (LSD) tests. Statistical significance was declared using P < 0.05 for all tests.
RESULTS
Experiment 1: Dose-dependence of nicotine overshadowing a light CS
Training
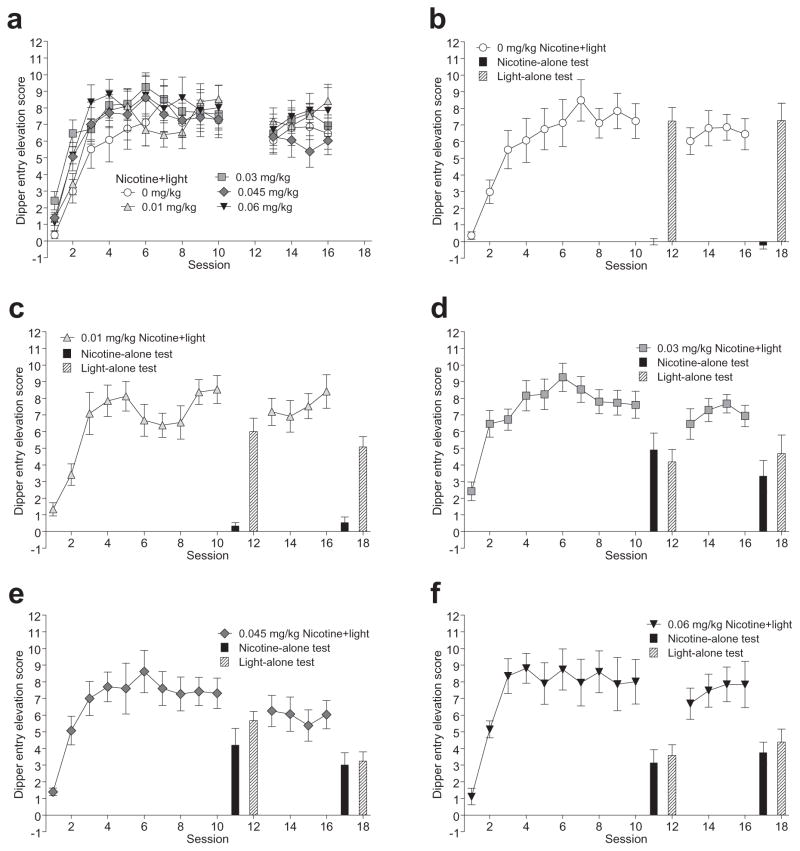
Figure 1 shows that rats in each group readily acquired the CR at a similar rate and to a similar level using the mean elevation scores of each session. The main effect of Session, F(9396) = 43.63, P < 0.001, MSE = 5.16, indicated that rats had higher elevation scores on all sessions compared with the first session and that there was no significant variation after session three, LSDminimum mean difference (mmd) = 0.899. There was no effect of Dose or Dose–Session interaction, Fs < 1. After the initial 10-session training, there was a set of test sessions (see later) followed by four more training sessions. During those four training sessions, rats retained a stable CR. There was no main effect of Dose or Session, Fs ≤ 1.54, Ps ≥ .201, or Dose–Session interaction, F < 1.
Figure 1.
Acquisition and maintenance of compound stimulus training. Mean conditioned responding [±1 standard error of the mean (SEM)] for each training session in all groups is shown (a). Conditioned responding in acquisition and maintenance phases of compound training with results of the element test sessions (+1 SEM) are shown for the individual training groups (b–f ).The order of element test presentation is for display purposes only; they were conducted in a counterbalanced manner (see Procedures). Significant results are described in the text
Testing
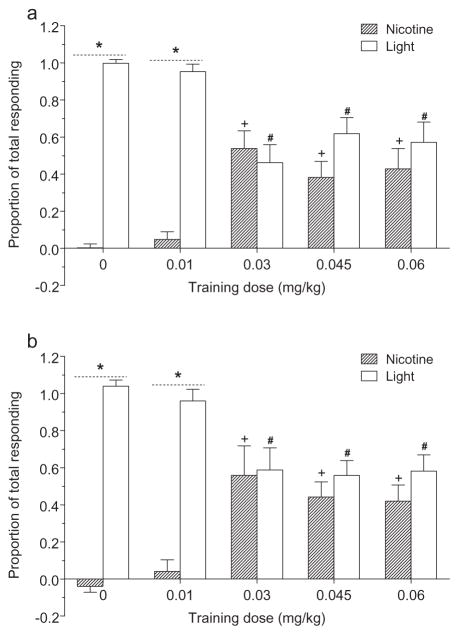
Figure 2 shows the response ratio for each element during testing (elevation scores are shown in Fig. 1). In the first element tests (Fig. 2a), there was a main effect of Element, F(1,44) = 38.07, P < 0.001, MSE = 4.74, with a greater proportion of responding on the light tests than on the nicotine tests. The ratio of responding on the light and nicotine trials varied as a function of nicotine training dose. This impression was supported by a significant Group–Element interaction, F(4,44) = 8.90, P < 0.001, MSE = 1.12. The 0 and 0.01 mg/kg nicotine groups had a greater proportion of responding on the light element than on the nicotine element, LSDmmd = 0.321. In the 0.03, 0.045, and 0.06 mg/kg groups, light and nicotine elements controlled an equivalent CR. Further, these groups had a greater proportion of nicotine-evoked responding and a lower proportion of light-evoked responding than that in the 0 mg/kg group. This data pattern indicates the CS effects of 0.03, 0.045, and 0.06 mg/kg nicotine overshadowed the light stimulus.
Figure 2.
Tests 1 and 2 (a and b, respectively) of the proportion of total elevation scores (+1 SEM) for responding on the nicotine and light test trials for each of the five groups are shown. * denotes significant difference between proportion of responding on the nicotine and light elements within the group. + denotes a significantly higher proportion of nicotine-evoked responding compared with the 0 mg/kg group. # denotes a significantly lower proportion of light-evoked responding compared with the 0 mg/kg group
The same pattern of responding was found in the second element tests (Fig. 2b). There was a significant main effect of Element, F(1,43) = 41.21, P < 0.001, MSE = 5.15, and a significant Group–Element interaction, F(4,43) = 9.15, P < 0.001, MSE = 1.14. The pattern of responding was the same as test 1, LSDmmd = 0.326.
Experiment 2: Assessing non-associative explanations for nicotine overshadowing
Training
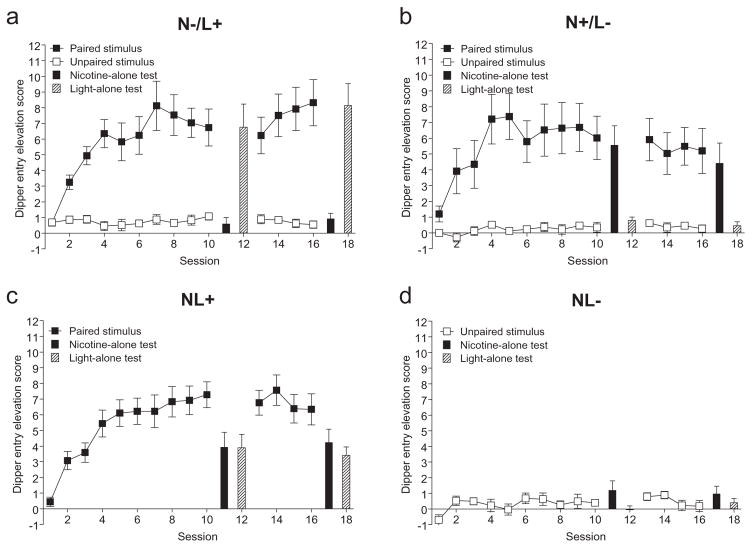
Conditioned responding appeared to develop to the paired stimulus at a similar rate and to a similar level regardless of training group (see Fig. 3). There were only minimal changes in responding to unpaired stimuli. For responding to paired stimuli in the first ten training sessions, there was no effect of Group or Group–Session interaction, Fs < 1. There was a main effect of Session, F(9207) = 26.37, P < 0.001, MSE = 4.13. Examining the marginal means of training sessions showed significant increases in responding until session four, LSDmmd = 1.116. For responding to unpaired stimuli, there was no main effect of Group, F(2,22) = 2.21, P = 0.134, or Group–Session interaction, F(18,198) = 1.49, P = 0.096. There was a main effect of Session, F(9198) = 2.42, P = 0.012, MSE = 0.397. Responding slightly increased across sessions, LSDmmd = 0.352. During the four training sessions following the first set of test sessions, responding remained stable to the paired stimuli, showing no main effects of Group or Session, Fs < 1, or Group–Session interaction, F(6,69) = 1.88, P = 0.097. For responding to the unpaired stimuli, there was no main effect of Group or Group–Session interaction, Fs < 1. However, there was a gradual decrease in dipper entries across the four sessions resulting in a main effect of Session, F(3,66) = 3.77, P = 0.015, MSE = 0.279, LSDmmd = 0.298.
Figure 3.
Acquisition and maintenance of stimulus training. Mean elevation scores (±1 standard error of the mean) of acquisition and element tests for the four training groups in Experiment 4 (a, b, c and d display the N−/L+, N+/L−, NL+, and NL− groups, respectively) are shown. The order of element test presentation is for display purposes only; they were conducted in a counterbalanced manner (see Procedures). Significant effects are described in the text
Testing
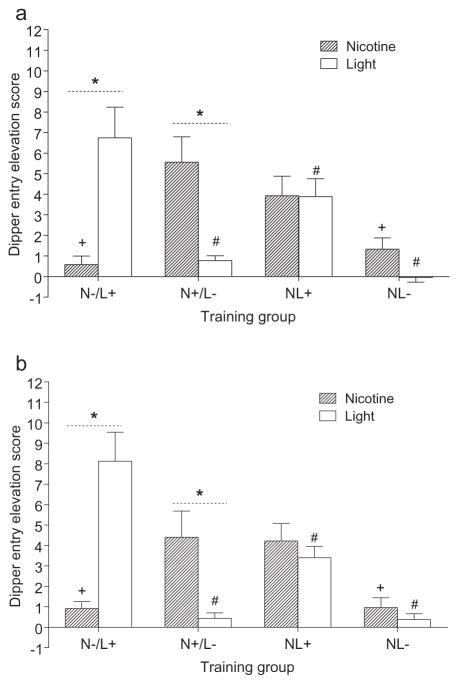
Figure 4 shows the mean dipper entry elevation scores for element testing in each group (also incorporated in Fig. 3). In the first element tests (Fig. 4a), there was no effect of Element, F < 1, but there was a main effect of Group, F(3,30), P = 0.002, MSE = 6.38, with the NL−group having lower response levels than all other groups, LSDmmd = 2.376. The significant Group–Element interaction, F(3,30) = 12.29, P < 0.001, MSE = 7.11, reflects differences between the nicotine and light elements in the N−/L+ and N+/L−groups, LSDmmd = 2.64. Additionally, light-evoked responding was higher in the N−/L+ group than in all other groups; nicotine-evoked responding was higher in the N+/L− group than in the N−/L+ and NL−groups. Unpaired stimuli were all statistically equivalent to zero.
Figure 4.
Tests 1 and 2 (a and b, respectively) of elevation scores (+1 standard error of the mean) for the nicotine and light test trials for each of the four groups are shown. * denotes significant difference between responding on the nicotine and light elements within the group. + denotes significantly lower nicotine-evoked responding compared with the N+/L− group. # denotes significantly lower light-evoked responding compared with the N−/L+ group
The findings were similar in the second element tests (Fig. 4b). There was no effect of Element, F < 1. The main effect of Group, F(3,30) = 8.43, P < 0.001, MSE = 5.57, indicated the NL− group had lower responding than the N−/L+ and NL+ groups, LSDmmd = 2.337. The Group–Element interaction, F(3,30) = 16.93, P < 0.001, MSE = 5.541, again suggested the only differences between light and nicotine elements occurred in the N−/L+ and N+/L− groups, LSDmmd = 2.337. Responding to the light element was higher in the N−/L+ group than all other groups; the N+/L− and NL+ groups responded higher on the nicotine element than the N−/L+ and NL− groups.
DISCUSSION
Previous research examining cue competition with nicotine has been limited. As described in the Introduction, there have been two cue-competition studies showing that nicotine can be overshadowed by midazolam in a two-lever drug discrimination task (Mariathasan & Stolerman 1993; White & Stolerman 1996). The present findings extend our understanding of the stimulus effects of nicotine in a couple important ways. For instance, this study shows that nicotine can overshadow a visual stimulus. Previously, overshadowing of an exteroceptive stimulus by an interoceptive stimulus has been shown with morphine (e.g, Krimmer et al. 1982) and with pentobarbital (e.g. Järbe & Johansson 1984). In one study, rats were trained with saline or pentobarbital consistently paired with either a lit or dark T-maze apparatus. The combination of the drug and visual cue indicated which turn would allow escape from shock. When each element was evaluated for control of the response, the group trained with the lower drug dose relied more heavily on the visual cue to indicate appropriate responding than the group trained with the higher drug dose (Järbe & Johansson 1984). Further, overshadowing of the light by nicotine may be particularly relevant given the importance of associative processes and nicotine-related stimuli in maintaining tobacco use (see Poulos et al. 1981; Niaura et al. 1988; Payne et al. 1991; Brandon et al. 1995; Drummond et al. 1995; Conklin & Tiffany 2001; Shiffman et al. 2002; Bevins & Palmatier 2004; Conklin 2006) and will be discussed below.
For theoretical and empirical reasons we have chosen to discuss nicotine using the terminology of the Pavlovian conditioning field (cf. Bevins & Palmatier 2004; Bevins 2009). Thus, we conceptualize nicotine in this discriminated goal-tracking task as a CS. In this way, we focus on the stimulus-reinforcer relations which have not received nearly the same attention in the study of drug states and addiction as the response-reinforcer relation. This is not meant to imply that such response-reinforcer relations are not or could not be important in this task. A head entry into the dipper must occur to retrieve the sucrose even though the actual programmed occurrence of the sucrose is done irrespective of ongoing behavior in this task [see Boakes (1977), Farwell & Ayres (1979) and Jenkins (1977) for a more detailed discussion of these issues]. Regardless of perspective, we have found evidence of neurochemical distinctions between the nicotine stimulus administered subcutaneously in the discriminated goal-tracking task when compared with previous publications of the nicotine stimulus in a two-lever, drug-discrimination task suggesting that further research regarding this distinction across tasks is warranted (Murray & Bevins 2007a; Murray et al. 2009).
In Experiment 1, overshadowing of the light by nicotine was evidenced by the higher training doses significantly reducing the proportion of light-evoked conditioned responding compared with the saline group. Notably, we have shown that 0.01 mg/kg nicotine can acquire control of a CR within 10 training sessions when not part of a nicotine-light compound CS (Bevins 2009), indicating this low dose can function as a CS. In the current experiment therefore, the 0.01 mg/kg nicotine was likely overshadowed by light.
The purpose of Experiment 2 was to provide a nicotine-alone control not evaluated in Experiment 1 and to control for total drug exposure across all groups. We only used the lowest effective dose to minimize the non-specific effects of the drug while maintaining a functional equivalence with the light under the current experimental conditions (0.03 mg/kg/infusion). That experiment found that stimuli had to be paired with the sucrose US in order to exhibit control of responding during element testing. This outcome confirms that contiguity with sucrose is necessary for the nicotine to evoke a CR (cf. Murray & Bevins 2009) and extends the necessity to the light CS. Further, by presenting nicotine unpaired in the N−/L+ group, Experiment 2 confirmed that the reduction in light-evoked conditioned responding seen in the 0.03 mg/kg group of Experiment 1 was in fact because of nicotine’s co-presentation with the light rather than an unconditioned (or non-associative) effect of the nicotine. We should note that in the current design, tests for rats in the N+/L− or N−/L+ group were identical to reinforced presentations during training; however, for rats in the NL+ group, test presentations of the elements were different from the compound experienced during training. This distinction may have resulted in a generalization decrement during testing rather than an overshadowing effect. More research will be required to parse apart these two explanations.
Interestingly, even though we did not explicitly look for it, we found limited evidence of overshadowing of the nicotine by light. That is, the nicotine-evoked responding in the N+/L− was the same as in the NL+ group. This result is unlikely because of a ceiling effect of responding because at test, the light-evoked response levels in the N−/L+ group appeared higher than nicotine-evoked responses in the N+/L− group. Acquisition was statistically similar for the paired stimuli between the N+/L− and N−/L+ groups; however, visual comparison of the graphs suggests the CR evoked by 0.03 mg/kg nicotine stimulus was drifting downward as the CR evoked by light was drifting upward. This pattern may explain the finding that nicotine overshadowed the light, but light did not overshadow the 0.03 mg/kg nicotine in our task. Because of the nature of the stimuli (i.e. nicotine is interoceptive, and the light is exteroceptive), the rat may learn to check that the light is on while its head is in the dipper, resulting in increased numbers of dipper entries during that CS presentation. Conversely, as nicotine is interoceptive, the rat has no need to check that the stimulus is ‘on’, and it may begin to reduce its number of dipper entries during the 30 seconds before sucrose, resulting in a post-asymptotic response decrement (see Farwell & Ayres 1979 for a similar distinction between noise and light CSs).
Another possible explanation for the results also involves the nature of the nicotine stimulus. In our experiments, nicotine presentations are an average of 11 minutes apart, meaning that there is likely a build-up of background brain levels of nicotine as the session progresses. The nicotine ‘stimulus’ in these experiments is the initial peak above baseline evoked by each infusion that subsequently fades back somewhat before the next infusion. Notably, the human tobacco user likely experiences similar patterns of nicotine exposure. There is a rapid increase in brain nicotine levels following a puff from a cigarette that drops relatively quickly after the bout of smoking (see Matta et al. 2007). Smokers spend the day titrating their blood nicotine levels to a fairly stable background. Perhaps then, having that background influences the impact of the discrete light stimulus on nicotine-evoked responding. Regardless of the explanation, under the current conditions in these experiments nicotine evoked the same CR regardless of the presence of the exteroceptive light stimulus, whereas control by the light was reduced by the presence of nicotine in the stimulus. If these results eventuate to generalize to tobacco use in humans, this pattern may have important therapeutic implications.
Because nicotine is considered to be the primary addictive component in tobacco (see Stolerman & Jarvis 1995; National Institute on Drug Abuse Research Report Series 2006), and because associative learning processes are inextricably entwined with tobacco consumption, craving, and relapse (see Conklin & Tiffany 2002; Bevins & Palmatier 2004), the ability of the interoceptive nicotine CS to overshadow conditioning to the exteroceptive stimulus may be particularly relevant to smoking cessation. Treatment approaches involving some form of cue-exposure therapy exploit the association between the US effects of nicotine and the predictive exteroceptive CS. Both discrete stimuli and contextual environmental stimuli have been shown to influence nicotine self-administration in rodents (e.g. Caggiula et al. 2001; Wing & Shoaib 2008) as well as tobacco use in human smokers (e.g. Conklin 2006; Juliano et al. 2006; Thewissen et al. 2006). Presentation of the drug-associated cue without the nicotine evokes a response to the cue (e.g. craving, drug seeking; Mucha et al. 2008). Repeated exposure without the nicotine can somewhat extinguish CS-evoked CRs (e.g. Marlatt 1990). This exposure is more effective at reducing craving CRs when the stimuli are personalized for the smoker rather than generic smoking imagery (Conklin & Tiffany 2001). Further, evaluating cue-evoked reactivity in the smoker’s natural environment has also recently been advanced and may prove useful in further personalizing the cue exposure therapy (Warthen & Tiffany 2009).
The long-term effectiveness of these sorts of therapies, however, has been limited (see Niaura et al. 1999; Monti & MacKillop 2007). If the stimulus properties of nicotine are capable of forming compound stimuli with the cues that are targeted during cessation therapy, then those target cues may be controlling a weaker CR than they would have been had there been no competition by nicotine. Further, similar to a generalization account of our current results, presentation of the exteroceptive cues alone may produce a response decrement from when the cues are experienced concurrently with nicotine, resulting in a similar effect. Finally, based on the results of the current study, the stimulus effects of nicotine may not be as impacted by the presence of an exteroceptive light stimulus as the exteroceptive light stimulus is by the presence of nicotine. Albeit speculative, perhaps in vivo exposure to nicotine could be presented as part of the target stimulus in exposure therapies to help extinguish cravings.
Urges to engage in drug use have been shown to be increased by a small dose of the target substance (see Niaura et al. 1988), and there is some precedent for drug inclusion in cue-exposure therapy. For example, presentations of priming doses of alcohol in alcoholics evoked craving CRs that gradually diminished with repeated presentations (Laberg & Ellertsen 1987). Similarly, the ability to resist drinking a second dose of alcohol increased with repeated trials, suggesting attenuation of the CR with experience (Rankin, Hodgson & Stockwell 1983). Controlled exposure to the stimulus effects of nicotine could be used in conjunction with cue-exposure therapy. Nicotine is already available in the form of over-the-counter nicotine replacement therapy (NRT). Indeed, there has been some success with NRT as a smoking cessation aid (e.g. Müller et al. 1990; Schneider et al. 1995). In a recent meta-analysis, approximately 17% of subjects on NRT remained abstinent for 6 months compared with about 10% of subjects on placebo or no treatment (Woolacott et al. 2002). At 12 months, 16% of NRT subjects remained abstinent compared with 10% of placebo/no-treatment subjects. The success rate varied depending on the route of administration and was related to pharmaco-kinetic similarity to cigarettes. The nasal spray delivery form of NRT was the most effective at both time points with quit rates of approximately 24%, whereas the least effective form was the patch with a 14 and 13% quit rate at 6 and 12 months, respectively. Further, when nicotine patches were given as treatment along with cue exposure in the form of smoking denicotinized cigarettes, that combination of therapy resulted in higher abstinence rates after 4 weeks than when placebo patches were used with the denicotinized cigarettes (Becker, Rose & Albino 2008). Whether this success was because of the NRT replacing ‘rewarding’ effects of nicotine for which NRT was designed, because of the extinction of nicotine-evoked CRs as speculated in this discussion, or some combination of the two, remains to be determined. Because there is some evidence that nicotine replacement does not completely eliminate cue-evoked craving (Waters et al. 2004), much more research is clearly needed at both the level of basic learning and at the level of clinical treatment to better understand the relation between nicotine and the predictive CSs. Based on the findings of these experiments, more consideration should be given to the nicotine CS and how this stimulus may affect the formation and expression of other smoking-related associations.
Acknowledgments
We thank Jessica D. Barr, Carmela M. Reichel, Amanda M. Struthers, and Jamie L. Wilkinson for their assistance with surgeries. JEM was supported by F31 DA025399. NRW was partially supported by UNL Undergraduate Creative Activities and Research Experiences (UCARE). The research was supported by DA018114 awarded to RAB and an American Psychological Association Dissertation Research Award and a Psi Chi Graduate Student Research Grant awarded to JEM. None of these sources had a role in the study design; the collection, analysis, and interpretation of data; in the writing of the paper; or the decision to submit the paper for publication. MED-PC programs used in the present article are available in a slightly modified version upon request to Rick A. Bevins, Department of Psychology, University of Nebraska-Lincoln, Lincoln NE USA 68588-0308, or rbevins1@unl.edu.
Footnotes
Authors Contribution
JEM and RAB designed the studies and wrote the protocols. JEM and NRW conducted the experiments. JEM conducted the statistical analyses and wrote the first draft of the manuscript. JEM, NRW, and RAB contributed to subsequent revisions. All authors critically reviewed content and approved final version for publication.
References
- Alessi SM, Roll JM, Reilly MP, Johanson C-E. Establishment of a diazepam preference in human volunteers following differential-conditioning history of placebo versus diazepam choice. Exp Clin Psychopharmacol. 2002;10:77–83. [PubMed] [Google Scholar]
- Becker KM, Rose JE, Albino AP. A randomized trial of nicotine replacement therapy in combination with reduced-nicotine replacement therapy in combination with reduced-nicotine cigarettes for smoking cessation. Nicotine Tob Res. 2008;10:1139–1148. doi: 10.1080/14622200802123294. [DOI] [PubMed] [Google Scholar]
- Besheer J, Palmatier MI, Metschke DM, Bevins RA. Nicotine as a signal for the presence or absence of sucrose reward: a Pavlovian drug appetitive conditioning preparation in rats. Psychopharmacology. 2004;172:108–117. doi: 10.1007/s00213-003-1621-9. [DOI] [PubMed] [Google Scholar]
- Bevins RA. The reference-dose place conditioning procedure yields a graded dose-effect function. Int J Comp Psychol. 2005;18:101–111. [Google Scholar]
- Bevins RA. Altering the motivational function of nicotine through conditioning processes. In: Bevins RA, Caggiula AR, editors. The Motivational Impact of Nicotine and Its Role in Tobacco Use: The 55th Nebraska Symposium on Motivation. New York: Springer; 2009. pp. 111–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevins RA, Palmatier MI. Extending the role of associative learning processes in nicotine addiction. Behav Cogn Neurosci Rev. 2004;3:143–158. doi: 10.1177/1534582304272005. [DOI] [PubMed] [Google Scholar]
- Boakes RA. Performance on learning to associate a stimulus with positive reinforcement. In: Davis H, Hurwitz HMB, editors. Operant-Pavlovian Interactions. Hillsdale, NJ: Erlbaum; 1977. pp. 67–97. [Google Scholar]
- Brandon TH, Piasecki TM, Quinn EP, Baker TB. Cue exposure treatment in nicotine dependence. In: Drummond DC, Tiffany ST, Glautier S, Remington B, editors. Addictive Behaviour: Cue Exposure Theory and Practice. West Sussex: John Wiley and Sons Ltd; 1995. pp. 211–227. [Google Scholar]
- Brooks DC, Bouton ME. A retrieval cue for extinction attenuates response recovery (renewal) caused by a return to the conditioning context. J Exp Psychol Anim Behav Process. 1994;20:366–379. doi: 10.1037//0097-7403.19.1.77. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF. Cue dependency of nicotine self-administration and smoking. Pharmacol Biochem Behav. 2001;70:515–530. doi: 10.1016/s0091-3057(01)00676-1. [DOI] [PubMed] [Google Scholar]
- Clements K, Glautier S, Stolerman IP, White J-AW, Taylor C. Classical conditioning in humans: nicotine as CS and alcohol as US. Hum Psychopharmacol. 1996;11:85–95. [Google Scholar]
- Conklin CA. Environments as cues to smoke: implications for human extinction-based research and treatment. Exp Clin Psychopharmacol. 2006;14:12–19. doi: 10.1037/1064-1297.14.1.12. [DOI] [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST. The impact of imagining personalized versus standardized urge scenarios on cigarette craving and autonomic reactivity. Exp Clin Psychopharmacol. 2001;9:399–408. doi: 10.1037//1064-1297.9.4.399. [DOI] [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST. Applying extinction research and theory to cue-exposure addiction treatments. Addiction. 2002;97:155–167. doi: 10.1046/j.1360-0443.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- Drummond DC, Tiffany ST, Glautier S, Remington B. Cue exposure in understanding and treating addictive behaviours. In: Drummond DC, Tiffany ST, Glautier S, Remington B, editors. Addictive Behaviour: Cue Exposure Theory and Practice. West Sussex: John Wiley and Sons Ltd; 1995. pp. 1–17. [Google Scholar]
- Duncan PM. The effect of training dose on discrimination of compound drug-exteroceptive stimuli. Psychopharmacology. 1986;90:543–547. doi: 10.1007/BF00174076. [DOI] [PubMed] [Google Scholar]
- Farwell BJ, Ayres JJB. Stimulus-reinforcer and response-reinforcer relations in the control of conditioned appetitive headpoking (‘goal-tracking’) in rats. Learn Motiv. 1979;10:295–312. [Google Scholar]
- Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, Dorfman SF, Froelicher ES, Goldstein MG, Healton CG, Henderson PN, Heyman RB, Koh HK, Kottke TE, Lando HA, Mecklenburg RE, Mermelstein RJ, Mullen PD, Orleans CT, Robinson L, Stitzer ML, Tommasello AC, Villejo L, Wewers ME. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services Public Health Service; 2008. Treating Tobacco Use and Dependence: 2008 Update. [Google Scholar]
- Järbe TUC, Johansson B. Interaction between drug discriminative stimuli and exteroceptive, sensory signals. Behav Neurosci. 1984;98:686–694. doi: 10.1037//0735-7044.98.4.686. [DOI] [PubMed] [Google Scholar]
- Jenkins HM. Sensitivity of different response systems to stimulus-reinforcer and response-reinforcer relations. In: Davis H, Hurwitz HMB, editors. Operant-Pavlovian Interactions. Hillsdale, NJ: Erlbaum; 1977. pp. 47–62. [Google Scholar]
- Juliano LM, Donny EC, Houtsmuller EJ, Stitzer ML. Experimental evidence for a causal relationship between smoking lapse and relapse. J Abnorm Psychol. 2006;115:166–173. doi: 10.1037/0021-843X.115.1.166. [DOI] [PubMed] [Google Scholar]
- Kim JA, Siegel S, Patenall VRA. Drug-onset cues as signals: intraadministration associations and tolerance. J Exp Psychol Anim Behav Process. 1999;25:491–504. [PubMed] [Google Scholar]
- Krimmer EC, Benson BJ, McGuire MS, Barry H., III . Conditional sensory stimuli during morphine discrimination. In: Colpaert FC, Slangen JL, editors. Drug Discrimination: Applications in CNS Pharmacology. Amsterdam: Elseiver Biomedical Press; 1982. pp. 135–145. [Google Scholar]
- Laberg JC, Ellertsen B. Psychophysiological indicators of craving in alcoholics: effects of cue exposure. Br J Addict. 1987;82:1341–1348. doi: 10.1111/j.1360-0443.1987.tb00437.x. [DOI] [PubMed] [Google Scholar]
- Mackintosh NJ. Overshadowing and stimulus intensity. Anim Learn Behav. 1976;4:186–192. doi: 10.3758/bf03214033. [DOI] [PubMed] [Google Scholar]
- Mariathasan EA, Stolerman IP. Overshadowing of nicotine discrimination in rats: a model for behavioural mechanisms of drug interactions? Behav Pharmacol. 1993;4:209–215. [PubMed] [Google Scholar]
- Marlatt GA. Cue exposure and relapse prevention in the treatment of addictive behaviors. Addict Behav. 1990;15:395–399. doi: 10.1016/0306-4603(90)90048-3. [DOI] [PubMed] [Google Scholar]
- Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj MI, Donny EC, Gardiner PS, Grady SR, Heberlein U, Leonard SS, Levin ED, Lukas RJ, Markou A, Marks MJ, McCallum SE, Parameswaran N, Perkins KA, Picciotto MR, Quik M, Rose JE, Rothenfluh A, Schafer WR, Stolerman IP, Tyndale RF, Wehner JM, Zirger JM. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology. 2007;190:269–319. doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- Matzel LD, Schachtman TR, Miller RR. Recovery of an overshadowed association achieved by extinction of the overshadowing stimulus. Learn Motiv. 1985;16:398–412. [Google Scholar]
- Miles CG, Jenkins HM. Overshadowing in operant conditioning as a function of discriminability. Learn Motiv. 1973;4:11–27. [Google Scholar]
- Monti PM, MacKillop J. Advances in the treatment of craving for alcohol and tobacco. In: Miller PM, Kavanagh D, editors. Translation of Addictions Science into Practice. New York: Elsevier Science; 2007. pp. 211–237. [Google Scholar]
- Mucha RF, Pauli P, Weber M, Winkler M. Smoking stimuli from the terminal phase of cigarette consumption may not be cues for smoking in healthy smokers. Psychopharmacology. 2008;201:81–95. doi: 10.1007/s00213-008-1249-x. [DOI] [PubMed] [Google Scholar]
- Müller P, Abelin T, Ehrsam R, Imhof P, Howald H, Mauli D. The use of transdermal nicotine in smoking cessation. Lung. 1990;168(Suppl):445–453. doi: 10.1007/BF02718163. [DOI] [PubMed] [Google Scholar]
- Murray JE, Bevins RA. Behavioral and neuropharmacological characterization of nicotine as a conditional stimulus. Eur J Pharmacol. 2007a;561:91–104. doi: 10.1016/j.ejphar.2007.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JE, Bevins RA. The conditional stimulus effects of nicotine vary as a function of training dose. Behav Pharmacol. 2007b;18:707–716. doi: 10.1097/FBP.0b013e3282f14ec6. [DOI] [PubMed] [Google Scholar]
- Murray JE, Bevins RA. Acquired appetitive responding to intravenous nicotine reflects a Pavlovian conditioned association. Behav Neurosci. 2009;123:97–108. doi: 10.1037/a0013735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JE, Li C, Palmatier MI, Bevins RA. The interoceptive Pavlovian stimulus effects of caffeine. Pharmacol Biochem Behav. 2007;86:838–846. doi: 10.1016/j.pbb.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JE, Wells NR, Lyford GD, Bevins RA. Investigation of endocannabinoid modulation of conditioned responding evoked by a nicotine CS and the Pavlovian stimulus effects of CP 55,940 in adult male ratas. Psychopharmacology. 2009;205:655–665. doi: 10.1007/s00213-009-1572-x. [DOI] [PubMed] [Google Scholar]
- National Institute on Drug Abuse Research Report Series. Tobacco Addiction. 2006. NIH Publication number 06-4342. [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academy Press; 1996. [Google Scholar]
- Niaura R, Abrams DB, Shadel WG, Rohsenow DJ, Monti PM, Sirota AD. Cue exposure treatment for smoking relapse prevention: a controlled clinical trial. Addiction. 1999;94:685–695. doi: 10.1046/j.1360-0443.1999.9456856.x. [DOI] [PubMed] [Google Scholar]
- Niaura RS, Rohsenow DJ, Binkoff JA, Monti PM, Pedraza M, Abrams DB. Relevance of cue reactivity to understanding alcohol and smoking relapse. J Abnorm Psychol. 1988;97:133–152. doi: 10.1037//0021-843x.97.2.133. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned Reflexes. London: Oxford University Press; 1927. [Google Scholar]
- Payne TJ, Schare ML, Levis DJ, Colletti G. Exposure to smoking-relavent cues: effects on desire to smoke and topographical components of smoking behavior. Addict Behav. 1991;16:467–479. doi: 10.1016/0306-4603(91)90054-l. [DOI] [PubMed] [Google Scholar]
- Poulos CX, Hinson RE, Siegel S. The role of Pavlovian processes in drug tolerance and dependence: implications for treatment. Addict Behav. 1981;6:205–211. doi: 10.1016/0306-4603(81)90018-6. [DOI] [PubMed] [Google Scholar]
- Rankin H, Hodgson R, Stockwell T. Cue exposure and response prevention with alcoholics: a controlled trial. Behav Res Ther. 1983;21:435–446. doi: 10.1016/0005-7967(83)90013-x. [DOI] [PubMed] [Google Scholar]
- Reichel CM, Murray JE, Grant KM, Bevins RA. Bupropion attenuates methamphetamine self-administration in adult male rats. Drug Alcohol Depend. 2009;100:52–62. doi: 10.1016/j.drugalcdep.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider NG, Olmstead R, Mody FV, Doan K, Franzon M, Jarvik ME, Steinberg C. Efficacy of a nicotine nasal spray in smoking cessation: a placebo-controlled, double-blind trial. Addiction. 1995;90:1671–1682. doi: 10.1046/j.1360-0443.1995.901216719.x. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Gwaltney CJ, Balabanis MH, Liu KS, Paty JA, Kassel JD, Hickcox M, Gnys M. Immediate antecedents of cigarette smoking: an analysis from ecological momentary assessment. J Abnorm Psychol. 2002;111:531–545. doi: 10.1037//0021-843x.111.4.531. [DOI] [PubMed] [Google Scholar]
- Simon NW, Setlow B. Post-training amphetamine administration enhances memory consolidation in appetitive Pavlovian conditioning: implications for drug addiction. Neurobiol Learn Mem. 2006;86:305–310. doi: 10.1016/j.nlm.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Sokolowska M, Siegel S, Kim JA. Intraadministration associations: conditional hyperalgesia elicited by morphine onset cues. J Exp Psychol Anim Behav Process. 2002;28:309–320. [PubMed] [Google Scholar]
- Stolerman IP, Jarvis MJ. The scientific case that nicotine is addictive. Psychopharmacology. 1995;117:2–10. doi: 10.1007/BF02245088. [DOI] [PubMed] [Google Scholar]
- Stout S, Arcediano F, Escobar M, Miller RR. Overshadowing as a function of trial number: dynamics of first- and second-order comparator effects. Learn Behav. 2003;31:85–97. doi: 10.3758/bf03195972. [DOI] [PubMed] [Google Scholar]
- Thewissen R, Snijders SJBD, Havermans RC, van den Hout M, Jansen A. Renewal of cue-elicited urge to smoke: implications for cue exposure treatment. Behav Res Ther. 2006;44:1441–1449. doi: 10.1016/j.brat.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Warthen MW, Tiffany ST. Evaluation of cue reactivity in the natural environment of smokers using ecological momentary assessment. Exp Clin Psychopharmacol. 2009;17:70–77. doi: 10.1037/a0015617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AJ, Shiffman S, Sayette MA, Patty JA, Gwaltney CJ, Balabanis MH. Cue-provoked craving and nicotine replacement therapy in smoking cessation. J Consult Clin Psychol. 2004;72:1136–1143. doi: 10.1037/0022-006X.72.6.1136. [DOI] [PubMed] [Google Scholar]
- White JA, Stolerman IP. Reversal of overshadowing in a drug mixture discrimination in rats. Psychopharmacology. 1996;123:46–54. doi: 10.1007/BF02246280. [DOI] [PubMed] [Google Scholar]
- Wing VC, Shoaib M. Contextual stimuli modulate extinction and reinstatement in rodents self-administering intravenous nicotine. Psychopharmacology. 2008;200:357–365. doi: 10.1007/s00213-008-1211-y. [DOI] [PubMed] [Google Scholar]
- Woolacott NF, Jones L, Forbes CA, Mather LC, Sowden AJ, Song FJ, Raftery JP, Aveyard PN, Hyde CJ, Barton PM. The clinical effectiveness and cost-effectiveness of bupropion and nicotine replacement therapy for smoking cessation: a systematic review and economic evaluation. Health Technol Assess. 2002;6:16. doi: 10.3310/hta6160. [DOI] [PubMed] [Google Scholar]