Abstract
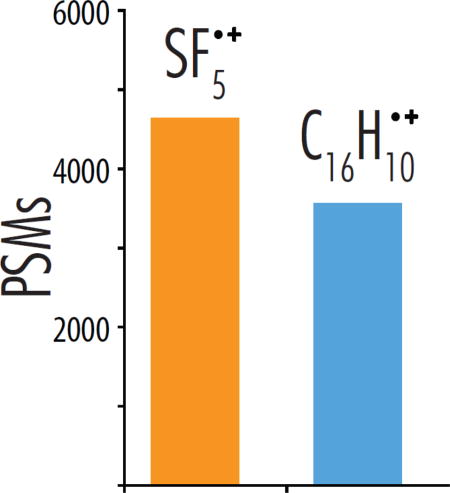
Negative mode proteome analysis offers access to unique portions of the proteome and several acidic post-translational modifications; however, traditional collision-based fragmentation methods fail to reliably provide sequence information for peptide anions. Negative electron transfer dissociation (NETD), on the other hand, can sequence precursor anions in a high-throughput manner. Similar to other ion-ion methods, NETD is most efficient with peptides of higher charge state due to the increased electrostatic interaction between reacting molecules. Here we demonstrate that NETD performance for lower charge state precursors can be improved by altering the reagent cation. Specifically, the recombination energy of the NETD reaction–largely dictated by the ionization energy (IE) of the reagent cation–can affect the extent of fragmentation. We compare the NETD reagent cations of C16H10•+ (IE = 7.9 eV) and SF5•+ (IE = 9.6 eV) on a set of standard peptides, concluding that SF5•+ yields greater sequence ion generation. Subsequent proteome-scale nLC-MS/MS experiments comparing C16H10•+ and SF5•+ further supported this outcome: analyses using SF5•+ yielded 4,637 peptide spectral matches (PSMs) and 2,900 unique peptides, whereas C16H10•+ produced 3,563 PSMs and 2,231 peptides. The substantive gain in identification power with SF5•+ was largely driven by improved identification of doubly deprotonated precursors, indicating that increased NETD recombination energy can increase product ion yield for low charge density precursors. This work demonstrates that SF5•+ is a viable, if not favorable, reagent cation for NETD, and provides improved fragmentation over the commonly used fluoranthene reagent.
Keywords: negative electron transfer dissociation, mass spectrometry, proteomics, negative mode, peptide anions, electron transfer dissociation, ion/ion reactions
Graphical abstract
INTRODUCTION
Modern proteome characterization relies on liquid chromatography coupled with tandem mass spectrometry (nLC-MS/MS) to detect and quantify proteins from complex biological samples [1–5]. Despite continual advances in proteomic depth achieved in such experiments, these technologies do not detect all the proteins present in a sample and, in fact, typically only monitor a portion of those proteins which are detected. More specifically, one or two peptides resulting from the enzymatic digestion of a protein can map uniquely to the parent protein and allow for its unambiguous detection and quantification. New approaches that can offer increased diversity of peptides measured are therefore of considerable significance as they can reveal new proteins and offer access to portions of proteins that were previously not detectable. One factor that may limit the scope of present technology is the unilateral use of positive electrospray ionization. Many proteins, and portions of most proteins, are acidic and thus are more easily ionized in the negative mode [6–8]. Negative electrospray ionization can generate multiply deprotonated peptide anions, but the commonly used collision-based dissociation methods are ineffective at producing sequence-informative fragmentation of negatively charged peptides [9, 10]. These limitations have driven the development of alternative dissociation methods for peptide anions that utilize electrons, photons, and metastable atoms [11–30].
Scott McLuckey, the recipient of the Award for Distinguished Contributions in Mass Spectrometry whom we honor in this issue of JASMS, has been a pioneer in this field, especially in the development of gas-phase ion-ion chemistry[31, 32]. Indeed, this investigation stems from foundational work his group and others described. Most notably, in 1995 McLuckey and co-workers first introduced ion-ion reactions of positive reagent cations with anionic oligonucleotides and have continued this innovating in this space[33–35]. Inspired by McLuckey’s experiment, we developed ion-ion reaction chemistries to abstract electrons from multiply deprotonated peptide anions using singly charged reagent cations, termed negative electron transfer dissociation (NETD) [11]. NETD has emerged as one of the most promising peptide anion dissociation methods and has been successfully utilized in nLC-MS/MS experiments to access the acidic proteome[11, 12, 16, 17, 30].
One challenge of using NETD in large scale proteomic experiments is its limited product ion yield for low charge density precursors. In NETD experiments, peptide anions are oxidized by positively charged reagent cations to initiate dissociation and production of a•- and x-type product ions. Sometimes electron transfer from the anion to the cation occurs without concomitant dissociation (i.e., non-dissociative negative electron transfer, or NETnoD)[36–39]. NETnoD frequently occurs and is one of the primary causes for reduced product ion yields in NETD, especially in the case of low charge density precursors [39]. In these cases, once electron transfer has occurred, peptide backbone cleavage may be achieved; however, the resultant product ions can be held together by non-covalent bonding and detected as a charge-reduced product. To maximize the production of NETD product ions, a reduction of NETnoD species can be accomplished by supplying the charge-reduced product with more energy, either concurrent with, or post, electron transfer. The additional energy can disrupt the non-covalent interactions holding these ions together, yielding sequence informative products[40, 41]. One such approach, termed activated-ion NETD (AI-NETD), has been implemented by concurrently irradiating the ions with infrared photons as they are interacting; however, this approach requires the addition of an IR laser to the system[16, 30].
An alternate approach to increase the energy of the system during electron transfer events is to alter the reaction exothermicity, which is determined by the difference between the ionization energy of the reagent cation and the electron affinity of the peptide anion. For example, the ionization energy of fluoranthene, C16H10•+, is 7.9 eV and the electron affinity of the carboxylate ion of a peptide is 3.4 eV, yielding a reaction enthalpy of 4.5 eV, known as the recombination energy. This energy surplus is redistributed into the peptide anion and drives fragmentation. Use of NETD reagent cations with higher ionization energies result in increased recombination energies and, potentially, an increase in NETD fragmentation efficiency [42]. The recombination energy of the reagent cation and its effect on NETD has been previously explored by Polfer and co-workers for use in determining phosphorylation sites on standard peptides[43], as well as by McLuckey et al. to investigate transition metal complexes and their interaction with peptide anions[44]. Polfer and colleagues compared two NETD reagents (fluoranthene and xenon) and determined that the increase in ionization energy of xenon led to considerable phosphate and side-chain neutral loss and therefore fluoranthene, having a lower ionization energy than xenon, should be used for sequencing phosphopeptides. Alternatively, McLuckey and co-workers showed that transition metal complexes yield both electron transfer as well as metal insertion reactions, allowing further control of the cation-anion interaction. To date, only xenon, fluoranthene, and phenanthroline complexes of Fe, Cu, and Co have been investigated as NETD reagent cations for peptide analysis, and fluoranthene remains by far the most commonly used NETD reagent.
The primary motivation of this work is to investigate the hypothesis that increasing the ionization energy of the NETD reagent cation will increase the NETnoD conversion to product ions thereby yielding greater peptide identification rates and protein sequence coverage in large-scale shotgun proteome analyses. To test this hypothesis, we used a set of synthetic peptides to compare the NETD fragmentation efficiency of sulfur pentafluoride cations (SF5•+, IE = 9.6 eV) and fluoranthene cations (C16H10•+, IE = 7.9 eV) over a range of available precursor charge states (z = −2 to −6). Concluding that SF5•+ cations provided increased sequence ion production for low charge state precursors, we then performed nanoflow liquid chromatography-tandem mass spectrometry (nLC-MS/MS) experiments employing high pH separations and optimized NETD reaction kinetics to compare C16H10•+ to SF5•+ NETD reagent ions for analysis of a complex mixture of yeast peptides. From these data we revealed that up to 40% more peptide spectral matches (PSMs) could be made when using reagent ions from sulfur pentafluoride compared to fluoranthene. The overall peptide spectral match and unique peptide identification numbers improved 30% when using SF5•+ as the NETD reagent instead of fluoranthene. From the data, we conclude that the use of SF5•+ offers a direct route to boosting the performance of NETD dissociation.
EXPERIMENTAL PROCEDURES
NETD on standard peptides
Synthetic peptides have the sequences SVFAVNWISYLASK, EEAQALEDLTGFK, and ELVNDDEDIDWVQTEK were obtained from New England Peptides (Gardner, MA) and were individually suspended in 3:1 methanol/water with 5 mM piperidine to a concentration of 10 ppm. The peptides were infused into a LTQ Velos mass spectrometer (Thermo Fisher Scientific, San Jose, CA) modified to perform NETD. For each peptide precursor, a 0 nce CAD MS/MS scan was performed, followed by a series of NETD MS/MS scans with increasing reaction time, encompassing the 2τ time point (see Supplementary Figure 1 for further experimental design diagram). This series was repeated at least 15 times. Once complete, the next precursor charge state was reacted until all accessible charge states were reacted. The precursor AGC target was set to 10,000 and the reagent AGC target was set to 1,000,000 for all MS/MS acquisitions. The q-value for the NETD reaction was kept at 0.4 for all experiments. This procedure was repeated using both SF5•+ and C16H10•+ reagent cations. The solid phase NETD reagent fluoranthene was introduced to the system using the standard glass vials contained in the ETD module’s reagent vial heater. The gaseous sulfur hexafluoride reagent was introduced to the system by connecting the high purity SF6 gas cylinder (Concorde Specialty Gases, Eatontown, NJ) to a precision regulator (Porter Instruments, Hatfield, PA) with 6 feet of 1/8 inch outer diameter and 0.065 inch inner diameter copper tubing. Then a 100 μm inner diameter capillary tube was attached to the out port of the precision regulator and fed directly into the vacuum manifold of the ETD module and the pressure reading of the precision regulator was adjusted to generate appropriate reagent cation signal (~2 psi). Reagent cations were generated via the EI/CI source in the presence of nitrogen gas, and reagent signal was optimized by varying lens voltages using an automated calibration routine. The filament emission current was set to 70 μA for both reagents. The nitrogen gas pressure was also optimized for maximum signal for each reagent cation. For fluoranthene, the reagent vial temperature was set to 108° C. For both reagents, the ion source, transfer line, and restrictor temperature were held at 160° C. The fluoranthene reagent produced ion radicals C16H10•+, while the predominant cation for sulfur hexafluoride was the fluorine-loss species SF5•+, shown in Supplementary Figure 2.
Yeast sample preparation
Tryptic yeast (Saccharomyces cerevisiae) peptides were prepared as previously described[16]. Briefly, cultured yeast cells were lysed by glass bead milling (Retsch GmnH, Germany), and proteins were reduced and alkylated using 5 mM dithiothreitol and 15 mM iodoacetimide, respectively. Trypsin digestion was performed during an overnight incubation at room temperature with a 1:50 (w/w) enzyme to protein ratio. A second trypsin addition was done the following morning at 1:100 (w/w) enzyme to protein ratio for 1 hour, followed by desalting over a C18 SepPak (Waters Corporation, Milford, MA).
High pH nLC-MS/MS
An ETD-enabled hybrid dual cell-quadrupole ion trap-Orbitrap mass spectrometer (Orbitrap Elite, Thermo Fisher Scientific) coupled to a nanoACQUITY UltraPerformance liquid chromatograph (Waters) was used for the nLC-MS/MS analyses. The mass spectrometer was modified to perform NETD as described previously[41, 45–47]. Briefly, the higher-energy collisional dissociation cell (HCD) was replaced with a multi-purpose dissociation cell (MDC) that can conduct ion-ion reactions, allowing for NETD to be performed within. Fluoranthene and sulfur hexafluoride reagents were introduced as is described above, with the exception of the SF6 pressure being adjusted to 10 psi. The solvent composition for liquid chromatography were mobile phase A (5 mM piperidine in water) and mobile phase B (5 mM piperidine in 85% ACN and 15% water). The reverse phase columns were prepared in house using 75 μm ID, 360 OD bare fused silica capillary tubing packed to a 30 cm length with 3.5 μm, 130 Å pore size, Ethylene Bridged Hybrid C18 particles (Waters). For each analysis, 1 μg of yeast digest was loaded onto the column equilibrated with 95% A at 400 nL/minute. The gradient elution was performed at 400 nL/min increasing from 5% mobile phase B to 30% B over 70 minutes, followed by an increase to 70% B at 76 minutes and a wash at 70% B for 4 more minutes. Peptides were ionized in the negative mode using electrospray ionization with a spray voltage of −1.5 kV. The inlet capillary temperature was set to 300° C. Survey MS scans were analyzed in the Orbitrap mass analyzer with a resolving power of 60,000 at 400 m/z and an AGC precursor ion target value of 1,000,000 over a mass range of 300–1250 m/z. Data dependent MS/MS events were triggered off of the 10 most intense peaks in the survey scan. Each MS/MS scan used a precursor AGC target of 100,000 ions and was analyzed in the Orbitrap with a resolving power of 15,000 at 400 m/z. Precursors were isolated at ±0.9 Th and an exclusion window of ±10 ppm was created around the monoisotopic peak of the precursor for 45 seconds. All nLC-MS/MS experiments were performed in duplicate.
Data analysis
Peptide standard infusion data was searched using an in-house C# script, which extracted ion current intensities directly from raw data files. These values were normalized relative to the ion current of the precursor from a 0 nce CAD scan collected previous to the NETD reacted spectra (see Supplementary Figure 1 for further experimental design diagram). The nLC-MS/MS raw data files were searched using the Open Mass Spectrometry Search Algorithm (OMSSA), modified to allow for anionic peptide a•- and x-type fragment ions to be searched. Search parameters included carbamidomethylation of cysteine as a fixed modification and oxidation of methionine as a variable modification[17, 48]. A multi-isotope search was employed using three isotopes with a mass tolerance of ±125 ppm for the precursors and a monoisotopic mass tolerance of ±0.02 Da for product ions. Three missed cleavages were allowed for the trypsin digestion. The data processing was done through the COMPASS software suite designed for OMSSA searching. A UniProt database for Saccharomyces cervisiae (downloaded September 29, 2014) was concatenated with reversed sequences and used to determine peptide spectral matches (PSMs). Scored spectra were filtered using a false discovery rate of 1% at the unique peptide level. False discovery rates for spectra from each set of duplicate nLC-MS/MS experiments were calculated for the combined set of spectra as opposed to separate calculations for each nLC-MS/MS run. Additionally, prior to OMSSA searching, each spectrum was preprocessed to remove the unreacted precursor ion (±3 Da) and neutral loss ions of the oxidized precursor by removing ions within a window of 55 to 5 Da below the oxidized precursor ion. The nLC-MS/MS experiments were also searched using an inhouse C# script to extract ion intensities for expected sequencing ions as well as ions resulting from neutral losses from both oxidized precursor ions and a•- and x-type product ions. Ion intensities from spectra in nLC-MS/MS experiments were normalized relative to the total ion current (TIC) of each individual MS/MS scan.
RESULTS AND DISCUSSION
NETD of standard peptides with alternative reagent cations
The primary metric in determining an effective reagent cation is the production of sequence informative fragment ions relative to all other product ions produced. In these experiments, reagent ions C16H10•+ and SF5•+ were tested for their effectiveness as NETD reagent cations using an ETD-enabled dual-pressure linear ion trap mass spectrometer. These species were reacted with three standard peptides (sequences given in top left of Figure 1), each with a C-terminal lysine to mimic those yielded from protein digestion with trypsin, and all were synthesized without additional post-translational modifications. The peptides had lengths of 13, 14, and 16 amino acid residues, isoelectric points of 3.62, 4.00, and 8.31, and generated peptide anions having charge states ranging from z = −2 to −6.
Figure 1.
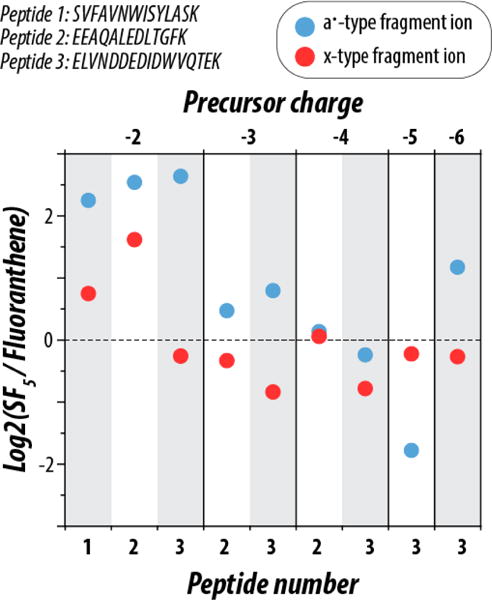
Comparison of product ion signal from NETD reactions using reagents C16H10•+ and SF5•+ for all precursor charge states of three synthetic peptides (sequences shown top left). The intensities of both a•- and x-type fragment ions were normalized to the total ion current of the unreacted precursor from a preceding scan as explained in the text. Following this normalization, the log2 fold change of the normalized fragment ion current intensity was calculated between SF5•+ and C16H10•+. All precursor ion populations were reacted to the 2τ time point (i.e., 13.5 ± 3% unreacted precursor ion current remaining in the MS/MS spectrum).
To provide a straightforward comparison of their product ion generation efficiencies the extent of reaction for the two reagent cations was carefully controlled. To do so, we standardized several conditions that dictate the number of electron transfer events that occur per reaction, isolating the chemistry of the reagent cations as the main variable of the experiment. The rate of ion-ion reactions, and thus the number of electron transfer events, are governed by a number of parameters including ion population, reaction q-value, reaction cell architecture, and reaction time. Previous work has shown that to maximize peptide identifications, the ideal electron transfer extent occurs when the precursor ion population has been reduced by 86% [49]. Such reaction conditions minimize the amount of secondary electron transfer while still offering sufficient sequence informative fragment ion production. Using this as a model, we created a method, illustrated in Supplementary Figure 1, where a series of increasing reaction times were employed surrounding the optimal reaction extent (13.5% unreacted precursor remaining), keeping all other reaction parameters constant. Figure 1 summarizes the production of sequence informative a•- and x-type fragment ions produced when all accessible charge states of the three standard peptides were reacted with SF5•+ or C16H10•+. Use of SF5•+ as the reagent cation more than quadrupled the a•-type ion signal relative to C16H10•+ for all doubly deprotonated precursors. An increase in x-type fragment ion production was observed for two out of three doubly deprotonated precursors.
As charge density increased, the difference in fragment ion production was reduced. In the case of triply deprotonated precursors, SF5•+ generated spectra with more a•-type fragment ions while use of C16H10•+ cations produced more x-type fragment ions, but the magnitude of the difference is considerably less than for the lower charge state precursors. Generally, the C16H10•+ reagent cation produced spectra with marginally more x-type fragment ions for higher charge state species, but the product ion signal is largely comparable between the two reagent cations for z ≥ 3 precursors. This suggests that the higher IE of SF5•+ can benefit fragmentation of low charge density precursors (i.e., z = −2) where the predominance of NETnoD can adversely affect dissociation product ion generation. Note, however, SF5•+ reagent cations retain the good performance of C16H10•+ for more highly charged ions. Supplementary Figure 3 also considers the distribution of even and odd electron fragment ions (odd electron species containing a radical electron and an additional hydrogen atom), showing that a•-type and x-type fragment ions are the predominant species formed upon NETD, and that there is no significant difference in the even and odd electron ratios between the two reagent cations.
nLC-MS/MS of yeast tryptic digest using SF5•+ and C16H10•+
To expand the scope of our study, we compared the performance of C16H10•+ and SF5•+ as reagent cations in nLC-MS/MS analyses of peptides derived following tryptic digestion of yeast proteins. Importantly, the majority of precursors sampled in negative mode nLC-MS/MS experiments are doubly deprotonated [16], suggesting the use of SF5•+ reagent cations could improve the depth of analysis in whole-proteome shotgun sequencing. Indeed, in 90-minute nLC-MS/MS experiments, NETD with SF5•+ generated 30% more PSM and unique peptide identifications than did NETD using C16H10•+ (Figure 2). Figure 2b displays the charge state distribution of peptide spectral matches (PSMs) following use of the two NETD reagents. We note a substantial increase in the identification of z = −2 peptides for analyses with SF5•+ (1,039 more z = −2 PSMs than those with C16H10•+), which comprised more than 70% of all PSMs identified in either dataset. This finding is consistent with the previous results using standard peptides, further showing that peptides with lower charge density benefit more from excess recombination energy, while higher charge density peptides are not as impacted.
Figure 2.
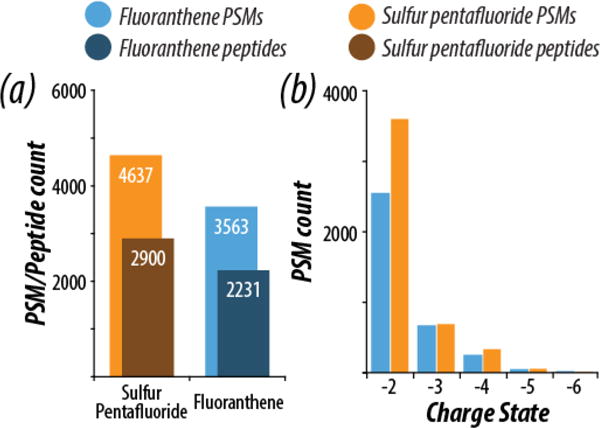
Summary of peptide spectral matches (PSM) and unique peptide identifications from nLC-MS/MS experiments using a yeast tryptic digest (a). Experiments were conducted using either SF5•+ or C16H10•+ as reagent cations. The charge state distributions of successfully sequenced PSMs from these experiments are also shown (b), revealing a significant increase in z = −2 peptides identified with SF5•+.
Figure 3 presents representative spectra from the nLC-MS/MS experiments illustrating the improved fragmentation afforded by use of SF5•+ reagent cations as compared to C16H10•+. For each spectrum, the doubly deprotonated precursor of the peptide ETAESYLGAK was reacted with either NETD reagent to a reaction extent of 37.9% and 38.4% for C16H10•+ and SF5•+, respectively. Reaction extent is defined as the ion current of the unreacted precursor divided by the total ion current of the scan. While in the standard peptide infusion data a 0 nce CAD scan was used to measure the total ion abundance for each peptide and fragment ion currents could be normalized to it, allowing direct calculation of the precursor to product ion conversion ratio, in the discovery nLC-MS experiments no 0 nce CAD scans were performed and therefore this normalization approach was not possible, and reaction extent was calculated instead. Comparing spectra with similar reaction extents ensures that the main contributor to the difference is the ion chemistries of the reagents themselves. The spectra show many similarities but with considerably more product ions generated when SF5•+ was used. Specifically, SF5•+ produced two more a•-type and three more x-type fragment ions than the corresponding NETD spectrum produced when C16H10•+ was used as the reagent, and it also generated three CO2 neutral losses from x-type fragment ions compared to only one when C16H10•+ cations were used. In all, use of SF5•+ as the reagent cation produced a spectrum with 100% peptide sequence coverage for ETAESYLGAK while the corresponding spectrum when C16H10•+ was used as the reagent yields only 55.5% coverage. Note, we define peptide sequence coverage as the ratio of the number of inter-residue positions broken to the total possible positions (residue length −1) for a given sequence, expressed here as a percentage.
Figure 3.
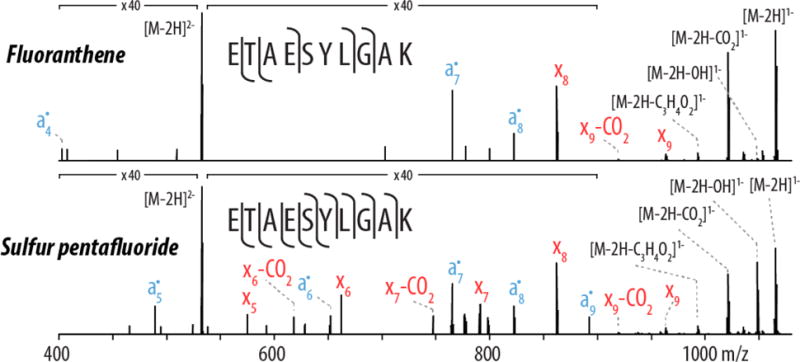
Single-scan spectra for the NETD fragmentation of the peptide ETAESYLGAK, z = −2. The unreacted precursor signal accounts for 37.9% and 38.4% of the total ion current in each MS/MS scan for C16H10•+ and SF5•+, respectively, indicating both precursors were reacted to similar extents. Use of SF5•+ as the NETD reagent provided greater sequence coverage than C16H10•+, yielding 11 sequence informative fragment ions compared to 5 for C16H10•+. The NETD reagent SF5•+ produced three CO2 product ion neutral loss species while the fluoranthene spectrum only contains a single CO2 product ion neutral loss fragment. Both spectra were acquired in the nLC-MS/MS experiments and represent a single scan (i.e., un-averaged).
Figure 4a expands on the change in peptide sequence coverage between reagent cations SF5•+ and C16H10•+ by showing the composite difference for all peptides identified in the NETD experiments. Only peptides found in both data sets were considered, and of the 1,427 peptides in common, 812 peptides yielded an increase in sequence coverage when SF5•+ was used as the NETD reagent while only a fourth of that (n = 202) showed an increase when C16H10•+ was used. On average, SF5•+ accounted for a nearly 10% improvement in peptide sequence coverage for all overlapping peptides.
Figure 4.
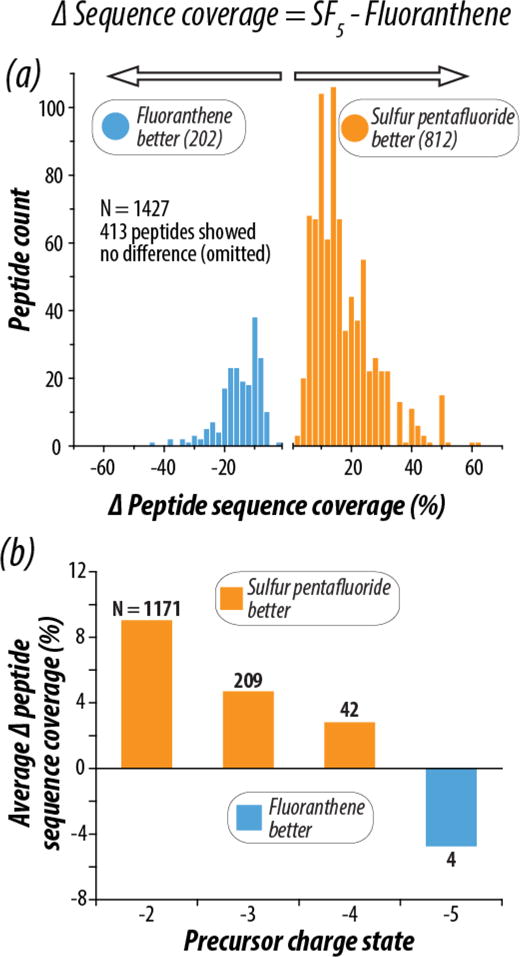
(a) Percent peptide sequence coverage was calculated for each of the 1427 peptides in common between the SF5•+ and C16H10•+ analyses. The difference between peptide sequence coverage with SF5•+ and C16H10•+ (Δ Peptide Sequence Coverage) was calculated for each peptide, and the distribution of the Δ Peptide Sequence Coverage values are shown. The orange distribution shows peptides with greater sequence coverage with SF5•+ (n = 812), and the blue shows peptides with better sequence coverage with C16H10•+ (n = 202). Panel (b) shows the average Δ Peptide Sequence Coverage for all peptides in common between the two analyses as a function of peptide precursor charge state. The number of precursors averaged is shown in black text above the bar for each charge state. Concordant with previously shown data, lower charged precursors benefit most from the use of SF5•+.
Differences in peptide sequence coverage are even more pronounced when delineating across charge states. Figure 4b displays peptides categorized by charge state, where charge states of z = −2, −3, −4, and −5 showed an average sequence coverage difference of 9.0%, 4.7%, 2.8%, and −4.7% respectively. Note, as indicated in the ΔSequence Coverage equation at the top of Figure 4, a positive value indicates higher sequence coverage with SF5•+. Interestingly, C16H10•+ as the NETD reagent only performs better on z = −5 precursors, which account for 1.3% of the total precursors sampled in the experiments. In all other cases, peptide sequence coverage was improved by use of SF5•+, especially for lower precursor charge states. Supplementary Figure 4 considers the impact of reagent cation on total protein sequence coverage for all proteins in common between the two data sets, as the increased number of peptide identifications with SF5•+ translates to high coverage of the proteins mapped in the experiments. Here protein sequence coverage is defined as the number of amino acid residues comprising each identified peptide divided by all amino acid residues in the protein sequence. Use of SF5•+ improved coverage of 298 proteins, C16H10•+ improved coverage of 125 proteins, and 84 showed no difference between the reagent cations, with an average improvement of 3.1% in favor of SF5•+.
Comparison of neutral losses
Utilizing the higher ionization energy SF5•+ reagent cation yields greater fragmentation in regards to sequence informative fragment ions. As the Polfer group noted with xenon, however, high ionization energies can drive the production of neutral losses from fragment ions. Using the same pool of doubly deprotonated peptides in common between the C16H10•+ and SF5•+ data sets discussed above, Figure 5 examines how the oxidized (i.e., charge reduced) precursor ions and their associated neutral losses are affected by the use of the two reagent cations. Figure 5a displays the average percent ion current accounted for by the oxidized precursor as well as the signal from corresponding neutral losses of CO2 and either NH3 or OH from this charge-reduced species. Doubly charged peptide precursors reacted with C16H10•+ show on average 8.5% more CO2 neutral loss while the SF5•+ data yielded greater NH3 and OH neutral loss. Figure 5b compares the abundance of amino-acid specific side chain neutral losses from the oxidized precursor [12]. The predominant differences are found in methionine, serine, and threonine residues. Interestingly, methionine and serine show a substantial increase in neutral loss when C16H10•+ is used, despite the lower recombination energy relative to SF5•+. However, in total, very little difference is found in amino-acid specific side chain neutral loss when comparing the two NETD reagent cations.
Figure 5.
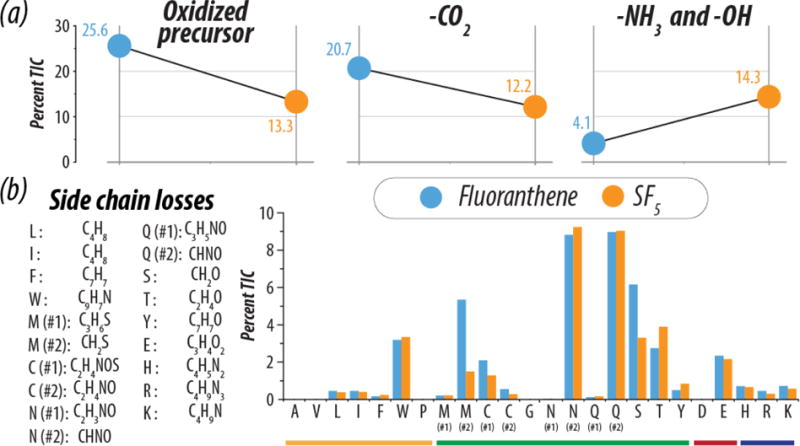
(a) The average percent of total ion current accounted for by the oxidized precursor ion and neutral losses of CO2 and either NH3 or OH from the oxidized precursor ion are shown for z = −2 PSMs from C16H10•+ (blue) and SF5•+ (orange) nLC-MS/MS analyses. (b) The average percent of total ion current is shown for side chain neutral losses from the oxidized precursor ion for z = −2 PSMs. In order for a spectrum to be searched for a given side chain loss, the PSM sequence had to contain that residue. Note, amino acids are organized by their side chain properties: non-polar (yellow), polar (green), acidic (red), and basic (blue).
Lastly, we investigated the a•- and x-type product ions generated in these experiments, including how CO2 neutral losses from product ions differed between the two reagent cations. Figure 6a provides a holistic look at the total number of a•- and x-type fragment ions produced in the NETD datasets. As shown with our standard peptide data above, SF5•+ yields more fragment ions species than C16H10•+, particularly more a•-type fragments, with an increase of 32%. Figure 6b shows the percentage of those sequence ions which also yield a CO2 neutral loss peak. SF5•+ cations generated 5.4% more x-type ion species with CO2 neutral losses. To investigate how the occurrence of these neutral losses from sequencing ions impacts data analysis, we searched our yeast peptide database allowing for a variety of different fragment ion combinations. No combination of product ions and neutral losses yielded a greater number of PSMs when searching the SF5•+ dataset than just a•- and x-type fragment ions alone, which concurs with the same analysis performed on the fluoranthene dataset (not shown). We conclude that the amount of CO2 neutral loss is not significant enough to impact or adversely affect automated spectral annotation.
Figure 6.

(a) The number of total a•- and x-type fragment ions generated from the peptides common to both C16H10•+ (blue) and SF5•+ (orange) analyses are shown. For both fragment ion types SF5•+ produces the greater number of sequencing ions and the percent gains over the number of ions from C16H10•+ analyses are shown in bold. Panel (b) compares the percentage of a•- and x-type product ions from Panel (a) that have a corresponding neutral loss of CO2. (c) Despite the small increase in CO2 neutral losses observed, incorporating CO2 neutral losses from a•- and x-type product ions as fragment ion types to query in a database search does not improve peptide identifications over using standard a•- and x-type product ions only for the SF5•+ data. The combination of product ion types used in the database searches are shown in black at the top, and the number of identified peptide spectral matches are shown in orange at the bottom. Similar results were obtained with the C16H10•+ analyses (data not shown).
CONCLUSION
NETD provides direct access to analysis of proteomes in the negative mode, but improvements are still needed to make NETD amenable to the large proportion of low charge density precursors generated in whole-proteome analyses. In this experiment, we sought to improve peptide anion fragmentation using SF5•+, a reagent cation with a higher IE of 9.6 eV, compared to C16H10•+, the most common NETD reagent cation with an IE of 7.9 eV. Using peptide fragmentation efficiency and unique peptide identifications as our primary metrics, we determined that SF5•+ significantly improves shotgun proteomic analyses with NETD, especially considering the fragmentation of doubly deprotonated precursors. We contribute this gain in identification power to the increase in ionization energy for SF5•+ compared to C16H10•+, which impacts the exothermicity of the ion-ion reaction and provides greater fragment ion yield and less non-dissociative negative electron transfer. The predominant gain in identification for low charge density precursors may also be useful when considering protein digestion using proteases other than trypsin, such as Lys-C, which often yields longer, less charge-dense peptides. Another benefit of using a gaseous reagent for NETD is the simplification of the ion source as no reagent vial heaters or heated transfer lines are required to volatilize and transfer the solid fluoranthene reagent.
While SF5•+ appears to be a favorable choice for large-scale negative mode proteomics analyses, its use in other types of NETD applications may benefit as well. While previous work has shown that lower recombination energy NETD reactions reduces the occurrence of labile PTM neutral loss [43], many other molecules might benefit from the more energetic reaction. In addition to peptides, NETD has been useful in the study of polynucleotides[35] and carbohydrates[50–53] and further improvements may be obtained through the utilization of SF5•+ as the reagent cation.
Supplementary Material
Supplementary Figure 1. The mass spectrometry method for reaction of standard peptides with both NETD reagents is shown. Each precursor of interest is reacted with 0 nce CAD followed by a series of NETD reactions with increasing reaction times encompassing the 2τ time point, (a). This sequence is repeated until at least 15 scans are completed for each reaction time. The reaction time closest to the 2τ time point, identified by the dotted orange line, is then analyzed to extract all ion currents, (b), which are normalized by taking the average ion current (I.C.) of an individual fragment ion and dividing by the average ion current of the 0 nce CAD scan, shown in (c). This allows for an accurate percent conversion from initial starting ion population to fragment ion population to be determined, labelled the % I.C.
Supplementary Figure 2. The EI ionization spectrum for both SF6 and fluoranthene are shown. Sulfur hexafluoride produces the fluorine loss product SF5+• as the primary ion, while fluoranthene produces the molecular ion C16H10+•.
Supplementary Figure 3. A selection of a and x fragment ions are shown from the NETD reaction of −3 charge state of the standard peptide EEAQALEDLTGFK, reacted using the reagent cation shown to the left. The distribution of a to a• fragment ions are very similar, with a• being the predominant species. The first isotope of the x fragment ions and the x• ion are indistinguishable at this resolution, however the isotope ratio is similar between reagents, indicating that recombination energy has little effect on the production of even and odd electron x fragment ion species.
Supplementary Figure 4. Protein sequence coverage for all proteins common to both analyses are shown. A Δ Protein Sequence Coverage value was calculated for all 507 proteins seen in both analyses, and each individual protein is shown as a semi-transparent bar. The average sequence coverage for all common proteins was 3.1% higher for SF5•+ (orange) compared to C16H10•+ (blue). Note the difference of peptide and protein sequence coverage discussed in the text.
Acknowledgments
The authors gratefully acknowledge support from Thermo Fisher Scientific and NIH grants R35 GM118110. N.M.R. was funded through an NSF Graduate Research Fellowship (DGE-1256259).
References
- 1.Kim MS, Pinto SM, Getnet D, Nirujogi RS, Manda SS, Chaerkady R, Madugundu AK, Kelkar DS, Isserlin R, Jain S, Thomas JK, Muthusamy B, Leal-Rojas P, Kumar P, Sahasrabuddhe NA, Balakrishnan L, Advani J, George B, Renuse S, Selvan LDN, Patil AH, Nanjappa V, Radhakrishnan A, Prasad S, Subbannayya T, Raju R, Kumar M, Sreenivasamurthy SK, Marimuthu A, Sathe GJ, Chavan S, Datta KK, Subbannayya Y, Sahu A, Yelamanchi SD, Jayaram S, Rajagopalan P, Sharma J, Murthy KR, Syed N, Goel R, Khan AA, Ahmad S, Dey G, Mudgal K, Chatterjee A, Huang TC, Zhong J, Wu X, Shaw PG, Freed D, Zahari MS, Mukherjee KK, Shankar S, Mahadevan A, Lam H, Mitchell CJ, Shankar SK, Satishchandra P, Schroeder JT, Sirdeshmukh R, Maitra A, Leach SD, Drake CG, Halushka MK, Prasad TSK, Hruban RH, Kerr CL, Bader GD, Iacobuzio-Donahue CA, Gowda H, Pandey A. A draft map of the human proteome. Nature. 2014;509:575–581. doi: 10.1038/nature13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilhelm M, Schlegl J, Hahne H, Moghaddas Gholami A, Lieberenz M, Savitski MM, Ziegler E, Butzmann L, Gessulat S, Marx H, Mathieson T, Lemeer S, Schnatbaum K, Reimer U, Wenschuh H, Mollenhauer M, Slotta-Huspenina J, Boese JH, Bantscheff M, Gerstmair A, Faerber F, Kuster B. Mass-spectrometry-based draft of the human proteome. Nature. 2014;509:582–7. doi: 10.1038/nature13319. [DOI] [PubMed] [Google Scholar]
- 3.Hebert AS, Richards AL, Bailey DJ, Ulbrich A, Coughlin EE, Westphall MS, Coon JJ. The one hour yeast proteome. Mol Cell Proteomics. 2014;13:339–47. doi: 10.1074/mcp.M113.034769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richards AL, Merrill AE, Coon JJ. Proteome sequencing goes deep. Curr Opin Chem Biol. 2015;24C:11–17. doi: 10.1016/j.cbpa.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riley NM, Hebert AS, Coon JJ. Proteomics Moves into the Fast Lane. Cell Syst. 2016;2:142–143. doi: 10.1016/j.cels.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Yamashita M, Fenn BJ. Negative ion production with the electrospray ion source. J Phys Chem. 1984;88:4671–4675. [Google Scholar]
- 7.Fenn JJBJ, Mann MM, Meng CKAIC, Wong SF, Whitehouse CM. Electrospray ionization for mass spectrometry of large biomolecules. Science. 1989;246:64–71. doi: 10.1126/science.2675315. 80- [DOI] [PubMed] [Google Scholar]
- 8.Straub RF, Voyksner RD, Voyksner D. Negative ion formation in electrospray mass spectrometry. J Am Soc Mass Spectrom. 1993;4:578–587. doi: 10.1016/1044-0305(93)85019-T. [DOI] [PubMed] [Google Scholar]
- 9.Brinkworth CS, Dua S, McAnoy AM, Bowie JH. Negative ion fragmentations of deprotonated peptides: backbone cleavages directed through both Asp and Glu. Rapid Commun Mass Spectrom. 2001;15:1965–73. doi: 10.1002/rcm.457. [DOI] [PubMed] [Google Scholar]
- 10.Bowie JH, Brinkworth CS, Dua S. Collision-induced fragmentations of the (M-H)-parent anions of underivatized peptides: An aid to structure determination and some unusual negative ion cleavages. Mass Spectrom Rev. 2002;21:87–107. doi: 10.1002/mas.10022. [DOI] [PubMed] [Google Scholar]
- 11.Coon JJ, Shabanowitz J, Hunt DF, Syka JEP. Electron transfer dissociation of peptide anions. J Am Soc Mass Spectrom. 2005;16:880–2. doi: 10.1016/j.jasms.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 12.Rumachik NG, McAlister GC, Russell JD, Bailey DJ, Wenger CD, Coon JJ. Characterizing Peptide Neutral Losses Induced by Negative Electron-Transfer Dissociation (NETD) J Am Soc Mass Spectrom. 2012;23:718–727. doi: 10.1007/s13361-011-0331-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flora JWJW, Muddiman DCDC. Selective, Sensitive, and Rapid Phosphopeptide Identification in Enzymatic Digests Using ESI-FTICR-MS with Infrared Multiphoton Dissociation proteolytic digests involving electrospray ionization Fou- Anal Chem. 2001;73:3305–3311. doi: 10.1021/ac010333u. [DOI] [PubMed] [Google Scholar]
- 14.Madsen JAJA, Kaoud TSTS, Dalby KNKN, Brodbelt JSJS. 193-nm photodissociation of singly and multiply charged peptide anions for acidic proteome characterization. Proteomics. 2011;11:1329–1334. doi: 10.1002/pmic.201000565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaw JB, Kaplan DA, Brodbelt JS. Activated ion negative electron transfer dissociation of multiply charged peptide anions. Anal Chem. 2013;85:4721–8. doi: 10.1021/ac4005315. [DOI] [PubMed] [Google Scholar]
- 16.Riley NM, Rush MJP, Rose CM, Richards AL, Kwiecien NW, Bailey DJ, Hebert AS, Westphall MS, Coon JJ. The Negative Mode Proteome with Activated Ion Negative Electron Transfer Dissociation. Mol Cell Proteomics. 2015;14:2644–2660. doi: 10.1074/mcp.M115.049726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McAlister GC, Russell JD, Rumachik NG, Hebert AS, Syka JEP, Geer LY, Westphall MS, Pagliarini DJ, Coon JJ. Analysis of the acidic proteome with negative electron-transfer dissociation mass spectrometry. Anal Chem. 2012;84:2875–2882. doi: 10.1021/ac203430u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cook SL, Jackson GP. Metastable atom-activated dissociation mass spectrometry of phosphorylated and sulfonated peptides in negative ion mode. J Am Soc Mass Spectrom. 2011;22:1088–1099. doi: 10.1007/s13361-011-0123-y. [DOI] [PubMed] [Google Scholar]
- 19.Smith SA, Kalcic CL, Cui L, Reid GE. Femtosecond laser-induced ionization/dissociation tandem mass spectrometry (fsLID-MS/MS) of deprotonated phosphopeptide anions. Rapid Commun Mass Spectrom. 2013;27:2807–2817. doi: 10.1002/rcm.6750. [DOI] [PubMed] [Google Scholar]
- 20.Jai-nhuknan J, Cassady CJ. Negative ion postsource decay time-of-flight mass spectrometry of peptides containing acidic amino acid residues. Anal Chem. 1998;70:5122–5128. doi: 10.1021/ac980577n. [DOI] [PubMed] [Google Scholar]
- 21.Larraillet V, Antoine R, Dugourd P, Lemoine J. Activated-electron photodetachment dissociation for the structural characterization of protein polyanions. Anal Chem. 2009;81:8410–8416. doi: 10.1021/ac901304d. [DOI] [PubMed] [Google Scholar]
- 22.Larraillet V, Vorobyev A, Brunet C, Lemoine J, Tsybin YO, Antoine R, Dugourd P. Comparative dissociation of peptide polyanions by electron impact and photo-induced electron detachment. J Am Soc Mass Spectrom. 2010;21:670–680. doi: 10.1016/j.jasms.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 23.Antoine R, Joly L, Tabarin T, Broyer M, Dugourd P, Lemoine J. Photo-induced formation of radical anion peptides. Electron photo-detachment dissociation experiments [1] 2007 doi: 10.1002/rcm.2810. [DOI] [PubMed] [Google Scholar]
- 24.Haselmann K, Budnik B, Kjeldsen F, Nielsen M, Olsen J, Zubarev R. Electronic excitation gives informative fragmentation of polypeptide cations and anions. Eur J Mass Spectrom. 2002;8:117. [Google Scholar]
- 25.Kjeldsen F, Silivra OA, Ivonin IA, Haselmann KF, Gorshkov M, Zubarev RA. Cα-C backbone fragmentation dominates in electron detachment dissociation of gas-phase polypeptide polyanions. Chem - A Eur J. 2005;11:1803–1812. doi: 10.1002/chem.200400806. [DOI] [PubMed] [Google Scholar]
- 26.Halim MA, Girod M, MacAleese L, Lemoine J, Antoine R, Dugourd P. 213 nm Ultraviolet Photodissociation on Peptide Anions: Radical-Directed Fragmentation Patterns. J Am Soc Mass Spectrom. 2016;27:474–486. doi: 10.1007/s13361-015-1297-5. [DOI] [PubMed] [Google Scholar]
- 27.Greer SM, Cannon JR, Brodbelt JS. Improvement of Shotgun Proteomics in the Negative Mode by Carbamylation of Peptides and Ultraviolet Photodissociation Mass Spectrometry. Anal Chem. 2014 doi: 10.1021/ac5035314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robinson MR, Moore KL, Brodbelt JS. Direct identification of tyrosine sulfation by using ultraviolet photodissociation mass spectrometry. J Am Soc Mass Spectrom. 2014;25:1461–1471. doi: 10.1007/s13361-014-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Madsen JAJa, Xu H, Robinson MRMR, Horton APAP, Shaw JBJB, Giles DKDK, Kaoud TSTS, Dalby KNKN, Trent MSS, Brodbelt JSJS. High-throughput database search and large-scale negative polarity liquid chromatography-tandem mass spectrometry with ultraviolet photodissociation for complex proteomic samples. Mol Cell Proteomics. 2013;12:2604–14. doi: 10.1074/mcp.O113.028258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riley NM, Bern M, Westphall MS, Coon JJ. A full-featured search algorithm for negative electron transfer dissociation. J Proteome Res. 2016;15:2768–2776. doi: 10.1021/acs.jproteome.6b00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prentice BM, McLuckey SA. Gas-phase ion/ion reactions of peptides and proteins: acid/base, redox, and covalent chemistries. Chem Commun (Camb) 2013;49:947–65. doi: 10.1039/c2cc36577d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McLuckey SA, Mentinova M. Ion/neutral, ion/electron, ion/photon, and ion/ion interactions in tandem mass spectrometry: Do we need them all? Are they enough? J Am Soc Mass Spectrom. 2011;22:3–12. doi: 10.1007/s13361-010-0004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.HERRON WJ, GOERINGER DE, McLuckey SA. Gas-Phase Electron-Transfer Reactions From Multiply-Charged Anions to Rare-Gas Cations. J Am Chem Soc. 1995;117:11555–11562. [Google Scholar]
- 34.Gao Y, Yang J, Cancilla M. Top-Down Interrogation of Chemically Modified Oligonucleotides by Negative Electron Transfer and Collision Induced Dissociation. Anal Chem. 2013 doi: 10.1021/ac400448t. [DOI] [PubMed] [Google Scholar]
- 35.Huang TY, McLuckey SA. Gas-phase ion/ion reactions of rubrene cations and multiply charged DNA and RNA anions. Int J Mass Spectrom. 2011;304:140–147. [Google Scholar]
- 36.Horn DM, Ge Y, McLafferty FW. Activated ion electron capture dissociation for mass spectral sequencing of larger (42 kDa) proteins. Anal Chem. 2000;72:4778–4784. doi: 10.1021/ac000494i. [DOI] [PubMed] [Google Scholar]
- 37.Xia Y, Gunawardena HP, Erickson DE, McLuckey SA. Effects of Cation ChargeSite Identity and Position on Electron-Transfer Dissociation of Polypeptide Cations. J Am Chem Soc. 2007;129:12232–12243. doi: 10.1021/ja0736764. [DOI] [PubMed] [Google Scholar]
- 38.Shaw JB, Madsen JA, Xu H, Brodbelt JS. Systematic comparison of ultraviolet photodissociation and electron transfer dissociation for peptide anion characterization. J Am Soc Mass Spectrom. 2012;23:1707–15. doi: 10.1007/s13361-012-0424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swaney DL, McAlister GC, Wirtala M, Schwartz JC, Syka JE, Coon JJ. Supplemental activation method for high-efficiency electron-transfer dissociation of doubly protonated peptide precursors. Anal Chem. 2007;79:477–485. doi: 10.1021/ac061457f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamidane H Ben, Chiappe D, Hartmer R, Vorobyev A, Moniatte M, Tsybin YO. Electron Capture and Transfer Dissociation: Peptide Structure Analysis at Different Ion Internal Energy Levels. J Am Soc Mass Spectrom. 2009;20:567–575. doi: 10.1016/j.jasms.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 41.Ledvina AR, Rose CM, McAlister GC, Syka JEP, Westphall MS, Griep-Raming J, Schwartz JC, Coon JJ. Activated ion ETD performed in a modified collision cell on a hybrid QLT-Oribtrap mass spectrometer. J Am Soc Mass Spectrom. 2013;24:1623–33. doi: 10.1007/s13361-013-0621-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gunawardena HP, He M, Chrisman PA, Pitteri SJ, Hogan JM, Hodges BDM, McLuckey SA. Electron transfer versus proton transfer in gas-phase ion/ion reactions of polyprotonated peptides. J Am Chem Soc. 2005;127:12627–12639. doi: 10.1021/ja0526057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huzarska M, Ugalde I, Kaplan DA, Hartmer R, Easterling ML, Polfer NC. Negative electron transfer dissociation of deprotonated phosphopeptide anions: choice of radical cation reagent and competition between electron and proton transfer. Anal Chem. 2010;82:2873–8. doi: 10.1021/ac9028592. [DOI] [PubMed] [Google Scholar]
- 44.Crizer DM, Xia Y, McLuckey SA. Transition Metal Complex Cations as Reagents for Gas-Phase Transformation of Multiply Deprotonated Polypeptides. J Am Soc Mass Spectrom. 2009;20:1718–1722. doi: 10.1016/j.jasms.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 45.Ledvina AR, Beauchene NA, McAlister GC, Syka JE, Schwartz JC, Griep-Raming J, Westphall MS, Coon JJ. Activated-ion electron transfer dissociation improves the ability of electron transfer dissociation to identify peptides in a complex mixture. Anal Chem. 2010;82:10068–10074. doi: 10.1021/ac1020358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rose CM, Russell JD, Ledvina AR, McAlister GC, Westphall MS, Griep-Raming J, Schwartz JC, Coon JJ, Syka JEP. Multipurpose dissociation cell for enhanced ETD of intact protein species. J Am Soc Mass Spectrom. 2013;24:816–27. doi: 10.1007/s13361-013-0622-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Riley NM, Westphall MS, Coon JJ. Activated Ion Electron Transfer Dissociation for Improved Fragmentation of Intact Proteins. Anal Chem. 2015;87:7109–7116. doi: 10.1021/acs.analchem.5b00881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Geer LY, Markey SP, Kowalak Ja, Wagner L, Xu M, Maynard DM, Yang X, Shi W, Bryant SH. Open mass spectrometry search algorithm. J Proteome Res. 2004;3:958–64. doi: 10.1021/pr0499491. [DOI] [PubMed] [Google Scholar]
- 49.Rose CM, Rush MJP, Riley NM, Merrill AE, Kwiecien NW, Holden DD, Mullen C, Westphall MS, Coon JJ. A Calibration Routine for Efficient ETD in Large-Scale Proteomics. J Am Soc Mass Spectrom. 2015;26:1848–1857. doi: 10.1007/s13361-015-1183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wolff JJJ, Leach FE, 3rd, Laremore TNTN, Kaplan DADA, Easterling MLML, Linhardt RJRJ, Amster IJJ, L FE, Iii, Laremore TNTN, Kaplan DADA, Easterling MLML, Linhardt RJRJ, Amster IJJ, Leach FE, Laremore TNTN, Kaplan DADA, Easterling MLML, Linhardt RJRJ, Amster IJJ. Negative Electron Transfer Dissociation of Glycosaminoglycans. Anal Chem. 2010;82:3460–3466. doi: 10.1021/ac100554a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leach FE, Wolff JJ, Xiao Z, Ly M, Laremore TN, Arungundram S, Al-Mafraji K, Venot A, Boons GJJ, Linhardt RJ, Amster IJ, Leach FE, 3rd, Wolff JJ, Xiao Z, Ly M, Laremore TN, Arungundram S, Al-Mafraji K, Venot A, Boons GJJ, Linhardt RJ, Amster IJ. Negative electron transfer dissociation Fourier transform mass spectrometry of glycosaminoglycan carbohydrates. Eur J Mass Spectrom (Chichester, Eng) 2011;17:167–176. doi: 10.1255/ejms.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang Y, Yu X, Mao Y, Costello CECE, Zaia J, Lin C. De novo sequencing of heparan sulfate oligosaccharides by electron-activated dissociation. Anal Chem. 2013;85:11979–86. doi: 10.1021/ac402931j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hu H, Huang Y, Yu X, Yongmei X, Liu J, Zong C, Boons GJ, Lin C, Xia Y, Zaia J. A Computational Framework for Heparan Sulfate Sequencing Using Highresolution Tandem Mass Spectra. Mol Cell Proteomics. 2014;13:2490–502. doi: 10.1074/mcp.M114.039560. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. The mass spectrometry method for reaction of standard peptides with both NETD reagents is shown. Each precursor of interest is reacted with 0 nce CAD followed by a series of NETD reactions with increasing reaction times encompassing the 2τ time point, (a). This sequence is repeated until at least 15 scans are completed for each reaction time. The reaction time closest to the 2τ time point, identified by the dotted orange line, is then analyzed to extract all ion currents, (b), which are normalized by taking the average ion current (I.C.) of an individual fragment ion and dividing by the average ion current of the 0 nce CAD scan, shown in (c). This allows for an accurate percent conversion from initial starting ion population to fragment ion population to be determined, labelled the % I.C.
Supplementary Figure 2. The EI ionization spectrum for both SF6 and fluoranthene are shown. Sulfur hexafluoride produces the fluorine loss product SF5+• as the primary ion, while fluoranthene produces the molecular ion C16H10+•.
Supplementary Figure 3. A selection of a and x fragment ions are shown from the NETD reaction of −3 charge state of the standard peptide EEAQALEDLTGFK, reacted using the reagent cation shown to the left. The distribution of a to a• fragment ions are very similar, with a• being the predominant species. The first isotope of the x fragment ions and the x• ion are indistinguishable at this resolution, however the isotope ratio is similar between reagents, indicating that recombination energy has little effect on the production of even and odd electron x fragment ion species.
Supplementary Figure 4. Protein sequence coverage for all proteins common to both analyses are shown. A Δ Protein Sequence Coverage value was calculated for all 507 proteins seen in both analyses, and each individual protein is shown as a semi-transparent bar. The average sequence coverage for all common proteins was 3.1% higher for SF5•+ (orange) compared to C16H10•+ (blue). Note the difference of peptide and protein sequence coverage discussed in the text.


