Abstract
Objective
To characterize anticipatory postural adjustments (APA) across a variety of step initiation tasks in people with Parkinson’s disease (PD) and healthy control (HC).
Design
Cross-sectional study. Step initiation was analyzed during a) self-initiated gait, b) perceptual cued gait, and c) compensatory forward stepping after platform perturbation. People with PD were assessed On and Off levodopa.
Setting
University research laboratory.
Participants
PD (n=19) and healthy aged matched controls (n=12).
Interventions
Not applicable.
Main Outcome Measures
Medio-lateral (ML) size of APA (calculated from center of pressure recordings), step kinematics and body alignment.
Results
With respect to self-initiated gait, the ML size of APAs were significantly larger during the cued condition and significantly smaller during the compensatory condition (p<0.001). HC and patients with PD did not differ in body alignment during the stance phase prior to stepping. No significant group effect was found for ML size of APA between HC and patients with PD. However, the reduction in APA size from cued to compensatory stepping was significantly less pronounced in PD Off Meds compared to HC, as indicated by a significant group by condition interaction effect (p<0.01). No significant differences were found comparing PD patients in On and Off meds.
Conclusions
Specific stepping conditions had a significant effect on the preparation and execution of step initiation. Thus, APA size has to be interpreted with respect to the specific stepping condition. Across-task changes in people with PD were less pronounced compared to HC. Antiparkinsonian medication did not significantly improve step initiation in this mildly affected PD cohort.
Keywords: step initiation, anticipatory postural adjustment, cue, compensatory, Parkinson’s disease
Introduction
Stepping from a stance position can occur during self-initiated gait or during compensatory stepping, when a step may be necessary to prevent a fall after a loss of balance. An anticipatory postural adjustment (APA) in preparation for a step involves a shift of the center of pressure (COP) posteriorly and laterally towards the stepping leg and causes the center of mass (COM) to accelerate forward and laterally towards the stance leg [1, 2]. APAs are typically reported during voluntary step initiation. However, the initiation of the first step is possible without performing any APA [3, 4] since a forward fall of the body can be initiated from normal postural sway during upright stance, without moving the COP posteriorly or laterally. [2]. In fact, Maki & colleagues showed that during compensatory stepping, healthy adults often do not exhibit APAs, as APAs could delay time of step initiation [5].
During gait initiation, the anterior-posterior (AP) size of APA is related to gait velocity [6], indicating that a larger posterior movement of the COP generates a higher moment of force and COM acceleration. Furthermore, the peak distance between the COP and the vertical projection of the COM to the ground is associated with improved dynamic postural control [7, 8]. Therefore, during self-initiated gait initiation a large APA is associated with better motor performance as it results in larger gait velocity and is related to improved postural control.
However, when gait initiation has to be performed quickly, as during compensatory stepping after a loss of balance, the performance of large APAs might be too time consuming so smaller or no APAs might be more beneficial to initiate the first step as quickly as possible [3, 4, 9]. During compensatory stepping, the first step needs to be performed within a limited temporal window and small or even no APA might be necessary to avoid a fall. Therefore, whether APAs are beneficial or detrimental may depend on the stepping condition [5].
Parkinsonian gait has been characterized as bradykinetic and unstable [10–12]. Analyzing APAs and step initiation of people with PD can help identify whether APAs are contributing to these stepping impairments. During self-initiated stepping patients with PD often exhibit hypometric APAs with a longer duration, larger step latency and reduced step speed in comparison to healthy control (HC) and this has been shown for self-initiated stepping in a comfortable pace and self-initiated stepping as quickly as possible [2, 13–17]. The difference in size of APAs in PD compared to HC is even more pronounced when initiating gait from a wide stance position compared to a narrow stance position [14]. One study found slightly differing results, showing that APA size in people with PD Off levodopa exhibited similar APA size to healthy adults prior to self-initiated gait when APAs are normalized to gait velocity [2]. Initiating gait in a comfortable pace after a cue increases the size of APA in both PD (On and Off levodopa) and HC subjects compared to a non-cued condition [15, 18]. During compensatory stepping in response to a perturbation, HC typically step with no or only a single APA whereas subjects with PD often use multiple APAs [3, 4]. Interestingly, while levodopa significantly increases the size [14, 15] and reduces the duration [15] of APAs during voluntary gait initiation, levodopa had little effect on the size of APAs during compensatory stepping [3, 14].
These previous studies provide considerable insight into APA impairments in people with PD. However, a direct comparison of the execution of APAs during different stepping conditions (self-initiated vs. cued vs. compensatory) has not been conducted so far and different methodological approaches limit the comparability of the results across existing studies. A more thorough characterization of altered APAs during different stepping conditions is necessary to identify potential rehabilitation targets.
This study has three aims. First, we characterize APAs across several gait initiation tasks (i.e. self-initiated, cued and compensatory stepping). We hypothesized that the size of APA will be smaller during compensatory stepping due to the limited temporal window to perform a step in comparison to the self-initiated and cued condition. Second, we will determine the degree to which PD affects APAs prior to each task by comparing HC with patients with PD. We hypothesized that patients with PD will exhibit smaller APAs during the self-initiated and cued condition and larger APAs during compensatory stepping compared to HC. Third, the effect of levodopa on step initiation during self-initiated, cued and compensatory stepping will be analyzed. We hypothesize that the dopaminergic medication will improve the conduction of stepping during voluntary and cued, but not during compensatory stepping that is externally triggered.
Methods
Participants
Twelve HC and 19 subjects with PD participated. Subjects with PD patients were diagnosed according Brain Bank Criteria for PD [19]. The following exclusion criteria were applied: any known neurological disorders other than PD, deep brain stimulation (PD patients only) and orthopedic injuries that interfere with gait or balance. Postural control was assessed with the Mini-Balance Evaluation Systems Test (Mini-BESTest) [20, 21]. The Montreal Cognitive Assessment (MoCA) was performed to estimate cognitive impairments. Furthermore, PD subjects’ disease severity was assessed with the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) part 3 [22]. Freezing of gait was assessed with the New Freezing of Gait Questionnaire [23] (patients were considered to be a freezer if they answered ‘yes’ at item 1). The study protocol was approved by the local ethics committee and all patients gave written informed consent prior to participating.
Testing Procedure
Subjects with PD were assessed during the OFF state of medication, i.e. after withdrawal from all anti-parkinsonian medication for at least 12 hours, and under the ON state of medication, i.e. within 2 hours of taking levodopa. These two sessions occurred over two days and the order of testing was counterbalanced.
Participants stood on 2 computer/servo-controlled hydraulic platforms. Step initiation was assessed during the following conditions: (a) self-initiated, (b) cued and (c) compensatory stepping. (a) For self-initiated stepping subjects were instructed to initiate gait whenever they were ready and with a comfortable speed. (b) For the cued condition, a small translational platform perturbation (6cm, 4cm/s) served as a perceptual cue. Participants were instructed to initiate gait as soon as they felt the cue. Despite the small and slow perturbation speed, all participants were able to identify platform movement. (c) Compensatory stepping was assessed during backward translational perturbations (15cm at 56cm/s) of the support surface causing the subjects to step forward. During the compensatory stepping, participants were instructed that the ground would move under their feet and were asked to do their best to maintain balance without anticipating the direction of movement. All participants required at least one compensatory step to maintain balance in response to these perturbations. The onset of perturbation was randomized between 2 and 10 seconds after the participant had taken the initial stance position. Forward perturbations were intermixed with backward perturbations to ensure participants were not anticipating the direction of movement. The order of forward and backward perturbation was randomized but for all subjects the same. 14 perturbations, delivered in each direction (forward, backward, leftward, and rightward), were administered prior to protective step perturbations to reduce the “first trial” effects.
Data were collected as part of a larger project assessing voluntary and reactive stepping in people with PD [24]. As part of this collection, feet were placed close together, but not touching, for all stepping conditions. During all conditions, participants were instructed to rest arms naturally at their sides with eyes open. Starting foot position was held constant across trials and participants. Five trials of stepping were recorded for the voluntary and cued condition, respectively. Ten trials were captured for the backward and forward reactive stepping, respectively.
Outcome measures
The size of APA prior to stepping was assessed by analyzing the center of pressure (COP) excursion during step initiation. Subjects stood on 2 independent force plates, one plate under each foot. The plates captured with a frequency of 480 Hz and the data were low pass filtered at 20 Hz with a 4th order Butterworth filter. Due to the variation of the anterio-posterior (AP) COP signal caused by the platform movements during the cued and compensatory condition, only medio-lateral (ML) APAs were analyzed for all conditions. APA detection began 75ms after platform movement (for cued and compensatory trials, or when the velocity of the center of mass reached 0.1m/s in the direction of the swing foot (for voluntary stepping trials). APA detection ceased at step initiation, defined as toe off, measured by the force plates. The ML size of APA was defined as the maximal ML distance from two consecutive local COP peaks towards the stepping leg (figure 1).
Figure 1.
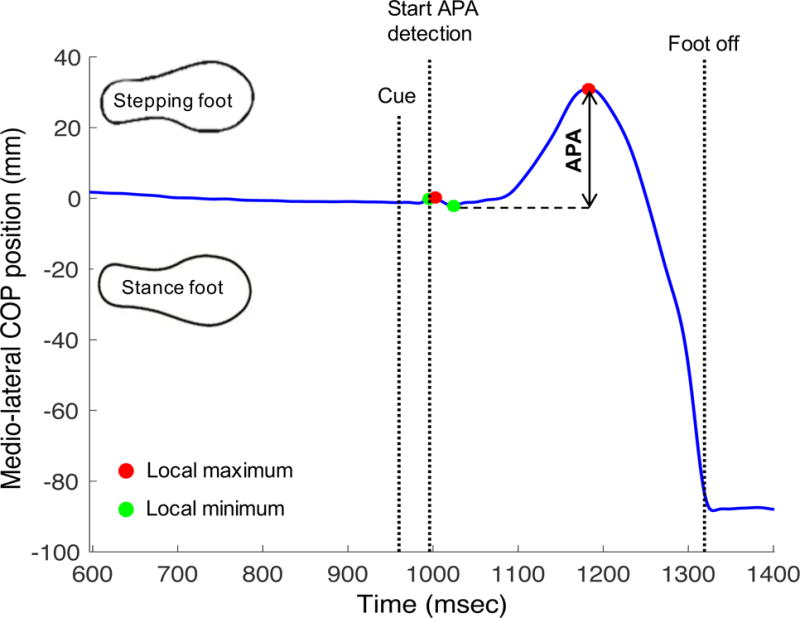
Calculation of medio-lateral size of APA in an example of multiple APA during cued condition.
After tracking the data with the motion analysis software (Motion Analysis Corporation, Santa Rosa, California)a, the 3D body markers position were analyzed with a custom made Matlab algorithm (R2016a)b. Step length (distance between the left and right heel markers at the moment of first foot contact), step duration (time from toe off to first foot contact), and step velocity (step length/step duration) of the first step were calculated. Body alignment prior to step initiation was analyzed by comparing the hip (angle of the knee-, hip- and shoulder markers), knee (angle of the ankle-, knee- and hip-markers) and ankle (angle of the 5th metatarsal-, ankle- and knee markers) angles of PD and HC subjects. Position of the center of mass of each segment was calculated using segment kinematics and anthropometric data from 36 reflective markers placed on anatomic landmarks and summed to establish the position of whole-body center of mass [25, 26]. The center of gravity position was then calculated as the vertical projection of the center of mass to the ground, and analyzed in relation to foot position (AP: % of foot length; ML: % of distance between left and right marker at 5th metatarsal). Capture frequency was 120 Hz and data were filtered with a 4th order Butterworth low pass filter with a frequency of 5 Hz.
Statistical Analysis
For statistical analysis SPSS (version 23)c was used. Independent sample Student’s T-Test was conducted to compare participant characteristics of both groups. Χ2-Test was used to test the gender distribution. The distributions of the data were analyzed and a logarithmic or square root transformation was used if the data were not normally distributed. For study aim I a one-way repeated measure ANOVA with dependent samples T-Test as post-hoc tests were conducted within the healthy control subjects to analyze the effect of stepping condition on the execution of step initiation. To analyze the effect of Parkinson’s disease (study aim II) a mixed measures ANOVA (between subject: PD vs. HC; within subject: stepping condition) with independent samples T-Tests as post-hoc tests were used. The effect of medication on stepping (study aim III) was analyzed with a repeated measures ANOVA within the PD group (factor 1: medication state; factor 2: stepping condition). The magnitude of adaptive change between two stepping conditions was correlated to different baseline variables with Spearman’s rank correlation. The pre-defined level of significance was set at p<0.05. A Bonferroni correction was used for multiple comparisons.
Results
Effects of different conditions on stepping
The different stepping conditions had a significant effect on ML size of APA, step length and step duration in HC subjects (p<0.0001; figure 2). Specifically, the ML APA amplitude significantly increased (p<0.001) from the self-initiated to the cued condition and significantly decreased (p<0.0001) when performing compensatory steps (figure 2A). During compensatory stepping, step length and step duration were significantly reduced compared to the self-initiated and cued condition (p<0.001) (figure 2). Step velocity did not differ among the stepping conditions. See supplemental material online for further details.
Figure 2.
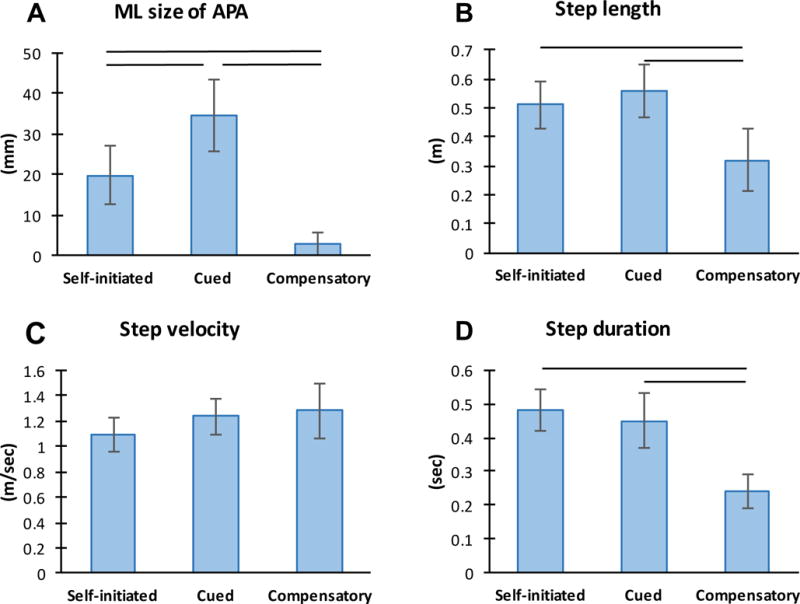
Effect of stepping condition on step initiation in healthy control subjects (n=12): A) medio-lateral (ML) size of APA; B) Step length; C) Step velocity; D) Step duration; bars indicate significant differences (p<0.001).
Effect of PD on stepping
No significant differences in participant characteristics were found between groups (table 1). There were no significant differences in height in PD and HC (PD: 168.6cm (SD 10.3); HC: 166.2cm (SD 9.2); p=0.53). The initial stance position prior to step initiation (hip-, knee-, ankle angles and average center of gravity position related to foot position) were also not different between groups (see supplemental material online).
Table 1.
Participant characteristics (n=31).
| PD (n=19) | HC (n=12) | p-value | |
|---|---|---|---|
| Age (y) | 65.7 (7.6) | 68.1 (6.7) | 0.383 |
| Gender (M/F) | 12/7 | 6/6 | 0.320# |
| BMI (kg/m2) | 27.6 (4.4) | 27.5 (2.3) | 0.988 |
| MoCA | 26.6 (3.5) | 27.2 (1.6) | 0.573 |
| Mini-BESTest (PD Med On) | 23.0 (4.2) | 25.3 (2.2) | 0.096 |
| Mini-BESTest (PD Med OFF) | 22.1 (3.7) | 25.3 (2.2) | 0.013* |
| Disease duration (y) | 6.6 (4.0) | n/a | |
| H&Y stage | 2.1 (0.3) | n/a | |
| H&Y stage (range) | 2.0 – 3.0 | n/a | |
| MDS-UPDRS III (ON) | 24.6 (9.7) | n/a | |
| MDS-UPDRS III (OFF) | 30.0 (9.1) | n/a | |
| LEDD | 556.8 (230.3) | n/a |
NOTE. Unless indicated differently, values are mean (SD) or number of participants; p-value of independent sample T-Test or
Chi-Square Test;
significantly different (p<0.05).
When people with PD (Off levodopa) were compared to healthy adults, a significant interaction effect was found for ML size of APA (p<0.01; Table 2; Figure 3A).
Table 2.
Comparison of step initiation between subjects with PD (Med Off, n=19) and healthy control (n=12) in three conditions.
| Variable | Group | Self-initiated | Cued | Compensatory | Group effect | Interaction effect | ||
|---|---|---|---|---|---|---|---|---|
| F | p-value | F | p-value | |||||
| ML size of APA (mm) | PD | 18.1 (7.0)ˆ | 26.8 (7.1)ˆ | 7.3 (6.7)ˆ | 0.00 | 0.983 | 5.91 | 0.009* |
| HC | 19.8 (7.0)ˆ | 34.4 (8.4)ˆ | 3.0 (2.6)ˆ | |||||
| Step length (m) | PD | 0.50 (0.09) | 0.49 (0.10) | 0.23 (0.04) | 5.63 | 0.025 | 2.32 | 0.127 |
| HC | 0.51 (0.08) | 0.56 (0.09) | 0.32 (0.11) | |||||
| Step velocity (m/sec) | PD | 1.07 (0.15)+ | 1.17 (0.12)+,ˆ | 1.01 (0.11)#,ˆ | 9.50 | 0.004* | 9.10 | 0.001* |
| HC | 1.09 (0.13) | 1.24 (0.14) | 1.28 (0.21)# | |||||
| Step duration (sec) | PD | 0.47 (0.04) | 0.41 (0.05) | 0.22 (0.03) | 3.25 | 0.082 | 0.543 | 0.584 |
| HC | 0.48 (0.06) | 0.45 (0.08) | 0.24 (0.05) | |||||
Note. significantly different (p<0.0125 (Bonferroni adjusted)).
significantly different (across groups; p<0.01) in post-hoc comparison (independent samples T-Test).
and
significantly different (across two conditions within one group; p<0.001) in post-hoc comparison (paired samples T-Test).
Figure 3.
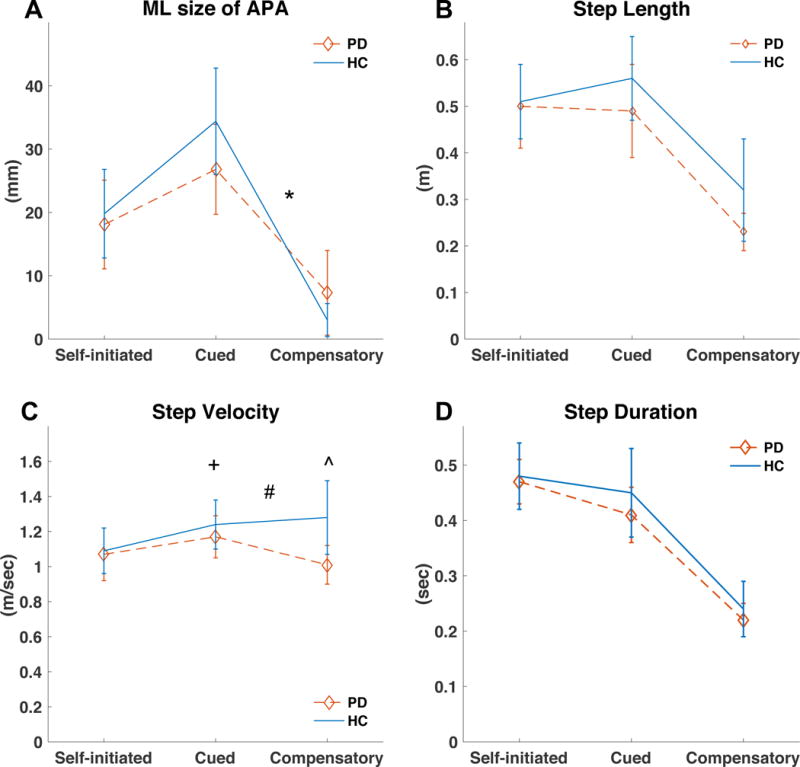
Comparison of patients with Parkinson’s disease (PD, n=19, Med Off) and healthy control (HC) subjects (n=12) during different stepping conditions: A) medio-lateral (ML) size of APA; B) Step length; C) Step velocity; D) Step duration; *, significant interaction from cued to compensatory (p<0.01); +, significant interaction from self-initiated to compensatory (p<0.01); #, significant interaction from cued to compensatory (p<0.01); ˆ, significant group effect (p<0.01).
Post-hoc comparisons confirmed this interaction, indicating that HC exhibited a more pronounced decrease in ML APA size from the cued to the compensatory stepping condition compared to PD (p<0.01) (Med OFF) (figure 3A). HC also exhibited a more pronounced increase in step velocity than people with PD (p<0.01; figure 3C). No significant group or interaction effect was found for step length and step duration. Although 7 of the patients with PD reported freezing of gait (FOG) during daily life, none of the patients with PD exhibited FOG during the assessment. Excluding the PD patients with FOG from the analysis did not change the results of the comparison between HC and PD patients.
The correlation between the magnitude of adaptive change of two stepping condition and different baseline variables within the PD group is shown in table 3.
Table 3.
Correlation (Spearman’s Rho) between the magnitude of adaptive change of two stepping conditions (Med Off) and different baseline variables within the PD group.
| Variable | Magnitude of change | Spearman’s rank correlation | Age | DD | UPDRS (OFF) | H&Y | Mini-BESTest | LEDD |
|---|---|---|---|---|---|---|---|---|
| ML size of APA | Cu – SI | Rho | −0.053 | −0.233 | −0.157 | −0.063 | 0.131 | −0.183 |
| p-value | 0.831 | 0.336 | 0.52 | 0.799 | 0.593 | 0.454 | ||
| Comp – Cu | Rho | 0.046 | 0.209 | 0.614 | 0.313 | −0.344 | 0.172 | |
| p-value | 0.853 | 0.391 | 0.005** | 0.192 | 0.15 | 0.481 | ||
| Comp – SI | Rho | 0.002 | −0.009 | 0.386 | 0.188 | −0.162 | −0.039 | |
| p-value | 0.994 | 0.972 | 0.103 | 0.441 | 0.507 | 0.875 | ||
| Step length | Cu – SI | Rho | −0.147 | −0.109 | −0.191 | 0.219 | 0.3 | −0.024 |
| p-value | 0.547 | 0.658 | 0.434 | 0.367 | 0.212 | 0.923 | ||
| Comp – Cu | Rho | 0.265 | 0.375 | 0.326 | 0.47 | −0.524 | −0.533 | |
| p-value | 0.273 | 0.113 | 0.173 | 0.042* | 0.021* | 0.019* | ||
| Comp – SI | Rho | 0.161 | 0.261 | 0.354 | 0.532 | −0.467 | −0.43 | |
| p-value | 0.509 | 0.28 | 0.137 | 0.019* | 0.043* | 0.066 | ||
| Step velocity | Cu – SI | Rho | 0 | −0.077 | 0.193 | 0.157 | −0.329 | 0.057 |
| p-value | 1 | 0.753 | 0.43 | 0.522 | 0.17 | 0.816 | ||
| Comp – Cu | Rho | 0.025 | −0.004 | −0.158 | 0.282 | 0.113 | −0.577 | |
| p-value | 0.92 | 0.989 | 0.518 | 0.242 | 0.644 | 0.01* | ||
| Comp – SI | Rho | −0.023 | −0.021 | 0.084 | 0.376 | −0.194 | −0.408 | |
| p-value | 0.926 | 0.932 | 0.731 | 0.113 | 0.426 | 0.083 | ||
| Step duration | Cu – SI | Rho | −0.116 | −0.056 | −0.302 | 0.125 | 0.471 | 0.048 |
| p-value | 0.637 | 0.819 | 0.208 | 0.609 | 0.042* | 0.846 | ||
| Comp – Cu | Rho | 0.402 | 0.263 | 0.304 | 0.407 | −0.673 | −0.34 | |
| p-value | 0.088 | 0.276 | 0.205 | 0.084 | 0.002** | 0.155 | ||
| Comp – SI | Rho | 0.3 | 0.288 | 0.108 | 0.47 | −0.333 | −0.483 | |
| p-value | 0.212 | 0.232 | 0.659 | 0.042* | 0.164 | 0.036* |
Note. Comp, compensatory; Cu, cued; DD, disease duration; SI, self-initiated;
significant correlation (p<0.05);
significant correlation (p<0.01).
Effect of levodopa on stepping
No significant effect of medication was found on ML size of APA, step length, step velocity and step duration when comparing PD patients in the On state of medication versus Off state of medication (table 4).
Table 4.
Effect of medication during different stepping condition (n=19).
| Variable | Group | self-initiated | cued | compensatory | Medication effect | Interaction effect | ||
|---|---|---|---|---|---|---|---|---|
| F | p-value | F | p-value | |||||
| ML size of APA (mm) | PD Off | 18.1 (7.0) | 26.8 (6.9) | 7.3 (6.7) | 1.63 | 0.219 | 1.11 | 0.320 |
| PD On | 21.0 (9.2) | 30.7 (10.8) | 6.3 (4.3) | |||||
| step length (m) | PD Off | 0.49 (0.09) | 0.49 (0.10) | 0.23 (0.04) | 4.9 | 0.04 | 0.22 | 0.759 |
| PD On | 0.52 (0.11) | 0.51 (0.12) | 0.24 (0.07) | |||||
| step velocity (m/s) | PD Off | 1.07 (0.15) | 1.17 (0.14) | 1.01 (0.02) | 1.96 | 0.179 | 0.02 | 0.957 |
| PD On | 1.09 (0.19) | 1.12 (0.20) | 1.04 (0.19) | |||||
| step duration (s) | PD Off | 0.47 (0.04) | 0.41 (0.05) | 0.22 (0.03) | 1.33 | 0.263 | 0.63 | 0.499 |
| PD On | 0.47 (0.06) | 0.42 (0.06) | 0.24 (0.05) | |||||
Discussion
This study provides a direct comparison of step execution during self-initiated, cued and compensatory stepping in healthy adults and patients with PD on and off levodopa. We observed three primary results, corresponding to each of our study aims. First, stepping condition significantly affected size of APA, step length and step duration. Specifically, compared to self-initiated gait, step initiation with a perceptual cue increases the size of ML APAs, while step initiation via balance recovery (i.e. compensatory steps) occurred with almost no APA. Step length and step duration were also reduced during compensatory stepping, indicating that, as noted previously, the limited temporal window during which a reactive step has to occur to avoid a fall does not allow a complete weight shift of the center of mass towards the stance leg [5]. Compensatory steps are performed as a quick lift of the stepping foot requiring explosive rate of force development whereas self-initiated and cued steps are a results of a coordinated weight shift of the center of mass towards the stance leg. The size of an APA must be interpreted with respect to the specific stepping condition.
Study aim II focused on the effect of Parkinson’s disease on step initiation. Our results show that patients with PD Off levodopa adapt the ML size of APAs to the different stepping conditions with the same pattern but not to the same extent as HC indicating a reduced adaptive capacity in patients with PD. This result supports postural inflexibility [27, 28] and reduced reactive adaptability [29] to changes in context in patients with PD compared to HC. It is unclear why people with PD exhibited less pronounced adaptive capacity compared to control subjects. Initial posture can affect the ability to produce APAs and effective steps. However, as noted above, foot position was consistent across groups and trials. In addition, we did not find any differences in the average center of gravity position or in the body alignment during the stance phase prior to stepping. Therefore, despite the fact that people with PD often exhibit altered posture, this was unlikely to contribute to the differences observed in the current study. Alternative explanations could include changes in proprioceptive deficits, rigidity, bradykinesia, strength, or sensory-motor-integration deficits; however these possibilities necessitate further investigation.
Other studies have shown that visual [18], auditory [30] or cutaneous [15] cueing lead to increased size of APA during voluntary gait initiation in PD. We used a small platform perturbation as perceptual cue and our results confirm these findings, indicating that cueing might be an effective rehabilitative strategy to improve preparation of gait initiation, independently of the type of cue. Further research is necessary to understand which type of cue best improves the preparation and execution of gait initiation and to explain which pathophysiological mechanisms are involved during cueing [31].
Although compensatory steps were slower in people with PD compared to controls, no falls were observed after perturbations in either group. Therefore, while steps were slower in those with PD, they were adequate to protect against a negative outcome (i.e. a fall). This raises the possibility that people with PD retain the ability to step faster, and perhaps similarly to healthy adults, but were not challenged enough to do so with the current protocol. Assessing speed of compensatory steps which are occasionally insufficient (i.e. result in a fall) are necessary to address this question. This could be achieved with larger perturbations and/or a more severe subject pool.
Surprisingly, in the present study, we did not find significant differences between HC and subjects with PD in the size of the APA during self-initiated steps, as previously reported by our group and others [13, 14]. One explanation might be that all subjects were instructed to stand with feet close together before initiating steps. In fact, it has been previously shown that the small APA size in people with PD compared to controls is less pronounced when stepping from a narrow stance width both Off and On levodopa [14].
In the PD group, age and disease duration did not relate to the magnitude of change across tasks. In contrast, balance performance (assessed with the Mini-BESTest) showed most often significant correlations to the adaptive change, indicating the better the subjects’ balance, the better the ability to flexibly adapt the motor pattern to different stepping tasks. This highlights the importance of rehabilitation programs targeting the enhancement of multiple aspects of postural control.
Our results show that differences between HC and people with PD were largest in the cued and compensatory stepping condition but not in the self-initiated condition. Cued and compensatory stepping therefore might be more sensitive to detect impaired gait initiation in PD than self-initiated stepping.
As we used a small backward platform perturbation as a perceptual cue, the cued condition differed from the compensatory condition only by size and velocity of the perturbation, but not by the type of cue. Our results have shown that a small and slow perturbation serving as a cue leads to increased size of APA but when applying a faster and larger perturbation requiring compensatory steps the size of APA decreased below the level of self-initiated steps. Future studies should analyze different sizes and velocities of perturbations to draw insights at which threshold subjects shift from increased to decreased size of APA.
Study aim III focused on the effect of levodopa medication on APAs in each condition. Levodopa did not affect APA size in our cohort. In contrast, other studies found that levodopa increased the size of APAs during self-initiated stepping [14, 15]. Again we believe that the initial stance position with feet close together might have resulted in small effect sizes when comparing medication On and Off [14]. Furthermore, our cohort of subjects with PD showed only a small, albeit significant, improvement in motor function when taking antiparkinsonian medication as measured by the UPDRS. Levodopa responsiveness might not have been large enough in this sample to cause significant effects on step execution.
Study limitations
The following limitations should be noted. As described above, our PD sample was relatively mild, and results may not generalize to more severe groups. Furthermore, the fact that feet were placed close together may have partially masked across group and across medication differences, as have been previously reported [3, 13]. However, the presence of significant task by group interaction, despite the small stance width and mildly affected participant, suggests that these differences are particularly robust. Finally, we only compared HC to PD subjects Off levodopa. This approach was chosen to analyze the true effect of disease on APA without the confound of differing medication doses. However, comparing healthy controls to people with PD in the On medication state would allow a different interpretation, as this condition is more relevant to everyday functioning of people with PD.
Conclusions
In summary, this study shows that anticipatory postural adjustments need to be interpreted with respect to the specific stepping condition. Compared to self-initiated steps, adding a perceptual cue leads to an increase of size of APA whereas size of APA decreases when performed during compensatory steps. Although the pattern of changes across tasks were similar in PD and HC groups, these changes were less pronounced in people with PD, consistent with reduced flexibility in adapting APAs for specific contexts.
Highlights.
APAs need to be interpreted with respect to specific stepping conditions
In comparison to self-initiated gait, adding a perceptual cue leads to an increase in size of ML APA
Size of ML APA decreases during compensatory stepping
Patients with PD adapt with the same pattern but less pronounced in comparison to HC
Acknowledgments
We thank Chad Murchison for his statistical advice and Heather Schlueter and Bauke Dijkstra for their help with subject recruitment and data collection.
The project was funded by by grants from Coppenrath-Stiftung, Geeste/Groß-Hesepe, Niedersachsen, Germany; The United States Department of Veteran’s Affairs Rehabilitation Research and Development Service (Career Development Award-1: #I01BX007080; PI: DP) & VA Merit Award (E1075-R; PI: FH), the National Institutes of Health (R01 AG006457 29 PI: FH), and the Medical Research Foundation of Oregon (Early Investigator Award; PI: DP). The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
List of abbreviations
- AP
anterior-posterior
- APA
anticipatory postural adjustment
- COM
center of mass
- COP
center of pressure
- FOG
freezing of gait
- HC
healthy control
- LEDD
Levodopa equivalent daily dose
- Mini-BESTest
Mini-Balance Evaluation Systems Test
- ML
medio-lateral
- MoCA
Montreal Cognitive Assessment
- PD
Parkinson’s disease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Suppliers:
a) Motion Analysis Corporation, Santa Rosa, California, USA.
b) Mathworks, Natick, Massachusetts, USA.
b) IBM, 1 New Orchard Rd, Armonk, NY 10504, USA.
Conflict of Interest Statement
Dr. Horak and OHSU have an equity/interest in APDM, a company that may have a commercial interest in the results of the study. This potential conflict of interest has been reviewed and managed by the Research & Development Committee at the Portland VA Medical Center and OHSU. No other authors declare any conflict of interest.
References
- 1.Winter DA. Human balance and posture control during standing and walking. Gait & Posture. 1995;3(4):193–214. [Google Scholar]
- 2.Halliday SE, et al. The initiation of gait in young, elderly, and Parkinson’s disease subjects. Gait Posture. 1998;8(1):8–14. doi: 10.1016/s0966-6362(98)00020-4. [DOI] [PubMed] [Google Scholar]
- 3.Jacobs JV, et al. Knee trembling during freezing of gait represents multiple anticipatory postural adjustments. Exp Neurol. 2009;215(2):334–41. doi: 10.1016/j.expneurol.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.King LA, et al. Preparation for compensatory forward stepping in Parkinson’s disease. Arch Phys Med Rehabil. 2010;91(9):1332–8. doi: 10.1016/j.apmr.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McIlroy WE, Maki BE. Do anticipatory postural adjustments precede compensatory stepping reactions evoked by perturbation? Neuroscience Letters. 1993;164(1–2):199–202. doi: 10.1016/0304-3940(93)90891-n. [DOI] [PubMed] [Google Scholar]
- 6.Breniere Y, Cuong Do M, Bouisset S. Are dynamic phenomena prior to stepping essential to walking? J Mot Behav. 1987;19(1):62–76. doi: 10.1080/00222895.1987.10735400. [DOI] [PubMed] [Google Scholar]
- 7.Martin M, et al. Gait initiation in community-dwelling adults with Parkinson disease: comparison with older and younger adults without the disease. Phys Ther. 2002;82(6):566–77. [PubMed] [Google Scholar]
- 8.Hass CJ, et al. Gait initiation and dynamic balance control in Parkinson’s disease. Arch Phys Med Rehabil. 2005;86(11):2172–6. doi: 10.1016/j.apmr.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 9.Peterson DS, et al. Characterization of Compensatory Stepping in People With Multiple Sclerosis. Arch Phys Med Rehabil. 2016;97(4):513–21. doi: 10.1016/j.apmr.2015.10.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giladi N, Horak FB, Hausdorff JM. Classification of gait disturbances: distinguishing between continuous and episodic changes. Movement disorders. 2013;28(11):1469–73. doi: 10.1002/mds.25672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fasano A, Bloem BR. Gait disorders. Continuum (Minneap Minn) 2013;19(5):1344–82. doi: 10.1212/01.CON.0000436159.33447.69. [DOI] [PubMed] [Google Scholar]
- 12.Schlenstedt C, et al. Postural control and freezing of gait in Parkinson’s disease. Parkinsonism Relat Disord. 2016;24:107–12. doi: 10.1016/j.parkreldis.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 13.Mancini M, et al. Anticipatory postural adjustments prior to step initiation are hypometric in untreated Parkinson’s disease: an accelerometer-based approach. Eur J Neurol. 2009;16(9):1028–34. doi: 10.1111/j.1468-1331.2009.02641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rocchi L, et al. Step initiation in Parkinson’s disease: influence of initial stance conditions. Neurosci Lett. 2006;406(1–2):128–32. doi: 10.1016/j.neulet.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 15.Burleigh-Jacobs A, et al. Step initiation in Parkinson’s disease: influence of levodopa and external sensory triggers. Mov Disord. 1997;12(2):206–15. doi: 10.1002/mds.870120211. [DOI] [PubMed] [Google Scholar]
- 16.Rogers MW, et al. Postural preparation prior to stepping in patients with Parkinson’s disease. J Neurophysiol. 2011;106(2):915–24. doi: 10.1152/jn.00005.2010. [DOI] [PubMed] [Google Scholar]
- 17.Rogers MW, et al. Perturbations of ground support alter posture and locomotion coupling during step initiation in Parkinson’s disease. Exp Brain Res. 2011;208(4):557–67. doi: 10.1007/s00221-010-2504-z. [DOI] [PubMed] [Google Scholar]
- 18.Plate A, et al. Anticipatory postural adjustments are unaffected by age and are not absent in patients with the freezing of gait phenomenon. Exp Brain Res. 2016 doi: 10.1007/s00221-016-4665-x. [DOI] [PubMed] [Google Scholar]
- 19.Hughes AJ, et al. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. Journal of neurology, neurosurgery, and psychiatry. 1992;55(3):181–4. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franchignoni F, et al. Using psychometric techniques to improve the Balance Evaluation Systems Test: the mini-BESTest. Journal of rehabilitation medicine. 2010;42(4):323–31. doi: 10.2340/16501977-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schlenstedt C, et al. Comparing the Fullerton Advanced Balance Scale With the Mini-BESTest and Berg Balance Scale to Assess Postural Control in Patients With Parkinson Disease. Arch Phys Med Rehabil. 2015;96(2):218–25. doi: 10.1016/j.apmr.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Goetz CG, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23(15):2129–70. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 23.Nieuwboer A, et al. Reliability of the new freezing of gait questionnaire: agreement between patients with Parkinson’s disease and their carers. Gait Posture. 2009;30(4):459–63. doi: 10.1016/j.gaitpost.2009.07.108. [DOI] [PubMed] [Google Scholar]
- 24.Peterson DS, Horak FB. The Effect of Levodopa on Improvements in Protective Stepping in People With Parkinson’s Disease. Neurorehabil Neural Repair. 2016;30(10):931–940. doi: 10.1177/1545968316648669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chandler RF. Investigation of inertial properties of the human body. Springfield, Va.: U.S. Dept of Commerce; 1975. [Google Scholar]
- 26.Vaughan CL, Davis BL, O’Connor JC. Dynamics of human gait. Human Kinetics Publishers; 1992. [Google Scholar]
- 27.Horak FB, Nutt JG, Nashner LM. Postural inflexibility in parkinsonian subjects. J Neurol Sci. 1992;111(1):46–58. doi: 10.1016/0022-510x(92)90111-w. [DOI] [PubMed] [Google Scholar]
- 28.Dimitrova D, Horak FB, Nutt JG. Postural muscle responses to multidirectional translations in patients with Parkinson’s disease. J Neurophysiol. 2004;91(1):489–501. doi: 10.1152/jn.00094.2003. [DOI] [PubMed] [Google Scholar]
- 29.Moreno Catala M, Woitalla D, Arampatzis A. Reactive but not predictive locomotor adaptability is impaired in young Parkinson’s disease patients. Gait Posture. 2016;48:177–82. doi: 10.1016/j.gaitpost.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 30.Delval A, et al. Auditory cueing of gait initiation in Parkinson’s disease patients with freezing of gait. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology. 2014;125(8):1675–81. doi: 10.1016/j.clinph.2013.12.101. [DOI] [PubMed] [Google Scholar]
- 31.Peterson DS, Smulders K. Cues and Attention in Parkinsonian Gait: Potential Mechanisms and Future Directions. Front Neurol. 2015;6:255. doi: 10.3389/fneur.2015.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]


