Abstract
The cell surface receptor for epidermal growth factor (EGFR), a receptor tyrosine kinase, is a key player in normal cell growth and proliferation. Mutations in this receptor often lead to oncological transformation and other pathologies. Because of its representation of the receptor tyrosine kinase family and its important role in health and disease, a broad range of studies have been carried out in many laboratories to investigate the structural basis for transmembrane receptor activation and the resulting assembly of cytosolic signaling components. This review highlights two approaches our laboratory has taken to gain more detailed information about both aspects: Surface patterned ligands to examine recruitment of the signaling machinery, and mutational analysis to examine the regulatory role of EGFR’s juxtamembrane segment.
Keywords: Receptor tyrosine kinase, surface patterned ligands, signaling complex, juxtamembrane segment, actin cytoskeleton
Graphical Abstract
I. Introduction
Epidermal growth factor receptor (EGFR) is a member of the ErbB family of receptor tyrosine kinases and is among the most studied cell surface receptors, both in terms of structure-function analysis, and as a prototype oncogene whose multiple mutations give rise to clinically relevant cancers in a number of different cell types. Recent reviews cover a broad range of these related topics [1, 2]. This review highlights two different approaches our laboratory is taking to characterize particular aspects of EGFR structure and signaling mechanisms. To address spatially defined assembly of signaling complexes resulting from activation of this receptor, we use micron-scale patterned ligands containing EGF. To characterize the juxtamembrane region of EGFR that is critical for its regulation in cells, we use mutational analysis. We summarize our studies in the context of existing literature on this receptor, with the goal to provide new insights into the mechanism by which this receptor mediates its complex signaling cascades and the regulation of this process.
II. Surface-patterned EGF reveals spatial recruitment of EGFR and signaling components
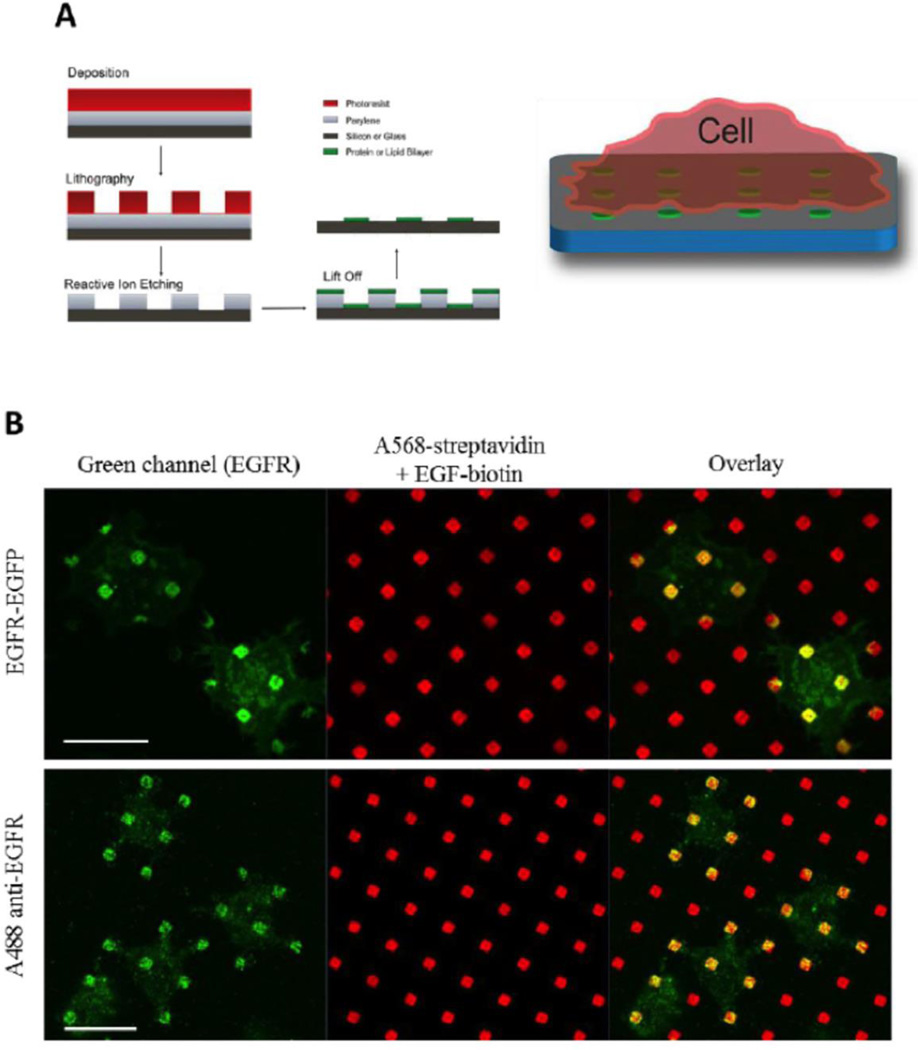
We investigated the association of signaling proteins with EGFR using biotinylated EGF bound to streptavidin that is covalently coupled in an ordered array of micron-sized (fluorescently labeled) features on silicon surfaces (Fig 1; [3]). For our general purposes we utilized an NIH-3T3 cell with a very low level of endogenous EGFR and created new lines stably expressing this receptor (or mutated variants; see Sec. III). We visualized EGFR and co-associating proteins with fluorescence confocal microscopy, using genetically encoded fluorescent proteins or fluorescently labeled specific antibodies or other probes. We previously showed that these stably expressed wt EGFR respond appropriately to soluble EGF and do not undergo spontaneous activation [13], which can be a consequence of over-expression in transiently transfected cells [19].
Figure 1.
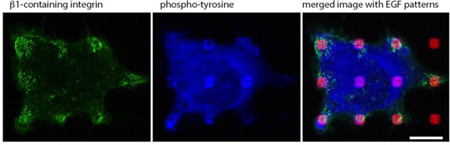
Fabrication of micron-scale patterned features of immobilized EGF (A) and localization of EGFR expressed in NIH 3T3 cells at these patterned features (red) as detected with genetically encoded fluorophores or fluorescently labeled, specific antibodies (green) (B). Adapted from [3].
For analysis, we quantified two-color colocalization by calculating Pearson’s cross-correlation coefficients or by a new radial analysis method we developed initially for this study [3]. The latter method takes advantage of the spatially defined patterned features and calculates the ratio of on-feature to off-feature fluorescence intensity, averaging over multiple features. This radial analysis allows these fluorescence ratios to be compared, such as features located under the cell periphery compared to those located
under the cell center. As expected, EGFR concentrates in the same pattern as the EGF-modified features (Figure 1B), and we also detected stimulated tyrosine phosphorylation that is spatially confined to these same regions. In addition, we observed recruitment of phosphorylated paxillin to activated EGFR at these patterned features, as well as recruitment of EGFP-Ras, MEK, and phosphorylated Erk. Recruitment of each of these proteins evidently occurs in a process that depends on actin polymerization and generation of phosphoinositides, as this recruitment is prevented in the presence of respective pharmacological inhibitors.
Consistent with dependence on actin polymerization, we found that F-actin also concentrates in these patterned regions, with somewhat greater concentration at more peripherally located patterned features under each cell. Interestingly, we also found that β1 integrins (e.g., α4β1, α5β1) co-cluster with patterned EGF, and this occurs to a much greater extent in the cell periphery compared to accumulation at patterned features near the cell center. By comparison, stimulated phosphorylation of tyrosines co-clusters with patterned EGF to the same extent in the center and at the periphery of the cell [3]. In preliminary experiments, we found evidence that eGFP-labeled β3 integrins also cluster at EGF patterned features, but the mechanism for redistribution of these integrins remain to be determined (D. Wakefield, unpublished results).
Our finding that phosphorylated Erk concentrates at the EGF-modified features is particularly notable, because association of this MAP kinase with activated EGF receptors had not been previously detected, athough EGF-stimulated activation of this Ras-MEK-Erk signaling cascade is well established [4]. That this association of stimulated phospho-Erk with activated EGFR depends on actin polymerization suggests EGFR-recruited F-actin serves to stabilize the signaling complex. The asymmetric distribution of β1 integrins to peripheral features often appears with the fluorescent label extending from those features towards the center of the cell. Using this same patterned ligand approach in a subsequent study on IgE receptor-mediated signaling pathways in RBL mast cells, we determined that β1 integrins are delivered from an intracellular pool of recycling endosomes to features at the cell periphery by a trafficking and exocytotic process that depends on Ca2+ influx [5]. This finding is reminiscent of evidence for intracellular trafficking of integrins to the leading edge of chemotaxing hematopoietic cells such as neutrophils [6].
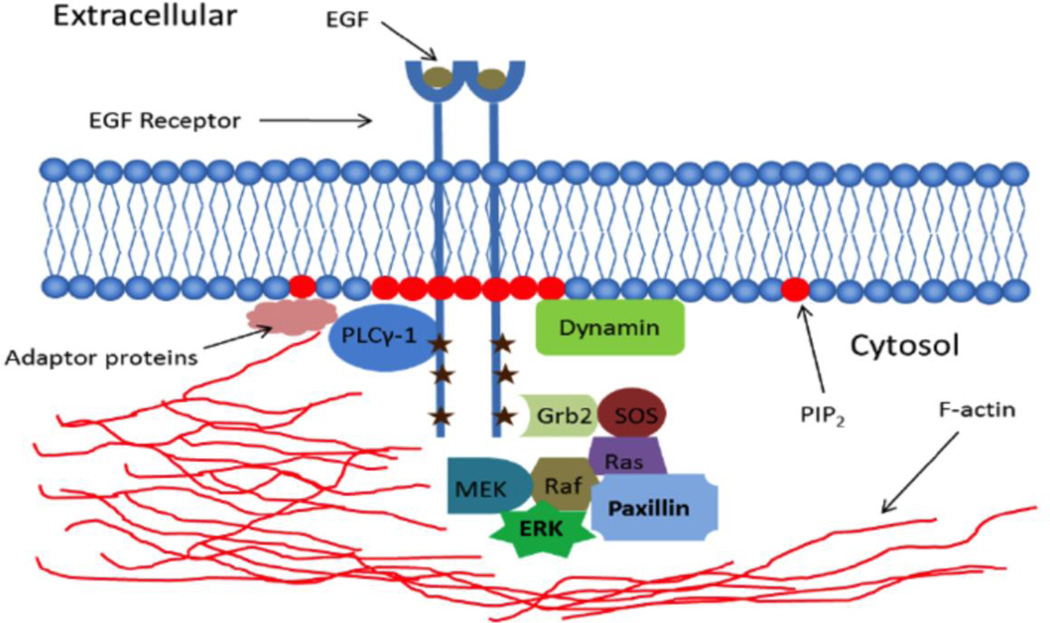
A model summarizing these findings for spatially defined assembly of signaling complexes by EGFR is shown in Figure 2. In this model, activation of EGFR by EGF presented at micron-sized patterned features stimulates tyrosine autophosphorylation by a mechanism similar to that achieved with soluble EGF, i.e., by EGF-induced dimerization and conformation changes of the EGFR extracellular segment, which causes the formation of an asymmetric tyrosine kinase domain dimer in the cytoplasmic segments of this pair [1, 2]. This activation recruits F-actin in a phosphoinositide-dependent manner, possibly mediated by dynamin bridging of these components [3]. F-actin localization at these activated EGFR complexes stabilizes the association of MAP kinase enzymes with these complexes, resulting in accumulation at the patterned features of detectable levels of phospho-ERK, as well as paxillin and PLCγ-1.
Figure 2.
Schematic of proteins and lipids associated with EGFR when activated by EGF localized to micron-scale patterned features. Components observed directly in these studies are described in text. Not shown are β1 integrins, which are trafficked from recycling endosomes to the plasma membrane at EGF-localized, peripheral features (see text).
We found that activated signaling partners are less readily detected in Western blots when EGFR is stimulated by soluble EGF. However, we found evidence for actin-mediated stabilization of signaling complexes, as an optimal concentration of cytochalasin D causes ~50% inhibition of stimulated phosphorylation of Erk by soluble EGF [3]. Thus, it appears that presentation of EGF in micron-scale patterned features stabilizes signaling complexes, and that these assemblies are smaller and more transient when activated by soluble EGF.
Unanswered Questions
The manner by which ligand is presented to specific receptors on cells may be important physiologically for any ligand-receptor pair and particular cell type. It will be interesting to determine whether assembly of signaling pathways initiated by EGFR activated by patterned and surface immobilized EGF is qualitatively different from that activated by EGF in solution. Our results with Erk phosphorylation indicate that actin polymerization contributes to this signaling step in both cases, but it is reasonable to posit that cells interacting with a surface will have different cytoskeletal (re)arrangements than will suspended cells. To gain some insight, the dependence of stimulated Erk phosphorylation on actin polymerization can be quantitatively compared for these two different modes of ligand presentation, as well as for ligand presentation on a small bead. EGFR stimulation by these different modes of ligand presentation have been previously described [21, 22], and the physiological relevance of surface-bound EGFR ligands has been considered [23].
Other downstream readouts, such as PLCγ1 activation, measured by its tyrosine phosphorylation, can also be compared. These assessments will help to determine whether the strong dependence on the actin cytoskeleton we observed for phospho-Erk and other signaling components with surface patterned ligands are indicative of a more general role of F-actin polymerization in regulating downstream signaling for EGFR and potentially for other types of cell surface receptors.
Also of interest is the mechanism by which integrins are recruited preferentially to EGF patterned features in the periphery in NIH-3T3 cells, when EGFR and early signaling complexes associate with patterned EGF ligands to similar extents in both peripheral and central cell regions [3]. We anticipate some dependence of this recruitment on the actin cytoskeleton, which may further facilitate intracellular membrane trafficking that is targeted to peripheral locations. This would be consistent with our recent results with RBL mast cells and IgE receptors binding to patterned ligands across the cell, which caused targeted trafficking of β1 integrins to more peripherally located patterned ligands [5]. In that study, we also found evidence for trafficking of β3-containing integrins to patterned ligands from and intracellular pool. We hypothesize that targeted localization observed with the EGF patterns could be a manifestation of a chemotactic response to EGF. Future experiments could test this hypothesis in a more direct manner by one of several different methods of migration analysis [7]. Engagement of integrins at a stimulated leading edge could drive directional movement by force generation via these interactions [24].
III. Mutagenesis reveals a regulatory role for the juxtamembrane polybasic sequence of EGFR
McLaughlin et al. [8] first proposed that EGFR is structurally constrained to prevent spontaneous activation of its tyrosine kinase activity by electrostatic interactions. These comprise positively charged basic residues, both in the cytoplasmic juxtamembrane segment and on the tyrosine kinase domain surface, interacting with negatively charged phospholipids at the inner leaflet of the plasma membrane. Supporting this hypothesis, the authors showed that a peptide corresponding this juxtamembrane segment (645–660) binds tightly to membrane vesicles containing negatively charged phosphatidyl inositol-4,5-bisphosphate (PIP2) and is displaced by Ca2+/calmodulin complexes, also exhibiting a net negative charge. They suggested that this calmodulin-dependent activation of EGFR is accomplished upon stimulated elevation of cytoplasmic Ca2+ in cells. Because EGF does not routinely mobilize cytoplasmic Ca2+ in all cell types in which it activates EGFR tyrosine kinase activity, it seems unlikely that this is a general mechanism for EGF-dependent activation of EGFR. For the same reason, it is unlikely that Ca2+ binding to PIP2 serves as a general mechanism for competition with PIP2/juxatamembrane interactions. Furthermore, deletion of this juxtamembrane segment results in a non-functional receptor [9, 10; 25], indicating some positive contributions of the juxtamembrane segment to signaling and making this deletion construct less useful for elucidating the normal functional roles of this segment.
Two other studies support the view that the juxtamembrane segment helps facilitate EGFR signaling. Michailidis et al. [11] mutated up to eight basic residues in the juxtamembrane segment to neutral asparagines, and they observed partial inhibition of EGF-stimulated autophosphorylation. Mutation of the first three arginines in this sequence (645–647) resulted in a ~50% decrease in stimulated EGFR tyrosine phosphorylation, and a similar decrease in a more downstream readout, the activation of a Ca2+-dependent chloride channel in Xenopus oocytes. Additional mutations in the eight basic residues in this juxtamembrane sequence did not further inhibit these responses significantly. Because a peptide containing this juxatmembrane sequence bound to PIP2 in a surface plasmon resonance assay, but failed to bind when these eight residues were neutralized to asparagines, the authors concluded that these basic residues in the intact receptor bind to membrane phosphoinositides. They then showed that stimulated phosphorylation of EGFR in oocytes is inhibited by wortmannin, a phosphoinositide (PI) kinase inhibitor, or co-expression of synaptojanin, a PI5-phosphatase. They further showed that co-expression of a PI5-kinase with EGFR enhances stimulated tyrosine phosphorylation of EGFR, altogether providing evidence that manipulation of PIP2 levels regulates stimulated EGFR activity. Importantly, they showed that EGFR in which the eight basic residues in the juxtamembrane region were mutated to asparagines fails to be activated by PI5-kinase overexpression, providing strong evidence that these residues (or a subset) are involved in functional regulation of EGFR by PIP2. Similar conclusions were reached for EGFR expressed in mammalian cells [11].
In a complementary study, molecular dynamics simulations predicted that, even in the EGFR activated dimer, these basic residues (645–647) can associate with PIP2 in the inner leaflet of the plasma membrane, supporting the notion that these associations contribute positively to EGF-stimulated EGFR activation [12]. This is despite the fact that the sequence containing these residues forms an anti-parallel helix between the activated dimers that also sequesters hydrophobic side chains, which normally insert into the inner leaflet of the plasma membrane in unstimulated EGFR [1].
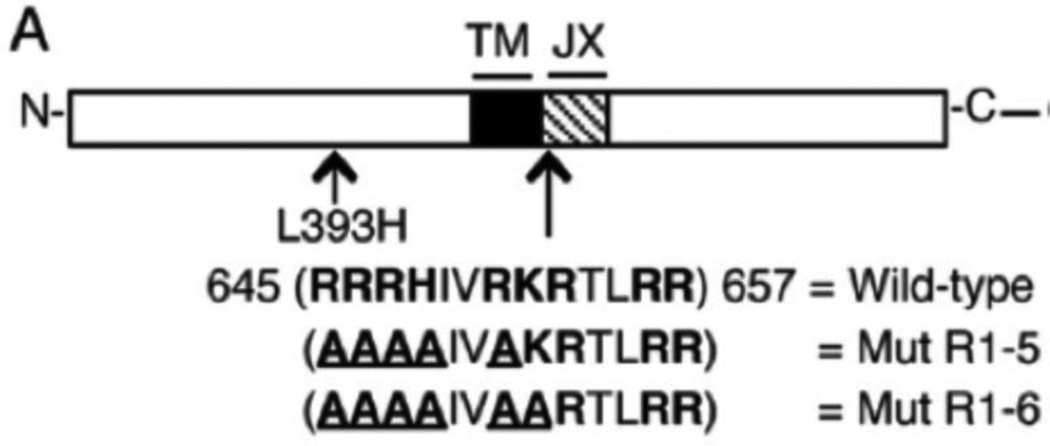
We investigated the structure-constraining regulatory role for basic residues in the juxtamembrane segment in activated EGFR, testing the hypothesis that these positively charged amino acids suppress basal EGFR activity in cells [8]. We began this study by sequentially mutating potentially positive amino acid residues in this juxtamembrane segment to alanines. Mutation of the first four arginines in this sequence, together with a histidine, did not detectably alter cell transformation by this receptor when stably expressed in mouse NIH 3T3 cells, but an additional mutation of the first lysine in this sequence to yield a construct that we named EGFR Mut R1-6 caused a dramatic change in receptor phenotype (Figure 3). This Mut R1-6 construct exhibited a low level of spontaneous tyrosine phosphorylation, and, more importantly, this mutation caused EGF-independent, anchorage-independent cell proliferation in a standard soft agar assay [13]. Thus, this construct effectively caused a transforming phenotype similar to that observed in EGFR with several single amino acid point mutations that originated from human cancers [14, 15].
Figure 3.
Wild-type and mutant EGFR constructs used in studies. The juxtamembrane (JX) sequence is shown, with basic amino acids in bold and mutated residues underlined TM = transmembrane. GFP indicates the C-terminal location of the eGFP tag, when present. From [13].
This Mut R1-6 construct was largely expressed at the plasma membrane, similar to the wild type EGFR. Thus, mutation of the first three arginines in this sequence to alanines did not alter its normal plasma membrane expression or its responsiveness to EGF [13]. We also identified a mutant similar in sequence to Mut R1-6, but with a single additional spontaneous mutation in residue L393 that caused the receptor to be fully retained in the endoplasmic reticulum (ER). This point mutation (L393H) was sufficient to cause full ER retention when introduced into a wt EGFR, and it did not cause any basal phosphorylation by itself. However, remarkably, the ER-retained version of Mut R1-6 was also transforming in the soft agar assay, indicating a lack of requirement for cell surface expression for this property. Dissection of the signaling pathways activated by Mut R1-6 indicated a strong dependence on PI3-kinase and mTOR, with some role for the MAP kinase signaling cascade [13]. In summary, these results established that basic residues in the juxtamembrane segment play a critical role in regulating the basal activity of EGFR, suggesting that their association with negatively charged phospholipids, including phosphoinositides, mediate this regulation.
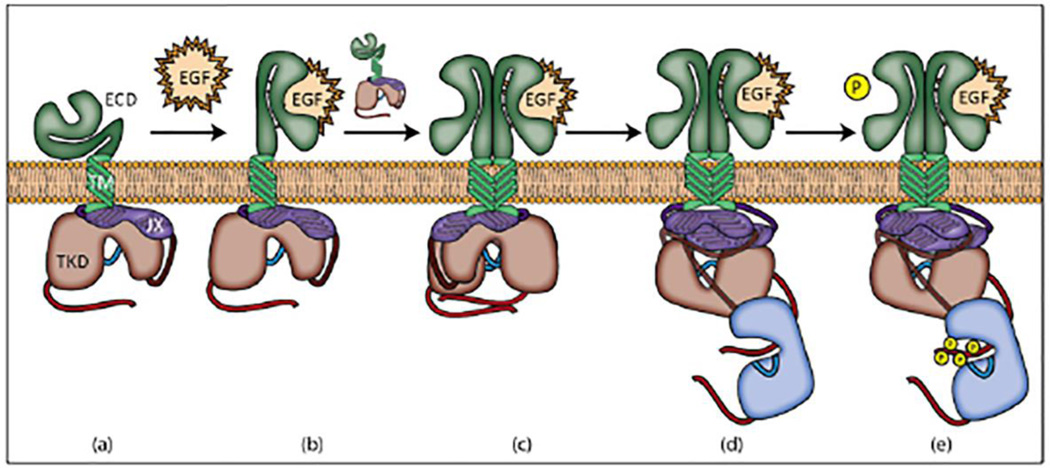
A model for EGFR activation that is consistent with these findings is depicted in Figure 4. In this model, binding of EGF to sites in domains I and III causes a conformational change that exposes the dimerization interface in domain II [16]. Dimerization with a second EGFR causes additional changes in the interaction between the transmembrane helices. These interactions lead to rearrangements in the juxatmembrane segments to form an anti-parallel alpha helix such that threonine side chains, which are buried in the inner leaflet of the plasma membrane prior to activation, become sequestered in the helix core, while at least some of the positively charged side chains of the basic amino acids in this sequence still associate with negatively charged phospholipids at the inner leaflet. This structural alteration is accompanied by conformational changes in the tyrosine kinase domains that lead to formation of an asymmetric dimer pair in which the C-terminus of the activating kinase inserts in the active site of the receiving kinase [1].
Figure 4.
Steps in EGFR activation. (a) Inactive EGFR. (b) EGFR is activated upon ligand binding. This induces a conformational change in extracellular domains I, II, and III. (c) The activated receptor dimerizes with another EGFR monomer driven by extracellular interactions. Domains I and II contribute to the binding site for EGF, and domain II contributes the dimerization interface; binding of a single EGF ligand to the activated dimer represents negative cooperativity when EGFR is in this state [2]. (d) Intracellular region then assumes the asymmetric kinase conformation. (e) The receiving receptor (blue) trans-phosphorylates tyrosine residues in the activating receptor’s (red) tail region.
Interestingly, the negative cooperativity that is normally observed for a second EGF binding to EGFR dimers appears to depend on the juxtamembrane segment. Tethering this region to the inner leaflet of the plasma membrane via palmitoylation abolishes the negative cooperativity and substantially reduces autophosphorylation (by ~80%) [20]. These findings further highlight regulation by, and structural changes occuring in, the juxtamembrane segment to facilitate coupling between the extracellular segment and the kinase domain during EGFR activation.
Unanswered Questions
The constitutive activation observed for EGFR Mut R1-6 raises the question of its oligomeric state. Although this mutant may spontaneously dimerize, some evidence indicates that the highly oncogenic deletion mutant EGFRvIII is both active as a monomer and intracellularly retained [17]. This question may be addressed for Mut R1-6 with chemical crosslinking approaches to capture potential dimers. A highly soluble, non-membrane-permeable crosslinker, such as bissulfosuccinimidyl suberate, can be used to capture EGFR dimers at the cell surface [18], while a membrane-permeable crosslinker, such as disuccinimydyl propionate, can be used to capture intracellular EGFR dimers. Analysis by western blotting should reveal dimers formed by Mut R1-6 in the presence or absence of its ligand, EGF. Appearance of dimers under these experimental conditions would indicate that the R1-6 mutation facilitates spontaneous EGFR dimers in an activated conformation. Comparing R1-6 and R1-6(L393H) mutations with permeable and non-permeable chemical crosslinkers is likely to yield interesting results, indicating whether dimers of these mutants form spontaneously at the plasma membrane compared to the ER membrane. No appearance of dimers under these experimental conditions would not be definitive, but would point to the possibility that R1-6 and/or R1-6(L393H) facilitate kinase activation in EGFR in the absence of dimerization.
IV. Concluding Remarks
EGFR and its interacting partners represent a complex molecular machine that plays fundamental roles in cell growth and division. As such, its activation is highly regulated, and the perturbation of this regulation by changes in its environment or by mutational changes that relieve structural constraints on spontaneous activation can give rise to uncontrolled cellular responses, which often lead to unregulated cell proliferation and oncogenesis. Patterned, immobilized EGF [3], has enabled visualization of stable complexes of activated EGFR with paxillin, PLCγ-1, and members of the MAP kinase casade, together with F-actin, and supports the view that molecular machines are assembled as a consequence of effective ligand-receptor engagement. Detection of phosphotyrosine on EGFR and phospho-Erk as part of these complexes confirms their functional activation. This approach can be further utilized to assess the activation status of associated PLCγ-1, and its capacity to initiate Ca2+ mobilization. Mechanical contributions to cellular responses, such as participation of integrins in responses of surface-adherent cells can also be further investigated with this approach.
Our mutational studies on EGFR provide direct evidence that basic residues in its cytoplasmic juxatmembrane segment play an important role in regulating the basal activity of this receptor in the absence of its ligand, EGF. Mutation of as few as five of these basic residues to uncharged amino acids is sufficient to convert the unliganded receptor from quiescent to active, conferring a transforming phenotype on the cells in which this receptor is expressed [13]. It is now clear that the balance between activated and quiescent EGFR is intricately regulated, both by charged amino acids in the juxtamembrane segment, and by effective dimerization of EGFR in the plasma membrane. Studies in which just a few additional mutations of positively charged amino acids in the juxtamembrane segment prevent EGFR activation [9, 11], with no detectable basal activation, indicate how delicately the charge balance in this region regulates receptor activity. At the same time, moderate overexpression of the wild type EGFR is sufficient to confer spontaneous activation, confirming that the formation of a threshold level of active dimers can be driven by mass action, independent of ligand binding [19].
Studies described in this review are necessarily limited in scope, and, in general, many of the complex features that contribute to activation of EGFR and consequent assembly of its signaling machine remain to be determined. Research efforts continue in many laboratories to delineate regulated activation of EGFR, as a prototype cell surface receptor and as a key player in health and disease. Future studies will undoubtedly uncover new aspects by which cell activation by EGFR is regulated in vivo, and how disruption of that regulation leads to cancer and other pathologies.
Highlights.
Micron-scale patterned EGF stimulates the formation of localized signaling complexes.
F-actin stabilizes these complexes, including the MAP kinase cascade.
β1-containing integrins are recruited preferentially to peripheral patterns.
Mutational analyses reveal a regulatory role for the polybasic juxtamembrane sequence
This charge neutralization causes constitutive EGFR activation & cell transformation.
Acknowledgments
Several members of our group and collaborators contributed to this work as shown in cited publications. Research described was supported at various times by grants from the National Institute of Health: R01AI022449, R01GM117552, and R01AI018306. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank Jordan Mohr for helpful discussions and Figure 4.
Abbreviations
- EGFR
epidermal growth factor receptor
- EGF
epidermal growth factor
- MAP kinase
mitogen-activated protein kinase
- PIP2
phosphatidylinositol-4,5-bisphosphate
- Mut R1-6
EGFR mutant with six residues mutated to alanines in the juxtamembrane segment
- PLCγ-1
phospholipase Cγ-1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Endres NF, Barros T, Cantor AJ, Kuriyan J. Emerging concepts in the regulation of the EGF receptor and other receptor tyrosine kinases. Trends in Biochemical Sciences. 2014;39:437–446. doi: 10.1016/j.tibs.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Lemmon MA, Schlessinger J, Ferguson KM. The EGFR family: not so prototypical receptor tyrosine kinases. Cold Spring Harbor Perspectives in Biology. 2014;6:a020768. doi: 10.1101/cshperspect.a020768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singhai A, Wakefield D, Bryant KL, Hammes SR, Holowka D, Baird B. Spatially defined EGF receptor activation reveals an F-actin-dependent phospho-Erk signaling complex. Biophysical Journal. 2014;107:2639–2651. doi: 10.1016/j.bpj.2014.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McKay MM, Morrison DK. Integrating signals from RTKs to ERK/MAPK. Oncogene. 2007;26:3113–3121. doi: 10.1038/sj.onc.1210394. [DOI] [PubMed] [Google Scholar]
- 5.Wakefield DL, Holowka D, Baird B. The FcεRI Signaling Cascade and Integrin Receptor Trafficking Converge at Patterned Ligand Surfaces. doi: 10.1091/mbc.E17-03-0208. Submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pierini LM, Lawson MA, Eddy RJ, Hendey B, Maxfield FR. Oriented endocytic recycling of α5β1 in motile neutrophils. Blood. 2000;95:2471–2480. [PubMed] [Google Scholar]
- 7.Lee J, Veatch SL, Baird B, Holowka D. Molecular mechanisms of spontaneous and directed mast cell motility. J Leukoc Biol. 2012;92:1029–1041. doi: 10.1189/jlb.0212091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McLaughlin S, Smith SO, Hayman MJ, Murray D. An electrostatic engine model for autoinhibition and activation of the epidermal growth factor receptor (EGFR/ErbB) family. J Gen Physiol. 2005;126:41–53. doi: 10.1085/jgp.200509274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aifa S, Aydin J, Nordvall G, Lundstrom I, Svensson SP, Hermanson O. A basic peptide within the juxtamembrane region is required for EGF receptor dimerization. Experimental Cell Research. 2005;302:108–114. doi: 10.1016/j.yexcr.2004.08.032. [DOI] [PubMed] [Google Scholar]
- 10.Jura N, Endres NF, Engel K, Deindl S, Das R, Lamers MH, Wemmer DE, Zhang X, Kuriyan J. Mechanism for activation of the EGF receptor catalytic domain by the juxtamembrane segment. Cell. 2009;137:1293–1307. doi: 10.1016/j.cell.2009.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michailidis IE, Rusinova R, Georgakopoulos A, Chen Y, Iyengar R, Robakis NK, Logothetis DE, Baki L. Phosphatidylinositol-4,5-bisphosphate regulates epidermal growth factor receptor activation. Pflugers Archiv : European Journal of Physiology. 2011;461:387–397. doi: 10.1007/s00424-010-0904-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abd Halim KB, Koldso H, Sansom MS. Interactions of the EGFR juxtamembrane domain with PIP2-containing lipid bilayers: Insights from multiscale molecular dynamics simulations. Biochimica et Biophysica ACTA. 2015;1850:1017–1025. doi: 10.1016/j.bbagen.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bryant KL, Antonyak MA, Cerione RA, Baird B, Holowka D. Mutations in the polybasic juxtamembrane sequence of both plasma membrane- and endoplasmic reticulum-localized epidermal growth factor receptors confer ligand-independent cell transformation. The Journal of Biological Chemistry. 2013;288:34930–34942. doi: 10.1074/jbc.M113.513333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, Louis DN, Christiani DC, Settleman J, Haber DA. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. The New England Journal of Medicine. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 15.Red Brewer M, Choi SH, Alvarado D, Moravcevic K, Pozzi A, Lemmon MA, Carpenter G. The juxtamembrane region of the EGF receptor functions as an activation domain. Molecular Cell. 2009;34:641–651. doi: 10.1016/j.molcel.2009.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dawson JP, Berger MB, Lin CC, Schlessinger J, Lemmon MA, Ferguson KM. Epidermal growth factor receptor dimerization and activation require ligand-induced conformational changes in the dimer interface. Molecular and Cellular Biology. 2005;25:7734–7742. doi: 10.1128/MCB.25.17.7734-7742.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ekstrand AJ, Liu L, He J, Hamid ML, Longo N, Collins VP, James CD. Altered subcellular location of an activated and tumour-associated epidermal growth factor receptor. Oncogene. 1995;10:1455–1460. [PubMed] [Google Scholar]
- 18.Fanger BO, Stephens JE, Staros JV. High-yield trapping of EGF-induced receptor dimers by chemical cross-linking. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1989;3:71–75. doi: 10.1096/fasebj.3.1.2783412. [DOI] [PubMed] [Google Scholar]
- 19.Endres NF, Das R, Smith AW, Arkhipov A, Kovacs E, Huang Y, Pelton JG, Shan Y, Shaw DE, Wemmer DE, Groves JT, Kuriyan J. Conformational coupling across the plasma membrane in activation of the EGF receptor. Cell. 2013;152:543–556. doi: 10.1016/j.cell.2012.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacDonald-Obermann JL, Pike LJ. The intracellular juxtamembrane domain of the epidermal growth factor (EGF) receptor is responsible for the allosteric regulation of EGF binding. The Journal of Biological Chemistry. 2009;284:13570–13576. doi: 10.1074/jbc.M109.001487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ito Y, Chen GP, Imanishi Y. Micropatterned immobilization of epidermal growth factor to regulate cell function. Bioconjugate Chem. 1998;9:277–282. doi: 10.1021/bc970190b. [DOI] [PubMed] [Google Scholar]
- 22.Verveer PJ, Wouters FS, Reynolds AR, Bastiaens PIH. Quantitative imaging of lateral ErbB1 receptor signal propagation in the plasma membrane. Science. 2000;290:1567–1570. doi: 10.1126/science.290.5496.1567. [DOI] [PubMed] [Google Scholar]
- 23.Stabley D, Retterer S, Marshall S, Salaita K. Manipulating the lateral diffusion of surface-anchored EGF demonstrates that receptor clustering modulates phosphorylation levels. Integr Biol-Uk. 2013;5:659–668. doi: 10.1039/c3ib20239a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stabley DR, Jurchenko C, Marshall SS, Salaita KS. Visualizing mechanical tension across membrane receptors with a fluorescent sensor. Nat Methods. 2012;9:64-U172. doi: 10.1038/nmeth.1747. [DOI] [PubMed] [Google Scholar]
- 25.Thiel KW, Carpenter G. Epidermal growth factor receptor juxtamembrane region regulates allosteric tyrosine kinase activation. P Natl Acad Sci USA. 2007;104:19238–19243. doi: 10.1073/pnas.0703854104. [DOI] [PMC free article] [PubMed] [Google Scholar]