Abstract
Pro-inflammatory reaction by the body occurs acutely in response to injury that is considered primarily beneficial. However, sustained pro-inflammatory cytokines observed with chronic pathologies such as metabolic syndrome, cancer, and arthritis are detrimental and in many cases is a major cardio-vascular risk factor. Pro-inflammatory cytokines such as interleulin-1 (IL-1), IL-6, and tumor necrosis factor α (TNFα) have long been implicated in cardiovascular risk and considered to be a major underlying cause for heart failure. The failure of the anti-TNFα therapy for heart failure indicates our elusive understanding on the dichotomous role of pro-inflammatory cytokines on acutely beneficial effects versus long-term deleterious effects. Despite these well-described observations, less is known about the mechanistic underpinnings of pro-inflammatory cytokines especially TNFα in pathogenesis of heart failure. Increasing evidence suggests the existence of an active cross-talk between the TNFα receptor signaling and G-protein coupled receptors (GPCRs) like β-adrenergic receptor (βAR). Given that βARs are the key regulators of cardiac function, the review will discuss current state of understanding on the role of pro-inflammatory cytokine TNFα in regulating βAR function.
Introduction
Pathophysiological consequences of inflammation have long been recognized1 but evidence of its involvement in contributing to and potentially mediating heart failure has been recognized in the last two decades2. Since then intense attention has been paid to pro-inflammatory cytokines that are involved in regulation of cardiac structure and function and more so on their critical role in progression of heart failure. The recognition of association between sustained elevated levels of tumor necrosis factor α (TNFα) and the stage of heart failure3 led to the rationale of therapeutically targeting TNFα in several clinical trials4–7. However, counterintuitive to the evidence, anti- TNFα treatment resulted in worsening of heart failure showing our incomplete understanding on the role of TNFα in cardiac remodeling and pathogenesis of heart failure. Given these observations, it becomes imperative to revisit the role of TNFα in pathogenesis of heart failure in the context of recent advances in understanding molecular mechanisms involved in TNFα signaling. Studies have shown that pro-inflammatory cytokines especially TNFα blunts the responsiveness of G protein coupled receptors (GPCRs) particularly beta-adrenergic receptors (βARs) impairing contractile function of the cardiac myocytes8–10. Similarly, studies have also shown that βAR receptor signaling can mediate beneficial cardiac effects through TNFα receptor 2 (TNFR2) in contrast to TNFR111. In addition, studies have also shown that the expression of TNFR associated factor 2 (TRAF2) could determine the outcome of the cardiac phenotype in response to TNFα12. Given the failure of the anti-TNFα therapy in heart failure4–7 and the key role βARs play in regulating cardiac function, we will summarize the recent advances in understanding the cross-talk between TNFα signaling and βAR, a prototypical G-protein coupled receptor that may provide new insights into the well-known role of inflammation in mediating cardiac dysfunction and asthma exacerbation13–18.
Cytokines and inflammation
Inflammatory response is a primordial reaction of the body to any kind of stress that could involve a simple injury to a complex infection. Despite the knowledge of the beneficial role inflammation plays, it is now recognized as a double-edged sword. The initial acute phase of the inflammatory response is multi-faceted involving synergistic activation of T and B cells in parallel with hepatic induction of acute phase proteins like interleukin 1 (IL-1), IL-6 and TNFα19. The acute phase is followed by a feed-forward pro-inflammatory loop that is selectively localized to the area of infection or smooth muscle injury wherein there is extravasation of leukocytes, erythrocytes, and plasma components into the injured tissue. This is classically associated with activation of macrophages, T lymphocytes and secretion of factors by activated smooth muscle cells including IL-1, C-reactive protein and TNFα leading to significant acute inflammation20, 21. Resolution of acute inflammation is dynamically driven by a tight interplay between anti- and pro-inflammatory cytokines. Major anti-inflammatory cytokines include IL-4, IL-6, IL-10, IL-11, and IL-13 while, transforming growth factor β (TGF-β), IL-1, TNFα, gamma-interferon (IFNγ), IL-12, IL-18 and granulocyte-macrophage colony stimulating factor are well known pro-inflammatory cytokines22–24. Acute phase is classically required for physiological effects of tissue repair, immune response and resolution of injury whereas often, chronic inflammatory response will lead to pathological effects. However, if left unchecked, this acute pro-inflammatory response can transition to chronic inflammation, a biochemical phenotype observed in conditions like cancer, arthritis, Alzheimer’s disease, auto-immune disease20, 25–28 including cardiovascular pathology21. Unlike acute inflammation, chronic inflammation is characterized primarily by tissue infiltration by lymphocytes and macrophages setting a stage and a site for generation of pro-inflammatory cytokines. As a consequence, there is an excessive production and sustained release of pro-inflammatory cytokines into the circulation. Apart from having the influence on the organ dysfunction at the site of production, higher levels of circulating pro-inflammatory cytokines can have deleterious effects on the remote organs. Since the heart is in close proximity to circulating factors, these high levels of cytokines can impact cardiac function via effects on both myocyte contractility and the extracellular matrix29, 30 involving molecular signaling mechanism are being intensely investigated. A vast majority of the co-morbid conditions like hypertension, diabetes, obesity and dyslipidemia that are annotated with cardiovascular risk have chronic inflammation associated with elevated levels of pro-inflammatory cytokines and, in particular TNFα which could have greater impact on cardiac function and remodeling31–36. In addition to co-morbid conditions associated with elevated cytokines, lung inflammation is a major cause for asthma exacerbation and less is known about how TNFα modulates lung remodeling and function.
Pro-inflammatory cytokines in comorbid conditions
Although pro-inflammatory cytokines including TNFα are elevated in chronic pathologies like cancer, arthritis, autoimmune disease37–40, these have not yet been categorized as cardiovascular risk or comorbid condition for heart failure. Interestingly, increasing evidence has shown that cancer therapy that uses anti-epidermal growth factor receptor seems to cause collateral deleterious cardiac remodeling41, 42. In contrast, asthma, hyperlipidemia, hypertension, atherosclerosis, diabetes and obesity are all characterized by subtle or overt elevation of pro-inflammatory cytokine TNFα and are co-morbid conditions due to their deleterious cardiovascular effects21, 43, 44. These observations suggest that all chronic pathologies that have elevated pro-inflammatory cytokines perhaps may not all be conditions for cardiovascular risk. In this context, research in heart failure (HF) has long been in the realm of neuro-hormonal activation and sympathetic systems involving both the animal studies and clinically in patient populations. Blockade of these pathways demonstrated significant beneficial outcomes in a variety of patient populations including HF with reduced ejection fraction45. However, these neuro-hormonal blockade therapies have unfortunately been ineffective in conditions of HF with preserved ejection fraction46, 47 and they tend to represent a major proportion of the patients admitted for HF. Interestingly, elevated serum pro-inflammatory cytokines and adverse clinical outcomes are common to both HF with reduced and preserved EF48–51. Given the inefficacy of the neuro-hormonal blockade in HF with preserved ejection fraction, understanding the mechanistic underpinnings of pro-inflammatory cytokines in contributing towards HF may hold a key to providing effective therapies as cytokines are universally elevated in all forms of HF. In fact, the magnitude of the elevation of pro-inflammatory cytokines in chronic HF is significantly less than what is classically observed in autoimmune diseases or acute infections. This suggests that low-grade chronic inflammation may be an important contributor to the maintenance and/or functional cardiac deterioration of patients with clinically established chronic HF7, 52, 53. These observations bring-to-fore the conundrum of the current understanding of some of the intricate and context-dependent mechanisms by inflammatory cells and pathways can influence HF in acute setting. However, few of these insights have been extended to assessing the role of inflammation once chronic HF has been established. In this context, a key question that remains to be addressed is whether pro-inflammatory cytokines are the “cause” or “effect” of HF. Therefore, it is important to dissect the cross-talk occurring between pro-inflammatory cytokines and neuro-hormonal signaling mechanisms as evidences show that both are elevated and underlie HF. Since βARs are one of the key regulators of cardiac function and as pro-inflammatory cytokine TNFα is universally upregulated in comorbid conditions, it is imperative to understand their interactive role, which may shed light on the key processes/pathways considered as “cardiovascular risk”.
Pro-inflammatory cytokines and cardiac dysfunction
In addition to the role of pro-inflammatory cytokines as key components of “cardiovascular risk” in comorbid conditions, broad range of cardiac diseases per se are also associated with elevated cytokines. These include HF54, 55, cardiac reperfusion injury56, myocarditis57, cardiac allograft rejection58, 59, and sepsis related cardiac dysfunction60–63. These observations indicate the importance of inflammation in HF, as biological effects of pro-inflammatory cytokines were sufficient to provoke a heart failure phenotype in experimental animals and in humans49. Thus, the evidence brings-to-fore the idea that heart failure progresses as a result of the deleterious effects exerted by endogenous cytokines signaling cascades on the heart and the secondary effects of the exogenous cytokines in circulation64. Hence, similar to sustained neuro-hormonal activation in heart failure, chronic inflammation may also contribute to worsening heart failure due to the deleterious effects of sustained inflammatory signaling. The pathophysiological effects of chronic pro-inflammatory cytokines have been reviewed extensively29, 54 including their role in myocyte function29 and tissue remodeling30, 49. Studies have documented that pro-inflammatory cytokines modulate contractile function and these effects can be classified into immediate and delayed effects. The immediate effects of pro-inflammatory cytokines are identified to be on EC coupling19, 28–40, on nitric oxide production by NOS65–82, on Sphingomyelinase-dependent signaling56, 71, 74, 76, 81–95, and/or on phospholipase A2 (PLA2) and arachidonic acid (AA) activation85, 96–102. In contrast, to these immediate effects, delayed effects that play a key role in modulating contractile function include altered βAR signaling and loss of βAR responsiveness to its cognate βAR agonist like isoproterenol10, 71, 103, 104.
Innate immunity in cardiac remodeling and cellular basis underlying transition to failure
Despite the idea that inflammatory cytokines could be key drivers in regulating cardiac remodeling, the role of innate immunity is starting to be appreciated49. Innate immunity is critical for providing defensive inflammatory response to onset of pathogen or tissue injury239. The protective role of this inflammatory response in the context of increased mechanical overload on the heart or cardiac injury is being recognized in its contribution to overall cardiac remodeling240. The concept that all inflammation in the realm of cardiac remodeling is deleterious needs to be re-visited given that innate immunity which mediates beneficial pro-inflammatory response may initially mitigate deleterious remodeling and provide benefit. However, with persistent inflammation the “adaptive inflammatory response” that occurs to maintain homeostasis can quickly become maladaptive. This graded inflammatory response or “parainflammation” occurs in the heart with the key purpose of maintaining homeostasis in response to cardiac injury which however with time transitions from being physiologic to pathological. Such a paradigm brings to fore the idea the idea that innate inflammatory response is reparative which becomes deleterious with time in presence of chronic cardiac tissue injury that occurs with increasing mechanical load. Currently, it is not known what determines this switch but the underpinning for such transition is thought to be mediated by the type of immune cells that infiltrate the myocardium and their ability to differentially activate fibroblasts to myofibroblasts241.
It is considered that the key aspect of the inflammatory response in the myocardium post injury involves active cross talk between the cardiomyocytes, vascular cells, fibroblasts and immune cells in that microenvironment. However, it is the necrotic cardiomyocytes that are thought to initiate the stimulus following tissue injury leading to the reparative response which could then transition to parainflammation. The charge of initial inflammatory response is thought to be mediated by resident monocytes associated with neutrophil infiltration in response to necrotic myocytes242, 243. These neutrophils at the sites of injury release proteolytic enzymes involved in clearance of debris from the wounded cells but may also cause collateral damage of targeting intact myocytes causing cytotoxicity. Though less is known about it, this phenomenon however has the potential for prolonging the inflammatory response providing a site for more monocyte recruitment which may decide the overall inflammatory outcome. Consistent with this idea, multiple groups have reported the observation of two key waves of monocyte infiltration into the injured myocardium244–246. Such a concept was based on the observation of early recruitment of Ly6ChiCCR2+CX3CR1lo monocytes followed later by Ly6CloCCR2-CX3CR1hi monocytes which are thought to be involved in injury response followed by low anti-inflammatory wound resolution response. Further studies have shown that in addition to recruitment there could be a switch from recruited Ly6Chi to Ly6Clo phenotype in the monocytes247. It is important to note that these Ly6Chi monocytes are mobilized from spleen as an immediate response and currently the mechanisms that allows for mobilization of the monocytes from spleen is being actively investigated248. However, in this context the key component that drives both the reparative as well as long term deleterious effects is due to pro-inflammatory cytokines which mediates cardiac dysfunction by both canonical and non-canonical signaling pathways.
Pro-inflammatory cytokines and GPCR dysfunction
The role of cytokines in determining GPCR function is clearly reflected by the dysfunction of proto-typical GPCR - βAR in the heart and lung airways. A key hallmark of the contractile dysfunction in heart induced by pro-inflammatory cytokines is impaired sensitivity of βARs to catecholamines8, 9, 105–107. Similarly, inflammation is a known instigator of acute asthmatic response due to loss in bronchodilator capacity of lungs108, 109. This is also associated with impaired ability of the βARs to responds to β-agonist suggesting a loss in βAR function due to inflammation. Though less is understood about the cross-talk in lungs, studies in the cardiac systems have shown that βAR sensitization is mediated both by NO-dependent mechanisms as well as by an apparently NO- and cGMP-independent functional uncoupling of the βAR to Adenylyl Cyclase8, 9. Prolonged exposure to IL-1β and TNFα resulted in reduced contractility augmentation and cAMP accumulation in response to βAR stimulation with isoproterenol. These phenomena occurred without changes in βAR density, binding affinity, or phosphodiesterase activity8, 9. Subsequent studies have also demonstrated uncoupling of βAR stimulation to both cAMP accumulation and Ca2+ transients after prolonged exposure to the cytokines IL-1β and TNFα110, 111.
Even though studies have implicated G-protein Gi mediated alterations in βAR signaling, the precise mechanism has not been fully defined8. Some studies have indicated accumulation of Gi proteins in cardiomyocyte membranes by prolonged exposure to cytokines such as TNFα112, 113 while, other studies have indicated otherwise114 suggesting that more indepth studies are required to better understand the role of Gi proteins. In addition to the role of Gi proteins, mechanistic underpinnings on the influence of pro-inflammatory cytokines specifically TNFα on βAR dysfunction was identified by our studies10. The studies showed that βARs are desensitized when exposed to TNFα through G-protein coupled receptor kinases (GRKs) mediated phosphorylation of the receptors. Normally, GRKs desensitize βARs by phosphorylating the receptors in response to its agonist epinephrine/norepinephrine. In contrast to this classical mechanism, TNFα non-canonically recruits GKR2 to the βAR complex and mediates desensitization accounting for the potential loss in contractile capability. Although broad deleterious effects of TNFα have long been known, this study shows that the mechanistic impact of cytokines occurs in close proximity to the βARs. Alternatively, structural and modeling studies have shown that alteration in the S-nitrosylation pathways may modulate G proteins coupling to βARs115. However, whether such a mechanism ensues following prolonged cytokine exposure is not known. Furthermore, in neonatal rat cardiomyocytes, it has been shown that prolonged exposure to low concentrations of TNFα is insufficient to induce iNOS or increase NO content and yet these cells show loss of βAR responsiveness116, 117, supporting a NO-independent mechanism for βAR dysfunction.
Several studies have implicated NO, derived from iNOS, as a mediator of pro-inflammatory cytokine induced loss of inotropic effect including βAR responsiveness either under basal conditions or post inotropic stimulation70, 75, 76, 78, 111, 114, 118–131. Series of investigations have revealed that prolonged exposure to conditioned medium of activated macrophages or to combinations of specific cytokines, adult rat ventricular myocytes lose βAR responsiveness but still maintain contraction114, 124–128 suggesting that NO generation and βAR responsiveness can have overlapping and yet independent effects on cardiac function. This paralleled very well with cardiomyocyte induction of iNOS125, 126, increased cGMP114, 124 and nitrite content in the medium, and directly measured NO release125. Furthermore, βAR hypo-responsiveness was reversed by treatment with NOS inhibitors strongly implicating iNOS-derived NO in the pathogenesis of delayed contractile dysfunction. Moreover, adult cardiomyocytes exposed to a combination of TNFα, IL-1β, and IFN-γ had iNOS induction, increased NO synthesis, and increased cell death132. Importantly, these effects were prevented by the co-treatment with NOS inhibitors127. The functional results using specific combinations of cytokines indicated the synergistic cardio-depressant effects of the pro-inflammatory cytokines IL-1β, TNFα, and IFNγ62, 75–77, 97, 119, 126–128, 133. This is important given that these cytokines in combination impart significantly greater negative inotropy at a substantially lower concentration than being delivered independently. NO-mediated depression of the βAR response is primarily dependent on cGMP mechanisms, including stimulation of phosphodiesterase II (PDE II) with attendant augmentation of cAMP degradation and activation of protein kinase G (PKG) with downregulation of L-type Ca2+ currents due to reduction in cAMP-dependent protein kinase A (PKA) activation65, 66, 114, 134, 135.
It has been observed that as compared with IL-1β, TNFα is a less potent inducer of iNOS78, 116, 126 and yet leads to significant contractile dysfunction suggesting alternative mechanisms of regulation. Given this observation, GRK mediated desensitization of βARs by TNFα may play a key role in regulating cardiac dysfunction. In that context, insights from transgenic mice with cardiomyocyte specific expression of TNFα further underscores the importance of TNFα-mediated βAR responsiveness in the pathogenesis of long-term cytokine-mediated contractile dysfunction10, 129, 130. Intriguingly, studies have also shown upregulation of pro-inflammatory cytokines by chronic stimulation of βAR136 suggesting a circulatory communication loop between GPCRs and receptors involved in inflammation particularly TNFα receptors. Increasing recognition of this cross-talk in the recent years has paved ways for mechanistic understanding and may hold the key for determining the cardio-vascular risk defined for co-morbid conditions that are always associated with chronic inflammation.
GPCR Kinases (GRKs) and inflammation
GRKs are classically known to play a key role in GPCR phosphorylation following agonist stimulation leading to GPCR desensitization137–143. GPCR phosphorylation is immediately followed by arrestin recruitment to the receptor mediating internalization144–146. However, increasing evidence from recent studies has established a role for GRKs in inflammation and inflammatory diseases wherein, GRKs in addition to regulating receptor function can directly alter signaling pathways that uniquely respond to inflammation. In a classical sense, GRKs can non-canonically regulate signaling pathways. Such an idea has been well described for NFκB signaling pathway that plays a key role in the production of inflammatory cytokines. GRK2, GRK5 and GRK6 have been shown to interact with IκB and NFκB-p105 mediating their phosphorylation147–154. Interestingly, TLR ligands enhance GRK2 expression in primary macrophages155. Similarly, immune cells from sepsis patients exhibit higher levels of GRK2 suggesting that GRK2 levels and the associated non-canonical signaling pathways regulated by GKR2 may have potential clinical relevance in inflammatory diseases156.
An alternative way GRKs can influence the production of inflammatory cytokines is by regulation of MAPK signaling pathways ERK, JNK and p38 MAPK156. Activation of ERK pathway leads to the induction of various inflammatory mediators (e.g. TNFα, IL-1, IL-8 and prostaglandin E2 (PGE2))156. However, GRK2 and 5 seem to negatively regulate lysophosphatidic acid (LPS)-induced ERK pathway in macrophages149, 151 suggesting a cross-talk between these pathways that needs indepth investigation. The requirement for indepth understanding of this cross-talk is apparent from the studies showing that overexpression of GRK5 and/or GRK6 enhances β-arrestin2-mediated ERK activation, whereas overexpression of GRK2 and/or GRK3 abolished β-arrestin2-mediated ERK activation157. Critically, these effects were observed with activation of β2 adrenergic receptor, cannabinoid receptor 2, and Angiotensin 1A receptor thereby, suggesting connection between GPCRs and differential regulation of pro-inflammatory cytokines by GRKs153, 158, 159.
The p38 MAP kinase pathway is also associated with inflammation. p38 MAPK mediates expression of many genes involved in inflammation, such as TNFα, IL-1β, IL-6, IL-8160. Inhibition of p38 MAPK reduced pro-inflammatory cytokine production161 and in that context, GRK2 and p38 MAPK have bidirectional functional roles. GRK2 inhibits p38 MAPK function by directly phosphorylating it and modulating levels of GRK2 expression in turn alters p38 MAPK activation and subsequent cytokines production162. Also, activation of p38 MAPK inhibits GRK2-mediated GPCR desensitization by directly phosphorylating GRK2 which blocks GRK2 translocation to the membrane163. Consistent with this role, GRK2+/− hemizygous macrophages have increased p38 MAPK activation162, 164. In addition, p38 MAPK inhibits GRK2-mediated desensitization by acting as a non-canonical GRK for the Formyl Peptide Receptor 1 (FPR1)163.
Similarly, JNK, another MAPK member is also activated by mitogens as well as by a variety of environmental stresses and they induce transcription of AP-1, c-Jun, ATF-2, and ELK-1, all of which are important mediators of inflammatory gene transcription165. JNK activation of AP-1 is important for synthesis of TNFα, as well as proliferation and differentiation of lymphocytes and hence plays a vital role in immune system166, 167. Role of GRKs in JNK signaling, particularly related to the immune system, is not well characterized. However, studies with transgenic mice overexpressing cardiac specific GRK5 showed attenuation of JNK activation compared to controls168. These observations suggest that more studies are needed to determine the non-canonical cross-regulation of MAPKs by GRKs which could have significant implications as GRKs can regulate immune cell chemotaxis, inflammatory signaling and cell apoptotic pathways. Dysregulation of any one these functions will have greater impact in altering the course of many diseases. A number of studies have examined and established the role of specific GRKs in various inflammatory diseases such as neurodegenerative, autoimmune, cardiovascular diseases and sepsis151, 152, 164, 169–204. GRKs play numerous physiological roles and are generally involved in maintaining homeostasis. However, derangement in the processes involving GRKs often leads to pathology. Pathophysiological role of GRKs are attributed to their canonical GPCR-dependent functions such as catecholamine mediated receptor desensitization and to their non-canonical functions such as regulation of cellular signaling and inflammation. Given the nodal role GRKs could play in calibrating the receptor function as well as the inflammatory response, more comprehensive studies are needed to understand both its canonical and non-canonical functions.
GPCR-βAR activation and TNFα signaling
βAR is a proto-typical GPCR and a major regulator of cardiac contractile function205. βAR dysfunction is one of the classical hallmarks in heart failure. However, studies have shown that chronic activation of βARs results in induction of pro-inflammatory response in the heart. The inflammatory response profile in response to βAR activation is characterized by increased expression of TNFα, IL-1β and IL-6206–209. Consistent with this observation, antagonizing βARs using the β-blockers markedly reduces myocardial TNFα and IL-1β expression in the hearts55. Correspondingly, studies in mouse models of asthma have shown that treatment with the β-blockers attenuates inflammatory response210 showing “quid-pro-quo” relationship between the βAR activation and induction of inflammatory response. Upregulation of TNFα, IL-1β and IL-6 in the myocardium is known to occur through the NFκB dependent mechanisms211, 212. Recent investigations have shown that upregulation of the inflammatory cytokines in response to β-agonist can be markedly blunted by treatment of epidermal growth factor receptor (EGFR) blocker gefitinib213 indicating a role for EGFR transactivation in mediating inflammatory response.
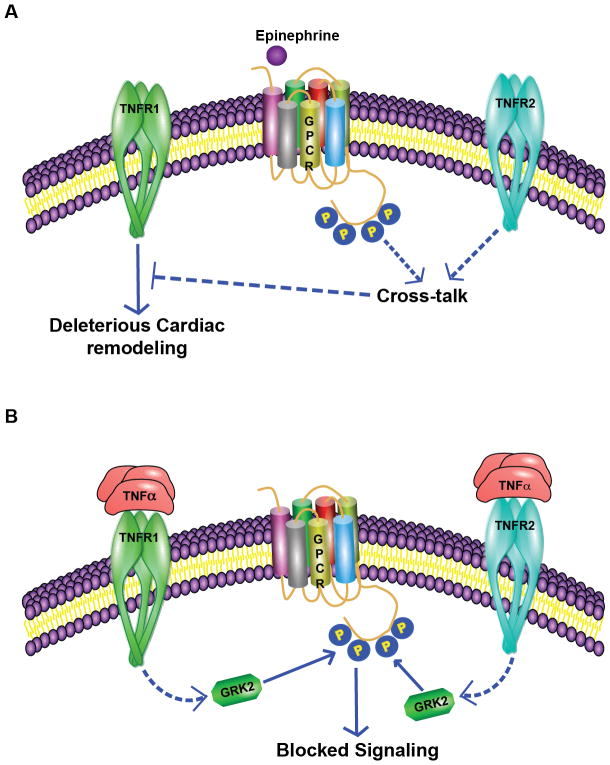
Sympathetic overdrive leads to production of inflammatory cytokines and TNFα induction is a consistent feature in various pathological conditions including obesity, rheumatoid arthritis, myocardial infarction, and kidney renal injury30, 43, 214. In this context, studies have shown that circulating levels of TNFα and soluble TNF receptors are independent predictors of mortality in patients with heart failure54. Induction of TNFα in response to sympathetic overdrive now in turn mediates cellular/physiological responses that are both beneficial acutely and deleterious chronically. TNFα that is generated in response to βAR activation is classically synthesized as a trans-membrane protein that is 26 kDa and is cleaved to 17 kDa soluble TNFα by TNFα-converting enzyme (TACE)214, 215. It is known that both the membrane and soluble TNFα initiate signals by binding to their receptors TNF R1 and R2. The underlying mechanism through which TNFα mediates cellular signaling and physiological effects have been detailed in multiple studies and reviews39, 43, 214, 216, 217. Increasing evidence has shown that downstream signaling effects mediated by TNFα are multifaceted due to differential effects that TNF R1 or R2 mediate in the cells. It is known from multiple studies that TNFR1 couples to deleterious death domain signaling pathways that mediate apoptosis39, 218, 219. However, it is also known that TNFR1 activation leads to NFκB activation and comprehensive studies have shown that TNFR1 can also couple to the beneficial NFκB pathway thereby “fine-tuning” the TNFα response in the cell. Such a concept is supported by the observation that TNFR1 interaction with the complex containing death domain TNFR1 protein (TRADD) and TRAF2 activates NFκB while TNFR1 interaction with TRADD-death domain containing Fas protein complex mediates apoptosis214, 216. In contrast to the divergent signals that can emanate from TNFR1 activation, it is understood that TNFR2 activation usually leads to beneficial signals from its interaction with TRAF2 that activates NFκB, MAPK and protein kinase B (AKT) – which are all pro-survival signals214, 220, 221. Given that TRAF2 is the nodal molecule that mediates the beneficial cellular signals downstream of both TNFR1 and R2, relative levels of TNFR1 and R2 expression in the tissues could determine the response to TNFα as observed in human alveolar epithelial cells219, human heart failure218 or renal injury38. However, in contrast to the idea that TRAF2 allows for beneficial signaling, high levels of myocyte-specific overexpression lead to deleterious cardiac remodeling12 while lower levels of TRAF2 provides beneficial remodeling ischemia reperfusion injury222. These studies suggest that not only are the levels of TNFR1 and R2 critical in cellular responses to TNFα but also the expression levels of TRAF2 in the cells may produce counterintuitive phenotypes in pathology. This is critical given that sympathetic overdrive leading to βAR stimulation results in significant increase in TNFα. Therefore, the TNFα driven inflammatory response would be determined by the relative ratios of TNFR1 and R2 expression associated with levels of TRAF2 in various cells as βARs are universally expressed in all cell types. Corresponding to this paradigm of signaling contribution based on TNFR1 and R2 expression, βAR agonist isoproterenol treatment in the mice with TNFR2 knockout showed deleterious remodeling and worsening cardiac function compared to TNFR1 knockout and wild type littermates223. These findings suggest that TNFR2 signaling provides beneficial effects that counter the deleterious TNFR1-mediated signaling as absence of TNFR2 results in increased fibrosis223 in response to βAR agonist isoproterenol (Fig 1A).
Figure 1. Intricate cross-talk between TNFα receptor signaling and βAR pathways.
A, The downstream effects of TNFα are mediated by both TNFR1 and R2 receptors. In this context, chronic administration of βAR agonist isoproterenol in mice leads to cardiac remodeling which is however worse in TNFR2 knockout mice. These observations suggest that cross-talk between βAR and TNFR2 signaling acts as a brake on the deleterious signals initiated by TNFR111. B, Correspondingly, chronic TNFα treatment results in loss of βAR response to its agonist isoproterenol. Studies have shown that TNFα is able to mediate non-canonical Gβγ-independent recruitment of GRK2 to the βAR complex mediating its phosphorylation even in the absence of βAR-agonist. Since pro-inflammatory cytokine TNFα is elevated in diabetes, hypertension, and obesity which are all cardiovascular risk factors, this unsuspected inhibition of βAR function by TNFα could underlie the cause of heart failure. βARs are one of the most powerful regulators of cardiac function and a key hallmark for heart failure is βAR dysfunction10.
In addition to the divergent signals that are initiated by activation of TNFR1 and R2, TNFR1 and R2 can be differentially and/or selectively activated by the ligand TNFα. The selectivity depends on whether TNFα is membrane bound or is in the soluble format. It is considered that membrane bound TNFα is the prime activating ligand for TNFR239, 217, 218 and is thought to primarily mediate the beneficial signals in response to TNFα. Such an idea is supported by the observation that cardiac-specific overexpression of non-cleavable TNFα leads to concentric hypertrophy while, overexpression of secreted TNFα leads to dilated cardiomyopathy17, 18. Furthermore, membrane bound TNFα in endothelial colony forming cells protects against senescence in inflammatory environment suggesting a key role in cell proliferation224. Consistently, significant reduction in apoptosis and amelioration in deleterious cardiac remodeling was observed in TNFα knockout mice (TNF−/−) following myocardial infarction225. In this context, it is important to note that myocardial infarction is associated with significant upregulation of sympathetic hormones epinephrine/norepinephrine, which would elevate TNFα. Given the absence of TNFα in the TNFα−/− mice, a major portion of the βAR agonist epinephrine mediated deleterious signals via TNFα are absent reflecting in better cardiac function and remodeling. Understanding the complexity in TNFα signaling is important as βAR-TNFα signaling axis underlies deleterious manifestation in many pathologies including cardiovascular disease. Since increase in sympathetic drive activates βAR downstream signaling, it is critical to delineate mechanistic pathways that mediate TNFα upregulation. Determination of these pathways would be key as it may provide insights into potentially novel therapeutic targets.
TNFα and βAR signaling
There is increasing appreciation that in response to increased sympathetic stimuli there is an upregulated pro-inflammatory response. However, there are circumstances wherein, activation of innate immune response occurs that is independent of sympathetic overdrive leading to release of potent cytokines like IL-1β, IL-6 and TNFα. It is known that upregulation of these cytokines is associated with pathogenesis of metabolic syndrome, airway disease such as asthma and heart failure. Both asthma and heart failure are associated with increased TNFα mRNA and protein226 and circulating levels of TNFα2, 29. In this context, it is known that TNFα treatment leads to cardio-depressant effects and previous studies have identified that TNFα may mediate these effects through βARs8, 9. The negative inotropic function mediated by TNFα is thought to involve both immediate and delayed signaling pathways, with immediate effects through altered intracellular Ca2+96, spingolipid mediators79, and nitric oxide synthase78. Despite these interesting observations, the mechanistic underpinnings for the TNFα-mediated βAR dysfunction are not well understood and whether any of the classical pathways that mediate βAR dysfunction are recruited in the TNFα-driven mechanisms.
βARs are one of the most powerful regulators of cardiac contractility and lung relaxation function205, 227. Diminished response of βAR signaling to catecholamines (sympathetic hormones) occurs through a process defined as βAR desensitization that contributes to the pathogenesis of heart failure and potentially to asthma exacerbation. Desensitization of βARs is mediated by phosphorylation of the C-terminal tail of the receptor by G-protein coupled receptor kinases (GRKs), protein kinase C (PKC) and protein kinase A (PKA). Among the plethora of kinases that regulate βAR phosphorylation and desensitization, GRK2 is a predominant player as inhibition of GRK2 recruitment to the βARs through a dominant negative strategy of using C-terminal of GRK2 (GRK2-Ct) results in beneficial cardiac remodeling228. Since GRK2 is consistently upregulated in response to pro-inflammatory cytokine TNFα in various tissues229, we assessed whether GRK2 is upregulated in the cardiac tissues to TNFα. Our studies revealed that cardiac GRK2 is upregulated in the transgenic mice with cardiac specific overexpression of TNFα even before identification of cardiac dysfunction by echocardiography10.
The observation of GRK2 upregulation in response to TNFα in the hearts suggested that GRK2 maybe the proximal link and the underlying mechanism for the observation of TNFα-mediated cardio-depressant effect through regulation of βAR dysfunction8, 9, 14. Our studies further showed that GRK2 is the key molecule involved in β2AR desensitization as cardiomyocyte specific GRK2 null mice have preserved β2AR function in response to TNFα. However, in contrast to GRK2-ct transgenic mice ameliorating cardiac dysfunction in response to transverse aortic constriction (TAC) or myocardial infraction (MI), chronic TNFα treatment resulted in cardiac dysfunction associated with βAR desensitization. These studies suggest the presence of a novel mechanism by which GRK2 can be recruited to the βAR complex to mediate phosphorylation instead of the classical G-protein beta-gamma (Gβγ) subunits 230–232. Interestingly, our studies further showed that the recruitment of GRK2 to the βAR complex could be mediated by TNFR2 as marked reduction in GRK2 recruitment was observed in TNFR2 knock out cells10. Thus, TNFα-induced βAR desensitization mediated by GRK2 in conditions of heart failure is observed to be independent of Gβγ subunits10 (Fig 1B). Correspondingly, a recent study reported elevated GRK2 and decreased β2AR expression in dendritic cell cytomembranes of adjuvant induced arthritis model233 further supporting the view that GRK2 function is regulated under conditions of inflammation. Accordingly, treatment of lung epithelial cells with glucocorticoids (anti-inflammatory agent) resulted in significant inhibition of GRK2 expression234. However, the involvement of TNFR2 in GRK2 recruitment to the plasma membranes in response to TNFα is counterintuitive10 given the beneficial role of TNFR2 signaling. A potential beneficial role that can be envisioned by this pathway could be the ability of TNFα signaling to dampen the cardiac function in the face of pre-existing sympathetic drive thus providing relief to the mechanically overloaded heart. Such a mechanism could be the integral part of the beneficial role TNFR2 could play in presence of chronic βAR agonist as absence of TNFR2 (TNFR2 knockout mice) leads to deleterious cardiac remodeling in response to β-agonist isoproterenol11, 223 (Fig 1A). These intriguing observations indicate that more comprehensive studies are warranted to better understand the signaling axis between TNFα/TNFR2 and βAR pathways, as less is known about the adaptor molecules involved and the mechanism of TNFR2 signal transduction235.
TNFα based therapy and clinical outcomes
Among the pro-inflammatory cytokines, TNFα is consistently elevated in majority of the chronic conditions such as inflammatory bowel disease, rheumatoid arthritis, psoriasis, asthma, and heart failure. Comprehensive studies have shown that circulating levels of TNFα and soluble TNFR could be independent predictors of heart failure54. Given the consistent elevation of TNFα in these pathologies, a logical idea would be to develop anti-TNFα therapies. Consistent with this idea, anti-TNFα therapies have worked and have been approved for treatment of inflammatory bowel disease, rheumatoid arthritis and psoriasis26. However, such therapies have been a failure in human heart failure patients as anti-TNFα resulted in time- and dose-related increase in death and heart failure hospitalization54. These negative clinical outcomes could be due to several reasons. Although TNFα levels are elevated in the HF, it may not be directly responsible for disease progression. Part of the complexity in the failure of anti-TNFα therapy for heart failure could be due to the inherently different and opposing signaling effects arising from the TNFR1 and TNFR2 receptors (Fig 1A). In that context, less is known about the detailed mechanism of TNFR2 signaling components and adaptor proteins, which could underlie/explain the opposing actions of TNF receptors in the heart. Furthermore, there may be several other pathways operating in congruence at the same time like pathways activated by elevated catecholamines. Another possibility is that anti-TNFα therapy is beneficial but is offset by unintended, unanticipated, and/or unrecognized mechanisms of actions, such as βAR desensitization by recruiting GRK2 to the receptor complex through non-canonical Gβγ-independent mechanisms10 (Fig 1B). Thus, in the context of heart failure, identification and validation of the molecules that may reflect specific perturbations of cardiac β-adrenergic system due to the elevated levels of TNFα is necessary. This will help in generation of precise therapeutic targets to improve the cardiac function altered due to chronic inflammation. Moreover, the effect of circulatory TNFα and localized generation of TNFα on the regulation of cardiac function has not been delineated. Given the role of TNFR1 and R2, further studies are warranted for clearly delineating the differential recruitment and activation of signaling components following activation of TNFR1 and R2. Such an idea may hold some promise given the observation in mouse models that TNFR1 and R2 have opposing effects on activation of NFκB in conditions of heart failure223, 236. These findings suggest a more nuanced approach for targeting TNF pathways in heart failure is required. The methods could be based on selective antagonism/blockade of TNFR1 associated signaling pathway and activation of TNFR2 thus harnessing the beneficial spatial, temporal and molecular components of TNFα signaling.
Summary and future outlook
Although understanding differential roles of TNFα on mediating TNFR1 and R2 signaling is important, it is also important to delineate the multiple arms of cross-talk that occurs with βARs in the context of cardiac function. Determining the underlying molecular mechanism between TNFα and βARs (Fig 1)is key since βARs are universally expressed and thus may have differential cellular consequences based on the cell type as circulating levels of TNFα is elevated in pathology (Fig 2). While all the studies on understanding the paradigm of cross-talk between TNFα-βARs is focused towards kinase-mediated desensitization of βARs, a key aspect that has not been studied in the cross-talk is the role of phosphatase-mediated resensitization of βARs. Indepth studies would be required as increasing evidence indicates that the phosphatase-driven mechanisms may not be passive homeostatic process but a tightly regulated mechanism237, 238 contributing to the pathology. Such observations open up to the possibility that TNFα-mediated signaling mechanisms could also regulate βAR function by modulating phosphatase functions and resensitization within the paradigm of cross-talk between TNFα and βARs. Consideration of these complex layers of regulation by TNFα signaling and the dissection of beneficial versus deleterious pathways mediated by TNFRs will hold the key for developing successful therapies that may harness the benefits of the TNFα (Figs 1 & 2).
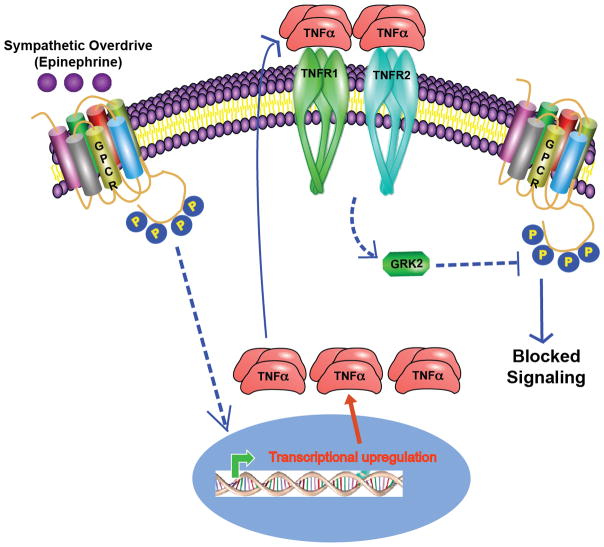
Figure 2. Sympathetic overdrive involves an active cross-talk between βAR and TNFα-mediated downstream signaling.
It is known that epinephrine binding to βAR activates downstream signaling playing a role in cardiac contraction. However, it is well established that sustained sympathetic overdrive leads to deleterious cardiac dysfunction and is associated with βAR dysfunction. In addition, increasing evidence shows that sympathetic overdrive leads to generation of pro-inflammatory cytokines including TNFα. Increased TNFα would bind to TNFR1 and R2 receptors that will non-canonically recruit GRK2 to βAR complex inhibiting βAR signaling. This inhibition of βAR signaling in other words represents βAR dysfunction, a phenotype observed in conditions of heart failure. Therefore, in this context it is important to note that sympathetic overdrive in addition to directly altering βAR function could have consequences that are mediated by altering TNFα and shows an additional pathway that regulate βAR function in various cell types.
References
- 1.Lower R. Tractatus de corde: De motu & colore sagnuinus et chyli in eum tranfitu. London, UK: 1669. [Google Scholar]
- 2.Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. The New England journal of medicine. 1990;323:236–241. doi: 10.1056/NEJM199007263230405. [DOI] [PubMed] [Google Scholar]
- 3.Dutka DP, Elborn JS, Delamere F, Shale DJ, Morris GK. Tumour necrosis factor alpha in severe congestive cardiac failure. British heart journal. 1993;70:141–143. doi: 10.1136/hrt.70.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT Anti TNFTACHFI. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: Results of the anti-tnf therapy against congestive heart failure (attach) trial. Circulation. 2003;107:3133–3140. doi: 10.1161/01.CIR.0000077913.60364.D2. [DOI] [PubMed] [Google Scholar]
- 5.Wood S. Renewal trial: No improvement in chf with ethanercept. HeartWire News; 2002. [Google Scholar]
- 6.Hughes S. Infliximab harmful in chf - final results of attach. HeartWire News; 2002. [Google Scholar]
- 7.Mann DL, McMurray JJ, Packer M, Swedberg K, Borer JS, Colucci WS, Djian J, Drexler H, Feldman A, Kober L, Krum H, Liu P, Nieminen M, Tavazzi L, van Veldhuisen DJ, Waldenstrom A, Warren M, Westheim A, Zannad F, Fleming T. Targeted anticytokine therapy in patients with chronic heart failure: Results of the randomized etanercept worldwide evaluation (renewal) Circulation. 2004;109:1594–1602. doi: 10.1161/01.CIR.0000124490.27666.B2. [DOI] [PubMed] [Google Scholar]
- 8.Chung MK, Gulick TS, Rotondo RE, Schreiner GF, Lange LG. Mechanism of cytokine inhibition of beta-adrenergic agonist stimulation of cyclic amp in rat cardiac myocytes. Impairment of signal transduction. Circulation research. 1990;67:753–763. doi: 10.1161/01.res.67.3.753. [DOI] [PubMed] [Google Scholar]
- 9.Gulick T, Chung MK, Pieper SJ, Lange LG, Schreiner GF. Interleukin 1 and tumor necrosis factor inhibit cardiac myocyte beta-adrenergic responsiveness. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:6753–6757. doi: 10.1073/pnas.86.17.6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vasudevan NT, Mohan ML, Gupta MK, Martelli EE, Hussain AK, Qin Y, Chandrasekharan UM, Young D, Feldman AM, Sen S, Dorn GW, 2nd, Dicorleto PE, Naga Prasad SV. Gbetagamma-independent recruitment of g-protein coupled receptor kinase 2 drives tumor necrosis factor alpha-induced cardiac beta-adrenergic receptor dysfunction. Circulation. 2013;128:377–387. doi: 10.1161/CIRCULATIONAHA.113.003183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garlie JB, Hamid T, Gu Y, Ismahil MA, Chandrasekar B, Prabhu SD. Tumor necrosis factor receptor 2 signaling limits beta-adrenergic receptor-mediated cardiac hypertrophy in vivo. Basic research in cardiology. 2011;106:1193–1205. doi: 10.1007/s00395-011-0196-6. [DOI] [PubMed] [Google Scholar]
- 12.Divakaran VG, Evans S, Topkara VK, Diwan A, Burchfield J, Gao F, Dong J, Tzeng HP, Sivasubramanian N, Barger PM, Mann DL. Tumor necrosis factor receptor-associated factor 2 signaling provokes adverse cardiac remodeling in the adult mammalian heart. Circulation Heart failure. 2013;6:535–543. doi: 10.1161/CIRCHEARTFAILURE.112.000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kubota T, McTiernan CF, Frye CS, Demetris AJ, Feldman AM. Cardiac-specific overexpression of tumor necrosis factor-alpha causes lethal myocarditis in transgenic mice. Journal of cardiac failure. 1997;3:117–124. doi: 10.1016/s1071-9164(97)90045-2. [DOI] [PubMed] [Google Scholar]
- 14.Kubota T, McTiernan CF, Frye CS, Slawson SE, Lemster BH, Koretsky AP, Demetris AJ, Feldman AM. Dilated cardiomyopathy in transgenic mice with cardiac-specific overexpression of tumor necrosis factor-alpha. Circulation research. 1997;81:627–635. doi: 10.1161/01.res.81.4.627. [DOI] [PubMed] [Google Scholar]
- 15.Li X, Moody MR, Engel D, Walker S, Clubb FJ, Jr, Sivasubramanian N, Mann DL, Reid MB. Cardiac-specific overexpression of tumor necrosis factor-alpha causes oxidative stress and contractile dysfunction in mouse diaphragm. Circulation. 2000;102:1690–1696. doi: 10.1161/01.cir.102.14.1690. [DOI] [PubMed] [Google Scholar]
- 16.Sivasubramanian N, Coker ML, Kurrelmeyer KM, MacLellan WR, DeMayo FJ, Spinale FG, Mann DL. Left ventricular remodeling in transgenic mice with cardiac restricted overexpression of tumor necrosis factor. Circulation. 2001;104:826–831. doi: 10.1161/hc3401.093154. [DOI] [PubMed] [Google Scholar]
- 17.Dibbs ZI, Diwan A, Nemoto S, DeFreitas G, Abdellatif M, Carabello BA, Spinale FG, Feuerstein G, Sivasubramanian N, Mann DL. Targeted overexpression of transmembrane tumor necrosis factor provokes a concentric cardiac hypertrophic phenotype. Circulation. 2003;108:1002–1008. doi: 10.1161/01.CIR.0000085203.46621.F4. [DOI] [PubMed] [Google Scholar]
- 18.Diwan A, Dibbs Z, Nemoto S, DeFreitas G, Carabello BA, Sivasubramanian N, Wilson EM, Spinale FG, Mann DL. Targeted overexpression of noncleavable and secreted forms of tumor necrosis factor provokes disparate cardiac phenotypes. Circulation. 2004;109:262–268. doi: 10.1161/01.CIR.0000109642.27985.FA. [DOI] [PubMed] [Google Scholar]
- 19.Ramadori G, Van Damme J, Rieder H, Meyer zum Buschenfelde KH. Interleukin 6, the third mediator of acute-phase reaction, modulates hepatic protein synthesis in human and mouse. Comparison with interleukin 1 beta and tumor necrosis factor-alpha. European journal of immunology. 1988;18:1259–1264. doi: 10.1002/eji.1830180817. [DOI] [PubMed] [Google Scholar]
- 20.Waters JP, Pober JS, Bradley JR. Tumour necrosis factor and cancer. The Journal of pathology. 2013;230:241–248. doi: 10.1002/path.4188. [DOI] [PubMed] [Google Scholar]
- 21.Willerson JT, Ridker PM. Inflammation as a cardiovascular risk factor. Circulation. 2004;109:II2–10. doi: 10.1161/01.CIR.0000129535.04194.38. [DOI] [PubMed] [Google Scholar]
- 22.Bone RC, Grodzin CJ, Balk RA. Sepsis: A new hypothesis for pathogenesis of the disease process. Chest. 1997;112:235–243. doi: 10.1378/chest.112.1.235. [DOI] [PubMed] [Google Scholar]
- 23.Liles WC, Van Voorhis WC. Review: Nomenclature and biologic significance of cytokines involved in inflammation and the host immune response. The Journal of infectious diseases. 1995;172:1573–1580. doi: 10.1093/infdis/172.6.1573. [DOI] [PubMed] [Google Scholar]
- 24.Lucey DR, Clerici M, Shearer GM. Type 1 and type 2 cytokine dysregulation in human infectious, neoplastic, and inflammatory diseases. Clinical microbiology reviews. 1996;9:532–562. doi: 10.1128/cmr.9.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feldmann M. Translating molecular insights in autoimmunity into effective therapy. Annual review of immunology. 2009;27:1–27. doi: 10.1146/annurev-immunol-082708-100732. [DOI] [PubMed] [Google Scholar]
- 26.Monaco C, Nanchahal J, Taylor P, Feldmann M. Anti-tnf therapy: Past, present and future. International immunology. 2015;27:55–62. doi: 10.1093/intimm/dxu102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. The Journal of clinical investigation. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lenart N, Brough D, Denes A. Inflammasomes link vascular disease with neuroinflammation and brain disorders. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2016 doi: 10.1177/0271678X16662043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prabhu SD. Cytokine-induced modulation of cardiac function. Circulation research. 2004;95:1140–1153. doi: 10.1161/01.RES.0000150734.79804.92. [DOI] [PubMed] [Google Scholar]
- 30.Prabhu SD, Frangogiannis NG. The biological basis for cardiac repair after myocardial infarction: From inflammation to fibrosis. Circulation research. 2016;119:91–112. doi: 10.1161/CIRCRESAHA.116.303577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodriguez-Hernandez H, Simental-Mendia LE, Rodriguez-Ramirez G, Reyes-Romero MA. Obesity and inflammation: Epidemiology, risk factors, and markers of inflammation. International journal of endocrinology. 2013;2013:678159. doi: 10.1155/2013/678159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Idris-Khodja N, Mian MO, Paradis P, Schiffrin EL. Dual opposing roles of adaptive immunity in hypertension. European heart journal. 2014;35:1238–1244. doi: 10.1093/eurheartj/ehu119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laaksonen DE, Niskanen L, Punnonen K, Nyyssonen K, Tuomainen TP, Salonen R, Rauramaa R, Salonen JT. Sex hormones, inflammation and the metabolic syndrome: A population-based study. European journal of endocrinology / European Federation of Endocrine Societies. 2003;149:601–608. doi: 10.1530/eje.0.1490601. [DOI] [PubMed] [Google Scholar]
- 34.Festa A, D’Agostino R, Jr, Howard G, Mykkanen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome: The insulin resistance atherosclerosis study (iras) Circulation. 2000;102:42–47. doi: 10.1161/01.cir.102.1.42. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki Y, Ruiz-Ortega M, Lorenzo O, Ruperez M, Esteban V, Egido J. Inflammation and angiotensin ii. The international journal of biochemistry & cell biology. 2003;35:881–900. doi: 10.1016/s1357-2725(02)00271-6. [DOI] [PubMed] [Google Scholar]
- 36.Solak Y, Afsar B, Vaziri ND, Aslan G, Yalcin CE, Covic A, Kanbay M. Hypertension as an autoimmune and inflammatory disease. Hypertension research : official journal of the Japanese Society of Hypertension. 2016;39:567–573. doi: 10.1038/hr.2016.35. [DOI] [PubMed] [Google Scholar]
- 37.Edrees AF, Misra SN, Abdou NI. Anti-tumor necrosis factor (tnf) therapy in rheumatoid arthritis: Correlation of tnf-alpha serum level with clinical response and benefit from changing dose or frequency of infliximab infusions. Clinical and experimental rheumatology. 2005;23:469–474. [PubMed] [Google Scholar]
- 38.Al-Lamki RS, Wang J, Vandenabeele P, Bradley JA, Thiru S, Luo D, Min W, Pober JS, Bradley JR. Tnfr1- and tnfr2-mediated signaling pathways in human kidney are cell type-specific and differentially contribute to renal injury. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2005;19:1637–1645. doi: 10.1096/fj.05-3841com. [DOI] [PubMed] [Google Scholar]
- 39.Cabal-Hierro L, Lazo PS. Signal transduction by tumor necrosis factor receptors. Cellular signalling. 2012;24:1297–1305. doi: 10.1016/j.cellsig.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 40.Faustman DL, Davis M. Tnf receptor 2 and disease: Autoimmunity and regenerative medicine. Frontiers in immunology. 2013;4:478. doi: 10.3389/fimmu.2013.00478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li A, Huang X, Song Y, Chen X, Sun J, Xu H, Wang Z. Anti-epidermal growth factor receptor-targeted therapy in upper gastrointestinal tract cancers: A meta-analysis. Growth factors. 2015;33:113–127. doi: 10.3109/08977194.2015.1010643. [DOI] [PubMed] [Google Scholar]
- 42.Sereno M, Brunello A, Chiappori A, Barriuso J, Casado E, Belda C, de Castro J, Feliu J, Gonzalez-Baron M. Cardiac toxicity: Old and new issues in anti-cancer drugs. Clinical & translational oncology : official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico. 2008;10:35–46. doi: 10.1007/s12094-008-0150-8. [DOI] [PubMed] [Google Scholar]
- 43.Hotamisligil GS. The role of tnfalpha and tnf receptors in obesity and insulin resistance. Journal of internal medicine. 1999;245:621–625. doi: 10.1046/j.1365-2796.1999.00490.x. [DOI] [PubMed] [Google Scholar]
- 44.Nieto-Vazquez I, Fernandez-Veledo S, Kramer DK, Vila-Bedmar R, Garcia-Guerra L, Lorenzo M. Insulin resistance associated to obesity: The link tnf-alpha. Archives of physiology and biochemistry. 2008;114:183–194. doi: 10.1080/13813450802181047. [DOI] [PubMed] [Google Scholar]
- 45.Francis GS. Neurohormonal control of heart failure. Cleveland Clinic journal of medicine. 2011;78(Suppl 1):S75–79. doi: 10.3949/ccjm.78.s1.13. [DOI] [PubMed] [Google Scholar]
- 46.Drazner MH. The progression of hypertensive heart disease. Circulation. 2011;123:327–334. doi: 10.1161/CIRCULATIONAHA.108.845792. [DOI] [PubMed] [Google Scholar]
- 47.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB American Heart Association Statistics C, Stroke Statistics S. Executive summary: Heart disease and stroke statistics--2014 update: A report from the american heart association. Circulation. 2014;129:399–410. doi: 10.1161/01.cir.0000442015.53336.12. [DOI] [PubMed] [Google Scholar]
- 48.Edelmann F, Holzendorf V, Wachter R, Nolte K, Schmidt AG, Kraigher-Krainer E, Duvinage A, Unkelbach I, Dungen HD, Tschope C, Herrmann-Lingen C, Halle M, Hasenfuss G, Gelbrich G, Stough WG, Pieske BM. Galectin-3 in patients with heart failure with preserved ejection fraction: Results from the aldo-dhf trial. European journal of heart failure. 2015;17:214–223. doi: 10.1002/ejhf.203. [DOI] [PubMed] [Google Scholar]
- 49.Mann DL. Innate immunity and the failing heart: The cytokine hypothesis revisited. Circulation research. 2015;116:1254–1268. doi: 10.1161/CIRCRESAHA.116.302317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Torre-Amione G, Kapadia S, Lee J, Durand JB, Bies RD, Young JB, Mann DL. Tumor necrosis factor-alpha and tumor necrosis factor receptors in the failing human heart. Circulation. 1996;93:704–711. doi: 10.1161/01.cir.93.4.704. [DOI] [PubMed] [Google Scholar]
- 51.Vasan RS, Sullivan LM, Roubenoff R, Dinarello CA, Harris T, Benjamin EJ, Sawyer DB, Levy D, Wilson PW, D’Agostino RB Framingham Heart S. Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: The framingham heart study. Circulation. 2003;107:1486–1491. doi: 10.1161/01.cir.0000057810.48709.f6. [DOI] [PubMed] [Google Scholar]
- 52.Selaas O, Nordal HH, Halse AK, Brun JG, Jonsson R, Brokstad KA. Serum markers in rheumatoid arthritis: A longitudinal study of patients undergoing infliximab treatment. International journal of rheumatology. 2015;2015:276815. doi: 10.1155/2015/276815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Surbatovic M, Popovic N, Vojvodic D, Milosevic I, Acimovic G, Stojicic M, Veljovic M, Jevdjic J, Djordjevic D, Radakovic S. Cytokine profile in severe gram-positive and gram-negative abdominal sepsis. Scientific reports. 2015;5:11355. doi: 10.1038/srep11355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mann DL. Inflammatory mediators and the failing heart: Past, present, and the foreseeable future. Circulation research. 2002;91:988–998. doi: 10.1161/01.res.0000043825.01705.1b. [DOI] [PubMed] [Google Scholar]
- 55.Prabhu SD, Chandrasekar B, Murray DR, Freeman GL. Beta-adrenergic blockade in developing heart failure: Effects on myocardial inflammatory cytokines, nitric oxide, and remodeling. Circulation. 2000;101:2103–2109. doi: 10.1161/01.cir.101.17.2103. [DOI] [PubMed] [Google Scholar]
- 56.Stamm C, Friehs I, Cowan DB, Moran AM, Cao-Danh H, Duebener LF, del Nido PJ, McGowan FX., Jr Inhibition of tumor necrosis factor-alpha improves postischemic recovery of hypertrophied hearts. Circulation. 2001;104:I350–355. doi: 10.1161/hc37t1.094851. [DOI] [PubMed] [Google Scholar]
- 57.Kanda T, McManus JE, Nagai R, Imai S, Suzuki T, Yang D, McManus BM, Kobayashi I. Modification of viral myocarditis in mice by interleukin-6. Circulation research. 1996;78:848–856. doi: 10.1161/01.res.78.5.848. [DOI] [PubMed] [Google Scholar]
- 58.Azzawi M, Hasleton P. Tumour necrosis factor alpha and the cardiovascular system: Its role in cardiac allograft rejection and heart disease. Cardiovascular research. 1999;43:850–859. doi: 10.1016/s0008-6363(99)00138-8. [DOI] [PubMed] [Google Scholar]
- 59.Azzawi M, Hasleton PS, Hutchinson IV. Tnf-alpha in acute cardiac transplant rejection. Cytokines, cellular & molecular therapy. 1999;5:41–49. [PubMed] [Google Scholar]
- 60.Tracey KJ, Fong Y, Hesse DG, Manogue KR, Lee AT, Kuo GC, Lowry SF, Cerami A. Anti-cachectin/tnf monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987;330:662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- 61.Kumar A, Haery C, Parrillo JE. Myocardial dysfunction in septic shock. Critical care clinics. 2000;16:251–287. doi: 10.1016/s0749-0704(05)70110-x. [DOI] [PubMed] [Google Scholar]
- 62.Kumar A, Thota V, Dee L, Olson J, Uretz E, Parrillo JE. Tumor necrosis factor alpha and interleukin 1beta are responsible for in vitro myocardial cell depression induced by human septic shock serum. The Journal of experimental medicine. 1996;183:949–958. doi: 10.1084/jem.183.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Landgarten MJ, Kumar A, Parrillo JE. Cardiovascular dysfunction in sepsis and septic shock. Current treatment options in cardiovascular medicine. 2000;2:451–459. doi: 10.1007/s11936-000-0040-z. [DOI] [PubMed] [Google Scholar]
- 64.Seta Y, Shan K, Bozkurt B, Oral H, Mann DL. Basic mechanisms in heart failure: The cytokine hypothesis. Journal of cardiac failure. 1996;2:243–249. doi: 10.1016/s1071-9164(96)80047-9. [DOI] [PubMed] [Google Scholar]
- 65.Massion PB, Feron O, Dessy C, Balligand JL. Nitric oxide and cardiac function: Ten years after, and continuing. Circulation research. 2003;93:388–398. doi: 10.1161/01.RES.0000088351.58510.21. [DOI] [PubMed] [Google Scholar]
- 66.Prabhu SD. Nitric oxide protects against pathological ventricular remodeling: Reconsideration of the role of no in the failing heart. Circulation research. 2004;94:1155–1157. doi: 10.1161/01.RES.0000129569.07667.89. [DOI] [PubMed] [Google Scholar]
- 67.Kojda G, Kottenberg K. Regulation of basal myocardial function by no. Cardiovascular research. 1999;41:514–523. doi: 10.1016/s0008-6363(98)00314-9. [DOI] [PubMed] [Google Scholar]
- 68.Finkel MS, Oddis CV, Jacob TD, Watkins SC, Hattler BG, Simmons RL. Negative inotropic effects of cytokines on the heart mediated by nitric oxide. Science. 1992;257:387–389. doi: 10.1126/science.1631560. [DOI] [PubMed] [Google Scholar]
- 69.McGowan FX, Jr, Takeuchi K, del Nido PJ, Davis PJ, Lancaster JR, Jr, Hattler BG. Myocardial effects of interleukin-2. Transplantation proceedings. 1994;26:209–210. [PubMed] [Google Scholar]
- 70.Kinugawa K, Takahashi T, Kohmoto O, Yao A, Aoyagi T, Momomura S, Hirata Y, Serizawa T. Nitric oxide-mediated effects of interleukin-6 on [ca2+]i and cell contraction in cultured chick ventricular myocytes. Circulation research. 1994;75:285–295. doi: 10.1161/01.res.75.2.285. [DOI] [PubMed] [Google Scholar]
- 71.Sugishita K, Kinugawa K, Shimizu T, Harada K, Matsui H, Takahashi T, Serizawa T, Kohmoto O. Cellular basis for the acute inhibitory effects of il-6 and tnf-alpha on excitation-contraction coupling. Journal of molecular and cellular cardiology. 1999;31:1457–1467. doi: 10.1006/jmcc.1999.0989. [DOI] [PubMed] [Google Scholar]
- 72.Goldhaber JI, Kim KH, Natterson PD, Lawrence T, Yang P, Weiss JN. Effects of tnf-alpha on [ca2+]i and contractility in isolated adult rabbit ventricular myocytes. The American journal of physiology. 1996;271:H1449–1455. doi: 10.1152/ajpheart.1996.271.4.H1449. [DOI] [PubMed] [Google Scholar]
- 73.Alloatti G, Penna C, De Martino A, Montrucchio G, Camussi G. Role of nitric oxide and platelet-activating factor in cardiac alterations induced by tumor necrosis factor-alpha in the guinea-pig papillary muscle. Cardiovascular research. 1999;41:611–619. doi: 10.1016/s0008-6363(98)00250-8. [DOI] [PubMed] [Google Scholar]
- 74.Hofmann U, Domeier E, Frantz S, Laser M, Weckler B, Kuhlencordt P, Heuer S, Keweloh B, Ertl G, Bonz AW. Increased myocardial oxygen consumption by tnf-alpha is mediated by a sphingosine signaling pathway. American journal of physiology Heart and circulatory physiology. 2003;284:H2100–2105. doi: 10.1152/ajpheart.00888.2002. [DOI] [PubMed] [Google Scholar]
- 75.Panas D, Khadour FH, Szabo C, Schulz R. Proinflammatory cytokines depress cardiac efficiency by a nitric oxide-dependent mechanism. The American journal of physiology. 1998;275:H1016–1023. doi: 10.1152/ajpheart.1998.275.3.H1016. [DOI] [PubMed] [Google Scholar]
- 76.Cain BS, Meldrum DR, Dinarello CA, Meng X, Joo KS, Banerjee A, Harken AH. Tumor necrosis factor-alpha and interleukin-1beta synergistically depress human myocardial function. Critical care medicine. 1999;27:1309–1318. doi: 10.1097/00003246-199907000-00018. [DOI] [PubMed] [Google Scholar]
- 77.Kumar A, Brar R, Wang P, Dee L, Skorupa G, Khadour F, Schulz R, Parrillo JE. Role of nitric oxide and cgmp in human septic serum-induced depression of cardiac myocyte contractility. The American journal of physiology. 1999;276:R265–276. doi: 10.1152/ajpregu.1999.276.1.R265. [DOI] [PubMed] [Google Scholar]
- 78.Stein B, Frank P, Schmitz W, Scholz H, Thoenes M. Endotoxin and cytokines induce direct cardiodepressive effects in mammalian cardiomyocytes via induction of nitric oxide synthase. Journal of molecular and cellular cardiology. 1996;28:1631–1639. doi: 10.1006/jmcc.1996.0153. [DOI] [PubMed] [Google Scholar]
- 79.Grandel U, Fink L, Blum A, Heep M, Buerke M, Kraemer HJ, Mayer K, Bohle RM, Seeger W, Grimminger F, Sibelius U. Endotoxin-induced myocardial tumor necrosis factor-alpha synthesis depresses contractility of isolated rat hearts: Evidence for a role of sphingosine and cyclooxygenase-2-derived thromboxane production. Circulation. 2000;102:2758–2764. doi: 10.1161/01.cir.102.22.2758. [DOI] [PubMed] [Google Scholar]
- 80.Yokoyama T, Vaca L, Rossen RD, Durante W, Hazarika P, Mann DL. Cellular basis for the negative inotropic effects of tumor necrosis factor-alpha in the adult mammalian heart. The Journal of clinical investigation. 1993;92:2303–2312. doi: 10.1172/JCI116834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Edmunds NJ, Lal H, Woodward B. Effects of tumour necrosis factor-alpha on left ventricular function in the rat isolated perfused heart: Possible mechanisms for a decline in cardiac function. British journal of pharmacology. 1999;126:189–196. doi: 10.1038/sj.bjp.0702294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oral H, Dorn GW, 2nd, Mann DL. Sphingosine mediates the immediate negative inotropic effects of tumor necrosis factor-alpha in the adult mammalian cardiac myocyte. The Journal of biological chemistry. 1997;272:4836–4842. doi: 10.1074/jbc.272.8.4836. [DOI] [PubMed] [Google Scholar]
- 83.Kolesnick R. The therapeutic potential of modulating the ceramide/sphingomyelin pathway. The Journal of clinical investigation. 2002;110:3–8. doi: 10.1172/JCI16127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu B, Andrieu-Abadie N, Levade T, Zhang P, Obeid LM, Hannun YA. Glutathione regulation of neutral sphingomyelinase in tumor necrosis factor-alpha-induced cell death. The Journal of biological chemistry. 1998;273:11313–11320. doi: 10.1074/jbc.273.18.11313. [DOI] [PubMed] [Google Scholar]
- 85.Jayadev S, Linardic CM, Hannun YA. Identification of arachidonic acid as a mediator of sphingomyelin hydrolysis in response to tumor necrosis factor alpha. The Journal of biological chemistry. 1994;269:5757–5763. [PubMed] [Google Scholar]
- 86.Friedrichs GS, Swillo RE, Jow B, Bridal T, Numann R, Warner LM, Killar LM, Sidek K. Sphingosine modulates myocyte electrophysiology, induces negative inotropy, and decreases survival after myocardial ischemia. Journal of cardiovascular pharmacology. 2002;39:18–28. doi: 10.1097/00005344-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 87.Dettbarn CA, Betto R, Salviati G, Palade P, Jenkins GM, Sabbadini RA. Modulation of cardiac sarcoplasmic reticulum ryanodine receptor by sphingosine. Journal of molecular and cellular cardiology. 1994;26:229–242. doi: 10.1006/jmcc.1994.1026. [DOI] [PubMed] [Google Scholar]
- 88.Schreur KD, Liu S. Involvement of ceramide in inhibitory effect of il-1 beta on l-type ca2+ current in adult rat ventricular myocytes. The American journal of physiology. 1997;272:H2591–2598. doi: 10.1152/ajpheart.1997.272.6.H2591. [DOI] [PubMed] [Google Scholar]
- 89.Torre-Amione G, Kapadia S, Lee J, Bies RD, Lebovitz R, Mann DL. Expression and functional significance of tumor necrosis factor receptors in human myocardium. Circulation. 1995;92:1487–1493. doi: 10.1161/01.cir.92.6.1487. [DOI] [PubMed] [Google Scholar]
- 90.Stamm C, Cowan DB, Friehs I, Noria S, del Nido PJ, McGowan FX., Jr Rapid endotoxin-induced alterations in myocardial calcium handling: Obligatory role of cardiac tnf-alpha. Anesthesiology. 2001;95:1396–1405. doi: 10.1097/00000542-200112000-00019. [DOI] [PubMed] [Google Scholar]
- 91.Edmunds NJ, Woodward B. Effects of tumour necrosis factor-alpha on the coronary circulation of the rat isolated perfused heart: A potential role for thromboxane a2 and sphingosine. British journal of pharmacology. 1998;124:493–498. doi: 10.1038/sj.bjp.0701863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cailleret M, Amadou A, Andrieu-Abadie N, Nawrocki A, Adamy C, Ait-Mamar B, Rocaries F, Best-Belpomme M, Levade T, Pavoine C, Pecker F. N-acetylcysteine prevents the deleterious effect of tumor necrosis factor-(alpha) on calcium transients and contraction in adult rat cardiomyocytes. Circulation. 2004;109:406–411. doi: 10.1161/01.CIR.0000109499.00587.FF. [DOI] [PubMed] [Google Scholar]
- 93.Robinson BS, Hii CS, Poulos A, Ferrante A. Activation of neutral sphingomyelinase in human neutrophils by polyunsaturated fatty acids. Immunology. 1997;91:274–280. doi: 10.1046/j.1365-2567.1997.d01-2227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mazurais D, Robert P, Gout B, Berrebi-Bertrand I, Laville MP, Calmels T. Cell type-specific localization of human cardiac s1p receptors. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2002;50:661–670. doi: 10.1177/002215540205000507. [DOI] [PubMed] [Google Scholar]
- 95.Morales-Ruiz M, Lee MJ, Zollner S, Gratton JP, Scotland R, Shiojima I, Walsh K, Hla T, Sessa WC. Sphingosine 1-phosphate activates akt, nitric oxide production, and chemotaxis through a gi protein/phosphoinositide 3-kinase pathway in endothelial cells. The Journal of biological chemistry. 2001;276:19672–19677. doi: 10.1074/jbc.M009993200. [DOI] [PubMed] [Google Scholar]
- 96.Amadou A, Nawrocki A, Best-Belpomme M, Pavoine C, Pecker F. Arachidonic acid mediates dual effect of tnf-alpha on ca2+ transients and contraction of adult rat cardiomyocytes. American journal of physiology Cell physiology. 2002;282:C1339–1347. doi: 10.1152/ajpcell.00471.2001. [DOI] [PubMed] [Google Scholar]
- 97.Liu SJ, McHowat J. Stimulation of different phospholipase a2 isoforms by tnf-alpha and il-1beta in adult rat ventricular myocytes. The American journal of physiology. 1998;275:H1462–1472. doi: 10.1152/ajpheart.1998.275.4.H1462. [DOI] [PubMed] [Google Scholar]
- 98.Damron DS, Summers BA. Arachidonic acid enhances contraction and intracellular ca2+ transients in individual rat ventricular myocytes. The American journal of physiology. 1997;272:H350–359. doi: 10.1152/ajpheart.1997.272.1.H350. [DOI] [PubMed] [Google Scholar]
- 99.de Bracco MM, Fink SB, Finiasz MR, Borda ES, Sterin-Borda L. Positive inotropic effect of interleukin-2. Role of phospholipases and protein kinase c. International journal of immunopharmacology. 1991;13:509–515. doi: 10.1016/0192-0561(91)90070-n. [DOI] [PubMed] [Google Scholar]
- 100.Fink SB, Finiasz M, Sterin-Borda L, Borda E, de Bracco MM. Stimulation of heart contractility by supernatants from lectin-activated lymphocytes. Role of il-2. International journal of immunopharmacology. 1989;11:367–370. doi: 10.1016/0192-0561(89)90082-9. [DOI] [PubMed] [Google Scholar]
- 101.Kang JX, Leaf A. Effects of long-chain polyunsaturated fatty acids on the contraction of neonatal rat cardiac myocytes. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:9886–9890. doi: 10.1073/pnas.91.21.9886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pavoine C, Magne S, Sauvadet A, Pecker F. Evidence for a beta2-adrenergic/arachidonic acid pathway in ventricular cardiomyocytes. Regulation by the beta1-adrenergic/camp pathway. The Journal of biological chemistry. 1999;274:628–637. doi: 10.1074/jbc.274.2.628. [DOI] [PubMed] [Google Scholar]
- 103.Kumar A, Kosuri R, Kandula P, Dimou C, Allen J, Parrillo JE. Effects of epinephrine and amrinone on contractility and cyclic adenosine monophosphate generation of tumor necrosis factor alpha-exposed cardiac myocytes. Critical care medicine. 1999;27:286–292. doi: 10.1097/00003246-199902000-00032. [DOI] [PubMed] [Google Scholar]
- 104.Liu SJ, Zhou W, Kennedy RH. Suppression of beta-adrenergic responsiveness of l-type ca2+ current by il-1beta in rat ventricular myocytes. The American journal of physiology. 1999;276:H141–148. doi: 10.1152/ajpheart.1999.276.1.H141. [DOI] [PubMed] [Google Scholar]
- 105.Blankenberg S, Luc G, Ducimetiere P, Arveiler D, Ferrieres J, Amouyel P, Evans A, Cambien F, Tiret L, Group PS. Interleukin-18 and the risk of coronary heart disease in european men: The prospective epidemiological study of myocardial infarction (prime) Circulation. 2003;108:2453–2459. doi: 10.1161/01.CIR.0000099509.76044.A2. [DOI] [PubMed] [Google Scholar]
- 106.Woldbaek PR, Sande JB, Stromme TA, Lunde PK, Djurovic S, Lyberg T, Christensen G, Tonnessen T. Daily administration of interleukin-18 causes myocardial dysfunction in healthy mice. American journal of physiology Heart and circulatory physiology. 2005;289:H708–714. doi: 10.1152/ajpheart.01179.2004. [DOI] [PubMed] [Google Scholar]
- 107.Boekstegers P, Kainz I, Giehrl W, Peter W, Werdan K. Subchronic exposure of cardiomyocytes to low concentrations of tumor necrosis factor alpha attenuates the positive inotropic response not only to catecholamines but also to cardiac glycosides and high calcium concentrations. Molecular and cellular biochemistry. 1996;156:135–143. doi: 10.1007/BF00426336. [DOI] [PubMed] [Google Scholar]
- 108.Fernandes DJ, Mitchell RW, Lakser O, Dowell M, Stewart AG, Solway J. Do inflammatory mediators influence the contribution of airway smooth muscle contraction to airway hyperresponsiveness in asthma? Journal of applied physiology. 2003;95:844–853. doi: 10.1152/japplphysiol.00192.2003. [DOI] [PubMed] [Google Scholar]
- 109.Allen ND, Davis BE, Cockcroft DW. Correlation between airway inflammation and loss of deep-inhalation bronchoprotection in asthma. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2008;101:413–418. doi: 10.1016/S1081-1206(10)60319-5. [DOI] [PubMed] [Google Scholar]
- 110.Bick RJ, Liao JP, King TW, LeMaistre A, McMillin JB, Buja LM. Temporal effects of cytokines on neonatal cardiac myocyte ca2+ transients and adenylate cyclase activity. The American journal of physiology. 1997;272:H1937–1944. doi: 10.1152/ajpheart.1997.272.4.H1937. [DOI] [PubMed] [Google Scholar]
- 111.Rozanski GJ, Witt RC. Il-1 inhibits beta-adrenergic control of cardiac calcium current: Role of l-arginine/nitric oxide pathway. The American journal of physiology. 1994;267:H1753–1758. doi: 10.1152/ajpheart.1994.267.5.H1753. [DOI] [PubMed] [Google Scholar]
- 112.Reithmann C, Gierschik P, Jakobs KH, Werdan K. Regulation of adenylyl cyclase by noradrenaline and tumour necrosis factor alpha in rat cardiomyocytes. European heart journal. 1991;12(Suppl F):139–142. doi: 10.1093/eurheartj/12.suppl_f.139. [DOI] [PubMed] [Google Scholar]
- 113.Reithmann C, Gierschik P, Werdan K, Jakobs KH. Tumor necrosis factor alpha up-regulates gi alpha and g beta proteins and adenylyl cyclase responsiveness in rat cardiomyocytes. European journal of pharmacology. 1991;206:53–60. doi: 10.1016/0922-4106(91)90146-9. [DOI] [PubMed] [Google Scholar]
- 114.Joe EK, Schussheim AE, Longrois D, Maki T, Kelly RA, Smith TW, Balligand JL. Regulation of cardiac myocyte contractile function by inducible nitric oxide synthase (inos): Mechanisms of contractile depression by nitric oxide. Journal of molecular and cellular cardiology. 1998;30:303–315. doi: 10.1006/jmcc.1997.0593. [DOI] [PubMed] [Google Scholar]
- 115.Lander HM, Hajjar DP, Hempstead BL, Mirza UA, Chait BT, Campbell S, Quilliam LA. A molecular redox switch on p21(ras). Structural basis for the nitric oxide-p21(ras) interaction. The Journal of biological chemistry. 1997;272:4323–4326. doi: 10.1074/jbc.272.7.4323. [DOI] [PubMed] [Google Scholar]
- 116.Muller-Werdan U, Schumann H, Fuchs R, Reithmann C, Loppnow H, Koch S, Zimny-Arndt U, He C, Darmer D, Jungblut P, Stadler J, Holtz J, Werdan K. Tumor necrosis factor alpha (tnf alpha) is cardiodepressant in pathophysiologically relevant concentrations without inducing inducible nitric oxide-(no)-synthase (inos) or triggering serious cytotoxicity. Journal of molecular and cellular cardiology. 1997;29:2915–2923. doi: 10.1006/jmcc.1997.0526. [DOI] [PubMed] [Google Scholar]
- 117.Muller-Werdan U, Schumann H, Loppnow H, Fuchs R, Darmer D, Stadler J, Holtz J, Werdan K. Endotoxin and tumor necrosis factor alpha exert a similar proinflammatory effect in neonatal rat cardiomyocytes, but have different cardiodepressant profiles. Journal of molecular and cellular cardiology. 1998;30:1027–1036. doi: 10.1006/jmcc.1998.0667. [DOI] [PubMed] [Google Scholar]
- 118.Oyama J, Shimokawa H, Momii H, Cheng X, Fukuyama N, Arai Y, Egashira K, Nakazawa H, Takeshita A. Role of nitric oxide and peroxynitrite in the cytokine-induced sustained myocardial dysfunction in dogs in vivo. The Journal of clinical investigation. 1998;101:2207–2214. doi: 10.1172/JCI986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schulz R, Panas DL, Catena R, Moncada S, Olley PM, Lopaschuk GD. The role of nitric oxide in cardiac depression induced by interleukin-1 beta and tumour necrosis factor-alpha. British journal of pharmacology. 1995;114:27–34. doi: 10.1111/j.1476-5381.1995.tb14901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Evans HG, Lewis MJ, Shah AM. Interleukin-1 beta modulates myocardial contraction via dexamethasone sensitive production of nitric oxide. Cardiovascular research. 1993;27:1486–1490. doi: 10.1093/cvr/27.8.1486. [DOI] [PubMed] [Google Scholar]
- 121.Ferdinandy P, Danial H, Ambrus I, Rothery RA, Schulz R. Peroxynitrite is a major contributor to cytokine-induced myocardial contractile failure. Circulation research. 2000;87:241–247. doi: 10.1161/01.res.87.3.241. [DOI] [PubMed] [Google Scholar]
- 122.Sun X, Delbridge LM, Dusting GJ. Cardiodepressant effects of interferon-gamma and endotoxin reversed by inhibition of no synthase 2 in rat myocardium. Journal of molecular and cellular cardiology. 1998;30:989–997. doi: 10.1006/jmcc.1998.0663. [DOI] [PubMed] [Google Scholar]
- 123.Tatsumi T, Matoba S, Kawahara A, Keira N, Shiraishi J, Akashi K, Kobara M, Tanaka T, Katamura M, Nakagawa C, Ohta B, Shirayama T, Takeda K, Asayama J, Fliss H, Nakagawa M. Cytokine-induced nitric oxide production inhibits mitochondrial energy production and impairs contractile function in rat cardiac myocytes. Journal of the American College of Cardiology. 2000;35:1338–1346. doi: 10.1016/s0735-1097(00)00526-x. [DOI] [PubMed] [Google Scholar]
- 124.Balligand JL, Ungureanu D, Kelly RA, Kobzik L, Pimental D, Michel T, Smith TW. Abnormal contractile function due to induction of nitric oxide synthesis in rat cardiac myocytes follows exposure to activated macrophage-conditioned medium. The Journal of clinical investigation. 1993;91:2314–2319. doi: 10.1172/JCI116461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Balligand JL, Ungureanu-Longrois D, Simmons WW, Pimental D, Malinski TA, Kapturczak M, Taha Z, Lowenstein CJ, Davidoff AJ, Kelly RA, et al. Cytokine-inducible nitric oxide synthase (inos) expression in cardiac myocytes. Characterization and regulation of inos expression and detection of inos activity in single cardiac myocytes in vitro. The Journal of biological chemistry. 1994;269:27580–27588. [PubMed] [Google Scholar]
- 126.Ungureanu-Longrois D, Balligand JL, Okada I, Simmons WW, Kobzik L, Lowenstein CJ, Kunkel SL, Michel T, Kelly RA, Smith TW. Contractile responsiveness of ventricular myocytes to isoproterenol is regulated by induction of nitric oxide synthase activity in cardiac microvascular endothelial cells in heterotypic primary culture. Circulation research. 1995;77:486–493. doi: 10.1161/01.res.77.3.486. [DOI] [PubMed] [Google Scholar]
- 127.Ungureanu-Longrois D, Balligand JL, Simmons WW, Okada I, Kobzik L, Lowenstein CJ, Kunkel SL, Michel T, Kelly RA, Smith TW. Induction of nitric oxide synthase activity by cytokines in ventricular myocytes is necessary but not sufficient to decrease contractile responsiveness to beta-adrenergic agonists. Circulation research. 1995;77:494–502. doi: 10.1161/01.res.77.3.494. [DOI] [PubMed] [Google Scholar]
- 128.Ungureanu-Longrois D, Balligand JL, Kelly RA, Smith TW. Myocardial contractile dysfunction in the systemic inflammatory response syndrome: Role of a cytokine-inducible nitric oxide synthase in cardiac myocytes. Journal of molecular and cellular cardiology. 1995;27:155–167. doi: 10.1016/s0022-2828(08)80015-6. [DOI] [PubMed] [Google Scholar]
- 129.Funakoshi H, Kubota T, Kawamura N, Machida Y, Feldman AM, Tsutsui H, Shimokawa H, Takeshita A. Disruption of inducible nitric oxide synthase improves beta-adrenergic inotropic responsiveness but not the survival of mice with cytokine-induced cardiomyopathy. Circulation research. 2002;90:959–965. doi: 10.1161/01.res.0000017632.83720.68. [DOI] [PubMed] [Google Scholar]
- 130.Funakoshi H, Kubota T, Machida Y, Kawamura N, Feldman AM, Tsutsui H, Shimokawa H, Takeshita A. Involvement of inducible nitric oxide synthase in cardiac dysfunction with tumor necrosis factor-alpha. American journal of physiology Heart and circulatory physiology. 2002;282:H2159–2166. doi: 10.1152/ajpheart.00872.2001. [DOI] [PubMed] [Google Scholar]
- 131.Yu X, Kennedy RH, Liu SJ. Jak2/stat3, not erk1/2, mediates interleukin-6-induced activation of inducible nitric-oxide synthase and decrease in contractility of adult ventricular myocytes. The Journal of biological chemistry. 2003;278:16304–16309. doi: 10.1074/jbc.M212321200. [DOI] [PubMed] [Google Scholar]
- 132.Pinsky DJ, Cai B, Yang X, Rodriguez C, Sciacca RR, Cannon PJ. The lethal effects of cytokine-induced nitric oxide on cardiac myocytes are blocked by nitric oxide synthase antagonism or transforming growth factor beta. The Journal of clinical investigation. 1995;95:677–685. doi: 10.1172/JCI117713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Okusawa S, Gelfand JA, Ikejima T, Connolly RJ, Dinarello CA. Interleukin 1 induces a shock-like state in rabbits. Synergism with tumor necrosis factor and the effect of cyclooxygenase inhibition. The Journal of clinical investigation. 1988;81:1162–1172. doi: 10.1172/JCI113431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Balligand JL. Regulation of cardiac beta-adrenergic response by nitric oxide. Cardiovascular research. 1999;43:607–620. doi: 10.1016/s0008-6363(99)00163-7. [DOI] [PubMed] [Google Scholar]
- 135.Ungureanu-Longrois D, Bezie Y, Perret C, Laurent S. Effects of exogenous and endogenous nitric oxide on the contractile function of cultured chick embryo ventricular myocytes. Journal of molecular and cellular cardiology. 1997;29:677–687. doi: 10.1006/jmcc.1996.0310. [DOI] [PubMed] [Google Scholar]
- 136.Murray DR, Prabhu SD, Chandrasekar B. Chronic beta-adrenergic stimulation induces myocardial proinflammatory cytokine expression. Circulation. 2000;101:2338–2341. doi: 10.1161/01.cir.101.20.2338. [DOI] [PubMed] [Google Scholar]
- 137.Benovic JL, Mayor F, Jr, Somers RL, Caron MG, Lefkowitz RJ. Light-dependent phosphorylation of rhodopsin by beta-adrenergic receptor kinase. Nature. 1986;321:869–872. doi: 10.1038/321869a0. [DOI] [PubMed] [Google Scholar]
- 138.Benovic JL, Strasser RH, Caron MG, Lefkowitz RJ. Beta-adrenergic receptor kinase: Identification of a novel protein kinase that phosphorylates the agonist-occupied form of the receptor. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:2797–2801. doi: 10.1073/pnas.83.9.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Benovic JL, Gomez J. Molecular cloning and expression of grk6. A new member of the g protein-coupled receptor kinase family. The Journal of biological chemistry. 1993;268:19521–19527. [PubMed] [Google Scholar]
- 140.Kim CM, Dion SB, Benovic JL. Mechanism of beta-adrenergic receptor kinase activation by g proteins. The Journal of biological chemistry. 1993;268:15412–15418. [PubMed] [Google Scholar]
- 141.Kim CM, Dion SB, Onorato JJ, Benovic JL. Expression and characterization of two beta-adrenergic receptor kinase isoforms using the baculovirus expression system. Receptor. 1993;3:39–55. [PubMed] [Google Scholar]
- 142.Kunapuli P, Benovic JL. Cloning and expression of grk5: A member of the g protein-coupled receptor kinase family. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:5588–5592. doi: 10.1073/pnas.90.12.5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hausdorff WP, Lohse MJ, Bouvier M, Liggett SB, Caron MG, Lefkowitz RJ. Two kinases mediate agonist-dependent phosphorylation and desensitization of the beta 2-adrenergic receptor. Symposia of the Society for Experimental Biology. 1990;44:225–240. [PubMed] [Google Scholar]
- 144.Lohse MJ, Benovic JL, Codina J, Caron MG, Lefkowitz RJ. Beta-arrestin: A protein that regulates beta-adrenergic receptor function. Science. 1990;248:1547–1550. doi: 10.1126/science.2163110. [DOI] [PubMed] [Google Scholar]
- 145.Menard L, Ferguson SS, Zhang J, Lin FT, Lefkowitz RJ, Caron MG, Barak LS. Synergistic regulation of beta2-adrenergic receptor sequestration: Intracellular complement of beta-adrenergic receptor kinase and beta-arrestin determine kinetics of internalization. Molecular pharmacology. 1997;51:800–808. [PubMed] [Google Scholar]
- 146.Zhang J, Ferguson SS, Barak LS, Aber MJ, Giros B, Lefkowitz RJ, Caron MG. Molecular mechanisms of g protein-coupled receptor signaling: Role of g protein-coupled receptor kinases and arrestins in receptor desensitization and resensitization. Receptors & channels. 1997;5:193–199. [PubMed] [Google Scholar]
- 147.Islam KN, Bae JW, Gao E, Koch WJ. Regulation of nuclear factor kappab (nf-kappab) in the nucleus of cardiomyocytes by g protein-coupled receptor kinase 5 (grk5) The Journal of biological chemistry. 2013;288:35683–35689. doi: 10.1074/jbc.M113.529347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ohba Y, Nakaya M, Watari K, Nagasaka A, Kurose H. Grk6 phosphorylates ikappabalpha at ser(32)/ser(36) and enhances tnf-alpha-induced inflammation. Biochemical and biophysical research communications. 2015;461:307–313. doi: 10.1016/j.bbrc.2015.04.027. [DOI] [PubMed] [Google Scholar]
- 149.Parameswaran N, Pao CS, Leonhard KS, Kang DS, Kratz M, Ley SC, Benovic JL. Arrestin-2 and g protein-coupled receptor kinase 5 interact with nfkappab1 p105 and negatively regulate lipopolysaccharide-stimulated erk1/2 activation in macrophages. The Journal of biological chemistry. 2006;281:34159–34170. doi: 10.1074/jbc.M605376200. [DOI] [PubMed] [Google Scholar]
- 150.Patial S, Luo J, Porter KJ, Benovic JL, Parameswaran N. G-protein-coupled-receptor kinases mediate tnfalpha-induced nfkappab signalling via direct interaction with and phosphorylation of ikappabalpha. The Biochemical journal. 2010;425:169–178. doi: 10.1042/BJ20090908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Patial S, Saini Y, Parvataneni S, Appledorn DM, Dorn GW, 2nd, Lapres JJ, Amalfitano A, Senagore P, Parameswaran N. Myeloid-specific gpcr kinase-2 negatively regulates nf-kappab1p105-erk pathway and limits endotoxemic shock in mice. Journal of cellular physiology. 2011;226:627–637. doi: 10.1002/jcp.22384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Patial S, Shahi S, Saini Y, Lee T, Packiriswamy N, Appledorn DM, Lapres JJ, Amalfitano A, Parameswaran N. G-protein coupled receptor kinase 5 mediates lipopolysaccharide-induced nfkappab activation in primary macrophages and modulates inflammation in vivo in mice. Journal of cellular physiology. 2011;226:1323–1333. doi: 10.1002/jcp.22460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sorriento D, Ciccarelli M, Santulli G, Campanile A, Altobelli GG, Cimini V, Galasso G, Astone D, Piscione F, Pastore L, Trimarco B, Iaccarino G. The g-protein-coupled receptor kinase 5 inhibits nfkappab transcriptional activity by inducing nuclear accumulation of ikappab alpha. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:17818–17823. doi: 10.1073/pnas.0804446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Valanne S, Myllymaki H, Kallio J, Schmid MR, Kleino A, Murumagi A, Airaksinen L, Kotipelto T, Kaustio M, Ulvila J, Esfahani SS, Engstrom Y, Silvennoinen O, Hultmark D, Parikka M, Ramet M. Genome-wide rna interference in drosophila cells identifies g protein-coupled receptor kinase 2 as a conserved regulator of nf-kappab signaling. Journal of immunology. 2010;184:6188–6198. doi: 10.4049/jimmunol.1000261. [DOI] [PubMed] [Google Scholar]
- 155.Loniewski K, Shi Y, Pestka J, Parameswaran N. Toll-like receptors differentially regulate gpcr kinases and arrestins in primary macrophages. Molecular immunology. 2008;45:2312–2322. doi: 10.1016/j.molimm.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 156.Packiriswamy N, Parameswaran N. G-protein-coupled receptor kinases in inflammation and disease. Genes and immunity. 2015;16:367–377. doi: 10.1038/gene.2015.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kim J, Ahn S, Ren XR, Whalen EJ, Reiter E, Wei H, Lefkowitz RJ. Functional antagonism of different g protein-coupled receptor kinases for beta-arrestin-mediated angiotensin ii receptor signaling. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:1442–1447. doi: 10.1073/pnas.0409532102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Franklin JM, Carrasco GA. G-protein receptor kinase 5 regulates the cannabinoid receptor 2-induced up-regulation of serotonin 2a receptors. The Journal of biological chemistry. 2013;288:15712–15724. doi: 10.1074/jbc.M113.454843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Guo J, Chen H, Ho J, Mancini J, Sontag T, Laporte SA, Richard DE, Lebrun JJ. Tgfbeta-induced grk2 expression attenuates angii-regulated vascular smooth muscle cell proliferation and migration. Cellular signalling. 2009;21:899–905. doi: 10.1016/j.cellsig.2009.01.037. [DOI] [PubMed] [Google Scholar]
- 160.Lee JC, Laydon JT, McDonnell PC, Gallagher TF, Kumar S, Green D, McNulty D, Blumenthal MJ, Heys JR, Landvatter SW, et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- 161.Marriott JB, Clarke IA, Dalgleish AG. Inhibition of p38 map kinase during cellular activation results in ifn-gamma-dependent augmentation of il-12 production by human monocytes/macrophages. Clinical and experimental immunology. 2001;125:64–70. doi: 10.1046/j.1365-2249.2001.01574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Peregrin S, Jurado-Pueyo M, Campos PM, Sanz-Moreno V, Ruiz-Gomez A, Crespo P, Mayor F, Jr, Murga C. Phosphorylation of p38 by grk2 at the docking groove unveils a novel mechanism for inactivating p38mapk. Current biology : CB. 2006;16:2042–2047. doi: 10.1016/j.cub.2006.08.083. [DOI] [PubMed] [Google Scholar]
- 163.Liu X, Ma B, Malik AB, Tang H, Yang T, Sun B, Wang G, Minshall RD, Li Y, Zhao Y, Ye RD, Xu J. Bidirectional regulation of neutrophil migration by mitogen-activated protein kinases. Nature immunology. 2012;13:457–464. doi: 10.1038/ni.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Nijboer CH, Heijnen CJ, Willemen HL, Groenendaal F, Dorn GW, 2nd, van Bel F, Kavelaars A. Cell-specific roles of grk2 in onset and severity of hypoxic-ischemic brain damage in neonatal mice. Brain, behavior, and immunity. 2010;24:420–426. doi: 10.1016/j.bbi.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Liu Y, Gorospe M, Yang C, Holbrook NJ. Role of mitogen-activated protein kinase phosphatase during the cellular response to genotoxic stress. Inhibition of c-jun n-terminal kinase activity and ap-1-dependent gene activation. The Journal of biological chemistry. 1995;270:8377–8380. doi: 10.1074/jbc.270.15.8377. [DOI] [PubMed] [Google Scholar]
- 166.Gomez del Arco P, Martinez-Martinez S, Calvo V, Armesilla AL, Redondo JM. Jnk (c-jun nh2-terminal kinase) is a target for antioxidants in t lymphocytes. The Journal of biological chemistry. 1996;271:26335–26340. doi: 10.1074/jbc.271.42.26335. [DOI] [PubMed] [Google Scholar]
- 167.Jung S, Yaron A, Alkalay I, Hatzubai A, Avraham A, Ben-Neriah Y. Costimulation requirement for ap-1 and nf-kappa b transcription factor activation in t cells. Annals of the New York Academy of Sciences. 1995;766:245–252. doi: 10.1111/j.1749-6632.1995.tb26672.x. [DOI] [PubMed] [Google Scholar]
- 168.Eckhart AD, Duncan SJ, Penn RB, Benovic JL, Lefkowitz RJ, Koch WJ. Hybrid transgenic mice reveal in vivo specificity of g protein-coupled receptor kinases in the heart. Circulation research. 2000;86:43–50. doi: 10.1161/01.res.86.1.43. [DOI] [PubMed] [Google Scholar]
- 169.Bychkov ER, Gurevich VV, Joyce JN, Benovic JL, Gurevich EV. Arrestins and two receptor kinases are upregulated in parkinson’s disease with dementia. Neurobiology of aging. 2008;29:379–396. doi: 10.1016/j.neurobiolaging.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Obrenovich ME, Palacios HH, Gasimov E, Leszek J, Aliev G. The grk2 overexpression is a primary hallmark of mitochondrial lesions during early alzheimer disease. Cardiovascular psychiatry and neurology. 2009;2009:327360. doi: 10.1155/2009/327360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sorensen SD, Conn PJ. G protein-coupled receptor kinases regulate metabotropic glutamate receptor 5 function and expression. Neuropharmacology. 2003;44:699–706. doi: 10.1016/s0028-3908(03)00053-4. [DOI] [PubMed] [Google Scholar]
- 172.Suo WZ, Li L. Dysfunction of g protein-coupled receptor kinases in alzheimer’s disease. TheScientificWorldJournal. 2010;10:1667–1678. doi: 10.1100/tsw.2010.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Vroon A, Kavelaars A, Limmroth V, Lombardi MS, Goebel MU, Van Dam AM, Caron MG, Schedlowski M, Heijnen CJ. G protein-coupled receptor kinase 2 in multiple sclerosis and experimental autoimmune encephalomyelitis. Journal of immunology. 2005;174:4400–4406. doi: 10.4049/jimmunol.174.7.4400. [DOI] [PubMed] [Google Scholar]
- 174.Willemen HL, Eijkelkamp N, Wang H, Dantzer R, Dorn GW, 2nd, Kelley KW, Heijnen CJ, Kavelaars A. Microglial/macrophage grk2 determines duration of peripheral il-1beta-induced hyperalgesia: Contribution of spinal cord cx3cr1, p38 and il-1 signaling. Pain. 2010;150:550–560. doi: 10.1016/j.pain.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Eijkelkamp N, Heijnen CJ, Lucas A, Premont RT, Elsenbruch S, Schedlowski M, Kavelaars A. G protein-coupled receptor kinase 6 controls chronicity and severity of dextran sodium sulphate-induced colitis in mice. Gut. 2007;56:847–854. doi: 10.1136/gut.2006.107094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tarrant TK, Rampersad RR, Esserman D, Rothlein LR, Liu P, Premont RT, Lefkowitz RJ, Lee DM, Patel DD. Granulocyte chemotaxis and disease expression are differentially regulated by grk subtype in an acute inflammatory arthritis model (k/bxn) Clinical immunology. 2008;129:115–122. doi: 10.1016/j.clim.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Degos V, Peineau S, Nijboer C, Kaindl AM, Sigaut S, Favrais G, Plaisant F, Teissier N, Gouadon E, Lombet A, Saliba E, Collingridge GL, Maze M, Nicoletti F, Heijnen C, Mantz J, Kavelaars A, Gressens P. G protein-coupled receptor kinase 2 and group i metabotropic glutamate receptors mediate inflammation-induced sensitization to excitotoxic neurodegeneration. Annals of neurology. 2013;73:667–678. doi: 10.1002/ana.23868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Eijkelkamp N, Heijnen CJ, Willemen HL, Deumens R, Joosten EA, Kleibeuker W, den Hartog IJ, van Velthoven CT, Nijboer C, Nassar MA, Dorn GW, 2nd, Wood JN, Kavelaars A. Grk2: A novel cell-specific regulator of severity and duration of inflammatory pain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:2138–2149. doi: 10.1523/JNEUROSCI.5752-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Eijkelkamp N, Wang H, Garza-Carbajal A, Willemen HL, Zwartkruis FJ, Wood JN, Dantzer R, Kelley KW, Heijnen CJ, Kavelaars A. Low nociceptor grk2 prolongs prostaglandin e2 hyperalgesia via biased camp signaling to epac/rap1, protein kinase cepsilon, and mek/erk. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:12806–12815. doi: 10.1523/JNEUROSCI.3142-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ferrari LF, Bogen O, Alessandri-Haber N, Levine E, Gear RW, Levine JD. Transient decrease in nociceptor grk2 expression produces long-term enhancement in inflammatory pain. Neuroscience. 2012;222:392–403. doi: 10.1016/j.neuroscience.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Liu J, Rasul I, Sun Y, Wu G, Li L, Premont RT, Suo WZ. Grk5 deficiency leads to reduced hippocampal acetylcholine level via impaired presynaptic m2/m4 autoreceptor desensitization. The Journal of biological chemistry. 2009;284:19564–19571. doi: 10.1074/jbc.M109.005959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Nakaya M, Tajima M, Kosako H, Nakaya T, Hashimoto A, Watari K, Nishihara H, Ohba M, Komiya S, Tani N, Nishida M, Taniguchi H, Sato Y, Matsumoto M, Tsuda M, Kuroda M, Inoue K, Kurose H. Grk6 deficiency in mice causes autoimmune disease due to impaired apoptotic cell clearance. Nature communications. 2013;4:1532. doi: 10.1038/ncomms2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sadot E, Gurwitz D, Barg J, Behar L, Ginzburg I, Fisher A. Activation of m1 muscarinic acetylcholine receptor regulates tau phosphorylation in transfected pc12 cells. Journal of neurochemistry. 1996;66:877–880. doi: 10.1046/j.1471-4159.1996.66020877.x. [DOI] [PubMed] [Google Scholar]
- 184.Wang H, Heijnen CJ, Eijkelkamp N, Garza Carbajal A, Schedlowski M, Kelley KW, Dantzer R, Kavelaars A. Grk2 in sensory neurons regulates epinephrine-induced signalling and duration of mechanical hyperalgesia. Pain. 2011;152:1649–1658. doi: 10.1016/j.pain.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 185.Wang H, Heijnen CJ, van Velthoven CT, Willemen HL, Ishikawa Y, Zhang X, Sood AK, Vroon A, Eijkelkamp N, Kavelaars A. Balancing grk2 and epac1 levels prevents and relieves chronic pain. The Journal of clinical investigation. 2013;123:5023–5034. doi: 10.1172/JCI66241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chen EP, Bittner HB, Akhter SA, Koch WJ, Davis RD. Myocardial function in hearts with transgenic overexpression of the g protein-coupled receptor kinase 5. The Annals of thoracic surgery. 2001;71:1320–1324. doi: 10.1016/s0003-4975(00)01754-9. [DOI] [PubMed] [Google Scholar]
- 187.Vinge LE, von Lueder TG, Aasum E, Qvigstad E, Gravning JA, How OJ, Edvardsen T, Bjornerheim R, Ahmed MS, Mikkelsen BW, Oie E, Attramadal T, Skomedal T, Smiseth OA, Koch WJ, Larsen TS, Attramadal H. Cardiac-restricted expression of the carboxyl-terminal fragment of grk3 uncovers distinct functions of grk3 in regulation of cardiac contractility and growth: Grk3 controls cardiac alpha1-adrenergic receptor responsiveness. The Journal of biological chemistry. 2008;283:10601–10610. doi: 10.1074/jbc.M708912200. [DOI] [PubMed] [Google Scholar]
- 188.Zhang Y, Matkovich SJ, Duan X, Gold JI, Koch WJ, Dorn GW., 2nd Nuclear effects of g-protein receptor kinase 5 on histone deacetylase 5-regulated gene transcription in heart failure. Circulation Heart failure. 2011;4:659–668. doi: 10.1161/CIRCHEARTFAILURE.111.962563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Brinks H, Boucher M, Gao E, Chuprun JK, Pesant S, Raake PW, Huang ZM, Wang X, Qiu G, Gumpert A, Harris DM, Eckhart AD, Most P, Koch WJ. Level of g protein-coupled receptor kinase-2 determines myocardial ischemia/reperfusion injury via pro- and anti-apoptotic mechanisms. Circulation research. 2010;107:1140–1149. doi: 10.1161/CIRCRESAHA.110.221010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Eckhart AD, Ozaki T, Tevaearai H, Rockman HA, Koch WJ. Vascular-targeted overexpression of g protein-coupled receptor kinase-2 in transgenic mice attenuates beta-adrenergic receptor signaling and increases resting blood pressure. Molecular pharmacology. 2002;61:749–758. doi: 10.1124/mol.61.4.749. [DOI] [PubMed] [Google Scholar]
- 191.Harding VB, Jones LR, Lefkowitz RJ, Koch WJ, Rockman HA. Cardiac beta ark1 inhibition prolongs survival and augments beta blocker therapy in a mouse model of severe heart failure. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:5809–5814. doi: 10.1073/pnas.091102398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Keys JR, Zhou RH, Harris DM, Druckman CA, Eckhart AD. Vascular smooth muscle overexpression of g protein-coupled receptor kinase 5 elevates blood pressure, which segregates with sex and is dependent on gi-mediated signaling. Circulation. 2005;112:1145–1153. doi: 10.1161/CIRCULATIONAHA.104.531657. [DOI] [PubMed] [Google Scholar]
- 193.Rengo G, Lymperopoulos A, Zincarelli C, Donniacuo M, Soltys S, Rabinowitz JE, Koch WJ. Myocardial adeno-associated virus serotype 6-betaarkct gene therapy improves cardiac function and normalizes the neurohormonal axis in chronic heart failure. Circulation. 2009;119:89–98. doi: 10.1161/CIRCULATIONAHA.108.803999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Shah AS, White DC, Emani S, Kypson AP, Lilly RE, Wilson K, Glower DD, Lefkowitz RJ, Koch WJ. In vivo ventricular gene delivery of a beta-adrenergic receptor kinase inhibitor to the failing heart reverses cardiac dysfunction. Circulation. 2001;103:1311–1316. doi: 10.1161/01.cir.103.9.1311. [DOI] [PubMed] [Google Scholar]
- 195.Sorriento D, Santulli G, Fusco A, Anastasio A, Trimarco B, Iaccarino G. Intracardiac injection of adgrk5-nt reduces left ventricular hypertrophy by inhibiting nf-kappab-dependent hypertrophic gene expression. Hypertension. 2010;56:696–704. doi: 10.1161/HYPERTENSIONAHA.110.155960. [DOI] [PubMed] [Google Scholar]
- 196.Raake PW, Vinge LE, Gao E, Boucher M, Rengo G, Chen X, DeGeorge BR, Jr, Matkovich S, Houser SR, Most P, Eckhart AD, Dorn GW, 2nd, Koch WJ. G protein-coupled receptor kinase 2 ablation in cardiac myocytes before or after myocardial infarction prevents heart failure. Circulation research. 2008;103:413–422. doi: 10.1161/CIRCRESAHA.107.168336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Otten JJ, de Jager SC, Kavelaars A, Seijkens T, Bot I, Wijnands E, Beckers L, Westra MM, Bot M, Busch M, Bermudez B, van Berkel TJ, Heijnen CJ, Biessen EA. Hematopoietic g-protein-coupled receptor kinase 2 deficiency decreases atherosclerotic lesion formation in ldl receptor-knockout mice. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2013;27:265–276. doi: 10.1096/fj.12-205351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wu JH, Zhang L, Fanaroff AC, Cai X, Sharma KC, Brian L, Exum ST, Shenoy SK, Peppel K, Freedman NJ. G protein-coupled receptor kinase-5 attenuates atherosclerosis by regulating receptor tyrosine kinases and 7-transmembrane receptors. Arteriosclerosis, thrombosis, and vascular biology. 2012;32:308–316. doi: 10.1161/ATVBAHA.111.239608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Arraes SM, Freitas MS, da Silva SV, de Paula Neto HA, Alves-Filho JC, Auxiliadora Martins M, Basile-Filho A, Tavares-Murta BM, Barja-Fidalgo C, Cunha FQ. Impaired neutrophil chemotaxis in sepsis associates with grk expression and inhibition of actin assembly and tyrosine phosphorylation. Blood. 2006;108:2906–2913. doi: 10.1182/blood-2006-05-024638. [DOI] [PubMed] [Google Scholar]
- 200.Alves-Filho JC, Sonego F, Souto FO, Freitas A, Verri WA, Jr, Auxiliadora-Martins M, Basile-Filho A, McKenzie AN, Xu D, Cunha FQ, Liew FY. Interleukin-33 attenuates sepsis by enhancing neutrophil influx to the site of infection. Nature medicine. 2010;16:708–712. doi: 10.1038/nm.2156. [DOI] [PubMed] [Google Scholar]
- 201.Melamed A, Sorvillo FJ. The burden of sepsis-associated mortality in the united states from 1999 to 2005: An analysis of multiple-cause-of-death data. Critical care. 2009;13:R28. doi: 10.1186/cc7733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Packiriswamy N, Lee T, Raghavendra PB, Durairaj H, Wang H, Parameswaran N. G-protein-coupled receptor kinase-5 mediates inflammation but does not regulate cellular infiltration or bacterial load in a polymicrobial sepsis model in mice. Journal of innate immunity. 2013;5:401–413. doi: 10.1159/000347002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Packiriswamy N, Parvataneni S, Parameswaran N. Overlapping and distinct roles of grk5 in tlr2-, and tlr3-induced inflammatory response in vivo. Cellular immunology. 2012;272:107–111. doi: 10.1016/j.cellimm.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Parvataneni S, Gonipeta B, Packiriswamy N, Lee T, Durairaj H, Parameswaran N. Role of myeloid-specific g-protein coupled receptor kinase-2 in sepsis. International journal of clinical and experimental medicine. 2011;4:320–330. [PMC free article] [PubMed] [Google Scholar]
- 205.Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415:206–212. doi: 10.1038/415206a. [DOI] [PubMed] [Google Scholar]
- 206.Tan KS, Nackley AG, Satterfield K, Maixner W, Diatchenko L, Flood PM. Beta2 adrenergic receptor activation stimulates pro-inflammatory cytokine production in macrophages via pka- and nf-kappab-independent mechanisms. Cellular signalling. 2007;19:251–260. doi: 10.1016/j.cellsig.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 207.Verhoeckx KC, Doornbos RP, Witkamp RF, van der Greef J, Rodenburg RJ. Beta-adrenergic receptor agonists induce the release of granulocyte chemotactic protein-2, oncostatin m, and vascular endothelial growth factor from macrophages. International immunopharmacology. 2006;6:1–7. doi: 10.1016/j.intimp.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 208.Kim MH, Gorouhi F, Ramirez S, Granick JL, Byrne BA, Soulika AM, Simon SI, Isseroff RR. Catecholamine stress alters neutrophil trafficking and impairs wound healing by beta2-adrenergic receptor-mediated upregulation of il-6. The Journal of investigative dermatology. 2014;134:809–817. doi: 10.1038/jid.2013.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Roth Flach RJ, Matevossian A, Akie TE, Negrin KA, Paul MT, Czech MP. Beta3-adrenergic receptor stimulation induces e-selectin-mediated adipose tissue inflammation. The Journal of biological chemistry. 2013;288:2882–2892. doi: 10.1074/jbc.M112.412346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Nguyen LP, Lin R, Parra S, Omoluabi O, Hanania NA, Tuvim MJ, Knoll BJ, Dickey BF, Bond RA. Beta2-adrenoceptor signaling is required for the development of an asthma phenotype in a murine model. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:2435–2440. doi: 10.1073/pnas.0810902106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Chandrasekar B, Marelli-Berg FM, Tone M, Bysani S, Prabhu SD, Murray DR. Beta-adrenergic stimulation induces interleukin-18 expression via beta2-ar, pi3k, akt, ikk, and nf-kappab. Biochemical and biophysical research communications. 2004;319:304–311. doi: 10.1016/j.bbrc.2004.04.185. [DOI] [PubMed] [Google Scholar]
- 212.Chandrasekar B, Vemula K, Surabhi RM, Li-Weber M, Owen-Schaub LB, Jensen LE, Mummidi S. Activation of intrinsic and extrinsic proapoptotic signaling pathways in interleukin-18-mediated human cardiac endothelial cell death. The Journal of biological chemistry. 2004;279:20221–20233. doi: 10.1074/jbc.M313980200. [DOI] [PubMed] [Google Scholar]
- 213.Grisanti LA, Repas AA, Talarico JA, Gold JI, Carter RL, Koch WJ, Tilley DG. Temporal and gefitinib-sensitive regulation of cardiac cytokine expression via chronic beta-adrenergic receptor stimulation. American journal of physiology Heart and circulatory physiology. 2015;308:H316–330. doi: 10.1152/ajpheart.00635.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kalliolias GD, Ivashkiv LB. Tnf biology, pathogenic mechanisms and emerging therapeutic strategies. Nature reviews Rheumatology. 2016;12:49–62. doi: 10.1038/nrrheum.2015.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S, Nelson N, Boiani N, Schooley KA, Gerhart M, Davis R, Fitzner JN, Johnson RS, Paxton RJ, March CJ, Cerretti DP. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- 216.Hsu H, Shu HB, Pan MG, Goeddel DV. Tradd-traf2 and tradd-fadd interactions define two distinct tnf receptor 1 signal transduction pathways. Cell. 1996;84:299–308. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- 217.Grell M, Douni E, Wajant H, Lohden M, Clauss M, Maxeiner B, Georgopoulos S, Lesslauer W, Kollias G, Pfizenmaier K, Scheurich P. The transmembrane form of tumor necrosis factor is the prime activating ligand of the 80 kda tumor necrosis factor receptor. Cell. 1995;83:793–802. doi: 10.1016/0092-8674(95)90192-2. [DOI] [PubMed] [Google Scholar]
- 218.Al-Lamki RS, Brookes AP, Wang J, Reid MJ, Parameshwar J, Goddard MJ, Tellides G, Wan T, Min W, Pober JS, Bradley JR. Tnf receptors differentially signal and are differentially expressed and regulated in the human heart. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2009;9:2679–2696. doi: 10.1111/j.1600-6143.2009.02831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Burvall K, Palmberg L, Larsson K. Expression of tnfalpha and its receptors r1 and r2 in human alveolar epithelial cells exposed to organic dust and the effects of 8-bromo-camp and protein kinase a modulation. Inflammation research : official journal of the European Histamine Research Society … [et al] 2005;54:281–288. doi: 10.1007/s00011-005-1356-7. [DOI] [PubMed] [Google Scholar]
- 220.Brenner D, Blaser H, Mak TW. Regulation of tumour necrosis factor signalling: Live or let die. Nature reviews Immunology. 2015;15:362–374. doi: 10.1038/nri3834. [DOI] [PubMed] [Google Scholar]
- 221.Bianchi K, Meier P. A tangled web of ubiquitin chains: Breaking news in tnf-r1 signaling. Molecular cell. 2009;36:736–742. doi: 10.1016/j.molcel.2009.11.029. [DOI] [PubMed] [Google Scholar]
- 222.Burchfield JS, Dong JW, Sakata Y, Gao F, Tzeng HP, Topkara VK, Entman ML, Sivasubramanian N, Mann DL. The cytoprotective effects of tumor necrosis factor are conveyed through tumor necrosis factor receptor-associated factor 2 in the heart. Circulation Heart failure. 2010;3:157–164. doi: 10.1161/CIRCHEARTFAILURE.109.899732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Hamid T, Gu Y, Ortines RV, Bhattacharya C, Wang G, Xuan YT, Prabhu SD. Divergent tumor necrosis factor receptor-related remodeling responses in heart failure: Role of nuclear factor-kappab and inflammatory activation. Circulation. 2009;119:1386–1397. doi: 10.1161/CIRCULATIONAHA.108.802918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Green LA, Njoku V, Mund J, Case J, Yoder M, Murphy MP, Clauss M. Endogenous transmembrane tnf-alpha protects against premature senescence in endothelial colony forming cells. Circulation research. 2016;118:1512–1524. doi: 10.1161/CIRCRESAHA.116.308332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Sun M, Dawood F, Wen WH, Chen M, Dixon I, Kirshenbaum LA, Liu PP. Excessive tumor necrosis factor activation after infarction contributes to susceptibility of myocardial rupture and left ventricular dysfunction. Circulation. 2004;110:3221–3228. doi: 10.1161/01.CIR.0000147233.10318.23. [DOI] [PubMed] [Google Scholar]
- 226.Berry M, Brightling C, Pavord I, Wardlaw A. Tnf-alpha in asthma. Current opinion in pharmacology. 2007;7:279–282. doi: 10.1016/j.coph.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 227.Pera T, Penn RB. Bronchoprotection and bronchorelaxation in asthma: New targets, and new ways to target the old ones. Pharmacology & therapeutics. 2016;164:82–96. doi: 10.1016/j.pharmthera.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Koch WJ, Rockman HA, Samama P, Hamilton RA, Bond RA, Milano CA, Lefkowitz RJ. Cardiac function in mice overexpressing the beta-adrenergic receptor kinase or a beta ark inhibitor. Science. 1995;268:1350–1353. doi: 10.1126/science.7761854. [DOI] [PubMed] [Google Scholar]
- 229.Garcia-Guerra L, Nieto-Vazquez I, Vila-Bedmar R, Jurado-Pueyo M, Zalba G, Diez J, Murga C, Fernandez-Veledo S, Mayor F, Jr, Lorenzo M. G protein-coupled receptor kinase 2 plays a relevant role in insulin resistance and obesity. Diabetes. 2010;59:2407–2417. doi: 10.2337/db10-0771. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 230.Koch WJ, Lefkowitz RJ, Rockman HA. Functional consequences of altering myocardial adrenergic receptor signaling. Annual review of physiology. 2000;62:237–260. doi: 10.1146/annurev.physiol.62.1.237. [DOI] [PubMed] [Google Scholar]
- 231.Lefkowitz RJ, Rockman HA, Koch WJ. Catecholamines, cardiac beta-adrenergic receptors, and heart failure. Circulation. 2000;101:1634–1637. doi: 10.1161/01.cir.101.14.1634. [DOI] [PubMed] [Google Scholar]
- 232.White DC, Hata JA, Shah AS, Glower DD, Lefkowitz RJ, Koch WJ. Preservation of myocardial beta-adrenergic receptor signaling delays the development of heart failure after myocardial infarction. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:5428–5433. doi: 10.1073/pnas.090091197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Wu H, Chen J, Song S, Yuan P, Liu L, Zhang Y, Zhou A, Chang Y, Zhang L, Wei W. Beta2-adrenoceptor signaling reduction in dendritic cells is involved in the inflammatory response in adjuvant-induced arthritic rats. Scientific reports. 2016;6:24548. doi: 10.1038/srep24548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Vroon A, Heijnen CJ, Kavelaars A. Grks and arrestins: Regulators of migration and inflammation. Journal of leukocyte biology. 2006;80:1214–1221. doi: 10.1189/jlb.0606373. [DOI] [PubMed] [Google Scholar]
- 235.Inoue M, Kamada H, Abe Y, Higashisaka K, Nagano K, Mukai Y, Yoshioka Y, Tsutsumi Y, Tsunoda S. Aminopeptidase p3, a new member of the tnf-tnfr2 signaling complex, induces phosphorylation of jnk1 and jnk2. Journal of cell science. 2015;128:656–669. doi: 10.1242/jcs.149385. [DOI] [PubMed] [Google Scholar]
- 236.Hamid T, Guo SZ, Kingery JR, Xiang X, Dawn B, Prabhu SD. Cardiomyocyte nf-kappab p65 promotes adverse remodelling, apoptosis, and endoplasmic reticulum stress in heart failure. Cardiovascular research. 2011;89:129–138. doi: 10.1093/cvr/cvq274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Vasudevan NT, Mohan ML, Goswami SK, Naga Prasad SV. Regulation of beta-adrenergic receptor function: An emphasis on receptor resensitization. Cell cycle. 2011;10:3684–3691. doi: 10.4161/cc.10.21.18042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Vasudevan NT, Mohan ML, Gupta MK, Hussain AK, Naga Prasad SV. Inhibition of protein phosphatase 2a activity by pi3kgamma regulates beta-adrenergic receptor function. Molecular cell. 2011;41:636–648. doi: 10.1016/j.molcel.2011.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 240.Mann DL. The emerging role of innate immunity in the heart and vascular system: for whom the cell tolls. Circulation Research. 2011;108:1133–1145. doi: 10.1161/CIRCRESAHA.110.226936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Hartupee J, Mann DL. Role of inflammatory cells fibroblast activation. Journal of Molecular and Cellular Cardiology. 2016;93:143–148. doi: 10.1016/j.yjmcc.2015.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Frangogiannis NG. Regulation of the inflammatory response in cardiac repair. Circulation Research. 2012;110:159–173. doi: 10.1161/CIRCRESAHA.111.243162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Yan X, Anzai A, Katsumata Y, Mtsuhashi T, Ito K, Endo J, Yamamoto T, Takeshima A, Shinmura K, Shen W, Fukuda K, Sano M. Temporal dynamics of cardiac immune cell accumulation following acute myocardial infarction. Journal of Molecular and Cellular Cardiology. 2013;62:24–35. doi: 10.1016/j.yjmcc.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 244.Nahrendorf M, Pittet MJ, Swirski FK. Monocytes: protagonists of infarct inflammation and repair after myocardial infarction. Circulation. 2010;121:2437–2445. doi: 10.1161/CIRCULATIONAHA.109.916346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Kain V, Prabhu SD, Halade GV. Inflammation revisited: inflammation versus resolution of inflammation and repair after myocardial infarction. Basic Research in Cardiology. 2014;109:444–461. doi: 10.1007/s00395-014-0444-7. [DOI] [PubMed] [Google Scholar]
- 246.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. The Journal of Experimental Medicine. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Hannna RN, Carlin LM, Hubberling HG, Nackiewicz D, Green AM, Punt JA, Geissmann F, Hedrrick CC. The transcription factor NR4A1 (Nur77) controls bone marrow differentiation and the survivla of Ly6C-monocytes. Nature Immunology. 2011;12:778–785. doi: 10.1038/ni.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Prabhu SD. It takes two to tango: Monocyte and Macrophage duality in the infarcted heart. Circulation Research. 2014;114:1558–1560. doi: 10.1161/CIRCRESAHA.114.303933. [DOI] [PMC free article] [PubMed] [Google Scholar]