Abstract
Purpose
To assess the incidence and predictors of post-suppression virologic rebound (VR) among adolescents on stable combination antiretroviral therapy (cART) in Asia.
Methods
Perinatally HIV-infected Asian adolescents (10-19 years) with documented virologic suppression [two consecutive viral loads (VL) <400 copies/ml >6 months apart] were included. Baseline was the date of the first VL <400 copies/ml at age >10 years, or the 10th birthday for those with prior suppression. Cox proportional hazards models were used to identify predictors of post-suppression VR (VL >1000 copies/ml).
Results
Of 1379 eligible adolescents, 47% were males. At baseline, 22% were receiving protease inhibitor (PI)-containing regimens; median CD4 cell count (IQR) was 685 (448-937) cells/mm3; 2% had pre-adolescent virologic failure (VF) before subsequent suppression. During adolescence, 180 individuals (13%) experienced post-suppression VR at a rate of 3.4 (95%CI: 2.9-3.9) per 100 person-years, which was consistent over time. Median time to VR during adolescence (IQR) was 3.3 (2.1-4.8) years. Wasting (weight-for-age z-score <-2.5), being raised by grandparents, receiving second-line PI-based regimens, starting cART after 2005, and having pre-adolescent VF were independent predictors of adolescent VR. At VR, median age, CD4 cell count, and VL (IQR) were 14.8 (13.2-16.4) years, 507 (325-723) cells/mm3, and 4.1 (3.5-4.7) log10 copies/ml, respectively.
Conclusions
A modest and consistent incidence of post-suppression VR was documented during adolescence in our cohort. Having poor weight, receiving second-line regimens, and prior VF were associated with an increased VR rate. Adolescents at higher risk of VR may benefit from more intensive VL monitoring to enhance adherence management.
Keywords: adolescents, Asia, children, highly active antiretroviral therapy, pediatric, perinatal HIV infection, treatment failure, virologic failure
Advances in combination antiretroviral therapy (cART) have made it possible for HIV infection to be a chronic and manageable life-long illness [1-3]. In countries where cART is available and accessible, people with HIV can achieve improved life expectancy and a better quality of life [4-6]. As a consequence, children with perinatally acquired HIV infection can grow up into adolescence and young adulthood, resulting in increased numbers of these populations worldwide [7,8].
Adolescence is a transitional stage from childhood to adulthood during which individuals experience accelerated physical growth, as well as cognitive, emotional, social and behavioral maturation. Adolescents express their needs for autonomy and independence, and can have a preoccupation with self-image, and a tendency to engage in risk-taking behaviors [9]. Since most perinatally HIV-infected adolescents currently in care largely initiated cART earlier in childhood, they frequently struggle with adherence to their life-long medications and risk discontinuing treatment during this critical period of emotional and neurocognitive development [10,11]. Thus, it is challenging to maintain continuous and virologically suppressive HIV treatment for this unique and vulnerable population.
Virologic rebound (VR) after suppression on cART has been reported in perinatally HIV-infected youth (cumulative incidence: 19%) and adults (cumulative incidence: 6-42%), using the definition of plasma viral load (VL) >400 copies/ml [12-15]. A study comparing the risk of VR between non-perinatally HIV-infected adolescents and adults found that adolescents had a significantly greater incidence than adults (6 months after initial suppression: cumulative incidence 31% vs. 17%; P = 0.02; and 12 months after initial suppression: cumulative incidence 42% vs. 20%; P = 0.004) [16]. Previously reported predictors of VR in HIV-infected individuals included younger age, female sex, being heavily treatment experienced, receiving complicated regimens, poor immunologic and virologic status, and having persistent low-level viremia [13-15,17]. However, these studies have rarely included adolescents in Asia. We therefore assessed the incidence and predictors of VR in perinatally HIV-infected Asian adolescents on stable cART with previously undetectable virus levels.
Methods
Study cohort and population
The TREAT Asia Pediatric HIV Observational Database (TApHOD) is a multicenter, longitudinal observational cohort of children and adolescents living with HIV in the Asia-Pacific region established in 2008. This cohort contributes to the US National Institutes of Health's International Epidemiology Databases to Evaluate AIDS (IeDEA) global consortium and the data collection methods have been previously described [18,19]. Almost all of youth in the cohort were enrolled during childhood and were followed through adolescence. Non-nucleoside reverse transcriptase inhibitor (NNRTI)-based cART is usually prescribed as the first-line regimen, whereas boosted protease inhibitor (PI)-based second-line regimens are recommended for these individuals failing their first-line treatment. In September 2014, TApHOD included data from 5609 children and adolescents who had ever received care from one of 16 pediatric clinical programs in Cambodia (n = 1), India (n = 1), Indonesia (n = 2), Malaysia (n = 4), Thailand (n = 5), or Vietnam (n = 3). These sites are predominantly public or university-based pediatric HIV referral clinics. The cohort is coordinated by TREAT Asia/amfAR (Bangkok, Thailand) with data management and statistical analysis support from the Kirby Institute, University of New South Wales (Sydney, Australia).
Perinatally HIV-infected Asian adolescents aged between 10 and 19 years enrolled in TApHOD through September 2014 were included in the analysis if they had been on cART (defined as a regimen containing at least three antiretroviral drugs), and had a documented period of virologic suppression (defined as two consecutive plasma VL measurements <400 copies/ml at least six months apart) before or during adolescence. Adolescents exposed to mono- or dual-therapy prior to cART initiation were excluded.
Data collection
Data included in this analysis were transferred to TApHOD through September 2014. Baseline was the date of the first of the two plasma VL measurement <400 copies/ml for adolescents experiencing their first period of virologic suppression at age 10 years or more, or the 10th birthday for those achieving prior suppression. Demographic characteristics, anthropometric measurements, and HIV-related parameters were abstracted from the database. The window period for weight, height, hemoglobin level, and baseline CD4 cell count was within three months of the date of interest. The measurement taken closest to that date was used in the analysis. Weight, height and body mass index [BMI, weight (kg)/height (m2)] measurements were transformed into age- and sex-standardized z-scores. Weight-for-age z-scores (WAZ) were calculated using the 1977 National Center for Health Statistics/World Health Organization (WHO) reference because the 2007 WHO weight charts are only for children up to 10 years [20]. Height-for-age (HAZ) and BMI-for-age z-scores were calculated using the 2007 WHO growth references [21]. Severity of anemia was defined by using the Division of AIDS toxicity table (version 2.0, November 2014) [22]. Primary caregiver, orphan status, residential status and school attendance, as well as the most severe WHO clinical stage, antiretroviral regimens and HIV disclosure status were based on the last recorded status at the date of interest. Pre-adolescent virologic failure (VF) was considered a documentation of a plasma VL >1000 copies/ml after a period of primary virologic suppression during childhood.
Endpoint definitions
Post-suppression VR was defined as a single plasma VL >1000 copies/ml while on cART after a previous documented history of undetectable virus levels. We selected the cut-off level of 1000 copies/ml to avoid misclassification with low-level viremia and viral blips. Loss to follow-up was defined as not presenting to care for at least 12 months without documentation of transfer to another clinic, or death. Mortality was defined as all-cause deaths notified from any source.
Statistical analysis
Baseline demographic and HIV-specific characteristics, as well as anthropometric parameters of participants were summarized with median and interquartile range [interquartile range (IQR)] for continuous variables, and number (n) and percentage (%) for categorical variables. Kaplan-Meier estimates were used to describe the cumulative probability of post-suppression VR. The incidence rate of post-suppression VR and 95% confidence intervals (CI) were calculated by dividing the number of adolescents with VR by the total number of person-years of follow-up (PYFU). The rates of loss to follow-up and mortality were calculated with a similar method.
Cox proportional hazards models, stratified by study center, were used to identify independent predictors of post-suppression VR. Time to VR was censored at the date of the last plasma VL measurement <1000 copies/ml for adolescents who did not experience VR during study follow-up, or at the 20th birthday for those who maintained viral suppression into adulthood. Times to loss to follow-up and death were censored at the last clinic visit for adolescents aged less than 20 years at the time of their last visit, or at the 20th birthday for those with a documented visit after this date. Co-variates were retained in multivariable model if one or more categories exhibited a P <0.05 in univariable analysis. The magnitudes of associations were summarized with hazard ratios (HR) and adjusted hazard ratios (aHR) together with 95% CI in univariable and multivariable analyses, respectively. A two-sided P <0.05 was considered to indicate statistical significance. All statistical analyses were performed using Stata statistical software, version 13.1 (StataCorp LP, College, Station, TX).
Ethical considerations
Ethical approval for this study was obtained at all participating sites, the study coordinating center (TREAT Asia/amfAR; Bangkok, Thailand), and the data management and statistical analysis center (the Kirby Institute, University of New South Wales; Sydney, Australia). Participant consent is deferred to the individual participating sites and their institutional review boards.
Results
Participant characteristics
Of 2271 adolescents on stable cART, 1379 (60.7%) experienced a documented period of viral suppression and were therefore eligible for this study. The analysis cohort was followed for a median (IQR) of 3.7 (2.0-5.4) years (Table 1). Among eligible adolescents, 47% were male and 72% were Thai. Notably, Indian patients in the cohort did not meet the eligibility criteria and were not included in the analysis. The majority of participants (57.1%) achieved virologic suppression before entering adolescence, and the median age at baseline (IQR) was 9.5 (9.0-11.6) years. Four-hundred and thirty-six (39.3%) and 312 (28.2%) adolescents were primarily being cared for by biological parents and grandparents, respectively. About half of them (53.9%) had the most severe WHO clinical stage of 3 and 4. At baseline, median CD4 cell count (IQR) was 685 (448-937) cells/mm3, median duration of cART use (IQR) was 3.4 (1.4-5.3) years, and approximately one-fifth of adolescents (21.2%) were receiving second-line PI-containing regimens. Twenty-four adolescents (1.7%), of whom 11 were currently on NNRTI-based and 13 were on PI-based regimens, had a documented history of pre-adolescent VF before subsequent suppression. CD4 cell count and plasma VL were routinely measured with a median frequency (IQR) of 2.2 (2.0-2.7) and 1.6 (1.2-2.2) times per patient per year.
Table 1. Baseline characteristics of perinatally HIV-infected Asian adolescents on stable combination antiretroviral therapy.
| Baseline characteristicsa | Total number of adolescents | Number (%) or median (IQR) |
|---|---|---|
| Demographic characteristics | ||
| Age at viral suppression, years | 1379 | 9.5 (9.0 - 11.6) |
| <10 | 787 (57.1) | |
| 10.0 to 13.9 | 458 (33.2) | |
| 14.0 to 19.9 | 134 (9.7) | |
| Sex | 1379 | |
| Male | 645 (46.8) | |
| Female | 734 (53.2) | |
| Country of residence | 1379 | |
| Cambodia | 228 (16.5) | |
| Indonesia | 15 (1.1) | |
| Malaysia | 93 (6.7) | |
| Thailand | 988 (71.7) | |
| Vietnam | 55 (4.0) | |
| Primary caregiver | 1108 | |
| Biological parents | 436 (39.3) | |
| Grandparents | 312 (28.2) | |
| Relatives/non-relative/foster | 360 (32.5) | |
| Orphan status | 1193 | |
| Both parents alive | 226 (19.0) | |
| Single parents alive | 363 (30.4) | |
| Neither parent alive | 604 (50.6) | |
| Residential status | 1166 | |
| Community | 1002 (85.9) | |
| Group home/ orphanage/ homeless | 164 (14.1) | |
| School attendance | 1170 | |
| Attending | 1055 (90.2) | |
| Not attending | 115 (9.8) | |
| HIV disclosure status | 902 | |
| Disclosed | 406 (45.0) | |
| Not disclosed | 496 (55.0) | |
| Anthropometric parameters | ||
| Weight-for-age z-score | 1297 | -1.9 (-2.8 to -1.1) |
| >-1.5 | 464 (35.8) | |
| -1.5 to -2.5 | 420 (32.4) | |
| <-2.5 | 413 (31.8) | |
| Height-for-age z-score | 1295 | -2.0 (-2.8 to -1.2) |
| >-1.5 | 456 (35.2) | |
| -1.5 to -2.5 | 440 (34.0) | |
| <-2.5 | 399 (30.8) | |
| BMI-for-age z-score | 1295 | -0.9 (-1.5 to -0.2) |
| >0 | 249 (19.2) | |
| 0 to -1.5 | 711 (54.9) | |
| <-1.5 | 335 (25.9) | |
| HIV-specific characteristics | ||
| Age at HIV diagnosis, years | 1148 | 6.1 (3.6 - 8.8) |
| <3 | 237 (20.6) | |
| 3 to 7 | 447 (39.0) | |
| >7 | 464 (40.4) | |
| Age at cART initiation, years | 1379 | 7.4 (5.1 - 9.9) |
| <7 | 636 (46.1) | |
| 7 to 10 | 415 (30.1) | |
| >10 | 328 (23.8) | |
| The most severe WHO clinical stage | 1379 | |
| Stage 1 and 2 | 636 (46.1) | |
| Stage 3 | 405 (29.4) | |
| Stage 4 | 338 (24.5) | |
| Haemoglobin, g/dl | 947 | 12.2 (11.4-13.0) |
| No anemia | 867 (91.6) | |
| Grade 1 to 2 anemia | 74 (7.8) | |
| Grade 3 to 4 anemia | 6 (0.6) | |
| Nadir CD4 cell count, cells/mm3 | 1379 | 168 (41 – 382) |
| >350 | 378 (27.4) | |
| 201 to 350 | 246 (17.9) | |
| 100 to 200 | 214 (15.5) | |
| <100 | 541 (39.2) | |
| Baseline CD4 cell count, cells/mm3 | 1286 | 685 (448 - 937) |
| >750 | 562 (43.7) | |
| 500 to 750 | 333 (25.9) | |
| <500 | 391 (30.4) | |
| Baseline cART regimen | 1379 | |
| First-line NNRTI-based | 1071 (77.7) | |
| Second-line PI-based | 293 (21.2) | |
| Other | 15 (1.1) | |
| Year of the first cART initiation | 1379 | |
| <2005 | 578 (41.9) | |
| 2005 to 2008 | 666 (48.3) | |
| >2008 | 135 (9.8) | |
| Duration of cART, years | 1379 | 3.4 (1.4 - 5.3) |
| <1 | 292 (21.2) | |
| 1 to 3 | 312 (22.6) | |
| >3 | 775 (56.2) | |
| Experienced pre-adolescent virologic failure | 1379 | |
| No | 1355 (98.3) | |
| Yes | 24 (1.7) |
Abbreviations: BMI, body mass index; cART, combination antiretroviral therapy; IQR, interquartile range; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; WHO, World Health Organization.
Baseline was defined as the date of the first plasma viral load <400 copies/mL for adolescents experiencing their first period of virologic suppression at age ≥10 years or the 10th birthday for those with prior suppression.
Incidence of post-suppression virologic rebound during adolescence
Over the follow-up period, 180 adolescents (cumulative incidence: 13.1%) experienced post-suppression VR, corresponding to an overall incidence rate of 3.4 (95% CI: 2.9-3.9) per 100 PYFU. The rate of VR was consistent over time (Figure 1). The median time to VR during adolescence (IQR) was 3.3 (2.1-4.8) years. The incidence rates of post-suppression VR (95% CI) by each clinical parameter are provided in Table 2.
Figure 1. Kaplan-Meier estimate of the cumulative probability of post-suppression virologic rebound over time.
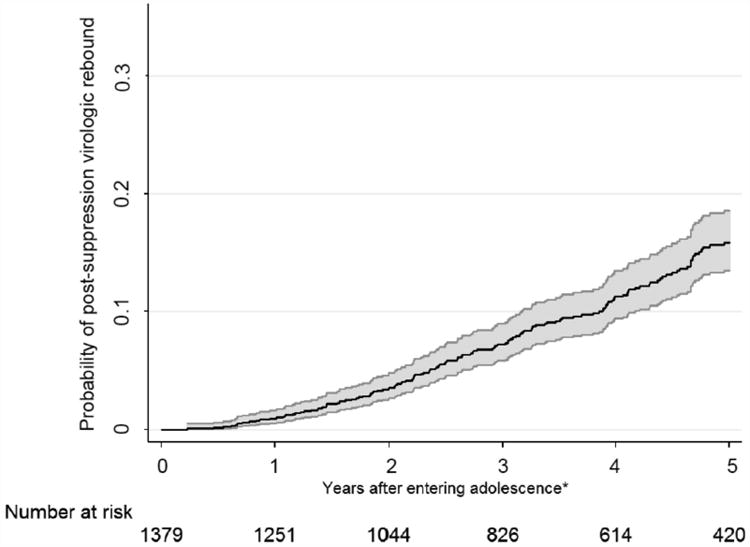
Note: Shaded area represents 95% confidence interval.
*Years after achieving viral suppression during adolescence for those having plasma viral load <400 copies/mL after 10 years of age.
Table 2. Predictors of post-suppression virologic rebound during adolescence among perinatally HIV-infected Asian adolescents on stable combination antiretroviral therapy.
| Clinical parameters | Virologic rebound, n (%) | Person-years of follow-up | Rate per 100 person-years (95% CI) | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| HR (95% CI) | P | Adjusted HR (95% CI) | P | ||||
| Age at viral suppression, years | |||||||
| <10 | 84 (10.7) | 2884 | 2.91 (2.35 - 3.61) | Ref | Ref | ||
| 10.0 to 13.9 | 78 (17.0) | 2095 | 3.72 (2.98 - 4.65) | 1.25 (0.91 - 1.72) | 0.18 | 1.00 (0.72-1.40) | 0.99 |
| 14.0 to 19.9 | 18 (13.4) | 381 | 4.72 (2.97 - 7.49) | 2.43 (1.41 - 4.18) | 0.001 | 1.53 (0.86-2.73) | 0.14 |
| Sex | |||||||
| Male | 81 (12.6) | 2479 | 3.27 (2.63 - 4.06) | Ref | |||
| Female | 99 (13.5) | 2881 | 3.44 (2.82 - 4.18) | 1.08 (0.80 - 1.47) | 0.61 | ||
| Primary caregiver | |||||||
| Biological parents | 31 (7.1) | 1314 | 2.36 (1.66 - 3.35) | Ref | Ref | ||
| Grandparents | 56 (17.9) | 1230 | 4.55 (3.50 - 5.92) | 1.79 (1.12 - 2.84) | 0.01 | 2.05 (1.27 - 3.30) | 0.003 |
| Relative/non-relative/foster | 56 (15.6) | 1507 | 3.72 (2.86 - 4.83) | 1.46 (0.88 - 2.41) | 0.14 | 1.53 (0.92 - 2.55) | 0.10 |
| Weight-for-age z-score | |||||||
| >-1.5 | 51 (11.0) | 1741 | 2.93 (2.23 - 3.86) | Ref | Ref | ||
| -1.5 to -2.5 | 49 (11.7) | 1683 | 2.91 (2.20 - 3.85) | 1.02 (0.68 - 1.53) | 0.92 | 1.06 (0.71 - 1.60) | 0.76 |
| <-2.5 | 70 (17.0) | 1673 | 4.18 (3.31 - 5.29) | 1.60 (1.10 - 2.33) | 0.01 | 1.49 (1.01 - 2.18) | 0.04 |
| Baseline CD4 cell counta, cells/mm3 | |||||||
| >750 | 61 (10.9) | 2042 | 2.99 (2.32 - 3.84) | Ref | |||
| 500 to 750 | 36 (10.8) | 1379 | 2.61 (1.88 - 3.62) | 0.85 (0.56 - 1.28) | 0.44 | ||
| <500 | 73 (18.7) | 1693 | 4.31 (3.43 - 5.42) | 1.37 (0.96 - 1.94) | 0.08 | ||
| Baseline cART regimena | |||||||
| First-line NNRTI-based | 119 (11.1) | 4444 | 2.68 (2.24 - 3.21) | Ref | Ref | ||
| Second-line PI-based | 57 (19.5) | 858 | 6.64 (5.12 - 8.61) | 2.66 (1.88 - 3.75) | <0.001 | 2.66 (1.86 - 3.79) | <0.001 |
| Year of the first cART regimen initiation | |||||||
| <2005 | 86 (14.9) | 2835 | 3.03 (2.46 - 3.75) | Ref | Ref | ||
| 2005 to 2008 | 80 (12.0) | 2214 | 3.61 (2.90 - 4.50) | 1.75 (1.22 - 2.50) | 0.002 | 1.89 (1.31 - 2.72) | 0.001 |
| >2008 | 14 (10.4) | 312 | 4.49 (2.66 - 7.58) | 2.96 (1.59 - 5.48) | 0.001 | 4.15 (2.15 - 7.99) | <0.001 |
| Experienced pre-adolescent virologic failure | |||||||
| No | 174 (12.8) | 5286 | 3.29 (2.84 - 3.82) | Ref | Ref | ||
| Yes | 6 (25.0) | 74 | 8.06 (3.62 - 17.95) | 2.72 (1.16 - 6.38) | 0.02 | 2.68 (1.11 - 6.48) | 0.03 |
Abbreviations: cART, combination antiretroviral therapy; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; 95% CI, 95% confidence interval; HR, hazard ratio.
Baseline was defined as the date of the first plasma viral load <400 copies/mL for adolescents experiencing their first period of virologic suppression at age ≥10 years or the 10th birthday for those with prior suppression.
Predictors of post-suppression virologic rebound during adolescence
In the multivariable analysis, adolescents having grandparents as a primary caregiver had significantly increased risk of post-suppression VR compared to those who had biological parents as a primary caregiver (aHR: 2.1; 95% CI: 1.3-3.3). Wasting (WAZ <-2.5; aHR: 1.5; 95% CI: 1.0-2.2, compared to WAZ >-1.5), receiving second-line PI-containing regimens (aHR: 2.7; 95% CI: 1.9-3.8, compared to first-line NNRTI-based regimens), starting their first cART regimen after the year 2005 (year 2005-2008; aHR: 1.9; 95% CI: 1.3-2.7, and post-2008; aHR: 4.2; 95% CI: 2.2-8.0, compared to pre-2005), and having an episode of pre-adolescent VF (aHR: 2.7; 95% CI: 1.1-6.5) were also associated with an increased risk of post-suppression VR during adolescence (Table 2). Notably, HIV disclosure status was not included in the multivariable analysis as it was not associated with post-suppression VR in the univariable analysis nor was it considered for inclusion a priori.
Characteristics of adolescents with post-suppression virologic rebound
At the time of VR, the median age (IQR) was 14.8 (13.2-16.4) years, and 81 (45.0%) were males. Approximately half of them (45.0%) were in WHO clinical stage 1 and 2 with the median CD4 cell count and plasma VL (IQR) of 507 (325-723) cells/mm3 and 4.1 (3.5-4.7) log10 copies/ml, respectively. Other characteristics of adolescents with post-suppression VR are demonstrated in Table 3.
Table 3. Characteristics of perinatally HIV-infected Asian adolescents with post-suppression virologic rebound.
| Characteristics at the time of virologic rebound | Number (%) or median (IQR) |
|---|---|
| Demographic characteristics | |
| Age, years (n=180) | 14.8 (13.2 - 16.4) |
| 10.0 to 13.9 | 63 (35.0) |
| 14.0 to 19.9 | 117 (65.0) |
| Sex (n=180) | |
| Male | 81 (45.0) |
| Female | 99 (55.0) |
| Anthropometric measurements | |
| Weight-for-age z-score (n=146) | -1.9 (-2.7 to -0.9) |
| >-1.5 | 56 (38.4) |
| -1.5 to -2.5 | 46 (31.5) |
| <-2.5 | 44 (30.1) |
| Height-for-age z-score (n=156) | -1.8 (-2.5 to -1.1) |
| >-1.5 | 63 (40.4) |
| -1.5 to -2.5 | 55 (35.3) |
| <-2.5 | 38 (24.4) |
| BMI-for-age z-score (n=141) | -0.9 (-1.5 to 0.0) |
| >0 | 36 (25.5) |
| 0 to -1.5 | 70 (49.6) |
| <-1.5 | 35 (24.8) |
| HIV-specific characteristics | |
| WHO clinical stage (n=180) | |
| Stage 1 and 2 | 81 (45.0) |
| Stage 3 | 54 (30.0) |
| Stage 4 | 45 (25.0) |
| Hemoglobin, g/dl (n=117) | 12.7 (11.5 - 13.6) |
| Grade 3 - 4 anemia | 1 (0.9) |
| No grade 3 - 4 anemia | 116 (99.1) |
| CD4 cell count, cells/mm3 (n=173) | 507 (325 - 723) |
| >750 | 40 (23.1) |
| 500 to 750 | 49 (28.3) |
| <500 | 84 (48.6) |
| Plasma viral load, copies/ml (n=180) | 13711 (2899 - 55989) |
| 1000 – 10000 | 83 (46.1) |
| >10000 | 97 (53.9) |
Abbreviations: BMI, body mass index; IQR, interquartile range; WHO, World Health Organization.
Loss to follow-up and mortality rates during adolescence
During the adolescent period (i.e., 10-19 years), 16 adolescents (1.2%) were lost to follow-up at an incidence rate of 0.3 (95% CI: 0.2-0.4) events per 100 PYFU. Eleven deaths (0.8%) occurred over the follow-up period at a mortality rate of 0.2 (95% CI: 0.1-0.3) deaths per 100 PYFU. Reported causes included cryptococcal meningitis (n = 1), Escherichia coli septicemia (n = 1), penicilliosis (n = 1), septic shock (n = 1), renal failure (n = 1), stroke (n = 1), trauma (n = 1), paraquat poisoning (n = 1), and unknown (n = 3).
Discussion
We found a modest and consistent 3.4 per 100 PYFU incidence rate and 13% cumulative incidence of post-suppression VR in our cohort of perinatally HIV-infected Asian adolescents. Individuals with wasting, being cared for by grandparents, receiving second-line PI-containing regimens, starting cART after 2005, or having a documented history of pre-adolescent VF were at higher risk of VR. Overall, during adolescence, the loss to follow-up and mortality rates among our adolescents were low.
The incidence rate of post-suppression VR in our study was lower than those documented in other adolescent cohorts, which is likely related in part to variations in the study populations in terms of age, mode of infection, and cART regimen sequence [23,24]. A South African study reported a post-suppression VR rate of 8.2 per 100 PYFU among their adolescents (aged 9-19 years; 72% perinatally infected) receiving standard first-line cART at a community-based ART clinic [23]. Another South African study showed a higher VR rate at a plasma VL threshold >400 copies/ml among younger adolescents (aged 10-14 years; 6.3 per 100 PYFU) compared with older adolescents (aged 15-19 years; 3.8 per 100 PYFU) on first-line cART [24]. Additionally, we found that our cumulative incidence of post-suppression VR was lower than a 19% incidence demonstrated in the Collaborative HIV Paediatric Study (CHIPS; using the definition of VR as a plasma VL >400 copies/ml), which was conducted in antiretroviral-naïve children and adolescents receiving first-line regimens, over a median duration of 1.8 years after treatment initiation in the UK and Ireland [12]. These results emphasize that although adolescents may have a childhood history of viral suppression, they remain at risk of developing VR later in life. Moreover, how adherence issues and VR are managed in childhood have long-term consequences, and point to the importance of support for standardized pediatric clinical guidance.
Several predictors were found to be associated with post-suppression VR during adolescence. Wasting has been a significant complication of HIV infection since early in the epidemic [25,26], and remains an important clinical problem, particularly among children and adolescents living in Asian and African countries [27,28]. Although there are no studies demonstrating the adverse consequences of wasting on VR, it is well-documented that low weight-for-age has been significantly correlated with clinical and immunologic failure in children and adolescents [27,29]. Unfortunately, in our analysis, we did not include incident opportunistic infections (OIs). This was primarily due to the lack of data on diagnosis and management of these OIs in our database. Nevertheless, opportunistic and other co-infections could have been factors in transient or long term changes in plasma VL due to acute illness and ART adherence problems.
Finding that second-line PI-containing cART and pre-adolescent VF were significant predictors of VR in this study reflect the importance of medication adherence on successful virologic outcomes [30,31]. In this region, PI-based cART is generally used as second-line treatment after failure of first-line NNRTI-based regimens, so patients receiving these regimens were more likely to have poorer earlier adherence to treatment compared with those who remained on traditionally first-line therapy. A cross-sectional study in HIV-infected Tanzanian adolescents and adults demonstrated that individuals who had prior suboptimal adherence (self-reported cumulative adherence <90%) to first-line NNRTI-containing regimens appeared to have suboptimal adherence to second-line PI-based therapy relative to those who had prior optimal adherence to first-line therapy (adjusted prevalence ratio: 2.4; 95% CI: 1.5-3.9) [32]. Furthermore, the limited availability of age- and weight-appropriate fixed-dose combination formulations and pill burden of PI-containing regimens may decrease adolescent adherence [33], contributing to the increased VR rate in these adolescents. Similarly, pre-adolescent VF may indicate nonadherence to treatment, resulting in a higher VR rate compared with individuals without prior VF.
We found that adolescents raised by grandparents demonstrated a higher VR rate compared with those cared for by biological parents. Although several studies have shown that grandparents play a key role in providing care and support for HIV-infected orphans and children [34-36], challenges with their health issues related to advancing age and social stress may limit their ability to optimally care for their grandchildren as they age into adolescence [37]. Additionally, half of our adolescents (50%) were double-orphans, putting them at risk of mental health conditions related to the social and family instability associated with orphanhood. A previous US study reported that children raised by custodial grandparents encountered greater risk of behavioural and emotional difficulties than other children [38]. These factors have the potential to impact medication adherence and the risk of VR in these individuals.
During the study period, our participating sites, which are almost exclusively government-supported treatment clinics, changed their prescribing practices as national guidelines were revised in response to the WHO treatment guidelines updates. While changes were made on a country-by-country basis, this had led to some common outcomes, such as near-complete phase-out of the use of d4T and changing the threshold for cART initiation to higher CD4 counts. We also noted the higher incidence rate of post-suppression VR among adolescents starting their first cART regimen after 2005 compared with those initiating pre-2005. However, this finding may be attributed to surveillance artifact, as there has been more frequent plasma VL testing in recent years in the region (data not shown). The relatively short duration of life-long cART use observed in this study (3.4 years) likely reflects delays in HIV diagnosis. Without knowing their child's status, caregivers are unaware of the need to start them on appropriate treatments until disease has progressed.
Our loss to follow-up and mortality rates seen during adolescence were lower compared with those reported in African cohorts, which may have further impacted our VR results. A South African study showed that the rates of loss to follow-up and mortality among a subgroup of perinatally HIV-infected adolescents on standard first-line cART were 3.9 and 0.8 per 100 PYFU, respectively [23]. Because our cohort included perinatally infected adolescents who were generally in continuous care at the study clinics since early childhood, they had the opportunity to develop strong relationships with their healthcare providers, which may have contributed to their retention in care. Additionally, our study settings primarily include HIV referral clinics with well-established pediatric HIV care infrastructure. This may have been a factor in the relatively lower mortality rate.
This study contains multiple limitations that restrict the generalizability of our findings. We included an adolescent survivor cohort of children who reached the age of 10 years with either pre-existing virologic suppression or suppression during adolescence. This prevents direct comparisons to studies that span from infancy, to childhood, and to adolescence. Additionally, our cohort primarily includes youth with perinatally acquired HIV infection and the majority of the study time was during the period of younger adolescence (i.e., 10-14 years). This may limit the generalizability of our findings to older adolescents and young adults, and those with behaviorally acquired infection. Moreover, because we defined the post-suppression VR as having a single plasma VL >1,000 copies/ml, this may not accurately reflect treatment failure. We were also not able to link VR to the emergence of HIV drug resistance as genotype testing was not available for this study. Furthermore, as our patients may have had plasma VL tests for both routine monitoring as well as confirmatory testing, this could potentially skew our results because patients in the latter category would have been experiencing immunologic and/or clinical failure when plasma VL measurements were performed. Lastly, our lack of adherence data meant that we were unable to link that to VR. Although we did limit our study population to those with a history of documented virologic suppression, which would be a proxy for good adherence, we are unable to comment on subsequent adherence behavior. Additional follow-up time may have shown higher VR rates as the cohort ages into late adolescence.
In conclusion, the incidence rate of post-suppression VR during adolescence was moderate and consistent in our cohort of perinatally HIV-infected adolescents in Asia. More frequent VL monitoring of adolescents, particularly for those at higher risk of treatment failure and VR, may be useful to promptly detect poor virologic outcomes and support long-term treatment success during this vulnerable period of life.
Implications and Contribution.
With cART, survival of perinatally HIV-infected adolescents has improved. Yet, maintaining successful treatment is challenging. This study demonstrated a modest and consistent incidence of post-suppression VR during adolescence. Wasting, receiving second-line regimens, and prior VF were associated with an increased VR rate. Adolescents with VR risk may benefit from intensive VL monitoring.
Acknowledgments
The TREAT Asia Pediatric HIV Network: PS Ly*, and V Khol, National Centre for HIV/AIDS, Dermatology and STDs, Phnom Penh, Cambodia;
J Tucker, New Hope for Cambodian Children, Phnom Penh, Cambodia;
N Kumarasamy*, S Saghayam, and E Chandrasekaran, YRGCARE Medical Centre, CART CRS, Chennai, India;
DK Wati*, LPP Atmikasari, and IY Malino, Sanglah Hospital, Udayana University, Bali, Indonesia;
N Kurniati*, and D Muktiarti, Cipto Mangunkusumo General Hospital, Jakarta, Indonesia;
SM Fong*,†, M Lim, and F Daut, Hospital Likas, Kota Kinabalu, Malaysia;
NK Nik Yusoff*, and P Mohamad, Hospital Raja Perempuan Zainab II, Kelantan, Malaysia;
KA Razali*, TJ Mohamed, and NADR Mohammed, Pediatric Institute, Hospital Kuala Lumpur, Kuala Lumpur, Malaysia;
R Nallusamy*, and KC Chan, Penang Hospital, Penang, Malaysia;
T Sudjaritruk*, V Sirisanthana, and L Aurpibul, Department of Pediatrics, Faculty of Medicine, Chiang Mai University, and Research Institute for Health Sciences, Chiang Mai, Thailand;
R Hansudewechakul*, S Denjanta, W Srisuk, and A Kongphonoi, Chiangrai Prachanukroh Hospital, Chiang Rai, Thailand;
P Lumbiganon*,‡, P Kosalaraksa, P Tharnprisan, and T Udomphanit, Department of Pediatrics, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand;
G Jourdain, PHPT-IRD UMI 174 (Institut de recherche pour le développement and Chiang Mai University), Chiang Mai, Thailand;
T Bunupuradah*, T Puthanakit, W Prasitsuebsai, and C Phadungphon, HIV-NAT, The Thai Red Cross AIDS Research Centre, Bangkok, Thailand;
K Chokephaibulkit*, K Lapphra, W Phongsamart, and S Sricharoenchai, Department of Pediatrics, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand;
KH Truong*, QT Du, and CH Nguyen, Children's Hospital 1, Ho Chi Minh City, Vietnam;
VC Do*, TM Ha, and VT An Children's Hospital 2, Ho Chi Minh City, Vietnam;
LV Nguyen*, DTK Khu, AN Pham, and LT Nguyen, National Hospital of Pediatrics, Hanoi, Vietnam;
ON Le, Worldwide Orphans Foundation, Ho Chi Minh City, Vietnam;
AH Sohn*, JL Ross, and C Sethaputra, TREAT Asia/amfAR -- The Foundation for AIDS Research, Bangkok, Thailand;
DA Cooper, MG Law*, and A Kariminia, The Kirby Institute, UNSW Australia, Sydney, Australia;
Sources of Funding: The TREAT Asia Pediatric HIV Observational Database is an initiative of TREAT Asia, a program of amfAR, The Foundation for AIDS Research, with support from the U.S. National Institutes of Health's National Institute of Allergy and Infectious Diseases, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Cancer Institute, National Institute of Mental Health, and National Institute on Drug Abuse as part of the International Epidemiology Databases to Evaluate AIDS (IeDEA; U01AI069907), and the AIDS Life Association. The Kirby Institute is funded by the Australian Government Department of Health and Ageing, and is affiliated with the Faculty of Medicine, UNSW Australia.
Abbreviations
- 95% CI
95% confidence intervals
- aHR
adjusted hazard ratio
- BMI
body mass index
- cART
combination antiretroviral therapy
- CHIPS
the Collaborative HIV Paediatric Study
- HAZ
height-for-age z-scores
- HR
hazard ratio
- IeDEA
The US National Institutes of Health's International Epidemiology Databases to Evaluate AIDS
- IQR
interquartile range
- NNRTI
non-nucleoside reverse transcriptase inhibitor
- OI
opportunistic infection
- PI
protease inhibitor
- PYFU
person-years of follow-up
- TApHOD
TREAT Asia Pediatric HIV Observational Database
- VF
virologic failure
- VL
viral load
- VR
virologic rebound
- WAZ
weight-for-age z-scores
- WHO
World Health Organization
Footnotes
TApHOD Steering Committee member
Current Steering Committee Chair;
co-Chair
Disclaimer: The views expressed are those of the authors and does not necessarily represent the official views of any of the governments or institutions mentioned in the Funding Sources.
Conflict of interest: The authors declare no conflict of interest related to this study.
Data were presented in part at the 2016 Conference on Retroviruses and Opportunistic Infections (CROI), February 22-25, 2016, Boston, United States of America.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brady MT, Oleske JM, Williams PL, et al. Declines in mortality rates and changes in causes of death in HIV-1-infected children during the HAART era. J Acquir Immune Defic Syndr. 2010;53:86–94. doi: 10.1097/QAI.0b013e3181b9869f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Judd A, Doerholt K, Tookey PA, et al. Morbidity, mortality, and response to treatment by children in the United Kingdom and Ireland with perinatally acquired HIV infection during 1996-2006: planning for teenage and adult care. Clin Infect Dis. 2007;45:918–24. doi: 10.1086/521167. [DOI] [PubMed] [Google Scholar]
- 3.Sudjaritruk T, Oberdorfer P, Puthanakit T, et al. Causes of first hospitalization among 1121 HIV-infected children: comparison of the pre-Pneumocystis jiroveci pneumonia prophylaxis, pre-antiretroviral therapy and antiretroviral therapy periods. Int J STD AIDS. 2012;23:335–9. doi: 10.1258/ijsa.2012.011203. [DOI] [PubMed] [Google Scholar]
- 4.Antiretroviral Therapy Cohort Collaboration. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008;372:293–9. doi: 10.1016/S0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samji H, Cescon A, Hogg RS, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One. 2013;8:e81355. doi: 10.1371/journal.pone.0081355.eCollection2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oguntibeju OO. Quality of life of people living with HIV and AIDS and antiretroviral therapy. HIV AIDS (Auckl) 2012;4:117–24. doi: 10.2147/HIV.S32321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The Joint United Nations Programme on HIV/AIDS (UNAIDS) [Accessed May 31, 2016];People living with HIV. Available at: http://aidsinfo.unaids.org/
- 8.The United Nations International Children's Emergency Fund. [Accessed May 31, 2016];Toward an AIDS free generation – Children and AIDS sixth stocktaking report. 2013 Available at: http://www.unaids.org/sites/default/files/media_asset/20131129_stocktaking_report_children_aids_en_0.pdf.
- 9.Sanders RA. Adolescent psychosocial, social, and cognitive development. Pediatr Rev. 2013;34:354–8. doi: 10.1542/pir.34-8-354. [DOI] [PubMed] [Google Scholar]
- 10.Haberer J, Mellins C. Pediatric adherence to HIV antiretroviral therapy. Curr HIV/AIDS Rep. 2009;6:194–200. doi: 10.1007/s11904-009-0026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim SH, Gerver SM, Fidler S, Ward H. Adherence to antiretroviral therapy in adolescents living with HIV: systematic review and meta-analysis. AIDS. 2014;28:1945–56. doi: 10.1097/QAD.0000000000000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Childs T, Shingadia D, Goodall R, et al. Outcomes after viral load rebound on first-line antiretroviral therapy in HIV-infected children in the UK/Ireland: an observational cohort study. Lancet HIV. 2015;2:e151–8. doi: 10.1016/S2352-3018(15)00021-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geretti AM, Smith C, Haberl A, et al. Determinants of virological failure after successful viral load suppression in first-line highly active antiretroviral therapy. Antivir Ther. 2008;13:927–36. [PubMed] [Google Scholar]
- 14.Phillips AN, Staszewski S, Lampe F, et al. Human immunodeficiency virus rebound after suppression to <400 copies/mL during initial highly active antiretroviral therapy regimens, according to prior nucleoside experience and duration of suppression. J Infect Dis. 2002;186:1086–91. doi: 10.1086/343801. [DOI] [PubMed] [Google Scholar]
- 15.Mocroft A, Ruiz L, Reiss P, et al. Virologic rebound after suppression on highly active antiretroviral therapy. AIDS. 2003;17:1741–51. doi: 10.1097/00002030-200308150-00003. [DOI] [PubMed] [Google Scholar]
- 16.Nachega JB, Hislop M, Nguyen H, et al. Antiretroviral therapy adherence, virologic and immunologic outcomes in adolescents compared with adults in southern Africa. J Acquir Immune Defic Syndr. 2009;51:65–71. doi: 10.1097/QAI.0b013e318199072e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith CJ, Phillips AN, Dauer B, et al. Factors associated with viral rebound among highly treatment-experienced HIV-positive patients who have achieved viral suppression. HIV Med. 2009;10:19–27. doi: 10.1111/j.1468-1293.2008.00650.x. [DOI] [PubMed] [Google Scholar]
- 18.Kariminia A, Chokephaibulkit K, Pang J, et al. Cohort profile: the TREAT Asia pediatric HIV observational database. Int J Epidemiol. 2011;40:15–24. doi: 10.1093/ije/dyp358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.IeDEA Pediatric Working Group. A survey of paediatric HIV programmatic and clinical management practices in Asia and sub-Saharan Africa - the International Epidemiology Databases to Evaluate AIDS (IeDEA) J Int AIDS Soc. 2013;16:17998. doi: 10.7448/IAS.16.1.17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Center for Health Statistics (NCHS) NCHS growth curves for children. [Accessed May 31, 2016]; Available at: http://dtic.mil/dtic/tr/fulltext/u2/a433981.pdf.
- 21.World Health Organization (WHO) The 2007 WHO growth reference for school-aged children and adolescents (5-19 years) [Accessed May 31, 2016]; Available at: http://www.who.int/growthref/en/
- 22.United States Department of Health and Human Services, National Institutes of Health, National Institute of Allergy and Infectious Diseases, Division of AIDS. Division of AIDS Table for grading the severity of adult and pediatric adverse events, version 2.0. [Accessed May 31, 2016]; Available from: http://rsc.tech-res.com/Document/safetyandpharmacovigilance/DAIDS_AE_GRADING_TABLE_v2_NOV2014.pdf.
- 23.Nglazi MD, Kranzer K, Holele P, et al. Treatment outcomes in HIV-infected adolescents attending a community-based antiretroviral therapy clinic in South Africa. BMC Infect Dis. 2012;12:21. doi: 10.1186/1471-2334-12-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Evans D, Menezes C, Mahomed K, et al. Treatment outcomes of HIV-infected adolescents attending public-sector HIV clinic across Gauteng and Mpumalanga, South Africa. AIDS Res Hum Retroviruses. 2013;29:892–900. doi: 10.1089/AID.2012.0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Serwadda D, Mugerwa RD, Sewankambo NK, et al. Slim disease: A new disease in Uganda and its association with HTLV-III infection. Lancet. 1985;2:849–52. doi: 10.1016/S0140-6736(85)90122-9. [DOI] [PubMed] [Google Scholar]
- 26.Mhiri C, Bélec L, Di Costanzo B, et al. The slim disease in African patients with AIDS. Trans R Soc Trop Med Hyg. 1992;86:303–6. doi: 10.1016/0035-9203(92)90323-5. [DOI] [PubMed] [Google Scholar]
- 27.Kariminia A, Durier N, Jourdain G, et al. Weight as predictors of clinical progression and treatment failure: results from the TREAT Asia Pediatric HIV Observational Database. J Acquir Immune Defic Syndr. 2014;67:71–6. doi: 10.1097/QAI.0000000000000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jesson J, Masson D, Adonon A, et al. Prevalence of malnutrition among HIV-infected children in Central and West-African HIV-care programmes supported by the Growing Up Programme in 2011: a cross-sectional study. BMC Infect Dis. 2015;15:216. doi: 10.1186/s12879-015-0952-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carvalho IR, Pinto JA, Cardoso CA, et al. Evaluation of haematological, virologic and anthropometric parameters as progression markers in HIV-1 infected children. J Pediatr (Rio J) 2009;85:149–56. doi: 10.2223/JPED.1880. [DOI] [PubMed] [Google Scholar]
- 30.Howard AA, Arnsten JH, Lo Y, et al. A prospective study of adherence and viral load in a large multi-center cohort of HIV-infected women. AIDS. 2002;16:2175–82. doi: 10.1097/00002030-200211080-00010. [DOI] [PubMed] [Google Scholar]
- 31.Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133:21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 32.Ramadhani HO, Bartlett JA, Thielman NM, et al. Association of first-line and second-line antiretroviral therapy adherence. Open Forum Infect Dis. 2014;1 doi: 10.1093/ofid/ofu079. ofu079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hazra R, Siberry GK, Mofenson LM. Growing up with HIV: children, adolescents, and young adults with perinatally acquired HIV infection. Annu Rev Med. 2010;61:169–85. doi: 10.1146/annurev.med.050108.151127. [DOI] [PubMed] [Google Scholar]
- 34.Williams N, Knodel J, Kiry Kim S, et al. Overlooked potential: older-age parents in the era of ART. AIDS Care. 2008;20:1169–76. doi: 10.1080/09540120701854642. [DOI] [PubMed] [Google Scholar]
- 35.Ntozi JPM, Ahimbishwe FE, Odwee JO, et al. Orphan care: the role of the extended family in Northern Uganda. Health Transition Review. 1999;8:225–36. [Google Scholar]
- 36.Nyambedha EO, Wandibba S, Aagaard-Hansen J. “Retirement lost”-the new role of the elderly as caretakers for orphans in Western Kenya. J Cross Cult Gerontol. 2003;18:33–52. doi: 10.1023/a:1024826528476. [DOI] [PubMed] [Google Scholar]
- 37.Skovdal M, Campbell C, Madanhire C, et al. Challenges faced by elderly guardians in sustaining the adherence to antiretroviral therapy in HIV-infected children in Zimbabwe. AIDS Care. 2011;23:957–64. doi: 10.1080/09540121.2010.542298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith GC, Palmieri PA. Risk of psychological difficulties among children raised by custodial grandparents. Psychiatr Serv. 2007;58:1303–10. doi: 10.1176/appi.ps.58.10.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]


