Abstract
Objectives
A case–control study was conducted at Omdurman Maternity Tertiary Hospital, Sudan, during the period from May to August 2014 to investigate AMH level in women with preeclampsia compared to healthy controls. The cases were women with preeclampsia and healthy pregnant women were the controls. The obstetrics and medical history was gathered using a questionnaire. AMH level was measured using ELISA.
Results
There was no significant difference between the two groups (40 in each arm of the study) in the age, parity and gestational age. Thirty-three of the 40 cases were patients with severe preeclampsia. There was no significant difference in median inter-quartile of the AMH level between the women with preeclampsia and the controls [0.700 (0.225–1.500) vs. 0.700 (0.400–1.275) ng/ml, P = 0.967]. In a linear regression model there was no association between the log of AMH and age, parity, gestational age, BMI, hemoglobin level and preeclampsia.
Keywords: Anti-Müllerian hormone, Preeclampsia, Pregnancy, Predictors, Sudan
Introduction
Preeclampsia is a worldwide major health problem characterized by the occurrence of hypertension and proteinuria after 20 weeks of pregnancy in previously normotensive women [1]. Preeclampsia is a main factor for maternal and perinatal morbidity and mortality, where it is responsible for at least 9% of the maternal mortality that occur in Africa [2, 3]. Moreover various long-term effects on patients’ health, e.g. cardiovascular risk factors and premature vascular aging that could modify the ovarian aging process have been reported with preeclampsia [4, 5].
Anti-Müllerian hormone (AMH) is a heavily glycosylated glycoprotein produced by the gonads and it is involve in the development and differentiation of the reproductive system [6, 7]. AMH is a reliable predictor of ovarian reserve, in assisted reproduction outcomes such as stillbirth, preeclampsia, gestational diabetes mellitus, delivery of small for gestational age [8–11]. The AMH levels have recently been investigated in preeclampsia with inconsistent results, where some reports showed high levels and others showed low levels of AMH among women with preeclampsia [12–14]. There is a need to investigate whether AMH level during pregnancy can be used as a predictor for preeclampsia and its poor maternal/perinatal outcomes. Preeclampsia/eclampsia is the leading cause of obstetric complications and maternal mortality in Sudan [15–18]. The current study was conducted at Omdurman Maternity Hospital, Sudan to determine the level of AMH in women with preeclampsia and to add on our recent research on preeclampsia in Sudan [19–21].
Main text
A case–control study was conducted at Omdurman Maternity Tertiary Hospital, Sudan during the period from May to August 2014. The cases were pregnant women diagnosed with preeclampsia. Preeclampsia was defined as the occurrence of hypertension (systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg) after 20 weeks of gestation in a woman who is normotensive before, and proteinuria (presence of 300 mg or more of protein in 24 hours urine sample or ≥2+ on dipstick) [22]. The cases of preeclampsia were considered mild or severe according to the diastolic blood pressure of <110, or ≥110 mmHg respectively. The controls were healthy pregnant women that matched with the cases for gestational age. Women with thyroid disease, hypertension, renal disease, diabetes, liver disease and those who received medication for hypertension were excluded from the study.
After signing an informed consent, the details of obstetrics history (age, parity, and gestational age) was gathered and recorded. Weight and height were measured and body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters. Systolic and diastolic blood pressures were assessed in supine position using mercury sphygmomanometer by the same investigator.
Five milliliters of venous blood were taken from each woman (case or control) in a plain tube, allowed to clot, centrifuged, and stored at −20 °C (for 6 months) until analyzed by Ultra-Sensitive AMH/MIS ELISA KIT, (bioactiva diagnostica GmbH—Bad Homburg, Germany), where the manufacturers` instructions were followed. The samples were withdrawn from the cases at presentation which was at mean (SD) of 36.9 (1.0) weeks. The samples were run in duplicate and the mean of the two readings was taken. The reference interval for the KIT was 0.13–13.01 ng/ml.
A total sample of 40 participants in each arm of the study was calculated to investigate the mean difference of the AMH. This sample size would provide 80% power to detect a 5% difference at α = 0.05, with an assumption that complete data might not be available for 10% of participants.
SPSS for Windows (version 16.0) was used for data analyses. Continuous variables were checked for normality and their difference was compared between two groups using T test and Mann–Whitney U when the data were normally and not normally distributed, respectively. Linear regression analyses were performed where the log of AMH (not normally distributed) was the dependent variable, and age, parity, gestational age, maternal BMI and hemoglobin were the independent variables. P < 0.05 was considered statistically significant.
The two groups (40 in each arm of the study) were matched in their basic characteristics, where there was no significant difference in the mean (SD) of the age, parity and gestational age [36.7 (1.9) vs. 36.9 (1.0) weeks, P = 0.625] between the studied groups. Thirty-three of the 40 cases were suffering from severe preeclampsia. All patients had late on-set preeclampsia (after 34 weeks of gestation). The BMI was marginally higher, while hemoglobin level was significantly lower in women with preeclampsia compared to the controls, Table 1. There was no significant difference in median inter-quartile of the AMH between the women with preeclampsia and the healthy controls [0.700 (0.225–1.500) vs. 0.700 (0.400–1.275) ng/ml, P = 0.967], Fig. 1.
Table 1.
Mean (SD) of the basic characteristics in the cases and controls
| Variables | Cases (N = 40) | Controls (N = 40) | P |
|---|---|---|---|
| Age, years | 27.7 (6.9) | 29.5 (6.7) | 0.254 |
| Parity | 2.2 (2.3) | 3.2 (2.8) | 0.089 |
| Gestational age, weeks | 36.7 (1.9) | 36.9 (1.0) | 0.625 |
| Body mass index, kg/m2 | 30.0 (5.8) | 27.2 (4.9) | 0.066 |
| Hemoglobin, g/dl | 10.9 (0.9) | 11.7 (1.3) | 0.004 |
Fig. 1.
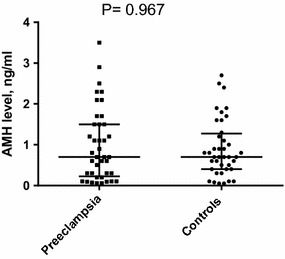
AMH level in women with preeclampsia and controls
In a linear regression model there was no association between the log of AMH and age, parity, gestational age, BMI, hemoglobin level and preeclampsia, Table 2.
Table 2.
Linear regression analysis of factors associated with log of anti-Müllerian hormone AMH
| Variable | Log of anti-Müllerian hormone | ||
|---|---|---|---|
| Coefficient | SE | P | |
| Age, years | −0.022 | 0.015 | 0.933 |
| Parity | 0.020 | 0.035 | 0.158 |
| Gestational age, weeks | −0.002 | 0.055 | 0.567 |
| Body mass index, kg/m2 | −0.005 | 0.015 | 0.977 |
| Hemoglobin, g/dl | 0.072 | 0.064 | 0.722 |
The main two findings of the current study were that first there was no significant difference in the AMH level between women with preeclampsia and the healthy controls. Secondly, significant association between AMH level and age, parity, gestational age and BMI was not established. This is similar with the findings of the recent studies that proved no significant difference in the AMH level between women with a history of preeclampsia and their matched control group [23, 24]. Furthermore, Birdir et al. [12] reported that at early gestation (11–13 weeks), the maternal serum (multiple of the expected median) values of AMH were not significantly different in pregnant women who developed preeclampsia and those who remained normotensive throughout pregnancy. They concluded that AMH was not an effective early predictor for the development of preeclampsia. Interestingly, in the later report the uncorrected median serum concentrations of AMH were significantly higher in the women with preeclampsia than in the controls [12].
Tokmak et al. [14], reported that AMH level (at delivery) was lower in preeclamptic patients than in normal pregnant women and there was no relationship between AMH level and adverse maternal (eclampsia, persistent hypertension and hemolysis, elevated liver enzyme and low platelets, and perinatal outcomes (prematurity, hypoglycemia, polycythemia, respiratory distress syndrome and perinatal deaths). It has been demonstrated that women with a history of preeclampsia had significantly lower AMH levels than women with normotensive pregnancies [5]. Remarkably, women with an AMH (in the first trimester) less than the 10th centile were at 3.3-fold increased risk of pregnancy-induced hypertension. However, there were no significant associations between low AMH concentration and adverse maternal or perinatal outcomes [13].
In the current study, AMH level was not associated with age, parity, gestational age and BMI, which is comparable with the findings of a recent report [12].
It is worth to be mentioned that, to our knowledge, this is the first published data on AMH serum level and preeclampsia in Africa. Maternal serum concentration of AMH was reported to be increased in Afro-Caribbean women [12], while African women (probably due to genetic or environmental factors) showed a relatively high ovarian reserve, AMH and oocyte yield compared to other races [25]. AMH has advantages over other ovarian reserve marker; it has no significant intra/inter-cycle variability, therefore it can be measured on any day of the cycle [26].
Thus the current study showed no significant difference in AMH level between women with preeclampsia and the controls. AMH level was not associated with age, parity, gestational and BMI.
Limitations
One of the limitations of the current study is that the patients were not followed-up to the postpartum period. The follow-up would have detected the post-partum changes of the AMH level and would have compared our results with the previous studies that were conducted following delivery. All women had late onset preeclampsia and none of them had early preeclampsia and this is the second limitation of the current study. Furthermore neither Doppler ultrasound nor intrauterine growth restriction were looked for.
Authors’ contributions
EA and IA involved in design of the study. HM and AEE were involved in data collection. EA, IA and HZH were involved in the design and interpretation of the analyses for this paper. HZH and IA carried out the analyses and wrote the first draft of the paper to which all authors contributed and approved the final version. All authors read and approved the final manuscript.
Acknowledgements
Authors are wishing to thank all women who participated in the study.
Competing interests
The authors declare that they have no competing interests. IA (correspondence) is one of the associate editors of this journal.
Availability of data and materials
The data are available within the manuscript.
Ethics approvals and consent to participate
The study received ethical clearance from the Research Board at the Department of Obstetrics and Gynaecology, Faculty of Medicine, University of Khartoum, Sudan. Informed consent was obtained from participants.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- AMH
anti-Müllerian hormone
- BMI
body mass index
Contributor Information
Eiman Agabain, Email: eimanagabein@qumed.edu.sa.
Hameed Mohamed, Email: kassala99@gmail.com.
Anas E. Elsheikh, Email: anaselshafia@gmail.com
Hamdan Z. Hamdan, Email: hamdanology@hotmail.com
Ishag Adam, Phone: +249912168988, Email: Ishagadam@hotmail.com.
References
- 1.Williams D. Long-term complications of preeclampsia. Semin Nephrol. 2011;31(1):111–122. doi: 10.1016/j.semnephrol.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 2.Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009;33(3):130–137. doi: 10.1053/j.semperi.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Khan KS, Wojdyla D, Say L, Gülmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367(9516):1066–1074. doi: 10.1016/S0140-6736(06)68397-9. [DOI] [PubMed] [Google Scholar]
- 4.Harskamp RE, Zeeman GG. Preeclampsia: at risk for remote cardiovascular disease. Am J Med Sci. 2007;334(4):291–295. doi: 10.1097/MAJ.0b013e3180a6f094. [DOI] [PubMed] [Google Scholar]
- 5.Yarde F, Maas AH, Franx A, Eijkemans MJ, Drost JT, van Rijn BB, van Eyck J, van der Schouw YT, Broekmans FJ. Serum AMH levels in women with a history of preeclampsia suggest a role for vascular factors in ovarian aging. J Clin Endocrinol Metab. 2014;99(2):579–586. doi: 10.1210/jc.2013-2902. [DOI] [PubMed] [Google Scholar]
- 6.Picard JY, Belville C. Genetics and molecular pathology of anti-Mullerian hormone and its receptor. J Soc Biol. 2002;196:217–221. doi: 10.1051/jbio/2002196030217. [DOI] [PubMed] [Google Scholar]
- 7.Deffieux X, Antoine JM. Inhibins, activins and anti-Mullerian hormone: structure, signalling pathways, roles and predictive value in reproductive medicine. Gynecol Obstet Fertil. 2003;31:900–911. doi: 10.1016/j.gyobfe.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 8.Fanchin R, Schona¨uer LM, Righini C, et al. Serum anti-Müllerian hormone is more strongly related to ovarian follicular status than serum inhibin B, estradiol, FSH and LH on day 3. Hum Reprod. 2003;18:323–327. doi: 10.1093/humrep/deg042. [DOI] [PubMed] [Google Scholar]
- 9.De Vet A, Laven JS, de Jong FH, et al. Anti-Müllerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril. 2002;77:357–362. doi: 10.1016/S0015-0282(01)02993-4. [DOI] [PubMed] [Google Scholar]
- 10.Ledger WL. Clinical utility of measurement of anti-Mullerian hormone in reproductive endocrinology. J Clin Endocrinol Metab. 2010;95:5144–5154. doi: 10.1210/jc.2010-0701. [DOI] [PubMed] [Google Scholar]
- 11.Chaveeva P, Carbone IF, Syngelaki A, et al. Contribution of method of conception on pregnancy outcome after the 11–13 weeks scan. Fetal Diagn Ther. 2011;30:9–22. doi: 10.1159/000323921. [DOI] [PubMed] [Google Scholar]
- 12.Birdir C, Fryze J, Vasiliadis H, Nicolaides KH, Poon LC. Maternal serum anti-Müllerian hormone at 11–13 weeks’ gestation in the prediction of preeclampsia. J Matern Fetal Neonatal Med. 2015;28(8):865–868. doi: 10.3109/14767058.2014.937418. [DOI] [PubMed] [Google Scholar]
- 13.Shand AW, Whitton K, Pasfield A, Nassar N, McShane M, Han X, Henry A. Evaluation of anti-Mullerian hormone in the first trimester as a predictor for hypertensive disorders of pregnancy and other adverse pregnancy outcomes. Aust N Z J Obstet Gynaecol. 2014;54(3):244–249. doi: 10.1111/ajo.12183. [DOI] [PubMed] [Google Scholar]
- 14.Tokmak A, Güney G, Aksoy RT, Guzel AI, Topcu HO, Keçecioğlu TS, Uygur D. May maternal anti-mullerian hormone levels predict adverse maternal and perinatal outcomes in preeclampsia? J Matern Fetal Neonatal Med. 2015;28(12):1451–1456. doi: 10.3109/14767058.2014.955007. [DOI] [PubMed] [Google Scholar]
- 15.Ali AA, Okud A, Khojali A, Adam I. High incidence of obstetric complications in Kassala Hospital, Eastern Sudan. J Obstet Gynaecol. 2012;32(2):148–149. doi: 10.3109/01443615.2011.637140. [DOI] [PubMed] [Google Scholar]
- 16.Ali AA, Khojali A, Okud A, Adam GK, Adam I. Maternal near-miss in a rural hospital in Sudan. BMC Pregnancy Childbirth. 2011;11:48. doi: 10.1186/1471-2393-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elhassan EM, Mirghani OA, Adam I. High maternal mortality and stillbirth in the Wad Medani Hospital, Central Sudan, 2003–2007. Trop Dr. 2009;39(4):238–239. doi: 10.1258/td.2009.090005. [DOI] [PubMed] [Google Scholar]
- 18.Adam I, Haggaz AD, Mirghani OA, Elhassan EM. Placenta previa and pre-eclampsia: analyses of 1645 cases at medani maternity hospital, Sudan. Front Physiol. 2013;4:32. doi: 10.3389/fphys.2013.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fadalallah ZM, Elhassan EM, Rayis DA, Abdullahi H, Adam I. Prospective cohort study of persistent hypertension following pre-eclampsia at Medani Hospital, Sudan. Int J Gynaecol Obstet. 2016;134:66–68. doi: 10.1016/j.ijgo.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 20.Elhaj ET, Adam I, Alim A, Elhassan EM, Lutfi MF. Thyroid function/antibodies in sudanese patients with preeclampsia. Front Endocrinol (Lausanne) 2015;11(6):87. doi: 10.3389/fendo.2015.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adam I, Elhassan EM, Mohmmed AA, Salih MM, Elbashir MI. Malaria and pre-eclampsia in an area with unstable malaria transmission in Central Sudan. Malar J. 2011;10:258. doi: 10.1186/1475-2875-10-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ task force on hypertension in pregnancy. Obstet Gynecol. 2013;122(5):1122–1131. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 23.Vårtun Å, Aune B, Flo K, Acharya G. PP079 ovarian reserve and function is preserved following severe preeclampsia. Pregnancy Hypertens. 2013;3(2):96. doi: 10.1016/j.preghy.2013.04.104. [DOI] [PubMed] [Google Scholar]
- 24.Bhide P, Vårtun Å, Aune B, Flo K, Basnet P, Acharya G. Ovarian reserve in women with a previous history of severe pre-eclampsia. Arch Gynecol Obstet. 2017;295(1):233–238. doi: 10.1007/s00404-016-4193-8. [DOI] [PubMed] [Google Scholar]
- 25.Gleicher N, Kim A, Weghofer A, Barad DH. Differences in ovarian aging patterns between races are associated with ovarian genotypes and sub-genotypes of the FMR1 gene. Reprod Biol Endocrinol. 2012;10:77. doi: 10.1186/1477-7827-10-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Rooij IA, Broekmans FJ, te Velde ER, Fauser BC, Bancsi LF, de Jong FH, et al. Serum anti-Müllerian hormone levels: a novel measure of ovarian reserve. Hum Reprod. 2002;17:3065–3071. doi: 10.1093/humrep/17.12.3065. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available within the manuscript.


