Abstract
Murine embryonic stem (ES) cells are pluripotent, but significant functional engraftment does not occur when they are introduced into the liver. However, here we demonstrate that functional liver engraftment does occur if the ES cells (from strain 129 mice) are first differentiated in vitro for 7 days in the presence of FGF. Strikingly, when these differentiated cells, termed putative endodermal precursors (PEPs), were injected into their livers, two of six C57BL/6 and four of eight BALB/c factor IX (F-IX)-deficient mice survived for >7 days, even though the recipients were of a different strain and, in the case of the BALB/c recipients, had a complete MHC mismatch. F-IX was detected in all six of the PEP-injected survivors. Two mice subsequently died of causes unrelated to F-IX; the others survived until death at 38 or 115 days after the transplantation. No uninjected control F-IX-deficient mice survived for >7 days. Large confluent regions of sinusoidal PEP engraftment were demonstrated by immunofluorescence in the long-term BALB/c survivors. The PEP engraftment was not associated with detectable cell fusion, and the transplantation was accompanied with only a low incidence of teratoma formation.
Keywords: gene therapy, in vitro differentiation, cellular transplantation, liver engraftment, hemophilia
Embryonic stem (ES) cells can differentiate into most cell types, thus leading to intense interest in their potential for therapeutic applications (1). Despite this interest, problems have arisen in translating the potential of ES-derived cells into therapeutic strategies. The dominant problems have been lack of function, allograft rejection, and teratoma formation (1–4). Yet, the therapeutic potential for ES-derived cells remains substantial. Thus, if ES-derived cells could be successfully engrafted within the hepatic parenchyma, they could serve as gene replacement vectors for correcting liver-based genetic defects such as hemophilia A and B. Cells with hepatocyte phenotypes have been derived from ES cells in vitro, using strategies involving isolating cells from dissociated embryoid bodies (5). However, achieving function with these cells has proven to be difficult (3, 6, 7), and no reports have been published of ES-derived cells persisting with function across allogeneic barriers.
We have previously demonstrated that murine ES cells removed from feeders can rapidly respond to in vitro inductive signals from chick cardiac mesoderm, resulting in differentiation into cells with a putative endodermal phenotype (8), thereby mimicking the transcriptional regulation for endodermal competence found in development (9–13). In our current experiments, we removed ES cells from their feeder layer and stimulated them with acidic FGF (100 μg/ml) for 7 days. We find that cells differentiated in this manner, termed putative endodermal precursors (PEPs), can engraft the liver and function in vivo in histocompatibility mismatched recipients sufficiently well enough to allow long-term survival of factor IX (F-IX)-deficient mice. No obvious histological evidence of allograft rejection was apparent in the recipient livers, and no alloantibodies were detectable. Teratoma formation was infrequent.
Materials and Methods
Enhanced GFP-Expressing ES Cells. To facilitate the identification of successful engraftment, we used PEPs differentiated from a murine ES cell line that constitutively expresses a single-copy transgene driven by the ubiquitously expressed β-actin promoter (8) encoding for an enhanced GFP (Stratagene) and localizes to the nucleus. This ES cell line is derived from a strain 129P2/Ola mouse. Cells were maintained in murine embryonic fibroblast feeder layers that produce leukemic inhibitory factor. To ensure viability and pluripotency, ES cell media were changed daily, and ES cells were passed every 2–3 days. Their karyotypes were checked at intervals.
ES Cell Culture. ES cell propagation media consisted of high-glucose DMEM (Sigma) supplemented with 15% FBS (Sigma)/0.1 mM 2-mercaptoethanol (Sigma)/100 units/ml penicillin/100 μg/ml streptomycin (GIBCO).
ES Cell Differentiation. Propagation media were supplemented with 100 ng/ml acidic FGF (Sigma). The cells were removed from their feeders and plated in P35 collagen-coated plates. The media were carefully changed daily, and the cells were allowed to obtain an ≈50% density. When the cells were ready for transplantation or analysis, they were removed from the plates with 0.05% trypsin (Sigma)/0.02% EDTA4Na in PBS, counted, and stained for viability with Trypan blue.
F-IX ELISA. The ELISA was performed as described by Gui et al. (14) with the following modifications. Both the capture and detection antibodies were sheep anti-mouse specific for F-IX (a kind gift of Affinity Biologicals, Ancaster, ON, Canada) with the detection antibody covalently linked to horseradish peroxidase. Purified mouse F-IX (produced and purified in the laboratory of D. Stafford, University of North Carolina) was used as the standard.
Quantitative RT-PCR. Real-time fluorescent quantitative PCR was performed by using an ABI PRISM 7700 (Applied Biosystems). The sequences of the oligonucleotides are listed in Supporting Text, which is published as supporting information on the PNAS web site.
Fluorescent Stereomicroscopy. To initially identify the engraftment, fresh liver explants were examined with a stereomicroscope (MZ16FA, Leica Microsystems, Wetzlar, Germany) using a GFP2 long-pass filter (100447084, Leica Microsystems) to detect the presence of GFP-positive cells.
Procedure Modifications for F-IX-Deficient Mice. The F-IX knockout mice used in our transplant studies were first confirmed to be F-IX-negative by PCR genotyping, and a day 0 F-IX baseline was collected. To perform the partial hepatectomy, the mice were given 100 units/kg recombinant human F-IX (15), both by intramuscular and s.c. injection after anesthesia, and another s.c. dose 4 h after the operation. At the time when the GFP-PEP injections were performed, on postoperative day 3, the intramuscular and s.c. doses of human F-IX were repeated. F-IX levels were assessed by ELISA on the plasma (14) 4, 7, and 10 days after injection and on a weekly basis thereafter. Recombinant human F-IX (stock concentration; 575 μg/ml) was from the laboratory of D. Stafford.
Partial Hepatectomy. Adult mice (C.129P2F9tm1Dws/Fre, B6.129P2tm1Dws/Fre) [F-IX, BALB/c and C57BL/6] 6–15 weeks of age were obtained from the laboratory of D.W.S. Wild-type BALB/c and C57BL/6 mice were obtained from The Jackson Laboratory. Mice were kept in a Department of Laboratory Animal Medicine facility and were watered and fed regular chow ad libitum. Mice were anesthetized with an s.c. injection of ketamine/xylazene mixture (60 mg/kg ketamine and 6 mg/kg xylazene). Once under anesthesia, the thoracic and abdominal surfaces were shaved by using appropriate animal clippers (Oster Golden A5), and the shaved area was decontaminated by applying betadine, followed by 70% ethanol. Once the mouse was anesthetized, the abdominal cavity was entered through a 2-cm incision starting just below the sternum and traveling straight down the abdominal wall. The median liver lobes (left and right) and the large left lateral lobe were isolated, tied off with 3-0 ties, and removed. After removing the lobes, the abdomen was closed with 4-0 prolene or silk suture. Before the abdominal wall was closed, 1 ml of room temperature PBS was placed in the abdomen. Mice then recovered in a clean, dry, warm cage under a warming lamp until they were mobile. Mice were given a 0.1 g/kg i.p. dose of buprenorphine every 12 h after surgery for 2 days and were monitored closely thereafter.
Hepatic Injection of ES-Derived Cells. PEPs (1 × 106) were suspended in 100 μl of PBS with 1% FBS and kept at 4°C for ≈30 min. The mice were anesthetized with an s.c. injection of ketamine/xylazene mixture (60 mg/kg ketamine and 6 mg/kg xylazene) by using a 28- or 31-gauge syringe. Once under anesthesia, a small incision was created below the right costal margin, and the liver lobe was delivered into view with a cotton swab. The cell suspension was injected directly into the parenchyma. Postoperative pain management was performed as above.
Tissue Processing. To further characterize cell engraftment, treated mice that showed significant levels of F-IX by ELISA were anesthetized with 60 mg/kg ketamine and 6 mg/kg xylazene and perfused transcardially with PBS, followed by 4% paraformaldehyde. The liver was removed and fixed overnight in 4% paraformaldehyde in PBS, transferred to 30% sucrose in PBS overnight, and then mounted in OCT compound (Tissue-Tek, Sakura Finetek, no. 4583) for quick-freezing. The liver samples were sliced into 10-μm-thick sections with a cryostat (Leica Microsystems, Bannockburn, IL), mounted onto clean superfrost/plus slides, air-dried, and stored at –80°C until processing. Tissue samples from positive (wild types) and negative (nontransplanted F-IX–/–) samples were obtained and processed identically to the experimental samples.
Histology. Samples were fixed in 4% paraformaldehyde overnight and transferred into 30% sucrose for a minimum of 24 h. Samples were then placed in a cryomold (Tissue-Tek, 25 × 20 × 5 mm) with OCT compound (Tissue Tek, no. 4583), frozen at –80° for a minimum of 24 h, and processed by cryosectioning in Leica no. CM 3050 S cryostat. Cryosections of 10- to 12-μm thickness were rehydrated in PBS, mounted with aqueous mounting medium (DAKO), and examined by fluorescence microscopy for the presence of GFP cells.
Immunofluorescence. Slides with tissue cryosections processed as above were washed twice in PBS, permeabilized, and blocked against nonspecific binding in blocking buffer (5% normal serum/2% BSA/0.1% Triton X-100 in PBS) for 30 min. Samples were then incubated with sheep anti-rat F-IX antibody (Haematologic Technologies, Essex Junction, VT) for 90 min at room temperature at a final dilution of 1:200 in blocking buffer. After the incubation, samples were washed three times with PBS-0.05% Triton X-100, followed by incubation with Texas red-labeled anti-sheep IG (Fab′2) IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) diluted 1:200 in blocking buffer for 30 min. After three washings in PBS-0.1% Triton X-100, samples were incubated with rabbit polyclonal GFP antibody conjugated to Alexa Fluor 488 (Molecular Probes) for 1 h at room temperature (1:400 dilution in blocking buffer) after three washings with PBS-0.1% Triton X-100 and mounted with Vectashield mounting media (Vector Laboratories). Processed slides were examined under a Zeiss Axiovert S100 fluorescent microscope, and representative slides were further analyzed by confocal laser-scanning microscopy performed on a Zeiss LSM5 Pascal microscope (Thornwood, NY).
Laser-Capture Microdissection (LCM). Slides containing frozen sections were placed on the stage of an Arcturus Pix-Cell II LCM system (Arcturus Engineering, Mountain View, CA). Either a standard CapSure or a new HS LCM cap was placed on the section. Desired areas of tissue were maneuvered under the target beam, and the IR laser was fired to melt the special plastic membrane from the cap into the tissue. After all desired areas were shot in this way, the cap was lifted off the tissue, bringing with it only the selected tissue. Fragments of loose tissue were removed from the cap by pressing the cap onto the adhesive surface of a new PostIt (3M, St. Paul) note. The cap was then placed into an Eppendorf tube containing extraction buffer. Images were made before capture, after capture, and of the cap containing captured material.
Antidonor Antibody Testing. Serum from graft recipient mice was tested for antibodies reactive to strain 129 spleen cells because PEPs were derived from strain 129 and share MHC with 129 splenocytes. Serum from BALB/c mice engrafted with PEPs was diluted and incubated with 129 target cells. Cells were subsequently washed and incubated with goat anti-mouse Ig. Cells were washed and run on a flow cytometer (FACSCalibur, B D Biosciences, Mountain View, CA), and the data were analyzed by using summit software (Cytomation, Fort Collins, CO). A polyclonal antiserum, (AXB10.D2), F1 anti-B10.A (5R), was used as a positive control.
Results
Directed in Vitro Differentiation of ES Cells.We stimulated ES cells, removed from their feeder layer, with acidic FGF (100 μg/ml) for 7 days, and we found by quantitative RT-PCR (16) that the cells display a statistically significant reduction in mRNA of Oct 4 (P < 0.01), and an increase in mRNA of α-fetoprotein (P < 0.01), GATA4 (P < 0.05), albumin, (P < 0.05), FoxA2 (P = 0.06), and Sox 17α (P = 0.06) (Fig. 4, which is published as supporting information on the PNAS web site). These molecular changes coincide with dramatic morphologic changes and the appearance of large cells with distinctive growth patterns (Fig. 1 A and B). We refer to the resulting cells as PEPs.
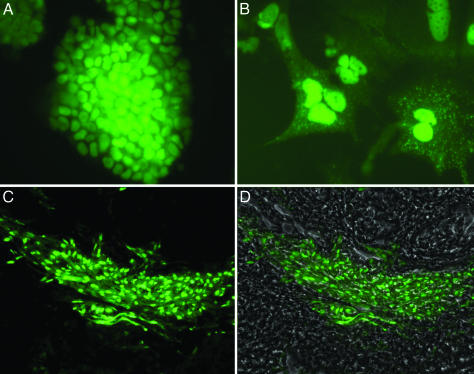
Fig. 1.
In vitro and in vivo postengraftment morphology of PEPs. (A) Undifferentiated ES cells on feeder layer in vitro.(B) Cell appearance after differentiation with FGF (100 ng/ml) in media for 7 days in vitro (magnification 200x). (C and D) Engraftment images 20 days after PEP injection into liver. With GFP filter only (C), image reveals engraftment of GFP PEPs; with phase overlay (D), image reveals GFP engraftment into hepatic parenchyma (magnification; ×63).
Cellular Transplantation of PEPs. To optimize the potential conditions for PEPs to engraft, we initially chose to partially hepatectomize the recipient mice. Numerous studies have explored possible routes of delivering cells for engraftment in the hepatic parenchyma (17). We performed preliminary injection studies (data not shown) that showed that direct injection of PEPs into the liver resulted in their dispersal throughout the parenchymal vasculature. This method was chosen as providing a direct delivery of PEPs to the intended engraftment location. When fresh explanted liver tissue is screened by 3D fluorescent microscopy, the exogenous GFP-ES-derived cells are easily visualized and distinguished from the endogenous hepatic parenchyma, which autofluoresces, allowing for immediate and accurate detection of engraftment. Fig. 1 C and D illustrates typical results 20 days after injecting strain 129 (H2b)-derived PEPs into a C57BL/6 recipient, also (H2b). The GFP-PEP hepatic engraftment displays a consistent pattern with the GFP-PEP cells coursing along the sinusoids within the space of Disse. Engraftment comparable to that shown in Fig. 1 C and D occurred in 10 of 19 mice (52%) injected with PEPs versus 0 of 14 mice injected with 1 × 106 undifferentiated ES cells (χ2 = 10.6; P < 0.01).
Having observed robust engraftment in a MHC type (H2b) recipient, we asked whether GFP-PEP could be transplanted across a major MHC barrier. Using the same in vitro and in vivo protocol as above, we partially hepatectomized BALB/c (H2d) mice, which are strain- and MHC-mismatched to the cells from which the PEPs, strain 129 (H2b), are derived. The PEPs were then injected as described, and this injection resulted in engraftment in 5 of 10 (50%) of the recipients 10 days after the GFP-PEP injection. We saw no histologic evidence of rejection in these grafts.
We tested for the possibility that cell fusion was the dominant mechanism of hepatic engraftment by analyzing the genetic composition of GFP-fluorescing cells in the hepatic parenchyma. LCM was used to isolate GFP-expressing cells, and quantitative DNA PCR was used to quantify the genomic DNA contribution from engrafted cells versus wild-type cells in the GFP+-fluorescing areas of parenchyma. The results (Fig. 5, which is published as supporting information on the PNAS web site) show no detectable colocalization of wild-type DNA with the GFP DNA in the engraftment areas, thereby excluding cell fusion as the dominant mechanism of the PEP hepatic engraftment and function in our experiments.
Teratoma formation is a major concern when using ES-derived cells for cellular engraftment. Including all of our data for 46 C57BL/6 mice and 18 BALB/c mice transplanted with PEPs, we find an overall teratoma rate of 6.2% (4 of 64 mice). Three of the 46 injected C57BL/6 mice developed teratoma, and none of them were engrafted mice. Of 18 BALB/c mice injected, an encapsulated teratoma developed in one engrafted mouse, adjacent to the liver but not associated directly with the engrafted regions of the liver.
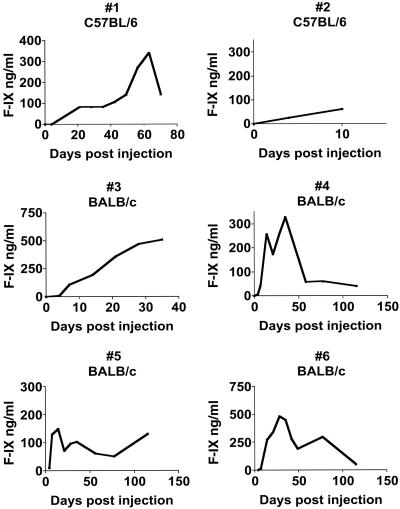
Engrafted GFP-PEP Produce F-IX. Initial experiments with C57BL/6 F-IX knockout mice, having only minor histocompatibility differences from the strain 129-derived PEPs, showed that, of the four mice surviving hepatectomy and injection, two survived beyond 7 days. Mouse no. 1 in Fig. 2 survived for 88 days after injection, achieving a F-IX level of 340 ng/ml, which is 10% of normal. This mouse was 18 months old at the time of death, and postmortem examination revealed no obvious cause of death or pathology related to the PEP engraftment. (The lifespan of a F-IX-deficient mouse is ≈18–24 months.) Mouse no. 2 in Fig. 2 showed typical hepatic histological engraftment with GFP-PEPs and substantial plasma concentrations of F-IX by day 10, but it died during anesthesia for blood drawing on day 10. We also subjected 18 F-IX-deficient mice to partial hepatectomies and human F-IX injections, but without subsequent PEP injections; none of these mice survived >7 days. We conclude that the injected PEPs provide a survival advantage in this model.
Fig. 2.
Plasma F-IX concentrations over time in C57BL/6 and BALB/c F-IX-deficient mice after engraftment with PEPs as determined by ELISA.
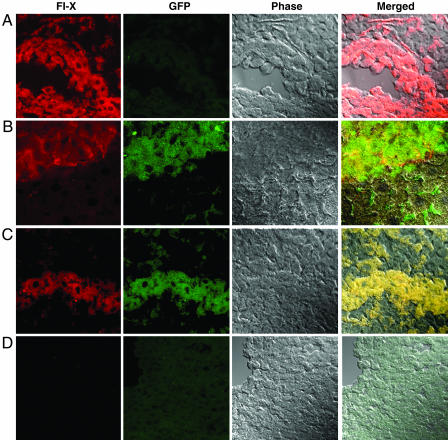
Based on the preceding experiments demonstrating that strain 129-derived GFP-PEPs can engraft across a complete MHC and non-MHC barrier, we subjected BALB/c F-IX-deficient mice to PEP transplantation with or without preceding hepatectomy. No immunosuppressive therapy was administered. Four of six mice survived surgery and showed continuous F-IX expression from 38 to 115 days. One mouse (mouse no. 3 in Fig. 2) had received hepatectomy before injection, and three mice (mice numbered 4–6 in Fig. 2) received PEP injection without hepatectomy. Mouse no. 3 was intentionally killed on day 38 because of the emergence of an abdominal mass adjacent to the liver capsule. Mice numbered 4–6 were killed on day 115. No evidence of neoplasm was observed in these mice at autopsy or histological sectioning of the explanted livers. GFP mRNA was detectable throughout the livers by PCR. Fig. 3 demonstrates large cords of GFP-positive cells within the hepatic parenchyma, from mouse no. 5 in Fig. 2, that colocalize F-IX by immunofluorescence. Because of this successful engraftment across an MHC barrier, we examined serum from the BALB/c recipients for 129/J-reactive antibodies by flow cytometry at 7 and 115 days after transfer. We found no detectable antibodies at either time directed at 129 or BALB/c spleen cells. Normal serum from BALB/c mice was also nonreactive.
Fig. 3.
Confocal analysis of immunofluorescence staining of liver sections from BALB/c F-IX knockout mice at day 115 after injection. (A) Liver sections from wild-type mice without PEP injection, demonstrating F-IX staining and no GFP staining. (B and C) Liver sections from F-IX knockout mice 115 days after PEP injection, demonstrating F-IX staining and GFP staining, with colocalization of F-IX and GFP staining demonstrated on phase overlay images. (D) Liver section from F-IX knockout mice without PEP injection, demonstrating neither F-IX staining or GFP staining (magnification: ×63).
Discussion
Our study demonstrates that ES-derived cells with early endodermal characteristics engraft, persist, and function in the liver. The cells we have described as PEPs have five features that make them highly promising in this setting. First, PEPs are obtained directly from ES cells by culture with FGF as an inductive stimulus. Second, when injected into the liver parenchyma, PEPs are able to engraft robustly and restore wild-type hepatocellular function in F-IX-deficient mice. F-IX-deficient mice (18, 19) have a phenotype that is similar to hemophilia B in humans, characterized by a severe bleeding diathesis caused by absence of F-IX protein (20, 21). F-IX has a short half-life requiring continuous replacement, and, consequently, any substantial production of mouse F-IX in the deficient mice indicates hepatocyte function derived from the engrafted PEPs. Third, PEPs engraft and persist across a complete mismatched MHC barrier. Fourth, PEPs exhibit only a low incidence of teratoma formation. Fifth, PEPs do not require host liver injury or regeneration to engraft and function.
Each of these five features requires further investigation. For example, it is important to characterize the changes that occur during the FGF inductive phase, which are demonstrably sufficient to produce transplantable cells. The occurrence of teratoma in some recipients also requires further study. Our experience suggests that teratoma formation is a separate event from that leading to functional PEP engraftment. Thus, in the three cases, it occurred in the mice that had no liver engraftment, and, in the one of 21 mice that had the described sinusoidal engraftment pattern, the teratoma was extra-hepatic. If this suggestion is correct, then purification of the differentiated PEPs before injection should reduce this complication. In this regard, it is also important to recall that strain 129 mice, from which the ES cells are derived, are notable for an unusually high incidence of spontaneous teratoma (22). Several strategies are available that may decrease or eliminate teratoma formation. One strategy is to use ES cells from another mouse strain that is less prone to spontaneous teratomas. A second strategy is based on our observation of a sharply increased expression of MHC class I antigens on the ES cells during their in vitro differentiation. A sorting strategy based on this marker should exclude any undifferentiated ES cells likely to give rise to teratomas.
The appearance of PEP engraftment in liver parenchyma illustrated here is different from that seen in other reports describing ES-derived cell engraftment in the liver (3, 6). We suspect that the cells possess an intrinsic rate of proliferation but that this rate subsides over time, as judged by the histologic appearance of the engraftment at 4 months; this phenomenon requires further investigation.
Cell fusion has been suggested as the dominant mechanism by which stem cells engraft in the liver, and numerous studies have shown that stem cells can fuse with the native hepatocytes and adopt a liver-specific phenotype (23, 24). These fusion events, however, have been in engraftment that involves individual cells in the liver parenchyma, rather than the diffuse engraftment of hundreds of contiguous cells in islands as seen in our model. Furthermore, pluripotent fractions of umbilical cord blood can engraft into the liver with low rates of fusion (25, 26) Although fusion as a primary mechanism of the large-scale engraftment seen in our model is excluded, more detailed future studies are required to see whether a low rate of fusion could be present.
Other questions include whether the biologic benefit we observe with PEPs is limited to deficiency states or can be generalized for the management of other hepatic diseases. Elucidation of factors important for engraftment, such as adhesion molecule expression, proliferation rates, and metabolism, will be critical. Whether hepatic engraftment with PEPs results in temporary or long-term allogeneic chimerism or induces tolerance to other allografts, as has been reported in another stem cell transplant model (27), also requires further study. Our observation of allogeneic graft survival is especially intriguing, given that mature allogeneic hepatocytes are vigorously rejected via CD4 and CD8 (nonclassical) pathways when transplanted into the liver, (28, 29). Even though the immunological mechanisms leading to graft persistence are not known, our finding that PEP engraftment occurs across a major MHC barrier is very encouraging for the use of allogeneic ES stem cells for treating human disease.
In summary, our demonstration of functional, persistent hepatic engraftment with PEPs serves as a proof of principle that directly differentiated ES cells are likely to have clinical relevance for the replacement of hepatic function. With concentrated efforts directed toward solving the various critical questions that we have discussed, it seems possible that effective clinical use of in vitro-differentiated ES cells may become a reality.
Supplementary Material
Acknowledgments
We thank Harold Roberts (University of North Carolina), Gil White (University of North Carolina), and Anna Mae Diehl (Duke University, Durham, NC) for assistance and advice. This work was supported by National Institutes of Health Grants K18 DK065013 (to J.H.F.), K08 GM 067147 (to B.A.C.), R01 HL037001 (to O.S.), R01 HL071266 (O.S.), R01 GM 67143 (to J.A.F.), and R01 AI 152435 (to J.A.F.) the North Carolina Jaycee Burn Center; and the Jefferson-Pilot Foundation.
Abbreviations: F-IX, factor IX; PEP, putative endodermal precursor; LCM, laser-capture microdissection.
References
- 1.Hwang, W. S., Ryu, Y. J., Park, J. H., Park, E. S., Lee, E. G., Koo, J. M., Jeon, H. Y., Lee, B. C., Kang, S. K., Kim, S. J., et al. (2004) Science 303, 1669–1674. [DOI] [PubMed] [Google Scholar]
- 2.Lu, S. J., Li, F., Vida, L. & Honig, G. R. (2002) Exp. Hematol. (Charlottesville, Va) 30, 58–66. [DOI] [PubMed] [Google Scholar]
- 3.Chinzei, R., Tanaka, Y., Shimizu-Saito, K., Hara, Y., Kakinuma, S., Watanabe, M., Teramoto, K., Arii, S., Takase, K., Sato, C., et al. (2002) Hepatology 36, 22–29. [DOI] [PubMed] [Google Scholar]
- 4.Drukker, M., Katz, G., Urbach, A., Schuldiner, M., Markel, G., Itskovitz-Eldor, J., Reubinoff, B., Mandelboim, O. & Benvenisty, N. (2002) Proc. Natl. Acad. Sci. USA 99, 9864–9869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamazaki, T., Iiboshi, Y., Oka, M., Papst, P. J., Meacham, A. M., Zon, L. I. & Terada, N. (2001) FEBS Lett. 497, 15–19. [DOI] [PubMed] [Google Scholar]
- 6.Yin, Y., Lim, Y. K., Salto-Tellez, M., Ng, S. C., Lin, C. S. & Lim, S. K. (2002) Stem Cells (Dayton) 20, 338–346. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto, H., Quinn, G., Asari, A., Yamanokuchi, H., Teratani, T., Terada, M. & Ochiya, T. (2003) Hepatology 37, 983–993. [DOI] [PubMed] [Google Scholar]
- 8.Fair, J. H., Cairns, B. A., Lapaglia, M., Wang, J., Meyer, A. A., Kim, H., Hatada, S., Smithies, O. & Pevny, L. (2003) Surgery (St. Louis) 134, 189–196. [DOI] [PubMed] [Google Scholar]
- 9.Ang, S. L., Wierda, A., Wong, D., Stevens, K. A., Cascio, S., Rossant, J. & Zaret, K. S. (1993) Development (Cambridge, U.K.) 119, 1301–1315. [DOI] [PubMed] [Google Scholar]
- 10.Kubo, A., Shinozaki, K., Shannon, J. M., Kouskoff, V., Kennedy, M., Woo, S., Fehling, H. J. & Keller, G. (2004) Development (Cambridge, U.K.) 131, 1651–1662. [DOI] [PubMed] [Google Scholar]
- 11.Zorn, A. M., Barish, G. D., Williams, B. O., Lavender, P., Klymkowsky, M. W. & Varmus, H. E. (1999) Mol. Cell 4, 487–498. [DOI] [PubMed] [Google Scholar]
- 12.Kanai-Azuma, M., Kanai, Y., Gad, J. M., Tajima, Y., Taya, C., Kurohmaru, M., Sanai, Y., Yonekawa, H., Yazaki, K., Tam, P. P. & Hayashi, Y. (2002) Development (Cambridge, U.K.) 129, 2367–2379. [DOI] [PubMed] [Google Scholar]
- 13.Zaret, K. S. (2000) Mech. Dev. 92, 83–88. [DOI] [PubMed] [Google Scholar]
- 14.Gui, T., Lin, H. F., Jin, D. Y., Hoffman, M., Straight, D. L., Roberts, H. R. & Stafford, D. W. (2002) Blood 100, 153–158. [DOI] [PubMed] [Google Scholar]
- 15.Ahmad, S. S., Rawala, R., Cheung, W. F., Stafford, D. W. & Walsh, P. N. (1995) Biochem. J. 310, 427–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim, H. S., Lee, G., John, S. W., Maeda, N. & Smithies, O. (2002) Proc. Natl. Acad. Sci. USA 99, 4602–4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta, S., Bhargava, K. K. & Novikoff, P. M. (1999) Semin. Liver Dis. 19, 15–26. [DOI] [PubMed] [Google Scholar]
- 18.Greenwood, R., Wang, B., Midkiff, K., White, G. C., II, Lin, H. F. & Frelinger, J. A. (2003) J. Thromb Haemost. 1, 95–102. [DOI] [PubMed] [Google Scholar]
- 19.Lin, H. F., Maeda, N., Smithies, O., Straight, D. L. & Stafford, D. W. (1997) Blood 90, 3962–3966. [PubMed] [Google Scholar]
- 20.Salier, J. P., Hirosawa, S. & Kurachi, K. (1990) J. Biol. Chem. 265, 7062–7068. [PubMed] [Google Scholar]
- 21.Snyder, R. O., Miao, C., Meuse, L., Tubb, J., Donahue, B. A., Lin, H. F., Stafford, D. W., Patel, S., Thompson, A. R., Nichols, T., et al. (1999) Nat. Med. 5, 64–70. [DOI] [PubMed] [Google Scholar]
- 22.Stevens, L. C. (1973) J. Natl. Cancer Inst. 50, 235–242. [DOI] [PubMed] [Google Scholar]
- 23.Willenbring, H., Bailey, A. S., Foster, M., Akkari, Y., Dorrell, C., Olson, S., Finegold, M., Fleming, W. H. & Grompe, M. (2004) Nat. Med. 10, 744–748. [DOI] [PubMed] [Google Scholar]
- 24.Wang, X., Willenbring, H., Akkari, Y., Torimaru, Y., Foster, M., Al-Dhalimy, M., Lagasse, E., Finegold, M., Olson, S. & Grompe, M. (2003) Nature 422, 897–901. [DOI] [PubMed] [Google Scholar]
- 25.Newsome, P. N., Johannessen, I., Boyle, S., Dalakas, E., McAulay, K. A., Samuel, K., Rae, F., Forrester, L., Turner, M. L., Hayes, P. C., et al. (2003) Gastroenterology 124, 1891–1900. [DOI] [PubMed] [Google Scholar]
- 26.Ishikawa, F., Drake, C. J., Yang, S., Fleming, P., Minamiguchi, H., Visconti, R. P., Crosby, C. V., Argraves, W. S., Harada, M., Key, L. L., Jr., et al. (2003) Ann. N.Y. Acad. Sci. 996, 174–185. [DOI] [PubMed] [Google Scholar]
- 27.Fandrich, F., Lin, X., Chai, G. X., Schulze, M., Ganten, D., Bader, M., Holle, J., Huang, D. S., Parwaresch, R., Zavazava, N. & Binas, B. (2002) Nat. Med. 8, 171–178. [DOI] [PubMed] [Google Scholar]
- 28.Bumgardner, G. L., Chen, S., Almond, P. S., Bach, F. H., Ascher, N. L. & Matas, A. J. (1992) Transplantation 53, 863–868. [DOI] [PubMed] [Google Scholar]
- 29.Bumgardner, G. L., Gao, D., Li, J., Baskin, J. H., Heininger, M. & Orosz, C. G. (2000) Transplantation 70, 1771–1780. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.