Abstract
Rheumatoid arthritis is a chronic inflammatory disease characterized by destruction of cartilage and bone that is mediated by synovial fibroblasts. To determine the mechanisms by which these cells are activated to produce matrix metalloproteinases (MMPs), the effects of microparticles were investigated. Microparticles are small membrane-bound vesicles whose release from immune cells is increased during activation and apoptosis. Because microparticles occur abundantly in the synovial fluid in rheumatoid arthritis, they could represent novel stimulatory agents. Microparticles derived from T cells and monocytes strongly induced the synthesis of MMP-1, MMP-3, MMP-9, and MMP-13 in fibroblasts. The induction was time-dependent, with effects primarily observed after 36 h; under these conditions, MMP-2, MMP-14, and tissue inhibitor of MMP-1 (TIMP-1), TIMP-2, and TIMP-3 were not induced. Microparticles also increased the synthesis of inflammatory mediators including IL-6, IL-8, monocyte chemoattractant protein 1 (MCP-1), and MCP-2. In Iκ-B-transfected synovial fibroblasts, MMPs were less inducible by microparticles compared with wild-type fibroblasts. Blocking of TNFα and IL-1β with antibodies against TNFα and with IL-1 receptor antagonist did not abrogate stimulation by microparticles. These data provide evidence for a novel mechanism by which vesicles derived from activated or apoptotic immune cells can promote the destructive activity of synovial fibroblasts in rheumatoid arthritis.
Keywords: rheumatoid arthritis, arthritis, T cells, macrophages
Rheumatoid arthritis (RA) is a chronic inflammatory disease characterized by inflammation and destruction of articular structures in association with extraarticular manifestations. Joint destruction in RA results from the invasion of cartilage and subchondral bone by the hyperplastic synovium (1), with synovial fibroblasts and inflammatory cells such as macrophages and T cells playing key roles in this process (1, 2). An important feature of rheumatoid arthritis synovial fibroblasts (RASF) is an increased production of matrix metalloproteinases (MMPs), likely the most important matrix-degrading enzymes in RA (3). RASF also produce proinflammatory cytokines, which promote critical events in both inflammation and destruction (1). Although various cytokines can affect RASF, the detailed mechanisms leading to the invasive phenotype of RASF are still unknown.
Among molecular structures that could impact on RA pathogenesis, microparticles have recently attracted interest as novel signaling structures. Microparticles are small, membrane-bound structures that are released from cells by exocytic budding of the membrane during activation or apoptosis (4). During this process, the membrane asymmetry is lost, and phosphatidylserine, which is normally confined to the inner leaflet, appears on the outer leaflet of the microparticle membrane (5). In addition to altered surface lipids, microparticles display cell surface markers from the parental cell from which they originate. As such, they provide a surface in which regulatory and triggering molecules are arrayed with potentially enhanced activity (6).
As shown by flow cytometric analysis of blood and other fluids, microparticle numbers are increased during a variety of inflammatory conditions (6, 7), with high levels of leukocyte-derived microparticles found in synovial fluid of inflamed joints of patients with RA (7). Because microparticles can display immunological activity (8, 9), we have investigated the possibility that these structures stimulate RASF and promote synovitis. The findings presented herein indicate that microparticles from lymphocytes and monocytes potently induce MMP and cytokine expression by RASF and could represent a novel signaling structure to promote the invasive and destructive phenotype of these cells in RA.
Materials and Methods
Cell Culture and Patients. All RA patients fulfilled the American College of Rheumatology criteria for RA. Synovial fibroblasts from patients with RA and osteoarthritis (OASF) as well as dermal fibroblasts were obtained and prepared as described (10). Normal synovial fibroblasts were obtained from trauma patients. Fibroblasts from passages 4–6 were used for the experiments. The number of cells was determined by using the CASY-1 cell-counter (Schärfe System, Reutlingen, Germany). All experiments were performed with protocols approved by the local ethics committee.
Isolation of Primary T Cells and Monocytes. For the isolation of primary T cells and monocytes, buffy coats from healthy donors were obtained from the local blood bank. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll/Hypaque (Pharmacia Biotech) density gradient centrifugation. After incubation with anti-human CD3-FITC or CD14-FITC antibodies (Becton Dickinson), primary T cells or monocytes were isolated by positive selection by using a fluorescence-activated cell sorter (FACStar Plus, Becton Dickinson).
Stimulation of Microparticle Release by Apoptosis and Activation. For the induction of apoptosis, U937 and Jurkat cells were stimulated with Fas ligand (50 μg/ml, Alexis, Lausen, Switzerland), actinomycin-D (5 μg/ml), or staurosporine (10 μM, all Sigma).
The release of microparticles was also induced by stimulation of Jurkat cells with IL-2 (10 ng/ml, R & D Systems), Con A (1 μg/ml), and a combination of phorbol 12-myristate 13-acetate (10 ng/ml) and ionomycin (0.5 ng/ml) (all Sigma). U937 cells were treated with LPS at a final concentration of 10 μg/ml or with TNFα (1 nM, R & D Systems). After 12 h of treatment, the supernatants were collected, and the microparticles were isolated immediately. For coculture experiments, 1.0 × 105 subconfluent fibroblasts were incubated for 6 h and 36 h with freshly isolated microparticles from Jurkat cells, U937 cells, HL60 cells, or primary cells. Levels of LPS were assessed with the Limulus Amebocyte Lysate kit QCL-1000 (BioWhittaker) and found to be below the lower detection limit of the kit (<0.1 endotoxin units/ml).
Isolation of Microparticles by Differential Centrifugation. Cell culture supernatants were centrifuged at 1,500 × g for 5 min. Afterward, the cell-free supernatants were centrifuged at 100,000 × g for 20 min by using a Centrikon T-1065 centrifuge with a TST28.38 head (Kontron Instruments, Munich, Germany). The supernatant was removed, and the pellet was washed twice with 10 ml of apop buffer (5 mM KCl/1 mM MgCl2/136 mM NaCl, pH 7.4) and finally resuspended in apop buffer.
Electron Microscopy. Concentrated microparticle pellets were fixed in 2.5% glutaraldehyde overnight. Thereafter, specimens were washed twice in phosphate buffer, postfixed with 1% osmium tetroxide, and dehydrated in ethanol. Epon-polymerized microparticle pellets were processed by semithin sections. Ultrathin sections (80 nm) were performed by using a Reichert ultra-microtome. Uranyl acetate and lead citrate served to enhance the contrast of the sections. Finally, specimens were analyzed with a Philips 300 electron microscope.
Flow Cytometry Analysis (FACS). For characterization and quantification, freshly isolated microparticles were incubated with FITC-labeled anti-human CD3 antibodies or FITC-labeled anti-human CD14 antibodies. Double staining was performed with phycoerythrin (PE)-labeled annexin V (all antibodies from Becton Dickinson). Unbound antibodies and annexin V were removed by two washing steps. Staining with isotype antibodies and annexin V in the absence of calcium was used as controls. The number of microparticles was determined by measuring 1 min at the “hi-flow” modus.
Real-Time PCR. Total RNA was isolated and converted into cDNA, and gene expression was quantified either by SYBR Green or TaqMan real-time PCR as described (11). Sequences of primers and probes are given in Tables 1 and 2, which are published as supporting information on the PNAS web site.
ELISA. ELISAs for IL-6 and IL-8 were performed by using DuoSet kits (R & D Systems). Data were analyzed by using revelation 4.22 software (Dynex Technologies, Denkendorf, Germany).
MMP Biotrak Activity Assays. The active forms of MMP-1 and MMP-3 were quantified with the MMP Biotrak Activity Assays according to the manufacturer's recommendations (Amersham Pharmacia Bioscience). The optical density was measured after 0 and 4 h with the MRX microplate reader (Dynex Technologies).
Effects of Antibodies Against TNFα and IL-1 Receptor Antagonist. The roles of TNFα and IL-1 in the induction of MMPs by microparticles were analyzed with neutralizing goat anti-human TNFα antibodies (5 μg/ml) (Becton Dickinson) or recombinant IL-1 receptor antagonist (IL-1Ra, kindly provided by Charles Dinarello, University of Colorado Health Sciences Center, Denver, CO) (12) (10 μg/ml). Synovial fibroblasts incubated only with apop buffer or protein controls and fibroblasts stimulated with microparticles in the absence of TNFα antibodies and IL-1Ra were used as controls. For positive control, synovial fibroblasts were stimulated with TNFα (1 or 10 ng/ml), IL-1α (5 ng/ml), or IL-1β (5 ng/ml). To demonstrate the ability of these antagonists to block TNF and IL-1 signaling, recombinant TNFα, IL-1α, or IL-1β was preincubated with TNFα antibodies or IL-1Ra for 1 h and then added to the synovial fibroblasts.
Transfection with pCMV-IκBαM. Before stimulation with microparticles, 2 × 105 cells were transfected with IκBαM pCMV vector DNA (Becton Dickinson) or empty vector for control (Becton Dickinson) by using the nucleofection system (Amaxa, Cologne, Germany) as described (13).
EMSA. Cells were collected by scratching in ice-cold PBS 10 min and 60 min after addition of the microparticles. Nuclear extracts were isolated and EMSA was performed as described (13).
Statistical Analysis. All data are expressed as mean ± standard deviation. The Wilcoxon signed-rank test for related samples and the Mann–Whitney test for nonrelated samples were used for statistical analyses. P values <0.05 were considered significant.
Results
The Release of Microparticles from T Cells and Macrophages During Apoptosis and Activation. The release of microparticles was first examined in T cells and macrophages in both the basal state and after treatments to induce apoptosis or activation. FACS analysis showed that 1 × 106 Jurkat cells released 1.1 × 104 ± 0.1 × 104 microparticles constitutively (Fig. 5a, which is published as supporting information on the PNAS web site). The number of microparticles released from Jurkat cells was significantly increased up to 3.5 × 104 ± 0.3 × 104 microparticles after stimulation with phorbol 12-myristate 13-acetate/ionomycin (Fig. 5a). Like Jurkat cells, primary T cells, as well as U937 monocytes and HL60 cells, released increased numbers of microparticles upon stimulation (data not shown).
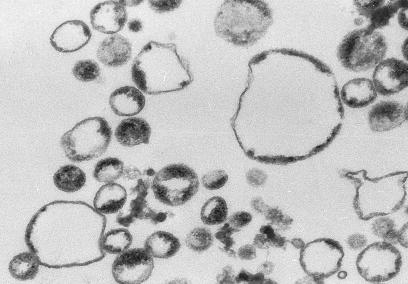
Morphological Analysis of Microparticles. Ultrastructural analysis of microparticles isolated from Jurkat cells showed numerous membrane-coated round vesicles (Fig. 1). The size of the vesicles ranged from 200–700 nm. The membranes of the microparticles showed the typical features of plasma membranes with intact bilayers in the large majority of vesicles.
Fig. 1.
Ultrastructural analysis of microparticles. Analysis of microparticles from Jurkat cells by transmission electron microscopy with an 80,000-fold magnification showed membrane-coated circular vesicles ranging in size from 200 to 700 nm.
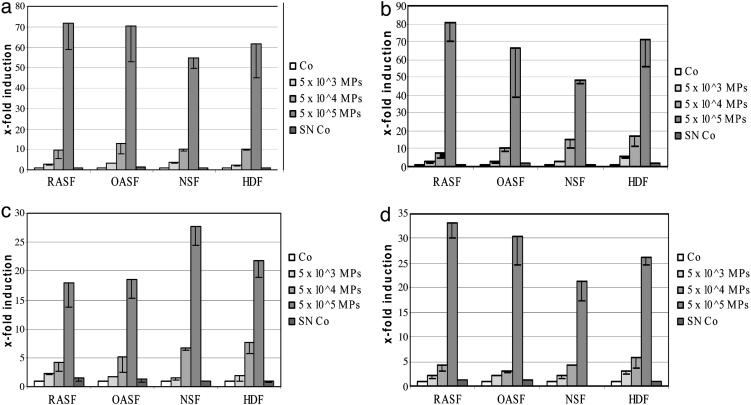
The Effects of Microparticles from Apoptotic and Activated Jurkat Cells on the Induction of MMP mRNA in Synovial Fibroblasts. A strong induction of MMP-1 mRNA was observed in RASF after stimulation with microparticles derived from staurosporine-stimulated Jurkat cells. As compared with buffer controls, incubation with microparticles increased the levels of MMP-1 mRNA up to 71.5 ± 12.5-fold (Fig. 2a) with the strongest effects seen with doses of 5.0 × 104 and 5.0 × 105 microparticles, whereas smaller effects were seen with lower doses. Similar results were obtained with microparticles from Jurkat cells, which were treated with other stimuli for apoptosis, including TNFα, Fas ligand, and actinomycin-D.
Fig. 2.
Up-regulation of MMP-1 (a), MMP-3 (b), MMP-9 (c), and MMP-13 (d) mRNA in fibroblasts by microparticles from Jurkat cells. Cultured RASF, OASF, NSF, and HDFs were stimulated with increasing amounts of microparticles for 36 h. A strong induction of MMP-1, MMP-3, MMP-9, and MMP-13 was observed in all types of fibroblasts. Co, control; SN Co, supernatant control (see The Effects of Microparticles from Apoptotic and Activated Jurkat Cells on the Induction of MMP mRNA in Synovial Fibroblasts).
As an additional control, supernatants of the first ultracentrifugation step were processed in the same way as the microparticle-pellets with two washing steps. Thus, although not containing microparticles, these control supernatants had the same concentration of soluble mediators as the microparticle suspensions. However, no induction of MMP-1 was observed with these controls (SN-Co in Fig. 2a).
The effects of microparticles from activated Jurkat cells on RASF were also investigated. Similar to the results with microparticles from apoptotic cells, an induction of MMP-1 mRNA of 55.2 ± 10.2-fold was observed after incubation with 5.0 × 105 microparticles from Jurkat cells activated with Con A. MMP-3, MMP-9, and MMP-13 mRNAs were also strongly up-regulated by microparticles from both apoptotic and activated Jurkat cells (Fig. 2 b–d). With 5.0 × 105 microparticles from staurosporine-treated Jurkat cells, inductions of 80.3 ± 10.0-fold for MMP-3, 17.9 ± 4.2-fold for MMP-9, and 36.8 ± 2.4-fold for MMP-13 were detected (Figs. 2 b–d). The same number of microparticles derived from Con A-stimulated Jurkat cells up-regulated MMP-3 by 52.1 ± 7.6-fold, MMP-9 by 24.8 ± 3.6-fold, and MMP-13 by 24.1 ± 3.5-fold.
Of note, the induction was selective for the aforementioned MMPs, because no induction was seen for other matrix-degrading enzymes including MMP-2, MMP-14, and cathepsin K. Likewise, microparticles failed to activate the expression of tissue inhibitor of MMP-1 (TIMP-1), TIMP-2, and TIMP-3 (data not shown). Furthermore, the effects of microparticles on the increase of MMPs were independent of the origin of the fibroblasts. When OASF, NSF, and healthy dermal fibroblasts (HDF) were stimulated with microparticles from apoptotic or activated Jurkat cells, MMP-1, MMP-3, MMP-9, and MMP-13 were induced in the same range as found for RASF (Fig. 2). However, the induction of MMPs seems to be specific for fibroblasts, because no significant effects were observed with MCF-7 and BT 549 breast cancer cells.
The Effect of Microparticles on Levels of Active MMP Proteins. To measure the biologically active form of MMP proteins, MMP Biotrak Activity Assays were used. Consistent with the findings on the mRNA levels, strong increases of active MMP-1 and MMP-3 protein were observed (Fig. 6, which is published as supporting information on the PNAS web site).
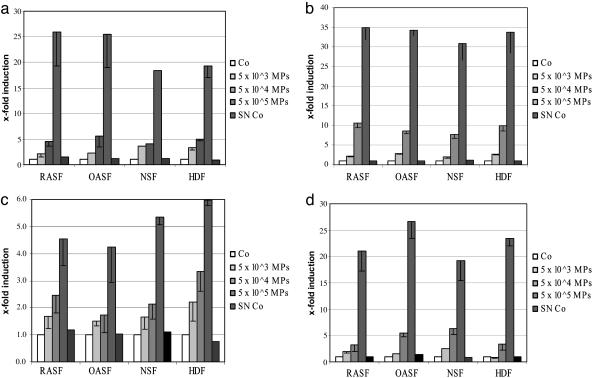
Stimulation of Cytokines by Microparticles. Microparticles derived from Jurkat cells stimulated with Con A dose-dependently induced the synthesis of IL-6, IL-8, monocyte chemoattractant protein (MCP)-1, and MCP-2 (Fig. 3). Microparticles from apoptotic Jurkat cells treated with staurosporine, TNFα, Fas ligand, or actinomycin-D induced IL-6, IL-8, MCP-1, and MCP-2 in RASF to a similar extent. Consistent with the findings for MMPs, there were no differences between RASF, OASF, NSF, and dermal fibroblasts (Figs. 3).
Fig. 3.
Induction of IL-6 (a), IL-8 (b), MCP-1 (c), and MCP-2 (d) by microparticles derived from Jurkat cells. These proinflammatory cytokines were up-regulated by microparticles. Comparable results were obtained with RASF, OASF, NSF, and NDF. Co, control; SN Co, supernatant control.
Stimulation of MMPs and Cytokines by Monocyte-Derived Microparticles. Because monocytes are a major component of the synovium, we tested whether monocyte microparticles can activate synovial fibroblasts. The induction of MMPs in fibroblasts was also observed with microparticles derived from the U937 monocytic cell line. Similar to results with T cell-derived microparticles, the induction of MMP-1 reached a 44.5 ± 6.4-fold induction (Fig. 7a, which is published as supporting information on the PNAS web site). Microparticles from U937 monocytes also strongly induced MMP-3, MMP-9, and MMP-13 (Fig. 7 b–d), whereas the levels of TIMP-1, TIMP-2, and TIMP-3 remained unchanged.
Like T cell microparticles, microparticles derived from U937 monocytes up-regulated IL-6, IL-8, MCP-1, and MCP-2 (Fig. 7 e–h). As observed with microparticles derived from Jurkat cells, similar results were obtained when OASF, NSF, and dermal fibroblasts were incubated with U937 microparticles. The induction of cytokines by microparticles from stimulated monocytes in synovial fibroblasts was confirmed on the protein level by ELISA (data not shown).
Stimulation of MMPs and Cytokines by Microparticles from Primary CD3- and CD14-Positive Cells. Microparticles from primary T cells and monocytes isolated from peripheral blood induced MMP-1, MMP-3, MMP-9 and MMP-13 was observed. IL-6, IL-8, MCP-1, and MCP-2 were also up-regulated by microparticles from primary cells (Fig. 8, which is published as supporting information on the PNAS web site).
Time Dependence of Stimulation of Cytokines and MMPs with Microparticles. After cocultivation for 6 h, there was only a weak increase of MMPs in synovial fibroblasts compared with the inductions observed after 36 h (Fig. 9a, which is published as supporting information on the PNAS web site). Similar results were obtained for MMP-3, MMP-9, and MMP-13. In contrast, the induction of cytokines after 6 h was comparable with that after 36 h (Fig. 9b).
The Effects of Antibodies Against TNFα and IL-1 Receptor Antagonist on the Activation of Fibroblasts by Microparticles. Neutralizing anti-TNFα did not reduce the induction of MMPs by microparticles. These antibodies abrogated completely the stimulatory effects of recombinant TNFα on MMPs in synovial fibroblasts, however, confirming their ability to block TNF signaling in our experimental setting.
In coincubation experiments of fibroblasts with microparticles and IL-1Ra, we observed a lower induction of MMP-1 than with microparticles alone (Fig. 10a, which is published as supporting information on the PNAS web site). The mean induction in the presence of IL-1Ra was 55.4 ± 7.3-fold compared with 82.7 ± 12.3-fold (mean reduction of 33%). This reduction could be explained by an effect on endogenous IL-1, produced by cultured RA synovial fibroblasts, on the synthesis of MMP-1. When IL-1Ra was added to cultured fibroblasts in the absence of any recombinant cytokines or microparticles, the production of MMP-1 was decreased by 39.3 ± 12.2% compared with unstimulated samples. Similar results were obtained for other MMPs and for inflammatory cytokines such as IL-6 (Fig. 10b).
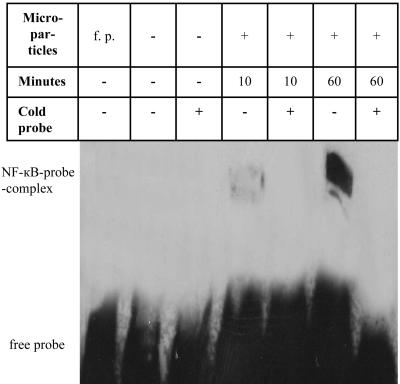
The Role of NF-κB-Dependent Pathways in the Induction of MMPs in Synovial Fibroblasts by Microparticles. The role of NF-κB in the induction of MMPs by microparticles was analyzed by EMSA (Fig. 4). No signal for the NF-κB/probe complex was detectable in unstimulated RASF after serum starvation. Sixty minutes after addition of the microparticles, a strong signal for the NF-κB/probe complex was observed in nuclear extracts from RASF. The specificity of the strong shift for NF-κB after stimulation was confirmed by the addition of an excess of unlabeled probe (cold probe), which inhibited completely the formation of the NF-κB/probe complex (Fig. 4).
Fig. 4.
EMSA demonstrates the activation of NF-κB in RASF after stimulation with microparticles. Lane 1, free probe without nuclear extracts; lane 2, nuclear extract from unstimulated RASF, with NF-κB probe; lane 3, nuclear extract from unstimulated RASF, with NF-κB probe and cold NF-κB probe; lane 4, nuclear extract from RASF stimulated with microparticles for 10 min, with NF-κB probe; lane 5, nuclear extract from RASF stimulated with microparticles for 10 min, with NF-κB probe and cold NF-κB probe; lane 6, nuclear extract from RASF stimulated with microparticles for 60 min, with NF-κB probe; lane 7, nuclear extract from RASF stimulated with microparticles for 60 min, with NF-κB probe and cold NF-κB probe. Signals for NF-κB–DNA complexes in lane 4 and especially lane 6 confirm an activation of the transcription factor NF-κB. No activation of NF-κB signaling was observed in nonstimulated, serum-starved fibroblasts (lanes 2 and 3). The signal of the NF-κB–DNA complexes disappeared completely after adding an excess of unlabeled cold NF-κB probe (lanes 5 and 7).
Because the NF-κB/probe complex detected by EMSA could be either repressive p50/p50 homodimers or stimulatory p50/p65 heterodimers, we next performed functional experiments by transfecting synovial fibroblasts with an IκBαM pCMV expression vector. As assessed by transfection with pEGFP-C1, the transfection efficiency of synovial fibroblasts with the nucleofection protocol was 64 ± 6%. In IκBαM-transfected fibroblasts, the induction of MMP-1 was 64.1 ± 20.3% lower compared with mock-transfected fibroblasts (Fig. 11, which is published as supporting information on the PNAS web site). The levels of MMP-3, MMP-9, and MMP-13 were also significantly reduced in IκBαM-transfected fibroblasts (Fig. 11). These results suggest that NF-κB accumulates in the nucleus of fibroblasts stimulated with microparticles and activates the transcription of MMP genes.
Discussion
The results of these studies demonstrate a pathway by which immune cell activation or death can modulate production of MMPs by RASF and promote the destruction of cartilage and bone in RA. MMPs are secreted in high levels by RASF and are thought to be key players in this process (2). Although almost all members of the MMP family have been detected in the RA synovium, MMP-1, MMP-3, and MMP-13 seem to be the most important for joint destruction (3, 14). In studies on RA and other fibroblast types presented herein, we show an induction of MMPs by T cell and monocyte microparticles, with massive up-regulation as high as 80-fold compared with controls. Importantly, the induced MMP proteins were biologically active as indicated by assays of enzymatic activity. In view of the dramatic up-regulation of MMP expression induced by the microparticles, these results suggest that microparticles released from immune cells could represent an important signaling element to trigger the destructive phenotype of RASF.
Immune cells, including T cells and macrophages, occur abundantly in synovium and synovial fluid of patients with RA and therefore could serve as a source of microparticles, whether induced by apoptosis or activation. In view of the abundance of these cells and their capacity to release microparticles, microparticles could serve as important triggering elements in RA to promote RASF activation. Evidence supporting this hypothesis comes from observations that activated T cells and macrophages release microparticles and that these microparticles induce the release of cytokines and chemokines, namely IL-6, IL-8, MCP-1, and MCP-2, from synovial fibroblasts. Importantly, these cytokines, which have all been linked to RA, have been demonstrated to attract leukocytes, thereby increasing the number of inflammatory cells in the joint (15, 16). Furthermore, results from the inhibition studies indicate that the effects of microparticles on synovial fibroblasts are independent of TNFα and IL-1 and may thus not be targeted by therapies directed against these cytokines.
To assess the pathways by which microparticles can activate RASF, we explored the role of NF-κB. NF-κB is a key regulator of proinflammatory mediator expression and plays a critical role in the induction of MMPs (17). NF-κB is also overexpressed in the inflamed synovium, especially in the intimal lining layer of RA patients, where it colocalizes with the expression of MMPs. Our results with the IκB expression vector and EMSA suggest that NF-κB also plays an important role in the induction of MMPs by microparticles in synovial fibroblasts. Activation of NF-κB is also consistent with the pattern of cytokine induction observed.
As shown by this and other studies (8, 9), microparticles may amplify as well as induce pathways of activation and destruction, occupying a place between direct cell–cell interaction and triggering by protein and small molecule mediators. Extensive work has been done during the past years on the identification and characterization of soluble mediators such as proinflammatory cytokines in rheumatoid arthritis (18). Similar to microparticles, resting T cells induce stromelysin, IL-6, and IL-8 in coculture with synovial fibroblasts (19), and plasma membrane preparations from stimulated T cells can induce the production of MMP-1 in synovial fibroblasts (20). The factors involved in these cell contact-mediated activation of synovial fibroblasts are not well defined (21), but membrane-bound surface IL-15 on synovial fibroblasts seems to be crucial for the initiation of the synovial fibroblast-T lymphocyte cross-talk by up-regulating CD69, CD25, IL-17, and TNFα in T cells that in turn induces secretion of IL-6 and IL-8 in synovial fibroblasts (22). However, in contrast to microparticles, this concept requires an intact T cell metabolism. Moreover, the cell contact-mediated effects are strongly dependent on cell membrane-associated IL-1α and TNFα (20). Other differences between microparticles and membrane preparations include for instance the morphology of microparticles as circular round vesicles. Because microparticles occur in blood, they could act at distant sites as well as locally. Notably, microparticles may also function as a transporter system for surface receptors, rendering the target cells susceptible for new, receptor-specific stimuli (23).
The specific factors that mediate the strong effects of microparticles on synovial fibroblasts are at this point speculative. The lack of effects in breast cancer tumor cells indicates that specific features of the target cells play an important role. Microparticles display surface molecules from their parental cell that could bind to mesenchymal cells and trigger activation. In addition, microparticle contents such as histones and DNA could contribute to stimulation, with the outcome depending on the cell of origin and the specific content of both internal and external molecules. In this regard, microparticles derived from T cells as well as macrophages, two key cell types in RA, were both active, although microparticles from other cell types may differ in properties.
In conclusion, our study provides evidence for a mechanism for the interaction of inflammatory cells and synovial fibroblasts in RA, with fragments released from T cells and monocytes inducing expression of MMPs and proinflammatory cytokines. Because leukocyte numbers and activation increase as synovitis progresses in RA, T cells and macrophages may release even more microparticles over time, triggering a vicious cycle of self-amplifying inflammation and destruction of cartilage and bone. Blocking this step in pathogenesis could therefore involve agents that modify the release of microparticles and their interaction with cells, as well as downstream activation events.
Supplementary Material
Acknowledgments
We thank the Central Laboratory for Cell Sorting, Institute of Biomedical Engineering, Eidgenössische Technische Hochschule, and University of Zurich, Switzerland, for the fluorescence-activated cell sorter service and Ferenc Pataky and Eva Niederer for excellent technical support.
Abbreviations: RA, rheumatoid arthritis; RASF, RA synovial fibroblast; MMP, matrix metalloproteinase; OASF, synovial fibroblasts from patients with RA and osteoarthritis; TIMP, tissue inhibitor of MMP; MCP, monocyte chemoattractant protein; NSF, normal synovial fibroblast; HDF, healthy dermal fibroblast.
References
- 1.Firestein, G. S. (2003) Nature 423, 356–361. [DOI] [PubMed] [Google Scholar]
- 2.Pap, T., Muller-Ladner, U., Gay, R. E. & Gay, S. (2000) Arthritis Res. 2, 361–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vincenti, M. P. & Brinckerhoff, C. E. (2002) Arthritis Res. 4, 157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Combes, V., Simon, A. C., Grau, G. E., Arnoux, D., Camoin, L., Sabatier, F., Mutin, M., Sanmarco, M., Sampol, J. & Dignat-George, F. (1999) J. Clin. Invest. 104, 93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zwaal, R. F. & Schroit, A. J. (1997) Blood 89, 1121–1132. [PubMed] [Google Scholar]
- 6.VanWijk, M. J., VanBavel, E., Sturk, A. & Nieuwland, R. (2003) Cardiovasc. Res. 59, 277–287. [DOI] [PubMed] [Google Scholar]
- 7.Berckmans, R. J., Nieuwland, R., Tak, P. P., Boing, A. N., Romijn, F. P., Kraan, M. C., Breedveld, F. C., Hack, C. E. & Sturk A. (2002) Arthritis Rheum. 46, 2857–2866. [DOI] [PubMed] [Google Scholar]
- 8.Mesri, M. & Altieri, D. C. (1998) J. Immunol. 161, 4382–4387. [PubMed] [Google Scholar]
- 9.Barry, O. P., Pratico, D., Savani, R. C. & FitzGerald, G. A. (1998) J. Clin. Invest. 102, 136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Distler, O., Distler, J. H., Scheid, A., Acker, T., Hirth, A., Rethage, J., Michel, B. A., Gay, R. E., Muller-Ladner, U., Matucci-Cerinic M., et al. (2004) Circ. Res. 95, 109–116. [DOI] [PubMed] [Google Scholar]
- 11.Jungel, A., Distler, J. H., Kurowska-Stolarska, M., Seemayer, C. A., Seibl, R., Forster, A., Michel, B. A., Gay, R. E., Emmrich, F., Gay, S., et al. (2004) Arthritis Rheum. 50, 1468–1476. [DOI] [PubMed] [Google Scholar]
- 12.Kaplanski, G., Farnarier, C., Kaplanski, S., Porat, R., Shapiro, L., Bongrand, P. & Dinarello, C. A. (1994) Blood 84, 4242–4248. [PubMed] [Google Scholar]
- 13.Distler, J. H., Hagen, C., Hirth, A., Muller-Ladner, U., Lorenz, H. M., del Rosso, A., Michel, B. A., Gay, R. E., Nanagara, R., Nishioka, K., et al. (2004) Mol. Pharmacol. 65, 389–399. [DOI] [PubMed] [Google Scholar]
- 14.Westhoff, C. S., Freudiger, D., Petrow, P., Seyfert, C., Zacher, J., Kriegsmann, J., Pap, T., Gay, S., Stiehl, P., Gromnica-Ihle, E., et al. (1999) Arthritis Rheum. 42, 1517–1527. [DOI] [PubMed] [Google Scholar]
- 15.Pierer, M., Rethage, J., Seibl, R., Lauener, R., Brentano, F., Wagner, U., Hantzschel, H., Michel, B. A., Gay, R. E., Gay, S., et al. (2004) J. Immunol. 172, 1256–1265. [DOI] [PubMed] [Google Scholar]
- 16.Firestein, G. S., Alvaro-Gracia, J. M., Maki, R. & Alvaro-Garcia, J. M. (1990) J. Immunol. 144, 3347–3353. [PubMed] [Google Scholar]
- 17.Tak, P. P. & Firestein, G. S. (2001) J. Clin. Invest. 107, 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miossec, P. (2000) Curr. Opin. Rheumatol. 12, 181–185. [DOI] [PubMed] [Google Scholar]
- 19.Yamamura, Y., Gupta, R., Morita, Y., He, X., Pai, R., Endres, J., Freiberg, A., Chung, K. & Fox, D. A. (2001) J. Immunol. 166, 2270–2275. [DOI] [PubMed] [Google Scholar]
- 20.Burger, D., Rezzonico, R., Li, J. M., Modoux, C., Pierce, R. A., Welgus, H. G. & Dayer, J. M. (1998) Arthritis Rheum. 41, 1748–1759. [DOI] [PubMed] [Google Scholar]
- 21.McInnes, I. B., Leung, B. P. & Liew, F. Y. (2000) Arthritis Res. 2, 374–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miranda-Carus, M. E., Balsa, A., Benito-Miguel, M., Perez de Ayala, C. & Martin-Mola, E. (2004) J. Immunol. 173, 1463–1476. [DOI] [PubMed] [Google Scholar]
- 23.Mack, M., Kleinschmidt, A., Bruhl, H., Klier, C., Nelson, P. J., Cihak, J., Plachy, J., Stangassinger, M., Erfle, V. & Schlondorff, D. (2000) Nat. Med. 6, 769–775. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.