Abstract
Exposure of the heart to ionizing radiation can cause adverse myocardial remodeling. In small animal models, local heart irradiation causes persistent alterations in cardiac mitochondrial function and swelling. POLY-MVA is a dietary supplement that contains a palladium lipoic acid complex that targets mitochondrial complex I and has been demonstrated to have greater redox potential than lipoic acid alone. POLY-MVA improves mitochondrial function and anti-oxidant enzyme activity in the aged rat heart. In this study, we tested whether POLY-MVA can mitigate cardiac effects of ionizing radiation. Adult male rats were exposed to local heart X rays with a daily dose of 9 Gy for 5 consecutive days. Eighteen weeks after irradiation, POLY-MVA was administered orally at 1 ml/kg bodyweight per day during weekdays, for 6 weeks. Alterations in cardiac function as measured with echocardiography coincided with enhanced mitochondrial swelling, a reduction in mitochondrial expression of complex II, manifestations of adverse remodeling such as a reduction in myocardial microvessel density and an increase in collagen deposition and mast cell numbers. POLY-MVA enhanced left ventricular expression of superoxide dismutase 2, but only in sham-irradiated animals. In irradiated animals, POLY-MVA caused a reduction in markers of inflammatory infiltration, CD2 and CD68. Moreover, POLY-MVA mitigated the effects of radiation on mitochondria. Nonetheless, POLY-MVA did not mitigate adverse cardiac remodeling, suggesting that this tissue remodeling may not be alleviated by altering cardiac mitochondria alone. However, we cannot exclude the possibility that an earlier onset of POLY-MVA administration may have more profound effects on radiation-induced cardiac remodeling.
INTRODUCTION
POLY-MVA is a liquid formula for oral administration that contains a palladium lipoic acid (PdLA) complex together with minerals (molybdenum, rhodium, ruthenium), vitamins (B1, B2 and B12) and amino acids (N-acetyl cysteine and formyl methionine). Using the electrochemistry technique of electronic impedance, one may demonstrate that the redox potential of the PdLA complex is significantly greater than a lipoic acid single molecule. PdLA targets complex I of the mitochondria, where traditionally lipoic acid and thiamine act as cofactors in the oxidation of pyruvate to acetyl co-enzyme A (1). When administered before whole-body irradiation in rodent models, POLY-MVA improved survival, reduced oxidative stress, enhanced spleen colony counts, enhanced DNA repair and improved erythrocyte membrane parameters, indicating POLY-MVA’s potential as a radiation protector (2–5).
Radiation-induced heart disease (RIHD) is a late and potentially severe side effect of radiation therapy, when all or part of the heart was situated in the radiation field (6, 7). Rodent models are commonly used to examine biological effects of local heart irradiation. In mice and rats, late manifestations of RIHD such as myocardial degeneration and fibrosis become apparent at about 6 months after local heart irradiation (8–10).
Studies in organ systems other than the heart have shown a clear contribution of mitochondria to normal tissue radiation injury (11, 12). Radiation-induced heart injury in rodent models has been shown to be associated with mitochondrial dysfunction (13, 14). Moreover, we have previously observed a reduction in mitochondrial and left ventricular tissue content of the mitochondrial complex II component, succinate dehydrogenase complex subunit A after local heart irradiation in a rat model (15). Nonetheless, the exact role of mitochondria in RIHD is still unknown. In addition to its radiation protective properties, POLY-MVA has previously been shown to enhance mitochondrial function and antioxidant enzyme activity in the aging rat heart (16, 17). In these studies, POLY-MVA administration enhanced the activities of mitochondrial complexes I, III and IV and the activity of superoxide dismutase 2 (SOD2), catalase and glutathione peroxidase (GPX). Therefore, we used a rat model of local heart irradiation to test the effects of POLY-MVA, when administered during the late phase of RIHD, on radiation-induced mitochondrial alterations, cardiac function and tissue injury.
METHODS
Rat Model of Local Heart Irradiation
All procedures in this study were approved by the Institutional Animal Care and Use Committee of the University of Arkansas for Medical Sciences. Male Sprague-Dawley rats were obtained from Charles River Laboratories and maintained in our Division of Laboratory Animal Medicine on a 12:12 light-to-dark cycle with free access to food and water. At a weight of 250–290 g (about 9 weeks of age), rats received local heart irradiation with the Small Animal Conformal Radiation Therapy Device (SACRTD) developed at our institution.
The SACRTD consists of a 225 kVp X-ray source (GE Isovolt Titan 225, GE Sensing and Inspection Technologies) mounted on a custom-made “gantry”, a stage mounted on a robotic arm for positioning (Viper™ s650 Adept Technology) and a digital X-ray detector (XRD 0820 CM3 Perkin Elmer). A brass and aluminum collimating assembly attached to the X-ray tube produced a field of 19 mm diameter at the isocenter (18).
Dosimetry was performed as described elsewhere (19). In short, the dose rate at the isocenter was measured using a pin-point ion chamber (PTW N301013, PTW; ADCL calibrated for 225 kV) following the TG-61 protocol of the American Association of Physicists in Medicine (20). In addition, dosimetry was performed with Gafchromic® EBT-2 films (Ashland Specialty Ingredients) that were calibrated with a Gamma Knife (Co-60) system (Elekta AB) and analyzed as described elsewhere (21). To measure relative depth dose, 11 pieces of film were placed in between 11 slabs of solid water phantom each 5 mm thick. The film on the top of the phantom was kept at the isocenter, normal to the beam direction and exposed to 5 Gy (225 kV, 13 mA).
For local heart irradiation, rats were anesthetized with 3% isoflurane and placed vertically in a cylindrical Plexiglas holder that was cut out such that no Plexiglas material was in between the radiation beam and the chest. The heart was exposed in three 19 mm-diameter fields (anterior-posterior and two lateral fields), given immediately after each other to a total dose of 9 Gy (225 kV, 13 mA, 0.5 mm-copper filtration, resulting in 1.92 Gy/min at 1 cm tissue depth). Before each exposure, the location of the heart was verified with the X-ray detector (70 kV, 5 mA, <1 cGy) and, if necessary, the position of the rat was adjusted with the use of the robotic arm to place the heart in the middle of the radiation field. This procedure was repeated for 5 consecutive days, to obtain a 5 × 9 Gy local heart irradiation.
POLY-MVA Administration
POLY-MVA was provided by Garnett McKeen Laboratory, Bohemia NY. POLY-MVA components are listed in Supplemental Table S1 (http://dx.doi.10.1667/RR14643.1.S1). Based on previous aging and radiation studies, a daily dose of 1 ml/kg bodyweight was selected, administered by oral gavage Monday–Friday for 6 consecutive weeks, starting at 18 weeks postirradiation. The experimental groups were: sham irradiation (5 × 0 Gy) + vehicle (tap water); irradiation (5 × 9 Gy) + vehicle; sham irradiation + POLY-MVA; irradiation + POLY-MVA. Each group contained 8–9 rats.
Echocardiography
Cardiac function was assessed with echocardiography at 18 weeks postirradiation, immediately before the start of POLY-MVA administration. Then, POLY-MVA administration was started, at a daily dose of 1 ml/kg bodyweight Monday–Friday for 6 weeks. Echocardiography was performed again at the end of the study, 24 weeks postirradiation.
A Vevo 2100 high-resolution in vivo micro imaging system (VisualSonics, Toronto, Canada) with the MS250 MicroScan transducer (13–24 MHz) was used for echocardiography. Animals were anesthetized with 2% isoflurane and all hair was removed from the chest with clippers followed by a lotion hair remover. Parasternal short axis M-mode recordings at the mid-left ventricular level were used to obtain parameters of cardiac function and wall thicknesses, using the Vevo 2100 cardiac analysis software package.
Tissue Collection
At 24 weeks postirradiation, rats were anesthetized with 3% isoflurane inhalation. The heart was collected and immediately processed. After a longitudinal cut, one half was placed in 5% formalin for 24 h. The other half was dissected into atria, right ventricle and left ventricle. Five hearts in each experimental group were used to determine mitochondrial alterations. For this purpose, a portion of the left ventricle was immediately processed to isolate mitochondria, as described below. The remaining left ventricular tissue was divided into smaller specimens, snap-frozen and stored at –80°C.
Mitochondrial Isolation and Swelling Assay
Specimens of left ventricular tissue (180–200 mg) were minced and homogenized in 10 mL of a 10 mM HEPES pH 7.4 containing 225 mM mannitol, 75 mM sucrose and 0.1 mM EGTA, using a mechanical Potter-Elvehjem homogenizer (Lab-Stirrer LR400C, Yamato, 30 strokes at 100 rpm and 15 strokes at 125 rpm) with a Teflon pestle. The homogenate was divided into 5 microcentrifuge tubes and centrifuged at 700g for 10 min at 4°C. The supernatant was removed and centrifuged at 12,500g for 30 min to obtain the mitochondrial pellet. Part of the pellet was snap-frozen and stored at −80°C for subsequent Western blot analyses. Part of the pellet was immediately resuspended in a 10 mM HEPES pH 7.4 containing 395 mM sucrose and 0.1 mM EGTA for the mitochondrial swelling assay.
The tendency of mitochondrial transition permeability pores (mPTP) to open was assessed by measuring calcium-induced swelling of isolated mitochondria. Isolated mitochondria were resuspended in swelling buffer pH 7.4 containing 120 mM KCl, 10 mM Tris HCl and 5 mM KH2PO4 to a final concentration of 150 μg/mL, and immediately exposed to vehicle, 250 μM CaCl2 or 250 μM CaCl2 in combination with 2 μM cyclosporin A (CsA) as an inhibitor of mPTP opening. Optical density at 540 nm (OD540) was measured with a Synergy 4 microplate reader (BioTek), immediately before the assay and every 2 min thereafter for a total of 20 min.
Histology
Formalin-fixed heart tissue was embedded in paraffin and 5 μm sections were prepared. For determination of collagen deposition, sections were incubated in Picrosirius red stain (American MasterTech, Lodi, CA) supplemented with Fast Green (0.01% w/v, Fisher Scientific, Pittsburgh, PA) for 2 h. Sections were scanned with a ScanScope CS2 slide scanner and analyzed with ImageScope 12 software (Leica Biosystems). The relative area of collagens was calculated as the Picrosirius red-stained area divided by the total tissue area of each section.
For determination of mast cell numbers, sections were incubated in 0.5% Toluidine Blue in 0.5 N HCl for 3 days at room temperature, followed by 0.7 N HCl for 10 min. Eosin was used as a counterstain. Stained sections were examined with an Axioskop transmitted light microscope (Carl Zeiss), and mast cells were counted.
A staining of the endothelium was used to determine microvascular density. Sections were deparaffinated and rehydrated, and antigen retrieval was performed by treating the sections with 10 mM sodium citrate (pH 6.0) at 95°C for 20 min. Sections were allowed to cool down prior to incubation in 1% H2O2 in methanol for 30 min to quench the endogenous peroxidase activity. Subsequently, sections were incubated with biotinylated Lycopersicon Esculentum (tomato) lectin (1:200, Vector Laboratories) for 90 min, followed by avidin-biotin-peroxidase complex (Vector Laboratories) for 45 min and 0.5 mg/ml DAB (Sigma-Aldrich) 0.003% H2O2 for 5 min. After counter staining with hematoxylin and mounting, sections were scanned with a ScanScope CS2 slide scanner and analyzed with ImageScope 12 software, using an optimized algorithm for vascular density measurement.
Western Blots
Left ventricular tissue was homogenized in radioimmunoprecipitation assay (RIPA) buffer with freshly added inhibitor cocktails of proteases (10 μL/mL) and phosphatases (10 μL/mL, both Sigma Aldrich), centrifuged at 20,000g at 4°C for 15 min and the supernatant was collected.
Mitochondria pellets, obtained as described above, were lysed with RIPA buffer containing protease and phosphatase inhibitors for 30 min with intermittent vortexing. The lysates were centrifuged at 14,000 rpm for 10 min and the supernatants were collected.
Protein concentrations of the lysates were determined with a BCA protein assay (Pierce). A total of 30 μg protein was prepared in Laemmli sample buffer containing β-mercaptoethanol (1:20 vol/vol) and boiled for 2–3 min.
Protein samples were separated in 4–20% gradient polyacrylamide gels (Bio-Rad) at 100 Volts and transferred to PVDF membranes at 20 Volts overnight at 4°C. Nonspecific antibody binding was reduced by TBS containing 0.05% Tween-20 and 5% nonfat dry milk. Membranes were then incubated overnight at 4°C with primary antibodies in TBS containing 5% nonfat dry milk and 0.1% Tween-20, followed by HRP conjugated secondary antibodies for 1 h at room temperature. Antibodies are listed in Supplemental Table S2 (http://dx.doi.10.1667/RR14643.1.S1). Protein loading was assessed with GAPDH (for left ventricular tissue) or F0–F1 ATPase (for mitochondria). Antibody binding was visualized with ECL™ Plus Western Blotting Detection reagent (GE Healthcare Life Sciences) on CL-Xposure Film (Thermo Scientific). Films were scanned using an AlphaImager® gel documentation system (Protein Simple) and protein bands were quantified with the public domain software ImageJ.
Statistics
Data are presented as average ± standard deviation (SD) and evaluated with the software package NCSS 8 (NCSS), using two-way ANOVA or repeated measures ANOVA (mitochondrial swelling assay), followed by Newman-Keuls individual comparisons. The criterion for significance was P < 0.05.
RESULTS
POLY-MVA Enhanced Left Ventricular Anti-Oxidant Enzyme Expression and Reversed the Effects of Radiation on Cardiac Mitochondria
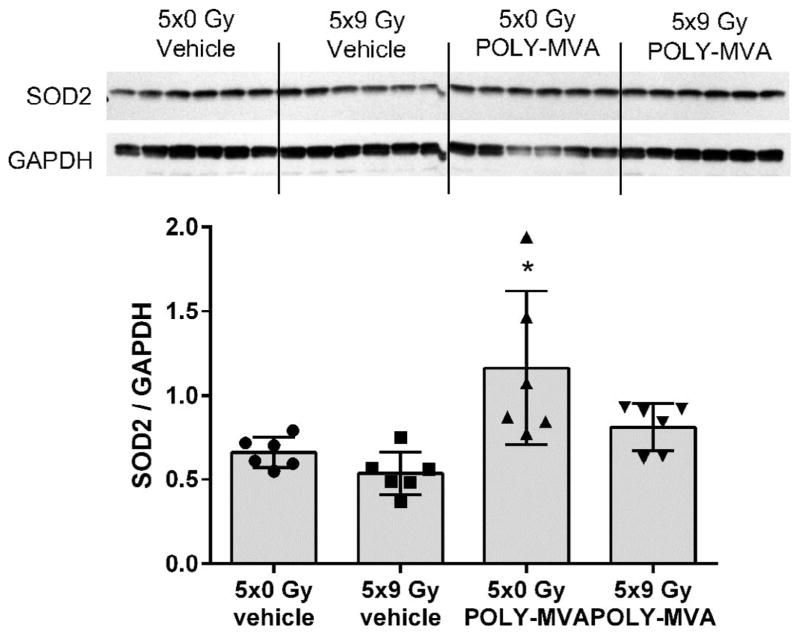
Since POLY-MVA had previously shown to enhance the anti-oxidant capacity of the aged rat heart, we examined left ventricular expression of SOD2. POLY-MVA enhanced SOD2 expression, but the effect was significant only in sham-irradiated animals (Fig. 1).
FIG. 1.
Effects of POLY-MVA on left ventricular SOD2 expression. Western blot image and densitometry results are presented. Western blot analysis revealed an increase in left ventricular SOD2 expression, but only in sham-irradiated animals. Densitometry results are given as average ± SD, n = 6. *P < 0.05 when compared to all other groups.
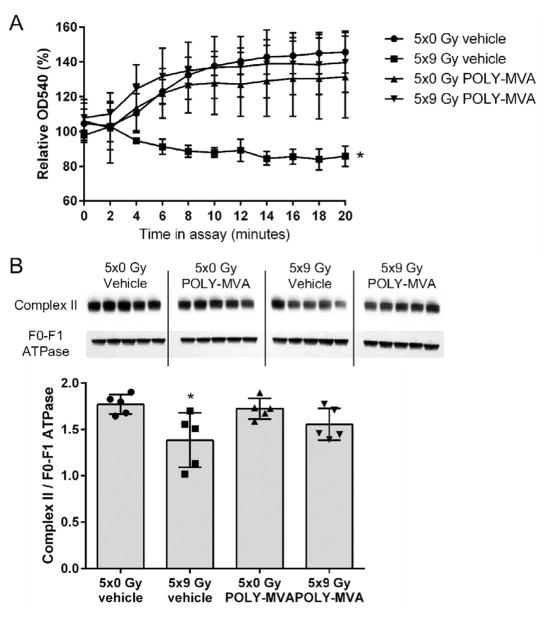
Analyses of cardiac mitochondria are shown in Fig. 2. At 24 weeks after local heart irradiation, cardiac mitochondria showed an increased susceptibility to swelling and a reduced expression of complex II. POLY-MVA significantly reduced the susceptibility to swelling and restored mitochondrial levels of complex II.
FIG. 2.
Effects of local heart irradiation and POLY-MVA on cardiac mitochondria. Panel A: Mitochondrial swelling was assessed as a reduction in the OD540 nm of suspensions of cardiac mitochondria, relative to the OD540 nm of the same mitochondrial samples immediately before the start of the assay. Shown here are samples incubated with CaCl2. Samples without CaCl2 or with CaCl2 in combination with the mPTP inhibitor CsA did not show enhanced swelling (data not shown). Mitochondria isolated after local heart irradiation in combination with POLY-MVA showed significantly reduced ex vivo swelling. Data are presented as average ± SD, n = 5. *P < 0.05 when compared to 5 × 0 Gy + vehicle and 5 × 9 Gy + vehicle. Panel B: Mitochondrial pellets were subjected to Western blotting to determine complex II expression. Western blot and densitometry results are shown. Please note that the order of experimental groups in the Western blot image is different from the order of the groups presented in the graph. Local heart irradiation reduced mitochondrial complex II, which was restored by POLY-MVA. Densitometry results are given as average ± SD, n = 5. *P < 0.05 when compared to 5 × 0 Gy + vehicle and 5 × 9 Gy + vehicle.
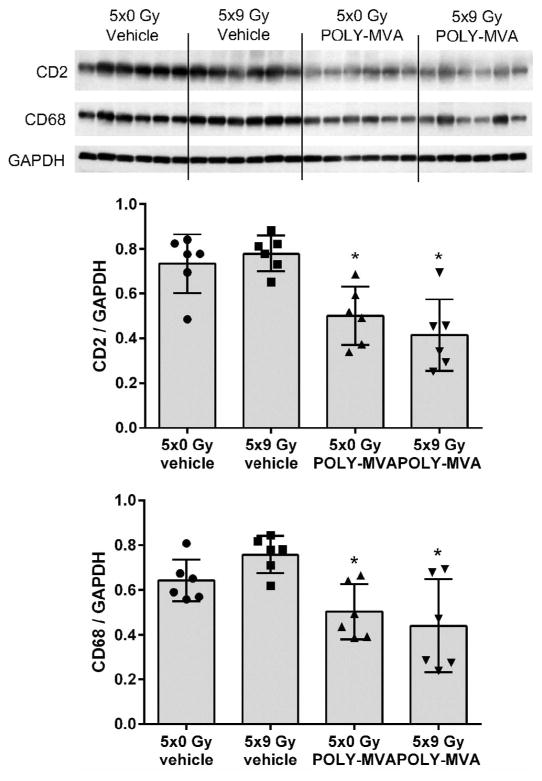
POLY-MVA Reduced Left Ventricular CD2 and CD68 Levels
Local heart irradiation did not modify left ventricular content of CD2 and CD68, cell surface markers of T-lymphocytes and monocytes/macrophages, respectively. The 6-weeks administration of POLY-MVA, on the other hand, reduced left ventricular content of CD2 and CD68, both in sham-irradiated and irradiated animals (Fig. 3).
FIG. 3.
Effects of local irradiation and POLY-MVA on left ventricular markers of inflammatory cells. Western blot image and densitometry results are presented. POLY-MVA reduced the left ventricular content of CD2 and CD68, both in sham-irradiated and irradiated rats. Densitometry results are given as average ± SD, n = 6. *P < 0.05 when compared to 5 × 0 Gy.
POLY-MVA Did Not Alter the Effects of Radiation on Cardiac Function or Remodeling
There were no significant differences in body weight or heart rate among any of the groups. At 18 weeks, effects of radiation on cardiac function started to emerge. A slight increase in systolic volume and decrease in diastolic volume caused a significant increase in stroke volume (SV), ejection fraction (EF), and fractional shortening (FS) (Table 1). These radiation-induced changes in cardiac function persisted at the week 24-time point. POLY-MVA did not have a significant effect on any of the alterations in cardiac function.
TABLE 1.
Effects of Local Heart Irradiation and POLY-MVA on Cardiac Function
| 18 weeks postirradiation (n = 18–20)
|
24 weeks postirradiation (n = 8–9)
|
|||||
|---|---|---|---|---|---|---|
| 5 × 0 Gy | 5 × 9 Gy | 5 × 0 Gy + vehicle | 5 × 9 Gy + vehicle | 5 × 0 Gy + POLY-MVA | 5 × 9 Gy + POLY-MVA | |
| Volume;s | 105.5 ± 25.9 | 85.1 ± 38.4 | 120.2 ± 22.1 | 113.0 ± 21.8 | 123.5 ± 30.6 | 93.8 ± 31.3 |
| Volume;d | 368.4 ± 61.0 | 379.0 ± 73.0 | 385.9 ± 42.4 | 430.0 ± 59.8 | 395.5 ± 41.1 | 414.0 ± 70.4 |
| CO (ml/mn) | 87.9 ± 16.8 | 94.4 ± 16.9 | 89.1 ± 21.3 | 103.1 ± 17.7 | 87.1 ± 12.4 | 99.1 ± 13.2 |
| SV (μl) | 262.9 ± 45.7 | 294.0 ± 42.1* | 265.7 ± 39.5 | 316.8 ± 44.2* | 272 ± 38.0 | 320.2 ± 47.6* |
| EF (%) | 71.7 ± 4.4 | 78.2 ± 6.2* | 68.8 ± 5.1 | 73.8 ± 3.1* | 69.0 ± 6.6 | 77.8 ± 5.1* |
| FS (%) | 42.6 ± 4.1 | 49.0 ± 5.9* | 40.3 ± 4.4 | 44.8 ± 2.7* | 40.5 ± 6.1 | 48.6 ± 5.2* |
| LVAW;s (mm) | 3.2 ± 0.3 | 3.4 ± 0.2 | 3.2 ± 0.2 | 3.6 ± 0.4 | 3.2 ± 0.4 | 3.4 ± 0.3 |
| LVAW;d (mm) | 2.0 ± 0.2 | 2.1 ± 0.2 | 2.1 ± 0.1 | 2.2 ± 0.2 | 2.1 ± 0.1 | 1.9 ± 0.2 |
| LVPW;s (mm) | 3.2 ± 0.3 | 3.2 ± 0.2 | 2.8 ± 0.4 | 3.0 ± 0.2 | 3.0 ± 0.2 | 3.0 ± 0.2 |
| LVPW;d (mm) | 2.2 ± 0.2 | 2.1 ± 0.2 | 2.0 ± 0.2 | 2.0 ± 0.2 | 2.1 ± 0.2 | 2.0 ± 0.2 |
| LV mass (g) | 1.4 ± 0.2 | 1.5 ± 0.2 | 1.4 ± 0.2 | 1.6 ± 0.2 | 1.5 ± 0.2 | 1.4 ± 0.1 |
P < 0.05 when compared to sham-irradiated control.
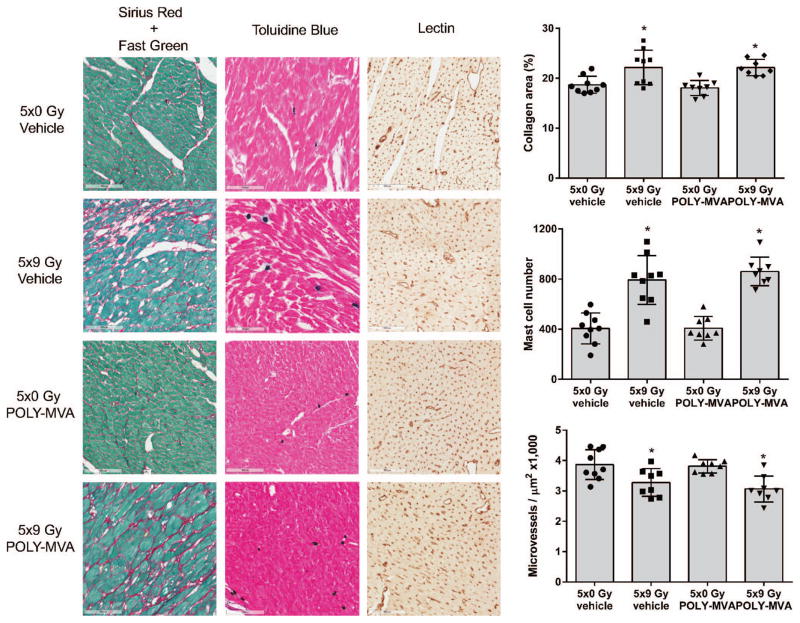
At 24 weeks after local irradiation, hearts showed an increase in cardiac collagen deposition, coinciding with increased cardiac mast cell numbers and reduced myocardial microvessel density. POLY-MVA did not alter these structural changes in the heart (Fig. 4).
FIG. 4.
Effects of local irradiation and POLY-MVA on cardiac structure. Representative micrographs of histology and the quantification of cardiac area occupied by collagens, cardiac mast cell numbers and microvessel density are shown. Radiation caused an increase in cardiac collagen deposition, cardiac mast cell numbers, and a decrease in myocardial microvessel density. POLY-MVA did not mitigate these effects. Data are presented as average ± SD, n = 8–9. *P < 0.05 when compared to 5 × 0 Gy.
DISCUSSION
Radiation-induced alterations such as adverse remodeling are very similar to manifestations of aging and are considered to have similar pathophysiology. POLY-MVA improved mitochondrial function and antioxidant status when administered once daily (0.05 ml/kg) for 30 days in aging rats (24–26 months of age) (16, 17). Although the rats in our current study were younger (8–9 months at tissue analysis), in accordance with the previous studies we found that POLY-MVA enhanced the left ventricular expression of SOD2. We also analyzed the effects of POLY-MVA on cardiac mitochondria. We have previously shown that local heart irradiation in the rat is associated with an enhanced tendency of mitochondrial swelling and reduced mitochondrial expression of complex II (15). These modifications occur as soon as 6 h postirradiation and last up to at least 9 months, the latest time point of investigation. These results suggest that radiation-induced alterations in mitochondria occur almost immediately and are not repaired. Alternatively, it is possible that for instance radiation-induced mitochondrial DNA damage or prolonged oxidative stress maintain a cycle of mitochondrial damage. While the role of mitochondrial alterations in RIHD are not yet known, studies in other organ systems have shown a clear contribution of mitochondria to normal tissue radiation injury. Hence, mitochondrially-targeted antioxidants and antioxidant enzyme mimetics serve as radiation protectors and mitigators (11, 12). To determine whether POLY-MVA could slow down or reverse the adverse effects of radiation in the heart, the current study was designed to start POLY-MVA administration at 18 weeks after local heart irradiation, a time point at which manifestations of RIHD have become apparent. When measured at 24 weeks postirradiation, cardiac mitochondria isolated from irradiated and POLY-MVA treated rats were less prone to swelling, and POLY-MVA had also restored the mitochondrial expression of complex II.
In addition to mitigating the effects of local heart irradiation on mitochondria, POLY-MVA reduced left ventricular content of inflammatory cell surface markers CD2 and CD68 in both sham-irradiated and irradiated animals. We cannot exclude that other components besides the PdLA complex of POLY-MVA contribute to these effects. Despite changes in cardiac mitochondria and inflammatory cells, POLY-MVA did not mitigate radiation-induced adverse cardiac remodeling, as indicated by ultrasound and measured by collagen deposition, mast cell numbers and myocardial microvessel density.
Even though POLY-MVA mitigated the effects of local heart irradiation on cardiac mitochondria and reduced left ventricular content of inflammatory cell surface markers when administered during the late phase of RIHD, this POLY-MVA schedule did not mitigate the adverse effects of radiation on cardiac function and fibrosis. It is possible that POLY-MVA would have had more profound effects on all manifestations of RIHD when administration had started before the onset of RIHD.
While mitochondrial alterations have long been described in animal models of local heart irradiation (15, 22), the role of mitochondria in RIHD has not been established. We previously investigated the effects of gamma-tocotrienol on RIHD in a rat model. A single dose of gamma-tocotrienol administered 24 h before irradiation reduced the effects of radiation on mitochondria, but late manifestations of RIHD were not modified (10). These results, together with the outcomes of the current study, suggest that mitochondria may not play a crucial role in the etiology of RIHD, and that a modification of radiation-induced alterations in cardiac mitochondria is not sufficient to reduce histological and functional manifestations of RIHD. These results could also indicate that there are manifestations of RIHD, not examined in the current study, that are a consequence of mitochondrial alterations. Hence, additional work is required to determine the roles of mitochondrial alterations in RIHD. The current study does not exclude the possibility that an earlier onset of POLY-MVA administration may have more profound effects on radiation-induced cardiac remodeling.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by the National Cancer Institute under grant number CA148679 and an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20 GM109005. Francis Antonawich and Merrill Garnett are supported by the Garnett McKeen Laboratory, Bohemia NY, the provider of POLY-MVA for this study.
References
- 1.Krishnan CV, Garnett M. Liquid crystal behavior in solutions, electrode passication, and impedance loci in four quadrants. In: Marcus P, Maurice V, editors. Passivation of Metals and Semiconductors and Properties of Thin Oxide Layers. 2006. pp. 389–94. [Google Scholar]
- 2.Ramachandran L, Krishnan CV, Nair CK. Radioprotection by alpha-lipoic acid palladium complex formulation (POLY-MVA) in mice. Cancer Biother Radiopharm. 2010;25:395–9. doi: 10.1089/cbr.2009.0744. [DOI] [PubMed] [Google Scholar]
- 3.Menon A, Nair CK. POLY MVA - a dietary supplement containing alpha-lipoic acid palladium complex, enhances cellular DNA repair. Int J Low Radiat. 2011;8:42–54. [Google Scholar]
- 4.Menon A, Krishnan CV. Protection from gamma-radiation insult to antioxidant defence and cellular DNA by POLY-MVA, a dietary supplement containing palladium-lipoic acid formulation. Int J Low Radiat. 2009;6:248–62. [Google Scholar]
- 5.El-Marakby SM, Selim NS, Desouky OS, Ashry HA, Sallam AM. Effects of poly-MVA on the rheological properties of blood after in-vivo exposure to gamma radiation. J Radiat Res Appl Sci. 2013;6:21–30. [Google Scholar]
- 6.Duma MN, Molls M, Trott KR. From heart to heart for breast cancer patients - cardiovascular toxicities in breast cancer radiotherapy. Strahlenther Onkol. 2014;190:5–7. doi: 10.1007/s00066-013-0465-4. [DOI] [PubMed] [Google Scholar]
- 7.Ogino I, Watanabe S, Iwahashi N, Kosuge M, Sakamaki K, Kunisaki C, et al. Symptomatic radiation-induced cardiac disease in long-term survivors of esophageal cancer. Strahlenther Onkol. 2016;192:359–67. doi: 10.1007/s00066-016-0956-1. [DOI] [PubMed] [Google Scholar]
- 8.Seemann I, Gabriels K, Visser NL, Hoving S, te Poele JA, Pol JF, et al. Irradiation induced modest changes in murine cardiac function despite progressive structural damage to the myocardium and microvasculature. Radiother Oncol. 2012;103:143–50. doi: 10.1016/j.radonc.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 9.van der Veen SJ, Ghobadi G, de Boer RA, Faber H, Cannon MV, Nagle PW, et al. ACE inhibition attenuates radiation-induced cardiopulmonary damage. Radiother Oncol. 2015;114:96–103. doi: 10.1016/j.radonc.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 10.Sridharan V, Tripathi P, Aykin-Burns N, Krager KJ, Sharma SK, Moros EG, et al. A tocotrienol-enriched formulation protects against radiation-induced changes in cardiac mitochondria without modifying late cardiac function or structure. Radiat Res. 2015;183:357–66. doi: 10.1667/RR13915.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fetisova EK, Antoschina MM, Cherepanynets VD, Izumov DS, Kireev II, Kireev RI, et al. Radioprotective effects of mitochondria-targeted antioxidant SkQR1. Radiat Res. 2015;183:64–71. doi: 10.1667/RR13708.1. [DOI] [PubMed] [Google Scholar]
- 12.Stoyanovsky DA, Jiang J, Murphy MP, Epperly M, Zhang X, Li S, et al. Design and Synthesis of a Mitochondria-Targeted Mimic of Glutathione Peroxidase, MitoEbselen-2, as a Radiation Mitigator. ACS Med Chem Lett. 2014;5:1304–7. doi: 10.1021/ml5003635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barjaktarovic Z, Schmaltz D, Shyla A, Azimzadeh O, Schulz S, Haagen J, et al. Radiation-induced signaling results in mitochondrial impairment in mouse heart at 4 weeks after exposure to x-rays. PLoS One. 2011;6:e27811. doi: 10.1371/journal.pone.0027811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boerma M, Singh P, Sridharan V, Tripathi P, Sharma S, Singh SP. Effects of Local Heart Irradiation in a Glutathione S-Transferase Alpha 4-Null Mouse Model. Radiat Res. 2015;183:610–9. doi: 10.1667/RR13979.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sridharan V, Aykin-Burns N, Tripathi P, Krager KJ, Sharma SK, Moros EG, et al. Radiation-induced alterations in mitochondria of the rat heart. Radiat Res. 2014;181:324–34. doi: 10.1667/RR13452.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sudheesh NP, Ajith TA, Janardhanan KK, Krishnan CV. Effect of POLY-MVA, a palladium alpha-lipoic acid complex formulation against declined mitochondrial antioxidant status in the myocardium of aged rats. Food Chem Toxicol. 2010;48:1858–62. doi: 10.1016/j.fct.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 17.Sudheesh NP, Ajith TA, Janardhanan KK, Krishnan CV. Palladium alpha-lipoic acid complex formulation enhances activities of Krebs cycle dehydrogenases and respiratory complexes I–IV in the heart of aged rats. Food Chem Toxicol. 2009;47:2124–8. doi: 10.1016/j.fct.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 18.Sharma S, Moros EG, Boerma M, Sridharan V, Han EY, Clarkson R, et al. A novel technique for image-guided local heart irradiation in the rat. TCRT Express. 2013:1. doi: 10.7785/tcrtexpress.2013.600256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sridharan V, Tripathi P, Sharma SK, Moros EG, Corry P, Lieblong BJ, et al. Cardiac inflammation after local irradiation is influenced by the kallikrein-kinin system. Cancer Res. 2012;72:4984–92. doi: 10.1158/0008-5472.CAN-12-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma CM, Coffey CW, DeWerd LA, Liu C, Nath R, Seltzer SM, et al. AAPM protocol for 40-300 kV x-ray beam dosimetry in radiotherapy and radiobiology. Med Phys. 2001;28:868–93. doi: 10.1118/1.1374247. [DOI] [PubMed] [Google Scholar]
- 21.Devic S. Precise radiochromic film dosimetry using a flat-bed document scanner. Med Phys. 2005;32:2245. doi: 10.1118/1.1929253. [DOI] [PubMed] [Google Scholar]
- 22.Cilliers GD, Harper IS, Lochner A. Radiation-induced changes in the ultrastructure and mechanical function of the rat heart. Radiother Oncol. 1989;16:311–26. doi: 10.1016/0167-8140(89)90044-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.