Abstract
AMP-activated protein kinase (AMPK) is a ubiquitously expressed metabolic sensor among various species. Specifically, cellular AMPK is phosphorylated and activated under certain stressful conditions, such as energy deprivation, in turn to activate diversified downstream substrates to modulate the adaptive changes and maintain metabolic homeostasis. Recently, emerging evidences have implicated the potential roles of AMPK signaling in tumor initiation and progression. Nevertheless, a comprehensive description on such topic is still in scarcity, especially in combination of its biochemical features with mouse modeling results to elucidate the physiological role of AMPK signaling in tumorigenesis. Hence, we performed this thorough review by summarizing the tumorigenic role of each component along the AMPK signaling, comprising of both its upstream and downstream effectors. Moreover, their functional interplay with the AMPK heterotrimer and exclusive efficacies in carcinogenesis were chiefly explained among genetically altered mice models. Importantly, the pharmaceutical investigations of AMPK relevant medications have also been highlighted. In summary, in this review, we not only elucidate the potential functions of AMPK signaling pathway in governing tumorigenesis, but also potentiate the future targeted strategy aiming for better treatment of aberrant metabolism-associated diseases, including cancer.
Keywords: AMPK, energy deprivation, kinase, LKB1, tumorigenesis, mouse models, metformin
1. Introduction
AMP-activated protein kinase (AMPK) is an evolutionally conserved serine/threonine protein kinase across species from yeast to mammal. Biologically, AMPK is regarded as a central switch of metabolic pathways, and directly senses cellular stresses and energy deprivation [1]. AMPK is initially identified due to its regulatory roles in lipid metabolism and cholesterol balance. Subsequently, accumulating evidences have elucidated that its energy-sensing efficacy covers, including but not limited to glucose, lipid or protein metabolism, either by transiently altering the phosphorylation status of various downstream metabolic kinases or modulating the chronic, long-term adaptation through transcriptional intervention [2].
It is well characterized that adenosine triphosphate (ATP) is the direct source of energy of cells in physiological status, which is mainly produced by oxidative phosphorylation inside the mitochondria. In order to guarantee the metabolic homeostasis, intracellular levels of ATP have to be maintained in a narrow physiological range [3]. However, following the excessive consumption of ATP in certain physiopathological stresses, the cytoplasmic AMP/ATP ratio is accordingly elevated, and acts as a sensitive indicator of nutritional abnormality. Subsequently, the increased ratio of AMP/ATP, as a result of relatively lower glucose supply, prolonged exercises, ischemia, hypoxia as well as medications, will trigger the activating phosphorylation of AMPK. This AMP-involved AMPK phosphorylation mainly depends on its upstream protein kinases including liver kinase B1 (LKB1), Calcium/calmodulin-dependent protein kinase II (CaMKKII) and TGF-beta-activated kinase 1 (TAK1) [4]. The major biological function of activated AMPK is to synergistically stimulate catabolic reactions and inhibit anabolic metabolism to supplement the deficiencies in ATP amount in variously above-mentioned conditions [5].
Structurally, all eukaryotes feature heterotrimeric components of the AMPK complex, containing a catalytic subunit α and two regulatory subunits, β and γ, respectively [6]. More importantly, the phosphorylation of Thr172 in the α subunit is required for AMPK enzymatic activation. Specifically, there are four tandem repeats identified within the subunit γ, in which include two theoretical binding sites either for AMP or for ATP. Notably, adenosine diphosphate (ADP), an intermediate of ATP and AMP, directly interacts with the γ subunit, implying a critical role for ADP under stressful circumstances [7]. Hence, according to current research findings, there are three possible mechanisms that mediate AMPK activation following the binding of AMP or ADP to γ subunit. Firstly, a conformational change occurs after the AMP binding, which makes it relatively easier for the AMPK holo-complex to become a substrate of upstream kinases. Secondly, the dephosphorylation effect on Thr172 by protein phosphatase 2Cα (PP2Cα) is antagonized after AMP is in physical association with the γ subunit. Thirdly, the allosteric activation of AMPK by the interaction of AMP with the γ subunit has been recognized as an auxiliary effect of LKB1 or CaMKKII-mediated phosphorylation events [8, 9].
Physiologically, diversified AMPK isoforms, derivative from transcriptional splice variants, including α1, α2, β1, β2, γ1, γ2 and γ3, are closely linked to different composition of the AMPK holo-complex, tissue distribution, subcellular localization, AMP/ATP sensitivity, downstream targets and upstream kinases [1]. Specifically, recent studies have elucidated that there are merely three distinct types of subunit assembly in human skeletal muscles (namely α2β2γ1, α2β2γ3 and α1β2γ1), rather than the 12 possible compositions theoretically. In contrast to subunit α1, complex constituted by subunit α2 is primarily anchored in cellular nucleus or cytoplasm, suggesting its dual functions of activating both transcriptional factors and cytoplasmic targets in these cellular compartments, respectively. Meanwhile, different isoforms of subunit α are specifically phosphorylated by their upstream kinases in a tissue-context-dependent manner. Taken together, different tissues prefer the specific AMPK complex, properly responding to systematic or cellular reactions through its own way to offer possible diversity in AMPK signaling pathway activation in different tissue or cellular contexts [10].
As a hallmark of cancer, the Warburg effect has primarily distinguished tumor cells from normal metabolism in healthy somatic cells. To this end, malignant cell has to remodel its metabolic pattern and therefore adapts to nutrient needs during rapid divisions, characterized by higher glycolysis rate and more production of lactates as well as macromolecules. TSC2, a well-known tumor suppressor, serving as a downstream effector of AMPK complex, connects AMPK-TSC2-mTOR as a pivotal pathway in cancer metabolism, which highlights the additional regulatory significance of AMPK besides its normal efficacy in energy homeostasis [11]. Moreover, in addition to the pivotal role of sensing energy or impacting cellular metabolism, AMPK could also module multiple cancer-associated pathways to play a potential role in tumorigenesis, including depressing inflammatory reactions and cell divisions, inducing cell cycle arrest, and facilitating the occurrence of apoptosis. In keeping with this notion, LKB1, the major AMPK upstream kinase, has also been identified to function as a tumor suppressor in various malignancies. Pathologically, mutation of LKB1 triggers carcinogenesis associated with the Peutz-Jeghers syndrome, predisposing patients to lung, liver as well as cervical cancers. Thus, all these evidences implicate that AMPK may similarly play a tumor suppressive role by governing the catabolic pathways, since synthesis of essential nutrients is the obligate step for cell growth and divisions [12].
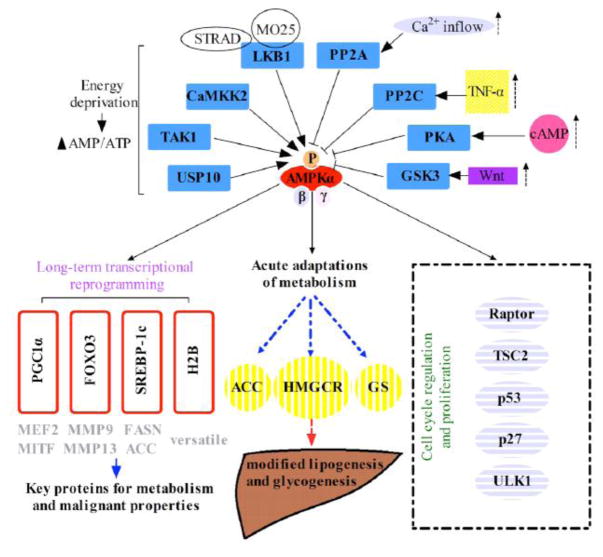
Taken together, there is currently lacking of a comprehensive summary discussing the interplay between AMPK signaling and carcinoma, especially in combination with its biochemical behaviors with the wealthy mouse modeling studies in elucidating the potentially critical role of AMPK signaling pathway in tumorigenesis. Therefore, we’re performing this review in order to summarize the tumorigenic role of each component within the AMPK pathway (Figure 1).
Figure 1.
The graphical demonstration of the AMPK complex and its signaling components including both upstream regulators and downstream effectors.
2. Functions of AMPK signaling components in tumorigenesis
2.1. AMPK isoforms
2.1.1. The α-subunit (α1, α2-catalytic subunits)
As stated above, the catalytic α-subunit of AMPK consists of two homogenous isoforms, AMPKα1 and AMPKα2, in which AMPKα1 is also termed “hepatic isoform” since it accounts for approximately 94% of catalytic activity of α-subunit in rat liver. On the contrary, AMPKα2 is most frequently expressed in skeletal muscles, with relatively lower levels of expression in liver and heart. Despite of their difference in tissue distribution and subcellular localization, both isoforms have similar and mutual efficacies, especially in regulation of various aspects of tumorigenesis, such as angiogenesis, cell multiplicity and chemosensitivity (Table 1) [13].
Table 1.
A summary of knockout phenotypes of major AMPK complex subunits in mice.
| AMPK subunits | Knockout mode | Phenotypic characteristics | Neoplastic implications | References |
|---|---|---|---|---|
| Ampkα1 | Ampkα1−/− | Splenomegaly and anemia | Context-dependent role in tumorigenesis | [230] |
| Ampkα1−/− | Shorter circadian period | [231] | ||
| Ampkα1−/− | Endothelial dysfunction and vascular inflammation | [232] | ||
| Ampkα1−/− | Spermatozoa abnormality and defective androgen production | [233] | ||
| Ampkα1−/− | Delayed liver regeneration | [234] | ||
| Ampkα1−/− | Decreased scar maturation and contractility following heart infarction | [235] | ||
| Conditional Ampkα1−/− (pancreatic beta cell) | Normal insulin secretion | [236] | ||
| Conditional Ampkα1−/− (myoblast) | Reduced myogenesis | [237] | ||
| Conditional Ampkα1−/− (spinal cord) | Thermal hyperalgesia and mechanical allodynia | [238] | ||
| Conditional Ampkα1−/− (satellite cell) | Dysfunctional myogenic differentiation | [239] | ||
| Conditional Ampkα1−/−(chondrocyte) | Normal bone growth | [240] | ||
| Conditional Ampkα1−/− (vascular smooth muscle) | Production of atherosclerotic calcification | [241] | ||
| Conditional Ampkα1−/− (macrophage) | Failed collateral remodeling and arteriogenesis | [242] | ||
| Conditional Ampkα1−/− (catecholaminergic cell) | Severer hypoventilation and apnea in the setting of hypoxia | [243] | ||
| Ampkα2 | Ampkα2−/− | Lowered insulin secretion and sensitivity | Context-dependent role in tumorigenesis | [244] |
| Ampkα2−/− | Longer circadian period | [231] | ||
| Ampkα2−/− | Increased microtubule proliferation in cardiac hypertrophy | [245] | ||
| Ampkα2−/− | Enhanced bone resorption and reduced bone mass | [246] | ||
| Ampkα2−/− | Impaired glycolysis in postmortem skeletal muscle | [247] | ||
| Ampkα2−/− | Attenuation of hepatic injury caused by metastasized tumor | [248] | ||
| Ampkα2−/− | Upregulation of mesenchymal transition of renal tubular epithelial cells | [249] | ||
| Ampkα2−/− | Aggravated left ventricular hypertrophy and dysfunction induced by hypertension | [250] | ||
| Ampkα2−/− | Atherosclerosis and endothelial dysfunction | [251, 252] | ||
| Ampkα2+/− | Reduced glucose uptake and normal fatty acid uptake | [253] | ||
| Conditional Ampkα2−/− (liver) | Decreased hepatic glucose production | [254] | ||
| Conditional Ampkα2−/− (pancreatic beta cell) | Impaired insulin secretion | [255] | ||
| Conditional Ampkα2−/− (vascular smooth muscle) | Elevated neointima formation and vascular smooth muscle cell migration | [256, 257] | ||
| Conditional Ampkα2−/− (skeletal muscle) | Normal insulin sensitivity and diminished adipose storage in skeletal muscle | [258] | ||
| Ampkβ | Ampkβ1−/− | Reduced bone density and normal osteoclast differentiation | Context-dependent role in tumorigenesis | [259] |
| Ampkβ2−/− | Reduced bone density and normal osteoclast differentiation | [259] | ||
| Ampkβ2−/− | Deficient exercise endurance and deteriorative glucose intolerance | [24] | ||
| Conditional Ampkβ1−/−β2−/− (skeletal muscle) | Decreased amount of capillary and resultant myopathy in nonpostural muscle | [260] | ||
| Conditional Ampkβ1−/−β2−/− (pancreatic beta cell) | Restricted insulin secretion | [261] | ||
| Ampkγ | Ampkγ1−/− | Hemolytic anemia and splenomegaly | Emerging tumor suppressive roles | [33] |
| Ampkγ3−/− | Attenuated adaptive ability of glucose metabolism in response to fasting | [262] | ||
| Ampkγ3−/− | Decreased post-workout glycogen restoration | [263] | ||
| Ampkγ3−/− | Damaged homeostasis of circadian oscillators | [264] |
Dysregulated AMPKα1/mTORC1 signaling axis has been verified via analyzing xenograft colon cancer in rodent models, in which the catalytic capability of AMPKα1 is greatly inhibited and consequently leads to activation on mTORC1 activity, following the attenuated inhibitory phosphorylation by AMPKα1 [14]. Reactivation of AMPKα1 could significantly decrease the proliferative ability of hepatocellular carcinoma cells [15]. Additionally, miR-301a-mediated depression of AMPKα1 is partially contributing to the chemoresistance of doxorubicin in osteosarcoma cells [16]. Nevertheless, different AMPKα1 functionality in tumorigenesis has been reported, revealing a versatile role of AMPKα1 that depends on tumor types and pathological phases. Notably, forced elevation of AMPKα1 expression exhibits potent impacts in triggering protective autophagy in chronic myelomonocytic leukemia cells, therefore enhancing its anti-apoptotic ability and longevity [17]. Similarly, amplified AMPKα1 expression has been also confirmed among cervical cancer, positively correlated to tumor progression and multiplicity. These mechanistic controversies implicate that AMPKα1 may also facilitate the survival of tumor cell under stressful circumstances, although there is lacking of enough evidence on how it works and how it distinguishes with physiological metabolic regulation in vivo [18].
Similar to AMPKα1, the actual role of AMPKα2 on carcinogenesis remains elusive according to current literatures. Ampkα2-knockout easily induces the malignant transformation among embryonic fibroblasts, implicating that AMPKα2 largely acts as a potentially vital suppressor of tumorigenesis (Table 1) [19]. Meanwhile, suppression of AMPKα2 isoform is commonly observed among murine models with breast cancer, resulting in repressed apoptosis and relieved tumor expansion among neoplastic cells [20]. Unlike AMPKα1, AMPKα2 is a favorable prognostic indicator amid cervical cancer patients, mainly due to its anti-tumor properties during cervical carcinogenesis [21]. Surprisingly, evidence from in vitro analysis has suggested that AMPKα2 serves as a strong promoter of vascular endothelial growth factor (VEGF) in glioblastoma, subsequently to stimulate its proliferative activity via increased blood supplies [22]. However, the physiological role of AMPKα2 in tumorigenesis and its relevance to VEGF expression warrants further investigations, especially in vivo.
2.1.2. The β-subunit (β1, β2-regulatory subunits)
As a regulatory subunit, AMPKβ, serving as a scaffold to anchor AMPKα and AMPKγ subunits, is essential for the assembly of AMPK complex. Additionally, the subcellular localization of AMPK trimer also largely depends on the activity of the β subunit. Conditional depletion of Ampkβ in transgenetic mice results in significant decrease of glucose uptake and adaptive alterations during exercises, suggesting the pivotal role of β-subunit in regulation of AMPK viability [23, 24]. Similar to the α subunit, there are two isomers of β subunit, AMPKβ1 and AMPKβ2, each with specific tissue distributions. Specifically, liver is the main habitat of AMPKβ1, while AMPKβ2 is primarily expressed in skeletal muscles [25].
Currently, evidences that discuss the oncogenic role of different AMPKβ isoforms remain inadequate, especially the in vivo results (Table 1). Notably, AMPKβ1 expression is dramatically depressed within lung carcinoma cells, while its forcing expression could conversely inhibit the malignant proliferation via a p53 dependent [26] or independent manner [27]. Moreover, its anti-tumor efficacy has also been confirmed in advanced ovarian cancer, in which reduced AMPKβ1 level indicates more metastatic tendency and worse clinical stages [28]. However, some controversies have emerged concerning the physiological effect of AMPKβ in tumorigenesis. According to a report from Li et al., higher expression of AMPKβ1 and AMPKβ2 were detected among human ovarian carcinoma, and further linked to poorer histological type and clinical stages [29]. These outcomes warrant us for the further investigations of the different roles of AMPKβ in tumorigenesis.
2.1.3. The γ-subunit (γ1, γ2, γ3-regulatory subunits)
Based on current knowledge, γ-subunit is a necessarily regulatory component of AMPK heterotrimer, since it directly senses and binds with cytoplasmic AMP/ADP in order to stimulate the enzymatic activity of AMPK. Structural analyses have identified three unique isoforms of γ-subunit, γ1, γ2 and γ3, in which γ1 is the most ubiquitously expressed isomer over the body. However, the other two subtypes, γ2 and γ3, have specific distributions and mainly exist in heart and skeletal muscles, respectively [30].
Consistent with the physiological role of AMPK, the expression of all three isotypes of γ-subunit could be elevated during the inhibition of the respiratory chain, while knockdown of γ-subunit displays unfavorable impacts on cell metabolism in rodent skeletal muscles (Table 1) [31]. Conversely, ectopic expression of the γ subunit may enhance the storage of glycogen, thus preventing cells from ischemic damage [32]. Furthermore, the γ1 isoform acts as a key factor in regulating erythrocyte membrane elasticity, and its deactivation triggers hemolytic anemia and splenomegaly in mouse models [33]. By far, the studies regarding tumorigenic role of AMPKγ remain inadequate, although it plays an inevitable role in AMPK activity. Clinically, missense mutation of AMPKγ1 has been observed in a small fraction of colorectal cancer specimens, indicating the potentially suppressive role of AMPKγ1 in tumorigenesis [34]. Meanwhile, amplification of AMPKγ2 is associated with better histological grade among ovarian cancer patients [29]. And also the identification of mutations in PRKAG2, which encodes the ubiquitously expressed γ2 subunit, characterized by increased unstimulated AMPK activity and resulting in heart muscle disease, provides an opportunity to investigate the metabolic consequences of AMPK activation in both mouse and man.
Hence, further in-depth evidence is urgently desired to clarify the physiological contribution of these γ isoforms to human diseases including cancer.
2.2. AMPK upstream regulators
2.2.1. LKB1
Liver kinase B1 (LKB1), also termed as serine/threonine kinase 11 (STK11), was initially discovered in the Peutz-Jeghers Syndrome due to its loss-of-function germline mutations, which features far-ranging hamartomas located in the entire gastrointestinal tract and with higher risk of transforming into invasive malignancies than sporadic polyps [35]. This enzyme remains structurally conserved across species, playing a vital role in mediating metabolic homeostasis, cell multiplicity, polarity and autophagic initiation [36].
LKB1 is ubiquitously detected inside versatile tissues and highest expression in embryonic digestive epithelium as well as testis, which gradually fades away along the process of cell maturity. Physiologically, LKB1 is predominantly anchored within cellular nucleus, owing to the presence of the nucleus localization region (NLS) inside its N-terminal domain [35]. More importantly, pseudokinase STRADα and scaffolding protein MO25 form a heterotrimer with LKB1, thus promoting the connection of exportins with LKB1, such that the holo-enzyme complex could be ultimately translocated into cytoplasm and act as a regulatory kinase to phosphorylate a spectrum of its downstream targets, including AMPK [37]. Moreover, the mitochondrial localization of LKB1 is proven to be pivotal for its role in modulating apoptosis [35]. These findings suggest that the cytoplasmic re-localization may be a prerequisite for LKB1 mediated biological behaviors.
Given its critical role in both metabolism and tumorigenesis, elucidating the regulatory network of LKB1 will greatly help us to understand its working mechanisms in suppressing tumorigenesis in vivo (Table 2). By far, AMPK is the most well-characterized substrate of LKB1, which is phosphorylated at the Thr172 residue of the catalytic subunit α to achieve enzymatic activation of AMPK [10]. LKB1 is the exclusive upstream activating kinase of AMPK following the energy deprivation in majority tissues, directly mediating AMPK-involved effects such as catabolic reactions and protective autophagy [38]. In support of this notion, genetic knockout of Lkb1 in murine models almost completely silences the phosphorylation on Thr172 as well as AMPK downstream efficacies among embryonic fibroblasts, despite in the setting of AMPK agonists or other functional stimulators [39].
Table 2.
A summary of knockout phenotypes of major AMPK upstream regulators in mice.
| Upstream regulators | Knockout mode | Phenotypic characteristics | Neoplastic implications | References |
|---|---|---|---|---|
| Lkb1 | Lkb1−/− | Early embryonic death and growth retardation | Versatile roles (mainly tumor suppressive) | [265] |
| Lkb1−/− | Pancytopenia and quiescence of hematopoietic stem cell | [266] | ||
| Lkb1+/− | Development of gastrointestinal hamartomas | [43, 267] | ||
| Lkb1+/− | Initiation of hepatocellular carcinoma | [268] | ||
| Lkb1+/− | Emergence of pancreatic adenocarcinoma | [269] | ||
| Lkb1+/− | Disabled exercise capacity and changed fibre types in diaphragm | [270] | ||
| Conditional Lkb1−/− (striated muscle) | Defective voluntary exercise and mitochondrial activity | [271] | ||
| Conditional Lkb1−/− (skeletal muscle) | Enhanced insulin sensitivity and ameliorated glucose metabolism | [272] | ||
| Conditional Lkb1−/− (pancreatic epithelium) | Impaired acinar polarity and initiation of cystic tumors | [273] | ||
| Conditional Lkb1−/− (endothelium) | Early embryonic death and vascular dysfunction | [274] | ||
| Conditional Lkb1−/− (pancreatic alpha cell) | Reduced glucagon secretion following hypoglycemia | [275] | ||
| Conditional Lkb1−/− (pancreatic beta cell) | Increased amount of beta cells and insulin secretion | [276] | ||
| Conditional Lkb1−/− (heart) | Cardiac hypertrophy and malfunction | [277] | ||
| Conditional Lkb1−/− (heart) | Spontaneous atrial fibrillation | [278] | ||
| Conditional Lkb1−/− (liver) | Damaged bile acid transport and abnormal accumulation | [279] | ||
| Conditional Lkb1−/− (spinal cord) | Disabled hind-limb and degenerative axon | [280] | ||
| Conditional Lkb1−/− (T cell) | Lowered level of peripheral T cells | [281] | ||
| Conditional Lkb1−/− (adipose tissue) | Improved thermogenesis and increased brown adipose tissue mass | [282] | ||
| Conditional Lkb1−/− (osteoblast) | Elevated density and porosity in trabecular and cortical bone respectively | [283] | ||
| Conditional Lkb1−/− (Schwann cell) | Developmental retardation in myelination and myelin maturity | [284] | ||
| Conditional Lkb1−/− (inner ear) | Malformed and dysfunctional stereocilia | [285] | ||
| Conditional Lkb1−/− (cerebellum) | Enhanced granule cell precursors proliferation, abnormal granule cell migration and impaired motor function | [286] | ||
| Conditional Lkb1−/− (oocyte) | Swollen ovaries and infertility | [287] | ||
| Camkk2 | Camkk2−/− | Impaired long-term and spatial memory in male mice but normal in female mice | Largely oncogenic roles | [288] |
| Camkk2−/− | Ameliorated glucose level and tolerance | [289] | ||
| Camkk2−/− | Damaged development of cerebellar granule cells | [290] | ||
| Camkk2−/− | Restricted inflammatory responses by macrophage | [291] | ||
| Camkk2−/− | Higher density in trabecular bone and activated osteoblast formation | [292] | ||
| Camkk2−/− | Dysfunctional blood-brain barrier | [293] | ||
| Conditional Camkk2−/− (hypothalamus) | Inhibited secretion of neuropeptide Y and food ingestion | [294] |
In addition to the classical LKB1/AMPK transduction, a total of 12 AMPK-related kinases are confirmative substrates of LKB1, including NUAKs, SIKs, BRSKs, MARKs, SNRK and MELK. Similarly, the phosphorylated T-loop by LKB1 is necessary to activate their kinase activities, whereas deficiency in LKB1-mediated phosphorylation event significantly restricts their physiological capabilities. Specifically, the LKB1/MARKs pathway has been characterized to regulate the intracellular microtubule dynamics, in addition to the myosin adjustment by LKB1/NUAKs and neuronal polarization by LKB1/BRSKs, all of which are physiologically reported to be responsible for regulating cell polarity, a critical process that runs a mock when cells undergo cellular transformation [35].
Meanwhile, LKB1 is capable of triggering transcriptional reprogramming via phosphorylation on remaining AMPK related substrates, in order to regulate cell adhesion, longevity and expansion [40]. On the other hand, the molecular basis that leads to LKB1 enzymatic activation remains to be rarely discussed. To this end, as mentioned above, the assembly of STRADα-MO25-LKB1 complex strongly stimulates the allosteric activation of LKB1. It is currently acknowledged that this effect is the core mechanism for activating LKB1, instead of other patterns of post-translational modifications such as phosphorylation [37]. Mechanistically, a recent study has reported that Skp2 directly polyubiquitinates LKB1, which facilitates the binding of LKB1 and MO25 and subsequently leads to the promotion of LKB1 kinase activity. As a result, Skp2-null mice display a significantly lowered ability to maintain the completeness of LKB1 complex in response to cellular stresses. The interaction of Skp2/LKB1 is crucial for holo-enzyme complex assembly and LKB1 activation, no matter under physiological or stressful situations [41]. In addition to Skp2-meidated regulation of LKB1 mechanism, there are also several identified upstream kinases of LKB1, including PKA, ATM and PKC that target either Thr366 or Ser325 residue of LKB1 for phosphorylation in murine models. These activating phosphorylations successfully activate the LKB1-AMPK-mTOR pathway or other similar signaling to trigger appropriate reactions [35]. However, the intrinsic mechanism of these kinases especially their physiological roles in affecting the complex formation of STRADα-MO25-LKB1 remains undefined, thus further clarifications should be anticipated to better understand their physiological contribution and underlying molecular mechanisms in activating LKB1 in response to various experimental challenges.
During the past decades, LKB1 has been well-described as a tumor suppressor, since its loss-of-function mutation is commonly detected and believed to directly induce the oncogenesis in a wide range of malignancies [42]. Knockout mice models have been extensively applied to study the molecular basis of LKB1 associated carcinogenesis. Specifically, germline biallelic depletion of Lkb1 induces embryonic lethality among engineering mice, while those characterized by heterozygous Lkb1+/− display multiple intestinal hamartomas, pathologically mimicking the alterations in the Peutz-Jeghers Syndrome (PJS) patients [43]. Following the selective knockout of both Lkb1 alleles in lung tissue, development of adenocarcinoma, squamous and large cell carcinoma have been early aroused, especially the KRAS-driven lung malignancies [44]. Moreover, conditional depression of Lkb1 in specific tissues has also led to occurrence of various cancers, including bone, prostate, pancreatic, breast as well as dermatological carcinomas [45]. Thus, these preclinical outcomes derived from genetically engineered mice have well verified and simulated the pathogenesis of human neoplasms, revealing an important role of LKB1 in clinical prognosis and potential treatment.
Clinically, analyses from human specimens have demonstrated that LKB1 depletion is closely correlated to more malignant behavior in various cancers [46]. Mechanistically, the LKB1 inactivation induced tumorigenesis is mainly attributed to metabolic reprogramming towards a more anabolic phenotype following the failure of the LKB1-AMPK pathway [47], Moreover, LKB1 silencing contributes to the redox imbalance and resultant chemoresistance in non-small cell lung carcinoma [48]. The CCL2-mediated macrophage recruitment is enhanced in part due to the loss-of-function mutation on LKB1, which partially explains the development of endometrial cancer [49]. Besides, LKB1 could likewise affect epithelial mesenchymal transition [50], Hippo/YAP signaling [51], proinflammatory transduction [52] as well as TGF-β pathway [53] to function as a tumor suppressor in multiple neoplasms.
On the contrary, several studies have reached controversial results that LKB1 may also play a pro-oncogenic role during diversified tumorigenesis, especially under particular environments such as energy deficiency [35]. Specifically maintenance of LKB1 is essential for the viability of ovarian cancer spheroids in vitro [54]. Furthermore, some researches on lung malignancy reveal that hyper-expression of LKB1 helps stressful cells to survive glucose-starvation induced apoptosis, meanwhile frustrating the cytotoxic impact by erlotinib [55]. These exceptional outcomes implicate that the functional range of LKB1 in tumorigenesis may be far beyond current cognition and depend on different cell types or developmental phases. Hence, more in-depth investigations are warranted to fully understand the physiological role of LKB1 in tumorigenesis.
2.2.2. CaMKK2
As a vital secondary messenger, Ca2+ and its signaling pathway have been verified to participate in various aspects of tumorigenesis, such as proliferation and malignant dissemination. Calmodulin could directly bind to calcium ions as their intracellular recipient, following the Ca2+ influx triggered by alterations on membrane potential. Consequently, the Ca2+-calmodulin complex will stimulate the family of calcium/calmodulin-dependent protein kinase kinases, which consists of calcium/calmodulin-dependent protein kinase kinase 1 (CaMKK1) and kinase 2 (CaMKK2). It has been currently clarified that calcium/calmodulin-dependent protein kinase I (CaMKI) and kinase IV (CaMKIV) are major downstream targets of CaMKK2. More importantly, CaMKK2 also activates its downstream molecule AMPK under certain physiological circumstances, in order to maintain the metabolic balances [56].
Recently, Nelson et al has identified CaMKK2 as an androgen-responsive gene, and its elevation by androgens leads to largely expressed in prostate cancer cells, favoring the migration and distant metastasis of prostate cancer [57][58]. Specifically, AMPK is believed to be a major downstream target of CaMKK2-mediated the carcinogenic role of androgens. In keeping with this notion, inhibiting CaMKK2 could dramatically reduce the metastatic property of prostate cancer [59]. Furthermore, Massie et al and Shima et al consistently confirmed that the presence of CaMKK2 is positively correlated to advanced stages and negatively linked to survival prognosis among patients of prostate cancer [60, 61]. Moreover, results derived from xenograft mouse models revealed that CaMKK2 aberrantly reemerged in the androgen-responsive or castration-resistant prostate cancer (CRPC) tissues and involved in the androgen-stimulated pathogenesis as a hub protein [61]. They further demonstrated that CaMKK2 displayed a higher expression level in castration-resistant xenograft models, and depletion of CaMKK2 could highly restrict the expression of downstream targets of androgen signaling. Nevertheless, in contrast to this finding, another group independently demonstrated that CaMKK2 significantly limited the transcriptional activity of AR in advanced CRPC as well as its proliferation, offering a controversial evidence of the potential role of CaMKK2 in CRPC development [61]. Thus, more investigations are still needed to clarify the physiological role of CaMKK2 in CRPC development (Table 2).
Similar to the prostate cancer setting, higher expression of CaMKK2 is observed in gastric cancer samples, leading to strengthen the gastric cancer cell proliferation and invasion by phosphorylating AMPK [62]. Therefore, by an overall consideration of prostate and gastric cancer, the CaMKK2-AMPK signaling axis may serve as an oncogenic pathway rather than the classically tumor-suppressive role of AMPK in most malignancies.
2.2.3. TAK1
Transforming growth factor-β activated kinase 1 (TAK1) is a member of MAP kinase kinase kinase (MAPKKK) family, regarded as a core effector in regulation of various cell behaviors including angiogenesis, inflammation, metabolism and carcinogenesis [63]. TAK1 has been demonstrated as an upstream kinase of AMPK by directly phosphorylating its Thr172 residue. Furthermore, AMPK is also observed to reciprocally activate TAK1, compromising a feedback loop in mediating inflammatory signals [64]. Importantly, activation of the TAK1/AMPK signaling axis plays vital roles in preventing malignant expansion and inducing protective autophagy, irrespective of pancreatic cancer [65] or hepatocellular carcinoma [63]. As such, genetic depletion of Tak1 in mouse models significantly promotes hepatic oncogenesis [66], while an elevation of apoptosis level is observed on KRAS-dependent colon cancer models, following the inhibited expression of TAK1 [67]. This paradox hints that the physiological function and downstream targets of TAK1 may vary among diverse malignancies, which warrant further in-depth investigation.
2.2.4. PKA
Protein kinase A (PKA) is a conserved hetero-tetramer kinase complex in mammalian cells, individually constituted by two catalytic subunits as well as two regulatory subunits. Structurally, there are totally four distinct isotypes of PKA with specific tissue distributions [68]. The intact heterotetrameric form arrests the biological function of PKA under physiological conditions. Once the intracellular cAMP level is elevated, this secondary messenger could directly bind the regulatory subunits of PKA to release its catalytic subunits, leading to phosphorylate their downstream targets for responding to cellular stresses [69]. Notably, the PKA catalytic subunit can interact with and directly phosphorylate AMPKα at the Ser173 residue, thus blocking its activating phosphorylation at Thr172 by other upstream kinases, such as LKB1. This PKA/AMPK regulatory pathway has been found to be involved in multiple cellular actions, especially in metabolic maintenance [70].
Given that AMPK is a well-known anti-oncogenic effector, the potentially tumorigenic role of PKA is therefore investigated. Experiments on murine models suggest that elevated PKA functionality significantly induces the malignant alteration of normal mammary epithelial cells, mechanistically through activating the Src pathway [71]. Meantime, the miR-33a/PDE8A/PKA signaling plays stimulatory role on glioma progression, in which the viability of PKA is regained following the inhibition on PDE8A activity by miR-33a [72]. Similar result is also observed in liver cancer cells, where PKA directly phosphorylates AMPK to reverse the cell cycle arrest [73]. Clinically, both regulatory subunits of PKA are overexpressed in thyroid carcinoma, correlating with stronger motility and proliferative ability [74].
On the other hand, novel studies have also demonstrated a possible tumor suppressive role of PKA among certain types of cancers. Surprisingly, PKA could activate AMPK to trigger cell cycle arrest and cellular apoptosis in myeloma, possibly through the phosphorylation on LKB1-Ser428 residue, which subsequently stimulates the AMPK activity [75]. Moreover, based on genetically modified mouse models, Pka knockout significantly accelerates the skin tumorigenesis via upregulation of transcriptional factors GLI1 and YAP1 [76]. Consistently, PKA activation is also effective to inverse the process of epithelial mesenchymal transition (EMT), thereby impeding the cancer initiation by targeting the histone demethylase PHF2 [77]. Taken together, these controversies reveal that the actual role of PKA in carcinogenesis remains obscure, even if in the same kind of human tissue such as mammary epithelium, where AMPK-independent pathway may partially involve in this process.
2.2.5. GSK3
As a pivotal enzyme in glucose metabolism, glycogen synthase kinase 3 (GSK3) is ubiquitously expressed in mammals and widely involved in numerous regulatory pathways by targeting over one hundred substrates [78]. Identified as an upstream kinase of AMPK, GSK3 binds with AMPKβ prior phosphorylating the AMPKα, which facilitates AMPKα binding to phosphatases and therefore inactivates AMPK [79]. Currently, the biological impacts of GSK3 have been extensively unveiled on metabolic dysregulation, neurodegenerative disorders as well as neoplastic onset [80]. The oncogenic properties of GSK3 have been confirmed among prostate and colon cancer models, acting as a downstream target of androgen-dependent [81], and the Wnt pathway, respectively [82]. On the contrary, engineered mice with Gsk3 depletion feature more aggressive phenotype of breast and skin cancer, which is attributed to stabilization of β-catenin and enhanced expression of c-Myc and survivin [80]. Hence, the physiological role and detailed molecular mechanisms of GSK3 in tumorigenesis await further investigation.
2.2.6. PP2A and PP2C
Phosphorylation is one of the most common forms of reversible protein post-translational modification, with up to 30% of all proteins being phosphorylated at any given time. To convert this process, the protein phosphatase is identified as an enzyme to remove the phosphate group from the phosphorylated amino acid residue of its substrate protein. Since around half of the intracellular serine/threonine kinase is controlled by Protein Phosphatase 2A (PP2A), which therefore modulates the major pathways of cell proliferation, metabolic homeostasis and cell survival [83]. Acting as a major antagonist of tumorigenic kinases, such as Akt, SRC and MEK, PP2A has been identified as a tumor suppressor in various malignancies, through decreasing cell proliferation, invasiveness and inducing apoptosis as well as senescence. Therefore, the inactivation of PP2A is commonly observed during neoplastic formation, and several explanatory mechanisms of which have been established in carcinogenesis, mainly including the elevated expression of PP2A inhibitors and genetic alterations on PP2A subunits [84].
Recently, the phosphorylation of AMPK has been observed largely inhibited by a ceramide-dependent activation of PP2A in the mice fed with high-fat diet [85]. Additionally, Park et al described a Ca2+-involved interplay between PP2A and AMPK, where the inflow calcium ions directly connected with the binding sites of PP2A and promoted its physiological activity, resulting in the depression of AMPK phosphorylation [86].
Moreover, the physiological role of PP2C has been disclosed to maintain homeostasis in the case of cellular stresses, through repressing the stress-responding signals especially the relevant kinases such as ASK1, TAK1, MKK3, JNK and ATM [87]. Meanwhile, the interplay between PP2C and AMPK has also been gradually disclosed. Moreover, PPM1A and PPM1B, the members of PP2C which serving as metal-dependent (Mg2+ or Mn2+) monomeric enzymes, were observed to involve TNF-α-mediated AMPK inactivation by dephosphorylating the AMPK Thr172 residue to trigger insulin resistance in skeletal muscle cells [88]. Likewise, PPM1D (WIP1) [89] and PPM1E [90] have been confirmed as upstream regulators of AMPKα, contributing to the participation of PP2C/AMPK pathway in various pathological procedures, including degenerative disorders and malignant formations. Nevertheless, further evidences are still needed to clarify whether the remaining isoenzymes of PP2A or PP2C family could directly interact with and dephosphorylate AMPK.
2.2.7. USP10
The ubiquitin specific proteases (USPs) are essential members of human deubiquitinases (DUBs), mainly functioning to facilitate the cellular deubiquitination process and therefore participate in various biological behaviors such as tumorigenesis and adaptive reprogramming [91]. USP10, also termed as UBPO, has been identified as a subtype of the USP sub-family of DUBs, of which the catalytic region recognizes the targeted ubiquitin and both terminal domains assist its subcellular localization [92].
p53 is the classical downstream target of USP10, whose activation under stressful circumstances leads to deubiquitination on p53, therefore inducing its stability and nuclear localization [93]. Meanwhile, experimental results have further concluded that AMPK is a novel substrate of USP10 in murine models. Mechanistically, following the energy deprivation, USP10 largely deubiquitinates AMPKα so that its activating phosphorylation by LKB1 could be greatly enhanced. Reversely, AMPK could also phosphorylate USP10 Ser76 residue, both of which construct a feedforward loop and thus help the exhaustive cells to survive extreme environments [94].
Biologically, silenced USP10 in lung cancer cells contributes to the elongated cell survival and depressed apoptosis, partially through deubiquitinating the mismatch repair protein MSH2 [95], or negatively modulating EIF4G1-mediated functions [96]. USP10 is also a proliferative inhibitor in pancreatic cancer, and negatively influenced by miR-191 [97]. Furthermore, USP10-mediated stabilization of SIRT6 attenuates the oncogenic effect of c-Myc activation and ultimately induces the neoplastic suppression investigated in a xenograft colon cancer model [98].
Clinically, relatively lower expression of USP10 is observed in gastric cancer specimens relative to the adjacent controls, and indicated the poor outcomes of the patients [92]. The USP10 responsible deubiquitination is able to stimulate the anti-oncogenic activity of p53 both in renal clear cell carcinoma [93] and colorectal cancer [99], highlighting the potential significance of USP10/p53 targeted cancer treatment. Although the majority of current viewpoints agree the tumor-inhibitory role of USP10, its possibly versatile functions require future in-depth studies.
2.2.8. LncRNA NBR2
Long non-coding RNA (LncRNA) is a subfamily of mammalian RNA featuring more than 200 constituted nucleotides. Since it is incapable of encoding functional proteins, this type of genomic product has long been recognized as transcriptional rubbish without actual cell merits [100]. Nevertheless, novel investigations have uncovered its core regulatory role on diverse processes, including cell multiplicity and neoplastic initiation [101].
Neighbor of BRCA1 gene 2 (NBR2) genetically locates alongside the well-known gene BRCA1, a frequently silenced tumoral suppressor in breast cancer and ovarian cancer [102]. It is experimentally verified that NBR2 tends to generate long non-coding RNA instead of parallel protein for exerting its biological impacts. Both in vivo and in vitro evidences have recently discovered a feed-forward loop of AMPK-LncRNA NBR2 axis under situations of chronic energy deprivation. After the initiation of AMPK activation by LKB1, the induced LncRNA NBR2 could directly bind to AMPKα and further stimulate the activation of AMPK as its novel upstream effector [103]. Mechanistically, the intensive rather than initiative functionality by LncRNA NBR2 seems to be a time-efficient manner for AMPK amplification especially under long-lasting starvation, the depletion of which significantly disrupts the signaling cascades of AMPK and ultimately leads to dysregulated cell cycle and apoptotic response. On the other hand, AMPK is probably not the only downstream target of LncRNA NBR2, since overexpressed LncRNA NBR2 remains effective in repressing cell proliferation despite of AMPK knockout [104].
Similar to its neighboring gene BRCA1, NBR2 is also believed to mainly serve tumor-inhibitory effect in several kinds of malignancies. Irrespective of in vivo (xenograft models) or in vitro (cell lines and clinical samples) experiments on renal and breast cancer, LncRNA NBR2 is greatly inhibited in contrast to adjacent controls, with involvement of AMPK and mTOR. Survival analysis of breast cancer has additionally revealed that the lower level LncRNA NBR2 has, the poorer prognosis patients will gain [104]. These results implicate that LncRNA NBR2 may be a novel therapeutic target although more in-depth investigations are still urgently needed, especially knockout evidences and mechanistical clarifications.
2.3. AMPK downstream substrates in tumorigenesis
2.3.1. AMPK downstream substrates in long-term transcriptional reprogramming
2.3.1.1. PGC1α
PPAR gamma co-activator 1α (PGC1α), as a transcriptional co-activator, regulates the faculty of genes involved in energy metabolism, and masters the regulation of mitochondrial biogenesis. Once activated, the ubiquitous expression of PGC1α interacts with certain transcriptional factors that correlate to metabolic regulation and cancer progression [105]. In detail, the N-terminal domain of PGC1α receives upstream activation signals and facilitates the formation of a transcriptional complex containing transcriptional factors and chromatin structure remodeling proteins such as p300 [106, 107]. Meantime, the C-terminal region of PGC1α helps to connect with vitamin D receptor (VDR), both of which accelerate the transcriptional initiation through an allosteric effect [108].
AMPK is a well-known upstream activator of PGC1α by phosphorylating its N-terminal residues. Cellular stresses that cause energy deprivation could easily induce the AMPK-PGC1α pathway activation to maintain internal homeostasis under physiological conditions, including exercises, fasting or hypothermy [109]. Mechanistically, PGC1α-involved adaptation is mainly achieved by a long-term transcriptional adjustment [105]. For instance, PGC1α responds to persistent endurance training by stimulating muscle-specific myocyte enhancer factor 2 (MEF2), which increases the oxidative capability of muscle fibers under stressful circumstances [110]. Meanwhile, the efficiency of oxidative phosphorylation is greatly improved inside mitochondria, owing to the activation of PGC1α as well as its subsequent co-activation of nuclear respiratory factor 1 (NRF1) [111].
Recently, the pathological linkage between PGC1α and neoplastic formation has drawn more attention due to the critical roles of PGC1α to make adaptive alterations on respiratory chain inside the cancer cell mitochondria [105]. Somatic depletion of Pgc1α significantly reduces the occurrence of colorectal and hepatic carcinoma among chemically induced mice (Table 3) [112]. Furthermore, aberrant positivity of PGC1α is detected in various cancer types with particular signal pathways. Specifically, Shiota et al stated that PGC1α could activate androgen receptor pathway to promote prostate cancer progression [113]. Moreover, PGC1α acts as a key downstream component of oncogene MITF, whose activation could largely boost the clearance of ROS inside melanoma cells and gear up the cancer metabolism and growth [114]. Taguchi et al reported that mutated p53 directly upregulated PGC1α level in a fraction of lung adenocarcinoma, resulting in tumor expansion and local progression [115]. Consequently, the above-mentioned evidences highlight the clinical potential of PGC1α as a therapeutic target among cancer sufferers.
Table 3.
A summary of knockout phenotypes of major AMPK downstream effectors in mice.
| Downstream regulators | Knockout mode | Phenotypic characteristics | Neoplastic implications | References |
|---|---|---|---|---|
| Pgc-1α | Pgc-1α−/− | Development of Huntington’s disease | Oncogenic roles | [295] |
| Pgc-1α−/− | Impaired early vasculogenesis in retina | [296] | ||
| Pgc-1α−/− | Striatal neurodegeneration and locomotive discordance | [297] | ||
| Pgc-1α−/− | Cortical hypereactivity | [298] | ||
| Conditional Pgc-1α−/− (endothelium) | Retarded wound healing and vascular dysfunction | [299] | ||
| Conditional Pgc-1α−/− (adipose tissue) | Insulin insensitivity | [300] | ||
| Conditional Pgc-1α−/− (skeletal muscle) | Development of glucose intolerance and whole-body inflammation | [301] | ||
| Conditional Pgc-1α−/− (renal tubule) | Normal function of kidney but elevated inflammatory levels | [302] | ||
| Srebp-1c | Srebp-1c−/− | Peripheral neuropathy | Oncogenic roles | [303] |
| Conditional Srebp-1c−/− (mammary epithelium) | Deficient lactation and lipid synthesis | [304] | ||
| Conditional Srebp-1c−/− (pancreatic beta cell) | Increased insulin responsiveness | [305] | ||
| Acc | Acc1−/− | Early embryonic death | Oncogenic roles | [306] |
| Conditional Acc1−/− (liver) | Decreased triglyceride storage in liver | [307] | ||
| Conditional Acc1−/− (adipose tissue) | Delayed growth and reduced level of adipose accumulation | [308] | ||
| Conditional Acc1−/− (T-cell) | Impaired peripheral distribution and proliferation of CD8+ T-cell | [309] | ||
| Acc2−/− | Elevated insulin sensitivity and ameliorated metabolic syndrome | [310] | ||
| Conditional Acc2−/− (heart) | Improvement on overloaded hypertrophy | [311] | ||
| Conditional Acc2−/− (skeletal muscle) | Unchanged glucose homeostasis and body weight | [312] | ||
| Conditional Acc1−/−Acc2−/− (liver) | Enhanced fat storage in liver | [313] | ||
| Hmgcr | Hmgcr−/− | Early embryonic death | Versatile roles (mainly oncogenic) | [314] |
| Conditional Hmgcr−/− (skeletal muscle) | Development of rhabdomyolysis | [315] | ||
| Conditional Hmgcr−/− (liver) | Hepatic steatosis and premature lethality | [316] | ||
| Gs | Conditional Gs−/− (brain) | Disruptive long-term memory and learning ability | Oncogenic roles | [317] |
| Conditional Gs−/− (skeletal muscle) | Normal exercise endurance and better glucose tolerance | [318] | ||
| Conditional Gs−/− (liver) | Elevated glucose intolerance | [319] |
2.3.1.2. FOXO3
With more than a hundred of members and evolutionarily conserved function, the forkhead-box family (FOX) plays key role in controlling cell fate, malignant transformation and internal metabolism [116]. FOXO3 is one of the four members (FOXO1, FOXO3, FOXO4 and FOXO6) of FOXO subfamily in mammals, which is ubiquitously expressed and serves as a transcriptional factor interacting with motif FHRE within target gene promoters [117]. Upstream signals ultimately lead to post-transcriptional modifications on FOXO3, including phosphorylation, acetylation and ubiquitination, in which six C-terminal residues of FOXO3 have been identified as phosphorylation sites by AMPK, including Thr179, Ser399, Ser413, Ser439, Ser555 and Ser588 [118]. Further studies have suggested that the AMPK-FOXO3 signaling exerts endurable and protective impact against skeletal atrophy [119] and keratinocytic senescence [120], mainly based on its transcriptional reprogramming. Additionally, its anti-tumor functionality has also been verified in breast and liver cancer in vitro, demonstrating a remarkable growth-inhibitory effect [121].
Besides its well-investigated role in aging-related and degenerative disorders, the physiological significance of FOXO3 in carcinogenesis has gradually surfaced. Germline knockout of both alleles (Foxo3−/−) triggers a late onset of tumor phenotype among mouse models. And conditional depletion on somatic cells directly results in the formation of hemangiomas and lymphoblastic lymphomas, implying the probably inhibitory activity of FOXO3 in human cancers [122]. Mechanistically, recent studies have disclosed that FOXO3 inactivation is mainly attributed to network regulation by diversified oncogenic pathways. Specifically, FOXO3 is a downstream target and negatively regulated by the PI3K-Akt pathway, which leads to cell cycle arrest and apoptosis among thyroid, cervival and breast cancer. In addition, the Ras-MEK-ERK, IKK pathway and several micro-RNAs such as miR-183, miR-96 and miR-182 are also capable of promoting the degradation or restricting the expression level of FOXO3 in various cancers respectively [123].
So far, although the majority of reports have regarded FOXO3 as a tumor suppressor, its pro-tumoral action may also exist. Analyses from in vivo experiments implicate that a simultaneous activation of both FOXO3 and β-catenin effectively elongates the cell survival and expedites the distant dissemination among colorectal cancer xenografts in murine models. Subsequent evidences from colon cancer shows a strong positive correlation between FOXO3 nuclear positivity with metastatic tendency and life expectancy among cancer patients [124]. Similarly, an oncogenic role of FOXO3 is also identified in breast cancer cells, straightly through upregulating the expression of metastatic promoters MMP-9 and MMP-13 in vitro and in vivo [125]. Hence, despite of the versatile functions that FOXO3 has involved in during cancer progression, more physiological evidences are still needed to clarify and consider its opposite role in tumorigenesis, especially for angiogenesis, metastasis, stemness maintenance and differentiation.
2.3.1.3. SREBP-1c
Sterol regulatory element binding protein (SREBP) is a transcriptional factor family involved in regulation of lipogenesis, in which SREBP-1c transcriptionally activates its downstream responsive genes in nucleus, promoting lipogenic activity in mammalian liver [126]. Specifically, the mature form of SREBP-1c moves towards nuclear genome and increases the expression of lipo-synthetic enzymes such as FASN and ACC. It has been discovered that AMPK serves as an upstream kinase of SREBP-1c by phosphorylating its Ser372 residue, leading to a significant reduction in SREBP-1c nuclear translocation and transcriptional activity, thereby resulting in attenuated lipo-synthetic ability among hepatic cells [127]. Hence, this behavior provides theoretical basis for AMPK agonists, including metformin and flavonoids, to be employed to cure fatty liver disorders.
Elevated lipogenesis is a metabolic hallmark of cancer cells, such that the oncogenic role of SREBP-1c has drawn much attention due to its predominant function in controlling lipid synthesis. Notably, the relevance of high-fat diet and tumorigenesis among breast, pancreas, prostate gland and endometrium has been clearly disclosed, which highlights the potential role of SREBP-1c on initiation of such malignancies. In terms of breast cancer development, the SREBP-1c-FASN axis is abnormally activated by oncoprotein HBXIP based on evidence from xenograft mice models [128]. In vivo and clinical experiments have respectively proven that SREBP-1c is a potent promoter of pancreatic cancer and its redundancy indicates worse prognosis (Table 3) [129]. Likewise, overexpression of SREBP-1c is ubiquitously detected in prostate cancer samples, and miR-185 and 342-mediated inhibition of SREBP-1c could greatly restrict the invasiveness and trigger apoptosis in prostate cancer cells [130]. Moreover, SREBP-1c-FASN has been recognized as a pivotal signaling that promotes the aggressive properties of endometrial carcinoma [131].
Sirtuin 1 (SIRT1), the most conserved NAD+-dependent protein deacetylase, has emerged as a key metabolic sensor, which activates SREBP-1c to facilitate its oncogenic properties during the malignant alteration of endometrium [132]. To this end, SREBP-1c does play a critical role in accelerating the metastasis of hepatocellular carcinoma due to its primary function to stimulate lipogenesis in liver [133]. Moreover, the correlations between SREBP-1c and neural oncogenesis have also been extensively investigated, in which aberrantly increased expression of SREBP-1c is found inside glioblastoma tissues, which positively relates to tumor growth and local spread by stimulating its downstream targets of ACC and FASN [134]. Additionally, upregulation of SOAT1 [135] and N-glycosylation of SCAP jointly contribute to SREBP-1c activation [136], while miR-132 suppressively targets SREBP-1c and diminishes its oncogenic properties in glioma cells [137]. In summary, based on current literatures, SREBP-1c targeted therapy has a bright future among cancer patients, especially for those with lipid-induced malignancies.
2.3.1.4. H2B
H2B is one of the core histones in eukaryotic cells that compositely construct chromatin along with condensed nuclear DNA [138]. Consisting of methylation, acetylation, phosphorylation and ubiquitination of specific residues, post-translational modifications on histones have been considered as a vital form among all epigenetic activities, which is essential for gene transcription, adaptive remodeling of chromatin and DNA replication. Specifically, the monoubiquitinated modification of H2B is not only serving as an activator of gene expression, but also involves in multiple DNA-processing procedures such as DNA repair [139]. More interestingly, AMPK could directly phosphorylate H2B in the Ser36 residue, to facilitate the transcriptional role of H2B on chromatin. Once the Ser36 residue has been replaced by alanine, the transcriptional action linked to AMPK activation could be largely inhibited, revealing a central role of H2B phosphorylation on AMPK-dependent transcriptional reprogramming for cellular stresses [140].
H2B does involve in multiple neoplastic procedures following various post-transcriptional modifications, among which monoubiquitination is of the most importance and has received the maximum attentions on its tumorous activity [141]. According to the classical facts, the ubiqutination cascade of CDK9-WAC-RNF20/40 signaling plays a unique role in monoubiquitinating H2B on the Lys120 residue. On the contrary, the deubiqutinase USP22 antagonizes the ubiquitin ligase complex RNF20/40 on H2B monoubiquitination. Mechanistically, the enhanced transcriptional elongation by H2Bub1 effectively reduces the possibility of DNA misparing and malignant mutation, which has been therefore classified as a nuclear indicator of tumor suppression among diversified malignancies. Collectively, downregulation of the H2B monoubiquitination level by altering any component of the CDK9-WAC-RNF20/40 signaling could lead to an increased tendency of cancerous transformation in vitro or in mouse models.
On the other hand, phosphorylation on H2B has been certified as an alternative mechanism mediating tumor initiation. Phosphorylated Ser32 residue on H2B by ribosomal S6 kinase 2 (RSK2) facilitates transformation from the normal skin epidermal cells towards squamous carcinoma in a genetic knockout model [142]. Nevertheless, a relative dephosphorylation status of the H2B Ser23 residue occurs in prostate cancer cells compared to normal controls, which reveals that the actual role of H2B phosphorylation on tumorigenesis depends on phosphorylation sites and developmental stages of malignancies [143]. Thus, as a promising therapeutic target, the exact mechanisms of H2B modifications on tumor progression should be supplementarily explained by additional updates and literatures.
2.3.2. AMPK downstream substrates in acute adaptations of metabolism
2.3.2.1. ACC
As a pivotal enzyme mediating fatty acid oxidation, acetyl-CoA carboxylase (ACC), triggering the transformation of cellular acetyl-CoA into malonyl-CoA, is an endogenous inhibitor of carnitine palmitoyltransferase-1 (CPT1) in mammals. Biologically, CPT1 acts as a direct promoter of mitochondrial β-oxidation of cytoplasmic fatty acids, therefore, activation of ACC effectively decreases the rate of fatty acid oxidation. Physiologically, ACC interchanges between its active and inactive form dependent on its dephosphorylation and phosphorylation status respectively. Two isoenzymes of ACC have been identified as ACC1 and ACC2, with a tissue-context expression manner, in which ACC2 has been elucidated as the major regulator of fatty acid oxidation in both cardiac and skeletal muscles, while similar function of ACC1 has been emphasized largely in renal tissue. Meanwhile, both isoenzymes during fatty acid oxidation share the functions in liver [144].
Mechanistically, AMPK could phosphorylate ACC1 on the Ser79 residue, resulting in the inhibition of enzymatic activity of ACC1 and subsequent to accelerate fatty acid oxidation in the setting of energy deprivation. Hence, ACC1, the downstream target of AMPK, primarily responds to metabolic stresses through adaptive adjustments instead of transcriptional alterations [145]. Similar to ACC1, AMPK is the major upstream kinase of ACC2 by phosphorylating its Ser212 residue to inhibit its physiological ability [146]. Moreover, PKA, as an alternative enzyme, could slightly deactivate ACC2 within cardiac muscles [147]. Collectively, all these evidence indicate that ACC2 is an acute adaptive responder under stressful conditions, with its vital role to regulate lipid synthesis inside the mitochondria.
Recently, accumulating investigations have also paid close attention to the actual role of ACC1 on carcinogenesis (Table 3), since elevated lipogenic activity is commonly occured in neoplastic cells. To this end, overexpression of phosphorylated ACC1 has been detected among diverse malignancies, including prostate cancer and breast cancer [148]. Furthermore, ACC1 is responsible for malignant transformation of skin epithelial cells, whose depletion greatly suppresses tumor burden and progression [149]. Moreover, the TNF-α-AMPK-ACC1 axis is aberrantly activated among pancreatic cancer models, implying the ACC1 involvement in inflammation-induced tumorigenesis [150]. On the contrary, inhibition of ACC1 by TOFA induces cell apoptosis amid colon cancer and lung cancer cells, which reflects its role on maintaining cell longevity [151]. Clinically, higher expression of ACC1 predicts poorer outcome of patients with certain types of tumors, such as head and neck, gastric cancer and colorectal cancer [152]. Thus, more evidences are still needed to clarify the in-depth mechanisms of ACC1 signaling in tumorigenesis in order to promote its clinical utilization.
2.3.2.2. HMGCR
HMG-CoA reductase (HMGCR), serving as the central enzyme to accelerate the de novo synthesis of cholesterol in liver, is governing the rate-limiting step of the transformation from HMG-CoA to mevalonate. It has been already clarified that AMPK is the major inhibitor of HMGCR in rat models by phosphorylating its Ser871 residue as protein kinase C (PKC) and calmodulin-activated protein kinase (CAMK) do [153][154]. Thus, activation of the AMPK-HMGCR axis is a direct respondence to cellular stresses via an acute adaptive pattern, restricting the cholesterol metabolism and promoting catabolic activity in liver (Table 3).
HMGCR has been widely regarded as a candidate that potentially participates in cancer metabolism due to its core role on stimulating the production of cholesterol. Specifically, Statins, well-known inhibitors of HMGCR, have been clinically correlated to prolonged survival expectancy among cancer sufferers, such as endometrial cancer [155] and esophageal adenocarcinoma [156]. Moreover, lower risk of tumor initiation or dissemination have been observed in a variety of cancer types following the administration of Statins, revealing a great possibility to expand its therapeutic indications towards tumorous field. Unexpectedly, the majority of tumors bearing over-expression of HMG-CoA reductase demonstrate modest phenotype and favorable prognosis, especially in breast [157] and colorectal malignancies [158]. However, there is in scarcity of statements that compare the expression disparity between active and inactive form of HMGCR. This indicates that the elevation of inactive form might contaminate the tumor-promoting role of HMGCR activation, since the excessively biological assembly of lipid and cholesterol is one of the major metabolic hallmarks of cancer cells. Therefore, those puzzles are waiting for more solid experimental evidence for further mechanistic interpretations.
2.3.2.3. Glycogen synthase
Glycogen synthase (GS) is a key promoter of glycogen biosynthesis in liver and skeletal muscles. Actually, there is a complex network governing the activity of glycogen synthase, including allosteric stimulation, inhibitory phosphorylation and negative feedback regulation. Glycogen synthase becomes activated under circumstances of glycogen deprivation, which typically occurs during endurance trainings via an insulin/GSK-3-independent manner [159].
Multiple kinases are capable of phosphorylating glycogen synthase, in which GSK-3-mediated continuous phosphorylation of glycogen synthase slightly restricts its activity under physiological baseline conditions. More interestingly, AMPK also directly phosphorylates glycogen synthase at the Ser7 residue to antagonize the producing of glycogen both in vivo and in vitro [159, 160]. As a result, aberrant regulation of the AMPK-GS axis modulates glycogen accumulation in cardiac and skeletal muscles as well as liver cells [161]. Conversely, despite of unclear upstream mechanisms, the aberrant glycogenesis in leukemic cells is concomitant with AMPK suppression and GS activation [162].
Abnormal glycogen storage is characterized as a feature of most malignancies, for example, ovarian clear cell carcinoma. In keeping with this notion, cellular glycogen accumulation is observed with highly expressed active glycogen synthase induced by hypoxia-HIF1α signals [163]. Notably, silencing of glycogen synthase leads to reduced glycogen production as well as invasiveness in xenograft glioma mice model, partially due to the abnormal PTEN-PI3K pathway [164]. Furthermore, over-activity of glycogen synthase is a strong catalyst of uncontrollable growth in prostate cancer [165] and renal clear cell carcinoma [166]. More importantly, the activation of glycogen synthase is an essential step for the initiation and development of hepatoma evidenced in vitro, in vivo or in clinical due to the fact that liver is the most important glycogenesis organ (Table 3) [167, 168]. However, its possibly oncogenic role in hepatocellular carcinoma has not yet been proven, anticipating for further investigation.
2.3.3. AMPK downstream substrates in cell cycle regulation and proliferation
2.3.3.1. Raptor
Raptor is a specific subunit of the mammalian target of rapamycin complex 1 (mTORC1), and functions to scaffold mTORC1 with its downstream substrates, including p70S6K1 and 4EBP1. The mTORC1 kinase complex is a well-conserved regulator of growth pathway following the upstream signals of metabolic alterations, specifically sensing the nutrient administration [169]. Nevertheless, Raptor could solely decrease the liver steatosis in rodent models by interacting with PHLPP2 in an mTORC1-independent manner [170]. This implicates that the actual scope of Raptor activity may be far beyond current knowledge.
At present, there are multiple recognized upstream kinases mediating the phosphorylation of Raptor to generate contrary efficacy. For example, GSK-3 could activate mTORC1 functionality via phosphorylating Raptor on Ser859 residue [171]; NLK, however, inhibits the mTORC1 activity by directly phosphorylating Raptor on the Ser863 residue under stressful conditions [169]. Besides, AMPK could largely depress the activity of mTORC1 by phosphorylation Raptor on Ser722 and Ser729, leading to cell cycle arrest and suppressed mitotic proliferation [172].
Clinically, higher expression of both Raptor and mTORC1 has been detected in colorectal cancer and linked to unfavorable prognosis [173]. Knockdown of Raptor significantly reverses the malignant properties of ovarian cancer, accompanied with the less recruitment of mTORC1 downstream substrates such as 4EBP1 exanimated in rodent models [174]. Silencing Raptor in xenograft mice results in elevated apoptosis and reduced growth potential in hepatocellular carcinoma, which is attributed to the inhibition on mTORC1 and its target effectors [175]. Raptor phosphorylation by the PI3K-PTEN axis in head and neck cancer leads to the connection between mTORC1 and its downstream substrate BMAL1, which is a pivotal clock gene inducing tumor multiplicity in mouse models [176]. Meanwhile, mTORC1-Raptor plays essential roles on maintaining the oncogenic characteristics of T-cell lymphoma, and its depletion obviously shortens cell longevity and triggers premature death [177]. On the other hand, since mTORC1 involves in liver steatosis, whether something similar happens in tumorigenesis remains undetermined, therefore in demand of further mechanistical discoveries.
2.3.3.2. TSC2
Tuberous sclerosis complex (TSC) clinically features systemic occurrence of hamartomas, which is caused by Tsc1 or Tsc2 genetic mutations among human patients. TSC2 has been identified as the central connector of adaptive and growth pathways, usually linking upstream kinase Akt and downstream target mTORC1. Mechanistically, inactivated phosphorylation of TSC2 by Akt could significantly suppress mTORC1 activity, leading to inactivation of 4EBP1 and p70S6K1, which serve as major regulators of cell growth and organ size. Conversely, AMPK could promote TSC2 functions to inhibit mTOCR1 in response to energy exhaustion, by direct phosphorylating TSC2 on Thr1227 and Ser1345 residues, hence protectively limiting cell expansion and reducing stress-induced apoptosis [178].
Conditional knockout of Tsc2 significantly elevates the possibility of tumor formation in vivo [179]. Specifically, silenced expression of TSC2 is observed in human hepatocellular carcinoma, and indicates well responsiveness to mTORC1-targeted therapy of Everolimus [180]. Furthermore, renal malignancies have been recognized as TSC2-associated, thus rapamycin is effective in restricting tumor development among conditional Tsc2 knockout mice [181]. Meanwhile, through an inhibitory nitrosylation on TSC2, nitric oxide synthase could inhibit TSC2 by nitrosylation modification, leading to activation of the mTORC1 pathway, resulting in enhanced cell invasiveness in xenograft melanoma models [182]. Notably, further evidence has implicated that TSC2 insufficiency is able to induce mesenchymal tumorigenesis by recruiting a transcriptional factor HMGA2, which is totally independent of mTOR activation [183]. Thus it gives us a hint that the anti-neoplastic role of TSC2 may be correlated to multiple underlying mechanisms other than the classical mTORC1 involvement.
2.3.3.3. p53
p53, an extensively studied transcriptional factor, plays pivotal role in monitoring and governing cell fate and mitotic cycle, especially notable as a tumor suppressor in a variety of cancers. Mechanically, p53 is activated and translocated into nucleus for transcription of target genes to maintain genomic stability and energy homeostasis under destructive conditions such as DNA breaking or metabolic imbalance. Pathologically, p53-deficiency demonstrates a remarkable decrease on cell viability in the setting of energy deprivation, despite of an increase on cell multiplicity [184].
Currently, the interaction of p53 and AMPK has been molecularly elucidated, in turn, AMPK-mediated phosphorylation of p53 at the Ser15 residue could trigger its transcriptional activity in the case of glucose exhaustion [185]. More interestingly, Sestrins 1/2, members of stress-sensitive gene governed by p53, could promote AMPK activated phosphorylation to comprise an AMPK-p53 feedback loop, which serves as the central mediator of adaptive regulation on cell growth and apoptosis. Besides, DAPK and CK1 also can phosphorylate p53 on the Ser20 residue to exert similar tumor suppressive functions [186].
Since gain-of-function mutations of P53 occurring nearly 50% of human tumors, including colorectal liver, breast and lung cancer, the anti-tumor activity has become the most investigative aspect of p53 function during the past decade. Generally, activated p53 interacts with its tumor-inhibitory partners to initiate the growth-restricting pathways and repair DNA mismatches [187]. Specifically, as the downstream target of RP-MDM2 axis, p53 directly antagonizes RAS-involved tumorigenesis in rodent melanoma models [188]. Moreover, restoration of p53 could enhance the chemosensitivity of skin cancer cells by inducing the production of NEAT1 paraspeckles [189]. p53 also exerts its tumor suppressive role via upregulating miR-139-5p, subsequently to inactivate oncoprotein PDE4D in xenograft colorectal carcinoma models [190]. Recently, nerve growth factor receptor (NGFR), the novel inactivator and downstream target of p53, has been characterized to inhibit tumor suppressor function of p53 in glioblastoma, lead to reduced apoptosis and enhanced migratory capability [191]. Hence, targeted p53 strategy seems to be a promising therapeutic option among patients refractory to routine treatments, either by p53 activator such as APR-246 or exogenous p53 supplements.
2.3.3.4. p27
p27 (also termed as Kip1), a member of the Cip/Kip family along with p21 and p57, functions as a vital inhibitor of cyclin dependent kinase (CDKs) in mammalian cells. To this end, p27 arrests cell cycle in G1 phase by targeting cyclin E-Cdk2 and cyclin D-Cdk4, and also manipulates cell migration and longevity independent its role on cell cycle [192].
p27 is manipulating both in transcriptional and post-transcriptional levels, including transcriptional adjustment, phosphorylation and ubiquitin-involved proteolysis. Thus, AMPK directly phosphorylates p27 on the Thr198 residue under certain stressful conditions, resulting in p27 cytoplasmic sequestration and stabilization [193], which facilitates cells to survive in energy-deprived environments, mainly via mediating autophagy and apoptosis [192].
p27 is considered to perform an anti-oncogenic effector within human malignancies due to its suppressive role on cell cycle regulation. Evidences from two meta-analyses reveal that the activity of p27 is largely depressed in oral squamous cell carcinoma [194] as well as non-small cell lung cancer [195]. In keeping with this notion, p27 expression is positively correlated with survival expectancy and inversely with tumor progression. p27 is also a downstream target of the Hippo signaling, which promotes breast cancer progression in murine models by inhibiting the transcriptional activity of p27 [196].
Multiple oncogenic factors including Skp2 [197] and Mdig [198] inhibiting p27 activation by accelerating its degradation, leading to higher cytoplasmic p27 expression, indicating poorer chemosensitivity and long-term survival among patients of non-small cell lung cancer [199]. Similarly, repressed p27 viability is likewisely identified as an inevitable pusher of neoplastic transformation in prostate. Both miR-150 [200] and miR-24 [201] suppresses the expression of p27 among xenograft mice, playing a contributing role on prostate oncogenesis and progression. Besides, administration of a novel compound CAPE displays beneficial anti-tumor effects in prostate cancer cells, primarily via reactivation and nuclear translocation of p27 [202]. Thus, all these evidences suggest that p27-targeted therapy is of great clinical potential as an anti-neoplastic strategy.
2.3.3.5. ULK1
Lysosomes act as intracellular scavengers by fusing with and then degrading the vesicles containing aberrant proteins and organelles, a process termed as autophagy. This catabolic process participates in a variety of biological situations, such as energy exhaustion, carcinogenesis and neurodegeneration. Degradation of defective components not only protects cells from secondary detriments, but also offers essential nutrients and elements under stressful circumstances [203]. Currently, over 30 autophagy-related genes (ATG) have been identified across species. The Unc-51-like kinase 1 (ULK1) is a mammalian ortholog of yeast ATG1, mechanistically serving as a core initiator of cellular autophagy along with its chaperones ATG13 and FIP200 to form a trimeric complex [204]. Four available residues within ULK1 are identified as the activating phosphorylation sites by AMPK, including Ser467, Ser555, Ser637 and Thr574 [205]. More interestingly, all three constitutive subunits of AMPK could be phosphorylated and inhibited by ULK1, indicating a feedback loop between both effectors [206]. Besides, ULK1 is also phosphorylated by mTOR on Ser757 to inhibit its functions in autophagy [207]. These results potently provide the central role of ULK1 in terms of linking metabolism with autophagy.
During the past decade, the relationship between autophagy and tumorigenesis has become a hot issue among scientific communities. Clinically, overexpression of ULK1 plays an oncogenic role in most of tumor types, including hepatocellular carcinoma [208], esophageal squamous cell carcinoma [209], nasopharygeal carcinoma [210] as well as colorectal cancer [211], which predicts poorer prognosis and severer progression. In addition, the protective autophagy induced by ULK1 is observed among mice with xenograft prostate cancer, thus preventing apoptosis and triggering neoplastic advancement [212].
Specially, the mTOR/ULK1 signaling also generates autophagic protection in chronic myeloid leukemia cells, antagonizing the chemotherapeutic benefits of regular regimen [213]. However, contrary outcome has been obtained in breast cancer specimens, among which lower ULK1 expression indicates unfavorable prognosis and local development [214]. Based on current perspectives, the exact interplay between autophagy and tumorigenesis mechanistically depends on different tumoral phases and types. Roughly, autophagic activity exerts inhibitory impact on tumor initiation by degrading misfold proteins while stimulatory effect during tumor development via decreasing lethal accumulations [215]. This may help us to comprehend the inconsistent role of ULK1 in various cancers, although the more specific explanations are still in deficiency.
3. Discussion and perspective
As we summarized above, accumulating investigations have outlined the hub-like role of AMPK in regulation of cell metabolism, autophagy, inflammation, proliferative cycle and tumorigenesis. Knockout and clinical evidences of AMPK subunits as well as its upstream and downstream molecules, listed in Table 1, Table 2 and Table 3, respectively, offer a more comprehensive perspective of the functional characteristics of AMPK signaling under both physiological and neoplastic circumstances. The translational medicine emphasizes the connection and transformation between basic research and clinical applications, trying to rectify the current dilemma that the published studies actually display little clinical significance despite of the absolute amount of literatures increase in a rapid rate annually [216]. On basis of our comprehensive review theming AMPK functionality, both in vivo and in vitro experiments have particularly declared its potential role as a tumor suppressor, implicating the underlying therapeutic worthiness of AMPK targeted strategy as an alternative option against routinely refractory malignancies.
To this end, metformin is the most widely prescribed anti-diabetic drug, and it’s function mainly through pharmaceutically targeting and stimulating the activity of AMPK heterotrimer (Table 4 and Figure 2). Besides its classical indications, several clinical trials have reported the comparative results of metformin usage within different cancers. In detail, neoadjuvant of metformin exhibits significantly anti-proliferative effects against ER-positive [217] and luminal B breast cancer [218]. However, the therapeutic benefits of metformin remain in controversies in digestive tumors, and the combination of metformin with gemcitabine or erlotinib does not improve the clinical prognosis of pancreatic cancer patients [219]. Nevertheless, employment of metformin patients could remarkably decrease the risk of malignant transformation for colon polyps [220] [221]. Taken together, the plausible interpretations of all these inconsistencies in metformin efficacy may mainly due to the distinctive activation status of the AMPK signaling. Moreover, the clinical trials towards neuroendocrine tumors as well as prostate cancer are still undergoing, which we believe will become vital additions to future analysis.
Table 4.
A list of major agonists and antagonists of AMPK and their potential clinical usage in human diseases
| Compounds | Function to AMPK | Clinical relevance | References |
|---|---|---|---|
| Metformin | AMPK agonist | Amelioration of diabetes and other relevant metabolic comorbidities | [320] |
| Anti-neoplastic efficacy in various cancers | [321–323] | ||
| Reduction of pulmonary fibrosis induced by Bleomycin | [324] | ||
| Protection against testicular ischemia | [325] | ||
| Neuroprotective impact after brain stroke | [326] | ||
| Survival benefit among patients with acute heart failure | [327] | ||
| Against several aging related and degenerative disorders | [328] | ||
| Improvement on multiple sclerosis | [329] | ||
| AICAR | AMPK agonist | Anti-diabetic effect | [330] |
| Reversal of alcohol-induced fatty liver | [331] | ||
| Pathological remission of severe spinal muscular atrophy | [332] | ||
| Suppression of multiple malignancies | [226, 333] | ||
| Improvement of dry eye | [334] | ||
| Alleviation of chemical hepatitis | [335] | ||
| Prevention from ischemia and reperfusion injury in diverse organs | [336, 337] | ||
| Enhanced cognition and motor coordination | [338] | ||
| A-769662 | AMPK agonist | Anti-obesity and diabetes | [339] |
| Amelioration of osteonecrosis | [340] | ||
| Reversal of inflammatory arthritis | [341] | ||
| Compound 13 | AMPK agonist | Anti-tumoral effect in melanoma | [342] |
| Protection efficacy in Helicobacter pylori infected gastric epithelium | [343] | ||
| Compound 7 | AMPK agonist | Improvement on diabetic nephropathy | [344] |
| DMC | AMPK agonist | Anti-diabetes | [345] |
| PT1 | AMPK agonist | Prevention from ischemic injury on cardiomyocytes | [346] |
| MT 63–78 | AMPK agonist | Inhibition on prostate cancer growth | [347] |
| Salicylate | AMPK agonist | Against diabetes | [348] |
| Decreasing the malignant properties of colorectal cancer, prostate cancer and lung cancer | [349, 350] | ||
| Compound C | AMPK antagonist | Inhibitory impact on several cancers including glioma, skin cancer, pancreatic cancer and colorectal cancer | [351–354] |
| Alleviation of obesity induced inflammatory reactions | [355] | ||
| Repression on circadian dysrhythmia | [356] |
Figure 2.
The clinical relevance of AMPK-targeted therapies in combating human disorders including diabetes, neoplasms, ischemia and inflammation.
5-Aminoimidazole-4-carboxamide riboside (AICAR or acadesine) is another well-investigated compound to structurally mimic AMP and functionally activate AMPK [222]. Accumulating evidences have demonstrated the therapeutic efficacy of AICAR administration in diverse malignancies. Clinically, AICAR intervention-mediated AMPK activation could significantly reverse the overexpression of HER2 and EGFR in vitro, hence triggering cell cycle arrest as well as shortening cell longevity in breast cancers [223, 224]. Meanwhile, AICAR synergizes with 5-fluorouracil (5-FU), a suicide inhibitor, to enhance apoptosis in colorectal [225] and gastric cancer cells [226]. Moreover, similar anti-neoplastic impact of AICAR is obtained within hepatocellular carcinoma [227], ovarian cancer [54] as well as hematological malignancy [225].
On the other hand, AMPK-independent mechanism of AICAR in regulation of tumorigenesis has been discovered. Specifically, AICAR generates programmed necrosis in prostate cancer cells via an N-acetylcysteine and cyclophilin-D dependent signals, instead of AMPK pathway [228]. Furthermore, AICAR is also able to inhibit the activation of the JNK/NANOG pathway to suppress its malignant properties in hepatocellular carcinoma [[229]. Overall, these results indicate the huge clinical potential of AICAR in the field of cancer treatment, although more clinical trials should be implemented for further safety and efficacy assessment.
Moreover, in addition to the above-mentioned activators, there are still various compounds serving as direct agonists or antagonists of AMPK, which exhibit great potentials for clinical applications (Table 4 and Figure 2). Taken together, AMPK and its signaling pathway has become a promising therapeutic target in terms of diverse human disorders, especially in metabolic syndrome and cancers.
Acknowledgments
The authors sincerely apologize to all those colleagues whose important work was not cited in this paper owing to space limitations. They thank the members of Wei laboratory for critical reading and discussion of the manuscript. W.W. is a Leukemia & Lymphoma Society (LLS) research scholar. J.G. is an NRSA T32 trainee and supported by 5T32HL007893-17. This work was supported in part by US National Institutes of Health (NIH) grants to W.W. (GM094777 and CA177910).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. NAT CELL BIOL. 2011;13:1016–1023. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bairwa SC, Parajuli N, Dyck JR. The role of AMPK in cardiomyocyte health and survival. Biochim Biophys Acta. 2016 doi: 10.1016/j.bbadis.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeon SM. Regulation and function of AMPK in physiology and diseases. EXP MOL MED. 2016;48:e245. doi: 10.1038/emm.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wall CE, Yu RT, Atkins AR, Downes M, Evans RM. Nuclear receptors and AMPK: can exercise mimetics cure diabetes? J MOL ENDOCRINOL. 2016;57:R49–R58. doi: 10.1530/JME-16-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hardie DG. AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes Dev. 2011;25:1895–1908. doi: 10.1101/gad.17420111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Novikova DS, Garabadzhiu AV, Melino G, Barlev NA, Tribulovich VG. AMP-activated protein kinase: structure, function, and role in pathological processes. Biochemistry (Mosc) 2015;80:127–144. doi: 10.1134/S0006297915020017. [DOI] [PubMed] [Google Scholar]
- 8.Rehman G, Shehzad A, Khan AL, Hamayun M. Role of AMP-activated protein kinase in cancer therapy. Arch Pharm (Weinheim) 2014;347:457–468. doi: 10.1002/ardp.201300402. [DOI] [PubMed] [Google Scholar]
- 9.Hardie DG, Alessi DR. LKB1 and AMPK and the cancer-metabolism link - ten years after. BMC BIOL. 2013;11:36. doi: 10.1186/1741-7007-11-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hardie DG. AMPK--sensing energy while talking to other signaling pathways. CELL METAB. 2014;20:939–952. doi: 10.1016/j.cmet.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salminen A, Kaarniranta K, Kauppinen A. AMPK and HIF signaling pathways regulate both longevity and cancer growth: the good news and the bad news about survival mechanisms. BIOGERONTOLOGY. 2016;17:655–680. doi: 10.1007/s10522-016-9655-7. [DOI] [PubMed] [Google Scholar]
- 12.Li W, Saud SM, Young MR, Chen G, Hua B. Targeting AMPK for cancer prevention and treatment. ONCOTARGET. 2015;6:7365–7378. doi: 10.18632/oncotarget.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stapleton D, Mitchelhill KI, Gao G, Widmer J, Michell BJ, Teh T, House CM, Fernandez CS, Cox T, Witters LA, Kemp BE. Mammalian AMP-activated protein kinase subfamily. J BIOL CHEM. 1996;271:611–614. doi: 10.1074/jbc.271.2.611. [DOI] [PubMed] [Google Scholar]
- 14.Lee YK, Park SY, Kim YM, Kim DC, Lee WS, Surh YJ, Park OJ. Suppression of mTOR via Akt-dependent and -independent mechanisms in selenium-treated colon cancer cells: involvement of AMPKalpha1. CARCINOGENESIS. 2010;31:1092–1099. doi: 10.1093/carcin/bgq040. [DOI] [PubMed] [Google Scholar]
- 15.Yie Y, Zhao S, Tang Q, Zheng F, Wu J, Yang L, Deng S, Hann SS. Ursolic acid inhibited growth of hepatocellular carcinoma HepG2 cells through AMPKalpha-mediated reduction of DNA methyltransferase 1. MOL CELL BIOCHEM. 2015;402:63–74. doi: 10.1007/s11010-014-2314-x. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Duan G, Feng S. MicroRNA-301a modulates doxorubicin resistance in osteosarcoma cells by targeting AMP-activated protein kinase alpha 1. Biochem Biophys Res Commun. 2015;459:367–373. doi: 10.1016/j.bbrc.2015.02.101. [DOI] [PubMed] [Google Scholar]
- 17.Obba S, Hizir Z, Boyer L, Selimoglu-Buet D, Pfeifer A, Michel G, Hamouda MA, Goncalves D, Cerezo M, Marchetti S, Rocchi S, Droin N, Cluzeau T, Robert G, Luciano F, Robaye B, Foretz M, Viollet B, Legros L, Solary E, Auberger P, Jacquel A. The PRKAA1/AMPKalpha1 pathway triggers autophagy during CSF1-induced human monocyte differentiation and is a potential target in CMML. AUTOPHAGY. 2015;11:1114–1129. doi: 10.1080/15548627.2015.1034406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang FY, Chiu PM, Tam KF, Kwok YK, Lau ET, Tang MH, Ng TY, Liu VW, Cheung AN, Ngan HY. Semi-quantitative fluorescent PCR analysis identifies PRKAA1 on chromosome 5 as a potential candidate cancer gene of cervical cancer. GYNECOL ONCOL. 2006;103:219–225. doi: 10.1016/j.ygyno.2006.02.028. [DOI] [PubMed] [Google Scholar]
- 19.Phoenix KN, Devarakonda CV, Fox MM, Stevens LE, Claffey KP. AMPKalpha2 Suppresses Murine Embryonic Fibroblast Transformation and Tumorigenesis. Genes Cancer. 2012;3:51–62. doi: 10.1177/1947601912452883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fox MM, Phoenix KN, Kopsiaftis SG, Claffey KP. AMP-Activated Protein Kinase alpha 2 Isoform Suppression in Primary Breast Cancer Alters AMPK Growth Control and Apoptotic Signaling. Genes Cancer. 2013;4:3–14. doi: 10.1177/1947601913486346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi CH, Chung JY, Cho H, Kitano H, Chang E, Ylaya K, Chung EJ, Kim JH, Hewitt SM. Prognostic Significance of AMP-Dependent Kinase Alpha Expression in Cervical Cancer. PATHOBIOLOGY. 2015;82:203–211. doi: 10.1159/000434726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neurath KM, Keough MP, Mikkelsen T, Claffey KP. AMP-dependent protein kinase alpha 2 isoform promotes hypoxia-induced VEGF expression in human glioblastoma. GLIA. 2006;53:733–743. doi: 10.1002/glia.20326. [DOI] [PubMed] [Google Scholar]
- 23.O’Neill HM, Maarbjerg SJ, Crane JD, Jeppesen J, Jorgensen SB, Schertzer JD, Shyroka O, Kiens B, van Denderen BJ, Tarnopolsky MA, Kemp BE, Richter EA, Steinberg GR. AMP-activated protein kinase (AMPK) beta1beta2 muscle null mice reveal an essential role for AMPK in maintaining mitochondrial content and glucose uptake during exercise. Proc Natl Acad Sci U S A. 2011;108:16092–16097. doi: 10.1073/pnas.1105062108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steinberg GR, O’Neill HM, Dzamko NL, Galic S, Naim T, Koopman R, Jorgensen SB, Honeyman J, Hewitt K, Chen ZP, Schertzer JD, Scott JW, Koentgen F, Lynch GS, Watt MJ, van Denderen BJ, Campbell DJ, Kemp BE. Whole body deletion of AMP-activated protein kinase {beta}2 reduces muscle AMPK activity and exercise capacity. J BIOL CHEM. 2010;285:37198–37209. doi: 10.1074/jbc.M110.102434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu WY, Jiang RS. Advances in the research of AMPK and its subunit genes. Pak J Biol Sci. 2013;16:1459–1468. doi: 10.3923/pjbs.2013.1459.1468. [DOI] [PubMed] [Google Scholar]
- 26.Feng Z, Hu W, de Stanchina E, Teresky AK, Jin S, Lowe S, Levine AJ. The regulation of AMPK beta1, TSC2, and PTEN expression by p53: stress, cell and tissue specificity, and the role of these gene products in modulating the IGF-1-AKT-mTOR pathways. CANCER RES. 2007;67:3043–3053. doi: 10.1158/0008-5472.CAN-06-4149. [DOI] [PubMed] [Google Scholar]
- 27.Li J, Jiang P, Robinson M, Lawrence TS, Sun Y. AMPK-beta1 subunit is a p53-independent stress responsive protein that inhibits tumor cell growth upon forced expression. CARCINOGENESIS. 2003;24:827–834. doi: 10.1093/carcin/bgg032. [DOI] [PubMed] [Google Scholar]
- 28.Li C, Liu VW, Chiu PM, Yao KM, Ngan HY, Chan DW. Reduced expression of AMPK-beta1 during tumor progression enhances the oncogenic capacity of advanced ovarian cancer. MOL CANCER. 2014;13:49. doi: 10.1186/1476-4598-13-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li C, Liu VW, Chiu PM, Chan DW, Ngan HY. Over-expressions of AMPK subunits in ovarian carcinomas with significant clinical implications. BMC CANCER. 2012;12:357. doi: 10.1186/1471-2407-12-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moffat C, Harper ME. Metabolic functions of AMPK: aspects of structure and of natural mutations in the regulatory gamma subunits. IUBMB LIFE. 2010;62:739–745. doi: 10.1002/iub.387. [DOI] [PubMed] [Google Scholar]
- 31.Jensen TE, Ross FA, Kleinert M, Sylow L, Knudsen JR, Gowans GJ, Hardie DG, Richter EA. PT-1 selectively activates AMPK-gamma1 complexes in mouse skeletal muscle, but activates all three gamma subunit complexes in cultured human cells by inhibiting the respiratory chain. BIOCHEM J. 2015;467:461–472. doi: 10.1042/BJ20141142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ofir M, Arad M, Porat E, Freimark D, Chepurko Y, Vidne BA, Seidman CE, Seidman JG, Kemp BE, Hochhauser E. Increased glycogen stores due to gamma-AMPK overexpression protects against ischemia and reperfusion damage. BIOCHEM PHARMACOL. 2008;75:1482–1491. doi: 10.1016/j.bcp.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 33.Foretz M, Hebrard S, Guihard S, Leclerc J, Do CM, Hamard G, Niedergang F, Gaudry M, Viollet B. The AMPKgamma1 subunit plays an essential role in erythrocyte membrane elasticity, and its genetic inactivation induces splenomegaly and anemia. FASEB J. 2011;25:337–347. doi: 10.1096/fj.10-169383. [DOI] [PubMed] [Google Scholar]
- 34.Choi MR, An CH, Yoo NJ, Lee SH. Frameshift mutations of PRKAG1 gene encoding an AMPK gamma subunit in colorectal cancers. J Gastrointestin Liver Dis. 2014;23:343–345. [PubMed] [Google Scholar]
- 35.Gan RY, Li HB. Recent progress on liver kinase B1 (LKB1): expression, regulation, downstream signaling and cancer suppressive function. INT J MOL SCI. 2014;15:16698–16718. doi: 10.3390/ijms150916698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Porat-Shliom N, Tietgens AJ, Van Itallie CM, Vitale-Cross L, Jarnik M, Harding OJ, Anderson JM, Gutkind JS, Weigert R, Arias IM. Liver kinase B1 regulates hepatocellular tight junction distribution and function in vivo. HEPATOLOGY. 2016 doi: 10.1002/hep.28724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Korsse SE, Peppelenbosch MP, van Veelen W. Targeting LKB1 signaling in cancer. Biochim Biophys Acta. 2013;1835:194–210. doi: 10.1016/j.bbcan.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 38.Dasgupta B, Chhipa RR. Evolving Lessons on the Complex Role of AMPK in Normal Physiology and Cancer. TRENDS PHARMACOL SCI. 2016;37:192–206. doi: 10.1016/j.tips.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci U S A. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Momcilovic M, Shackelford DB. Targeting LKB1 in cancer - exposing and exploiting vulnerabilities. Br J Cancer. 2015;113:574–584. doi: 10.1038/bjc.2015.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee SW, Li CF, Jin G, Cai Z, Han F, Chan CH, Yang WL, Li BK, Rezaeian AH, Li HY, Huang HY, Lin HK. Skp2-dependent ubiquitination and activation of LKB1 is essential for cancer cell survival under energy stress. MOL CELL. 2015;57:1022–1033. doi: 10.1016/j.molcel.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andrade-Vieira R, Goguen D, Bentley HA, Bowen CV, Marignani PA. Pre-clinical study of drug combinations that reduce breast cancer burden due to aberrant mTOR and metabolism promoted by LKB1 loss. ONCOTARGET. 2014;5:12738–12752. doi: 10.18632/oncotarget.2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bardeesy N, Sinha M, Hezel AF, Signoretti S, Hathaway NA, Sharpless NE, Loda M, Carrasco DR, DePinho RA. Loss of the Lkb1 tumour suppressor provokes intestinal polyposis but resistance to transformation. NATURE. 2002;419:162–167. doi: 10.1038/nature01045. [DOI] [PubMed] [Google Scholar]
- 44.Ji H, Ramsey MR, Hayes DN, Fan C, McNamara K, Kozlowski P, Torrice C, Wu MC, Shimamura T, Perera SA, Liang MC, Cai D, Naumov GN, Bao L, Contreras CM, Li D, Chen L, Krishnamurthy J, Koivunen J, Chirieac LR, Padera RF, Bronson RT, Lindeman NI, Christiani DC, Lin X, Shapiro GI, Janne PA, Johnson BE, Meyerson M, Kwiatkowski DJ, Castrillon DH, Bardeesy N, Sharpless NE, Wong KK. LKB1 modulates lung cancer differentiation and metastasis. NATURE. 2007;448:807–810. doi: 10.1038/nature06030. [DOI] [PubMed] [Google Scholar]
- 45.Shackelford DB. Unravelling the connection between metabolism and tumorigenesis through studies of the liver kinase B1 tumour suppressor. J Carcinog. 2013;12:16. doi: 10.4103/1477-3163.116323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun J, Ling B, Xu X, Ma R, Li G, Cao X, Ling W, Yang Z, Hoffman RM, Lu J. Decreased Expression of Tumor-suppressor Gene LKB1 Correlates with Poor Prognosis in Human Gastric Cancer. ANTICANCER RES. 2016;36:869–875. [PubMed] [Google Scholar]
- 47.Barbier-Torres L, Delgado TC, Garcia-Rodriguez JL, Zubiete-Franco I, Fernandez-Ramos D, Buque X, Cano A, Gutierrez-de JV, Fernandez-Dominguez I, Lopitz-Otsoa F, Fernandez-Tussy P, Boix L, Bruix J, Villa E, Castro A, Lu SC, Aspichueta P, Xirodimas D, Varela-Rey M, Mato JM, Beraza N, Martinez-Chantar ML. Stabilization of LKB1 and Akt by neddylation regulates energy metabolism in liver cancer. ONCOTARGET. 2015;6:2509–2523. doi: 10.18632/oncotarget.3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li F, Han X, Li F, Wang R, Wang H, Gao Y, Wang X, Fang Z, Zhang W, Yao S, Tong X, Wang Y, Feng Y, Sun Y, Li Y, Wong KK, Zhai Q, Chen H, Ji H. LKB1 Inactivation Elicits a Redox Imbalance to Modulate Non-small Cell Lung Cancer Plasticity and Therapeutic Response. CANCER CELL. 2015;27:698–711. doi: 10.1016/j.ccell.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pena CG, Nakada Y, Saatcioglu HD, Aloisio GM, Cuevas I, Zhang S, Miller DS, Lea JS, Wong KK, DeBerardinis RJ, Amelio AL, Brekken RA, Castrillon DH. LKB1 loss promotes endometrial cancer progression via CCL2-dependent macrophage recruitment. J CLIN INVEST. 2015;125:4063–4076. doi: 10.1172/JCI82152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yao YH, Cui Y, Qiu XN, Zhang LZ, Zhang W, Li H, Yu JM. Attenuated LKB1-SIK1 signaling promotes epithelial-mesenchymal transition and radioresistance of non-small cell lung cancer cells. Chin J Cancer. 2016;35:50. doi: 10.1186/s40880-016-0113-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ma LG, Bian SB, Cui JX, Xi HQ, Zhang KC, Qin HZ, Zhu XM, Chen L. LKB1 inhibits the proliferation of gastric cancer cells by suppressing the nuclear translocation of Yap and beta-catenin. INT J MOL MED. 2016;37:1039–1048. doi: 10.3892/ijmm.2016.2494. [DOI] [PubMed] [Google Scholar]
- 52.Koyama S, Akbay EA, Li YY, Aref AR, Skoulidis F, Herter-Sprie GS, Buczkowski KA, Liu Y, Awad MM, Denning WL, Diao L, Wang J, Parra-Cuentas ER, Wistuba II, Soucheray M, Thai T, Asahina H, Kitajima S, Altabef A, Cavanaugh JD, Rhee K, Gao P, Zhang H, Fecci PE, Shimamura T, Hellmann MD, Heymach JV, Hodi FS, Freeman GJ, Barbie DA, Dranoff G, Hammerman PS, Wong KK. STK11/LKB1 Deficiency Promotes Neutrophil Recruitment and Proinflammatory Cytokine Production to Suppress T-cell Activity in the Lung Tumor Microenvironment. CANCER RES. 2016;76:999–1008. doi: 10.1158/0008-5472.CAN-15-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li NS, Zou JR, Lin H, Ke R, He XL, Xiao L, Huang D, Luo L, Lv N, Luo Z. LKB1/AMPK inhibits TGF-beta1 production and the TGF-beta signaling pathway in breast cancer cells. Tumour Biol. 2016;37:8249–8258. doi: 10.1007/s13277-015-4639-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peart T, Ramos VY, Correa RJ, Fazio E, Bertrand M, McGee J, Prefontaine M, Sugimoto A, DiMattia GE, Shepherd TG. Intact LKB1 activity is required for survival of dormant ovarian cancer spheroids. ONCOTARGET. 2015;6:22424–22438. doi: 10.18632/oncotarget.4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Whang YM, Park SI, Trenary IA, Egnatchik RA, Fessel JP, Kaufman JM, Carbone DP, Young JD. LKB1 deficiency enhances sensitivity to energetic stress induced by erlotinib treatment in non-small-cell lung cancer (NSCLC) cells. ONCOGENE. 2016;35:856–866. doi: 10.1038/onc.2015.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Racioppi L, Means AR. Calcium/calmodulin-dependent protein kinase kinase 2: roles in signaling and pathophysiology. J BIOL CHEM. 2012;287:31658–31665. doi: 10.1074/jbc.R112.356485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Racioppi L. CaMKK2: a novel target for shaping the androgen-regulated tumor ecosystem. TRENDS MOL MED. 2013;19:83–88. doi: 10.1016/j.molmed.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nelson PS, Clegg N, Arnold H, Ferguson C, Bonham M, White J, Hood L, Lin B. The program of androgen-responsive genes in neoplastic prostate epithelium. Proc Natl Acad Sci U S A. 2002;99:11890–11895. doi: 10.1073/pnas.182376299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Frigo DE, Howe MK, Wittmann BM, Brunner AM, Cushman I, Wang Q, Brown M, Means AR, McDonnell DP. CaM kinase kinase beta-mediated activation of the growth regulatory kinase AMPK is required for androgen-dependent migration of prostate cancer cells. CANCER RES. 2011;71:528–537. doi: 10.1158/0008-5472.CAN-10-2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Massie CE, Lynch A, Ramos-Montoya A, Boren J, Stark R, Fazli L, Warren A, Scott H, Madhu B, Sharma N, Bon H, Zecchini V, Smith DM, Denicola GM, Mathews N, Osborne M, Hadfield J, Macarthur S, Adryan B, Lyons SK, Brindle KM, Griffiths J, Gleave ME, Rennie PS, Neal DE, Mills IG. The androgen receptor fuels prostate cancer by regulating central metabolism and biosynthesis. EMBO J. 2011;30:2719–2733. doi: 10.1038/emboj.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shima T, Mizokami A, Miyagi T, Kawai K, Izumi K, Kumaki M, Ofude M, Zhang J, Keller ET, Namiki M. Down-regulation of calcium/calmodulin-dependent protein kinase kinase 2 by androgen deprivation induces castration-resistant prostate cancer. PROSTATE. 2012;72:1789–1801. doi: 10.1002/pros.22533. [DOI] [PubMed] [Google Scholar]
- 62.Subbannayya Y, Syed N, Barbhuiya MA, Raja R, Marimuthu A, Sahasrabuddhe N, Pinto SM, Manda SS, Renuse S, Manju HC, Zameer MA, Sharma J, Brait M, Srikumar K, Roa JC, Vijaya KM, Kumar KV, Prasad TS, Ramaswamy G, Kumar RV, Pandey A, Gowda H, Chatterjee A. Calcium calmodulin dependent kinase kinase 2 - a novel therapeutic target for gastric adenocarcinoma. CANCER BIOL THER. 2015;16:336–345. doi: 10.4161/15384047.2014.972264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Inokuchi-Shimizu S, Park EJ, Roh YS, Yang L, Zhang B, Song J, Liang S, Pimienta M, Taniguchi K, Wu X, Asahina K, Lagakos W, Mackey MR, Akira S, Ellisman MH, Sears DD, Olefsky JM, Karin M, Brenner DA, Seki E. TAK1-mediated autophagy and fatty acid oxidation prevent hepatosteatosis and tumorigenesis. J CLIN INVEST. 2014;124:3566–3578. doi: 10.1172/JCI74068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim SY, Jeong S, Jung E, Baik KH, Chang MH, Kim SA, Shim JH, Chun E, Lee KY. AMP-activated protein kinase-alpha1 as an activating kinase of TGF-beta-activated kinase 1 has a key role in inflammatory signals. CELL DEATH DIS. 2012;3:e357. doi: 10.1038/cddis.2012.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang B, Wang XB, Chen LY, Huang L, Dong RZ. Belinostat-induced apoptosis and growth inhibition in pancreatic cancer cells involve activation of TAK1-AMPK signaling axis. Biochem Biophys Res Commun. 2013;437:1–6. doi: 10.1016/j.bbrc.2013.05.090. [DOI] [PubMed] [Google Scholar]
- 66.Yang L, Inokuchi S, Roh YS, Song J, Loomba R, Park EJ, Seki E. Transforming growth factor-beta signaling in hepatocytes promotes hepatic fibrosis and carcinogenesis in mice with hepatocyte-specific deletion of TAK1. GASTROENTEROLOGY. 2013;144:1042–1054. doi: 10.1053/j.gastro.2013.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Singh A, Sweeney MF, Yu M, Burger A, Greninger P, Benes C, Haber DA, Settleman J. TAK1 inhibition promotes apoptosis in KRAS-dependent colon cancers. CELL. 2012;148:639–650. doi: 10.1016/j.cell.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sigloch FC, Burk UC, Biniossek ML, Brabletz T, Schilling O. miR-200c dampens cancer cell migration via regulation of protein kinase A subunits. ONCOTARGET. 2015;6:23874–23889. doi: 10.18632/oncotarget.4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Keil MF, Briassoulis G, Stratakis CA, Wu TJ. Protein Kinase A and Anxiety-Related Behaviors: A Mini-Review. Front Endocrinol (Lausanne) 2016;7:83. doi: 10.3389/fendo.2016.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Djouder N, Tuerk RD, Suter M, Salvioni P, Thali RF, Scholz R, Vaahtomeri K, Auchli Y, Rechsteiner H, Brunisholz RA, Viollet B, Makela TP, Wallimann T, Neumann D, Krek W. PKA phosphorylates and inactivates AMPKalpha to promote efficient lipolysis. EMBO J. 2010;29:469–481. doi: 10.1038/emboj.2009.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Beristain AG, Molyneux SD, Joshi PA, Pomroy NC, Di Grappa MA, Chang MC, Kirschner LS, Prive GG, Pujana MA, Khokha R. PKA signaling drives mammary tumorigenesis through Src. ONCOGENE. 2015;34:1160–1173. doi: 10.1038/onc.2014.41. [DOI] [PubMed] [Google Scholar]
- 72.Wang H, Sun T, Hu J, Zhang R, Rao Y, Wang S, Chen R, McLendon RE, Friedman AH, Keir ST, Bigner DD, Li QJ, Wang H, Wang XF. miR-33a promotes glioma-initiating cell self-renewal via PKA and NOTCH pathways. J CLIN INVEST. 2014;124:4489–4502. doi: 10.1172/JCI75284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ferretti AC, Tonucci FM, Hidalgo F, Almada E, Larocca MC, Favre C. AMPK and PKA interaction in the regulation of survival of liver cancer cells subjected to glucose starvation. ONCOTARGET. 2016;7:17815–17828. doi: 10.18632/oncotarget.7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Del GA, Peverelli E, Treppiedi D, Lania A, Mantovani G, Ferrero S. Expression of protein kinase A regulatory subunits in benign and malignant human thyroid tissues: A systematic review. EXP CELL RES. 2016;346:85–90. doi: 10.1016/j.yexcr.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 75.Medina EA, Oberheu K, Polusani SR, Ortega V, Velagaleti GV, Oyajobi BO. PKA/AMPK signaling in relation to adiponectin’s antiproliferative effect on multiple myeloma cells. LEUKEMIA. 2014;28:2080–2089. doi: 10.1038/leu.2014.112. [DOI] [PubMed] [Google Scholar]
- 76.Iglesias-Bartolome R, Torres D, Marone R, Feng X, Martin D, Simaan M, Chen M, Weinstein LS, Taylor SS, Molinolo AA, Gutkind JS. Inactivation of a Galpha(s)-PKA tumour suppressor pathway in skin stem cells initiates basal-cell carcinogenesis. NAT CELL BIOL. 2015;17:793–803. doi: 10.1038/ncb3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pattabiraman DR, Bierie B, Kober KI, Thiru P, Krall JA, Zill C, Reinhardt F, Tam WL, Weinberg RA. Activation of PKA leads to mesenchymal-to-epithelial transition and loss of tumor-initiating ability. SCIENCE. 2016;351:d3680. doi: 10.1126/science.aad3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Beurel E, Grieco SF, Jope RS. Glycogen synthase kinase-3 (GSK3): regulation, actions, and diseases. Pharmacol Ther. 2015;148:114–131. doi: 10.1016/j.pharmthera.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Suzuki T, Bridges D, Nakada D, Skiniotis G, Morrison SJ, Lin JD, Saltiel AR, Inoki K. Inhibition of AMPK catabolic action by GSK3. MOL CELL. 2013;50:407–419. doi: 10.1016/j.molcel.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McCubrey JA, Steelman LS, Bertrand FE, Davis NM, Sokolosky M, Abrams SL, Montalto G, D’Assoro AB, Libra M, Nicoletti F, Maestro R, Basecke J, Rakus D, Gizak A, Demidenko ZN, Cocco L, Martelli AM, Cervello M. GSK-3 as potential target for therapeutic intervention in cancer. ONCOTARGET. 2014;5:2881–2911. doi: 10.18632/oncotarget.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li B, Thrasher JB, Terranova P. Glycogen synthase kinase-3: a potential preventive target for prostate cancer management. Urol Oncol. 2015;33:456–463. doi: 10.1016/j.urolonc.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tejeda-Munoz N, Robles-Flores M. Glycogen synthase kinase 3 in Wnt signaling pathway and cancer. IUBMB LIFE. 2015;67:914–922. doi: 10.1002/iub.1454. [DOI] [PubMed] [Google Scholar]
- 83.Rincon R, Cristobal I, Zazo S, Arpi O, Menendez S, Manso R, Lluch A, Eroles P, Rovira A, Albanell J, Garcia-Foncillas J, Madoz-Gurpide J, Rojo F. PP2A inhibition determines poor outcome and doxorubicin resistance in early breast cancer and its activation shows promising therapeutic effects. ONCOTARGET. 2015;6:4299–4314. doi: 10.18632/oncotarget.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Seshacharyulu P, Pandey P, Datta K, Batra SK. Phosphatase: PP2A structural importance, regulation and its aberrant expression in cancer. CANCER LETT. 2013;335:9–18. doi: 10.1016/j.canlet.2013.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wu Y, Song P, Xu J, Zhang M, Zou MH. Activation of protein phosphatase 2A by palmitate inhibits AMP-activated protein kinase. J BIOL CHEM. 2007;282:9777–9788. doi: 10.1074/jbc.M608310200. [DOI] [PubMed] [Google Scholar]
- 86.Park S, Scheffler TL, Rossie SS, Gerrard DE. AMPK activity is regulated by calcium-mediated protein phosphatase 2A activity. CELL CALCIUM. 2013;53:217–223. doi: 10.1016/j.ceca.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 87.Salminen A, Kaarniranta K, Kauppinen A. Age-related changes in AMPK activation: Role for AMPK phosphatases and inhibitory phosphorylation by upstream signaling pathways. AGEING RES REV. 2016;28:15–26. doi: 10.1016/j.arr.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 88.Steinberg GR, Michell BJ, van Denderen BJ, Watt MJ, Carey AL, Fam BC, Andrikopoulos S, Proietto J, Gorgun CZ, Carling D, Hotamisligil GS, Febbraio MA, Kay TW, Kemp BE. Tumor necrosis factor alpha-induced skeletal muscle insulin resistance involves suppression of AMP-kinase signaling. CELL METAB. 2006;4:465–474. doi: 10.1016/j.cmet.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 89.Zhu Y, Demidov ON, Goh AM, Virshup DM, Lane DP, Bulavin DV. Phosphatase WIP1 regulates adult neurogenesis and WNT signaling during aging. J CLIN INVEST. 2014;124:3263–3273. doi: 10.1172/JCI73015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Voss M, Paterson J, Kelsall IR, Martin-Granados C, Hastie CJ, Peggie MW, Cohen PT. Ppm1E is an in cellulo AMP-activated protein kinase phosphatase. CELL SIGNAL. 2011;23:114–124. doi: 10.1016/j.cellsig.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 91.Nijman SM, Luna-Vargas MP, Velds A, Brummelkamp TR, Dirac AM, Sixma TK, Bernards R. A genomic and functional inventory of deubiquitinating enzymes. CELL. 2005;123:773–786. doi: 10.1016/j.cell.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 92.Zeng Z, Wu HX, Zhan N, Huang YB, Wang ZS, Yang GF, Wang P, Fu GH. Prognostic significance of USP10 as a tumor-associated marker in gastric carcinoma. Tumour Biol. 2014;35:3845–3853. doi: 10.1007/s13277-013-1509-1. [DOI] [PubMed] [Google Scholar]
- 93.Yuan J, Luo K, Zhang L, Cheville JC, Lou Z. USP10 regulates p53 localization and stability by deubiquitinating p53. CELL. 2010;140:384–396. doi: 10.1016/j.cell.2009.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Deng M, Yang X, Qin B, Liu T, Zhang H, Guo W, Lee SB, Kim JJ, Yuan J, Pei H, Wang L, Lou Z. Deubiquitination and Activation of AMPK by USP10. MOL CELL. 2016;61:614–624. doi: 10.1016/j.molcel.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang M, Hu C, Tong D, Xiang S, Williams K, Bai W, Li GM, Bepler G, Zhang X. Ubiquitin-specific Peptidase 10 (USP10) Deubiquitinates and Stabilizes MutS Homolog 2 (MSH2) to Regulate Cellular Sensitivity to DNA Damage. J BIOL CHEM. 2016;291:10783–10791. doi: 10.1074/jbc.M115.700047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cao Y, Wei M, Li B, Liu Y, Lu Y, Tang Z, Lu T, Yin Y, Qin Z, Xu Z. Functional role of eukaryotic translation initiation factor 4 gamma 1 (EIF4G1) in NSCLC. ONCOTARGET. 2016 doi: 10.18632/oncotarget.8168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu H, Xu XF, Zhao Y, Tang MC, Zhou YQ, Lu J, Gao FH. MicroRNA-191 promotes pancreatic cancer progression by targeting USP10. Tumour Biol. 2014;35:12157–12163. doi: 10.1007/s13277-014-2521-9. [DOI] [PubMed] [Google Scholar]
- 98.Lin Z, Yang H, Tan C, Li J, Liu Z, Quan Q, Kong S, Ye J, Gao B, Fang D. USP10 antagonizes c-Myc transcriptional activation through SIRT6 stabilization to suppress tumor formation. CELL REP. 2013;5:1639–1649. doi: 10.1016/j.celrep.2013.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Oi N, Yuan J, Malakhova M, Luo K, Li Y, Ryu J, Zhang L, Bode AM, Xu Z, Li Y, Lou Z, Dong Z. Resveratrol induces apoptosis by directly targeting Ras-GTPase-activating protein SH3 domain-binding protein 1. ONCOGENE. 2015;34:2660–2671. doi: 10.1038/onc.2014.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ulitsky I, Bartel DP. lincRNAs: genomics, evolution, and mechanisms. CELL. 2013;154:26–46. doi: 10.1016/j.cell.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xiao ZD, Zhuang L, Gan B. Long non-coding RNAs in cancer metabolism. BIOESSAYS. 2016 doi: 10.1002/bies.201600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xu CF, Brown MA, Nicolai H, Chambers JA, Griffiths BL, Solomon E. Isolation and characterisation of the NBR2 gene which lies head to head with the human BRCA1 gene. HUM MOL GENET. 1997;6:1057–1062. doi: 10.1093/hmg/6.7.1057. [DOI] [PubMed] [Google Scholar]
- 103.Liu X, Xiao ZD, Gan B. An lncRNA switch for AMPK activation. CELL CYCLE. 2016;15:1948–1949. doi: 10.1080/15384101.2016.1184515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu X, Xiao ZD, Han L, Zhang J, Lee SW, Wang W, Lee H, Zhuang L, Chen J, Lin HK, Wang J, Liang H, Gan B. LncRNA NBR2 engages a metabolic checkpoint by regulating AMPK under energy stress. NAT CELL BIOL. 2016;18:431–442. doi: 10.1038/ncb3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tan Z, Luo X, Xiao L, Tang M, Bode AM, Dong Z, Cao Y. The Role of PGC1alpha in Cancer Metabolism and its Therapeutic Implications. MOL CANCER THER. 2016;15:774–782. doi: 10.1158/1535-7163.MCT-15-0621. [DOI] [PubMed] [Google Scholar]
- 106.Puigserver P, Adelmant G, Wu Z, Fan M, Xu J, O’Malley B, Spiegelman BM. Activation of PPARgamma coactivator-1 through transcription factor docking. SCIENCE. 1999;286:1368–1371. doi: 10.1126/science.286.5443.1368. [DOI] [PubMed] [Google Scholar]
- 107.Wallberg AE, Yamamura S, Malik S, Spiegelman BM, Roeder RG. Coordination of p300-mediated chromatin remodeling and TRAP/mediator function through coactivator PGC-1alpha. MOL CELL. 2003;12:1137–1149. doi: 10.1016/s1097-2765(03)00391-5. [DOI] [PubMed] [Google Scholar]
- 108.Monsalve M, Wu Z, Adelmant G, Puigserver P, Fan M, Spiegelman BM. Direct coupling of transcription and mRNA processing through the thermogenic coactivator PGC-1. MOL CELL. 2000;6:307–316. doi: 10.1016/s1097-2765(00)00031-9. [DOI] [PubMed] [Google Scholar]
- 109.Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. NATURE. 2002;418:797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- 111.Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R, Landaker EJ, Goldfine AB, Mun E, DeFronzo R, Finlayson J, Kahn CR, Mandarino LJ. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc Natl Acad Sci U S A. 2003;100:8466–8471. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bhalla K, Hwang BJ, Dewi RE, Ou L, Twaddel W, Fang HB, Vafai SB, Vazquez F, Puigserver P, Boros L, Girnun GD. PGC1alpha promotes tumor growth by inducing gene expression programs supporting lipogenesis. CANCER RES. 2011;71:6888–6898. doi: 10.1158/0008-5472.CAN-11-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shiota M, Yokomizo A, Tada Y, Inokuchi J, Tatsugami K, Kuroiwa K, Uchiumi T, Fujimoto N, Seki N, Naito S. Peroxisome proliferator-activated receptor gamma coactivator-1alpha interacts with the androgen receptor (AR) and promotes prostate cancer cell growth by activating the AR. MOL ENDOCRINOL. 2010;24:114–127. doi: 10.1210/me.2009-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vazquez F, Lim JH, Chim H, Bhalla K, Girnun G, Pierce K, Clish CB, Granter SR, Widlund HR, Spiegelman BM, Puigserver P. PGC1alpha expression defines a subset of human melanoma tumors with increased mitochondrial capacity and resistance to oxidative stress. CANCER CELL. 2013;23:287–301. doi: 10.1016/j.ccr.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Taguchi A, Delgado O, Celiktas M, Katayama H, Wang H, Gazdar AF, Hanash SM. Proteomic signatures associated with p53 mutational status in lung adenocarcinoma. PROTEOMICS. 2014;14:2750–2759. doi: 10.1002/pmic.201400378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chiacchiera F, Simone C. The AMPK-FoxO3A axis as a target for cancer treatment. CELL CYCLE. 2010;9:1091–1096. doi: 10.4161/cc.9.6.11035. [DOI] [PubMed] [Google Scholar]
- 117.Furuyama T, Nakazawa T, Nakano I, Mori N. Identification of the differential distribution patterns of mRNAs and consensus binding sequences for mouse DAF-16 homologues. BIOCHEM J. 2000;349:629–634. doi: 10.1042/0264-6021:3490629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Greer EL, Oskoui PR, Banko MR, Maniar JM, Gygi MP, Gygi SP, Brunet A. The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J BIOL CHEM. 2007;282:30107–30119. doi: 10.1074/jbc.M705325200. [DOI] [PubMed] [Google Scholar]
- 119.Liu J, Peng Y, Wang X, Fan Y, Qin C, Shi L, Tang Y, Cao K, Li H, Long J, Liu J. Mitochondrial Dysfunction Launches Dexamethasone-Induced Skeletal Muscle Atrophy via AMPK/FOXO3 Signaling. Mol Pharm. 2016;13:73–84. doi: 10.1021/acs.molpharmaceut.5b00516. [DOI] [PubMed] [Google Scholar]
- 120.Ido Y, Duranton A, Lan F, Weikel KA, Breton L, Ruderman NB. Resveratrol prevents oxidative stress-induced senescence and proliferative dysfunction by activating the AMPK-FOXO3 cascade in cultured primary human keratinocytes. PLOS ONE. 2015;10:e115341. doi: 10.1371/journal.pone.0115341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shrestha A, Nepal S, Kim MJ, Chang JH, Kim SH, Jeong GS, Jeong CH, Park GH, Jung S, Lim J, Cho E, Lee S, Park PH. Critical Role of AMPK/FoxO3A Axis in Globular Adiponectin-Induced Cell Cycle Arrest and Apoptosis in Cancer Cells. J CELL PHYSIOL. 2016;231:357–369. doi: 10.1002/jcp.25080. [DOI] [PubMed] [Google Scholar]
- 122.Paik JH, Kollipara R, Chu G, Ji H, Xiao Y, Ding Z, Miao L, Tothova Z, Horner JW, Carrasco DR, Jiang S, Gilliland DG, Chin L, Wong WH, Castrillon DH, DePinho RA. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. CELL. 2007;128:309–323. doi: 10.1016/j.cell.2006.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Coomans DBA, Demoulin JB. FOXO transcription factors in cancer development and therapy. CELL MOL LIFE SCI. 2016;73:1159–1172. doi: 10.1007/s00018-015-2112-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tenbaum SP, Ordonez-Moran P, Puig I, Chicote I, Arques O, Landolfi S, Fernandez Y, Herance JR, Gispert JD, Mendizabal L, Aguilar S, Ramon YCS, Schwartz SJ, Vivancos A, Espin E, Rojas S, Baselga J, Tabernero J, Munoz A, Palmer HG. beta-catenin confers resistance to PI3K and AKT inhibitors and subverts FOXO3a to promote metastasis in colon cancer. NAT MED. 2012;18:892–901. doi: 10.1038/nm.2772. [DOI] [PubMed] [Google Scholar]
- 125.Sisci D, Maris P, Cesario MG, Anselmo W, Coroniti R, Trombino GE, Romeo F, Ferraro A, Lanzino M, Aquila S, Maggiolini M, Mauro L, Morelli C, Ando S. The estrogen receptor alpha is the key regulator of the bifunctional role of FoxO3a transcription factor in breast cancer motility and invasiveness. CELL CYCLE. 2013;12:3405–3420. doi: 10.4161/cc.26421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kohjima M, Higuchi N, Kato M, Kotoh K, Yoshimoto T, Fujino T, Yada M, Yada R, Harada N, Enjoji M, Takayanagi R, Nakamuta M. SREBP-1c, regulated by the insulin and AMPK signaling pathways, plays a role in nonalcoholic fatty liver disease. INT J MOL MED. 2008;21:507–511. [PubMed] [Google Scholar]
- 127.Li Y, Xu S, Mihaylova MM, Zheng B, Hou X, Jiang B, Park O, Luo Z, Lefai E, Shyy JY, Gao B, Wierzbicki M, Verbeuren TJ, Shaw RJ, Cohen RA, Zang M. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. CELL METAB. 2011;13:376–388. doi: 10.1016/j.cmet.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhao Y, Li H, Zhang Y, Li L, Fang R, Li Y, Liu Q, Zhang W, Qiu L, Liu F, Zhang X, Ye L. Oncoprotein HBXIP modulates abnormal lipid metabolism and growth of breast cancer cells by activating the LXRs/SREBP-1c/FAS signaling cascade. CANCER RES. 2016 doi: 10.1158/0008-5472.CAN-15-1734. [DOI] [PubMed] [Google Scholar]
- 129.Sun Y, He W, Luo M, Zhou Y, Chang G, Ren W, Wu K, Li X, Shen J, Zhao X, Hu Y. SREBP1 regulates tumorigenesis and prognosis of pancreatic cancer through targeting lipid metabolism. Tumour Biol. 2015;36:4133–4141. doi: 10.1007/s13277-015-3047-5. [DOI] [PubMed] [Google Scholar]
- 130.Li X, Chen YT, Josson S, Mukhopadhyay NK, Kim J, Freeman MR, Huang WC. MicroRNA-185 and 342 inhibit tumorigenicity and induce apoptosis through blockade of the SREBP metabolic pathway in prostate cancer cells. PLOS ONE. 2013;8:e70987. doi: 10.1371/journal.pone.0070987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Qiu C, Dongol S, Lv QT, Gao X, Jiang J. Sterol regulatory element-binding protein-1/fatty acid synthase involvement in proliferation inhibition and apoptosis promotion induced by progesterone in endometrial cancer. INT J GYNECOL CANCER. 2013;23:1629–1634. doi: 10.1097/IGC.0000000000000004. [DOI] [PubMed] [Google Scholar]
- 132.Lin L, Zheng X, Qiu C, Dongol S, Lv Q, Jiang J, Kong B, Wang C. SIRT1 promotes endometrial tumor growth by targeting SREBP1 and lipogenesis. ONCOL REP. 2014;32:2831–2835. doi: 10.3892/or.2014.3521. [DOI] [PubMed] [Google Scholar]
- 133.Li C, Yang W, Zhang J, Zheng X, Yao Y, Tu K, Liu Q. SREBP-1 has a prognostic role and contributes to invasion and metastasis in human hepatocellular carcinoma. INT J MOL SCI. 2014;15:7124–7138. doi: 10.3390/ijms15057124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Guo D, Bell EH, Mischel P, Chakravarti A. Targeting SREBP-1-driven lipid metabolism to treat cancer. Curr Pharm Des. 2014;20:2619–2626. doi: 10.2174/13816128113199990486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Geng F, Cheng X, Wu X, Yoo JY, Cheng C, Guo JY, Mo X, Ru P, Hurwitz B, Kim S, Otero J, Puduvalli VK, Lefai E, Ma J, Nakano I, Horbinski C, Kaur B, Chakravarti A, Guo D. Inhibition of SOAT1 suppresses glioblastoma growth via blocking SREBP-1-mediated lipogenesis. CLIN CANCER RES. 2016 doi: 10.1158/1078-0432.CCR-15-2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cheng C, Ru P, Geng F, Liu J, Yoo JY, Wu X, Cheng X, Euthine V, Hu P, Guo JY, Lefai E, Kaur B, Nohturfft A, Ma J, Chakravarti A, Guo D. Glucose-Mediated N-glycosylation of SCAP Is Essential for SREBP-1 Activation and Tumor Growth. CANCER CELL. 2015;28:569–581. doi: 10.1016/j.ccell.2015.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li Y, Zhang J, He J, Zhou W, Xiang G, Xu R. MicroRNA-132 cause apoptosis of glioma cells through blockade of the SREBP-1c metabolic pathway related to SIRT1. BIOMED PHARMACOTHER. 2016;78:177–184. doi: 10.1016/j.biopha.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 138.Xu Q, Yang C, Du Y, Chen Y, Liu H, Deng M, Zhang H, Zhang L, Liu T, Liu Q, Wang L, Lou Z, Pei H. AMPK regulates histone H2B O-GlcNAcylation. NUCLEIC ACIDS RES. 2014;42:5594–5604. doi: 10.1093/nar/gku236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Johnsen SA. The enigmatic role of H2Bub1 in cancer. FEBS LETT. 2012;586:1592–1601. doi: 10.1016/j.febslet.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 140.Bungard D, Fuerth BJ, Zeng PY, Faubert B, Maas NL, Viollet B, Carling D, Thompson CB, Jones RG, Berger SL. Signaling kinase AMPK activates stress-promoted transcription via histone H2B phosphorylation. SCIENCE. 2010;329:1201–1205. doi: 10.1126/science.1191241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Thompson LL, Guppy BJ, Sawchuk L, Davie JR, McManus KJ. Regulation of chromatin structure via histone post-translational modification and the link to carcinogenesis. Cancer Metastasis Rev. 2013;32:363–376. doi: 10.1007/s10555-013-9434-8. [DOI] [PubMed] [Google Scholar]
- 142.Lau AT, Lee SY, Xu YM, Zheng D, Cho YY, Zhu F, Kim HG, Li SQ, Zhang Z, Bode AM, Dong Z. Phosphorylation of histone H2B serine 32 is linked to cell transformation. J BIOL CHEM. 2011;286:26628–26637. doi: 10.1074/jbc.M110.215590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cang S, Xu X, Ma Y, Liu D, Chiao JW. Hypoacetylation, hypomethylation, and dephosphorylation of H2B histones and excessive histone deacetylase activity in DU-145 prostate cancer cells. J HEMATOL ONCOL. 2016;9:3. doi: 10.1186/s13045-016-0233-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fraser SA, Choy SW, Pastor-Soler NM, Li H, Davies MR, Cook N, Katerelos M, Mount PF, Gleich K, McRae JL, Dwyer KM, van Denderen BJ, Hallows KR, Kemp BE, Power DA. AMPK couples plasma renin to cellular metabolism by phosphorylation of ACC1. Am J Physiol Renal Physiol. 2013;305:F679–F690. doi: 10.1152/ajprenal.00407.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zordoky BN, Nagendran J, Pulinilkunnil T, Kienesberger PC, Masson G, Waller TJ, Kemp BE, Steinberg GR, Dyck JR. AMPK-dependent inhibitory phosphorylation of ACC is not essential for maintaining myocardial fatty acid oxidation. CIRC RES. 2014;115:518–524. doi: 10.1161/CIRCRESAHA.115.304538. [DOI] [PubMed] [Google Scholar]
- 146.Munday MR, Campbell DG, Carling D, Hardie DG. Identification by amino acid sequencing of three major regulatory phosphorylation sites on rat acetyl-CoA carboxylase. Eur J Biochem. 1988;175:331–338. doi: 10.1111/j.1432-1033.1988.tb14201.x. [DOI] [PubMed] [Google Scholar]
- 147.Boone AN, Rodrigues B, Brownsey RW. Multiple-site phosphorylation of the 280 kDa isoform of acetyl-CoA carboxylase in rat cardiac myocytes: evidence that cAMP-dependent protein kinase mediates effects of beta-adrenergic stimulation. BIOCHEM J. 1999;341(Pt 2):347–354. [PMC free article] [PubMed] [Google Scholar]
- 148.Wang C, Rajput S, Watabe K, Liao DF, Cao D. Acetyl-CoA carboxylase-a as a novel target for cancer therapy. Front Biosci (Schol Ed) 2010;2:515–526. doi: 10.2741/s82. [DOI] [PubMed] [Google Scholar]
- 149.Li W, Zhang C, Du H, Huang V, Sun B, Harris JP, Richardson Q, Shen X, Jin R, Li G, Kevil CG, Gu X, Shi R, Zhao Y. Withaferin A suppresses the up-regulation of acetyl-coA carboxylase 1 and skin tumor formation in a skin carcinogenesis mouse model. Mol Carcinog. 2015 doi: 10.1002/mc.22423. [DOI] [PubMed] [Google Scholar]
- 150.Al-Zoubi M, Chipitsyna G, Saxena S, Sarosiek K, Gandhi A, Kang CY, Relles D, Andrelsendecki J, Hyslop T, Yeo CJ, Arafat HA. Overexpressing TNF-alpha in pancreatic ductal adenocarcinoma cells and fibroblasts modifies cell survival and reduces fatty acid synthesis via downregulation of sterol regulatory element binding protein-1 and activation of acetyl CoA carboxylase. J GASTROINTEST SURG. 2014;18:257–268. 268. doi: 10.1007/s11605-013-2370-7. [DOI] [PubMed] [Google Scholar]
- 151.Wang C, Xu C, Sun M, Luo D, Liao DF, Cao D. Acetyl-CoA carboxylase-alpha inhibitor TOFA induces human cancer cell apoptosis. Biochem Biophys Res Commun. 2009;385:302–306. doi: 10.1016/j.bbrc.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Luo DX, Peng XH, Xiong Y, Liao DF, Cao D, Li L. Dual role of insulin-like growth factor-1 in acetyl-CoA carboxylase-alpha activity in human colon cancer cells HCT-8: downregulating its expression and phosphorylation. MOL CELL BIOCHEM. 2011;357:255–262. doi: 10.1007/s11010-011-0896-0. [DOI] [PubMed] [Google Scholar]
- 153.Zhang X, Song Y, Feng M, Zhou X, Lu Y, Gao L, Yu C, Jiang X, Zhao J. Thyroid-stimulating hormone decreases HMG-CoA reductase phosphorylation via AMP-activated protein kinase in the liver. J LIPID RES. 2015;56:963–971. doi: 10.1194/jlr.M047654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gillespie JG, Hardie DG. Phosphorylation and inactivation of HMG-CoA reductase at the AMP-activated protein kinase site in response to fructose treatment of isolated rat hepatocytes. FEBS LETT. 1992;306:59–62. doi: 10.1016/0014-5793(92)80837-7. [DOI] [PubMed] [Google Scholar]
- 155.Yoon LS, Goodman MT, Rimel BJ, Jeon CY. Statin use and survival in elderly patients with endometrial cancer. GYNECOL ONCOL. 2015;137:252–257. doi: 10.1016/j.ygyno.2015.01.549. [DOI] [PubMed] [Google Scholar]
- 156.Alexandre L, Clark AB, Bhutta HY, Chan SS, Lewis MP, Hart AR. Association Between Statin Use After Diagnosis of Esophageal Cancer and Survival: A Population-Based Cohort Study. GASTROENTEROLOGY. 2016;150:854–865. doi: 10.1053/j.gastro.2015.12.039. [DOI] [PubMed] [Google Scholar]
- 157.Borgquist S, Jogi A, Ponten F, Ryden L, Brennan DJ, Jirstrom K. Prognostic impact of tumour-specific HMG-CoA reductase expression in primary breast cancer. BREAST CANCER RES. 2008;10:R79. doi: 10.1186/bcr2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bengtsson E, Nerjovaj P, Wangefjord S, Nodin B, Eberhard J, Uhlen M, Borgquist S, Jirstrom K. HMG-CoA reductase expression in primary colorectal cancer correlates with favourable clinicopathological characteristics and an improved clinical outcome. DIAGN PATHOL. 2014;9:78. doi: 10.1186/1746-1596-9-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Halse R, Fryer LG, McCormack JG, Carling D, Yeaman SJ. Regulation of glycogen synthase by glucose and glycogen: a possible role for AMP-activated protein kinase. DIABETES. 2003;52:9–15. doi: 10.2337/diabetes.52.1.9. [DOI] [PubMed] [Google Scholar]
- 160.Nielsen JN, Wojtaszewski JF, Haller RG, Hardie DG, Kemp BE, Richter EA, Vissing J. Role of 5′AMP-activated protein kinase in glycogen synthase activity and glucose utilization: insights from patients with McArdle’s disease. J Physiol. 2002;541:979–989. doi: 10.1113/jphysiol.2002.018044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bultot L, Guigas B, Von Wilamowitz-Moellendorff A, Maisin L, Vertommen D, Hussain N, Beullens M, Guinovart JJ, Foretz M, Viollet B, Sakamoto K, Hue L, Rider MH. AMP-activated protein kinase phosphorylates and inactivates liver glycogen synthase. BIOCHEM J. 2012;443:193–203. doi: 10.1042/BJ20112026. [DOI] [PubMed] [Google Scholar]
- 162.Bhanot H, Reddy MM, Nonami A, Weisberg EL, Bonal D, Kirschmeier PT, Salgia S, Podar K, Galinsky I, Chowdary TK, Neuberg D, Tonon G, Stone RM, Asara J, Griffin JD, Sattler M. Pathological glycogenesis through glycogen synthase 1 and suppression of excessive AMP kinase activity in myeloid leukemia cells. LEUKEMIA. 2015;29:1555–1563. doi: 10.1038/leu.2015.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Iida Y, Aoki K, Asakura T, Ueda K, Yanaihara N, Takakura S, Yamada K, Okamoto A, Tanaka T, Ohkawa K. Hypoxia promotes glycogen synthesis and accumulation in human ovarian clear cell carcinoma. INT J ONCOL. 2012;40:2122–2130. doi: 10.3892/ijo.2012.1406. [DOI] [PubMed] [Google Scholar]
- 164.Beckner ME, Gobbel GT, Abounader R, Burovic F, Agostino NR, Laterra J, Pollack IF. Glycolytic glioma cells with active glycogen synthase are sensitive to PTEN and inhibitors of PI3K and gluconeogenesis. LAB INVEST. 2005;85:1457–1470. doi: 10.1038/labinvest.3700355. [DOI] [PubMed] [Google Scholar]
- 165.Schnier JB, Nishi K, Gumerlock PH, Gorin FA, Bradbury EM. Glycogen synthesis correlates with androgen-dependent growth arrest in prostate cancer. BMC UROL. 2005;5:6. doi: 10.1186/1471-2490-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mayer D, Bannasch P. Activity of glycogen synthase and phosphorylase and glucose 6-phosphate content in renal clear cell carcinomas. J Cancer Res Clin Oncol. 1988;114:369–372. doi: 10.1007/BF02128180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hammond KD, Balinsky D. Activities of key gluconeogenic enzymes and glycogen synthase in rat and human livers, hepatomas, and hepatoma cell cultures. CANCER RES. 1978;38:1317–1322. [PubMed] [Google Scholar]
- 168.Fujita N, Kaku K, Okubo M, Nagasaka Y, Kaneko T. Insulin stimulates protein synthesis of glycogen synthase in rat hepatoma H4 cells associated with acceleration of translation rate. ENDOCR J. 1996;43:313–320. doi: 10.1507/endocrj.43.313. [DOI] [PubMed] [Google Scholar]
- 169.Yuan HX, Wang Z, Yu FX, Li F, Russell RC, Jewell JL, Guan KL. NLK phosphorylates Raptor to mediate stress-induced mTORC1 inhibition. Genes Dev. 2015;29:2362–2376. doi: 10.1101/gad.265116.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kim K, Qiang L, Hayden MS, Sparling DP, Purcell NH, Pajvani UB. mTORC1-independent Raptor prevents hepatic steatosis by stabilizing PHLPP2. NAT COMMUN. 2016;7:10255. doi: 10.1038/ncomms10255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Stretton C, Hoffmann TM, Munson MJ, Prescott A, Taylor PM, Ganley IG, Hundal HS. GSK3-mediated raptor phosphorylation supports amino-acid-dependent mTORC1-directed signalling. BIOCHEM J. 2015;470:207–221. doi: 10.1042/BJ20150404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. MOL CELL. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Shuhua W, Chenbo S, Yangyang L, Xiangqian G, Shuang H, Tangyue L, Dong T. Autophagy-related genes Raptor, Rictor, and Beclin1 expression and relationship with multidrug resistance in colorectal carcinoma. HUM PATHOL. 2015;46:1752–1759. doi: 10.1016/j.humpath.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 174.Montero JC, Chen X, Ocana A, Pandiella A. Predominance of mTORC1 over mTORC2 in the regulation of proliferation of ovarian cancer cells: therapeutic implications. MOL CANCER THER. 2012;11:1342–1352. doi: 10.1158/1535-7163.MCT-11-0723. [DOI] [PubMed] [Google Scholar]
- 175.Watari K, Nishitani A, Shibata T, Noda M, Kawahara A, Akiba J, Murakami Y, Yano H, Kuwano M, Ono M. Phosphorylation of mTOR Ser2481 is a key target limiting the efficacy of rapalogs for treating hepatocellular carcinoma. ONCOTARGET. 2016 doi: 10.18632/oncotarget.10161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Matsumoto CS, Almeida LO, Guimaraes DM, Martins MD, Papagerakis P, Papagerakis S, Leopoldino AM, Castilho RM, Squarize CH. PI3K-PTEN dysregulation leads to mTOR-driven upregulation of the core clock gene BMAL1 in normal and malignant epithelial cells. ONCOTARGET. 2016 doi: 10.18632/oncotarget.9877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Witzig TE, Reeder C, Han JJ, LaPlant B, Stenson M, Tun HW, Macon W, Ansell SM, Habermann TM, Inwards DJ, Micallef IN, Johnston PB, Porrata LF, Colgan JP, Markovic S, Nowakowski GS, Gupta M. The mTORC1 inhibitor everolimus has antitumor activity in vitro and produces tumor responses in patients with relapsed T-cell lymphoma. BLOOD. 2015;126:328–335. doi: 10.1182/blood-2015-02-629543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. CELL. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 179.Kwiatkowski DJ. Tuberous sclerosis: from tubers to mTOR. ANN HUM GENET. 2003;67:87–96. doi: 10.1046/j.1469-1809.2003.00012.x. [DOI] [PubMed] [Google Scholar]
- 180.Huynh H, Hao HX, Chan SL, Chen D, Ong R, Soo KC, Pochanard P, Yang D, Ruddy D, Liu M, Derti A, Balak MN, Palmer MR, Wang Y, Lee BH, Sellami D, Zhu AX, Schlegel R, Huang A. Loss of Tuberous Sclerosis Complex 2 (TSC2) Is Frequent in Hepatocellular Carcinoma and Predicts Response to mTORC1 Inhibitor Everolimus. MOL CANCER THER. 2015;14:1224–1235. doi: 10.1158/1535-7163.MCT-14-0768. [DOI] [PubMed] [Google Scholar]
- 181.Yang J, Kalogerou M, Samsel PA, Zhang Y, Griffiths DF, Gallacher J, Sampson JR, Shen MH. Renal tumours in a Tsc2(+/−) mouse model do not show feedback inhibition of Akt and are effectively prevented by rapamycin. ONCOGENE. 2015;34:922–931. doi: 10.1038/onc.2014.17. [DOI] [PubMed] [Google Scholar]
- 182.Lopez-Rivera E, Jayaraman P, Parikh F, Davies MA, Ekmekcioglu S, Izadmehr S, Milton DR, Chipuk JE, Grimm EA, Estrada Y, Aguirre-Ghiso J, Sikora AG. Inducible nitric oxide synthase drives mTOR pathway activation and proliferation of human melanoma by reversible nitrosylation of TSC2. CANCER RES. 2014;74:1067–1078. doi: 10.1158/0008-5472.CAN-13-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.D’Armiento J, Shiomi T, Marks S, Geraghty P, Sankarasharma D, Chada K. Mesenchymal Tumorigenesis Driven by TSC2 Haploinsufficiency Requires HMGA2 and Is Independent of mTOR Pathway Activation. CANCER RES. 2016;76:844–854. doi: 10.1158/0008-5472.CAN-15-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Humpton TJ, Vousden KH. Regulation of Cellular Metabolism and Hypoxia by p53. Cold Spring Harb Perspect Med 6. 2016 doi: 10.1101/cshperspect.a026146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Jones RG, Plas DR, Kubek S, Buzzai M, Mu J, Xu Y, Birnbaum MJ, Thompson CB. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. MOL CELL. 2005;18:283–293. doi: 10.1016/j.molcel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 186.Zhang XD, Qin ZH, Wang J. The role of p53 in cell metabolism. ACTA PHARMACOL SIN. 2010;31:1208–1212. doi: 10.1038/aps.2010.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Haupt S, Raghu D, Haupt Y. Mutant p53 Drives Cancer by Subverting Multiple Tumor Suppression Pathways. Front Oncol. 2016;6:12. doi: 10.3389/fonc.2016.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Meng X, Tackmann NR, Liu S, Yang J, Dong J, Wu C, Cox AD, Zhang Y. RPL23 links oncogenic RAS signaling to p53-mediated tumor suppression. CANCER RES. 2016 doi: 10.1158/0008-5472.CAN-15-3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Adriaens C, Standaert L, Barra J, Latil M, Verfaillie A, Kalev P, Boeckx B, Wijnhoven PW, Radaelli E, Vermi W, Leucci E, Lapouge G, Beck B, van den Oord J, Nakagawa S, Hirose T, Sablina AA, Lambrechts D, Aerts S, Blanpain C, Marine JC. p53 induces formation of NEAT1 lncRNA-containing paraspeckles that modulate replication stress response and chemosensitivity. NAT MED. 2016 doi: 10.1038/nm.4135. [DOI] [PubMed] [Google Scholar]
- 190.Cao B, Wang K, Liao JM, Zhou X, Liao P, Zeng SX, He M, Chen L, He Y, Li W, Lu H. Inactivation of oncogenic cAMP-specific phosphodiesterase 4D by miR-139–5p in response to p53 activation. ELIFE 5. 2016 doi: 10.7554/eLife.15978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zhou X, Hao Q, Liao P, Luo S, Zhang M, Hu G, Liu H, Zhang Y, Cao B, Baddoo M, Flemington EK, Zeng SX, Lu H. Nerve growth factor receptor negates the tumor suppressor p53 as a feedback regulator. ELIFE 5. 2016 doi: 10.7554/eLife.15099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Liang J, Shao SH, Xu ZX, Hennessy B, Ding Z, Larrea M, Kondo S, Dumont DJ, Gutterman JU, Walker CL, Slingerland JM, Mills GB. The energy sensing LKB1-AMPK pathway regulates p27(kip1) phosphorylation mediating the decision to enter autophagy or apoptosis. NAT CELL BIOL. 2007;9:218–224. doi: 10.1038/ncb1537. [DOI] [PubMed] [Google Scholar]
- 193.Short JD, Houston KD, Dere R, Cai SL, Kim J, Johnson CL, Broaddus RR, Shen J, Miyamoto S, Tamanoi F, Kwiatkowski D, Mills GB, Walker CL. AMP-activated protein kinase signaling results in cytoplasmic sequestration of p27. CANCER RES. 2008;68:6496–6506. doi: 10.1158/0008-5472.CAN-07-5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Gao L, Gu W, Zheng J, Ren W, Chang S, Wang X, Li S, Song T, Huang C, Zhi K. Clinicopathological and prognostic significance of p27 expression in oral squamous cell carcinoma: a meta-analysis. Int J Biol Markers. 2013;28:e329–e335. doi: 10.5301/jbm.5000035. [DOI] [PubMed] [Google Scholar]
- 195.Zhuang Y, Yin HT, Yin XL, Wang J, Zhang DP. High p27 expression is associated with a better prognosis in East Asian non-small cell lung cancer patients. CLIN CHIM ACTA. 2011;412:2228–2231. doi: 10.1016/j.cca.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 196.Wang C, Nie Z, Zhou Z, Zhang H, Liu R, Wu J, Qin J, Ma Y, Chen L, Li S, Chen W, Li F, Shi P, Wu Y, Shen J, Chen C. The interplay between TEAD4 and KLF5 promotes breast cancer partially through inhibiting the transcription of p27Kip1. ONCOTARGET. 2015;6:17685–17697. doi: 10.18632/oncotarget.3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Li Z, Huang J, Yuan H, Chen Z, Luo Q, Lu S. SIRT2 inhibits non-small cell lung cancer cell growth through impairing Skp2-mediated p27 degradation. ONCOTARGET. 2016;7:18927–18939. doi: 10.18632/oncotarget.7816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ma D, Guo D, Li W, Zhao H. Mdig, a lung cancer-associated gene, regulates cell cycle progression through p27(KIP1) Tumour Biol. 2015;36:6909–6917. doi: 10.1007/s13277-015-3397-z. [DOI] [PubMed] [Google Scholar]
- 199.Lin TC, Tsai LH, Chou MC, Chen CY, Lee H. Association of cytoplasmic p27 expression with an unfavorable response to cisplatin-based chemotherapy and poor outcomes in non-small cell lung cancer. Tumour Biol. 2016;37:4017–4023. doi: 10.1007/s13277-015-4272-7. [DOI] [PubMed] [Google Scholar]
- 200.Liu DZ, Zhang HY, Long XL, Zou SL, Zhang XY, Han GY, Cui ZG. MIR-150 promotes prostate cancer stem cell development via suppressing p27Kip1. Eur Rev Med Pharmacol Sci. 2015;19:4344–4352. [PubMed] [Google Scholar]
- 201.Lynch SM, McKenna MM, Walsh CP, McKenna DJ. miR-24 regulates CDKN1B/p27 expression in prostate cancer. PROSTATE. 2016;76:637–648. doi: 10.1002/pros.23156. [DOI] [PubMed] [Google Scholar]
- 202.Lin HP, Lin CY, Huo C, Hsiao PH, Su LC, Jiang SS, Chan TM, Chang CH, Chen LT, Kung HJ, Wang HD, Chuu CP. Caffeic acid phenethyl ester induced cell cycle arrest and growth inhibition in androgen-independent prostate cancer cells via regulation of Skp2, p53, p21Cip1 and p27Kip1. ONCOTARGET. 2015;6:6684–6707. doi: 10.18632/oncotarget.3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Fan XY, Tian C, Wang H, Xu Y, Ren K, Zhang BY, Gao C, Shi Q, Meng G, Zhang LB, Zhao YJ, Shao QX, Dong XP. Activation of the AMPK-ULK1 pathway plays an important role in autophagy during prion infection. Sci Rep. 2015;5:14728. doi: 10.1038/srep14728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wong PM, Puente C, Ganley IG, Jiang X. The ULK1 complex: sensing nutrient signals for autophagy activation. AUTOPHAGY. 2013;9:124–137. doi: 10.4161/auto.23323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor R, Asara JM, Fitzpatrick J, Dillin A, Viollet B, Kundu M, Hansen M, Shaw RJ. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. SCIENCE. 2011;331:456–461. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Loffler AS, Alers S, Dieterle AM, Keppeler H, Franz-Wachtel M, Kundu M, Campbell DG, Wesselborg S, Alessi DR, Stork B. Ulk1-mediated phosphorylation of AMPK constitutes a negative regulatory feedback loop. AUTOPHAGY. 2011;7:696–706. doi: 10.4161/auto.7.7.15451. [DOI] [PubMed] [Google Scholar]
- 207.Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. NAT CELL BIOL. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Xu H, Yu H, Zhang X, Shen X, Zhang K, Sheng H, Dai S, Gao H. UNC51-like kinase 1 as a potential prognostic biomarker for hepatocellular carcinoma. Int J Clin Exp Pathol. 2013;6:711–717. [PMC free article] [PubMed] [Google Scholar]
- 209.Jiang S, Li Y, Zhu YH, Wu XQ, Tang J, Li Z, Feng GK, Deng R, Li DD, Luo RZ, Zhang MF, Qin W, Wang X, Jia WH, Zhu XF. Intensive expression of UNC-51-like kinase 1 is a novel biomarker of poor prognosis in patients with esophageal squamous cell carcinoma. CANCER SCI. 2011;102:1568–1575. doi: 10.1111/j.1349-7006.2011.01964.x. [DOI] [PubMed] [Google Scholar]
- 210.Yun M, Bai HY, Zhang JX, Rong J, Weng HW, Zheng ZS, Xu Y, Tong ZT, Huang XX, Liao YJ, Mai SJ, Ye S, Xie D. ULK1: a promising biomarker in predicting poor prognosis and therapeutic response in human nasopharygeal carcinoma. PLOS ONE. 2015;10:e117375. doi: 10.1371/journal.pone.0117375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Zou Y, Chen Z, He X, He X, Wu X, Chen Y, Wu X, Wang J, Lan P. High expression levels of unc-51-like kinase 1 as a predictor of poor prognosis in colorectal cancer. ONCOL LETT. 2015;10:1583–1588. doi: 10.3892/ol.2015.3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Wang L, Wang J, Xiong H, Wu F, Lan T, Zhang Y, Guo X, Wang H, Saleem M, Jiang C, Lu J, Deng Y. Co-targeting hexokinase 2-mediated Warburg effect and ULK1-dependent autophagy suppresses tumor growth of PTEN- and TP53-deficiency-driven castration-resistant prostate cancer. EBioMedicine. 2016;7:50–61. doi: 10.1016/j.ebiom.2016.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.He W, Ye X, Huang X, Lel W, You L, Wang L, Chen X, Qian W. Hsp90 inhibitor, BIIB021, induces apoptosis and autophagy by regulating mTOR-Ulk1 pathway in imatinib-sensitive and -resistant chronic myeloid leukemia cells. INT J ONCOL. 2016;48:1710–1720. doi: 10.3892/ijo.2016.3382. [DOI] [PubMed] [Google Scholar]
- 214.Tang J, Deng R, Luo RZ, Shen GP, Cai MY, Du ZM, Jiang S, Yang MT, Fu JH, Zhu XF. Low expression of ULK1 is associated with operable breast cancer progression and is an adverse prognostic marker of survival for patients. Breast Cancer Res Treat. 2012;134:549–560. doi: 10.1007/s10549-012-2080-y. [DOI] [PubMed] [Google Scholar]
- 215.Schmukler E, Kloog Y, Pinkas-Kramarski R. Ras and autophagy in cancer development and therapy. ONCOTARGET. 2014;5:577–586. doi: 10.18632/oncotarget.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Galluzzi L, Vacchelli E, Bravo-San PJ, Buque A, Senovilla L, Baracco EE, Bloy N, Castoldi F, Abastado JP, Agostinis P, Apte RN, Aranda F, Ayyoub M, Beckhove P, Blay JY, Bracci L, Caignard A, Castelli C, Cavallo F, Celis E, Cerundolo V, Clayton A, Colombo MP, Coussens L, Dhodapkar MV, Eggermont AM, Fearon DT, Fridman WH, Fucikova J, Gabrilovich DI, Galon J, Garg A, Ghiringhelli F, Giaccone G, Gilboa E, Gnjatic S, Hoos A, Hosmalin A, Jager D, Kalinski P, Karre K, Kepp O, Kiessling R, Kirkwood JM, Klein E, Knuth A, Lewis CE, Liblau R, Lotze MT, Lugli E, Mach JP, Mattei F, Mavilio D, Melero I, Melief CJ, Mittendorf EA, Moretta L, Odunsi A, Okada H, Palucka AK, Peter ME, Pienta KJ, Porgador A, Prendergast GC, Rabinovich GA, Restifo NP, Rizvi N, Sautes-Fridman C, Schreiber H, Seliger B, Shiku H, Silva-Santos B, Smyth MJ, Speiser DE, Spisek R, Srivastava PK, Talmadge JE, Tartour E, Van Der Burg SH, Van Den Eynde BJ, Vile R, Wagner H, Weber JS, Whiteside TL, Wolchok JD, Zitvogel L, Zou W, Kroemer G. Classification of current anticancer immunotherapies. ONCOTARGET. 2014;5:12472–12508. doi: 10.18632/oncotarget.2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kim J, Lim W, Kim EK, Kim MK, Paik NS, Jeong SS, Yoon JH, Park CH, Ahn SH, Kim LS, Han S, Nam SJ, Kang HS, Kim SI, Yoo YB, Jeong J, Kim TH, Kang T, Kim SW, Jung Y, Lee JE, Kim KS, Yu JH, Chae BJ, Jung SY, Kang E, Choi SY, Moon HG, Noh DY, Han W. Phase II randomized trial of neoadjuvant metformin plus letrozole versus placebo plus letrozole for estrogen receptor positive postmenopausal breast cancer (METEOR) BMC CANCER. 2014;14:170. doi: 10.1186/1471-2407-14-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Bonanni B, Puntoni M, Cazzaniga M, Pruneri G, Serrano D, Guerrieri-Gonzaga A, Gennari A, Trabacca MS, Galimberti V, Veronesi P, Johansson H, Aristarco V, Bassi F, Luini A, Lazzeroni M, Varricchio C, Viale G, Bruzzi P, Decensi A. Dual effect of metformin on breast cancer proliferation in a randomized presurgical trial. J CLIN ONCOL. 2012;30:2593–2600. doi: 10.1200/JCO.2011.39.3769. [DOI] [PubMed] [Google Scholar]
- 219.Kordes S, Pollak MN, Zwinderman AH, Mathot RA, Weterman MJ, Beeker A, Punt CJ, Richel DJ, Wilmink JW. Metformin in patients with advanced pancreatic cancer: a double-blind, randomised, placebo-controlled phase 2 trial. LANCET ONCOL. 2015;16:839–847. doi: 10.1016/S1470-2045(15)00027-3. [DOI] [PubMed] [Google Scholar]
- 220.Tseng CH. Diabetes, metformin use, and colon cancer: a population-based cohort study in Taiwan. EUR J ENDOCRINOL. 2012;167:409–416. doi: 10.1530/EJE-12-0369. [DOI] [PubMed] [Google Scholar]
- 221.Higurashi T, Takahashi H, Endo H, Hosono K, Yamada E, Ohkubo H, Sakai E, Uchiyama T, Hata Y, Fujisawa N, Uchiyama S, Ezuka A, Nagase H, Kessoku T, Matsuhashi N, Yamanaka S, Inayama Y, Morita S, Nakajima A. Metformin efficacy and safety for colorectal polyps: a double-blind randomized controlled trial. BMC CANCER. 2012;12:118. doi: 10.1186/1471-2407-12-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Vincent EE, Coelho PP, Blagih J, Griss T, Viollet B, Jones RG. Differential effects of AMPK agonists on cell growth and metabolism. ONCOGENE. 2015;34:3627–3639. doi: 10.1038/onc.2014.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Fodor T, Szanto M, Abdul-Rahman O, Nagy L, Der A, Kiss B, Bai P. Combined Treatment of MCF-7 Cells with AICAR, Methotrexate Arrests Cell Cycle and Reverses Warburg Metabolism through AMP-Activated Protein Kinase (AMPK) and FOXO1. PLOS ONE. 2016;11:e150232. doi: 10.1371/journal.pone.0150232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Jhaveri TZ, Woo J, Shang X, Park BH, Gabrielson E. AMP-activated kinase (AMPK) regulates activity of HER2 and EGFR in breast cancer. ONCOTARGET. 2015;6:14754–14765. doi: 10.18632/oncotarget.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Sui X, Xu Y, Yang J, Fang Y, Lou H, Han W, Zhang M, Chen W, Wang K, Li D, Jin W, Lou F, Zheng Y, Hu H, Gong L, Zhou X, Pan Q, Pan H, Wang X, He C. Use of metformin alone is not associated with survival outcomes of colorectal cancer cell but AMPK activator AICAR sensitizes anticancer effect of 5-fluorouracil through AMPK activation. PLOS ONE. 2014;9:e97781. doi: 10.1371/journal.pone.0097781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Wu Y, Qi Y, Liu H, Wang X, Zhu H, Wang Z. AMPK activator AICAR promotes 5-FU-induced apoptosis in gastric cancer cells. MOL CELL BIOCHEM. 2016;411:299–305. doi: 10.1007/s11010-015-2592-y. [DOI] [PubMed] [Google Scholar]
- 227.Cheng J, Huang T, Li Y, Guo Y, Zhu Y, Wang Q, Tan X, Chen W, Zhang Y, Cheng W, Yamamoto T, Jing X, Huang J. AMP-activated protein kinase suppresses the in vitro and in vivo proliferation of hepatocellular carcinoma. PLOS ONE. 2014;9:e93256. doi: 10.1371/journal.pone.0093256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Guo F, Liu SQ, Gao XH, Zhang LY. AICAR induces AMPK-independent programmed necrosis in prostate cancer cells. Biochem Biophys Res Commun. 2016;474:277–283. doi: 10.1016/j.bbrc.2016.04.077. [DOI] [PubMed] [Google Scholar]
- 229.Shen C, Ka SO, Kim SJ, Kim JH, Park BH, Park JH. Metformin and AICAR regulate NANOG expression via the JNK pathway in HepG2 cells independently of AMPK. Tumour Biol. 2016 doi: 10.1007/s13277-016-5007-0. [DOI] [PubMed] [Google Scholar]
- 230.Zhu H, Foretz M, Xie Z, Zhang M, Zhu Z, Xing J, Leclerc J, Gaudry M, Viollet B, Zou MH. PRKAA1/AMPKalpha1 is required for autophagy-dependent mitochondrial clearance during erythrocyte maturation. AUTOPHAGY. 2014;10:1522–1534. doi: 10.4161/auto.29197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Um JH, Pendergast JS, Springer DA, Foretz M, Viollet B, Brown A, Kim MK, Yamazaki S, Chung JH. AMPK regulates circadian rhythms in a tissue- and isoform-specific manner. PLOS ONE. 2011;6:e18450. doi: 10.1371/journal.pone.0018450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Schuhmacher S, Foretz M, Knorr M, Jansen T, Hortmann M, Wenzel P, Oelze M, Kleschyov AL, Daiber A, Keaney JJ, Wegener G, Lackner K, Munzel T, Viollet B, Schulz E. alpha1AMP-activated protein kinase preserves endothelial function during chronic angiotensin II treatment by limiting Nox2 upregulation. Arterioscler Thromb Vasc Biol. 2011;31:560–566. doi: 10.1161/ATVBAHA.110.219543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Tartarin P, Guibert E, Toure A, Ouiste C, Leclerc J, Sanz N, Briere S, Dacheux JL, Delaleu B, McNeilly JR, McNeilly AS, Brillard JP, Dupont J, Foretz M, Viollet B, Froment P. Inactivation of AMPKalpha1 induces asthenozoospermia and alters spermatozoa morphology. ENDOCRINOLOGY. 2012;153:3468–3481. doi: 10.1210/en.2011-1911. [DOI] [PubMed] [Google Scholar]
- 234.Merlen G, Gentric G, Celton-Morizur S, Foretz M, Guidotti JE, Fauveau V, Leclerc J, Viollet B, Desdouets C. AMPKalpha1 controls hepatocyte proliferation independently of energy balance by regulating Cyclin A2 expression. J HEPATOL. 2014;60:152–159. doi: 10.1016/j.jhep.2013.08.025. [DOI] [PubMed] [Google Scholar]
- 235.Noppe G, Dufeys C, Buchlin P, Marquet N, Castanares-Zapatero D, Balteau M, Hermida N, Bouzin C, Esfahani H, Viollet B, Bertrand L, Balligand JL, Vanoverschelde JL, Beauloye C, Horman S. Reduced scar maturation and contractility lead to exaggerated left ventricular dilation after myocardial infarction in mice lacking AMPKalpha1. J MOL CELL CARDIOL. 2014;74:32–43. doi: 10.1016/j.yjmcc.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 236.Sun G, Tarasov AI, McGinty J, McDonald A, Da SXG, Gorman T, Marley A, French PM, Parker H, Gribble F, Reimann F, Prendiville O, Carzaniga R, Viollet B, Leclerc I, Rutter GA. Ablation of AMP-activated protein kinase alpha1 and alpha2 from mouse pancreatic beta cells and RIP2.Cre neurons suppresses insulin release in vivo. DIABETOLOGIA. 2010;53:924–936. doi: 10.1007/s00125-010-1692-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Fu X, Zhao JX, Zhu MJ, Foretz M, Viollet B, Dodson MV, Du M. AMP-activated protein kinase alpha1 but not alpha2 catalytic subunit potentiates myogenin expression and myogenesis. MOL CELL BIOL. 2013;33:4517–4525. doi: 10.1128/MCB.01078-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Maixner DW, Yan X, Gao M, Yadav R, Weng HR. Adenosine Monophosphate-activated Protein Kinase Regulates Interleukin-1beta Expression and Glial Glutamate Transporter Function in Rodents with Neuropathic Pain. ANESTHESIOLOGY. 2015;122:1401–1413. doi: 10.1097/ALN.0000000000000619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Fu X, Zhu MJ, Dodson MV, Du M. AMP-activated protein kinase stimulates Warburg-like glycolysis and activation of satellite cells during muscle regeneration. J BIOL CHEM. 2015;290:26445–26456. doi: 10.1074/jbc.M115.665232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Yang C, Li Z, Lai P, Bai X, Jin D. Chondrocyte-Specific Ablation of AMPKalpha1 Does Not Affect Bone Development or Pathogenesis of Osteoarthritis in Mice. DNA CELL BIOL. 2016;35:156–162. doi: 10.1089/dna.2015.3074. [DOI] [PubMed] [Google Scholar]
- 241.Cai Z, Ding Y, Zhang M, Lu Q, Wu S, Zhu H, Song P, Zou MH. Ablation of Adenosine Monophosphate-Activated Protein Kinase alpha1 in Vascular Smooth Muscle Cells Promotes Diet-Induced Atherosclerotic Calcification In Vivo. CIRC RES. 2016;119:422–433. doi: 10.1161/CIRCRESAHA.116.308301. [DOI] [PubMed] [Google Scholar]
- 242.Zhu H, Zhang M, Liu Z, Xing J, Moriasi C, Dai X, Zou MH. AMP-Activated Protein Kinase alpha1 in Macrophages Promotes Collateral Remodeling and Arteriogenesis in Mice In Vivo. Arterioscler Thromb Vasc Biol. 2016 doi: 10.1161/ATVBAHA.116.307743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Mahmoud AD, Lewis S, Juricic L, Udoh UA, Hartmann S, Jansen MA, Ogunbayo OA, Puggioni P, Holmes AP, Kumar P, Navarro-Dorado J, Foretz M, Viollet B, Dutia MB, Marshall I, Evans AM. AMP-activated Protein Kinase Deficiency Blocks the Hypoxic Ventilatory Response and Thus Precipitates Hypoventilation and Apnea. Am J Respir Crit Care Med. 2016;193:1032–1043. doi: 10.1164/rccm.201508-1667OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Viollet B, Andreelli F, Jorgensen SB, Perrin C, Geloen A, Flamez D, Mu J, Lenzner C, Baud O, Bennoun M, Gomas E, Nicolas G, Wojtaszewski JF, Kahn A, Carling D, Schuit FC, Birnbaum MJ, Richter EA, Burcelin R, Vaulont S. The AMP-activated protein kinase alpha2 catalytic subunit controls whole-body insulin sensitivity. J CLIN INVEST. 2003;111:91–98. doi: 10.1172/JCI16567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Fassett JT, Hu X, Xu X, Lu Z, Zhang P, Chen Y, Bache RJ. AMPK attenuates microtubule proliferation in cardiac hypertrophy. Am J Physiol Heart Circ Physiol. 2013;304:H749–H758. doi: 10.1152/ajpheart.00935.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Kang H, Viollet B, Wu D. Genetic deletion of catalytic subunits of AMP-activated protein kinase increases osteoclasts and reduces bone mass in young adult mice. J BIOL CHEM. 2013;288:12187–12196. doi: 10.1074/jbc.M112.430389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Liang J, Yang Q, Zhu MJ, Jin Y, Du M. AMP-activated protein kinase (AMPK) alpha2 subunit mediates glycolysis in postmortem skeletal muscle. MEAT SCI. 2013;95:536–541. doi: 10.1016/j.meatsci.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 248.Qiu SL, Xiao ZC, Piao CM, Xian YL, Jia LX, Qi YF, Han JH, Zhang YY, Du J. AMP-activated protein kinase alpha2 protects against liver injury from metastasized tumors via reduced glucose deprivation-induced oxidative stress. J BIOL CHEM. 2014;289:9449–9459. doi: 10.1074/jbc.M113.543447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Qiu S, Xiao Z, Piao C, Zhang J, Dong Y, Cui W, Liu X, Zhang Y, Du J. AMPKalpha2 reduces renal epithelial transdifferentiation and inflammation after injury through interaction with CK2beta. J PATHOL. 2015;237:330–342. doi: 10.1002/path.4579. [DOI] [PubMed] [Google Scholar]
- 250.Zhang P, Hu X, Xu X, Fassett J, Zhu G, Viollet B, Xu W, Wiczer B, Bernlohr DA, Bache RJ, Chen Y. AMP activated protein kinase-alpha2 deficiency exacerbates pressure-overload-induced left ventricular hypertrophy and dysfunction in mice. HYPERTENSION. 2008;52:918–924. doi: 10.1161/HYPERTENSIONAHA.108.114702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Dong Y, Zhang M, Liang B, Xie Z, Zhao Z, Asfa S, Choi HC, Zou MH. Reduction of AMP-activated protein kinase alpha2 increases endoplasmic reticulum stress and atherosclerosis in vivo. CIRCULATION. 2010;121:792–803. doi: 10.1161/CIRCULATIONAHA.109.900928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Wang S, Zhang M, Liang B, Xu J, Xie Z, Liu C, Viollet B, Yan D, Zou MH. AMPKalpha2 deletion causes aberrant expression and activation of NAD(P)H oxidase and consequent endothelial dysfunction in vivo: role of 26S proteasomes. CIRC RES. 2010;106:1117–1128. doi: 10.1161/CIRCRESAHA.109.212530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Abbott MJ, Bogachus LD, Turcotte LP. AMPKalpha2 deficiency uncovers time dependency in the regulation of contraction-induced palmitate and glucose uptake in mouse muscle. J Appl Physiol (1985) 2011;111:125–134. doi: 10.1152/japplphysiol.00807.2010. [DOI] [PubMed] [Google Scholar]
- 254.Andreelli F, Foretz M, Knauf C, Cani PD, Perrin C, Iglesias MA, Pillot B, Bado A, Tronche F, Mithieux G, Vaulont S, Burcelin R, Viollet B. Liver adenosine monophosphate-activated kinase-alpha2 catalytic subunit is a key target for the control of hepatic glucose production by adiponectin and leptin but not insulin. ENDOCRINOLOGY. 2006;147:2432–2441. doi: 10.1210/en.2005-0898. [DOI] [PubMed] [Google Scholar]
- 255.Beall C, Piipari K, Al-Qassab H, Smith MA, Parker N, Carling D, Viollet B, Withers DJ, Ashford ML. Loss of AMP-activated protein kinase alpha2 subunit in mouse beta-cells impairs glucose-stimulated insulin secretion and inhibits their sensitivity to hypoglycaemia. BIOCHEM J. 2010;429:323–333. doi: 10.1042/BJ20100231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Song P, Zhou Y, Coughlan KA, Dai X, Xu H, Viollet B, Zou MH. Adenosine monophosphate-activated protein kinase-alpha2 deficiency promotes vascular smooth muscle cell migration via S-phase kinase-associated protein 2 upregulation and E-cadherin downregulation. Arterioscler Thromb Vasc Biol. 2013;33:2800–2809. doi: 10.1161/ATVBAHA.113.301869. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 257.Song P, Wang S, He C, Wang S, Liang B, Viollet B, Zou MH. AMPKalpha2 deletion exacerbates neointima formation by upregulating Skp2 in vascular smooth muscle cells. CIRC RES. 2011;109:1230–1239. doi: 10.1161/CIRCRESAHA.111.250423. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 258.Chen T, Li Z, Zhang Y, Feng F, Wang X, Wang X, Shen QW. Muscle-selective knockout of AMPKalpha2 does not exacerbate diet-induced obesity probably related to altered myokines expression. Biochem Biophys Res Commun. 2015;458:449–455. doi: 10.1016/j.bbrc.2015.01.075. [DOI] [PubMed] [Google Scholar]
- 259.Quinn JM, Tam S, Sims NA, Saleh H, McGregor NE, Poulton IJ, Scott JW, Gillespie MT, Kemp BE, van Denderen BJ. Germline deletion of AMP-activated protein kinase beta subunits reduces bone mass without altering osteoclast differentiation or function. FASEB J. 2010;24:275–285. doi: 10.1096/fj.09-137158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Thomas MM, Wang DC, D’Souza DM, Krause MP, Layne AS, Criswell DS, O’Neill HM, Connor MK, Anderson JE, Kemp BE, Steinberg GR, Hawke TJ. Muscle-specific AMPK beta1beta2-null mice display a myopathy due to loss of capillary density in nonpostural muscles. FASEB J. 2014;28:2098–2107. doi: 10.1096/fj.13-238972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Sun G, Tarasov AI, McGinty JA, French PM, McDonald A, Leclerc I, Rutter GA. LKB1 deletion with the RIP2.Cre transgene modifies pancreatic beta-cell morphology and enhances insulin secretion in vivo. Am J Physiol Endocrinol Metab. 2010;298:E1261–E1273. doi: 10.1152/ajpendo.00100.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Long YC, Barnes BR, Mahlapuu M, Steiler TL, Martinsson S, Leng Y, Wallberg-Henriksson H, Andersson L, Zierath JR. Role of AMP-activated protein kinase in the coordinated expression of genes controlling glucose and lipid metabolism in mouse white skeletal muscle. DIABETOLOGIA. 2005;48:2354–2364. doi: 10.1007/s00125-005-1962-5. [DOI] [PubMed] [Google Scholar]
- 263.Barnes BR, Long YC, Steiler TL, Leng Y, Galuska D, Wojtaszewski JF, Andersson L, Zierath JR. Changes in exercise-induced gene expression in 5′-AMP-activated protein kinase gamma3-null and gamma3 R225Q transgenic mice. DIABETES. 2005;54:3484–3489. doi: 10.2337/diabetes.54.12.3484. [DOI] [PubMed] [Google Scholar]
- 264.Vieira E, Nilsson EC, Nerstedt A, Ormestad M, Long YC, Garcia-Roves PM, Zierath JR, Mahlapuu M. Relationship between AMPK and the transcriptional balance of clock-related genes in skeletal muscle. Am J Physiol Endocrinol Metab. 2008;295:E1032–E1037. doi: 10.1152/ajpendo.90510.2008. [DOI] [PubMed] [Google Scholar]
- 265.Jishage K, Nezu J, Kawase Y, Iwata T, Watanabe M, Miyoshi A, Ose A, Habu K, Kake T, Kamada N, Ueda O, Kinoshita M, Jenne DE, Shimane M, Suzuki H. Role of Lkb1, the causative gene of Peutz-Jegher’s syndrome, in embryogenesis and polyposis. Proc Natl Acad Sci U S A. 2002;99:8903–8908. doi: 10.1073/pnas.122254599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Gan B, Hu J, Jiang S, Liu Y, Sahin E, Zhuang L, Fletcher-Sananikone E, Colla S, Wang YA, Chin L, Depinho RA. Lkb1 regulates quiescence and metabolic homeostasis of haematopoietic stem cells. NATURE. 2010;468:701–704. doi: 10.1038/nature09595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Miyoshi H, Nakau M, Ishikawa TO, Seldin MF, Oshima M, Taketo MM. Gastrointestinal hamartomatous polyposis in Lkb1 heterozygous knockout mice. CANCER RES. 2002;62:2261–2266. [PubMed] [Google Scholar]
- 268.Nakau M, Miyoshi H, Seldin MF, Imamura M, Oshima M, Taketo MM. Hepatocellular carcinoma caused by loss of heterozygosity in Lkb1 gene knockout mice. CANCER RES. 2002;62:4549–4553. [PubMed] [Google Scholar]
- 269.Morton JP, Jamieson NB, Karim SA, Athineos D, Ridgway RA, Nixon C, McKay CJ, Carter R, Brunton VG, Frame MC, Ashworth A, Oien KA, Evans TR, Sansom OJ. LKB1 haploinsufficiency cooperates with Kras to promote pancreatic cancer through suppression of p21-dependent growth arrest. GASTROENTEROLOGY. 2010;139:586–597. 591–597. doi: 10.1053/j.gastro.2010.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Miura S, Kai Y, Tadaishi M, Tokutake Y, Sakamoto K, Bruce CR, Febbraio MA, Kita K, Chohnan S, Ezaki O. Marked phenotypic differences of endurance performance and exercise-induced oxygen consumption between AMPK and LKB1 deficiency in mouse skeletal muscle: changes occurring in the diaphragm. Am J Physiol Endocrinol Metab. 2013;305:E213–E229. doi: 10.1152/ajpendo.00114.2013. [DOI] [PubMed] [Google Scholar]
- 271.Thomson DM, Porter BB, Tall JH, Kim HJ, Barrow JR, Winder WW. Skeletal muscle and heart LKB1 deficiency causes decreased voluntary running and reduced muscle mitochondrial marker enzyme expression in mice. Am J Physiol Endocrinol Metab. 2007;292:E196–E202. doi: 10.1152/ajpendo.00366.2006. [DOI] [PubMed] [Google Scholar]
- 272.Koh HJ, Arnolds DE, Fujii N, Tran TT, Rogers MJ, Jessen N, Li Y, Liew CW, Ho RC, Hirshman MF, Kulkarni RN, Kahn CR, Goodyear LJ. Skeletal muscle-selective knockout of LKB1 increases insulin sensitivity, improves glucose homeostasis, and decreases TRB3. MOL CELL BIOL. 2006;26:8217–8227. doi: 10.1128/MCB.00979-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Hezel AF, Gurumurthy S, Granot Z, Swisa A, Chu GC, Bailey G, Dor Y, Bardeesy N, Depinho RA. Pancreatic LKB1 deletion leads to acinar polarity defects and cystic neoplasms. MOL CELL BIOL. 2008;28:2414–2425. doi: 10.1128/MCB.01621-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Londesborough A, Vaahtomeri K, Tiainen M, Katajisto P, Ekman N, Vallenius T, Makela TP. LKB1 in endothelial cells is required for angiogenesis and TGFbeta-mediated vascular smooth muscle cell recruitment. DEVELOPMENT. 2008;135:2331–2338. doi: 10.1242/dev.017038. [DOI] [PubMed] [Google Scholar]
- 275.Sun G, Da SXG, Gorman T, Priest C, Solomou A, Hodson DJ, Foretz M, Viollet B, Herrera PL, Parker H, Reimann F, Gribble FM, Migrenne S, Magnan C, Marley A, Rutter GA. LKB1 and AMPKalpha1 are required in pancreatic alpha cells for the normal regulation of glucagon secretion and responses to hypoglycemia. Mol Metab. 2015;4:277–286. doi: 10.1016/j.molmet.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Fu A, Ng AC, Depatie C, Wijesekara N, He Y, Wang GS, Bardeesy N, Scott FW, Touyz RM, Wheeler MB, Screaton RA. Loss of Lkb1 in adult beta cells increases beta cell mass and enhances glucose tolerance in mice. CELL METAB. 2009;10:285–295. doi: 10.1016/j.cmet.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 277.Ikeda Y, Sato K, Pimentel DR, Sam F, Shaw RJ, Dyck JR, Walsh K. Cardiac-specific deletion of LKB1 leads to hypertrophy and dysfunction. J BIOL CHEM. 2009;284:35839–35849. doi: 10.1074/jbc.M109.057273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Ozcan C, Battaglia E, Young R, Suzuki G. LKB1 knockout mouse develops spontaneous atrial fibrillation and provides mechanistic insights into human disease process. J AM HEART ASSOC. 2015;4:e1733. doi: 10.1161/JAHA.114.001733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Woods A, Heslegrave AJ, Muckett PJ, Levene AP, Clements M, Mobberley M, Ryder TA, Abu-Hayyeh S, Williamson C, Goldin RD, Ashworth A, Withers DJ, Carling D. LKB1 is required for hepatic bile acid transport and canalicular membrane integrity in mice. BIOCHEM J. 2011;434:49–60. doi: 10.1042/BJ20101721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Sun G, Reynolds R, Leclerc I, Rutter GA. RIP2-mediated LKB1 deletion causes axon degeneration in the spinal cord and hind-limb paralysis. DIS MODEL MECH. 2011;4:193–202. doi: 10.1242/dmm.006833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.MacIver NJ, Blagih J, Saucillo DC, Tonelli L, Griss T, Rathmell JC, Jones RG. The liver kinase B1 is a central regulator of T cell development, activation, and metabolism. J IMMUNOL. 2011;187:4187–4198. doi: 10.4049/jimmunol.1100367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Shan T, Xiong Y, Zhang P, Li Z, Jiang Q, Bi P, Yue F, Yang G, Wang Y, Liu X, Kuang S. Lkb1 controls brown adipose tissue growth and thermogenesis by regulating the intracellular localization of CRTC3. NAT COMMUN. 2016;7:12205. doi: 10.1038/ncomms12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Lai LP, Lotinun S, Bouxsein ML, Baron R, McMahon AP. Stk11 (Lkb1) deletion in the osteoblast lineage leads to high bone turnover, increased trabecular bone density and cortical porosity. BONE. 2014;69:98–108. doi: 10.1016/j.bone.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Shen YA, Chen Y, Dao DQ, Mayoral SR, Wu L, Meijer D, Ullian EM, Chan JR, Lu QR. Phosphorylation of LKB1/Par-4 establishes Schwann cell polarity to initiate and control myelin extent. NAT COMMUN. 2014;5:4991. doi: 10.1038/ncomms5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Men Y, Zhang A, Li H, Zhang T, Jin Y, Li H, Zhang J, Gao J. LKB1 Is Required for the Development and Maintenance of Stereocilia in Inner Ear Hair Cells in Mice. PLOS ONE. 2015;10:e135841. doi: 10.1371/journal.pone.0135841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Men Y, Zhang A, Li H, Jin Y, Sun X, Li H, Gao J. LKB1 Regulates Cerebellar Development by Controlling Sonic Hedgehog-mediated Granule Cell Precursor Proliferation and Granule Cell Migration. Sci Rep. 2015;5:16232. doi: 10.1038/srep16232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Jiang ZZ, Hu MW, Ma XS, Schatten H, Fan HY, Wang ZB, Sun QY. LKB1 acts as a critical gatekeeper of ovarian primordial follicle pool. ONCOTARGET. 2016;7:5738–5753. doi: 10.18632/oncotarget.6792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Mizuno K, Antunes-Martins A, Ris L, Peters M, Godaux E, Giese KP. Calcium/calmodulin kinase kinase beta has a male-specific role in memory formation. NEUROSCIENCE. 2007;145:393–402. doi: 10.1016/j.neuroscience.2006.11.056. [DOI] [PubMed] [Google Scholar]
- 289.Anderson KA, Lin F, Ribar TJ, Stevens RD, Muehlbauer MJ, Newgard CB, Means AR. Deletion of CaMKK2 from the liver lowers blood glucose and improves whole-body glucose tolerance in the mouse. MOL ENDOCRINOL. 2012;26:281–291. doi: 10.1210/me.2011-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Kokubo M, Nishio M, Ribar TJ, Anderson KA, West AE, Means AR. BDNF-mediated cerebellar granule cell development is impaired in mice null for CaMKK2 or CaMKIV. J NEUROSCI. 2009;29:8901–8913. doi: 10.1523/JNEUROSCI.0040-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Racioppi L, Noeldner PK, Lin F, Arvai S, Means AR. Calcium/calmodulin-dependent protein kinase kinase 2 regulates macrophage-mediated inflammatory responses. J BIOL CHEM. 2012;287:11579–11591. doi: 10.1074/jbc.M111.336032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Cary RL, Waddell S, Racioppi L, Long F, Novack DV, Voor MJ, Sankar U. Inhibition of Ca(2)(+)/calmodulin-dependent protein kinase kinase 2 stimulates osteoblast formation and inhibits osteoclast differentiation. J BONE MINER RES. 2013;28:1599–1610. doi: 10.1002/jbmr.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.McCullough LD, Tarabishy S, Liu L, Benashski S, Xu Y, Ribar T, Means A, Li J. Inhibition of calcium/calmodulin-dependent protein kinase kinase beta and calcium/calmodulin-dependent protein kinase IV is detrimental in cerebral ischemia. STROKE. 2013;44:2559–2566. doi: 10.1161/STROKEAHA.113.001030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Anderson KA, Ribar TJ, Lin F, Noeldner PK, Green MF, Muehlbauer MJ, Witters LA, Kemp BE, Means AR. Hypothalamic CaMKK2 contributes to the regulation of energy balance. CELL METAB. 2008;7:377–388. doi: 10.1016/j.cmet.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 295.Rona-Voros K, Weydt P. The role of PGC-1alpha in the pathogenesis of neurodegenerative disorders. CURR DRUG TARGETS. 2010;11:1262–1269. doi: 10.2174/1389450111007011262. [DOI] [PubMed] [Google Scholar]
- 296.Saint-Geniez M, Jiang A, Abend S, Liu L, Sweigard H, Connor KM, Arany Z. PGC-1alpha regulates normal and pathological angiogenesis in the retina. AM J PATHOL. 2013;182:255–265. doi: 10.1016/j.ajpath.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Lucas EK, Dougherty SE, McMeekin LJ, Trinh AT, Reid CS, Cowell RM. Developmental alterations in motor coordination and medium spiny neuron markers in mice lacking pgc-1alpha. PLOS ONE. 2012;7:e42878. doi: 10.1371/journal.pone.0042878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Dougherty SE, Bartley AF, Lucas EK, Hablitz JJ, Dobrunz LE, Cowell RM. Mice lacking the transcriptional coactivator PGC-1alpha exhibit alterations in inhibitory synaptic transmission in the motor cortex. NEUROSCIENCE. 2014;271:137–148. doi: 10.1016/j.neuroscience.2014.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Sawada N, Jiang A, Takizawa F, Safdar A, Manika A, Tesmenitsky Y, Kang KT, Bischoff J, Kalwa H, Sartoretto JL, Kamei Y, Benjamin LE, Watada H, Ogawa Y, Higashikuni Y, Kessinger CW, Jaffer FA, Michel T, Sata M, Croce K, Tanaka R, Arany Z. Endothelial PGC-1alpha mediates vascular dysfunction in diabetes. CELL METAB. 2014;19:246–258. doi: 10.1016/j.cmet.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Kleiner S, Mepani RJ, Laznik D, Ye L, Jurczak MJ, Jornayvaz FR, Estall JL, Chatterjee BD, Shulman GI, Spiegelman BM. Development of insulin resistance in mice lacking PGC-1alpha in adipose tissues. Proc Natl Acad Sci U S A. 2012;109:9635–9640. doi: 10.1073/pnas.1207287109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Sczelecki S, Besse-Patin A, Abboud A, Kleiner S, Laznik-Bogoslavski D, Wrann CD, Ruas JL, Haibe-Kains B, Estall JL. Loss of Pgc-1alpha expression in aging mouse muscle potentiates glucose intolerance and systemic inflammation. Am J Physiol Endocrinol Metab. 2014;306:E157–E167. doi: 10.1152/ajpendo.00578.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Tran M, Tam D, Bardia A, Bhasin M, Rowe GC, Kher A, Zsengeller ZK, Akhavan-Sharif MR, Khankin EV, Saintgeniez M, David S, Burstein D, Karumanchi SA, Stillman IE, Arany Z, Parikh SM. PGC-1alpha promotes recovery after acute kidney injury during systemic inflammation in mice. J CLIN INVEST. 2011;121:4003–4014. doi: 10.1172/JCI58662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Cermenati G, Audano M, Giatti S, Carozzi V, Porretta-Serapiglia C, Pettinato E, Ferri C, D’Antonio M, De Fabiani E, Crestani M, Scurati S, Saez E, Azcoitia I, Cavaletti G, Garcia-Segura LM, Melcangi RC, Caruso D, Mitro N. Lack of sterol regulatory element binding factor-1c imposes glial Fatty Acid utilization leading to peripheral neuropathy. CELL METAB. 2015;21:571–583. doi: 10.1016/j.cmet.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 304.Rudolph MC, Monks J, Burns V, Phistry M, Marians R, Foote MR, Bauman DE, Anderson SM, Neville MC. Sterol regulatory element binding protein and dietary lipid regulation of fatty acid synthesis in the mammary epithelium. Am J Physiol Endocrinol Metab. 2010;299:E918–E927. doi: 10.1152/ajpendo.00376.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Shimano H, Amemiya-Kudo M, Takahashi A, Kato T, Ishikawa M, Yamada N. Sterol regulatory element-binding protein-1c and pancreatic beta-cell dysfunction. DIABETES OBES METAB 9 Suppl. 2007;2:133–139. doi: 10.1111/j.1463-1326.2007.00779.x. [DOI] [PubMed] [Google Scholar]
- 306.Abu-Elheiga L, Matzuk MM, Kordari P, Oh W, Shaikenov T, Gu Z, Wakil SJ. Mutant mice lacking acetyl-CoA carboxylase 1 are embryonically lethal. Proc Natl Acad Sci U S A. 2005;102:12011–12016. doi: 10.1073/pnas.0505714102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Mao J, DeMayo FJ, Li H, Abu-Elheiga L, Gu Z, Shaikenov TE, Kordari P, Chirala SS, Heird WC, Wakil SJ. Liver-specific deletion of acetyl-CoA carboxylase 1 reduces hepatic triglyceride accumulation without affecting glucose homeostasis. Proc Natl Acad Sci U S A. 2006;103:8552–8557. doi: 10.1073/pnas.0603115103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Mao J, Yang T, Gu Z, Heird WC, Finegold MJ, Lee B, Wakil SJ. aP2-Cre-mediated inactivation of acetyl-CoA carboxylase 1 causes growth retardation and reduced lipid accumulation in adipose tissues. Proc Natl Acad Sci U S A. 2009;106:17576–17581. doi: 10.1073/pnas.0909055106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Lee J, Walsh MC, Hoehn KL, James DE, Wherry EJ, Choi Y. Regulator of fatty acid metabolism, acetyl coenzyme a carboxylase 1, controls T cell immunity. J IMMUNOL. 2014;192:3190–3199. doi: 10.4049/jimmunol.1302985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Abu-Elheiga L, Wu H, Gu Z, Bressler R, Wakil SJ. Acetyl-CoA carboxylase 2-/- mutant mice are protected against fatty liver under high-fat, high-carbohydrate dietary and de novo lipogenic conditions. J BIOL CHEM. 2012;287:12578–12588. doi: 10.1074/jbc.M111.309559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Kolwicz SJ, Olson DP, Marney LC, Garcia-Menendez L, Synovec RE, Tian R. Cardiac-specific deletion of acetyl CoA carboxylase 2 prevents metabolic remodeling during pressure-overload hypertrophy. CIRC RES. 2012;111:728–738. doi: 10.1161/CIRCRESAHA.112.268128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Olson DP, Pulinilkunnil T, Cline GW, Shulman GI, Lowell BB. Gene knockout of Acc2 has little effect on body weight, fat mass, or food intake. Proc Natl Acad Sci U S A. 2010;107:7598–7603. doi: 10.1073/pnas.0913492107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Chow JD, Lawrence RT, Healy ME, Dominy JE, Liao JA, Breen DS, Byrne FL, Kenwood BM, Lackner C, Okutsu S, Mas VR, Caldwell SH, Tomsig JL, Cooney GJ, Puigserver PB, Turner N, James DE, Villen J, Hoehn KL. Genetic inhibition of hepatic acetyl-CoA carboxylase activity increases liver fat and alters global protein acetylation. Mol Metab. 2014;3:419–431. doi: 10.1016/j.molmet.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Ohashi K, Osuga J, Tozawa R, Kitamine T, Yagyu H, Sekiya M, Tomita S, Okazaki H, Tamura Y, Yahagi N, Iizuka Y, Harada K, Gotoda T, Shimano H, Yamada N, Ishibashi S. Early embryonic lethality caused by targeted disruption of the 3-hydroxy-3-methylglutaryl-CoA reductase gene. J BIOL CHEM. 2003;278:42936–42941. doi: 10.1074/jbc.M307228200. [DOI] [PubMed] [Google Scholar]
- 315.Osaki Y, Nakagawa Y, Miyahara S, Iwasaki H, Ishii A, Matsuzaka T, Kobayashi K, Yatoh S, Takahashi A, Yahagi N, Suzuki H, Sone H, Ohashi K, Ishibashi S, Yamada N, Shimano H. Skeletal muscle-specific HMG-CoA reductase knockout mice exhibit rhabdomyolysis: A model for statin-induced myopathy. Biochem Biophys Res Commun. 2015;466:536–540. doi: 10.1016/j.bbrc.2015.09.065. [DOI] [PubMed] [Google Scholar]
- 316.Nagashima S, Yagyu H, Ohashi K, Tazoe F, Takahashi M, Ohshiro T, Bayasgalan T, Okada K, Sekiya M, Osuga J, Ishibashi S. Liver-specific deletion of 3-hydroxy-3-methylglutaryl coenzyme A reductase causes hepatic steatosis and death. Arterioscler Thromb Vasc Biol. 2012;32:1824–1831. doi: 10.1161/ATVBAHA.111.240754. [DOI] [PubMed] [Google Scholar]
- 317.Duran J, Saez I, Gruart A, Guinovart JJ, Delgado-Garcia JM. Impairment in long-term memory formation and learning-dependent synaptic plasticity in mice lacking glycogen synthase in the brain. J Cereb Blood Flow Metab. 2013;33:550–556. doi: 10.1038/jcbfm.2012.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Pederson BA, Schroeder JM, Parker GE, Smith MW, DePaoli-Roach AA, Roach PJ. Glucose metabolism in mice lacking muscle glycogen synthase. DIABETES. 2005;54:3466–3473. doi: 10.2337/diabetes.54.12.3466. [DOI] [PubMed] [Google Scholar]
- 319.Irimia JM, Meyer CM, Peper CL, Zhai L, Bock CB, Previs SF, McGuinness OP, DePaoli-Roach A, Roach PJ. Impaired glucose tolerance and predisposition to the fasted state in liver glycogen synthase knock-out mice. J BIOL CHEM. 2010;285:12851–12861. doi: 10.1074/jbc.M110.106534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Palmer SC, Mavridis D, Nicolucci A, Johnson DW, Tonelli M, Craig JC, Maggo J, Gray V, De Berardis G, Ruospo M, Natale P, Saglimbene V, Badve SV, Cho Y, Nadeau-Fredette AC, Burke M, Faruque L, Lloyd A, Ahmad N, Liu Y, Tiv S, Wiebe N, Strippoli GF. Comparison of Clinical Outcomes and Adverse Events Associated With Glucose-Lowering Drugs in Patients With Type 2 Diabetes: A Meta-analysis. JAMA. 2016;316:313–324. doi: 10.1001/jama.2016.9400. [DOI] [PubMed] [Google Scholar]
- 321.Bruno S, Ledda B, Tenca C, Ravera S, Orengo AM, Mazzarello AN, Pesenti E, Casciaro S, Racchi O, Ghiotto F, Marini C, Sambuceti G, DeCensi A, Fais F. Metformin inhibits cell cycle progression of B-cell chronic lymphocytic leukemia cells. ONCOTARGET. 2015;6:22624–22640. doi: 10.18632/oncotarget.4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Al-Wahab Z, Mert I, Tebbe C, Chhina J, Hijaz M, Morris RT, Ali-Fehmi R, Giri S, Munkarah AR, Rattan R. Metformin prevents aggressive ovarian cancer growth driven by high-energy diet: similarity with calorie restriction. ONCOTARGET. 2015;6:10908–10923. doi: 10.18632/oncotarget.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Ge R, Wang Z, Wu S, Zhuo Y, Otsetov AG, Cai C, Zhong W, Wu CL, Olumi AF. Metformin represses cancer cells via alternate pathways in N-cadherin expressing vs. N-cadherin deficient cells. ONCOTARGET. 2015;6:28973–28987. doi: 10.18632/oncotarget.5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Choi SM, Jang AH, Kim H, Lee KH, Kim YW. Metformin Reduces Bleomycin-induced Pulmonary Fibrosis in Mice. J KOREAN MED SCI. 2016;31:1419–1425. doi: 10.3346/jkms.2016.31.9.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Asghari A, Akbari G, Meghdadi A, Mortazavi P. Protective effect of metformin on testicular ischemia/reperfusion injury in rats. ACTA CIR BRAS. 2016;31:411–416. doi: 10.1590/S0102-865020160060000008. [DOI] [PubMed] [Google Scholar]
- 326.Deng T, Zheng YR, Hou WW, Yuan Y, Shen Z, Wu XL, Chen Y, Zhang LS, Hu WW, Chen Z, Zhang XN. Pre-stroke Metformin Treatment is Neuroprotective Involving AMPK Reduction. NEUROCHEM RES. 2016 doi: 10.1007/s11064-016-1988-8. [DOI] [PubMed] [Google Scholar]
- 327.Facila L, Fabregat-Andres O, Bertomeu V, Navarro JP, Minana G, Garcia-Blas S, Valero E, Morell S, Sanchis J, Nunez J. Metformin and risk of long-term mortality following and admission for acute heart failure. J Cardiovasc Med (Hagerstown) 2016 doi: 10.2459/JCM.0000000000000420. [DOI] [PubMed] [Google Scholar]
- 328.Barzilai N, Crandall JP, Kritchevsky SB, Espeland MA. Metformin as a Tool to Target Aging. CELL METAB. 2016;23:1060–1065. doi: 10.1016/j.cmet.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Negrotto L, Farez MF, Correale J. Immunologic Effects of Metformin and Pioglitazone Treatment on Metabolic Syndrome and Multiple Sclerosis. JAMA NEUROL. 2016;73:520–528. doi: 10.1001/jamaneurol.2015.4807. [DOI] [PubMed] [Google Scholar]
- 330.Boon H, Bosselaar M, Praet SF, Blaak EE, Saris WH, Wagenmakers AJ, McGee SL, Tack CJ, Smits P, Hargreaves M, van Loon LJ. Intravenous AICAR administration reduces hepatic glucose output and inhibits whole body lipolysis in type 2 diabetic patients. DIABETOLOGIA. 2008;51:1893–1900. doi: 10.1007/s00125-008-1108-7. [DOI] [PubMed] [Google Scholar]
- 331.Tomita K, Tamiya G, Ando S, Kitamura N, Koizumi H, Kato S, Horie Y, Kaneko T, Azuma T, Nagata H, Ishii H, Hibi T. AICAR, an AMPK activator, has protective effects on alcohol-induced fatty liver in rats. ALCOHOL CLIN EXP RES. 2005;29:240S–245S. doi: 10.1097/01.alc.0000191126.11479.69. [DOI] [PubMed] [Google Scholar]
- 332.Cervero C, Montull N, Tarabal O, Piedrafita L, Esquerda JE, Caldero J. Chronic Treatment with the AMPK Agonist AICAR Prevents Skeletal Muscle Pathology but Fails to Improve Clinical Outcome in a Mouse Model of Severe Spinal Muscular Atrophy. NEUROTHERAPEUTICS. 2016;13:198–216. doi: 10.1007/s13311-015-0399-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Montraveta A, Xargay-Torrent S, Lopez-Guerra M, Rosich L, Perez-Galan P, Salaverria I, Bea S, Kalko SG, de Frias M, Campas C, Roue G, Colomer D. Synergistic anti-tumor activity of acadesine (AICAR) in combination with the anti-CD20 monoclonal antibody rituximab in in vivo and in vitro models of mantle cell lymphoma. ONCOTARGET. 2014;5:726–739. doi: 10.18632/oncotarget.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Sung MS, Li Z, Cui L, Choi JS, Choi W, Park MJ, Park SH, Yoon KC. Effect of Topical 5-Aminoimidazole-4-carboxamide-1-beta-d-Ribofuranoside in a Mouse Model of Experimental Dry Eye. Invest Ophthalmol Vis Sci. 2015;56:3149–3158. doi: 10.1167/iovs.14-16153. [DOI] [PubMed] [Google Scholar]
- 335.Yang C, Gong X, Ai Q, Ge P, Lin L, Zhang L. 5-Aminoimidazole-4-carboxamide-1-beta-d-ribofuranoside alleviated carbon tetrachloride-induced acute hepatitis in mice. INT IMMUNOPHARMACOL. 2015;25:393–399. doi: 10.1016/j.intimp.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 336.Matsiukevich D, Piraino G, Klingbeil LR, Hake PW, Wolfe V, O’Connor M, Zingarelli B. The Ampk Activator Aicar Ameliorates Age-Dependent Myocardial Injury in Murine Hemorrhagic Shock. SHOCK. 2016 doi: 10.1097/SHK.0000000000000730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Idrovo JP, Yang WL, Jacob A, Aziz M, Nicastro J, Coppa GF, Wang P. AICAR attenuates organ injury and inflammatory response after intestinal ischemia and reperfusion. MOL MED. 2014;20:676–683. doi: 10.2119/molmed.2014.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Kobilo T, Guerrieri D, Zhang Y, Collica SC, Becker KG, van Praag H. AMPK agonist AICAR improves cognition and motor coordination in young and aged mice. Learn Mem. 2014;21:119–126. doi: 10.1101/lm.033332.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Zhou Y, Wang D, Zhu Q, Gao X, Yang S, Xu A, Wu D. Inhibitory effects of A-769662, a novel activator of AMP-activated protein kinase, on 3T3-L1 adipogenesis. BIOL PHARM BULL. 2009;32:993–998. doi: 10.1248/bpb.32.993. [DOI] [PubMed] [Google Scholar]
- 340.Zhu Y, Zhou J, Ao R, Yu B. A-769662 protects osteoblasts from hydrogen dioxide-induced apoptosis through activating of AMP-activated protein kinase (AMPK) INT J MOL SCI. 2014;15:11190–11203. doi: 10.3390/ijms150611190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Guma M, Wang Y, Viollet B, Liu-Bryan R. AMPK Activation by A-769662 Controls IL-6 Expression in Inflammatory Arthritis. PLOS ONE. 2015;10:e140452. doi: 10.1371/journal.pone.0140452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Hu X, Jiang F, Bao Q, Qian H, Fang Q, Shao Z. Compound 13, an alpha1-selective small molecule activator of AMPK, potently inhibits melanoma cell proliferation. Tumour Biol. 2016;37:1071–1078. doi: 10.1007/s13277-015-3854-8. [DOI] [PubMed] [Google Scholar]
- 343.Zhao H, Zhu H, Lin Z, Lin G, Lv G. Compound 13, an alpha1-selective small molecule activator of AMPK, inhibits Helicobacter pylori-induced oxidative stresses and gastric epithelial cell apoptosis. Biochem Biophys Res Commun. 2015;463:510–517. doi: 10.1016/j.bbrc.2015.05.059. [DOI] [PubMed] [Google Scholar]
- 344.Cameron KO, Kung DW, Kalgutkar AS, Kurumbail RG, Miller R, Salatto CT, Ward J, Withka JM, Bhattacharya SK, Boehm M, Borzilleri KA, Brown JA, Calabrese M, Caspers NL, Cokorinos E, Conn EL, Dowling MS, Edmonds DJ, Eng H, Fernando DP, Frisbie R, Hepworth D, Landro J, Mao Y, Rajamohan F, Reyes AR, Rose CR, Ryder T, Shavnya A, Smith AC, Tu M, Wolford AC, Xiao J. Discovery and Preclinical Characterization of 6-Chloro-5-[4-(1-hydroxycyclobutyl)phenyl]-1H-indole-3-carboxylic Acid (PF-06409577), a Direct Activator of Adenosine Monophosphate-activated Protein Kinase (AMPK), for the Potential Treatment of Diabetic Nephropathy. J MED CHEM. 2016 doi: 10.1021/acs.jmedchem.6b00866. [DOI] [PubMed] [Google Scholar]
- 345.Choi JW, Kim M, Song H, Lee CS, Oh WK, Mook-Jung I, Chung SS, Park KS. DMC (2′,4′-dihydroxy-6′-methoxy-3′,5′-dimethylchalcone) improves glucose tolerance as a potent AMPK activator. METABOLISM. 2016;65:533–542. doi: 10.1016/j.metabol.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 346.Huang L, Dai K, Chen M, Zhou W, Wang X, Chen J, Zhou W. The AMPK Agonist PT1 and mTOR Inhibitor 3HOI-BA-01 Protect Cardiomyocytes After Ischemia Through Induction of Autophagy. J Cardiovasc Pharmacol Ther. 2016;21:70–81. doi: 10.1177/1074248415581177. [DOI] [PubMed] [Google Scholar]
- 347.Zadra G, Photopoulos C, Tyekucheva S, Heidari P, Weng QP, Fedele G, Liu H, Scaglia N, Priolo C, Sicinska E, Mahmood U, Signoretti S, Birnberg N, Loda M. A novel direct activator of AMPK inhibits prostate cancer growth by blocking lipogenesis. EMBO MOL MED. 2014;6:519–538. doi: 10.1002/emmm.201302734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Hawley SA, Fullerton MD, Ross FA, Schertzer JD, Chevtzoff C, Walker KJ, Peggie MW, Zibrova D, Green KA, Mustard KJ, Kemp BE, Sakamoto K, Steinberg GR, Hardie DG. The ancient drug salicylate directly activates AMP-activated protein kinase. SCIENCE. 2012;336:918–922. doi: 10.1126/science.1215327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.O’Brien AJ, Villani LA, Broadfield LA, Houde VP, Galic S, Blandino G, Kemp BE, Tsakiridis T, Muti P, Steinberg GR. Salicylate activates AMPK and synergizes with metformin to reduce the survival of prostate and lung cancer cells ex vivo through inhibition of de novo lipogenesis. BIOCHEM J. 2015;469:177–187. doi: 10.1042/BJ20150122. [DOI] [PubMed] [Google Scholar]
- 350.Jung YR, Kim EJ, Choi HJ, Park JJ, Kim HS, Lee YJ, Park MJ, Lee M. Aspirin Targets SIRT1 and AMPK to Induce Senescence of Colorectal Carcinoma Cells. MOL PHARMACOL. 2015;88:708–719. doi: 10.1124/mol.115.098616. [DOI] [PubMed] [Google Scholar]
- 351.Liu X, Chhipa RR, Nakano I, Dasgupta B. The AMPK inhibitor compound C is a potent AMPK-independent antiglioma agent. MOL CANCER THER. 2014;13:596–605. doi: 10.1158/1535-7163.MCT-13-0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Huang SW, Wu CY, Wang YT, Kao JK, Lin CC, Chang CC, Mu SW, Chen YY, Chiu HW, Chang CH, Liang SM, Chen YJ, Huang JL, Shieh JJ. p53 modulates the AMPK inhibitor compound C induced apoptosis in human skin cancer cells. Toxicol Appl Pharmacol. 2013;267:113–124. doi: 10.1016/j.taap.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 353.Yang WL, Perillo W, Liou D, Marambaud P, Wang P. AMPK inhibitor compound C suppresses cell proliferation by induction of apoptosis and autophagy in human colorectal cancer cells. J SURG ONCOL. 2012;106:680–688. doi: 10.1002/jso.23184. [DOI] [PubMed] [Google Scholar]
- 354.Duong HQ, Hwang JS, Kim HJ, Seong YS, Bae I. BML-275, an AMPK inhibitor, induces DNA damage, G2/M arrest and apoptosis in human pancreatic cancer cells. INT J ONCOL. 2012;41:2227–2236. doi: 10.3892/ijo.2012.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Lee Y, Park BH, Bae EJ. Compound C inhibits macrophage chemotaxis through an AMPK-independent mechanism. Biochem Biophys Res Commun. 2016;469:515–520. doi: 10.1016/j.bbrc.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 356.Oosterman JE, Belsham DD. Glucose Alters Per2 Rhythmicity Independent of AMPK, Whereas AMPK Inhibitor Compound C Causes Profound Repression of Clock Genes and AgRP in mHypoE-37 Hypothalamic Neurons. PLOS ONE. 2016;11:e146969. doi: 10.1371/journal.pone.0146969. [DOI] [PMC free article] [PubMed] [Google Scholar]