Abstract
For more than a century, chemists have endeavored to discover and develop reaction processes that enable the selective oxidation of hydrocarbons. In the 1970s, Abramovitch and Yamada described the synthesis and electrophilic reactivity of sulfonyliminoiodinanes (RSO2N=IPh), demonstrating the utility of this new class of reagents to function as nitrene equivalents. Subsequent investigations by Breslow, Mansuy, and Müller would show such oxidants to be competent for alkene and saturated hydrocarbon functionalization when combined with transition metal salts or metal complexes, namely those of Mn, Fe, and Rh. Here, we trace our own studies to develop N-atom transfer technologies for C–H and π-bond oxidation. This Account discusses advances in both intra- and intermolecular amination processes mediated by dirhodium and diruthenium complexes, as well as the mechanistic foundations of catalyst reactivity and arrest. Explicit reference is given to questions that remain unanswered and to problem areas that are rich for discovery.
A fundamental advance in amination technology has been the recognition that iminoiodinane oxidants can be generated in situ in the presence of a metal catalyst that elicits subsequent N-atom transfer. Under these conditions, both dirhodium and diruthenium lantern complexes function as competent catalysts for C–H bond oxidation with a range of nitrogen sources (e.g., carbamates, sulfamates, sulfamides, etc.), many of which will not form isolable iminoiodinane equivalents. Practical synthetic methods and applications thereof have evolved in parallel with inquiries into the operative reaction mechanism(s). For the intramolecular dirhodium-catalyzed process, the body of experimental and computational data is consistent with a concerted asynchronous C–H insertion pathway, analogous to the consensus mechanism for Rh-carbene transfer. Other studies reveal that the bridging tetracarboxylate ligand groups, which shroud the dirhodium core, are labile to exchange under standard reaction conditions. This information has led to the generation of chelating dicarboxylate dinuclear rhodium complexes, exemplified by Rh2(esp)2. The performance of this catalyst system is unmatched by other dirhodium complexes in both intra- and intermolecular C–H amination reactions.
Tetra-bridged, mixed-valent diruthenium complexes function as effective promoters of sulfamate ester oxidative cyclization. These catalysts can be crafted with ligand sets other than carboxylates and are more resistant to oxidation than their dirhodium counterparts. A range of experimental and computational mechanistic data amassed with the tetra-2-oxypyridinate diruthenium chloride complex, [Ru2(hp)4Cl], has established the insertion event as a stepwise pathway involving a discrete radical intermediate. These data contrast dirhodium-catalyzed C–H amination and offer a cogent model for understanding the divergent chemoselectivity trends observed between the two catalyst types. This work constitutes an important step toward the ultimate goal of achieving predictable, reagent-level control over product selectivity.
Graphical Abstract
Introduction
Significant advances in reaction technology over the past two decades have rendered the C–H bond a functional synthon for polar amine and alcohol groups.1 In many cases, these methods operate at a level of efficiency suitable for application in complex molecule synthesis. The continued development of C–H oxidation reactions necessitates a deeper understanding of the elements that influence chemoselectivity in substrates possessing multiple, differentially substituted C–H centers. Evaluation of catalyst stability and the off-pathway reactions that lead to catalyst arrest is also warranted. Ultimately, such knowledge should give way to the invention of high performance catalyst systems that offer superior reagent-level control over product selectivity. These research efforts are stimulated by the manifold challenges associated with the development of catalytic redox processes that enable selective functionalization of inert chemical bonds and the potential of such methods to transform strategic approaches to chemical synthesis.
For some time, our lab has been invested in the development of heteroatom-transfer methods for C–H bond oxidation, with an emphasis on technologies for the facile amination and hydroxylation of hydrocarbons (Figure 1).2,3 In this Account, we discuss our investigations of both intra- and intermolecular catalytic C–H and π-bond amination, focusing on our efforts to develop chemoselective and diastereoselective insertion reactions and to understand operative mechanisms for N-atom transfer and catalyst inhibition. An exhaustive overview of the historical traces of this technology and the salient contributions from many others who work in this field has been presented elsewhere and will be largely omitted from this text.1,4
FIGURE 1.
Selective C–H oxidation as a general method for C–N bond formation.
C–H Amination in Synthesis
The potential for C–H amination in synthesis had been recognized by the 1960s in the work of Edwards, Meyer, and Masamune (Figure 2).5 These early investigations highlight the unique capabilities of such a method as well as the challenges associated with achieving efficient, site-selective C–H bond functionalization using hyper-reactive N-based oxidants. To this latter point, the recognition that sulfonamide-derived aryliodinanes (ArSO2N=IPh) could function as nitrene precursors is among the most important advances in the development of N-atom transfer technologies.6,7 Such oxidants, when combined with metal catalysts, will react with saturated and unsaturated hydrocarbons to give sulfonamide products. A singular example by Breslow and more extensive studies by Müller on the reactivity of ArSO2N=IPh established dirhodium tetracarboxylate complexes as highly effective catalysts for promoting selective C–H bond functionalization.7a,8
FIGURE 2.
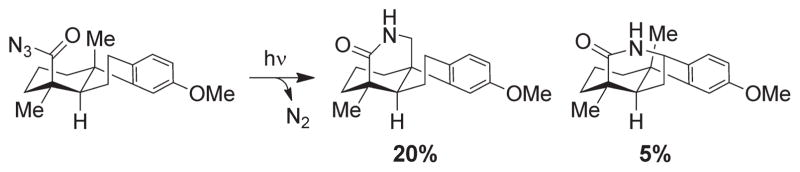
C–H amination through photolytic azide decomposition.
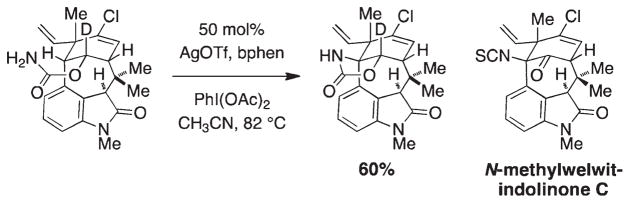
Both Che’s group and ours recognized the limitations of iminoiodinane preparation (i.e., scope, stability) and found that the isolation of these species could be superseded by simply mixing an appropriate amine derivative with PhI-(OAc)2.9–11 This advance has led to the development of a number of amination methods using nitrogen sources for which there is no known iodoimine equivalent. Amination technologies have also found a place in total synthesis, examples of which include the preparation of (−)-tetrodotoxin,12 manzacidins A and C,13 (−)-agelastatin A,14 and aminosugars, 15 among others. Garg and co-workers have recently featured an intramolecular C–H amination reaction to install a critical bridgehead aminomoiety in anelegant synthesis of (−)-N-methylwelwitindolinone C isothiocyanate (Figure 3).16 These works underscore the power of selective C–H oxidation reactions for streamlining total synthesis.
FIGURE 3.
Intramolecular C–H amination en route to (−)-N-methylwelwitindolinone C isothiocyanate.16
Initial Reaction Development
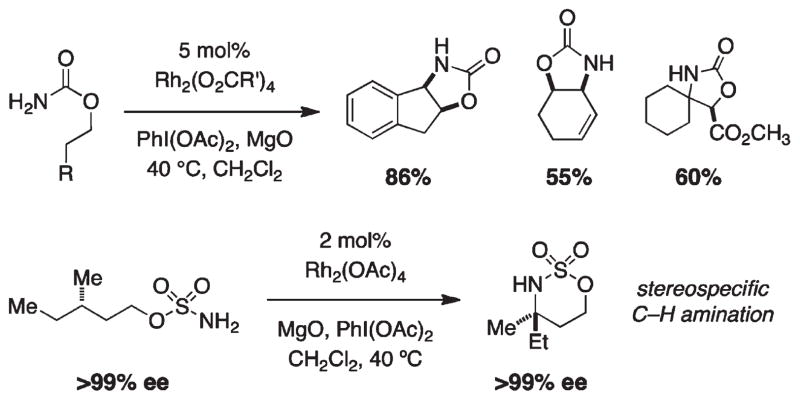
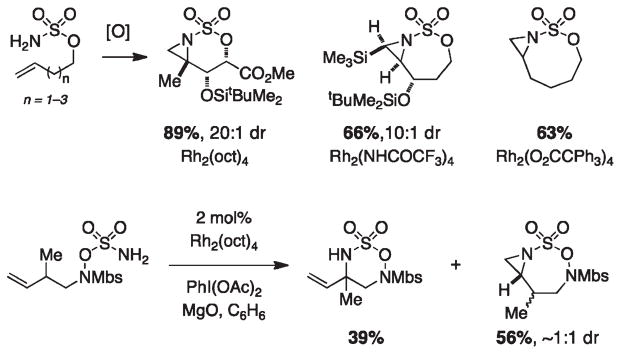
In our earliest efforts to develop a versatile rhodium-catalyzed C–H amination process, we identified both primary carbamate and sulfamate ester derivatives as viable substrates (Figure 4).2 The combination of either carbamate or sulfamate with PhI(OAc)2 and a commercial dirhodium catalyst, Rh2(OAc)4 or Rh2(oct)4 (typically 2–5 mol %), results in oxazolidin-2-one or [1,2,3]-oxathiazinane-2,2-dioxide formation, respectively. In certain instances, reaction performance is enhanced using the Ikegami/Hashimoto complex Rh2(O2CCPh3)4, which is easily prepared from Rh2(OAc)4.10,17 Moderate to high catalyst turnover numbers are achieved with the inclusion of MgO, which serves as a basic additive to scavenge the carboxylic acid byproduct generated from PhI-(OAc)2. Product yields are usually in excess of 70% for substrates possessing either a tertiary or benzylic C–H center. Reactions with chiral sulfamate esters often give high levels of diastereoinduction (≥10:1); substrate-based stereochemical control can be rationalized by invoking an energy-minimized chairlike transition structure in the bond-forming event.18,19 Levels of diastereocontrol in reactions with acyclic carbamate esters are considerably more modest.10,20 Heterocyclic products furnished through these oxidative cyclization processes function as useful surrogates for 1,2-amino alcohols and 1,3-difunctionalized amine derivatives.21
FIGURE 4.
Oxidative cyclization of carbamate and sulfamate esters.
Dirhodium-catalyzed amination can be performed on carbamate and sulfamate substrates possessing a number of common functional groups (alkynes, acetals, silyl ethers, esters, 2° and 3° amides). Alternative N-derivatives, including ureas, guanidines, sulfonamides, hydroxylamine sulfamates, sulfamides, and phosphoramidates, will also engage in intramolecular oxidation reactions (Figure 5).2,20,22,23 Among these nitrogen sources, only a small subset of sulfonyliminoiodinanes have been prepared and characterized; all other functional equivalents are generated in situ and are postulated as transient intermediates in the catalytic cycle.
FIGURE 5.
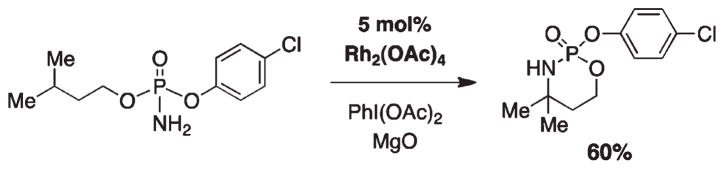
Oxazaphosphinane synthesis through rhodium-catalyzed amination.
Five- versus Six-Membered Ring Formation
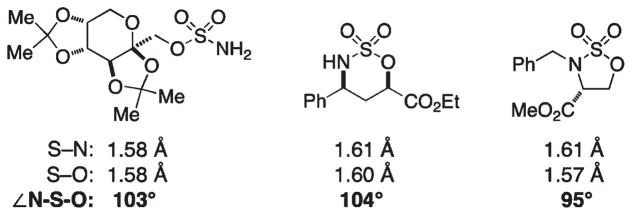
The geometrical parameters of the tethered nitrogen-bearing functional group appear to dictate the ring size of the corresponding heterocycle. With carbamates as starting materials, five-membered oxazolidinones form preferentially over the six-membered homologues. Reactions with sulfamates and phosphoramidates are predisposed toward the generation of six-membered ring oxathiazinanes and oxazaphosphinanes, respectively, and are complementary to carbamate oxidation in this regard. For sulfamate cyclization, the strong bias for oxathiazinane formation can be rationalized by comparing metrical parameters (in particular, the N–S–O bond angle) between the starting sulfamate and product heterocycle (Figure 6).20 This preference for oxathiazinane production can be overridden by designing substrates that preclude six-membered ring formation, in which case five- or seven-membered ring products are furnished. Che et al. have demonstrated that a Ru(II)-porphyrin catalyst will engage phenethyl alcohol-derived sulfamates to furnish five-membered heterocycles in modest yields (31–61%).24 In our hands, dirhodium tetracarboxylate catalysts have typically underperformed with these same substrates.
FIGURE 6.
Metrical parameters for alkoxysulfonamide derivatives.
Mechanistic Investigations
Intramolecular C–H insertion technologies have evolved in tandem with inquiries into operative reaction mechanisms. These studies have focused primarily on sulfamate ester oxidation, as this process does not require MgO to achieve reasonable catalyst turnover numbers.25 As such, homogeneous reaction mixtures can be analyzed by spectroscopic methods. Our collective studies provide insight into the interaction between sulfamate and hypervalent iodine oxidant, the influence of catalyst architecture on product selectivity, the transition structure at the C–N bond forming event, and catalyst decomposition pathways. Relevant mechanisms using other nitrogen sources (e.g., carbamates, ureas, sulfonamides, sulfamides) are drawn by analogy in the absence, to date, of any direct experimental data. Che, Phillips, and co-workers have disclosed a theoretical investigation of carbamate-based C–H amination, the findings from which accord with experimental mechanistic data obtained for sulfamate oxidation.26
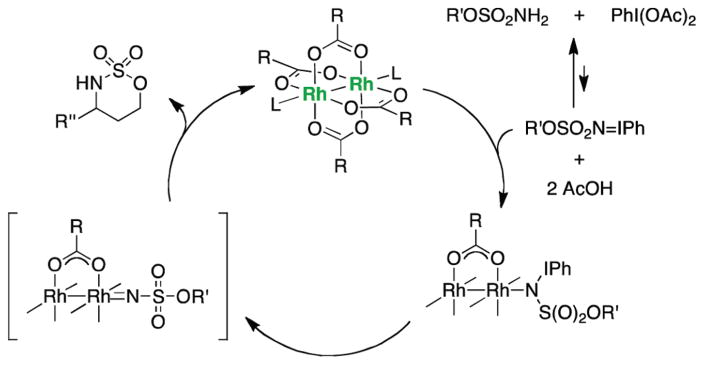
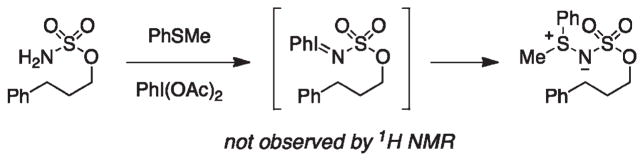
The amination reaction purportedly initiates with the formation of iminoiodinane ROSO2N=IPh (Figure 7).25 NMR studies in which sulfamate is combined with either PhI(OAc)2 or PhI(O2CtBu)2 give no evidence for the generation of this intermediate, except when Cl3CCH2OSO2NH2 is used as the nitrogen source. Compelling indirect evidence for the formation of ROSO2N=IPh follows from reactions with a PhSMe additive, which is oxidized by the transient iminoiodinane to give the corresponding sulfilimine (Figure 8). In the C–H amination pathway, coordination of the iminoiodinane to the dirhodium catalyst facilitates the extrusion of PhI and generates the active nitrenoid.20,25,27 C–H bond oxidation proceeds through a direct insertion event to afford the heterocyclic product and to regenerate the catalyst. Kinetic data from NMR experiments show no variance in product formation rates within the first 100 s of oxidant addition across 0.5–5.0 mol % catalyst loadings, implying that the initial reaction rate is limited by the production of ROSO2N=IPh. Our efforts to identify the rate-determining step in the proposed reaction pathway have not reached a definitive conclusion. Given the rapid rate of product formation, low-temperature kinetic data is needed to obtain a higher resolution understanding of the earliest mechanistic time points. Initial rate investigations with sulfonylated N-hydroxycarbamate derivatives, which do not require added oxidant, could provide greater insight into the nitrenoid-forming step in this process.27b
FIGURE 7.
Proposed mechanism for dirhodium-catalyzed C–H amination.
FIGURE 8.
Sulfilimine formation supports intermediacy of iminoiodinane oxidant.
A substantial body of mechanistic data on the rhodium-catalyzed amination process shapes our understanding of the product-determining C–H insertion event. For studies with Rh2(OAc)4, an intramolecular cyclopropane clock having an estimated fragmentation rate constant of 1011 s−1 fails to provide any indication of ring-opened products.25 Evidence for a concerted asynchronous C–H oxidation transition state is supported by a measured kinetic isotope value of 2.6 and a Hammett ρ value of −0.55.25,28 Additionally, stereochemical retention in the amination of optically active tertiary C–H bonds has been noted for both carbamate and sulfamate esters.10,13 While these collective findings appear consistent with a singlet-type nitrene C–H insertion, recent studies on the intermolecular C–H amination reaction suggest that a stepwise C–H abstraction/radical rebound pathway may be operative.29
For some time, we have endeavored to understand the fate of the dirhodium catalyst in reactions that underperform. Kinetic experiments show a clear difference in rate data following the first 100 s of the reaction, evidencing a change in catalyst order. These observations indicate a possible change in reaction mechanism and/or in situ modification of catalyst structure. We have found that simple dirhodium tetracarboxylate complexes (i.e., Rh2(OAc)4, Rh2-(O2CtBu)4) undergo facile ligand exchange reactions: within 60 s of diacetoxyiodobenzene addition, the isotopically labeled complex Rh2(O2 13CMe)4 liberates Me13CO2H. Coordination of PhI(OAc)2 to one of the two axial sites on the dirhodium catalyst is believed to initiate this ligand exchange process. Although ligand metathesis between PhI-(OAc)2 and Rh2(O2 13CMe)4 should be degenerate, we hypothesize that the exchange process renders the dirhodium(II) centers susceptible to oxidative degradation.
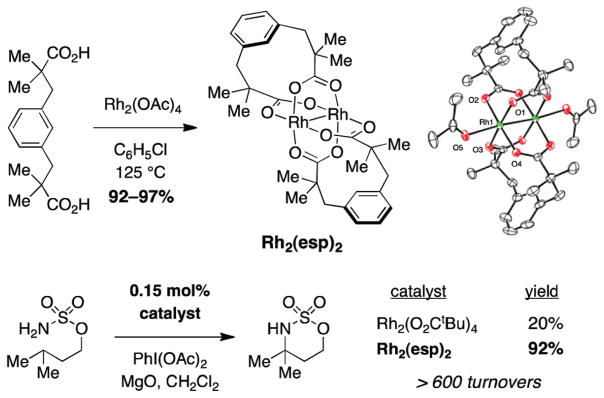
The evident lability of Rh2(OAc)4 and related tetracarboxylate complexes under the reaction conditions has encouraged the design of alternative, chelating dicarboxylate-based architectures.30 The complex Rh2(esp)2, born from these studies and inspired by work of Taber and Davies, has demonstrated exemplary performance in a wide variety of amination reactions (Figure 9).31 Critical design elements include both a strapped four-point binding motif and gem-dimethylated α-carbon centers, which confer a more conformationally rigid catalyst structure. In the presence of PhI(OAc)2, no ligand exchange takes place with Rh2(esp)2 over a period of hours to days.32 This catalyst offers unmatched performance relative to the more common tetradic carboxylate systems. In select examples, intramolecular sulfamate ester insertion reactions have been effected in high yield with catalyst loadings as low as 0.15 mol % (Figure 9).30
FIGURE 9.
Efficient preparation and high performance of Rh2(esp)2.
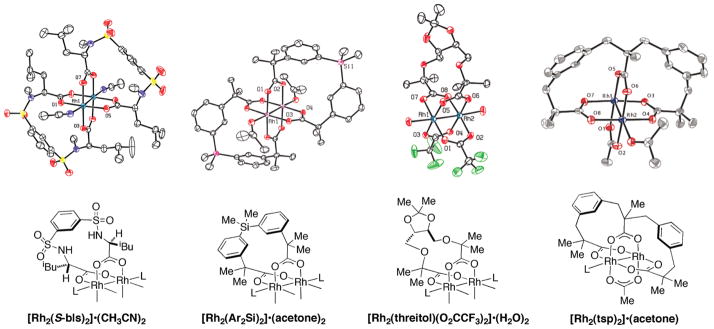
Rh2(esp)2 is only one of a number of strapped dicarboxylate catalyst designs that we have explored, several of which are pictured in Figure 10. While preparation of these complexes is straightforward, no other chelated dicarboxylate system has surpassed the performance and scope of Rh2(esp)2 for mediating C–H amination reactions.33 Crystallographic analysis of these structures gives insight into the underlying reasons for their modest performance and poor stability under the reaction conditions. In the preferred arrangement, four bridging carboxylate groups coordinate the dirhodium core at right angle connections.34 For most strapped complexes, the ligand design does not precisely conform to this geometry, forcing a degree of twisting and torsional strain that presumably results in greater lability toward exchange. The esp ligand appears to have an optimal geometry and chelate size for spanning the two rhodium centers. To this point, it is interesting to note that one of the most effective strapped dicarboxylate catalysts for Rh-carbene reactions, Rh2(bi-TBSP)2, is particularly poor at promoting amination reactions.31b
FIGURE 10.
Novel dirhodium strapped carboxylate catalysts.
In addition to demonstrating improved stability and catalyst turnover numbers, Rh2(esp)2 has proven effective in facilitating nitrogen-transfer reactions from a broad range of nitrogen sources, including ureas, guanidines, sulfamides, and hydroxylamine-derived sulfamates.19,22,23 For many of these substrate types, other dirhodium complexes either fail to catalyze the reaction or severely underperform. To our knowledge, oxidative cyclization reactions with urea and guanidine substrates have not been demonstrated with other, non-dirhodium metal catalysts.
Despite the evident advantages afforded by Rh2(esp)2, several of these oxidation methods require additional development. With a few notable exceptions, the formation of five-membered ring products using carbamate, urea, and guanidine substrates is generally limited to insertion at tertiary and benzylic C–H centers.35 In addition, secondary alcohol-derived carbamates typically afford ketone products when subjected to the amination conditions.16,20 Some improvement in the overall performance of the carbamate oxidation, however, has been witnessed with the use of a silver(I) catalyst, findings that should encourage future efforts in reaction development.16,36
Chemoselectivity in Intramolecular Reactions
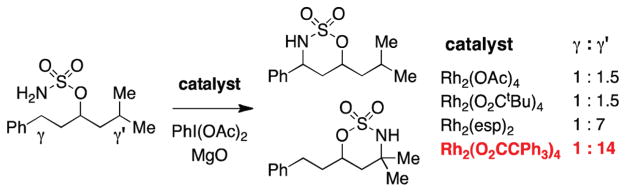
Our desire to perform oxidation reactions on substrates bearing multiple C–H bonds has motivated studies to assess the influence of substrate and catalyst structure on chemoselectivity. In intramolecular reactions of sulfamate substrates with Rh2(OAc)4, relative rate data for C–H insertion follow the trend: 3° > α-ethereal, α-aminal, benzylic > 2° ≫ 1° (Figure 11).25 The choice of catalyst structure and the nature of the protecting groups, however, can significantly influence positional selectivity. Looking forward, a grand challenge for C–H amination reaction development is to better appreciate the nuanced details of the noncovalent interactions between substrate and catalyst that influence product ratios, information that should lead to greater predictive power with respect to both substrate design and catalyst selection.
FIGURE 11.
Catalyst choice influences positional selectivity.
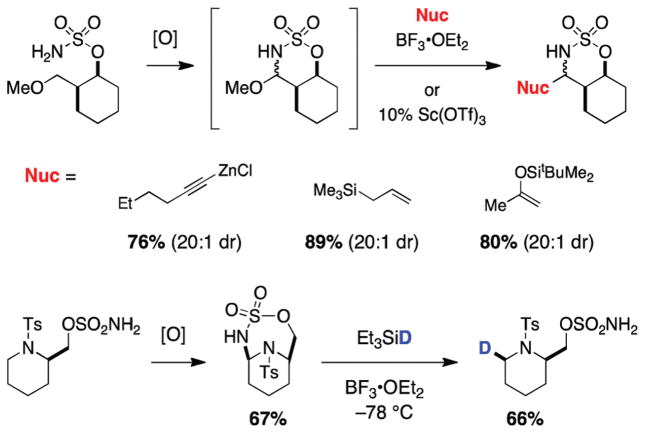
The formal products of methylene or methyl oxidation may be accessed in two steps by taking advantage of the facility of α-ethereal (or α-aminal) C–H bond oxidation using sulfamate esters (Figure 12). The resultant N,O- and N,N-acetals can be directly converted (generally without intermediate purification) to cyclic and acyclic products through Lewis acid-mediated addition reactions. A wide range of common nucleophiles (allylsilanes, alkynylzinc reagents, silyl enol ethers, silylhydrides, tethered phenols) will engage in this process.37,38 Product diastereoselectivity in many instances is exceptional (>20:1), and has been rationalized through a combined Stevens/Felkin-Ahn model.
FIGURE 12.
Heterocyclic acetals available through α-ethereal and α-aminal C–H insertion.
One of the principle challenges in amination methods development is achieving chemoselectivity that favors C–H insertion products in reactions of unsaturated compounds.39 Substituted alkenes are particularly adept substrates for reactions with electrophilic nitrenoids, and the prevailing tendency of unsaturated sulfamates and carbamates is to generate aziridine product(s).14,23,25,28 Examples include reactions of bis- and tris-homoallylic sulfamate esters, which preferentially furnish the 7- and 8-membered fused bicyclic structures, respectively (Figure 13). For intermolecular amination reactions, alkene aziridination dominates. Motivated by this problem and given the utility of allylic amines in synthesis, we have sought catalyst systems that can override the inherent bias of the nitrenoid toward olefin oxidation (vide infra).
FIGURE 13.
Competitive aziridination and C–H insertion reactions.
Diastereoselectivity in Intramolecular Reactions
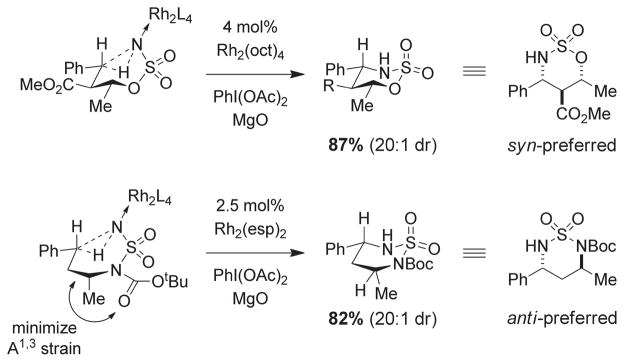
A predictive model has been put forth to rationalize diastereochemical selectivity in C–H oxidation reactions with chiral sulfamate and sulfamide derivatives (Figure 14). α-Substitution at the sulfamate results in preferential formation of the corresponding 1,3-syn oxathiazinane, whereas substitution at the β-carbon favors the 1,2-anti isomer.18 The sense of induction may be rationalized by invoking a putative chairlike transition structure in which substituent groups are disposed in pseudoequatorial arrangements in response to A-strain. Few sulfamate reactions have been identified that do not accord with this stereochemical model. Notably, cyclization reactions of α-branched, N-Boc sulfamide derivatives favor formation of the anti-isomer with moderate levels of stereocontrol.19 This preference can be rationalized using the same cyclic transition state model in which A1,3-strain between the N-Boc group and the α-substituent forces the latter into a pseudoaxial alignment, thereby favoring the anti-diastereomer.
FIGURE 14.
Divergent stereochemical outcomes in sulfamate and sulfamide C–H insertion reactions.
The interaction of optically active amination substrates with enantiomeric dirhodium catalysts has not been extensively explored. The design and examination of new catalyst systems that could override substrate-biased stereocontrol could aid efforts to better understand catalyst–substrate interactions and would have considerable utilitarian value in synthesis.
Asymmetric C–H Amination
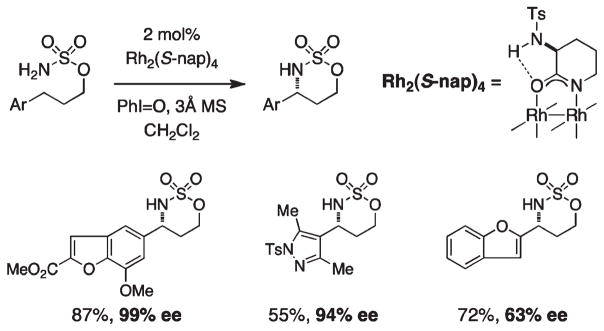
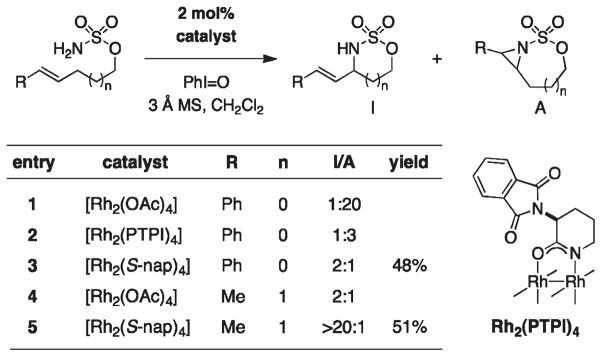
Enantioselective C–H amination has been accomplished using the dirhodium carboxamidate complex, Rh2(S-nap)4 (Figure 15).40 This particular catalyst is unique in its effectiveness at promoting amination, as most other amide-based rhodium dimers (an exception being the electron-withdrawn tetrakis-trifluoroacetamide catalyst) are susceptible to rapid oxidative degradation under the reaction conditions. A ligand-based hydrogen bond interaction in Rh2(S-nap)4 appears to be an important design feature; N-methylation of the α-amino moiety significantly reduces the performance of this catalyst system.
FIGURE 15.
Asymmetric intramolecular C–H amination of aryl-substituted sulfamate esters.
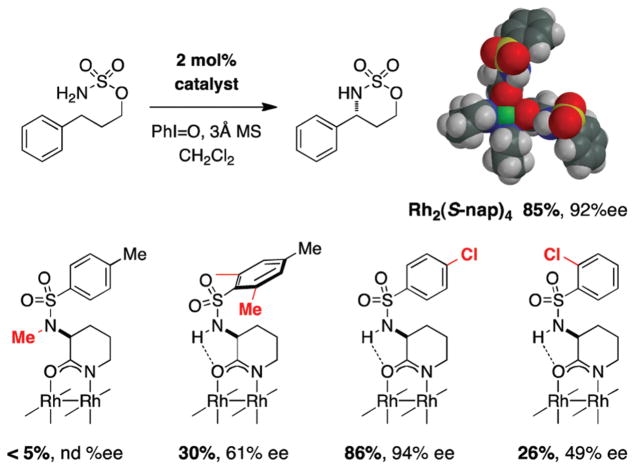
Despite progress in asymmetric C–H amination with the advent of Rh2(S-nap)4, the substrate scope of these reactions remains limited. To date, only benzylic and allylic substrates afford products in acceptable yields (40–90%) and only the former class of substrates can be oxidized with reasonable asymmetric control (er ~ 10:1). An especially curious feature of the S-nap ligand system is that changes to the sulfonamide moiety, ostensibly remote to the metal binding site, affect both catalyst performance and product enantiomeric ratios (Figure 16). Findings such as these give a sense as to why the development of new catalysts for enantioselective C–H amination remains an outstanding challenge in this field.
FIGURE 16.
Catalyst SAR data highlights the influence of remote steric groups on reaction performance.
A rather surprising feature of Rh2(S-nap)4 is its predilection to favor formation of C–H insertion products over the isomeric bicyclic aziridines in oxidation reactions of bis-homoallylic sulfamates (Figure 17). We are unaware of any other dirhodium system that exhibits such a preference in intramolecular reactions. Other metal complexes, including Mn- and Ru-porphyrin catalysts and Ru(pybox) have been shown to favor allylic insertion pathways.7b,9,41,42 With Rh2(S-nap)4, the substrate scope is narrowly defined and product yields are moderate; the bias this dirhodium catalyst has for allylic C–H insertion, however, is rather remarkable such that even 5-membered ring formation is favored with homoallylsulfamate. The unique capabilities of Rh2(S-nap)4 should inspire continued efforts to investigate the mechanism for substrate oxidation by this catalyst system and the influence of the ligand architecture on chemoselectivity.
FIGURE 17.
Competitive aziridination versus allylic C–H amination. Product ratios were estimated by 1H NMR integration.
Ruthenium Catalysts for Intramolecular Allylic C–H Insertion
Rh2(tfacam)4 and Rh2(S-nap)4 complexes notwithstanding, effective dirhodium systems for C–H amination are largely restricted to a subset of tetracarboxylate designs.1a,2,4 Dirhodium complexes supported by strongly donating amidate ligands appear to be quickly oxidized under the reaction conditions, while electron-withdrawn carboxylate frameworks (e.g., α-amino acid derivatives, trifluoroacetate) are labile to ligand exchange. We have for some time sought non-rhodium metal complexes that promote competent N-atom transfer. While much of this work has met with limited success, the efforts of Che and Blakey have encouraged further analysis of Ru-based catalyst systems.41,43
Initial reports on the synthesis of mixed valent diruthenium(II,III) paddlewheel complexes can be found as early as 1966.44 Despite intensive studies on the coordination chemistry and spectroscopy of these complexes and their obvious parallels to dirhodium structures, these systems have found almost no utility as catalysts for organic transformations. 45,46 We have investigated the viability of mixed-valent diruthenium(II,III) complexes for catalyzing intra- and intermolecular C–H amination. The higher oxidation potential of the diruthenium core vis-à-vis analogous dirhodium complexes was viewed as a potential advantage that would allow greater flexibility in ligand design by permitting the use of amidate and other strongly donating ligand sets.28
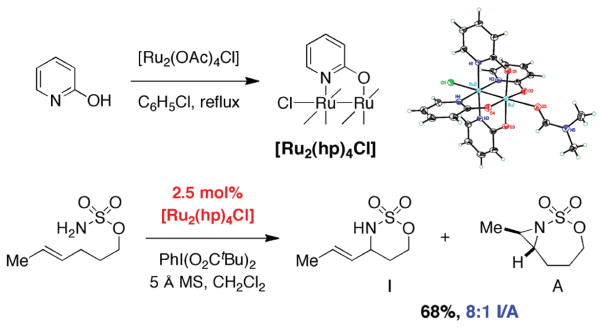
We have disclosed the first examples of diruthenium tetracarboxylate- and tetra-2-oxypyridinate-mediated intramolecular sulfamate C–H amination reactions (Figure 18).28 The strapped dicarboxylate complex, [Ru2(esp)2]SbF6, boasts outstanding catalytic efficiency, and oxidizes isoamyl sulfamate to the corresponding oxathiazinane in excess of 600 turnovers. Empirical examination of a variety of ligand architectures, and their efficacy in promoting useful levels of conversion on a range of functionalized substrates, highlights one particular complex, [Ru2(hp)4Cl]. This tetrakis(2-oxypyridinato) adduct readily promotes sulfamate cyclization, while the dirhodium analogue Rh2(hp)4 expectedly fails, presumably due to its low solubility and rapid oxidative degradation under the reaction conditions. For most substrates, low catalyst loadings of [Ru2(hp)4Cl] may be utilized (2.5 mol %); substrates possessing an assortment of different functional groups will engage, and 6-membered ring oxathiazinanes are generally furnished in 60–75% yield. The uniqueness of [Ru2(hp)4Cl] is displayed in reactions of bis-homoallylic sulfamates, which strongly favor products of allylic C–H insertion over the corresponding aziridines. This catalyst complex is superior to Rh2(S-nap)4 for chemoselective allylic amination.
FIGURE 18.
[Ru2(hp)4Cl] for selective, intramolecular allylic C–H amination.
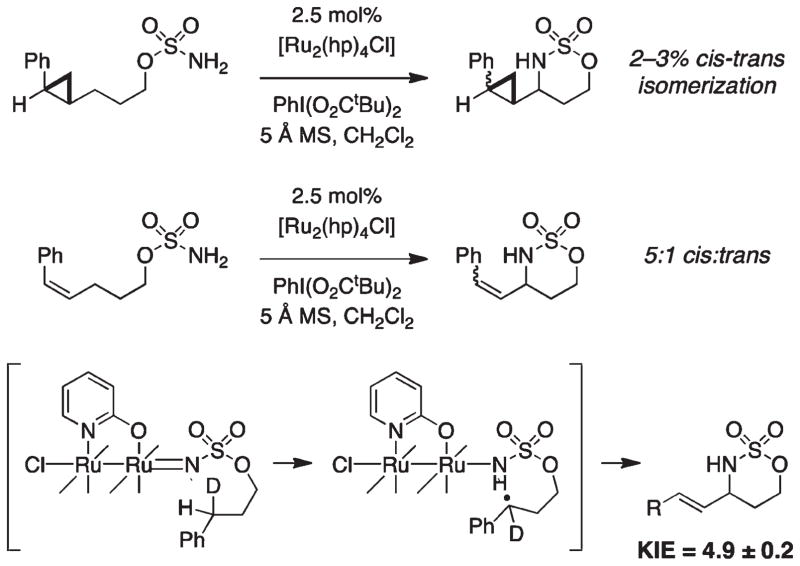
The differential performance of [Ru2(hp)4Cl] for allylic C–H bond oxidation has compelled mechanistic inquiry.28 A large body of physical organic data on the intramolecular rhodium-catalyzed amination reaction intimates a concerted insertion process through an asynchronous transition state. Stereochemical retention in amination reactions of optically active tertiary C–H centers, cyclopropyl clock studies, computational studies, Hammett data, and KIE values collectively support this conclusion. Experiments with [Ru2(hp)4Cl], which include partial isomerization of a cis-cyclopropane radical clock and cis–trans alkene isomerization, as well as density functional theory (DFT) calculations, however, reveal an alternative oxidation pathway (Figure 19). This catalyst appears to operate through a discrete two-step mechanism involving homolytic C–H bond scission followed by fast radical recombination. Hammett and kinetic isotope data show significant disparities between dirhodium- and diruthenium-mediated amination, the latter displaying a larger Hammett ρ value (−0.55 vs −0.90, respectively) and KIE (2.6 vs 4.9, respectively). The stepwise nature of the [Ru2(hp)4Cl]-catalyzed reaction accounts for preferential allylic C–H bond functionalization over alkene aziridination. Ongoing studies should continue to reveal differences between dirhodium and diruthenium systems as well as the mechanism(s) for [Ru2(hp)4Cl] arrest and/or decomposition. Such information will enable next-generation catalyst designs that function with other substrates types (e.g., carbamates) and that mediate intermolecular allylic insertion reactions.
FIGURE 19.
Results support a stepwise C–H abstraction/radical rebound mechanism for [Ru2(hp)4Cl]-catalyzed amination.
Intermolecular Benzylic C–H Insertion
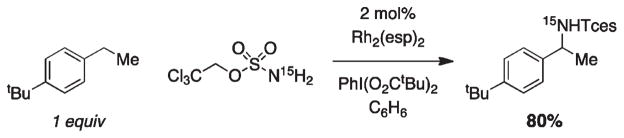
Chemoselective intermolecular C–H functionalization is an exceptional challenge for oxidative insertion technology. The effectiveness of Rh2(esp)2 as an amination catalyst has made possible an earnest exploration of such a method.47 With a limiting amount of starting alkane and a simple sulfamate nitrogen source, 2,2,2-trichloroethoxysulfonamide (TcesNH2), moderate to high isolated yields (50–80%) of benzylic amine products are attainable. In combination with TcesNH2, these reactions employ 2 mol % Rh2-(esp)2 and an organic-soluble oxidant, PhI(O2CtBu)2. A salient feature of this method is that 15N-labeled TcesNH2 can be prepared and used to generate isotopically enriched amine derivatives (Figure 20).
FIGURE 20.
Generation of 15N-isotopomers through intermolecular C–H amination.
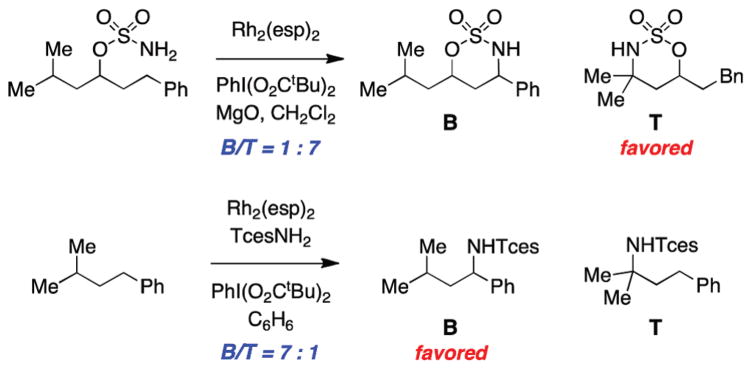
Conditions for intermolecular amination that employ TcesNH2 favor oxidation of benzylic C–H bonds over tertiary sites (Figure 21). These results contrast intramolecular insertion reactions, which show a clear preference for tertiary C–H functionalization.25 The mechanism of dirhodium-catalyzed intermolecular C–H amination is under active investigation in an effort to illuminate the underlying reason(s) for the change in chemoselectivity between intra- and intermolecular regimes. The evident disparity between these processes is, admittedly, one of the more perplexing observations that we have been trying to decode.
FIGURE 21.
Intermolecular oxidation with TcesNH2 favors benzylic C–H centers.
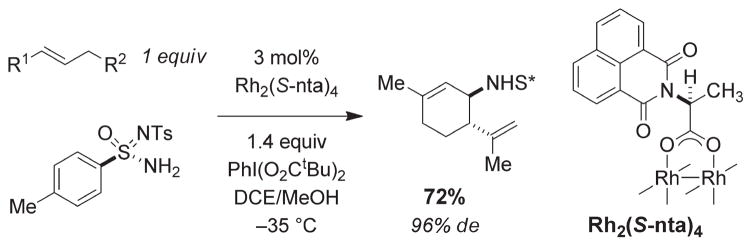
A small number of reports have outlined protocols for asymmetric intermolecular C–H amination using simple hydrocarbons such as indane and tetralin.48 Dauban et al. have offered an impressive method for diastereoselective C–H amination reactions of benzylic and allylic substrates that relies on a combination of an optically active dirhodium tetracarboxylate catalyst and a chiral sulfonimidamide nitrogen source (Figure 22).49 Remarkably, the reaction occurs at −35 ° C and provides high levels of chemo- and diastereoselectivity for intermolecular allylic insertion. This process is currently unrivaled for both efficiency and stereocontrol.
FIGURE 22.
Chemo- and diastereoselective intermolecular amination of allylic C–H bonds.
With the exception of the Dauban and Dodd disclosure, intermolecular amination reactions on alkene-bearing substrates are often complicated by product mixtures arising from indiscriminant π-bond and allylic C–H oxidation. In some cases, it is possible to bias selectivity in favor of aziridine formation; for this purpose, the trifluoroacetamide catalyst Rh2(tfacam)4 and the analogous rhodium perfluorobutyramide have proven indispensible, furnishing azaheterocycles stereospecifically in 65–95%yield (Figure 23).50 A number of non-dirhodium catalysts have been employed for intermolecular aziridination reactions, but few match the catalytic performance of Rh2(tfacam)4.
FIGURE 23.
Intermolecular olefin aziridination.
Mechanistic Investigations of the Intermolecular Process
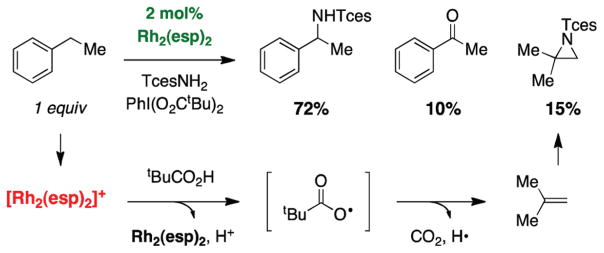
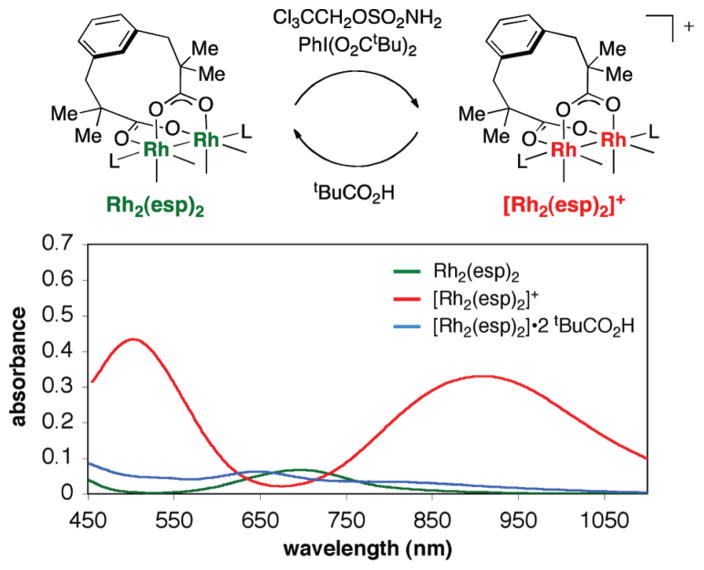
Product analysis of intermolecular C–H amination reactions has given way to a number of mechanistic discoveries. Examination of reaction mixtures consisting of ethylbenzene, TcesNH2, PhI(O2CtBu)2, and catalytic Rh2(esp)2 reveal acetophenone and the aziridine of isobutylene as isolable side products (Figure 24).33,47 These early observations led to speculation that a one-electron oxidation pathway operates either in parallel with or in lieu of the concerted two-electron amination mechanism. The evident change in solution color from bright green to red suggested a change in the redox state of the dirhodium catalyst. Subsequent spectroscopic and spectrometric experiments have identified this red species as a one-electron oxidized complex, [Rh2(esp)2]+ (Figure 25).32,51
FIGURE 24.
Ketone and aziridine byproducts identified in intermolecular C–H oxidation.
FIGURE 25.
Spectroscopic characterization of a mixed-valent Rh2(II,III) dimer.
We have proposed that [Rh2(esp)2]+ forms through a secondary process during nitrenoid decomposition. Empirically, the onset of the red color appears to correlate with the difficulty associated with oxidizing a particular hydrocarbon substrate. The mixed-valent dimer, [Rh2(esp)2]+, has a prolonged lifetime under the reaction conditions as compared to other, simple dirhodium tetracarboxylates such as [Rh2-(O2CtBu)4]+.32 A transient red solution of [Rh2(O2CtBu)4]+ fades rapidly (<60 s) to a pale yellow color, presumably consisting of Rh3+ monomeric and/or multimeric species. By contrast, [Rh2(esp)2]+ persists for a much longer duration owing to the chelating nature of the ligand set; this complex can be reduced under the reaction conditions to the corresponding dirhodium(II,II) adduct by the carboxylic acid byproduct, tBuCO2H. These findings have led us to propose that the differential advantage of the esp ligand is its ability to stabilize the mixed-valent dirhodium species, thereby allowing one-electron reduction to take place to return the active (II,II) dimer. Our results also explain the improved performance of PhI(O2CtBu)2 as a terminal oxidant over PhI(OAc)2. We have found that the use of other carboxylic acids (e.g., dimethylphenylacetic acid), known to be more effective one-electron reducing agents, can improve catalytic turnover numbers in intermolecular amination reactions.29,33b
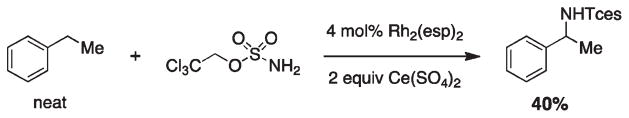
Berry and Kornecki have identified and characterized a red mixed-valent complex as [Rh2(esp)2(HNTces)] and have proposed an alternative amination pathway that operates through proton-coupled one-electron oxidation of the catalyst to give the reactive dirhodium(II,II)-nitrene.51 Formation of [Rh2(esp)2(HNTces)] is suggested to arise from H-atom loss by the dirhodium TcesNH2 adduct, [Rh2(esp)2] · (H2NTces). This provocative proposal implies a pathway for C–H amination that may be mediated by single-electron oxidants such as Ce4+ salts, which Berry and Kornecki have in fact used to effect catalytic turnover (Figure 26). Whether the rate of a sequential proton-coupled electron transfer or H-atom abstraction mechanism is competitive with carboxylic acid-induced [Rh2(esp)2]+ reduction under the normal operating reaction conditions is a question that remains to be answered. The fate of this intermediate mixed-valent dirhodium complex is the subject of active investigations in our laboratory.
FIGURE 26.
Intermolecular C–H amination mediated by Ce4+ oxidant.
Conclusion
This Account outlines our recent contributions to the advancement of intra- and intermolecular rhodium- and ruthenium-catalyzed amination reactions, specifically highlighting the many unique challenges posed by problems of selectivity (both chemo and stereo) and the complexities of the underlying catalytic reaction mechanism. Despite the progress of other laboratories and ours, absolute reagent control over chemo- and stereoselective C–H bond oxidation remains beyond reach. A deeper understanding of the pathway(s) for catalyst arrest is needed to inform the rational design of next-generation systems that extend the capabilities of current amination technologies. The subtle interplay between catalyst structure and substrate can dramatically influence reaction performance and product distributions, but the molecular details of such interactions remain opaque at this time. Undoubtedly, there is much to learn as we attempt to unravel these mysteries.
Acknowledgments
We are grateful to the many co-workers whose works are described in this report and to our collaborator, Dr. Jamal Musaev (Emory University), for many stimulating conversations. Funding has been provided by the National Science Foundation Center for Stereoselective C–H Functionalization (CSCHF) and the National Institutes of Health (NIH). J.L.R. is supported as a NIH Fellow (5F32GM089033-02).
Biographies
Jennifer L. Roizen received a B.A. from Williams College in 2003 and earned her Ph.D. from Caltech in 2010 with Brian M. Stoltz. She is currently a NIH Postdoctoral Fellow at Stanford.
Mark Edwin Harvey received a B.A. in chemistry from Boston University in 2007 and is currently working toward his Ph.D. at Stanford.
Justin Du Bois received a B.S. degree from UC Berkeley in 1992 and earned his Ph.D. from Caltech in 1997 with Erick Carreira. Following a NIH postdoctoral appointment at MIT with Stephen Lippard, he began his independent career at Stanford University.
Footnotes
The authors declare no competing financial interest.
References
- 1.(a) Yu J-Q, Shi Z, editors. Topics in Current Chemistry. Vol. 292. Springer-Verlag; Berlin: 2010. C–H Activation; pp. 1–384. [PubMed] [Google Scholar]; (b) Dyker G, editor. Handbook of C–H Transformations: Applications in Organic Synthesis. Wiley-VCH; Weinheim: 2005. pp. 1–688. [Google Scholar]
- 2.Du Bois J. Rhodium-Catalyzed C–H Amination. An Enabling Method for Chemical Synthesis. Org Process Res Dev. 2011;15:758–762. doi: 10.1021/op200046v. and references therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) McNeill E, Du Bois J. Ruthenium-Catalyzed Hydroxylation of Unactivated 3° C–H Bonds. J Am Chem Soc. 2010;132:10202–10204. doi: 10.1021/ja1046999. [DOI] [PubMed] [Google Scholar]; (b) Litvinas ND, Brodsky BH, Du Bois J. C–H Hydroxylation Using a Heterocyclic Catalyst and Aqueous H2O2. Angew Chem, Int Ed. 2009;48:4513–4516. doi: 10.1002/anie.200901353. [DOI] [PubMed] [Google Scholar]
- 4.For recent reviews, see: Lebel H. In: Catalyzed Carbon-Heteroatom Bond Formation. Yudin A, editor. Wiley-VCH; Weinheim: 2011. pp. 137–156.Collet F, Dodd RH, Dauban P. Catalytic C–H amination: recent progress and future directions. Chem Commun. 2009:5061–5074. doi: 10.1039/b905820f.
- 5.(a) Masamune S. Total Syntheses of Diterpenes and Diterpene Alkaloids. IV. Garryine. J Am Chem Soc. 1964;86:290–291. [Google Scholar]; (b) Meyer WL, Levinson AS. Photolysis of 1,1-Dimethyl-trans-decalin-10-carbonyl Azide. Proc Chem Soc. 1963;1:15. [Google Scholar]; (c) ApSimon JW, Edwards OE. A New Photochemical Reaction: The Structure and Absolute Stereochemistry of Atisine. Can J Chem. 1962;40:896–902. [Google Scholar]
- 6.(a) Abramovitch RA, Bailey TD, Takaya T, Uma V. The Reaction of Methanesulfonyl Nitrene with Benzene. Attempts to Generate Sulfonyl Nitrenes from Sources Other than the Azides. J Org Chem. 1974;39:340–345. [Google Scholar]; (b) Yamada Y, Yamamoto T, Okawara M. Synthesis and Reaction of New Type I–N Ylide, N-Tosyliminoiodinane. Chem Lett. 1975:361–362. [Google Scholar]
- 7.(a) Breslow R, Gellman SH. Intramolecular Nitrene C–H Insertions Mediated by Transition-Metal Complexes as Nitrogen Analogues of Cytochrome P-450 Reactions. J Am Chem Soc. 1983;105:6728–6729. [Google Scholar]; (b) Mansuy D, Mahy JP, Dureault A, Bedi G, Battioni P. Iron- and Manganese-porphyrin Catalysed Aziridination of Alkenes by Tosyl- and Acyl-iminoiodobenzene. J Chem Soc, Chem Commun. 1984:1161–1163. [Google Scholar]
- 8.Müller P. Transition metal-catalyzed nitrene transfer: aziridination and insertion. Adv Catal Processes. 1997;2:113–151. and references therein. [Google Scholar]
- 9.Yu XQ, Huang YS, Zhou XG, Che CM. Amidation of Saturated C–H Bonds Catalyzed by Electron-Deficient Ruthenium and Manganese Porphyrins. Org Lett. 2000:2233–2236. doi: 10.1021/ol000107r. [DOI] [PubMed] [Google Scholar]
- 10.Espino CG, Du Bois J. A Rh-Catalyzed C–H Insertion Reaction for the Oxidative Conversion of Carbamates to Oxazolidinones. Angew Chem, Int Ed. 2001;40:598–600. doi: 10.1002/1521-3773(20010202)40:3<598::AID-ANIE598>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 11.As originally noted by Dauban et al., PhI=O functions in combination with sulfonamides for in situ aziridination, see: Dauban P, Sanièere L, Tarrade A, Dodd RH. Copper-Catalyzed Nitrogen Transfer Mediated by Iodosylbenzene PhI=O. J Am Chem Soc. 2001;123:7707–7708. doi: 10.1021/ja010968a.
- 12.Hinman A, Du Bois J. A Stereoselective Synthesis of (−)-Tetrodotoxin. J Am Chem Soc. 2003;125:11510–11511. doi: 10.1021/ja0368305. [DOI] [PubMed] [Google Scholar]
- 13.Wehn PM, Du Bois J. Enantioselective Synthesis of the Bromopyrrole Alkaloids Manzacidin A and C by Stereospecific C–H Bond Oxidation. J Am Chem Soc. 2002;124:12950–12951. doi: 10.1021/ja028139s. [DOI] [PubMed] [Google Scholar]
- 14.Wehn PM, Du Bois J. A Stereoselective Synthesis of the Bromopyrrole Natural Product (−)-Agelastatin A. Angew Chem, Int Ed. 2009;48:3802–3805. doi: 10.1002/anie.200806292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.For a leading example, see: Parker KA, Chang W. A Synthesis of L-Vancosamine Derivatives from Non-Carbohydrate Precursors by a Short Sequence Based on the Marshall, McDonald, and Du Bois Reactions. Org Lett. 2003;5:3891–3893. doi: 10.1021/ol035479p.
- 16.Quasdorf KW, Huters AD, Lodewyk MW, Tantillo DJ, Garg NK. Total Synthesis of Oxidized Welwitindolinones and (−)-N-Methylwelwitindolinone C Isonitrile. J Am Chem Soc. 2012;134:1396–1399. doi: 10.1021/ja210837b. and references therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hashimoto S-i, Watanabe N, Ikegami S. Dirhodium(II) Tetra(triphenylacetate): A Highly Efficient Catalyst for the Site-selective Intramolecular C–H Insertion Reactions. Tetrahedron Lett. 1992;33:2709–2712. [Google Scholar]
- 18.Wehn PM, Lee J, Du Bois J. Stereochemical Models for Rh-Catalyzed Amination Reactions of Chiral Sulfamates. Org Lett. 2003;5:4823–4826. doi: 10.1021/ol035776u. [DOI] [PubMed] [Google Scholar]
- 19.Kurokawa T, Kim M, Du Bois J. Synthesis of 1,3-Diamines Through Rhodium-Catalyzed C–H Insertion. Angew Chem, Int Ed. 2009;48:2777–2779. doi: 10.1002/anie.200806192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Espino CG. PhD Thesis. Stanford University; Stanford, CA: 2004. C–H Amination Reactions for Organic Synthesis: Discovery, Scope, and Mechanism. [Google Scholar]
- 21.For a leading reference on cyclic sulfamates, see: Bower JF, Rujirawanich J, Gallagher T. N-Heterocycle construction via cyclic sulfamidates. Org Biomol Chem. 2010;8:1505–1519. doi: 10.1039/b921842d.
- 22.Kim M, Mulcahy JV, Espino CG, Du Bois J. Expanding the Substrate Scope for C–H Amination Reactions: Oxidative Cyclization of Urea and Guanidine Derivatives. Org Lett. 2006;8:1073–1076. doi: 10.1021/ol052920y. [DOI] [PubMed] [Google Scholar]
- 23.Olson DE, Maruniak A, Malhotra S, Trost BM, Du Bois J. Synthesis and Reactivity of Unique Heterocyclic Structures en Route to Substituted Diamines. Org Lett. 2011;13:3336–3339. doi: 10.1021/ol2010769. and references therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liang JL, Yuan SX, Huang JS, Yu WY, Che CM. Highly Diastereo- and Enantioselective Intramolecular Amidation of Saturated C–H Bonds Catalyzed by Ruthenium Porphyrins. Angew Chem, Int Ed. 2002;41:3465–3468. doi: 10.1002/1521-3773(20020916)41:18<3465::AID-ANIE3465>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 25.Williams Fiori K, Espino CG, Brodsky BH, Du Bois J. A mechanistic analysis of the Rh-catalyzed intramolecular C–H amination reaction. Tetrahedron. 2009;65:3042–3051. [Google Scholar]
- 26.Lin X, Zhao C, Che CM, Ke Z, Phillips DL. A DFT Study on the Mechanism of Rh2II/II-Catalyzed Intramolecular Amidation of Carbamates. Chem–Asian J. 2007;2:1101–1108. doi: 10.1002/asia.200700068.. Also, see: Lin X, Sun J, Xi Y, Pang B. Computational interpretation of the stereoselectivity for a dirhodium tetracarboxylate-catalyzed amidation reaction. Comput Theor Chem. 2011;963:284–289.
- 27.(a) Müller P, Baud C, Nägeli I. Rhodium(II)-catalyzed nitrene transfer with phenyliodonium ylides. J Phys Org Chem. 1998;11:597–601. [Google Scholar]; (b) Huard K, Lebel H. N-Tosyloxycarbamates as Reagents in Rh-Catalyzed C–H Amination Reactions. Chem–Eur J. 2008;14:6222–6230. doi: 10.1002/chem.200702027. [DOI] [PubMed] [Google Scholar]
- 28.Harvey ME, Musaev DG, Du Bois J. A Diruthenium Catalyst for Selective, Intramolecular Allylic C–H Amination: Reaction Development and Mechanistic Insight Gained through Experiment and Theory. J Am Chem Soc. 2011;133:17207–17216. doi: 10.1021/ja203576p. [DOI] [PubMed] [Google Scholar]
- 29.Roizen JL, Zalatan DN, Du Bois J. Manuscript in preparation. [Google Scholar]
- 30.Espino CG, Williams Fiori K, Kim M, Du Bois J. Expanding the Scope of C–H Amination through Catalyst Design. J Am Chem Soc. 2004;126:15378–15379. doi: 10.1021/ja0446294. [DOI] [PubMed] [Google Scholar]
- 31.(a) Taber DF, Meagley RP, Louey JP, Rheingold AL. Molecular complex design: bridging the tetrakiscarboxylatodirhodium core. Inorg Chim Acta. 1995;239:25–28. [Google Scholar]; (b) Davies HML, Venkataramani C. Dirhodium Tetraprolinate-Catalyzed Asymmetric Cyclopropanations with High Turnover Numbers. Org Lett. 2003;5:1403–1406. doi: 10.1021/ol034002a. and references therein. [DOI] [PubMed] [Google Scholar]
- 32.Zalatan DN, Du Bois J. Understanding the Differential Performance of Rh2(esp)2 as a Catalyst for C–H Amination. J Am Chem Soc. 2009;131:7558–7559. doi: 10.1021/ja902893u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.(a) Williams Fiori K. PhD Thesis. Stanford University; Stanford, CA: 2007. [Google Scholar]; (b) Zalatan DN. PhD Thesis. Stanford University; Stanford, CA: 2009. [Google Scholar]
- 34.Cotton FA, Felthouse TR. Structural Studies of Three Tetrakis(carboxylato)dirhodium(II) Adducts in which Carboxylate Groups and Axial Ligands are Varied. Inorg Chem. 1980;19:323–328. and references therein. [Google Scholar]
- 35.Grigg RD, Rigoli JW, Pearce SD, Schomaker JM. Synthesis of Progargylic and Allenic Carbamates via the C–H Amination of Alkynes. Org Lett. 2012;14:280–283. doi: 10.1021/ol203055v. and references therein. [DOI] [PubMed] [Google Scholar]
- 36.Cui Y, He C. A Silver-Catalyzed Intramolecular Amidation of Saturated C–H Bonds. Angew Chem, Int Ed. 2004;43:4210–4212. doi: 10.1002/anie.200454243. [DOI] [PubMed] [Google Scholar]
- 37.Williams Fiori K, Fleming JJ, Du Bois J. Rh-Catalyzed Amination of Ethereal Cα–H Bonds: A Versatile Strategy for the Synthesis of Complex Amines. Angew Chem, Int Ed. 2004;43:4349–4352. doi: 10.1002/anie.200460791. [DOI] [PubMed] [Google Scholar]
- 38.Toumieux S, Compain P, Martin OR. Iterative Multifunctionalization of Unactivated C–H Bonds in Piperidines by way of Intramolecular Rh(II)-Catalyzed Aminations. J Org Chem. 2008;73:2155–2162. doi: 10.1021/jo702350u. [DOI] [PubMed] [Google Scholar]
- 39.Guthikonda K, Wehn PM, Caliando BJ, Du Bois J. Rh-catalyzed alkene oxidation. Tetrahedron. 2006;62:11331–11342. and references therein. [Google Scholar]
- 40.Zalatan DN, Du Bois J. A Chiral Rhodium Carboxamidate Catalyst for Enantioselective C–H Amination. J Am Chem Soc. 2008;130:9220–9221. doi: 10.1021/ja8031955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Milczek E, Boudet N, Blakey S. Enantioselective C–H Amination Using Cationic Ruthenium(II)-pybox Catalysts. Angew Chem, Int Ed. 2008;47:6825–6828. doi: 10.1002/anie.200801445. [DOI] [PubMed] [Google Scholar]
- 42.Liang JL, Huang JS, Yu XQ, Zhu N, Che CM. Metalloporphyrin-Mediated Asymmetric Nitrogen-Atom Transfer to Hydrocarbons. Chem– Eur J. 2002;8:1563–1572. doi: 10.1002/1521-3765(20020402)8:7<1563::aid-chem1563>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 43.Au SM, Huang JS, Che CM, Yu WY. Amidation of Unfunctionalized Hydrocarbons Catalyzed by Ruthenium Cyclic Amine or Bipyridine Complexes. J Org Chem. 2000;65:7858–7864. doi: 10.1021/jo000881s. [DOI] [PubMed] [Google Scholar]
- 44.Cotton FA, Murillo CA, Walton RA, editors. Multiple Bonds Between Metal Atoms. 3. Springer; New York: 2005. pp. 377–430. [Google Scholar]
- 45.(a) Barker JE, Ren T. Diruthenium(II,III) Bis(tetramethyl-1,3-benzenedipropionate) as a Novel Catalyst for tert-Butyl Hydroperoxide Oxygenation. Inorg Chem. 2008;47:2264–2266. doi: 10.1021/ic800035w. [DOI] [PubMed] [Google Scholar]; (b) Murahashi SI, Okano Y, Sato H, Nakae T, Komiya N. Aerobic Ruthenium-Catalyzed Oxidative Transformation of Secondary Amines to Imines. Synlett. 2007;11:1675–1678. [Google Scholar]
- 46.Musch Long AK, Yu RP, Timmer GH, Berry JF. Aryl C–H Bond Amination by an Electrophilic Diruthenium Nitride. J Am Chem Soc. 2010;132:12228–12230. doi: 10.1021/ja1062955. and references therein. [DOI] [PubMed] [Google Scholar]
- 47.Williams Fiori K, Du Bois J. Catalytic Intermolecular Amination of C–H Bonds: Method Development and Mechanistic Insights. J Am Chem Soc. 2007;129:562–568. doi: 10.1021/ja0650450.. Also, see: Norder A, Herrmann P, Herdtweck E, Bach T. Acyclic Stereo-control in the Catalytic C–H Amination of Benzylic Methylene Groups. Org Lett. 2010;12:3690–3692. doi: 10.1021/ol101517v.
- 48.Reddy RP, Davies HML. Dirhodium Tetracarboxylates Derived from Adamantylglycine as Chiral Catalysts for Enantioselective C–H Aminations. Org Lett. 2006;8:5013–5016. doi: 10.1021/ol061742l. and references therein. [DOI] [PubMed] [Google Scholar]
- 49.Collet F, Lescot C, Liang C, Dauban P. Studies in catalytic C–H amination involving nitrene C–H insertion. Dalton Trans. 2010;39:10401–10413. doi: 10.1039/c0dt00283f. [DOI] [PubMed] [Google Scholar]
- 50.Keaney GF, Wood JL. Rhodium perfluorobutyramide (Rh2(pfm)4): a synthetically useful catalyst for olefin aziridinations. Tetrahedron Lett. 2005;46:4031–4034. [Google Scholar]
- 51.Kornecki KP, Berry JF. Evidence for a One-Electron Mechanistic Regime in Dirhodium-Catalyzed Intermolecular C–H Amination. Chem—Eur J. 2011;17:5827–5832. doi: 10.1002/chem.201100708. [DOI] [PubMed] [Google Scholar]