Abstract
Macrophage activation is increased in diabetes and correlated with the onset and progression of vascular complications. To identify drugs that could inhibit macrophage activation, we developed a cell-based assay and screened a 1,040 compound library for anti-inflammatory effects. Beta2 adrenergic receptor (β2AR) agonists were identified as the most potent inhibitors of phorbol myristate acetate-induced tumor necrosis factor-α production in rat bone marrow macrophages. In peripheral blood mononuclear cells isolated from streptozotocin-induced diabetic rats, β2AR agonists inhibited diabetes-induced tumor necrosis factor-α production, which was prevented by co-treatment with a selective β2AR blocker. To clarify the underlying mechanisms, THP-1 cells and bone marrow macrophages were exposed to high glucose. High glucose reduced β-arrestin2, a negative regulator of NF-κB activation, and its interaction with IκBα. This subsequently enhanced phosphorylation of IκBα and activation of NF-κB. The β2AR agonists enhanced β-arrestin2 and its interaction with IκBα, leading to down-regulation of NF-κB. A siRNA specific for β-arrestin2 reversed β2AR agonist-mediated inhibition of NF-κB activation and inflammatory cytokine production. Treatment of Zucker Diabetic Fatty rats with a β2AR agonist for 12 weeks attenuated monocyte activation as well as pro-inflammatory and pro-fibrotic responses in the kidneys and heart. Thus, β2AR agonists might have protective effects against diabetic renal and cardiovascular complications.
Keywords: diabetes, macrophages, inflammation, fibrosis
Introduction
It has become clear that inflammatory processes play an important role in vascular complications in diabetes. Previous studies have shown that hyperglycemia activates differentiation of circulating monocytes into macrophages, resulting in their adherence to endothelial cells and migration into cardiovascular and renal tissues1,2. Once localized, the activated macrophages develop into foam cells and produce oxidants, oxidized lipids, and proinflammatory and profibrotic cytokines3. There is a great deal of evidence to support the critical role of monocytes/macrophages in facilitating some of the diabetic complications. It has been shown that monocytes from patients with both type 14,5 and type 25–7 diabetes exhibit increased proatherogenic activity, and the number of macrophages are increased in the renal tissues of these patients8,9. Similarly, increased adhesion of leukocytes or monocytes has been observed in the retinal circulation of diabetic animals10,11. Although the precise cascade that leads to tissue injury has yet to be determined, several lines of evidence support the idea that anti-inflammatory interventions such as specific antagonists of monocyte chemoattractant protein (MCP)-1 may inhibit the progression of diabetic vascular complications12,13.
The present study is designed to identify drugs with potential for use in targeting macrophage activation associated with diabetic vascular complications. We established a cell-based assay to assess macrophage activation and screened for anti-inflammatory effect in a 1,040 compound library of the US Food and Drug Administration (FDA)-approved drugs obtained from the National Institutes of Health (NIH). Beta2 adrenergic receptor (β2AR) agonists were discovered to have considerable anti-inflammatory effects in primary rat bone marrow (BM)-derived macrophages (BMMs) and this effect was further confirmed using experimental diabetic animal models.
RESULTS
High glucose (HG) and diabetes increased tumor necrosis factor (TNF)-α production and PKC activity in BMMs
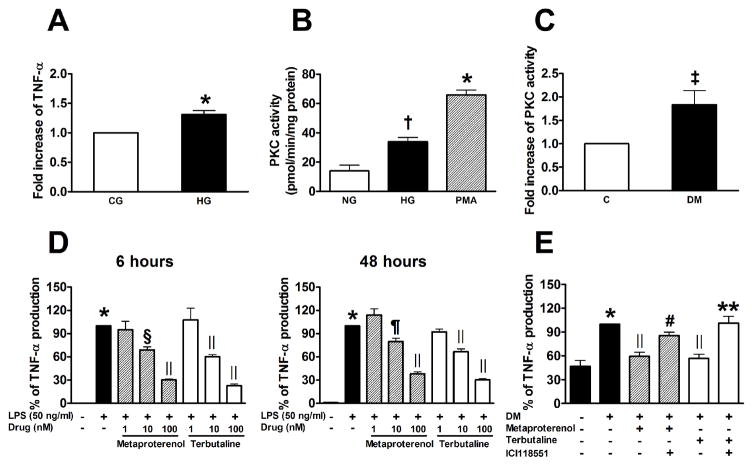
We established a cell-based assay to assess macrophage activation by first determining if HG increases TNF-α production. Rat BMMs incubated with HG (25 mmol/L D-glucose) for 72 h showed a 31% increase in TNF-α production (Figure 1A). Although the effect was statistically significant, the difference was too small for the screening purpose. In contrast, exposure to phorbol myristate acetate (PMA) for 48 h increased TNF-α production by 20-fold more than the control levels and a chemical inhibitor of conventional and novel PKC isoforms, GF109203X (1 μM) completely suppressed its effect (data not shown). Furthermore, HG significantly enhanced PKC activity in rat BMMs (Figure 1B). We also observed higher PKC activity in BMMs isolated from diabetic mice at 12 weeks of disease than in those from normal controls (Figure 1C). Based on these and previous results suggesting that PKC is an important mediator of the activation of monocytes/macrophages in diabetes14–16, we decided to use PMA as a stimulant in the initial screening.
Figure 1.
High glucose (HG) and diabetes increase TNF-α production and PKC activity in BM-derived macrophages (A–C). A: Rat BM-derived macrophages were cultured under control glucose (CG, 5.6 mM) or HG (25 mM) for 72 h. TNF-α production was measured in conditioned medium (n=17). B: PKC activity in rat BM-derived macrophages stimulated with HG for 72 h or PMA (100 nM) for 30 min (n=3). C: PKC activity in BM-derived macrophages isolated from control and diabetic mice at 12 weeks of diabetes (n=4, also indicates the number of mice studied individually). Diabetes was induced in 6-week-old male C57Bl6/J mice fasted for 12 h, with intraperitoneal injections of STZ in citrate buffer (90 mg/kg) for 2 consecutive days. D: β2AR agonists decreased LPS-induced TNF-α production in rat BM-derived macrophages. Cells were preincubated with metaproterenol or terbutaline hemisulfate for 1 h and then stimulated with LPS (50 ng/mL) for 6 or 48 h (n=3). E: β2AR agonists decreased diabetes-induced TNF-α production in rat PBMCs isolated from control and diabetic rats at 4 weeks of diabetes. Cells were incubated in RPMI medium with 10% FBS for 1 h, and then treated with 500 nM metaproterenol or terbutaline hemisulfate for an additional 16 h with or without 1 h pretreatment with ICI118551 (100 nM), a selective β2AR blocker (n=9~11, also indicates the number of rats studied individually). * p < 0.001, †p < 0.01, and ‡p < 0.05 vs. control, §p < 0.01, ||p < 0.001, and ¶p < 0.005 vs. LPS or DM, #p < 0.05, and ** p < 0.001 vs. corresponding control without ICI118551.
Identification of positive hits
Out of 1,040 compounds, 145 affected cell viability and were considered toxic while 54 increased TNF-α production by more than 2-fold without any stimulation. We analyzed 895 compounds after excluding 145 toxic compounds. Of these, 33 were identified as positive hits since they inhibited PMA-induced TNF-α levels by ≥ 95%, 197 increased TNF-α production, and the rest inhibited by < 95%. The assignment of each compound to these groups was carried out before the identities of the compounds were revealed. Interestingly, 23 compounds out of 33 positive hits were corticosteroids, known potent anti-inflammatory drugs, validating the reliability of our assay to detect compounds with anti-inflammatory actions. For the 10 non-corticosteroid positive hits, their effects were retested at five different doses from 1 nM–10 μM with PMA stimulation. After exclusion of compounds that had half-maximal inhibitory concentration (IC50) >100 nM, two remaining compounds were β2AR agonists, metaproterenol and terbutaline hemisulfate (IC50 68 and 45 nM, respectively) which were studied further.
The anti-inflammatory actions of the compounds were confirmed by testing their effects to neutralize lipopolysaccharide (LPS) stimulation of rat BMMs. We observed that TNF-α production was significantly increased after a 6- and 48-h incubation with LPS. Both metaproterenol and terbutaline hemisulfate decreased LPS-induced TNF-α production dose-dependently (Figure 1D). We evaluated the relevance of these findings to diabetes by determining the effects of β2AR agonists on TNF-α production in peripheral blood mononuclear cells (PBMCs) isolated from streptozotocin (STZ)-induced diabetic rats vs control rats. As shown in Figure 1E, TNF-α production was significantly increased by 2.1-fold in PBMCs from diabetic rats compared with control rats (P<0.001). Metaproterenol and terbutaline hemisulfate decreased diabetes-induced TNF-α production by 40 and 43%, respectively. When metaproterenol and terbutaline hemisulfate were administered in the presence of a selective β2AR blocker ICI118551 (Sigma-Aldrich, St Louis, MO), both compounds failed to reduce TNF-α production, suggesting that their effects were mediated by the β2AR pathway.
β2AR agonists inhibit HG-induced proinflammatory responses via β-arrestin2-nuclear factor (NF)-κB pathway in THP-1 cells and BMMs
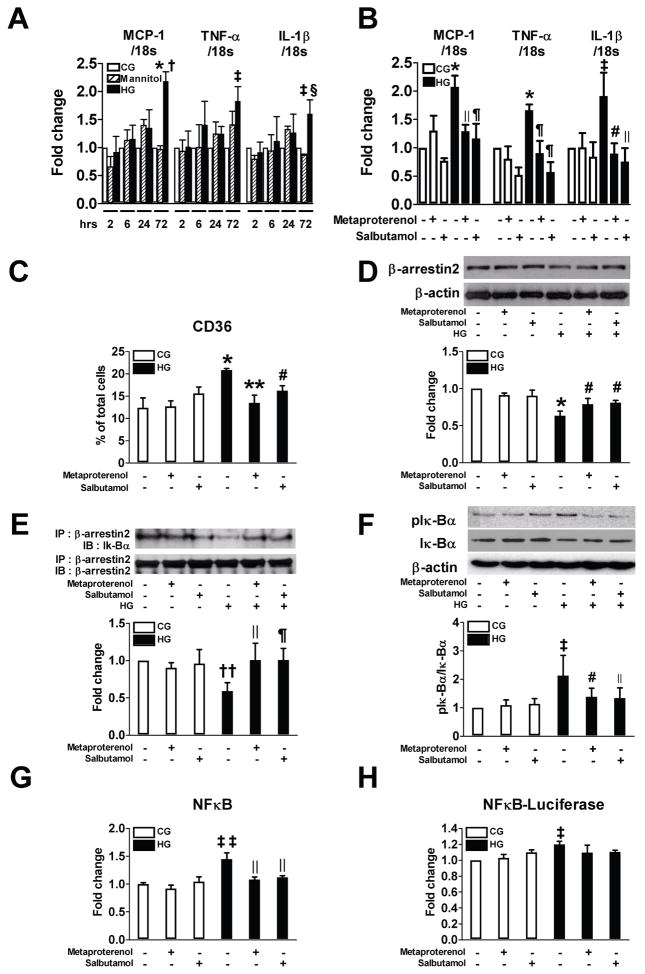
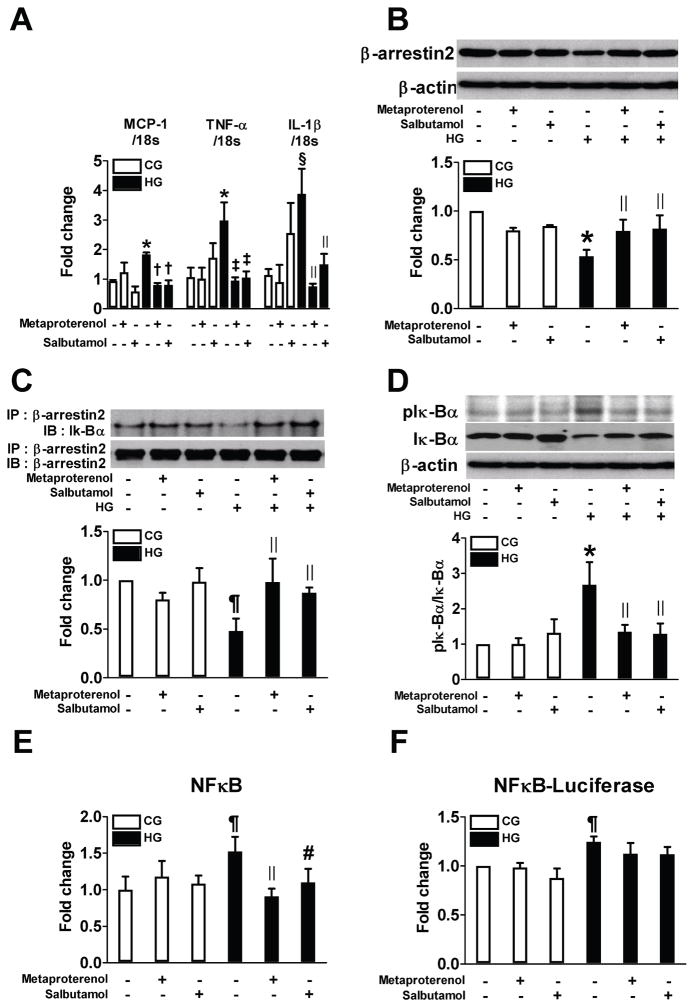
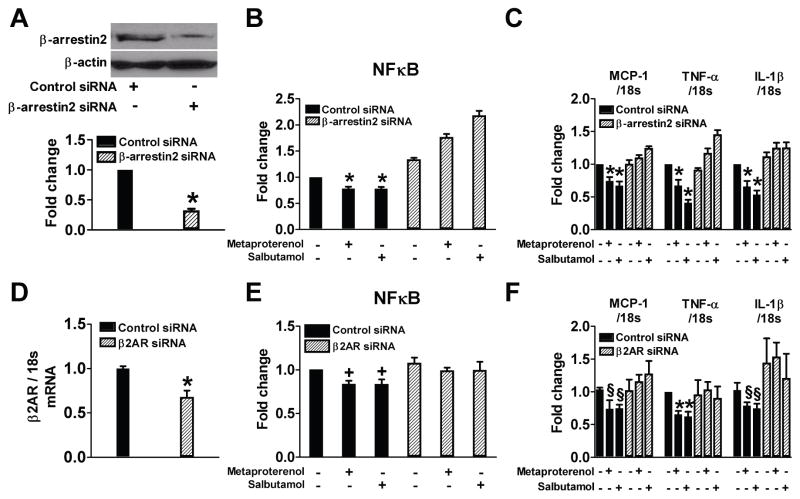
To characterize the potential mechanisms, THP-1 cells were exposed to HG and the mRNA expressions of MCP-1, TNF-α, and interleukin (IL)-1β and protein expression of CD36 at 72 h were measured. Metaproterenol or salbutamol (10 μM) significantly inhibited the effects of HG (Figure 2A–C). Since β-arrestin2 has been identified to be activated by G protein-coupled receptor (GPCR) to regulate NF-κB activation17,18, we examined the involvement of β-arrestin2 in the anti-inflammatory effect of β2AR agonists. Exposure of THP-1 cells to HG decreased β-arrestin2 protein expression and its interaction with inhibitor of NF-κB (IκB)α, which induced phosphorylation of IκBα and activation of NF-κB (Figure 2D–H). Both metaproterenol and salbutamol significantly restored these changes by increasing β-arrestin2 protein expression and decreasing the activation of NF-κB. In rat BMMs, similar findings were also observed (Figure 3). The role of β-arrestin2 and β2AR in these changes was further confirmed by the findings that siRNA-mediated knockdown of β-arrestin2 or β2AR abolished the effects of the β2AR agonists (Figure 4).
Figure 2.
β2AR agonists inhibit high glucose (HG)-induced proinflammatory responses via β-arrestin2-NF-κB pathway in THP-1 cells. A: Cells were treated with control glucose (CG, 5.6 mM), HG (25 mM), or mannitol (25 mM) and real-time RT-PCR was performed to measure the level of gene expression (n=3). B–H: Cells were preincubated with 10 μM metaproterenol or salbutamol for 2 h and then stimulated with HG for 72 h (n=3–7). Real time RT-PCR and flow cytometry were performed to measure the level of mRNA expressions of MCP-1, TNF-α and IL-1β (B) and CD36 positive cells (C). Representative western blots show the protein levels of β-arrestin2 (D) and phospho-IκBα (F). Equal amounts of protein were subjected to immunoprecipitation with anti-β-arrestin2 antibody followed by immunoblotting with antibody against β-arrestin2 or IκBα (E). NF-κB activity was measured in nuclear protein extract (G). Cells were transfected with luciferase reporter vector for NF-κB and luciferase activity was measured (H). * p < 0.001, ‡p < 0.05, ††p < 0.01, and ‡‡p < 0.005 vs. CG, †p < 0.001, and §p < 0.05 vs. mannitol, ||p < 0.01, ¶p < 0.005, #p < 0.05, and ** p < 0.001 vs. HG.
Figure 3.
β2AR agonists inhibit high glucose (HG)-induced proinflammatory responses via β-arrestin2-NF-κB pathway in rat BM-derived macrophages. Cells were preincubated with 10 μM metaproterenol or salbutamol for 2 h and then stimulated with HG for 72 h (n=3–6). Real time RT-PCR was performed to measure the level of mRNA expressions of MCP-1, TNF-α and IL-1β (A). Representative western blots show the protein levels of β-arrestin2 (B) and phospho-IκBα (D). Equal amounts of protein were subjected to immunoprecipitation with anti-β-arrestin2 antibody followed by immunoblotting with antibody against β-arrestin2 or IκBα (C). NF-κB activity was measured in nuclear protein extract (E). Cells were transfected with luciferase reporter vector for NF-κB and luciferase activity was measured (F). * p < 0.005, § p < 0.01, and ¶p < 0.05 vs. CG, †p < 0.001, ‡p < 0.005, ||p < 0.05, and #p < 0.01 vs. HG.
Figure 4.
Suppression of β-arrestin2 or β2AR using siRNA significantly blocks effects of β2AR agonists on NF-κB activation and proinflammatory cytokine production. THP-1 cells were treated with high glucose (HG) for 72 h in the presence or absence of 10 μM metaproterenol or salbutamol pretreatment for 2 h (n=4–8). A: Representative western blot shows that protein level of β-arrestin2 was decreased by siRNA transfection. B and E: NF-κB activity was measured in nuclear protein extract. C and F: Real time RT-PCR was performed to measure the level of mRNA expressions of MCP-1, TNF-α and IL-1β. D: Quantitative RT-PCR shows that mRNA level of β2AR was decreased by siRNA transfection. * p < 0.001, †p < 0.01, and § p < 0.05 vs. control siRNA.
Effect of β2AR agonist on monocyte activation in Zucker diabetic fatty (ZDF) rats
As shown in Table 1, both vehicle-treated and salbutamol-treated ZDF rats developed similar levels of hyperglycemia accompanied by elevated total cholesterol, triglyceride, and high-density lipoprotein (HDL)-cholesterol levels. Plasma insulin and HOMA-IR (homeostatic model assessment-insulin resistance) were increased in ZDF rats without significant difference by salbutamol. Blood urea nitrogen was significantly higher in ZDF rats than the levels in control rats; however, creatinine and cystatin C levels were not different in all four groups. Urine volume as well as albumin/creatinine and MCP-1/creatinine ratios were significantly increased in ZDF rats compared with Zucker fatty (ZF) lean rats. Chronic treatment with salbutamol partially prevented the increase in albumin/creatinine and MCP-1/creatinine ratios. The kidney/body weight and heart/body weight were higher in ZDF rats than they were in control rats. The heart/body weight ratio was reduced by salbutamol treatment. Blood pressure (BP) was not affected by salbutamol treatment.
TABLE 1.
Characteristics of ZF lean and ZDF rats with or without salbutamol treatment
| ZF lean+V | ZF lean+S | ZDF+V | ZDF+S | |
|---|---|---|---|---|
| N | 6 | 6 | 6 | 9 |
| BW (g) | 376.4±6.6 | 402.0±7.0 | 405.0±9.2* | 436.7±14.3† |
| Kidneys/BW (%) | 0.74±0.05 | 0.80±0.02 | 1.11±0.06‡ | 1.06±0.06‡ |
| Heart/BW (%) | 0.31±0.01 | 0.31±0.01 | 0.36±0.01* | 0.29±0.02§ |
| Systolic BP (mmHg) | 115.0±1.2 | 106.5±7.6 | 109.0±9.0 | 104.0±4.6 |
| Diastolic BP (mmHg) | 93.0±2.1 | 77.0±13.4 | 87.0±8.2 | 78.0±8.0 |
| Blood glucose (mg/dL) | 106.6±3.4 | 106.6±2.9 | 507.8±35.5‡ | 525.0±66.6‡ |
| HbA1C (%) | 4.5±0.1 | 4.7±0.1 | 14.9±0.9‡ | 13.1±1.6‡ |
| Insulin (ng/ml) | 0.01±0.00 | 0.03±0.01 | 0.10±0.01‡ | 0.16±0.05* |
| HOMA-IR | 0.5±0.2 | 2.4±1.1 | 24.0±3.2* | 29.5±6.0|| |
| Total cholesterol (mg/dL) | 95.0±4.6 | 104.3±5.6 | 260.2±33.6‡ | 218.9±27.7‡ |
| Triglyceride (mg/dL) | 84.2±26.5 | 29.8±8.2 | 199.4±51.6* | 169.0±49.7 |
| HDL (mg/dL) | 33.0±0.9 | 34.3±0.8 | 86.6±8.4‡ | 74.6±9.0‡ |
| BUN (mg/dL) | 20.9±0.8 | 24.0±0.8 | 58.7±18.5* | 42.5±13.0 |
| Cr (mg/dL) | 0.28±0.01 | 0.36±0.02 | 0.49±0.17 | 0.40±0.10 |
| Cystatin C (mg/L) | 0.15±0.02 | 0.11±0.01 | 0.18±0.04 | 0.10±0.02 |
| Urine volume (ml/d) | 7.6±0.3 | 10.5±1.2 | 80.0±30.0‡ | 52.4±7.8|| |
| Urine Alb/Cr (μg/mg) | 0.18±0.01 | 0.22±0.06 | 29.87±18.95|| | 11.90±3.04||† |
| Urine MCP-1/Cr (pg/mg) | 90.9±7.1 | 155.8±32.6 | 12674.6±8125.1|| | 5127.4±1330.5||† |
Abbreviations: V, vehicle; S, salbutamol; BW, body weight; BP, blood pressure; HbA1C, glycated hemoglobin; HOMA-IR, homeostatic model assessment-insulin resistance; HDL, high-density lipoprotein; BUN, blood urea nitrogen; Cr, creatinine; Alb, albumin; MCP, monocyte chemoattractant protein; data are mean ± SE,
p < 0.05,
p < 0.001, and
p < 0.005 vs. ZF lean+V,
p < 0.05, and
p < 0.005 vs. ZDF+V.
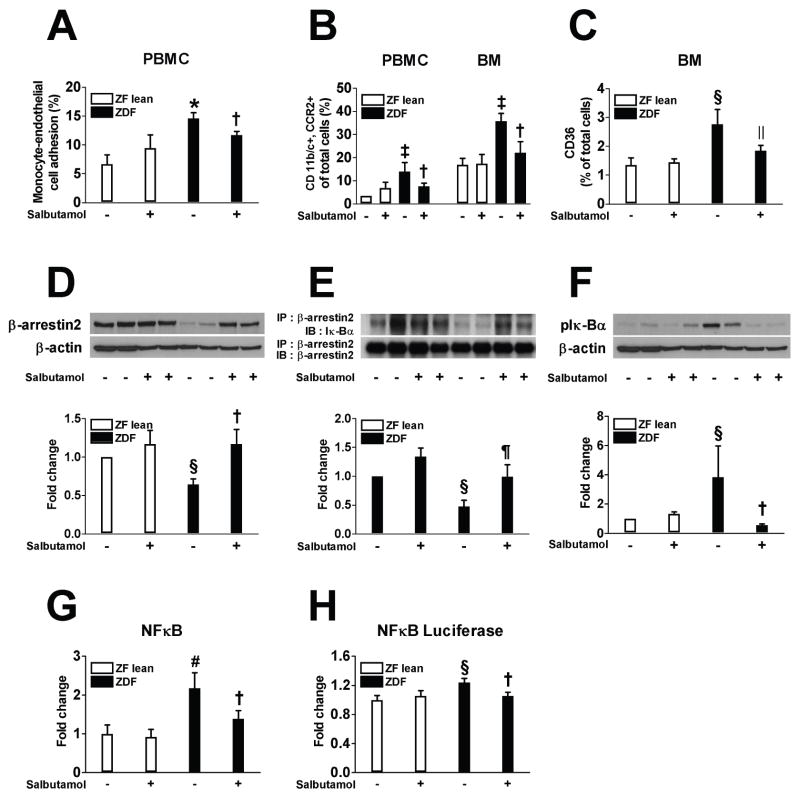
We determined the reactivity of PBMCs from ZF lean and ZDF rats by performing monocyte adhesion test. Vehicle-treated ZDF rats showed a significant increase in monocyte attachment to the endothelial monolayers compared to the controls (Figure 5A). Furthermore, immunopositivity of both CD11b/c and C-C chemokine receptor (CCR) 2 and the LPS-induced upregulation of CD36 expression in PBMCs and BM mononuclear cells from ZDF rats were higher than those in cells from the lean controls by 4.0-, 2.1-, and 2.1-fold, respectively; salbutamol effectively prevented all of these changes (Figure 5B and C). The role of β-arrestin2 was further studied by assessing the expression of β-arrestin2 and its association with IκBα in BMMs from ZF lean and ZDF rats. Compared with controls, the vehicle-treated ZDF rats showed decreased protein expression of β-arrestin2 as well as its interaction with IκBα and increased phosphorylation of IκBα in the BMMs following LPS treatment (Figure 5D–F). BMMs from vehicle-treated ZDF rats displayed increased NF-κB activity compared to control, which were all markedly attenuated by salbutamol treatment (Figure 5G and H).
Figure 5.
β2AR agonist inhibits monocyte activation in ZDF rats (A–C). A: Monocyte adhesion to human microvascular endothelial cells was assessed in PBMCs isolated from ZF lean and ZDF rats with or without salbutamol treatment. B: CD11b/c and CCR2-positive cells were measured using flow cytometry in PBMCs and BM mononuclear cells. C: CD36 positive cells were measured in BM mononuclear cells after 24 h stimulation with LPS (1 μg/mL). β2AR agonist inhibits NF-κB pathway in BM-derived macrophages in ZDF rats (D–H). Rat BM-derived macrophages were differentiated for 7 days in RPMI medium containing L929 cells conditioned medium and then stimulated with LPS (1 μg/mL) for an additional 24 h. Representative western blots show the protein levels of β-arrestin2 (D) and phospho-IκBα (F). Equal amounts of protein were subjected to immunoprecipitation with anti-β-arrestin2 antibody followed by immunoblotting with antibody against β-arrestin2 or IκBα (E). NF-κB activity was measured in nuclear protein extract (G). Cells were transfected with luciferase reporter vector for NF-κB and luciferase activity was measured (H). * p < 0.001, ‡p < 0.005, §p < 0.05, and #p < 0.01 vs. vehicle-treated ZF lean, †p < 0.05, ||p < 0.001, and ¶p < 0.005 vs. vehicle-treated ZDF, n=6–9.
Effect of β2AR agonist on renal changes in ZDF rats
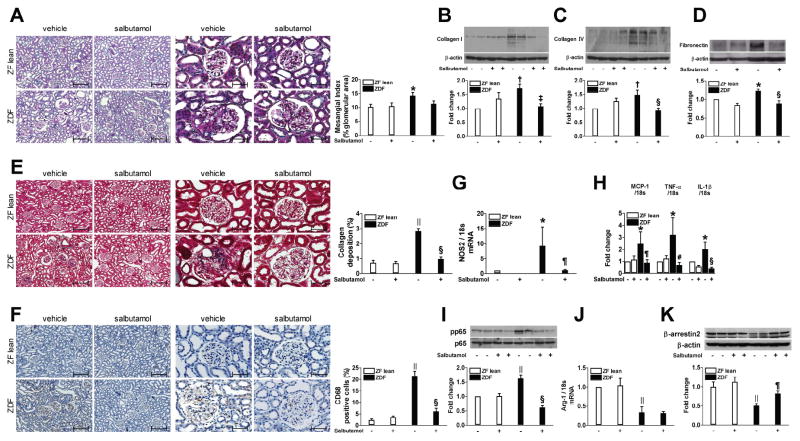
The effects of the β2AR agonist on renal changes induced by diabetes were studied. Histological analysis exhibited an increase in the mesangial matrix area in the vehicle-treated ZDF rats compared to ZF lean rats at 20 weeks of age. Although there was a trend toward less mesangial matrix, the effect of salbutamol was not statistically significant (Figure 6A). The expression of type I/IV collagen and fibronectin was significantly higher in the renal cortex from ZDF rats compared with ZF lean controls (Figure 6B–D). Masson’s trichrome staining confirmed a greater deposition of collagen in ZDF than in ZF lean rats (Figure 6E). Salbutamol treatment almost completely normalized these changes (Figure 6E). As shown in Figure 6F–I, ZDF rats exhibited a significantly higher number of CD68 positive cells and elevated levels of nitric oxide synthase (NOS) 2, MCP-1, TNF-α, and IL-1β mRNA as well as phosphorylation of p65 subunit of NF-κB than ZF lean controls. These changes were attenuated in salbutamol-treated ZDF accompanied by enhancement of β-arrestin2 (Fig 6K). In contrast, the expression of arginase-1 was decreased in vehicle-treated ZDF rats without significant difference following salbutamol treatment (Figure 6J).
Figure 6.
β2AR agonist reduces renal changes in ZDF rats. Representative kidney sections stained with PAS (A), Masson’s trichrome (E) and immunohistochemical staining for CD68 positive macrophages (F), original magnification ×100 and ×400, scale bar: 200 and 50 μm. B–D, I, K: Protein expression of collagen I and IV, fibronectin, phosphorylated p65, and β-arrestin2 was analyzed using immunoblot analysis. G, H, J: Real-time RT-PCR was performed to measure the mRNA expression of NOS2, MCP-1, TNF-α, IL-1β, and arginase-1. * p < 0.05, †p < 0.005, and ||p < 0.001 vs. vehicle-treated ZF lean, ‡p < 0.005, §p < 0.001, ¶p < 0.05, and #p < 0.01 vs. vehicle-treated ZDF, n=6–9.
Effect of β2AR agonist on cardiac changes in ZDF rats
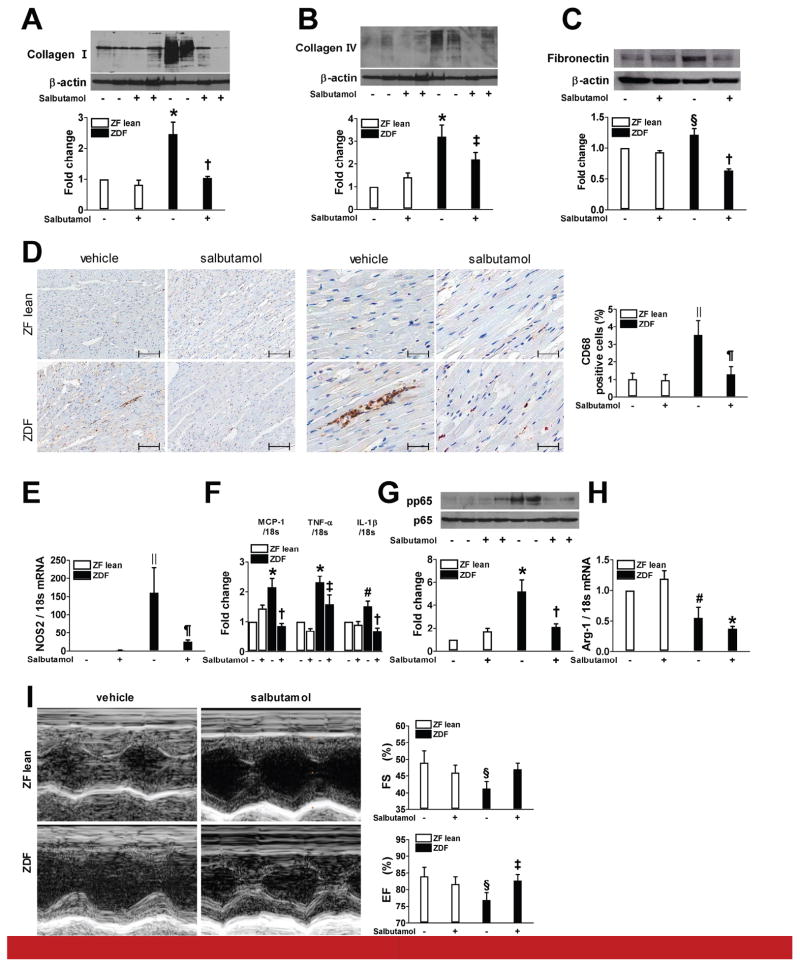
The actions of β2AR agonist on cardiac changes associated with diabetes were studied. Compared with ZF lean rats, vehicle-treated ZDF showed an increased cardiac expression of collagen I/IV and fibronectin while salbutamol treatment significantly attenuated these changes (Figure 7A–C). Similarly, salbutamol treatment reduced ZDF-induced CD68 positive macrophage infiltration, elevated levels of NOS2, MCP-1, TNF-α, and IL-1β mRNA expression, and phosphorylation of p65 subunit of NF-κB in the myocardium (Figure 7D–G). In contrast, no significant difference in the mRNA expression of arginase-1 was observed between the ZDF and salbutamol-treated ZDF groups (Figure 7H). The functional cardiac consequences of diabetes and the treatments with salbutamol were evaluated using echocardiography. As shown in Figure 7I, ejection fraction (EF) and fractional shortening (FS) significantly decreased in vehicle-treated ZDF group compared to the ZF control group. However, salbutamol treatment improved both of these parameters.
Figure 7.
β2AR agonist reduces cardiac changes in ZDF rats. A–C, G: Protein expression of collagen I and IV, fibronectin, and phosphorylated p65 was analyzed using immunoblot analysis. Representative heart sections stained for CD68 positive macrophages by immunohistochemistry (D, original magnification ×100 and ×400). Scale bar: 200 and 50 μm. E, F, H: Real-time RT-PCR was performed to measure mRNA expression of NOS2, MCP-1, TNF-α, IL-1β, and arginase-1. I: Representative M-mode echocardiograms and quantification of left ventricular ejection fraction and fractional shortening. * p < 0.001, §p < 0.05, ||p < 0.005, and #p < 0.01 vs. vehicle-treated ZF lean, †p < 0.001, ‡p < 0.05, and ¶p < 0.005 vs. vehicle-treated ZDF, n=6–9.
Effect of short-term treatment with β2AR agonist on monocyte activation and renal and cardiac changes in ZDF rats
To test whether short-term treatment with β2AR agonist has similar protective effects, 15-week-old ZDF rats were treated with salbutamol for 5 weeks (Supplementary Table S1). Five-week-treatment with salbutamol induced a qualitatively similar effect to 12-week treatment on ZDF-induced monocyte activation (Supplementary Figure S1), enhanced proinflammatory cytokine production, and influx of macrophages in renal and cardiac tissues (Supplementary Figure S2A and B and S3A and B). However, the increased expression of type I/IV collagen and fibronectin in ZDF renal cortex and albuminuria were not attenuated by salbutamol (Supplementary Table S1 and Figure S2C–E). Similarly, cardiac expression of type IV collagen and LV systolic dysfunctions were not significantly changed following short-term treatment with salbutamol (Supplementary Figure S3C and D).
DISCUSSION
In the present study, drug screen revealed that β2AR agonists were potential therapeutic agents targeting macrophage activation. The findings that β2AR agonists may have anti-inflammatory actions were confirmed by multiple ways including inhibition of PMA or LPS-induced TNF-α production in rat BMMs and diabetes-induced TNF-α production in PBMCs isolated from STZ-induced diabetic rats. Furthermore, we demonstrated that β2AR agonists significantly reduced the expression of proinflammatory cytokines and CD36 in HG-treated THP-1 cells and rat BMMs. Beta adrenergic actions on the macrophages have been reported by Abrass et al. In 1985, Abrass et al. characterized βARs in rat peritoneal macrophages and suggested that beta adrenergic hormones may inhibit macrophage function19. Recently, Wang et al. reported that β2AR agonists can inhibit LPS-induced activation of THP-1 cells, similar to our findings20. However, the inhibition of HG and diabetes-induced macrophage activation has not been reported with β2AR activation especially associated with improvement in renal and cardiac abnormalities related to diabetes. The results from our study suggested that enhancement of β2AR pathway can decrease diabetes or HG-induced activation of macrophage.
Monocytes/macrophages accumulation may contribute to renal and cardiovascular complications of diabetes. For example, there are correlations between the degree of macrophage accumulation in renal tissue and severity of kidney damage21,22. Studies performed in patients with acute myocardial infarction, diabetic patients have shown increased levels of macrophage in the vascular wall23. In addition, diabetic cardiomyopathy, a distinct entity independent of coronary atherosclerosis, also shows infiltration of macrophages in cardiac tissue24,25. The salient finding of the present study is that β2AR agonists improve both renal and cardiac inflammation. This was accompanied by decreased renal fibrosis in diabetic rodents, reduced albuminuria, and improved cardiac dysfunction.
Activated macrophages can be roughly categorized into two distinct phenotypes of M1 and M226,27. Activated M1 macrophages are generally characterized by high production of proinflammatory cytokines while alternatively activated M2 macrophages secret anti-inflammatory cytokines. The expression of the M1 marker NOS2 was significantly elevated in vehicle-treated ZDF rats compared to ZF lean control but interestingly, it was reduced by salbutamol treatment. On the other hand, the expression of the M2 marker arginase-1 was decreased in ZDF rats without significant changes following salbutamol treatment. Our results showing an increase in the M1/M2 ratio have been similarly reported in the STZ-induced diabetic kidney28 and peripheral blood of patients with diabetes29. These results suggest that the protective action of β2AR agonists involves abolishing the recruitment of M1-activated macrophages into the renal and cardiac tissues rather than induction of a phenotypic switch from M1 to M2.
The NF-κB pathway is a key factor linking inflammation and diabetic vascular complications30. In unstimulated states, the regulatory mechanism of the NF-κB pathway is its interaction with endogenous inhibitors, IκB proteins, which retain it in the cytoplasm31. However, following stimulation, the inhibitory proteins are phosphorylated and degraded via the ubiquitin-proteasome pathway31. This process induces NF-κB activation. Therefore, strategies targeting NF-κB activation have included stabilization of endogenous inhibitors. Recent evidence suggests that β-arrestin2 serves as an endogenous regulator of the NF-κB pathways17. This protein was originally discovered as a desensitizing molecule for GPCRs32; however, it interacts with IκBα and, thereby prevents the phosphorylation and degradation of IκBα17. In the present study, we demonstrated that HG and diabetes decreased the protein expression of β-arrestin2 and its association with IκBα while the β2AR agonists significantly reversed these changes. The involvement of β-arrestin2 in these changes was further confirmed by the finding that suppression of β-arrestin2 using siRNA significantly blocked the effects of β2AR agonists on NF-κB activation and proinflammatory cytokine production. It appears that our observations are consistent with a previous report33 where β2AR stimulation was shown to inhibit advanced glycation end products-induced adhesion molecule expression and cytokine production in human PBMCs.
The interplay between inflammation and tissue fibrosis has been observed in various disease models. Data from numerous studies suggest that macrophages are invariably found at sites of tissue fibrosis and that the levels of macrophage accumulation significantly correlate with tissue fibrosis34–36. Our results indicated that β2AR agonist was able to reduce both inflammation and extracellular matrix deposition in renal and cardiac tissues, further support the association between inflammation and fibrosis. It is unclear whether the lack of anti-fibrotic response with short-term treatment of ZDF rats was attributed to the short duration or late initiation of treatment.
It has been reported that β2AR agonist could activate renin angiotensin system37. However, in our experiment, renin levels were not detectable in all four groups and angiotensin II levels were significantly higher in ZDF rats (0.49±0.19 vs. 0.03±0.02 pg/ml, p<0.05) without difference following salbutamol treatment (0.48±0.29 and 0.06±0.01 pg/ml in ZDF+salbutamol and ZF lean+salbutamol, respectively).
A limitation of our study is that we did not directly test whether macrophage activation is necessary for the β2AR agonist-mediated protection. Since macrophage depletion itself delays the onset of diabetes38 and attenuates diabetic renal injury28, there would be technical difficulties to demonstrate whether macrophage depletion abrogates the protective effects of salbutamol upon diabetic vascular injury in our experimental setting. Future experiments will need to address this issue.
In conclusion, we have identified and demonstrated the function of β2AR agonists as potential novel therapeutic agents targeting monocytes/macrophages activation in the treatment of diabetic renal and cardiovascular complications. These drugs are currently used for other medical purposes, indicating that they have already passed extensive toxicity testing. Therefore, we believe that further well-designed mechanistic animal studies and human trials including investigation of off-target tissue effects39 could lead to the rapid development of potentially effective therapies for diabetic renal and cardiovascular diseases.
MATERIALS AND METHODS
PART I: Drug screening
Isolation of BMMs
BMMs were prepared by flushing the dissected femur and tibia of 8–12-week-old Sprague-Dawley (SD) rats (Charles River Laboratories (CRL), Wilmington, MA). Cells were cultured in RPMI containing 10% fetal bovine serum (FBS) and 20% L929 cell (American Type Culture Collection [ATCC], Manassas, VA) conditioned media as a source of macrophage colony stimulating factor40. After 7 days, at least 97% of the cells were double positive for CD68 (Serotec, Oxford, UK) and CD11b/c (BD Biosciences, San Diego, CA). Mouse BMMs were similarly prepared. All experiments were conducted in accordance with the Joslin Diabetes Center’s Animal Care and Use Committee guidelines.
PKC activity
PKC activity was measured using a modified in situ PKC assay41.
Screening for anti-inflammatory effects
The NIH library of 1,040 FDA-approved compounds was screened to identify potential anti-inflammatory effects. BMMs were plated in 24-well plates (5×104 cells/well) and subconfluent cells were stimulated with PMA (100 nM). The compounds were treated 1 h before stimulation. After 48 h, conditioned media were analyzed for TNF-α using enzyme-linked immunosorbent assay (ELISA, Pierce, Rockford, IL). The library was screened in duplicate with test compounds at a final concentration of 10 μM. For each drug, the effect of the drug itself without stimulation was also tested. Data were expressed as a relative % of TNF-α production compared to the effects of PMA. The drugs that inhibited PMA-induced TNF-α production by ≥ 95% were defined as positive hits and were retested at five different doses (1, 10, and 100 nM and 1 and 10 μM) with PMA stimulation and further confirmed with LPS (50 ng/mL) stimulation.
Isolation of PBMCs
Diabetes was induced in 8-week-old male SD rats by intraperitoneal injection of citrate-buffered STZ (70 mg/kg, Sigma-Aldrich). After 4 weeks, PBMCs were isolated using Histopaque 1077 (Sigma-Aldrich).
PART II: β2AR agonists study
Animals and drug treatment
Male 6-week-old ZDF rats and their normal littermates (ZF lean) purchased from CRL (Yokohama, Japan) were housed in a pathogen-free facility set on a 12-h light-dark cycle and given free access to water and Purina 5008 diet. From the age of 8 weeks, either salbutamol (0.5 mg·kg−1·d−1, Sigma-Aldrich), a β2AR agonist or vehicle was administered for 12 weeks using an osmotic minipump (Durect, Cupertino, CA). In some experiments, 15-week-old rats were treated for 5 weeks. All animal studies were conducted with the approval of the Institutional Care and Use Committee of the Soon Chun Hyang University Hospital, and our study complied with the NIH Guidelines for the Care and Use of Experimental Animals.
Blood and urine chemistry
Glucose and glycated hemoglobin levels were determined in whole blood using Gluco Dr. Plus2 (Allmedicus, Korea) and Quo-Lab (Quotient Diagnostics, Surrey, UK) analyzers, respectively. Plasma or urine creatinine, urea nitrogen, total cholesterol, triglyceride, and HDL-cholesterol were measured using a colorimetric method with a Hitachi 7600-110 analyzer (Hitachi, Tokyo, Japan). Plasma insulin (EMD Millipore, Temecula, CA) and renin (MyBiosource, San Diego, CA), and urinary albumin (Abcam, Cambridge, MA) and MCP-1 (Sigma-Aldrich) were measured using an ELISA kit. Plasma angiotensin II was measured using an enzyme immunoassay (EIA) kit (Sigma-Aldrich). HOMA-IR was calculated as follows: fasting serum glucose (mmol/L) × fasting serum insulin (μU/ml)/22.542.
Cell culture
THP-1 monocytic cells (ATCC) were cultured in RPMI under 5.6 or 25 mmol/L D-glucose (HG). BMMs and PBMCs were isolated as described previously.
NF-κB activity and luciferase assay
NF-κB activity was measured in nuclear protein extract using a TransAM™ NF-κB p65 protein assay (Active Motif, Carlsbad, CA). In some experiments, cells were transfected with luciferase reporter vector for NF-κB (Thermo Scientific, Rockford, IL) and luciferase activity was measured using a luminometer.
siRNA
An effective predesigned siRNA of β-arrestin2 or β2AR (Applied Biosystems, Carlsbad, CA) was selected in a preliminary study. The sequence was as follows: β-arrestin2, sense 5′-AGCCUUCUGUGCUAAAUCATT-3′ and antisense 5′-UGAUUUAGCACAGAAGGCUCG-3′; β2AR, sense 5′-GGGAGGAAUUGUAGUACAATT-3′ and antisense 5′-UUGUACUACAAUUCCUCCCTT-3′. Cells were transfected with siRNA (50 nM/well) using Lipofectamine RNAiMAX (Invitrogen, Carlsbad, CA) reagent under serum- and antibiotic-free condition for 24 h.
RT-PCR
Total RNA was prepared using TRIzol reagent (Sigma-Aldrich). The cDNA product was synthesized and amplified by PCR using a 7900HT Fast Real-Time PCR System (Applied Biosystems). For PCR reactions, we used TaqMan Assays-on-Demand Gene Expression Products from Applied Biosystems: MCP-1, Hs00234140_m1 and Rn00580555_m1; TNF-α, Hs00174128_m1 and Rn01525859_g1; IL-1β, Hs01555410_m1 and Rn00580432_m1; NOS 2, Rn00561646_m1; arginase-1, Rn00691090_m1; and β2AR, Hs00240532_s1. As an internal control, 18S ribosomal RNA expression, Hs99999901_s1 and Rn03928990_g1, was quantified with the target genes.
Western blot analysis
Tissue and cell lysates were subjected to western blot analysis using a standard procedure. Membranes were immunoblotted with antibodies against β-arrestin2 (Cell Signaling, Beverly, MA), phospho-IκBα (Cell Signaling), collagen I/IV (Southern Biotech, Birmingham, AL), fibronectin (DAKO, Glostrup, Denmark), p65 (Cell Signaling), and phosphorylated p65 (Cell Signaling) followed by appropriate secondary antibodies.
Immunoprecipitation
Approximately 1 mg of total proteins were incubated overnight at 4°C with anti-β-arrestin2 antibody (Santa Cruz) followed by precipitation with 20 μL of protein A/G-Plus-Agarose (Santa Cruz) for 4 h at 4°C. The precipitated complexes were immunoblotted with anti-β-arrestin2 or anti-IκBα (Cell Signaling).
Flow cytometry
Cells were exposed to monoclonal antibodies against CD36 (Abcam), CD11b/c, or CCR2 (Novus Biologicals, Littleton, CO) followed by fluorescein isothiocyanate (FITC) or phycoerythrin (PE)-conjugated secondary antibody. Cells were analyzed using flow cytometry.
Monocyte adhesion assay
Human microvascular endothelial cells (HMVECs, Invitrogen) were allowed to grow to confluent monolayers. Then, 5-chloromethylfluorescein diacetate (Invitrogen)-labeled PBMCs were incubated with HMVECs for 30 min at 37°C. After incubation, the nonadherent cells were washed away with phosphate-buffered saline and the adherent cells were counted using an inverted microscope.
BP measurement
BP was measured by tail cuff method using automatic noninvasive BP system (AD Instruments, Sydney, Australia). Tail cuff and signal transducer were equipped to rats placed on holder. Average BP value was acquired from two readings. All measurements were performed between 2 and 4 PM.
Echocardiography
Rats were anesthetized with tiletamine (25 mg/kg, Virbac, Carros, France)/zolazepam (25 mg/kg, Virbac)/xylazine (10 mg/kg, Bayer, Seoul, Korea) by intraperitoneal injection. An experienced cardiologist who was shielded to the treatments performed the echocardiography (iE33, Philips, Andover, MA).
Histology
Paraffin-embedded sections (3-μm) were subjected to periodic acid-Schiff (PAS) and Masson’s trichrome staining. On PAS-stained kidney sections, 150 different superficial glomeruli were randomly sampled for morphometric analysis and the percentage mesangial area was determined using the Image Scope software (Aperio, Vista, CA). Collagen deposition was determined on the trichrome-stained kidney sections. The positive area was quantitatively measured using the Image Scope software. For analysis of macrophage infiltration, paraffin-embedded sections were stained for CD68 (EMD Millipore). Diaminobenzidine was used for visualization of immunoreactivity followed by hematoxylin for nuclear counterstaining.
Statistical analyses
The mean values were compared using ANOVA followed by the Fisher’s least significant difference method. Unpaired two-tailed Student’s t-tests were used where appropriate. Data are presented as mean±standard error (SE). A P value <0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank Drs. HJ Han (College of Veterinary Medicine and Research Institute for Veterinary Science, Seoul National University) and SH Lee (Medical Science Research Institute, Soon Chun Hyang University Seoul Hospital) for technical assistance with blood pressure measurement. This study was supported by NIH grants 5P30DK036836, 2R01DK053105, NIDDK/NIH grant 1R21DK063000 (to G.L.K), National Research Foundation of Korea Grant funded by the Korean Government (2011-0022380), and Soon Chun Hyang University Research Fund (to H.N.).
Footnotes
DISCLOSURE
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sassy-Prigent C, Heudes D, Mandet C, et al. Early glomerular macrophage recruitment in streptozotocin-induced diabetic rats. Diabetes. 2000;49:466–475. doi: 10.2337/diabetes.49.3.466. [DOI] [PubMed] [Google Scholar]
- 2.Tsao PS, Niebauer J, Buitrago R, et al. Interaction of diabetes and hypertension on determinants of endothelial adhesiveness. Arterioscler Thromb Vasc Biol. 1998;18:947–953. doi: 10.1161/01.atv.18.6.947. [DOI] [PubMed] [Google Scholar]
- 3.Li AC, Glass CK. The macrophage foam cell as a target for therapeutic intervention. Nat Med. 2002;8:1235–1242. doi: 10.1038/nm1102-1235. [DOI] [PubMed] [Google Scholar]
- 4.Devaraj S, Cheung AT, Jialal I, et al. Evidence of increased inflammation and microcirculatory abnormalities in patients with type 1 diabetes and their role in microvascular complications. Diabetes. 2007;56:2790–2796. doi: 10.2337/db07-0784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cipolletta C, Ryan KE, Hanna EV, et al. Activation of peripheral blood CD14+ monocytes occurs in diabetes. Diabetes. 2005;54:2779–2786. doi: 10.2337/diabetes.54.9.2779. [DOI] [PubMed] [Google Scholar]
- 6.Devaraj S, Jialal I. Low-density lipoprotein postsecretory modification, monocyte function, and circulating adhesion molecules in type 2 diabetic patients with and without macrovascular complications: the effect of alpha-tocopherol supplementation. Circulation. 2000;102:191–196. doi: 10.1161/01.cir.102.2.191. [DOI] [PubMed] [Google Scholar]
- 7.Devaraj S, Jialal I. Alpha tocopherol supplementation decreases serum C-reactive protein and monocyte interleukin-6 levels in normal volunteers and type 2 diabetic patients. Free Radic Biol Med. 2000;29:790–792. doi: 10.1016/s0891-5849(00)00420-2. [DOI] [PubMed] [Google Scholar]
- 8.Furuta T, Saito T, Ootaka T, et al. The role of macrophages in diabetic glomerulosclerosis. Am J Kidney Dis. 1993;21:480–485. doi: 10.1016/s0272-6386(12)80393-3. [DOI] [PubMed] [Google Scholar]
- 9.Bohle A, Wehrmann M, Bogenschutz O, et al. The pathogenesis of chronic renal failure in diabetic nephropathy. Investigation of 488 cases of diabetic glomerulosclerosis. Pathol Res Pract. 1991;187:251–259. doi: 10.1016/s0344-0338(11)80780-6. [DOI] [PubMed] [Google Scholar]
- 10.Joussen AM, Poulaki V, Le ML, et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004;18:1450–1452. doi: 10.1096/fj.03-1476fje. [DOI] [PubMed] [Google Scholar]
- 11.Abiko T, Abiko A, Clermont AC, et al. Characterization of retinal leukostasis and hemodynamics in insulin resistance and diabetes: role of oxidants and protein kinase-C activation. Diabetes. 2003;52:829–837. doi: 10.2337/diabetes.52.3.829. [DOI] [PubMed] [Google Scholar]
- 12.Tesch GH. MCP-1/CCL2: a new diagnostic marker and therapeutic target for progressive renal injury in diabetic nephropathy. Am J Physiol Renal Physiol. 2008;294:F697–F701. doi: 10.1152/ajprenal.00016.2008. [DOI] [PubMed] [Google Scholar]
- 13.Kolattukudy PE, Niu J. Inflammation, endoplasmic reticulum stress, autophagy, and the monocyte chemoattractant protein-1/CCR2 pathway. Circ Res. 2012;110:174–189. doi: 10.1161/CIRCRESAHA.111.243212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Devaraj S, Venugopal SK, Singh U, et al. Hyperglycemia induces monocytic release of interleukin-6 via induction of protein kinase c-{alpha} and -{beta} Diabetes. 2005;54:85–91. doi: 10.2337/diabetes.54.1.85. [DOI] [PubMed] [Google Scholar]
- 15.Shanmugam N, Reddy MA, Guha M, et al. High glucose-induced expression of proinflammatory cytokine and chemokine genes in monocytic cells. Diabetes. 2003;52:1256–1264. doi: 10.2337/diabetes.52.5.1256. [DOI] [PubMed] [Google Scholar]
- 16.Ceolotto G, Gallo A, Miola M, et al. Protein kinase C activity is acutely regulated by plasma glucose concentration in human monocytes in vivo. Diabetes. 1999;48:1316–1322. doi: 10.2337/diabetes.48.6.1316. [DOI] [PubMed] [Google Scholar]
- 17.Gao H, Sun Y, Wu Y, et al. Identification of β-arrestin2 as a G protein-coupled receptor-stimulated regulator of NF-κB pathways. Mol Cell. 2004;14:303–317. doi: 10.1016/s1097-2765(04)00216-3. [DOI] [PubMed] [Google Scholar]
- 18.Farmer P, Pugin J. β-Adrenergic agonists exert their “anti-inflammatory” effects in monocytic cells through the IκB/NF-κB pathway. Am J Physiol Lung Cell Mol Physiol. 2000;279:L675–L682. doi: 10.1152/ajplung.2000.279.4.L675. [DOI] [PubMed] [Google Scholar]
- 19.Abrass CK, O’Connor SW, Scarpace PJ, et al. Characterization of the beta-adrenergic receptor of the rat peritoneal macrophage. J Immunol. 1985;135:1338–1341. [PubMed] [Google Scholar]
- 20.Wang W, Zhang Y, Xu M, et al. Fenoterol inhibits LPS-induced AMPK activation and inflammatory cytokine production through β-arrestin-2 in THP-1 cell line. Biochem Biophys Res Commun. 2015;462:119–123. doi: 10.1016/j.bbrc.2015.04.097. [DOI] [PubMed] [Google Scholar]
- 21.Chow F, Ozols E, Nikolic-Paterson DJ, et al. Macrophages in mouse type 2 diabetic nephropathy: Correlation with diabetic state and progressive renal injury. Kidney Int. 2004;65:116–128. doi: 10.1111/j.1523-1755.2004.00367.x. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen DUY, Ping FU, Mu WEI, et al. Macrophage accumulation in human progressive diabetic nephropathy. Nephrology. 2006;11:226–231. doi: 10.1111/j.1440-1797.2006.00576.x. [DOI] [PubMed] [Google Scholar]
- 23.Fukumoto H, Naito Z, Asano G, et al. Immunohistochemical and morphometric evaluations of coronary atherosclerotic plaques associated with myocardial infarction and diabetes mellitus. J Atheroscler Thromb. 1998;5:29–35. doi: 10.5551/jat1994.5.29. [DOI] [PubMed] [Google Scholar]
- 24.Westermann D, Van Linthout S, Dhayat S, et al. Cardioprotective and anti-inflammatory effects of interleukin converting enzyme inhibition in experimental diabetic cardiomyopathy. Diabetes. 2007;56:1834–1841. doi: 10.2337/db06-1662. [DOI] [PubMed] [Google Scholar]
- 25.Van Linthout S, Riad A, Dhayat N, et al. Anti-inflammatory effects of atorvastatin improve left ventricular function in experimental diabetic cardiomyopathy. Diabetologia. 2007;50:1977–1986. doi: 10.1007/s00125-007-0719-8. [DOI] [PubMed] [Google Scholar]
- 26.Mantovani A, Sica A, Sozzani S, et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 27.Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol. 2011;11:750–761. doi: 10.1038/nri3088. [DOI] [PubMed] [Google Scholar]
- 28.You H, Gao T, Cooper TK, et al. Macrophages directly mediate diabetic renal injury. Am J Physiol Renal Physiol. 2013;305:F1719–F1727. doi: 10.1152/ajprenal.00141.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fadini GP, de Kreutzenberg SV, Boscaro E, et al. An unbalanced monocyte polarisation in peripheral blood and bone marrow of patients with type 2 diabetes has an impact on microangiopathy. Diabetologia. 2013;56:1856–1866. doi: 10.1007/s00125-013-2918-9. [DOI] [PubMed] [Google Scholar]
- 30.Tornatore L, Thotakura AK, Bennett J, et al. The nuclear factor kappa B signaling pathway: integrating metabolism with inflammation. Trends Cell Biol. 2012;22:557–566. doi: 10.1016/j.tcb.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Karin M, Yamamoto Y, Wang QM. The IKK NF-kappa B system: a treasure trove for drug development. Nat Rev Drug Discov. 2004;3:17–26. doi: 10.1038/nrd1279. [DOI] [PubMed] [Google Scholar]
- 32.Ferguson SSG, Downey WE, III, Colapietro A-M, et al. Role of beta-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science. 1996;271:363. doi: 10.1126/science.271.5247.363. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi HK, Mori S, Liu K, et al. β2-adrenoceptor stimulation inhibits advanced glycation end products-induced adhesion molecule expression and cytokine production in human peripheral blood mononuclear cells. Eur J Pharmacol. 2010;627:313–317. doi: 10.1016/j.ejphar.2009.10.034. [DOI] [PubMed] [Google Scholar]
- 34.Yang N, Isbel NM, Nikolic-Paterson DJ, et al. Local macrophage proliferation in human glomerulonephritis. Kidney Int. 1998;54:143–151. doi: 10.1046/j.1523-1755.1998.00978.x. [DOI] [PubMed] [Google Scholar]
- 35.Lan HY, Nikolic-Paterson DJ, Mu W, et al. Local macrophage proliferation in the progression of glomerular and tubulointerstitial injury in rat anti-GBM glomerulonephritis. Kidney Int. 1995;48:753–760. doi: 10.1038/ki.1995.347. [DOI] [PubMed] [Google Scholar]
- 36.Meng X-M, Nikolic-Paterson DJ, Lan HY. Inflammatory processes in renal fibrosis. Nat Rev Nephrol. 2014;10:493–503. doi: 10.1038/nrneph.2014.114. [DOI] [PubMed] [Google Scholar]
- 37.Millar EA, McInnes GT, Thomson NC. Investigation of the mechanism of β2-agonist-induced activation of the renin—angiotensin system. Clin Sci. 1995;88:433–437. doi: 10.1042/cs0880433. [DOI] [PubMed] [Google Scholar]
- 38.Jourdan T, Godlewski G, Cinar R, et al. Activation of the Nlrp3 inflammasome in infiltrating macrophages by endocannabinoids mediates beta cell loss in type 2 diabetes. Nat Med. 2013;19:1132–1140. doi: 10.1038/nm.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim D, Lee J, Lee S, et al. Predicting unintended effects of drugs based on off-target tissue effects. Biochem Biophys Res Commun. 2016;469:399–404. doi: 10.1016/j.bbrc.2015.11.095. [DOI] [PubMed] [Google Scholar]
- 40.Englen MD, Valdez YE, Lehnert NM, et al. Granulocyte/macrophage colony-stimulating factor is expressed and secreted in cultures of murine L929 cells. J Immunol Methods. 1995;184:281–283. doi: 10.1016/0022-1759(95)00136-x. [DOI] [PubMed] [Google Scholar]
- 41.Koya D, Lee IK, Ishii H, et al. Prevention of glomerular dysfunction in diabetic rats by treatment with d-alpha-tocopherol. J Am Soc Nephrol. 1997;8:426–435. doi: 10.1681/ASN.V83426. [DOI] [PubMed] [Google Scholar]
- 42.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.