Abstract
The relative importance of sarcopenia and its individual components as independent predictors of mortality in the dialysis population have not been determined. We estimated whole-body muscle mass using pre-dialysis bioimpedance spectroscopy measurements in 645 ACTIVE/ADIPOSE enrolled prevalent hemodialysis patients from San Francisco and Atlanta. Low muscle mass was defined as two standard deviations below sex-specific means for young adults from NHANES and indexed to height2, body weight, body surface area, or body mass index. We evaluated the association of sarcopenia (low muscle mass) by four indexing methods, weak handgrip strength, and slow gait speed with mortality. Seventy-eight deaths were observed during a mean follow-up of 1.9 years. Sarcopenia was not significantly associated with mortality after adjusting for covariates. No muscle mass criteria were associated with death, regardless of indexing metrics. In contrast, having weak grip strength or slow walking speed was associated with mortality in the adjusted model. Only gait slowness significantly improved the predictive accuracy for death with an increase in C-statistic from 0.63 to 0.68. However, both gait slowness and hand grip weakness significantly improved the net reclassification index compared to models without performance measures (50.5% for slowness and 33.7% for weakness), whereas models with muscle size did not. Neither sarcopenia nor low muscle mass by itself was a better predictor of mortality than functional limitation alone in patients receiving hemodialysis. Thus, physical performance measures, including slow gait speed and weak hand grip strength, were associated with mortality even after adjustment for muscle size and other confounders.
Keywords: mortality, sarcopenia, muscle mass, handgrip strength, gait speed, hemodialysis
INTRODUCTION
Although muscle mass is a major determinant of muscle function, muscle strength and size are not solely dependent upon each other.1 Therefore, sarcopenia is currently defined as generalized loss of skeletal muscle mass combined with reduced strength or physical performance.2–4 The rationale for use of two criteria is that decline in muscle strength may occur much more rapidly than the concomitant loss of muscle mass, and maintenance or even gain of muscle mass might not prevent functional deficits (in strength or speed).5 Accordingly, defining sarcopenia only in terms of reduced muscle size is probably inadequate. The accelerated process of protein catabolism induced by metabolic acidosis, (unresolved) uremia, pro-inflammatory cytokines, and the dialysis procedure itself may result in accelerated degradation of lean mass and lead to sarcopenia in patients with ESRD.6 Sarcopenia has been associated with adverse clinical outcomes including physical disability, functional limitation, and all-cause mortality in community-dwelling older people.7–9 However, whether and how the recommended methods of defining sarcopenia in the healthy elderly population should be best applied to patients on dialysis has not been carefully examined. Specifically, the extent to which low muscle mass and functional limitations contribute to higher mortality in the dialysis population is not clear.
Preliminary data suggest that muscle size may be less closely associated with mortality than functional status,10 but this result might depend on how muscle mass is measured and low muscle mass defined. Various normalization metrics, reference populations (e.g., young healthy individuals versus age-matched controls), and instruments to estimate muscle quantity (e.g., bioelectrical impedance analysis, BIA; dual x-ray absorptiometry, DXA) have been applied by investigators around the world.11 Although height-squared is commonly employed as an indexing metric of muscle mass,2, 3, 12 overweight or obese individuals with low muscle mass relative to their size may not be classified as sarcopenic by this method,13, 14 and low muscle mass based on criteria that adjust for both height and weight may be more strongly associated with weakness and poor physical performance than low muscle mass based on methods using either height or weight alone.15 Therefore, some experts have recommended indexing muscle mass to height- and weight-adjusted metrics of body size (e.g., body mass index [BMI], body surface area [BSA]). The Foundation for the National Institutes of Health (FNIH) Sarcopenia Project recently published suggested cutpoints for clinically relevant low muscle mass adjusted for BMI, derived from a large, diverse sample of older individuals.16
We conducted a prospective cohort study of prevalent hemodialysis patients and analyzed data that includes measurements of height and weight, as well as estimation of muscle mass using bioelectrical impedance spectroscopy (BIS) and tests of physical performance. The goal of the study was to compare the relative importance of sarcopenia, low muscle mass by four indexing methods (height2, percentage of body weight, BSA, and BMI), reduced muscle strength, and slow walking speed as predictors of mortality risk.
RESULTS
Characteristics of participants
Characteristics of the participants are shown in Table 1. The average age was 56.7±14.5 years; 41.4% were women. Sixty-two percent of participants were black, 24% were white, and 44% had diabetes. Median dialysis vintage was 2.8 (25th, 75th percentile 1.3, 5.4) years. Muscle mass, handgrip strength, and gait speed were significantly higher among men. The prevalence of low muscle mass ranged from 12.2 to 37.3% in men and 2.3 to 25.5% in women depending on the criterion used to index total-body muscle mass. The prevalence of weakness was not significantly different between men and women (30.6% vs 28.8%, respectively, p=0.63). In contrast, slow walking speed was almost twice as common among women as among men (24.7% vs 48.3%, p<0.001). Patients with sarcopenia (low muscle mass by all indexing methods accompanied by weak handgrip strength or slow gait speed by FNIH criteria) were older and had significantly lower muscle contents and serum creatinine concentrations than those without sarcopenia (Table 2 and Supplemental Table 1).
Table 1.
Patient characteristics
| Parameters | Total (n=645) | Men (n=378) | Women (n=267) | P value |
|---|---|---|---|---|
| Age, years | 56.7 (14.5) | 55.5 (14.3) | 58.5 (14.5) | 0.01 |
| Black, % | 61.5 | 59.0 | 65.2 | 0.11 |
| Diabetes, % | 43.9 | 39.4 | 50.2 | 0.01 |
| CAD, % | 8.8 | 10.3 | 6.7 | 0.11 |
| CHF, % | 18.8 | 19.6 | 17.6 | 0.53 |
| Dialysis vintage, years | 2.8 (1.3–5.4) | 2.6 (1.2–5.2) | 2.9 (1.4–5.9) | 0.20 |
| BMI, kg/m2 | 28.1 (6.9) | 27.4 (6.4) | 29.2 (7.6) | 0.001 |
| BSA, m2 | 1.9 (0.3) | 1.9 (0.2) | 1.8 (0.2) | <0.001 |
| Body fat, % | 29.9 (10.2) | 25.5 (9.2) | 36.1 (8.2) | <0.001 |
| Total muscle mass (kg) | 26.6 (6.5) | 29.0 (6.0) | 23.1 (5.6) | <0.001 |
| Handgrip strength, kg | 26.4 (10.6) | 31.5 (10.2) | 19.2 (5.9) | <0.001 |
| Gait speed, m/s | 0.9 (0.3) | 1.0 (0.3) | 0.9 (0.3) | <0.001 |
| Serum creatinine, mg/dl | 8.4 (2.7) | 8.9 (2.9) | 7.6 (2.3) | <0.001 |
| Serum albumin, g/dl | 4.0 (0.4) | 4.0 (0.4) | 4.0 (0.3) | 0.08 |
| Serum prealbumin, g/dl | 29.9 (7.3) | 30.2 (7.7) | 29.4 (6.6) | 0.14 |
| Prevalence of low muscle mass* by each indexing method, % | ||||
| Muscle mass/height2 | 8.1 | 12.2 | 2.3 | <0.001 |
| Muscle mass/BW (*100) | 25.3 | 27.8 | 21.7 | 0.08 |
| Muscle mass/BSA | 32.4 | 37.3 | 25.5 | 0.002 |
| Muscle mass/BMI | 25.0 | 24.9 | 25.1 | 0.95 |
| Prevalence of low muscle strength**, % (n) | 29.9 | 30.6 | 28.8 | 0.63 |
| Prevalence of slow gait speed† ,% (n) | 34.5 | 24.7 | 48.3 | <0.001 |
BMI, body mass index; BSA, body surface area; BW, body weight; CAD, coronary artery disease; CHF, congestive heart failure
Data are presented as mean (SD) and median (25th to 75th).
p<0.05 consider significantly different between men and women
Presence of low muscle mass defined as muscle mass ≥2SDs below normal mean of young adults. The mean-2SD values for men and women are 7.89 and 6.05 kg/m2 for muscle mass/height2, 32.68 and 27.85 % for muscle mass/body weight (%), 14.31 and 11.64 kg/m2 for muscle mass/BSA, 0.97 and 0.72 m2 for muscle mass/BMI, respectively.
Defined as handgrip strength <26 and <16 kg in men and women, respectively.
Defined as gait speed ≤ 0.8 m/s.
Table 2.
Characteristics of sarcopenic patients (low muscle mass groups according to each indexing method and weakness) and deaths during follow-up
| Variables | Low muscle mass by height2 and weakness | Low muscle mass by BW and weakness | Low muscle mass by BSA and weakness | Low muscle mass by BMI and weakness | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||
| non-sarcopenic (n=618) | sarcopenic (n=25) | P | non-sarcopenic (n=570) | sarcopenic (n=73) | P | non-sarcopenic (n=541) | sarcopenic (n=102) | P | non-sarcopenic (n=553) | sarcopenic (n=90) | P | |
| Black (%) | 62.5 | 36.0 | 0.01 | 66.0 | 26.0 | <0.001 | 66.4 | 35.3 | <0.001 | 67.3 | 25.6 | <0.001 |
| Male (%) | 57.4 | 84.0 | 0.01 | 58.1 | 61.6 | 0.56 | 56.6 | 68.6 | 0.02 | 58.4 | 58.9 | 0.93 |
| Diabetes (%) | 43.5 | 56.0 | 0.22 | 41.9 | 60.3 | 0.003 | 42.0 | 54.9 | 0.02 | 41.4 | 60.0 | 0.001 |
| Age (year) | 55.9±14.1 | 75.9±9.9 | <0.001 | 54.3±13.3 | 75.2±9.1 | <0.001 | 53.8±13.4 | 72.2±9.5 | <0.001 | 54.1±13.4 | 72.6±9.9 | <0.001 |
| Muscle metric (units vary) | 9.5±1.9 | 7.1±0.7 | <0.001 | 33.7±4.2 | 29.2±2.3 | <0.001 | 14.4±1.8 | 12.4±1.3 | <0.001 | 1.0±0.2 | 0.8±0.1 | <0.001 |
| BMI (kg/m2) | 28.4±7.0 | 21.8±2.0 | <0.001 | 28.0±7.0 | 29.5±6.4 | 0.09 | 28.8±7.3 | 25.0±3.7 | <0.001 | 28.1±7.1 | 28.6±6.3 | 0.52 |
| Percent fat (%) | 30.0±10.2 | 25.9±10.7 | 0.045 | 29.1±10.4 | 35.6±6.3 | <0.001 | 29.8±10.5 | 30.4±8.9 | 0.56 | 29.2±10.4 | 33.8±8.2 | 0.0001 |
| Serum creatinine (g/dl) | 8.5±2.7 | 6.6±2.2 | 0.001 | 8.6±2.7 | 6.6±2.0 | <0.001 | 8.7±2.8 | 7.0±2.1 | <0.001 | 8.7±2.7 | 6.8±2.1 | <0.001 |
| Serum albumin (g/dl) | 4.0±0.4 | 3.8±0.3 | 0.03 | 4.0±0.4 | 3.9±0.4 | 0.01 | 4.0±0.3 | 3.9±0.4 | 0.005 | 4.0±0.4 | 4.0±0.3 | 0.31 |
| Death during follow-up (%) | 69 (11.2) | <10 (<40.0) | <0.001 | 61 (10.7) | 17 (23.3) | 0.002 | 55 (10.2) | 23 (22.5) | <0.001 | 58 (10.5) | 20 (22.2) | 0.002 |
| Death rate (per 100 patient- years) | 5.9 [4.6, 7.4] | 24.7 [12.8, 47.4] | <0.001 | 5.6 [4.4, 7.2] | 13.9 [8.7, 22.4] | 0.001 | 5.3 [4.1, 6.9] | 13.4 [8.9, 20.2] | 0.0001 | 5.5 [4.2, 7.1] | 13.3 [8.6, 20.6] | 0.0004 |
Data are presented as mean ± SD, n (%), and [95% confidence interval]
Patients who were classified as having low muscle mass by percentage of body weight, BSA, and BMI combined with weakness were significantly less likely to be black and had a higher prevalence of diabetes. Sarcopenic patients with low muscle mass by height2 and BSA accompanied with either weakness or slowness had significantly lower BMI than those without sarcopenia. In contrast, weak patients with low muscle mass indexed to body weight and BMI had significantly higher percent body fat (35.6±6.3 vs 29.1±10.4%, and 33.8±8.2 vs 29.2±10.4%, p<0.001) than those with no weakness and normal muscle mass by these criteria.
Association of sarcopenia, muscle mass, strength, and physical performance with mortality
Seventy-eight deaths (12.1%) were observed during a mean follow-up period of 1.9 years (range 0.1–3.2 years). The incidence rate of death was 6.4 per 100 person-years; 95% CI 5.1–8.0. Sarcopenic patients had significantly higher mortality rate compared with those without sarcopenia (Table 2 and Supplemental Table 1) and had a significantly higher risk of death in unadjusted models (Table 3). However, in adjusted models, none of the definitions of sarcopenia that included weakness were significantly associated with death, whereas patients with low muscle mass indexed to height2 and BMI combined with slowness were more than twice as likely to die relative to individuals with normal muscle mass and no slowness in the fully adjusted model (HR 2.92; 95% CI 1.33–6.41, p=0.01 and HR 2.51; 95% CI 1.41–4.66, p=0.002, respectively).
Table 3.
Association analysis of sarcopenia and death
| Methods | Unadjusted | Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| HR (95%CI) | p | HR (95%CI) | p | HR (95%CI) | p | HR (95%CI) | p | |
| Low muscle mass by each criteria and low grip strength | ||||||||
|
| ||||||||
| Low muscle mass by Height2 | 4.23 (2.11–8.49) | 0.001 | 2.61 (1.18–5.73) | 0.02 | 2.83 (1.27–6.33) | 0.01 | 2.23 (0.99–5.00) | 0.05 |
| + weakness | C = 0.55 | C = 0.64 | C = 0.65 | C = 0.73 | ||||
| Low muscle mass by BW | 2.51 (1.46–4.31) | 0.001 | 1.60 (0.82–3.10) | 0.17 | 1.59 (0.82–3.07) | 0.17 | 1.24 (0.63–2.43) | 0.54 |
| + weakness | C = 0.56 | C = 0.63 | C = 0.64 | C = 0.72 | ||||
| Low muscle mass by BSA | 2.58 (1.58–4.20) | 0.001 | 1.77 (0.97–3.22) | 0.06 | 1.83 (1.00–3.33) | 0.05 | 1.53 (0.84–2.78) | 0.16 |
| + weakness | C = 0.58 | C = 0.64 | C = 0.65 | C = 0.73 | ||||
| Low muscle mass by BMI | 2.46 (1.48–4.09) | 0.001 | 1.69 (0.91–3.14) | 0.10 | 1.72 (0.92–3.20) | 0.09 | 1.65 (0.88–3.08) | 0.12 |
| + weakness | C = 0.56 | C = 0.62 | C = 0.64 | C = 0.72 | ||||
|
| ||||||||
| Low muscle mass by each criteria and slow gait speed | ||||||||
|
| ||||||||
| Low muscle mass by Height2 | 4.86 (2.42–9.76) | 0.001 | 3.23 (1.52–6.85) | 0.002 | 3.31 (1.54–7.12) | 0.002 | 2.92 (1.33–6.41) | 0.01 |
| + slowness | C = 0.56 | C = 0.64 | C = 0.65 | C = 0.73 | ||||
| Low muscle mass by BW | 2.67 (1.61–4.40) | 0.001 | 1.94 (1.09–3.45) | 0.03 | 1.85 (1.03–3.31) | 0.04 | 1.56 (0.85–2.83) | 0.15 |
| + slowness | C = 0.57 | C = 0.64 | C = 0.64 | C = 0.72 | ||||
| Low muscle mass by BSA | 2.63 (1.59–4.33) | 0.001 | 1.84 (1.04–3.27) | 0.04 | 1.79 (1.01–3.17) | 0.04 | 1.46 (0.83–2.58) | 0.19 |
| + slowness | C = 0.58 | C = 0.64 | C = 0.64 | C = 0.73 | ||||
| Low muscle mass by BMI | 3.35 (2.05–5.45) | 0.001 | 2.62 (1.48–4.66) | 0.001 | 2.55 (1.43–4.54) | 0.002 | 2.51 (1.41–4.66) | 0.002 |
| + slowness | C = 0.58 | C = 0.64 | C = 0.64 | C = 0.73 | ||||
BW, body weight; BMI, body mass index; BSA, body surface area; C, C-statistic; HR, hazard ratio; 95% CI, 95% confidence interval.
Model 1: Adjusted for age, sex, and race; Model 2: Adjusted for age, sex, race, and comorbidities (diabetes mellitus, congestive heart failure, coronary artery disease); Model 3: Further adjusted for serum albumin.
We then assessed whether individual components of sarcopenia (reduced muscle mass, weakness, or slowness) were independent predictors of mortality. In unadjusted analysis, low muscle mass by all indexing methods was associated with significantly higher mortality compared with normal muscle mass, with the lowest and highest HR being observed for muscle mass indexed to body weight (HR 1.75; 95% CI 1.10–2.78, p=0.02) and muscle mass indexed to height2 (HR 2.71; 95% CI 1.52–4.83, p=0.001), respectively. However, all of the low muscle mass criteria were attenuated after adjusting for covariates, and only muscle mass/height2 (HR 2.03; 95% CI 1.00–4.10, p=0.05) and muscle mass/BMI (HR 1.70; 95% CI 0.94–3.05, p=0.08) were of borderline statistical significance in adjusted models (Table 4). In contrast, having low grip strength (HR 1.68; 95% CI 1.01–2.79, p=0.04) or slow walking speed (HR 2.25; 95% CI 1.36–3.74, p=0.002) was associated with mortality risk even after adjusting for potential confounders including age, sex, race, comorbidities, and serum albumin concentration. Patients with weakness had a significantly higher rate of death (11.1 per 100 person-years; 95% CI 8.1–15.2) than those with normal grip strength (4.6 per 100 person-years; 95% CI 3.4–6.3, p<0.001). The death rate of patients with slow walking speed were 11.9; 95% CI 8.9–15.8 compared with 3.9 per 100 person-years; 95% CI 2.7–5.5, p<0.001 in patients having normal gait speed. The associations of sarcopenia, low muscle mass, strength, and gait speed with mortality were not modified by sex, the presence of diabetes, or obesity (p>0.1 for all interactions, Supplemental Table 2–4). However, non-elderly patients (<65 years) who were classified as having sarcopenia based on low muscle mass by height2 and BSA combined with weakness had significantly higher risk of death compared with those without sarcopenia (Supplemental Table 5).
Table 4.
Association analysis of low muscle mass by each criteria, weakness, and slowness (as categorical variables) and total muscle mass, handgrip strength, and gait speed (as continuous variables) with death
| Methods | Unadjusted | Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P | |
| Low muscle mass by each criteria, weakness, and slowness (as categorical variables) | ||||||||
|
| ||||||||
| Low muscle mass by Height2 | 2.71 (1.52–4.83) | 0.001 | 1.93 (1.00–3.72) | 0.05 | 2.22 (1.13–4.38) | 0.02 | 2.03 (1.00–4.10) | 0.05 |
| C = 0.56 | C = 0.64 | C = 0.65 | C = 0.74 | |||||
| Low muscle mass by BW | 1.75 (1.10–2.78) | 0.02 | 1.09 (0.62–1.91) | 0.76 | 1.05 (0.60–1.83) | 0.86 | 0.98 (0.56–1.74) | 0.96 |
| C = 0.56 | C = 0.63 | C = 0.63 | C = 0.72 | |||||
| Low muscle mass by BSA | 1.77 (1.14–2.77) | 0.01 | 1.06 (0.60–1.88) | 0.84 | 1.11 (0.63–1.98) | 0.71 | 1.06 (0.60–1.86) | 0.85 |
| C = 0.58 | C = 0.63 | C = 0.64 | C = 0.72 | |||||
| Low muscle mass by BMI | 2.20 (1.39–3.46) | 0.001 | 1.54 (0.87–2.73) | 0.14 | 1.52 (0.86–2.68) | 0.15 | 1.70 (0.94–3.05) | 0.08 |
| C = 0.58 | C = 0.62 | C = 0.63 | C = 0.73 | |||||
| Weakness* | 2.42 (1.55–3.77) | 0.001 | 2.04 (1.24–3.36) | 0.01 | 2.10 (1.27–3.49) | 0.004 | 1.68 (1.01–2.79) | 0.04 |
| C = 0.60 | C = 0.65 | C = 0.66 | C = 0.73 | |||||
| Slowness** | 3.09 (1.97–4.86) | 0.001 | 2.94 (1.79–4.80) | 0.001 | 2.83 (1.72–4.65) | 0.001 | 2.25 (1.36–3.74) | 0.002 |
| C = 0.63 | C = 0.68 | C = 0.68 | C = 0.74 | |||||
|
| ||||||||
| Total muscle mass, handgrip strength, and gait speed (as continuous variables)† | ||||||||
|
| ||||||||
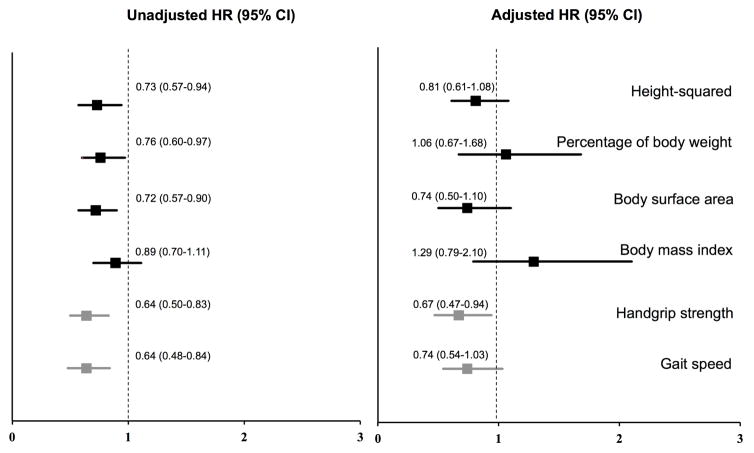
| Muscle mass/Height2, kg/m2 | 0.73 (0.57–0.94) | 0.02 | 0.85 (0.64–1.12) | 0.25 | 0.79 (0.59–1.07) | 0.12 | 0.81 (0.61–1.08) | 0.15 |
| C = 0.60 | C = 0.64 | C = 0.64 | C = 0.73 | |||||
| Muscle mass/BW (x100), % | 0.76 (0.60–0.97) | 0.03 | 0.93 (0.60–1.45) | 0.75 | 1.01 (0.64–1.60) | 0.96 | 1.06 (0.67–1.68) | 0.81 |
| C = 0.57 | C = 0.63 | C = 0.63 | C = 0.72 | |||||
| Muscle mass/BSA, kg/m2 | 0.72 (0.57–0.90) | 0.004 | 0.78 (0.52–1.16) | 0.21 | 0.71 (0.47–1.08) | 0.11 | 0.74 (0.50–1.10) | 0.14 |
| C = 0.59 | C = 0.63 | C = 0.64 | C = 0.73 | |||||
| Muscle mass/BMI, m2 | 0.89 (0.70–1.11) | 0.31 | 1.30 (0.80–2.10) | 0.28 | 1.39 (0.85–2.26) | 0.19 | 1.29 (0.79–2.10) | 0.31 |
| C = 0.52 | C = 0.64 | C = 0.65 | C = 0.73 | |||||
| Handgrip strength, kg | 0.64 (0.50–0.83) | 0.001 | 0.57 (0.41–0.79) | 0.001 | 0.55 (0.39–0.78) | 0.001 | 0.67 (0.47–0.94) | 0.02 |
| C = 0.60 | C = 0.67 | C = 0.68 | C = 0.74 | |||||
| Gait speed, m/s | 0.64 (0.48–0.84) | 0.001 | 0.66 (0.48–0.91) | 0.01 | 0.66 (0.48–0.91) | 0.01 | 0.74 (0.54–1.03) | 0.07 |
| C = 0.62 | C = 0.66 | C = 0.66 | C = 0.72 | |||||
Model 1: Adjusted for age, sex, and race; Model 2: Adjusted for age, sex, race, and comorbidities (diabetes mellitus, congestive heart failure, coronary artery disease); Model 3: Further adjusted for serum albumin.
Weakness defined as handgrip strength <26 and <16 kg in men and women, respectively.
Slowness defined as gait speed ≤0.8 m/s.
HR per 1 standard deviation increase of muscle mass indexed to each criteria, handgrip strength, and gait speed
When we compared the discrimination of the Cox models using C-statistics, only slowness significantly improved the predictive accuracy for death beyond demographic characteristics with an increase in the C-statistic from 0.63 to 0.68, p=0.004 (Table 5). However, slowness and weakness each individually improved discrimination compared to the adjusted models without performance measures (overall continuous NRI of 50.5%; 95% CI 24.3–73.0% for slowness and 33.7%; 95% CI 9.8–62.7% for weakness).
Table 5.
The comparison of Harrell’ C statistics of the adjusted Cox proportional hazard model and continuous net reclassification improvement (NRI) of baseline and enhanced model for predicting risk of death.
| Models | Harrell’C statistics | Continuous NRI | |
|---|---|---|---|
|
|
|
||
| C-statistic | P value† | Overall (%) with 95% CI | |
| Base model: age, sex, race, comorbidities* | 0.63 | reference | |
|
| |||
| Base model + low muscle mass, weakness, and slowness as dichotomous variables | |||
|
| |||
| low muscle mass by height2 | 0.65 | 0.17 | 4.7 (−22.9, 29.2) |
| low muscle mass by BW | 0.63 | 0.56 | −6.0 (−33.5, 27.2) |
| low muscle mass by BSA | 0.64 | 0.30 | 13.8 (−34.6, 35.0) |
| low muscle mass by BMI | 0.63 | 0.49 | 15.4 (−28.5, 42.4) |
| weakness | 0.66 | 0.56 | 33.7 (9.8, 62.7) |
| slowness | 0.68 | 0.004 | 50.5 (24.3, 73.0) |
|
| |||
| Base model + sarcopenia, defined as low muscle mass combined with weakness | |||
|
| |||
| low muscle mass by height2 and weakness | 0.65 | 0.31 | −8.4 (−28.5, 20) |
| low muscle mass by BW and weakness | 0.64 | 0.28 | 2.8 (−32.1, 27.5) |
| low muscle mass by BSA and weakness | 0.65 | 0.41 | 14.3 (−25.5, 37.6) |
| low muscle mass by BMI and weakness | 0.64 | 0.18 | 1.7 (−26.7, 31.5) |
|
| |||
| Base model + sarcopenia, defined as low muscle mass combined with slowness | |||
|
| |||
| low muscle mass by height2 and slowness | 0.65 | 0.28 | −7.1 (−28.1, 19.7) |
| low muscle mass by BW and slowness | 0.64 | 0.23 | 0.2 (−28.1, 32.0) |
| low muscle mass by BSA and slowness | 0.64 | 0.33 | 5.2 (−25.5, 31.1) |
| low muscle mass by BMI and slowness | 0.64 | 0.74 | 11.8 (−10.5, 39.0) |
|
| |||
| Base model + total muscle mass, handgrip strength, and gait speed as continuous variables | |||
|
| |||
| muscle mass/height2 | 0.64 | 0.28 | 9.9 (−25.9, 34.2) |
| muscle mass/BW | 0.63 | 0.15 | −28.5 (−32.7, 35.7) |
| muscle mass/BSA | 0.64 | 0.70 | 10.3 (−24.1, 38.6) |
| muscle mass/BMI | 0.65 | 0.17 | −5.7 (−31.3, 31.6) |
| handgrip strength | 0.68 | 0.03 | 32.0 (6.8, 59.2) |
| gait speed | 0.66 | 0.01 | 18.8 (−12.5, 49.9) |
BMI, body mass index; BSA, body surface area; b.s.CI, bootstrap confidence interval
Comorbidities including diabetes mellitus, congestive heart failure, and coronary artery disease.
p<0.05 consider significantly different between each model and the based model.
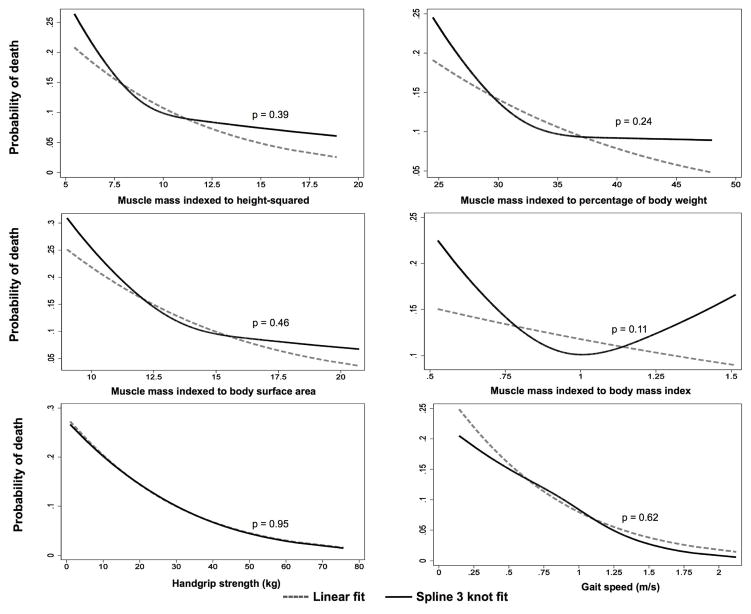
When we examined associations of total muscle mass by each indexing method and risk of death using cubic splines, we found them to be fairly linear without obvious cutpoints. The relations between probability of death and muscle mass indexed by each method were not significantly different from linear models without splines (Figure 1). Similarly, the probability of death across the range of handgrip strength and gait speed demonstrated relatively linear associations without thresholds suitable for dichotomizing (Figure 1). We therefore performed analyses in which the measures of muscle size and function were treated as continuous variables. Higher total muscle mass (per 1 SD) according to muscle mass indexed to height2, body weight, and BSA were all associated with lower hazard of death. However, these associations were no longer significant in adjusted models (Table 4; Figure 2). By contrast, higher muscle strength and faster gait speed were associated with lower likelihood of death after adjusting for covariates. Each 1 SD of stronger handgrip strength and faster gait speed was associated with a HR of death of 0.64; 95% CI 0.50–0.83, p=0.001 and 0.64; 95% CI 0.48–0.84, p=0.001, respectively). Models with handgrip strength as a linear predictor had significantly higher C-statistics than the base model adjusted for demographic characteristics and comorbidities (0.68 vs 0.63, p=0.03) and improved the overall continuous NRI (30.2; 95% CI 6.8–59.2%), but models with gait speed and muscle size did not (Table 5).
Figure 1.
Linear and restricted cubic spline analyses of association of probability of death and total muscle mass by each indexing method, handgrip strength and gait speed. P-values are for the comparison between linear and spline curves of associations of the probability of death with total muscle mass by each indexing method, handgrip strength and gait speed.
Figure 2.
Unadjusted and fully adjusted hazard ratio of death (age, race, sex, diabetes mellitus, coronary artery disease, congestive heart failure, and serum albumin) per 1 SD of total muscle mass by each criterion, handgrip strength, and gait speed among all participants.
DISCUSSION
We found that sarcopenia by most definitions and low muscle mass, regardless of indexing method, were not significantly associated with mortality after adjustment for covariates. In contrast, slow gait speed and low muscle strength were significantly associated with mortality among patients on maintenance hemodialysis.
The lack of association of most sarcopenia definitions with all-cause mortality in the adjusted model in our study differed from the results of a 7-year follow-up study in a very elderly population,17 in which sarcopenic participants had a 2–3 fold higher risk of death compared with their non-sarcopenic counterparts. Albumin appeared to mitigate the association between sarcopenia and survival. It is possible that albumin serves as a marker of nutritional status or general health and may capture some of the same information as sarcopenia. However, our results are in agreement with a previous study by Kim et al.,18 which reported that sarcopenia was not associated with higher risk of death in community-dwelling elderly women. The lack of unified criteria and consensus cutpoints of low muscle mass, weak grip strength, and slow gait speed might account for the conflicting results among studies addressing sarcopenia and its associations with adverse outcomes. Furthermore, we found some indication that sarcopenia may be worse among younger individuals, which might be expected as they would not have accrued as much age-related muscle mass loss and therefore may have experienced greater disease-related loss.
The most appropriate method of indexing muscle mass remains unknown, although recent evidence suggests that low muscle mass indexed to body size has more robust associations with poor physical performance than criteria adjusted only for height or body weight.13, 15, 16 Because having low muscle mass is a fundamental component of sarcopenia, we sought to find the low muscle mass criterion that was most suitable based on its performance as an independent predictor of mortality. We hypothesized that height2 might not be the best method to index muscle mass among patients receiving dialysis, particularly among obese individuals as it would not recognize patients with “sarcopenic obesity,” a condition recently identified as sarcopenia that co-occurs with excess adiposity.19 However, contrary to our expectations, our study showed that although low muscle mass based on other indexing metrics identified a higher proportion and categorized different patients as “sarcopenic,”13 no indexing method appeared superior in terms of discrimination, at least with respect to survival, after adjusting for demographic and case mix factors. Although previous epidemiologic studies20, 21 in hemodialysis patients have reported that higher muscle mass, representing by higher quartile of mid-arm muscle circumference, was significantly associated with greater survival, those studies analyzed the data by using arbitrary cutpoints for high muscle mass derived from within their cohort rather than cutpoints derived from normative data from young, healthy individuals as recommended in the definition of sarcopenia.2, 3, 11 In addition, reliance on anthropometric measures in those studies could lead to misclassification because of interobserver variation22 and fatty infiltration of muscle compartments that occurs with atrophy.23 Isoyama and colleagues10 also recently found that patients new to dialysis with low muscle mass (defined as appendicular muscle mass by DXA/height2 at least 2 SDs below the sex-specific mean of young adults) were not at higher risk of mortality.
We considered that the lack of association of muscle size with mortality could be because thresholds determined in healthy elderly populations are not appropriate in the dialysis population. If loss of muscle mass occurs by different mechanisms or in different patterns among patients on dialysis than among healthy elderly, it may not be as well detected by some methods of assessment and may not have the same association with outcomes. For example, atrophy preferentially affects type 2 muscle fibers compared to type 1 muscle fibers in patients on dialysis, shifting the fiber type distribution towards slower fibers,24 and atrophy can be accompanied by replacement of muscle with fatty and fibrotic infiltration.25 In addition, there is evidence that the hydration of muscle varies across the dialysis cycle, at least in the calf muscles.26 Such variation would add variability to measures of muscle mass by all techniques, but perhaps more by BIS than others, and likely contributes to the difficulty of applying a particular threshold of muscle mass in defining sarcopenia in the dialysis population. Perhaps for these reasons, we were not able to identify alternative optimal cutpoints to define levels of atrophy associated with higher mortality by any indexing method. In addition, insufficient study power may be an issue, as our study had relatively few deaths and a limited period of follow-up. A longer period of time with larger sample size may be needed in order to better determine the association of muscle size with mortality.
Our data suggest that muscle strength and gait speed may be more relevant predictors of survival than muscle size. Patients with slow walking speed and low handgrip strength had significantly higher hazard of death in the adjusted analysis compared with having low muscle mass alone. In particular, models with grip strength as a linear predictor and slow gait speed as a dichotomous predictor significantly improved the C-statistic and continuous NRI compared to the base model. Our results are in agreement with those of Yoda and colleagues,27 who reported that diminished handgrip strength per unit of muscle mass was a significant predictor of death in dialysis patients regardless of total arm muscle mass. Likewise, Vogt and colleagues28 found that grip strength was an independent risk factor of all-cause mortality, whereas muscle mass assessed by mid-arm muscle circumference did not differ significantly among surviving and non-surviving patients on dialysis. Prospective studies have shown that both weak handgrip strength10, 29 and decreased lower extremity muscle strength30 were independent risk factors for mortality among patients undergoing hemodialysis, although various cutpoints have been applied to define weakness. Given that handgrip strength is easy and inexpensive to measure and is not affected by hydration status, it could be assessed in dialysis facilities to improve predictions of survival.
Slowness was also consistently associated with mortality in our cohort when we defined slowness as <0.8 m/s, in accordance with other analyses in healthy elderly individuals31 and the FNIH-adopted definition of mobility limitation.32 Moreover, models containing slowness significantly improved the predictive power of the models based on both the C-statistics and continuous NRI. In a pooled analysis of several large cohorts among community-dwelling older adults,33 gait speed <0.8 m/s was significantly associated with 5- and 10-year survival in all studies. Our finding that the association between gait speed and mortality was fairly linear in our dialysis population is consistent with the observation that slow gait speed using a different cutpoint of walking speed less than 0.6 m/s was also associated with higher risk of mortality within one year.34
Taken together, functional limitations (in strength or speed) were associated with mortality among patients receiving hemodialysis, whereas muscle size appeared to be less important with regard to survival. Handgrip strength and gait speed, both easy to perform in clinical practice, provide integrated information about muscle size, strength, activation, and neural control, with the possible additional component of balance for gait speed.
Some limitations of our study should be taken into account. We used total-body rather than appendicular muscle mass, which may be have a more robust association with muscle function. Compared with DXA, BIS is an inexpensive and clinically feasible method of estimation of muscle mass. However, this technique has some drawbacks, mainly due to patients’ hydration status in the predialysis setting, which may lead to overestimation of muscle mass and thus lower the number of patients classified as having low muscle mass in our cohort. Nevertheless, we performed BIS at a midweek session and used an equation to calculate muscle mass that was derived from pre-dialysis BIS measures and separately estimates intracellular and extracellular fluid, allowing for excess extracellular fluid. However, we did not further validate this equation in our study population. Furthermore, we used a single measurement of muscle mass and physical performance to predict the subsequent risk of death, whereas changes in muscle mass or physical performance over time or other methods of estimating muscle mass, such as DXA or serum creatinine, might be more informative.
In conclusion, neither sarcopenia nor low muscle mass by itself was a better predictor of mortality than functional limitation alone in patients receiving hemodialysis. Physical performance measures, including slow gait speed and weak grip strength, were associated with mortality even after adjustment for muscle size and other confounders.
MATERIALS AND METHODS
Study design and participants
ACTIVE/ADIPOSE (A Cohort To Investigate the Value of Exercise/Analyses Designed to Investigate the Paradox of Obesity and Survival in ESRD) was a United States Renal Data System (USRDS) Special Study conducted by the Nutrition and Rehabilitation/Quality of Life Special Studies Centers35 that enrolled 771 prevalent adult hemodialysis patients from seven dialysis centers in the San Francisco Bay Area and seven centers from the Atlanta, Georgia metropolitan area from June 2009 and August 2011. Eligible participants were over 18 years of age, receiving maintenance hemodialysis for at least 3 months, English or Spanish-speaking, and able to provide informed consent. The exclusion criteria were participants scheduled for living donor kidney transplantation or planning to relocate to another center or change to peritoneal dialysis within the next 6 months. The study was approved by the Institutional Review Boards (No.10-02719) at the University of California San Francisco and Emory University, and all participants provided written informed consent.
Study coordinators interviewed participants, abstracted recent clinical and laboratory data from medical records, and measured body composition by BIS and physical performance on the same day prior to the start of the dialysis session. We collected a blood sample at the time of testing. Serum albumin was measured in duplicate using a Polychem Nephelometer (Walpole, MA, USA) and the values averaged. We linked patients’ ACTIVE/ADIPOSE data to data from the ESRD Medical Evidence Report (Center for Medicare & Medicaid Services Form 2728) available in the USRDS at the time of dialysis initiation. In addition, we used information from coordinators’ chart review, and participants were considered to have comorbid conditions if they were listed on the 2728 or in the medical record. Participants who had data for body composition and muscle strength available (n=645, 84%) were included in these analyses.
Measurement of Body Composition
Study personnel measured height using a stadiometer and recorded weight to the nearest 0.1 kg immediately prior to a midweek dialysis session. We evaluated total-body muscle mass using multifrequency whole-body BIS, performed immediately before the same midweek dialysis session, using a portable device that scans 256 frequencies between 4 and 1000 kHz (SFB7; ImpediMed, San Diego, CA). All jewelry, metallic equipment, and socks were removed prior to the testing. Patients were placed in a supine position at least 10 minutes before measurement. We placed electrodes in a tetrapolar configuration using the wrist and ankle on the side opposite the dialysis vascular access with proximal and distal electrodes 5 cm apart and avoided contacting the patients’ arms and legs with one another. Ten consecutive measures were performed within a 1-minute period. We used a program based on the Cole-Cole model to calculate extracellular and intracellular resistance. We estimated total body water and extracellular volume using the resistance extrapolated to infinite and zero frequency, respectively.36 The equation for calculation of total-body muscle mass (kg) was 9.52 + 0.331 x whole body BIS-derived intracellular volume (L) + 0.180 x pre dialysis weight (kg) + 2.77 (if male) – 0.113 x age (years). The results of this equation gave a value of R2 = 0.937, p<0.0001 compared to muscle mass from whole-body MRI in a cohort of patients on hemodialysis.37
Definitions of low muscle mass, weakness, and physical performance
We indexed the BIS-derived total-body muscle mass to height2, body weight, BSA, and BMI. For these calculations, we used the mean of the last three post-dialysis weight measurements within the past 7 days. Body surface area was derived from the Du Bois formula using post- dialysis weight.38 Body mass index was calculated as post-dialysis weight divided by height in meters squared. We defined low muscle mass as muscle mass of two standard deviations (SD) or more below sex-specific means of healthy young adults (18–49 years) based on each indexing strategy as recommend by the European Working Group on Sarcopenia in Older People.2 We obtained reference populations and cutoff points of BIS-derived whole body muscle mass from the National Health and Nutrition Examination Survey (NHANES) 2003–200439 using a Stata specific survey command that accounts for the stratified, multistage, probability sampling survey design of NHANES.
Weakness was based on measurement of handgrip strength using a hydraulic hand dynamometer (BASELINE®; Fabrication Enterprise, Inc., Irvington, NY, USA) immediately before a dialysis session. For participants with an indwelling dialysis catheter, we tested handgrip strength on both sides and used the higher of the two. We performed three trials with a 15-second rest period between each trial. We discarded the first trial as a “warm up” session, and the highest force exerted in the latter two trials was recorded. We defined low muscle strength as handgrip strength of less than 26 kg in men and 16 kg in women.40
We asked participants to walk a marked 15-foot course at their usual pace before a dialysis session and used the faster of the two walks for analysis. We defined low physical performance as gait speed of less than or equal to 0.8 m/sec according to the FNIH criteria.32
Mortality follow-up
We evaluated the association of weakness, poor physical performance, and low muscle mass according to different indexing metrics with all-cause mortality. We obtained vital status and date of death from the USRDS. We censored follow-up time at recovery of renal function, kidney transplantation, or end of study (August 31, 2013).
Statistical analysis
We described patient characteristics using mean ± SD for normally distributed or median (interquartile range) for non-normally distributed variables and proportions for categorical variables. We compared patients’ characteristics using chi squared, unpaired t-tests, and Mann-Whitney U test as appropriate. We used Cox proportional hazards models to estimate the association between sarcopenia based on low muscle mass by each definition and its components (i.e., low muscle mass, low muscle strength, and low physical performance individually) as categorical variables with mortality. Because appropriate cutpoints have not been established in the dialysis population, we also assessed the associations of total muscle mass, handgrip strength, and gait speed as continuous variables with mortality. To explore non-linear associations among muscle mass, grip strength and gait speed and mortality, we created cubic splines for the continuous variables associations and used the Wald test to test whether these associations deviated from a linear relationship. We examined a model that included age, sex, and race (model 1). We then added diabetes mellitus, congestive heart failure, and coronary artery disease as comorbidities (model 2) and serum albumin concentration (model 3). We tested for interactions among muscle mass, handgrip strength, and gait speed as categorical and continuous variables and sex. We used Harrell’s C and net reclassification index (NRI) to compare the discrimination of the survival models.41, 42 We calculated the continuous NRI with 95% bootstrap CI to quantify the improvement in discrimination offered by adding each new predictor on the base model adjusted for comorbidities. We conducted all analyses in Stata 13 (StataCorp LP, College Station, TX), and P values less than 0.05 were considered statistically significant.
Supplementary Material
Supplemental Table 1 Characteristics of sarcopenic patients (low muscle mass groups according to each indexing method and slowness) and deaths during follow-up
Supplemental Table 2 Association between sarcopenia, low muscle mass by each criterion, weakness, and slowness with mortality among men and women
Supplemental Table 3 Association between sarcopenia, low muscle mass by each criterion, weakness, and slowness with mortality among patients with and without diabetes
Supplemental Table 4 Association between sarcopenia, low muscle mass by each criterion, weakness, and slowness with mortality among non-obese (BMI<30 kg/m2) and obese (BMI≥30 kg/m2) patients
Supplemental Table 5 Association between sarcopenia, low muscle mass by each criterion, weakness, and slowness with mortality among non-elderly (age <65 years) and elderly patients (age ≥65 years)
Acknowledgments
Disclaimer: The data reported here have been supplied by the USRDS. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of the US government.
Funding/Support: This work was supported through contracts N01-DK-7-0005 and KD-7-5004 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).This work has been made possible in part by an International Society of Nephrology (ISN) funded Fellowship to Dr. Kittiskulnam. Dr. Johansen’s effort was also supported by K24DK085153 from the NIDDK. Dr. Carrero-Roig acknowledges grant support from Stockholm County Council and the Swedish Research Council. Dr. Delgado’s work is supported by the Department of Veterans Affairs, Clinical Science Research and Development Program under Career Development Award 1IK2CX000527-01A2.
Footnotes
DISCLOSURE
All the authors declared no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stenvinkel P, Carrero JJ, von Walden F, et al. Muscle wasting in end-stage renal disease promulgates premature death: established, emerging and potential novel treatment strategies. Nephrol Dial Transplant. 2016;31:1070–1077. doi: 10.1093/ndt/gfv122. [DOI] [PubMed] [Google Scholar]
- 2.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12:249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muscaritoli M, Anker SD, Argiles J, et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) "cachexia-anorexia in chronic wasting diseases" and "nutrition in geriatrics". Clin Nutr. 2010;29:154–159. doi: 10.1016/j.clnu.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 6.Carrero JJ, Stenvinkel P, Cuppari L, et al. Etiology of the protein-energy wasting syndrome in chronic kidney disease: a consensus statement from the International Society of Renal Nutrition and Metabolism (ISRNM) J Ren Nutr. 2013;23:77–90. doi: 10.1053/j.jrn.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Arango-Lopera VE, Arroyo P, Gutierrez-Robledo LM, et al. Mortality as an adverse outcome of sarcopenia. J Nutr Health Aging. 2013;17:259–262. doi: 10.1007/s12603-012-0434-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Landi F, Liperoti R, Fusco D, et al. Sarcopenia and mortality among older nursing home residents. J Am Med Dir Assoc. 2012;13:121–126. doi: 10.1016/j.jamda.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Rolland Y, Czerwinski S, Abellan Van Kan G, et al. Sarcopenia: its assessment, etiology, pathogenesis, consequences and future perspectives. J Nutr Health Aging. 2008;12:433–450. doi: 10.1007/BF02982704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Isoyama N, Qureshi AR, Avesani CM, et al. Comparative associations of muscle mass and muscle strength with mortality in dialysis patients. Clin J Am Soc Nephrol. 2014;9:1720–1728. doi: 10.2215/CJN.10261013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carrero JJ, Johansen KL, Lindholm B, et al. Screening for muscle wasting and dysfunction in patients with chronic kidney disease. Kidney Int. 2016;90:53–66. doi: 10.1016/j.kint.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 12.Morley JE, Abbatecola AM, Argiles JM, et al. Sarcopenia with limited mobility: an international consensus. J Am Med Dir Assoc. 2011;12:403–409. doi: 10.1016/j.jamda.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kittiskulnam P, Carrero JJ, Chertow GM, et al. Sracopenia among patients receiving hemodialysis: weighing the evidence. J Cachexia Sarcopenia Muscle. 2016 doi: 10.1002/jcsm.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newman AB, Kupelian V, Visser M, et al. Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc. 2003;51:1602–1609. doi: 10.1046/j.1532-5415.2003.51534.x. [DOI] [PubMed] [Google Scholar]
- 15.Meng NH, Li CI, Liu CS, et al. Sarcopenia Defined by Combining Height- and Weight-Adjusted Skeletal Muscle Indices is Closely Associated With Poor Physical Performance. J Aging Phys Act. 2015;23:597–606. doi: 10.1123/japa.2014-0036. [DOI] [PubMed] [Google Scholar]
- 16.Cawthon PM, Peters KW, Shardell MD, et al. Cutpoints for low appendicular lean mass that identify older adults with clinically significant weakness. J Gerontol A Biol Sci Med Sci. 2014;69:567–575. doi: 10.1093/gerona/glu023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Landi F, Cruz-Jentoft AJ, Liperoti R, et al. Sarcopenia and mortality risk in frail older persons aged 80 years and older: results from ilSIRENTE study. Age Ageing. 2013;42:203–209. doi: 10.1093/ageing/afs194. [DOI] [PubMed] [Google Scholar]
- 18.Kim JH, Lim S, Choi SH, et al. Sarcopenia: an independent predictor of mortality in community-dwelling older Korean men. J Gerontol A Biol Sci Med Sci. 2014;69:1244–1252. doi: 10.1093/gerona/glu050. [DOI] [PubMed] [Google Scholar]
- 19.Honda H, Qureshi AR, Axelsson J, et al. Obese sarcopenia in patients with end-stage renal disease is associated with inflammation and increased mortality. Am J Clin Nutr. 2007;86:633–638. doi: 10.1093/ajcn/86.3.633. [DOI] [PubMed] [Google Scholar]
- 20.Noori N, Kopple JD, Kovesdy CP, et al. Mid-arm muscle circumference and quality of life and survival in maintenance hemodialysis patients. Clin J Am Soc Nephrol. 2010;5:2258–2268. doi: 10.2215/CJN.02080310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang CX, Tighiouart H, Beddhu S, et al. Both low muscle mass and low fat are associated with higher all-cause mortality in hemodialysis patients. Kidney Int. 2010;77:624–629. doi: 10.1038/ki.2009.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubbieri G, Mossello E, Di Bari M. Techniques for the diagnosis of sarcopenia. Clin Cases Miner Bone Metab. 2014;11:181–184. [PMC free article] [PubMed] [Google Scholar]
- 23.Sakkas GK, Kent-Braun JA, Doyle JW, et al. Effect of diabetes mellitus on muscle size and strength in patients receiving dialysis therapy. Am J Kidney Dis. 2006;47:862–869. doi: 10.1053/j.ajkd.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 24.Andersen JL. Muscle fibre type adaptation in the elderly human muscle. Scand J Med Sci Sports. 2003;13:40–47. doi: 10.1034/j.1600-0838.2003.00299.x. [DOI] [PubMed] [Google Scholar]
- 25.Johansen KL, Shubert T, Doyle J, et al. Muscle atrophy in patients receiving hemodialysis: effects on muscle strength, muscle quality, and physical function. Kidney Int. 2003;63:291–297. doi: 10.1046/j.1523-1755.2003.00704.x. [DOI] [PubMed] [Google Scholar]
- 26.Sawant A, House AA, Chesworth BM, et al. Association between muscle hydration measures acquired using bioelectrical impedance spectroscopy and magnetic resonance imaging in healthy and hemodialysis population. Physiol Rep. 2015 doi: 10.14814/phy2.12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoda M, Inaba M, Okuno S, et al. Poor muscle quality as a predictor of high mortality independent of diabetes in hemodialysis patients. Biomed Pharmacother. 2012;66:266–270. doi: 10.1016/j.biopha.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Vogt BP, Borges MC, Goes CR, et al. Handgrip strength is an independent predictor of all-cause mortality in maintenance dialysis patients. Clin Nutr. 2016 doi: 10.1016/j.clnu.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 29.Matos CM, Silva LF, Santana LD, et al. Handgrip strength at baseline and mortality risk in a cohort of women and men on hemodialysis: a 4-year study. J Ren Nutr. 2014;24:157–162. doi: 10.1053/j.jrn.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 30.Matsuzawa R, Matsunaga A, Wang G, et al. Relationship between lower extremity muscle strength and all-cause mortality in Japanese patients undergoing dialysis. Phys Ther. 2014;94:947–956. doi: 10.2522/ptj.20130270. [DOI] [PubMed] [Google Scholar]
- 31.Cheung CL, Lam KS, Cheung BM. Evaluation of Cutpoints for Low Lean Mass and Slow Gait Speed in Predicting Death in the National Health and Nutrition Examination Survey 1999–2004. J Gerontol A Biol Sci Med Sci. 2016;71:90–95. doi: 10.1093/gerona/glv112. [DOI] [PubMed] [Google Scholar]
- 32.McLean RR, Shardell MD, Alley DE, et al. Criteria for clinically relevant weakness and low lean mass and their longitudinal association with incident mobility impairment and mortality: the foundation for the National Institutes of Health (FNIH) sarcopenia project. J Gerontol A Biol Sci Med Sci. 2014;69:576–583. doi: 10.1093/gerona/glu012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kutner NG, Zhang R, Huang Y, et al. Gait Speed and Mortality, Hospitalization, and Functional Status Change Among Hemodialysis Patients: A US Renal Data System Special Study. Am J Kidney Dis. 2015;66:297–304. doi: 10.1053/j.ajkd.2015.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.US Renal Data System. USRDS 2011 Annual Data Report B, MD. National Institute of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2011. [Google Scholar]
- 36.De Lorenzo A, Andreoli A, Matthie J, et al. Predicting body cell mass with bioimpedance by using theoretical methods: a technological review. J Appl Physiol (1985) 1997;82:1542–1558. doi: 10.1152/jappl.1997.82.5.1542. [DOI] [PubMed] [Google Scholar]
- 37.Kaysen GA, Zhu F, Sarkar S, et al. Estimation of total-body and limb muscle mass in hemodialysis patients by using multifrequency bioimpedance spectroscopy. Am J Clin Nutr. 2005;82:988–995. doi: 10.1093/ajcn/82.5.988. [DOI] [PubMed] [Google Scholar]
- 38.Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. Nutrition. 1989;5:303–311. [PubMed] [Google Scholar]
- 39.National Health and Nutrition Examination Survey. [cited 12 November 2015]. Available from: http://wwwn.cdc.gov/nchs/nhanes/search/nhanes03_04.aspx.
- 40.Alley DE, Shardell MD, Peters KW, et al. Grip strength cutpoints for the identification of clinically relevant weakness. J Gerontol A Biol Sci Med Sci. 2014;69:559–566. doi: 10.1093/gerona/glu011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Newson RB. Comparing the predictive powers of survival models using Harrell's C or Somers'D. The Stata Journal. 2010;10:339–358. [Google Scholar]
- 42.Pencina MJ, D'Agostino RB, Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Statist Med. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1 Characteristics of sarcopenic patients (low muscle mass groups according to each indexing method and slowness) and deaths during follow-up
Supplemental Table 2 Association between sarcopenia, low muscle mass by each criterion, weakness, and slowness with mortality among men and women
Supplemental Table 3 Association between sarcopenia, low muscle mass by each criterion, weakness, and slowness with mortality among patients with and without diabetes
Supplemental Table 4 Association between sarcopenia, low muscle mass by each criterion, weakness, and slowness with mortality among non-obese (BMI<30 kg/m2) and obese (BMI≥30 kg/m2) patients
Supplemental Table 5 Association between sarcopenia, low muscle mass by each criterion, weakness, and slowness with mortality among non-elderly (age <65 years) and elderly patients (age ≥65 years)