Abstract
Tumor-induced osteomalacia (TIO) is a rare paraneoplastic syndrome caused by small benign tumors of mesenchymal origin also known as phosphaturic mesenchymal tumors mixed connective tissue variant. Excellent prognosis is expected with eradication of the culprit tumor. These small tumors are notoriously difficult to localize with conventional radiographic studies; this often leads to an extensive work up and prolonged morbidity. We report a patient with clinical diagnosis of TIO whose culprit tumor was localized with Ga-68 DOTATOC PET/CT and MRI. Biopsy and cryoablation were performed under Ga-68 DOTATOC PET/CT guidance. Autoradiography of the biopsy specimen was performed and showed in situ correlation between Ga-68 DOTATOC uptake and histopathology with millimeter resolution.
Case report
A 45-year-old female presented with a 5-year history of diffuse body pain and fractures of several bones including the right calcaneus, over 10 vertebrae and several ribs. None of the fractures were associated with trauma. She was wheelchair-bound for a prolonged period and ambulated with a cane at baseline. Patient did not have history of excess vitamin A use. About 10 years prior to start of her diffuse bone pain, she had history of resection of a papillary serous tumor of the left fallopian tube with low malignant potential.
On physical exam, the most painful areas included the left hip trochanteric region, lower back, bilateral knees and right ankle. She had antalgic gait, slight weakness of motor strength in extremities and decreased range of motion of the left hip with pain at terminal flexion of approximately 125 degrees and internal and external rotation to 20 degrees. Laboratory values were as follows: negative rheumatoid factor (12 IU/mL), positive antinuclear antibody at 1:80, normal serum calcium (8.8 mg/dL), high serum alkaline phosphatase (230 U/L), low serum phosphorus (0.9 mg/dL), urine phosphorus (139.4 mg/dL), high fibroblast growth factor-23 (FGF-23) (279 RU/mL), low serum 1, 25 vitamin D (13 pg/mL), normal 25-hydroxyD (63 ng/mL), low normal 24 hour urine calcium (49mg / 24 hours), normal calcium and parathyroid hormone (PTH) (8.9 mg/dL and 83.5pg/mL) and normal thyroid stimulating hormone (0.74 mIU/L). The fractional excretion of phosphate was calculated at 25% (inappropriately high in the presence of hypophosphatemia). Diagnosis of TIO was made based on high urine phosphate excretion in the setting of low serum phosphorus, low 1, 25 vitamin D and high FGF-23. Patient was treated with: high dose calcitriol 1.25 mcg orally twice daily and elemental phosphorus 250 mg three tablets every six hours ergocalciferol 50,000 IU once weekly and cinacalcet 30mg daily while investigating the location of the culprit tumor. This medical regimen increased the serum phosphate to the low normal range (2.6 mg/dL [2.5–4.2]), reduced serum calcium and parathyroid hormone (to 8.5 mg/dL and 15.5 pg/mL, respectively), did not induce hypercalciuria and did produce some symptomatic improvement such that the patient was able to ambulate independently and did not suffer further clinical fractures. As with prior reports, the addition of cinacalcet appeared to be a key part of the medical regimen (Geller JL et al JBMR 2007). The patient did admit to interruption in her quality of life due to the high pill burden.
Comprehensive imaging workup was performed. Dual-Energy x-ray absorptiometry (DXA) scan showed normal bone density. Plain x-rays, contrast enhanced CT scan, radionuclide bone scan, In-111 pentetreotide scan (OctreoScan) and FDG PET CT scan all failed to show a definite culprit tumor. Gynecologic surgical oncology team evaluated the patient to assess any role for further surgery based on patient’s remote history of a benign left pelvic mass. A pelvic MRI performed for this reason was unremarkable. No further gynecologic surgery was advised. The patient underwent complete dermatologic exam. A punch biopsy of a tender nodule in the left ankle revealed angiolipoma. To further evaluate questionable findings on FDG PET/CT, the patient underwent dedicated MR examinations of the right femur, left tibia/fibula, left foot and pancreas. None of these examinations revealed evidence of a mass lesion while multiple healing stress fractures were identified. Ga-68 DOTATOC PET/CT showed focal increased uptake in a small focus in the right anterolateral sixth rib (SUV 18.5) with no CT correlate (Figure 1). To further investigate this finding MRI of the chest was performed and confirmed a small enhancing marrow lesion in the same location. Orthopedic surgical oncology advised image guided biopsy for confirmation. Due to suboptimal design of the only available MR-compatible bone biopsy needle (InVivo, Schwerin, Germany) for a non-expansile rib and also because of inability to treat intra procedural pneumothorax in interventional MR suite, it was decided to perform the biopsy using Ga-68 DOTATOC PET/CT guidance. Due to logistical issues with this tracer, it was planned to perform ablation in the same session after confirmation of an adequate biopsy specimen. Autoradiography of biopsy specimen was added to standard on-site cytopathologic assessment to increase diagnostic yield of biopsy specimen prior to ablation.
Figure 1.
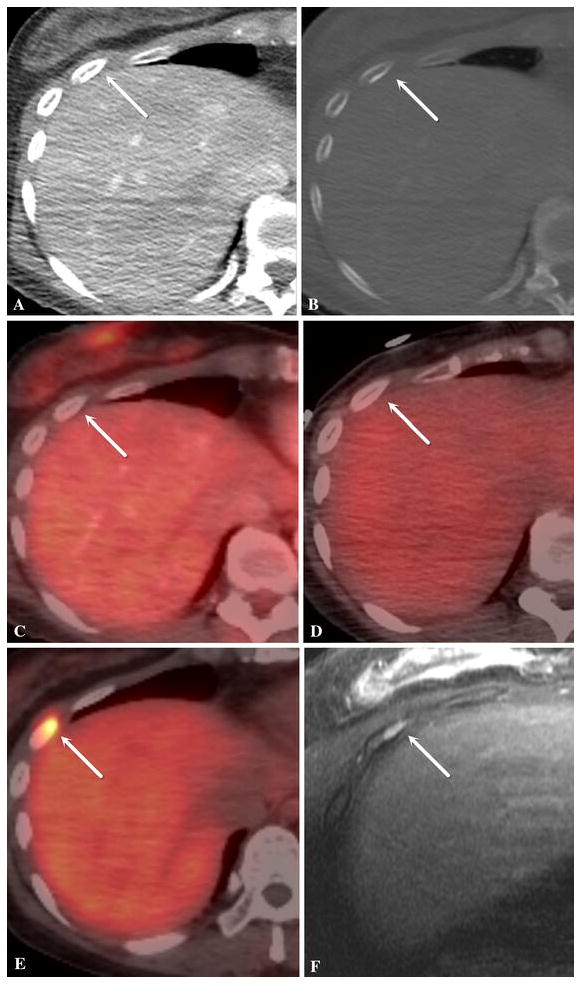
Axial images of contrast enhanced CT scan in soft tissue window (a) and bone window (b), FDG PET/ CT (c), In-111 pentetreotide scan (OctreoScan) SPECT/CT (d), Ga-68 DOTATOC PET/CT (e) and contrast-enhanced T1-weighted with fat saturation MRI (f) of the right anterolateral sixth rib (white arrows). The MR examination was done in prone position. The lesion is seen only on Ga-68 DOTATOC PET/CT and MRI. It had no CT correlate.
Intervention
Following intravenous injection of 4.99 mCi Ga-68 DOTATOC and an approximately 46 minute uptake period, low-dose CT and PET images of the lower chest/upper abdomen (one field of view) were acquired with a Discovery 690 PET/CT scanner (GE Healthcare, Waukesha, WI, USA) with the patient in the fasted state. No contrast material was administered. This intervention was performed under general anesthesia with a single prophylactic dose of Ancef to cover skin flora as part of institutional standards. Using Ga-68 DOTATOC PET images fused with CT and CT fluoroscopic images, a 13-gauge Madison Bone Biopsy MiniKit (Laurane Medical, Le Pradet, France) was advanced into the lesion via an anterior medial approach. A total of two core specimens were obtained while the rib was drilled through-and-through using the biopsy needle (Figure 2). Autoradiography was performed on the first specimen by using an imaging plate (Fujifilm, BAS, MS20-25) which was scanned using a Typhoon FLA 7000 laser scanner (GE Healthcare) 20 minutes after the exposure. Green or blue stains were applied to each end of the biopsy specimen to help retain orientation between PET/CT biopsy image, autoradiography and surgical pathology slides (Figure 3). After on-site cytopathologic confirmation of lesional specimen and confirmation of tracer activity in the specimen with the autoradiography a single Perc-17 cryo applicator (Endocare Inc., Austin, TX, USA) was advanced into the lesion through the introducer needle of the biopsy needle. The introducer needle was then retracted back over the applicator to stay a few centimeters away from the ice ball. Pneumodissection and local warm compress were used to protect the overlying skin. Two freeze cycles were performed at 100% power for 12 and 8 minutes respectively. Each freeze cycle was followed by a 6-minute thaw cycle. The first thaw cycle was passive and the second cycle was active. Target temperatures of minus 145 and minus147 degrees centigrade were recorded in the first and second freeze cycles respectively. Postprocedural chest x-rays showed no evidence of pneumothorax.
Figure 2.
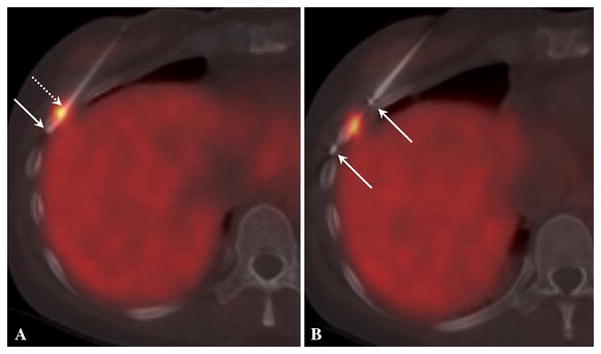
a) Ga-68 DOTATOC PET/CT guided biopsy. The arrows depict the first biopsy specimen within the needle. The specimen ends were stained green (dotted arrow) and blue (solid arrow) to retain orientation between this image, autoradiography and surgical pathology. b) Ga-68 DOTATOC PET/CT guided cryoablation. A new PET acquisition is used for this fused image. The arrows depict the markers at both ends of active tip of cryoapplicator. The ice ball properly covered the lesion and intended margin during both freeze cycles (not shown).
Figure 3.
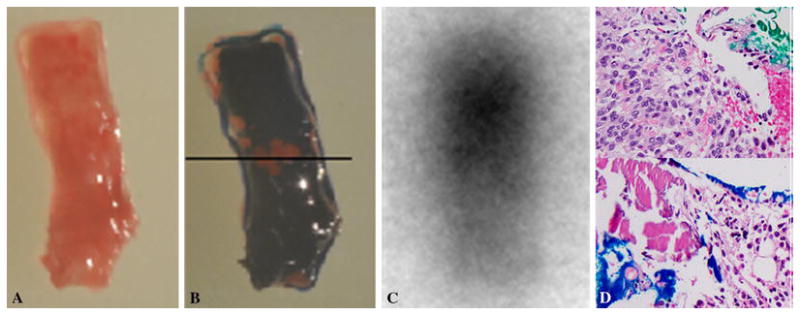
a) color photograph of biopsy specimen in an orientation close to figure 2a. b) The end of the specimen close to the tip of needle (solid arrow on figure 2a) is stained blue at the bottom of this image and the other end (dotted arrow on figure 2a) is stained green on top of the image. The black line depicts the two stained ends. c) autoradiography of biopsy specimen in the same orientation shows intense activity toward the end stained green. d) Select images of each end of specimen show tumor cells on the end stained green and normal marrow elements on the blue stained end. The green end contains monomorphic spindle cells with no atypia or abnormal mitotic activity representing phosphaturic mesenchymal tumor mixed connective tissue variant. The findings on Ga-68 DOTATOC PET/CT guided biopsy, autoradiography and surgical pathology are concordant.
Outcomes
The patient was discharged home the next day after an uneventful recovery. She was taken off all oral supplements at the time of discharge. Surgical pathology confirmed phosphaturic mesenchymal tumor mixed connective tissue variant. The location of tumor cells on the first specimen was concordant with findings on Ga-68 DOTATOC PET/CT images and QABS. By immunohistochemistry, the neoplastic cells did not express PAX8, cytokeratin and CD34 (Figure 3). Serum FGF-23 was normal on blood work drawn the next day (82 RU/mL; normal range < 180). Over the course of several days after ablation the patient progressively felt better with complete resolution of diffuse bone pain in less than 2 weeks. All biochemical abnormalities including serum phosphorus (3.4 mg/dL), alkaline phosphatase (54 U/L) and serum 1, 25 vitamin D level (70.6 pg/mL) were normal on the first available blood work drawn between 5–14 days after cryoablation. Routine follow-up contrast enhanced CT scan obtained 6 weeks after cryoablation showed a small mixed lytic-sclerotic focus in the right anterolateral sixth rib representing cryoablation effect. The tumor was deemed to be in remission.
Discussion
Tumor-induced osteomalacia is a rare acquired paraneoplastic syndrome caused by overexpression of fibroblast growth factor-23 (FGF-23) which inhibits renal phosphate reabsorption and also directly affects bone mineralization, causing severe osteomalacia [1, 2]. Small benign tumors of mesenchymal origin (bones 53%, soft tissues 45% and skin 3%) also known as phosphaturic mesenchymal tumor mixed connective tissue (PMTMCT) variant are the causative agents [3]. Patients present with progressive musculoskeletal pain, fractures and muscular weakness. The diagnosis is clinical and biochemical based on high alkaline phosphatase, normal calcium and parathyroid hormone, high FGF-23, normal or low 1, 25 vitamin D, low serum phosphorus with concomitant high fractional excretion of phosphate in the urine. Rare malignant cases are reported [4]. Localization of culprit tumor confirms the diagnosis and is essential for definitive treatment. Difficulty in localizing the culprit tumor due to conspicuity and nonspecific symptoms can lead to prolonged morbidity. We found, similar to other reports, that cinacalcet has an important role in the medical management of this disorder, which may be the only viable option if tumors localization fails [5]. Cinacalcet induces relative hypoparathyroidism and was initially investigated based on the hypothesis that the phosphaturic effect of FGF-23 is partially dependent on PTH. The small size and low grade nature of PMTMCT often make it difficult to localize the culprit tumor by conventional imaging studies. FDG PET/CT, whole body MRI and In-111 pentetreotide SPECT/CT are among the proposed diagnostic methods. Somatostatin tumor receptor 2A (SSTR2A) is highly expressed in PMTMCT [6, 7]. Negative staining for both FGF-23 and SSTR2A is suggested as a definitive rule-out test for PMTMCT [7]. Galium-68 DOTATOC is a radiolabeled DOTA-conjugated peptide that together with Ga-68 DOTATATE and Ga-68 DOTANOC represent a new generation of radiolabeled somatostatin analogues. The TATE, TOC or NOC peptides selectively bind to SSTR2 and Ga-68, a positron emitter enables PET imaging. DOTATATE (TOC or NOC) PET/CT is emerging as an ideal diagnostic method for PMTMCT compared to FDG PET/CT and In-111 octreotide SPECT/CT due to its superior detection rate [8, 9]. Quantitative autoradiography of biopsy specimens (QABS) obtained by F-18 FDG PET/CT guidance has been shown to accurately correlate with histopathologic findings [10]. Due to the established correlation between autoradiography and histopathology, utilization of QABS is promising in increasing the diagnostic yield for this type of treatment where definitive diagnosis is needed prior to same-session cryoablation. This justifies efforts to reduce the time needed for specimen autoradiography and to calibrate the detector response for Ga-68 in order to quantify the activity in specimens extracted under Ga-68 DOTATOC PET/CT. A supplemental treatment regimen with phosphate and vitamin D is maintained while investigating the location of culprit tumor. Surgical resection is the treatment of choice [11]. Radiation treatment is considered for unresectable tumors [12]. A few successful image guided ablation of PMTMCT have been reported [13, 14]. Image guided ablation has been shown effective for small benign musculoskeletal tumors such as osteoblastoma and osteoid osteoma [15]. Excellent prognosis is expected with complete eradication of culprit tumor and metastasis or local recurrence are rare [11].
This case shows successful utilization of Ga-68 DOTATOC PET/CT for imaging guidance of biopsy and cryoablation of an otherwise radiographically occult PMTMCT. Although surgical resection is the treatment of choice, image guided biopsy and ablation may be considered for select patients with tumor-induced osteomalacia.
Acknowledgments
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
68Ga-DOTATOC was provided by Memorial Sloan Kettering Cancer Center institutional protocol 14-226 “68Ga-DOTATOC for imaging of Neuroendocrine tumors: Expanded access trial”.
Quantitative autobiography of biopsy specimen was made available by Memorial Sloan Kettering Cancer Center institutional protocol 12-072 “Evaluation and Improvement of PET Accuracy for Tumor Targeting Using Autoradiography and Pathology Tests of Biospecimens from Patients Undergoing PET/CT Guided Biopsy”.
Footnotes
Conflict of Interest: On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical Approval: All procedures performed were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent: Informed consent was obtained for all procedures.
Contributor Information
Majid Maybody, Memorial Sloan-Kettering Cancer Center, Interventional Radiology Service, 1275 York Avenue, M276C, New York, NY, USA 10065.
Ravinder K Grewal, Memorial Sloan-Kettering Cancer Center, Department of Radiology, Molecular Imaging and Therapy Service.
John H Healey, Memorial Sloan-Kettering Cancer Center, Department of Surgery, Orthopedic Surgical Oncology Service.
Cristina R Antonescu, Memorial Sloan-Kettering Cancer Center, Department of Pathology.
Louise Fanchon, Memorial Sloan-Kettering Cancer Center, Department of Physics.
Sinchun Hwang, Memorial Sloan-Kettering Cancer Center, Department of Radiology.
Jorge A Carrasquillo, Memorial Sloan-Kettering Cancer Center, Department of Radiology, Molecular Imaging and Therapy Service.
Assen Kirov, Memorial Sloan-Kettering Cancer Center, Department of Physics.
Azeez Farooki, Memorial Sloan-Kettering Cancer Center, Department of Medicine.
References
- 1.de Jan Beur SM. Tumor-induced osteomalacia. JAMA: J Am Med Assoc. 2005;294(10):1260–7. doi: 10.1001/jama.294.10.1260. [DOI] [PubMed] [Google Scholar]
- 2.Imanishi Y, Hashimoto J, Ando W, et al. Matrix extracellular phosphoglycoprotein is expressed in causative tumors of oncogenic osteomalacia. J Bone Miner Metab. 2012;30(1):93–9. doi: 10.1007/s00774-011-0290-8. [DOI] [PubMed] [Google Scholar]
- 3.Folpe AL, Fanburg-Smith JC, Billings SD, et al. Most osteomalacia-associated mesenchymal tumors are a single histopathologic entity: an analysis of 32 cases and a comprehensive review of the literature. Am J Surg Pathol. 2004;28(1) doi: 10.1097/00000478-200401000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Honda R, Kawabata Y, Ito S, et al. Phosphaturic mesenchymal tumor, mixed connective tissue type, non-phosphaturic variant: report of a case and review of 32 cases from the Japanese published work. J Dermatol. 2014;41(9):845–9. doi: 10.1111/1346-8138.12602. [DOI] [PubMed] [Google Scholar]
- 5.Geller JL, Khosravi A, Kelly MH, et al. Cinacalcet in the management of tumor-induced osteomalacia. J Bone Miner Res. 2007 Jun;22(6):931–7. doi: 10.1359/jbmr.070304. [DOI] [PubMed] [Google Scholar]
- 6.Duet M, Kerkeni S, Sfar R, et al. Clinical impact of somatostatin receptor scintigraphy in the management of tumor-induced osteomalacia. Clin Nucl Med. 2008;33(11) doi: 10.1097/RLU.0b013e31818866bf. [DOI] [PubMed] [Google Scholar]
- 7.Houang M, Clarkson A, Sioson L, et al. Phosphaturic mesenchymal tumors show positive staining for somatostatin receptor 2A (SSTR2A) Hum Pathol. 2013;44(12):2711–8. doi: 10.1016/j.humpath.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 8.Agrawal K, Bhadada S, Mittal BR, et al. Comparison of 18F-FDG and 68Ga DOTATATE PET/CT in localization of tumor causing oncogenic osteomalacia. Clin Nucl Med. 2015;40(1):e6–e10. doi: 10.1097/RLU.0000000000000460. [DOI] [PubMed] [Google Scholar]
- 9.Breer S, Brunkhorst T, Beil FT, et al. 68Ga DOTA-TATE PET/CT allows tumor localization in patients with tumor-induced osteomalacia but negative 111In-octreotide SPECT/CT. Bone. 2014;64:222–7. doi: 10.1016/j.bone.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 10.Fanchon LM, Dogan S, Moreira AL, et al. Feasibility of in situ, high-resolution correlation of tracer uptake with histopathology by quantitative autoradiography of biopsy specimens obtained under 18F-FDG PET/CT guidance. J Nucl Med. 2015 Apr;56(4):538–44. doi: 10.2967/jnumed.114.148668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hautmann AH, Hautmann MG, Kölbl O, Herr W. Martin Fleck3,4 Tumor-Induced Osteomalacia: an Up-to-Date Review. Curr Rheumatol Rep. 2015;17:512. doi: 10.1007/s11926-015-0512-5. [DOI] [PubMed] [Google Scholar]
- 12.Hautmann AH, Schroeder J, Wild P, et al. Tumor-induced osteomalacia: increased level of FGF-23 in a patient with a phosphaturic mesenchymal tumor at the tibia expressing periostin. Case Rep Endocrinol. 2014;2014:729387. doi: 10.1155/2014/729387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hesse E, Rosenthal H, Bastian L. Radiofrequency ablation of a tumor causing oncogenic osteomalacia. N Engl J Med. 2007;357:422–424. doi: 10.1056/NEJMc070347. (letter) [DOI] [PubMed] [Google Scholar]
- 14.Tutton S, Olson E, King D, Shaker JL. Successful treatment of tumor-induced osteomalacia with CT-guided percutaneous ethanol and cryoablation. J Clin Endocrinol Metab. 2012 Oct;97(10):3421–5. doi: 10.1210/jc.2012-1719. [DOI] [PubMed] [Google Scholar]
- 15.Weber MA, Sprengel SD, Omlor GW, Lehner B, Wiedenhöfer B, Kauczor HU, Rehnitz C. Clinical long-term outcome, technical success, and cost analysis of radiofrequency ablation for the treatment of osteoblastomas and spinal osteoid osteomas in comparison to open surgical resection. Skeletal Radiology. 2015 Jul;44(7):981–993. doi: 10.1007/s00256-015-2139-z. [DOI] [PubMed] [Google Scholar]


