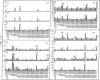
Abstract
In myasthenia gravis an autoimmune response against the nicotinic acetylcholine receptor (AChR) occurs. The alpha subunit of the AChR contains both the epitope(s) that dominates the antibody response (main immunogenic region) and epitopes involved in T helper cell sensitization. In this study, overlapping synthetic peptides corresponding to the complete AChR alpha-subunit sequence were used to propagate polyclonal AChR-specific T helper cell lines from four myasthenic patients of different HLA types. Response of the T helper lines to the individual peptides was studied. Four immunodominant sequence segments were identified--i.e., residues 48-67, 101-120, 304-322, and 419-437. These regions did not include residues known to form the main immunogenic region or the cholinergic binding site, and they frequently contained sequence motifs that have been proposed to be related to T-epitope formation.
Full text
PDF

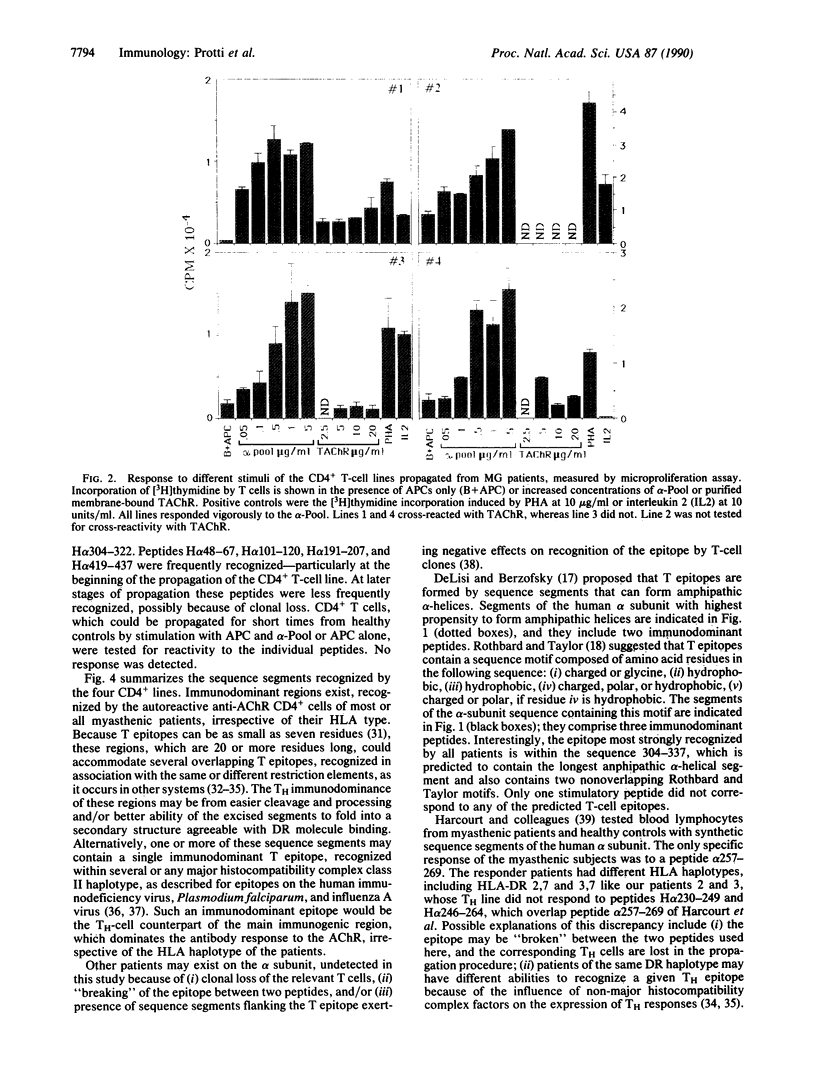
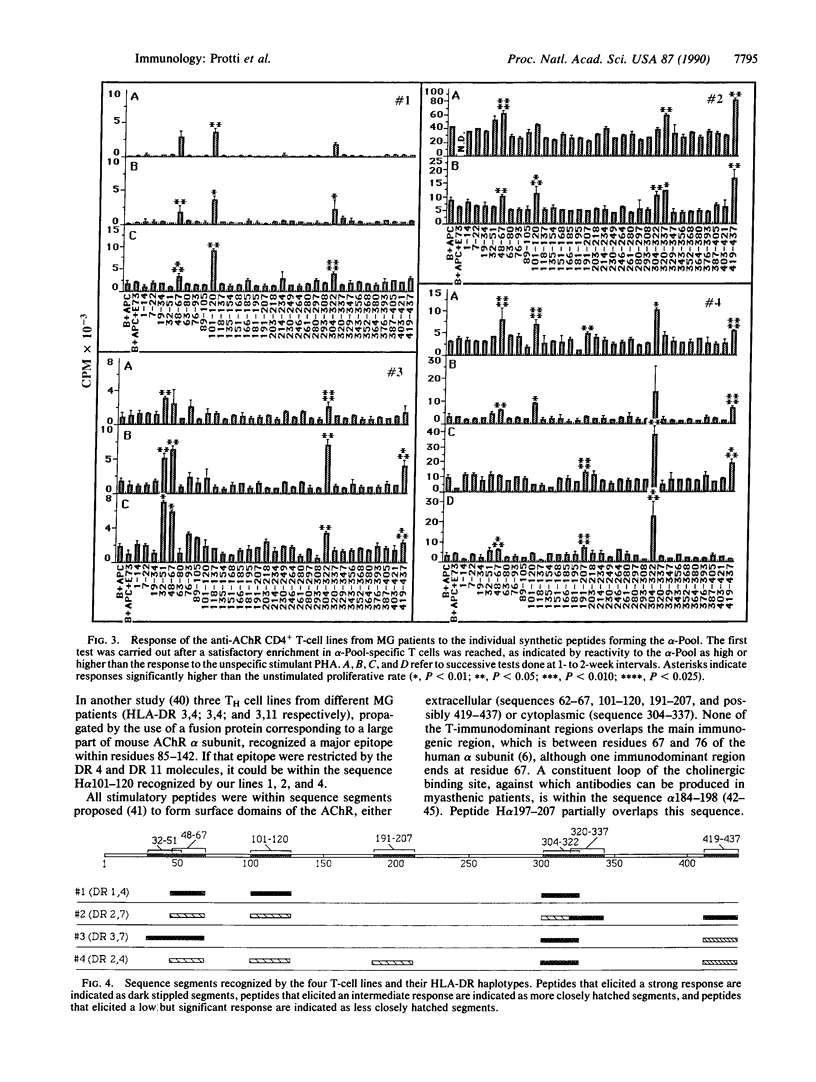

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellone M., Tang F., Milius R., Conti-Tronconi B. M. The main immunogenic region of the nicotinic acetylcholine receptor. Identification of amino acid residues interacting with different antibodies. J Immunol. 1989 Dec 1;143(11):3568–3579. [PubMed] [Google Scholar]
- Blanchard S. G., Quast U., Reed K., Lee T., Schimerlik M. I., Vandlen R., Claudio T., Strader C. D., Moore H. P., Raftery M. A. Interaction of [125I]-alpha-bungarotoxin with acetylcholine receptor from Torpedo californica. Biochemistry. 1979 May 15;18(10):1875–1883. doi: 10.1021/bi00577a005. [DOI] [PubMed] [Google Scholar]
- Brett S. J., Cease K. B., Ouyang C. S., Berzofsky J. A. Fine specificity of T cell recognition of the same peptide in association with different I-A molecules. J Immunol. 1989 Aug 1;143(3):771–779. [PubMed] [Google Scholar]
- Clark D. G., Macmurchie D. D., Elliott E., Wolcott R. G., Landel A. M., Raftery M. A. Elapid neurotoxins. Purification, characterization, and immunochemical studies of -bungarotoxin. Biochemistry. 1972 Apr 25;11(9):1663–1668. doi: 10.1021/bi00759a020. [DOI] [PubMed] [Google Scholar]
- Clerici M., Stocks N. I., Zajac R. A., Boswell R. N., Bernstein D. C., Mann D. L., Shearer G. M., Berzofsky J. A. Interleukin-2 production used to detect antigenic peptide recognition by T-helper lymphocytes from asymptomatic HIV-seropositive individuals. Nature. 1989 Jun 1;339(6223):383–385. doi: 10.1038/339383a0. [DOI] [PubMed] [Google Scholar]
- DeLisi C., Berzofsky J. A. T-cell antigenic sites tend to be amphipathic structures. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7048–7052. doi: 10.1073/pnas.82.20.7048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott J., Blanchard S. G., Wu W., Miller J., Strader C. D., Hartig P., Moore H. P., Racs J., Raftery M. A. Purification of Torpedo californica post-synaptic membranes and fractionation of their constituent proteins. Biochem J. 1980 Mar 1;185(3):667–677. doi: 10.1042/bj1850667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott J., Dunn S. M., Blanchard S. G., Raftery M. A. Specific binding of perhydrohistrionicotoxin to Torpedo acetylcholine receptor. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2576–2579. doi: 10.1073/pnas.76.6.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel A. G. Myasthenia gravis and myasthenic syndromes. Ann Neurol. 1984 Nov;16(5):519–534. doi: 10.1002/ana.410160502. [DOI] [PubMed] [Google Scholar]
- Gotti C., Frigerio F., Bolognesi M., Longhi R., Racchetti G., Clementi F. Nicotinic acetylcholine receptor: a structural model for alpha-subunit peptide 188-201, the putative binding site for cholinergic agents. FEBS Lett. 1988 Feb 8;228(1):118–122. doi: 10.1016/0014-5793(88)80598-2. [DOI] [PubMed] [Google Scholar]
- Harcourt G. C., Sommer N., Rothbard J., Willcox H. N., Newsom-Davis J. A juxta-membrane epitope on the human acetylcholine receptor recognized by T cells in myasthenia gravis. J Clin Invest. 1988 Oct;82(4):1295–1300. doi: 10.1172/JCI113729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrikson R. L., Meredith S. C. Amino acid analysis by reverse-phase high-performance liquid chromatography: precolumn derivatization with phenylisothiocyanate. Anal Biochem. 1984 Jan;136(1):65–74. doi: 10.1016/0003-2697(84)90307-5. [DOI] [PubMed] [Google Scholar]
- Hohlfeld R., Kalies I., Kohleisen B., Heininger K., Conti-Tronconi B., Toyka K. V. Myasthenia gravis: stimulation of antireceptor autoantibodies by autoreactive T cell lines. Neurology. 1986 May;36(5):618–621. doi: 10.1212/wnl.36.5.618. [DOI] [PubMed] [Google Scholar]
- Hohlfeld R., Toyka K. V., Heininger K., Grosse-Wilde H., Kalies I. Autoimmune human T lymphocytes specific for acetylcholine receptor. Nature. 1984 Jul 19;310(5974):244–246. doi: 10.1038/310244a0. [DOI] [PubMed] [Google Scholar]
- Hohlfeld R., Toyka K. V., Tzartos S. J., Carson W., Conti-Tronconi B. M. Human T-helper lymphocytes in myasthenia gravis recognize the nicotinic receptor alpha subunit. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5379–5383. doi: 10.1073/pnas.84.15.5379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghten R. A. General method for the rapid solid-phase synthesis of large numbers of peptides: specificity of antigen-antibody interaction at the level of individual amino acids. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5131–5135. doi: 10.1073/pnas.82.15.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani H., Takada S., Ueda Y., Murakawa Y., Suzuki N., Sakane T. Activation of immune regulatory circuits among OKT4+ cells by autologous mixed lymphocyte reactions. Clin Exp Immunol. 1984 May;56(2):390–398. [PMC free article] [PubMed] [Google Scholar]
- Kurnick J. T., Ostberg L., Stegagno M., Kimura A. K., Orn A., Sjöberg O. A rapid method for the separation of functional lymphoid cell populations of human and animal origin on PVP-silica (Percoll) density gradients. Scand J Immunol. 1979;10(6):563–573. doi: 10.1111/j.1365-3083.1979.tb01391.x. [DOI] [PubMed] [Google Scholar]
- Lambert L. E., Unanue E. R. Analysis of the interaction of peptide hen egg white lysozyme (34-45) with the I-Ak molecule. J Immunol. 1989 Aug 1;143(3):802–807. [PubMed] [Google Scholar]
- Levinson A. I., Zweiman B., Lisak R. P. Immunopathogenesis and treatment of myasthenia gravis. J Clin Immunol. 1987 May;7(3):187–197. doi: 10.1007/BF00915723. [DOI] [PubMed] [Google Scholar]
- Lider O., Reshef T., Beraud E., Ben-Nun A., Cohen I. R. Anti-idiotypic network induced by T cell vaccination against experimental autoimmune encephalomyelitis. Science. 1988 Jan 8;239(4836):181–183. doi: 10.1126/science.2447648. [DOI] [PubMed] [Google Scholar]
- Lindstrom J., Einarson B., Tzartos S. Production and assay of antibodies to acetylcholine receptors. Methods Enzymol. 1981;74(Pt 100):432–460. doi: 10.1016/0076-6879(81)74031-x. [DOI] [PubMed] [Google Scholar]
- Livingstone A. M., Fathman C. G. The structure of T-cell epitopes. Annu Rev Immunol. 1987;5:477–501. doi: 10.1146/annurev.iy.05.040187.002401. [DOI] [PubMed] [Google Scholar]
- Lohse A. W., Mor F., Karin N., Cohen I. R. Control of experimental autoimmune encephalomyelitis by T cells responding to activated T cells. Science. 1989 May 19;244(4906):820–822. doi: 10.1126/science.2471264. [DOI] [PubMed] [Google Scholar]
- McCarthy M. P., Earnest J. P., Young E. F., Choe S., Stroud R. M. The molecular neurobiology of the acetylcholine receptor. Annu Rev Neurosci. 1986;9:383–413. doi: 10.1146/annurev.ne.09.030186.002123. [DOI] [PubMed] [Google Scholar]
- Melms A., Schalke B. C., Kirchner T., Müller-Hermelink H. K., Albert E., Wekerle H. Thymus in myasthenia gravis. Isolation of T-lymphocyte lines specific for the nicotinic acetylcholine receptor from thymuses of myasthenic patients. J Clin Invest. 1988 Mar;81(3):902–908. doi: 10.1172/JCI113401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalek M. T., Benacerraf B., Rock K. L. Two genetically identical antigen-presenting cell clones display heterogeneity in antigen processing. Proc Natl Acad Sci U S A. 1989 May;86(9):3316–3320. doi: 10.1073/pnas.86.9.3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann D., Barchan D., Fridkin M., Fuchs S. Analysis of ligand binding to the synthetic dodecapeptide 185-196 of the acetylcholine receptor alpha subunit. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9250–9253. doi: 10.1073/pnas.83.23.9250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas J. A., Levely M. E., Mitchell M. A., Smith C. W. A 16-amino acid peptide of respiratory syncytial virus 1A protein contains two overlapping T cell-stimulating sites distinguishable by class II MHC restriction elements. J Immunol. 1989 Nov 1;143(9):2790–2796. [PubMed] [Google Scholar]
- Noda M., Furutani Y., Takahashi H., Toyosato M., Tanabe T., Shimizu S., Kikyotani S., Kayano T., Hirose T., Inayama S. Cloning and sequence analysis of calf cDNA and human genomic DNA encoding alpha-subunit precursor of muscle acetylcholine receptor. 1983 Oct 27-Nov 2Nature. 305(5937):818–823. doi: 10.1038/305818a0. [DOI] [PubMed] [Google Scholar]
- Protti M. P., Manfredi A. A., Straub C., Howard J. F., Jr, Conti-Tronconi B. M. CD4+ T cell response to the human acetylcholine receptor alpha subunit in myasthenia gravis. A study with synthetic peptides. J Immunol. 1990 Feb 15;144(4):1276–1281. [PubMed] [Google Scholar]
- Protti M. P., Manfredi A. A., Straub C., Wu X. D., Howard J. F., Jr, Conti-Tronconi B. M. Use of synthetic peptides to establish anti-human acetylcholine receptor CD4+ cell lines from myasthenia gravis patients. J Immunol. 1990 Mar 1;144(5):1711–1720. [PubMed] [Google Scholar]
- Ralston S., Sarin V., Thanh H. L., Rivier J., Fox J. L., Lindstrom J. Synthetic peptides used to locate the alpha-bungarotoxin binding site and immunogenic regions on alpha subunits of the nicotinic acetylcholine receptor. Biochemistry. 1987 Jun 16;26(12):3261–3266. doi: 10.1021/bi00386a004. [DOI] [PubMed] [Google Scholar]
- Romain P. L., Morimoto C., Daley J. F., Palley L. S., Reinherz E. L., Schlossman S. F. Reactivity of inducer cell subsets and T8-cell activation during the human autologous mixed lymphocyte reaction. Clin Immunol Immunopathol. 1984 Jan;30(1):117–128. doi: 10.1016/0090-1229(84)90012-6. [DOI] [PubMed] [Google Scholar]
- Rothbard J. B., Taylor W. R. A sequence pattern common to T cell epitopes. EMBO J. 1988 Jan;7(1):93–100. doi: 10.1002/j.1460-2075.1988.tb02787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K., Sinha A. A., Mitchell D. J., Zamvil S. S., Rothbard J. B., McDevitt H. O., Steinman L. Involvement of distinct murine T-cell receptors in the autoimmune encephalitogenic response to nested epitopes of myelin basic protein. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8608–8612. doi: 10.1073/pnas.85.22.8608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J., Raftery M. A. A simple assay for the study of solubilized acetylcholine receptors. Anal Biochem. 1973 Apr;52(2):349–354. doi: 10.1016/0003-2697(73)90036-5. [DOI] [PubMed] [Google Scholar]
- Schoepfer R., Luther M., Lindstrom J. The human medulloblastoma cell line TE671 expresses a muscle-like acetylcholine receptor. Cloning of the alpha-subunit cDNA. FEBS Lett. 1988 Jan 4;226(2):235–240. doi: 10.1016/0014-5793(88)81430-3. [DOI] [PubMed] [Google Scholar]
- Sinigaglia F., Guttinger M., Kilgus J., Doran D. M., Matile H., Etlinger H., Trzeciak A., Gillessen D., Pink J. R. A malaria T-cell epitope recognized in association with most mouse and human MHC class II molecules. Nature. 1988 Dec 22;336(6201):778–780. doi: 10.1038/336778a0. [DOI] [PubMed] [Google Scholar]
- Sun D., Qin Y., Chluba J., Epplen J. T., Wekerle H. Suppression of experimentally induced autoimmune encephalomyelitis by cytolytic T-T cell interactions. Nature. 1988 Apr 28;332(6167):843–845. doi: 10.1038/332843a0. [DOI] [PubMed] [Google Scholar]
- Tzartos S. J., Kokla A., Walgrave S. L., Conti-Tronconi B. M. Localization of the main immunogenic region of human muscle acetylcholine receptor to residues 67-76 of the alpha subunit. Proc Natl Acad Sci U S A. 1988 May;85(9):2899–2903. doi: 10.1073/pnas.85.9.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzartos S. J., Seybold M. E., Lindstrom J. M. Specificities of antibodies to acetylcholine receptors in sera from myasthenia gravis patients measured by monoclonal antibodies. Proc Natl Acad Sci U S A. 1982 Jan;79(1):188–192. doi: 10.1073/pnas.79.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban J. L., Horvath S. J., Hood L. Autoimmune T cells: immune recognition of normal and variant peptide epitopes and peptide-based therapy. Cell. 1989 Oct 20;59(2):257–271. doi: 10.1016/0092-8674(89)90288-2. [DOI] [PubMed] [Google Scholar]
- Vacchio M. S., Berzofsky J. A., Krzych U., Smith J. A., Hodes R. J., Finnegan A. Sequences outside a minimal immunodominant site exert negative effects on recognition by staphylococcal nuclease-specific T cell clones. J Immunol. 1989 Nov 1;143(9):2814–2819. [PubMed] [Google Scholar]
- Vandenbark A. A., Hashim G., Offner H. Immunization with a synthetic T-cell receptor V-region peptide protects against experimental autoimmune encephalomyelitis. Nature. 1989 Oct 12;341(6242):541–544. doi: 10.1038/341541a0. [DOI] [PubMed] [Google Scholar]
- Wilson P. T., Lentz T. L., Hawrot E. Determination of the primary amino acid sequence specifying the alpha-bungarotoxin binding site on the alpha subunit of the acetylcholine receptor from Torpedo californica. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8790–8794. doi: 10.1073/pnas.82.24.8790. [DOI] [PMC free article] [PubMed] [Google Scholar]