Abstract
Pseudomonas aeruginosa is a nosocomial pathogen colonizing patients with chronic infectious diseases and has gained resistance to all the known broad spectrum antibiotics available today. The present study showcases the antibiofilm potential of an essential oil (EO) from an underexplored Cinnamomum species namely, C. tamala, against P. aeruginosa biofilms. Furthermore, the synergistic effects of the EO along with a commercially available DNase (DNaseI) and a DNase (MBD) isolated from a marine bacterium were explored for its antibiofilm activity. The results showed that the synergized action has maximum efficacy in inhibiting young and preformed biofilms. The synergized effect of EO and DNaseI showed 70% inhibition against matured biofilms of P. aeruginosa. The EO from C. tamala also showed quorum sensing inhibitory potential as it could inhibit the swarming motility behavior of P. aeruginosa. The synergistic action of EO and DNases offers a novel alternate therapeutic strategy for combating P. aeruginosa biofilm associated infections.
Keywords: biofilm, Cinnamomum essential oil, synergism, DNase, Almora
Introduction
Essential oils (EOs) are natural plant secondary metabolites which constitute a complex mixture of volatile components found to have multiple antimicrobial properties (Ogunwande et al., 2005; Matan et al., 2006; Millezi et al., 2013). EOs containing aldehydes or phenols, such as cinnamaldehyde, citral, carvacrol, eugenol, and thymol as major components have exhibited the highest antibacterial activity, wherein the phenolic components present in the EOs are considered to be responsible for the antimicrobial property (Bassole and Juliani, 2012). Since the action of EOs and their constituents bring forth research into the development of novel biocides with broad spectrum activity to combat antimicrobial resistance, several studies are taking place worldwide in bioprospecting EOs for its anti-virulent properties (Gupta et al., 2013; Nazzaro et al., 2013; Utchariyakiat et al., 2016).
Pseudomonas aeruginosa is a notorious nosocomial pathogen which is ubiquitous in health care settings where it causes persistent and chronic infection in immunocompromised individuals (Sydnor and Perl, 2011). A majority of P. aeruginosa infections are difficult to treat since the bacterial cells protect themselves by a self-produced exopolymeric matrix called biofilms (Ribeiro et al., 2016). The exopolysaccharide (EPS) matrix of both bacterial and fungal biofilms delays the infiltration of antimicrobial agents and act as a barrier thus endorsing intrinsic resistance to several antibiotics which leads to recalcitrant infections (Chung and Toh, 2014; Nithyanand et al., 2015a,b; Frieri et al., 2016). Proteins, nucleic acids and lipids make up the major portion of the biofilm matrix and recently the presence of extracellular DNA (eDNA) has also been documented within the biofilm matrix (Okshevsky and Meyer, 2015). eDNA helps in the adhesion of the biofilm on to any solid surface (Regina et al., 2014), promotes bacterial aggregation (Das and Manefield, 2012) and stabilizes the biofilm architecture (Kreth et al., 2009). Most importantly, a recent study shows that eDNA solely supports biofilm formation by P. aeruginosa which caused Catheter associated Urinary Tract Infection (Cole et al., 2014). Biofilm formation and swarming motility are quorum sensing (QS) controlled virulence factors in P. aeruginosa. The release of eDNA and swarming motility is mediated through QS dependent mechanism involving N-acyl homoserine lactones (AHL) and Pseudomonas quinolone signaling (PQS) molecule (Rasamiravaka et al., 2015). Swarming motility is responsible for surface colonization and dissemination of the pathogen which contribute to the formation of biofilms (O’May and Tufenkji, 2011). The failure of antimicrobial therapy to eliminate biofilm associated infections (Davies, 2003) has pushed the scientific fraternity to look for alternative solutions to suppress this key virulent trait of pathogens. Natural compounds from plants and other sources have currently gained importance (Koh et al., 2013) and EOs from aromatic plants is considered as alternative antimicrobial and antibiofilm agents (Nithyanand et al., 2015c). Since deoxyribonucleases have been used to degrade eDNA in biofilms (Pressler, 2008; Nijland et al., 2010), we envisioned that the combination of EO and DNases might have an enhanced biofilm disruption activity. So the present study focuses on the exploration of the synergistic effects of the EO of Cinnamomum tamala (Buch.-Ham.) T. Nees and Eberm (Indian bay leaf/Indian cassia) and DNases to disrupt biofilms. The literature scrutiny reveals that till date, the antibiofilm potential of only the commercial cinnamon/Ceylon cinnamon (Cinnamomum zeylanicum) has so far been reported (Gupta et al., 2013; Kalia et al., 2015). The present study is the first of its kind to showcase the biofilm inhibition as well as QS inhibition potential of C. tamala, which broadens the pharmacological prospects of this wild species and the synergistic effects of EO with DNases have better biofilm disruption ability.
Materials and Methods
Test Organism
Pseudomonas aeruginosa (PAO1) wild-type strain and two clinical P. aeruginosa isolates AU07 (GenBank Accession No: JN871909) and AU09 (GenBank Accession No: JN871910) (Sethupathy et al., 2016) which were obtained as a generous gift from Dr. S. Karutha Pandian, Alagappa University were maintained in Luria Bertani (LB) agar (HiMedia, India). Prior to the experiment, the cultures were grown overnight in LB broth in Orbital shaker at 150 rpm, at 37°C. From this, 100 μl of the suspension containing 107 cells ml-1 was used for all the assays (Musthafa et al., 2011).
Source of Enzymes
Bovine pancreatic DNaseI was purchased from HiMedia and Marine Bacterial DNase (MBD) was isolated and purified from the marine bacterium Vibrio alginolyticus (GenBank Accession Number: KX458518).
Source and Analyses of Essential Oil
The leaves of C. tamala were collected from Almora, Uttarakhand, India. The leaves were partially shade dried and used for extraction of EO. On hydro-distillation, the partially shade dried leaves yielded 1.2% pale yellow color EO. After distillation, the EO was dried over by adding a pinch of anhydrous sodium sulfate and stored at 4°C until further analysis and experiments. The EO was quantitative and qualitatively analyzed by GC-FID and GC-MS following the methods and conditions used by Sriramavaratharajan et al. (2016). The individual component of the EO such as cinnamaldehyde (#W228613) and linalool (#L2602) was purchased from Sigma–Aldrich.
Determination of Minimum Inhibitory Concentration (MIC)
The Minimum Inhibitory Concentration (MIC) of the EOs were determined as per Clinical and Laboratory Standards Institute (2006) guidelines. Various concentrations of EO were prepared by mixing proportions of EO with 1.25% dimethyl sulfoxide (DMSO). Serial two-fold dilutions of the EO ranging from 1 to 20% v/v were prepared in 96-well titer plate supplemented with test culture and incubated for 24 h at 37°C. Following incubation, microtiter plates were read spectrophotometrically at 620 nm. The lowest concentration inhibiting the growth of the pathogen was determined as the MIC. Similarly, the individual component of EO such as cinnamaldehyde and linalool was tested for their respective MICs against PAO1 and the clinical isolates.
Growth Curve Analysis
In order to examine that the EO did not have bactericidal effect against P. aeruginosa planktonic cells, growth curve analysis was done. LB broth containing 1% overnight culture of P. aeruginosa was supplemented with Biofilm Inhibitory Concentration (BIC) of the EO and the flask was incubated for 24 h at 37°C. The flask containing overnight culture without EO served as control. The optical density was read spectrophotometrically at every 1 h interval up to 24 h (Packiavathy et al., 2013).
Antibiofilm Activity of EO, DNases
Biofilm Inhibition Assay
The effect of EO to inhibit the young biofilms was assessed using the crystal violet assay (Nithyanand et al., 2015c). The assay was carried out in 24-well microtiter plate (NEST Biotechnology, Korea) containing 1ml of media, 100 μl of cell suspension (107 cells ml-1) and EO at its sub-MIC (5% v/v). The well without EO and only DMSO (1.25%) served as a control. Likely, the effect of commercial and MBDs and synergism of EO and the respective DNases were assessed as above to determine the maximum efficacy in inhibiting the biofilm.
Matured Biofilm Disruption Ability of EO and DNases
To determine the potency of the EO to inhibit mature biofilms, the BIC of EO was added to the preformed biofilms and incubated further for 24 h at 37°C. After incubation, the spent media was discarded and the wells were washed with sterile distilled water. After air drying the wells were stained with 0.4% crystal violet and the absorbance was quantified spectrophotometrically at 595 nm (Nithyanand et al., 2015c). Similarly, the potency of DNases and synergism of EO and DNases over mature biofilm dispersal was also determined.
Microscopic Visualization of Antibiofilm Activity
Light Microscopy
Biofilms were allowed to form on 1 cm × 1 cm glass slides which were placed into the wells of the 24-well titer plates. The BIC of EO (5%v/v) was added to the wells and the plates were incubated at 37°C for 24 h. After incubation, the biofilm formed on the glass slides was stained using crystal violet dye for 5 min. It was then gently washed with de-ionized water and allowed to dry for 5 min. Then, the slides were viewed under light microscope at a magnification of 40× (Nikon Eclipse Ti 100, Japan) (Thenmozhi et al., 2009).
Confocal Laser Scanning Microscopy (CLSM)
Confocal Laser scanning microscopy (CLSM) was used to determine the action of EO on mature biofilms. The biofilms were allowed to form on 1 cm × 1 cm glass slide placed in a 24-well titer plates. After 24 h incubation, the BIC of EO was added to the preformed biofilms and the plates were further incubated at 37°C for 24 h. The three dimensional architecture of the preformed biofilm treated with BIC of EO was assessed by CLSM (Nithyanand et al., 2015c). Images were captured and processed by using Zeiss LSM Image Examiner Version 4.2.0.121. The parameters such as biomass, average thickness and surface volume ratio of the control and treated biofilms were evaluated using COMSTAT software (Heydorn et al., 2000).
Scanning Electron Microscopy (SEM)
In order to visualize the reduction in EPS of P. aeruginosa biofilm, SEM analysis was performed. Briefly, P. aeruginosa biofilms were grown in the presence and absence of enzymes (DNaseI and MBD) on the glass slides. The biofilms on the glass slides were fixed with 2.5% glutaraldehyde were washed in 0.1M sodium acetate buffer (pH 7.3). Finally, the dehydrated samples were dried, gold sputtered and examined with VEGA3 TESCAN (Thenmozhi et al., 2009).
eDNA Staining
In order to investigate the accumulation of eDNA in P. aeruginosa biofilm, eDNA staining was performed. The P. aeruginosa strains were inoculated in LB broth and grown in the presence of DNaseI and MBD onto 1 cm × 1 cm glass slide within a 24-well titer plate and was incubated for 24 h at 37°C. After 24 h, the glass slides were gently rinsed with PBS and stained with 20 μM propidium iodide (PI) (Sigma), which stains the DNA present within a biofilm. Accumulation of eDNA were visualized under a fluorescence microscope (Nikon eclipse Ni, Japan) at excitation and emission wavelengths of 540 and 525 nm for PI and photographed digitally (Rajendran et al., 2013).
Virulence Assays
All the QS mediated virulence assays were performed with the EO against P. aeruginosa (PA01) as well as the clinical P. aeruginosa isolates. In addition, cinnamaldehyde and linalool which were the major components of EO were also assayed individually against the QS mediated virulence factors of PA01 and the clinical isolates.
EPS Inhibition Assay
Bacterial biofilm consists of self-generated extra polymeric substances which plays a distinct role during biofilm formation. EPS from EO treated and untreated test pathogen was quantified by the method of Nithya et al. (2011). The test pathogen was grown in glass slides placed in 24 well microtiter plates with and without the addition of EO and incubated for 16 h. The glass slides were removed and washed with 0.5 ml of 0.9% NaCl. Equal volume of 5% phenol was added to the cell suspension, to which five volume of concentrated H2SO4 was also added. The mixture was incubated for 1 h in the dark and centrifuged at 10,000 rpm for 10 min. The absorbance of the supernatant was measured at 490 nm.
Alginate Assay
Alginate is an abundant polysaccharide in P. aeruginosa biofilms which aids in the adhesion of EPS that promote cell attachment and shields the cell surface structure. Alginate was quantified from the EO treated and untreated cell suspension. 600 μl of boric acid – H2SO4 (4: 1) was added to 70 μl cell suspension and the cell suspension was vortexed for 10 s. Further, 20 μl of 0.2% carbazole solution was added to the cell suspension which was incubated for 30 min at 55°C. After incubation, absorbance was measured spectrophotometrically at 530 nm (Owlia et al., 2007).
LasA Staphylolytic Assay
Pseudomonas aeruginosa uses las QS system which consists of lasI that interact with the LasR transcriptional activator to activate the lasA virulence gene which leads to the production of LasA protease. The ability of culture supernatant to lyse the boiled Staphylococcus aureus cells was determined by LasA protease activity (Kessler et al., 1993). A 30 ml overnight culture of S. aureus cells were centrifuged at 7,000 rpm for 3 min and the pellet was suspended in 0.02 M Tris-HC1 (pH 8.5). The suspension was boiled for 10 min and then diluted with the same buffer to an OD of 0.8 at 595 nm. To 900 μl of diluted S. aureus suspension, 100 μl of cell free culture supernatant of the strains cultured with or without EO was added. OD was measured at 595 nm after every 15 min interval for 60 min using a UV–visible spectrophotometer. Similarly, the LasA staphylolytic assay was carried out using cinnamaldehyde and linalool against P. aeruginosa and the clinical isolates.
Pyocyanin Assay
Pseudomonas aeruginosa uses rhl QS system that consists of rhlI which in conjunction with RhlR activates the expression of pyocyanin pigment production. Cell free supernatants of PA01 and the clinical isolates cultured with the presence and absence of EO at their respective sub-MIC concentrations (5 and 7% v/v) was extracted for pyocyanin quantification (Essar et al., 1990). The extracted pyocyanin was quantified spectrophotometrically at OD 520 nm. Cinnamaldehyde and linalool were also tested for their ability to inhibit pyocyanin produced by PA01 and the clinical isolates.
Swarming Motility
Pseudomonas aeruginosa uses QS to regulate several functions such as biofilm formation, motility, and pigment production. QS regulated swarming motility is characterized as a form of flagella dependent movement on a viscous environment such as semisolid agar surface (Krishnan et al., 2012). Initially, 10 ml of swarming agar [(1% w/v) glucose, (0.5% w/v) agar, (0.5% w/v) peptone and (0.2% w/v) yeast extract] was overlaid on to the sterile petri plate. The 5 ml of swarming media seeded with BIC of EO was poured as an additional layer and was allowed to solidify. The 2 μl of overnight grown P. aeruginosa cells (2 × 107 CFU) was inoculated in the middle of the agar and incubated for 16 h at 37°C (Krishnan et al., 2012). Cinnamaldehyde and linalool were tested for its inhibition of swarming motility by the same method.
Statistical Analysis
All experiments were carried out in triplicates. The statistical analogy among control and treated samples were determined using Graph Pad prism version 5. Multivariate analysis such as Tukey test was carried out to show the significance.
Results
Chemical Constituents of EO
GC-FID and GC-MS analysis of leaf EO of C. tamala enabled the identification of 40 volatile constituents amounting to 97.7% of the EO. Only two components namely, Linalool (42.5%) and (E)-cinnamaldehyde (31.2%) were the major compounds of the oil (Table 1).
Table 1.
The chemical composition of essential oil extracted from Cinnamomum tamala.
| S. No. | Compound | RI | Relative amount % |
|---|---|---|---|
| 1 | (2E)-Hexenal | 852 | 0.1 |
| 2 | α-Thujene | 928 | 0.4 |
| 3 | α-Pinene | 937 | 3.1 |
| 4 | Camphene | 952 | 1.2 |
| 5 | Benzaldehyde | 962 | 1.3 |
| 6 | β-Pinene | 981 | 1.4 |
| 7 | Myrcene | 992 | 0.4 |
| 8 | α-Phyllandrene | 1004 | 0.8 |
| 9 | δ-3-Carene | 1013 | 0.4 |
| 10 | ρ-Cymene | 1027 | 3.6 |
| 11 | Limonene | 1032 | 0.9 |
| 12 | 1,8-Cineole | 1035 | 0.6 |
| 13 | Salicylaldehyde | 1044 | 0.6 |
| 14 | (Z)-Linalool oxide | 1075 | 0.2 |
| 15 | Terpinolene | 1093 | 0.2 |
| 16 | Linalool | 1100 | 42.5 |
| 17 | Benzenepropanal | 1164 | 0.9 |
| 18 | Borneol | 1172 | 0.6 |
| 19 | Terpinen-4-ol | 1179 | 0.5 |
| 20 | α-Terpineol | 1189 | 0.2 |
| 21 | (Z)-Cinnamaldehyde | 1222 | 0.3 |
| 22 | Nerol | 1232 | 0.1 |
| 23 | (E)-Cinnamaldehyde | 1272 | 31.2 |
| 24 | Bornyl acetate | 1387 | 0.1 |
| 25 | Thymol | 1291 | 0.1 |
| 26 | α-Ylangene | 1378 | 0.2 |
| 27 | α-Copaene | 1380 | 0.1 |
| 28 | Geranyl acetate | 1384 | 0.1 |
| 29 | (Z)-Cinnamyl acetate | 1392 | 0.1 |
| 30 | (E)-Caryophyllene | 1422 | 1.0 |
| 31 | Coumarin | 1435 | 0.2 |
| 32 | (E)-Cinnamyl acetate | 1449 | 2.6 |
| 33 | α-Humulene | 1456 | 0.1 |
| 34 | γ-Muurolene | 1480 | 0.1 |
| 35 | δ-Cadinene | 1527 | 0.1 |
| 36 | α-Cadinene | 1537 | 0.1 |
| 37 | α-Calacorene | 1547 | 0.1 |
| 38 | (E)-Nerolidol | 1566 | 0.1 |
| 39 | Spathulenol | 1579 | 0.1 |
| 40 | Caryophyllene oxide | 1590 | 1.0 |
| Total | 97.7 | ||
Bolded values indicate the compounds present in high percentage.
Determination of Minimal Inhibitory Concentration of EO
The MIC of the EO against PAO1 and the he clinical isolates AU07 and AU09 was found to be 10 and 15% v/v from the various concentrations tested (1–20% v/v). The MIC of cinnamaldehyde against PAO1 was found to be 0.36 mg/ml and for clinical isolates it showed MIC of about 2 mg/ml. Likely, the MIC of linalool against PAO1 was found to be 0.36 mg/ml and for Clinical isolates it showed MIC of about 2 mg/ml. It was observed that chemical composition of volatile oils varied significantly among the different Cinnamomum sp. The major components of the EO obtained from the bark of Ceylon type C. zeylanicum were eugenol and (E)-cinnamaldehyde which represent 82.5% of the total composition (Dagli et al., 2015). Linalool was found to be the highest (42.5%) in underexplored C. tamala. As linalool is found to be a major component, we envisage that it might enhance the antimicrobial effect against P. aeruginosa. Previously, there was a report that EO in combination with linalool significantly enhances the antimicrobial effectiveness (Herman et al., 2016).
Growth Curve Analysis
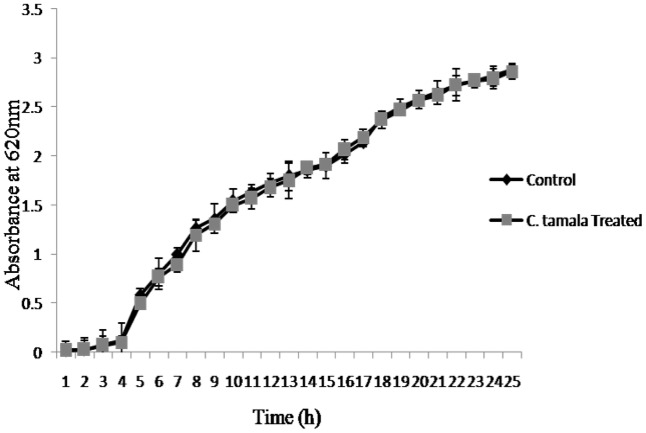
Growth curve analysis was performed to ensure that the EO did not show any antibacterial activity at its sub-MIC (5%) concentration. The results no reduction in optical density of cells between control and treated even after 24 h of incubation (Figure 1).
FIGURE 1.
Growth curve of P. aeruginosa treated with EO of C. tamala after 24 h incubation.
Inhibition of Young and Matured Biofilms
The results showed that difference was observed in reduction of the biofilms treated with the combination of EO and DNases than EO and DNases treated individually. Biofilm inhibition of about 75% ± 1.09 was seen when EO and DNases were used in combination. On the other hand, EO showed only 58% ± 2.9 of inhibition when used individually (Figure 2). The results were in similar lines with matured biofilms. A maximum inhibition of about 70% ± 1.09 was seen in synergism of EO and DNases. EO alone on the other hand could inhibit only 50% ± 2.07 of the mature biofilm and 65% ± 2.8 inhibition was noticed for DNases alone (Figure 3).
FIGURE 2.
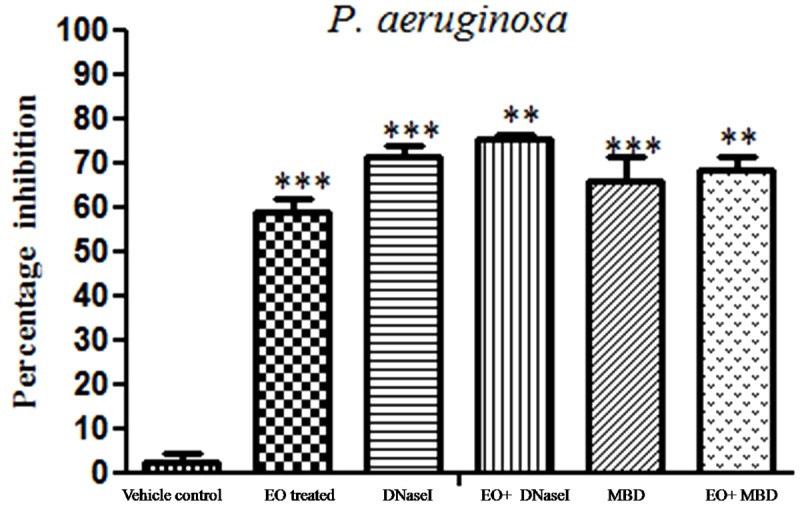
Percentage inhibition of P. aeruginosa young biofilm. Mean values of triplicate of independent experiments ± SD are shown. One-way ANOVA test demonstrates significant difference between the control and the test. Double asterisk indicates significant at P ≤ 0.01 and triple asterisk indicates significant at P ≤ 0.005.
FIGURE 3.

Percentage inhibition of P. aeruginosa matured biofilm. Mean values of triplicate independent experiments ± SD are shown. One-way ANOVA test demonstrates significant difference between the control and the test. Double asterisk indicates significant at P ≤ 0.01 and triple asterisk indicates significant at P ≤ 0.005.
Microscopic Visualization of Antibiofilm Activity
Light Microscopy
To clearly demonstrate the synergized effect of EO and DNases on young biofilms, microscopic examinations were performed in the presence of sub-MIC (5% v/v) of EO. The microscopy analysis revealed maximum reduction of young biofilm upon treatment with EO and DNases. There was also reduction in EO treated biofilm but to a lesser extent on comparison with combination of EO and DNases (Figure 4).
FIGURE 4.
Light microscopic images at 40× demonstrate the inhibition of P. aeruginosa young biofilm. (A) Control, (B) EO treated, (C) DNaseI treated, (D) MBD treated, (E) EO + DNaseI, (F) EO + MBD.
Examination of Matured Biofilm Architecture by Confocal Laser Scanning Microscope
Confocal Laser scanning microscopy was further done to confirm that synergized effect has maximum ability to disrupt matured biofilm than EO and DNases alone and to study the architecture of the biofilm before and after treatment. From the CLSM images (Figure 5), it is evident that there was a disintegration of biofilms in the treated samples on comparing with the control, which showed the presence of a dense biofilm. COMSTAT analysis was carried to determine the three dimensional features like biomass, average thickness and surface volume ratio of matured biofilms before and after treatment. The results show that there was a substantial reduction in different parameters of biofilm architecture such as biomass and average thickness, whereas there was an increase in surface to volume ratio which indicates the detachment of cells from biofilm matrix upon treatment (Table 2). From the Table 2, it is evident that the increase in surface to volume ratio (μm2/μm3) of around 0.043 and decrease in biomass (μm) (32.22) and average thickness (μm) (30.08) is due to the synergistic effect of both EO and DNase.
FIGURE 5.
CLSM reveals the architecture of P. aeruginosa matured biofilm dispersal. (A) Control, (B) EO treated, (C) DNaseI treated (D) MBD treated, (E) EO + DNaseI, (F) EO + MBD.
Table 2.
COMSTAT analysis of treated and untreated P. aeruginosa biofilms.
| Strain | Component | Biomass (μm) | Average thickness (μm) | Surface volume ratio (μm2/μm3) |
|---|---|---|---|---|
| Control | 85.09 | 82.77 | 0.021 | |
| EO treated | 62.89∗∗∗ | 60.12∗∗∗ | 0.026 | |
| P. aeruginosa | DNaseI | 48.35∗∗∗ | 49.4∗∗∗ | 0.039 |
| EO + DNaseI | 32.22∗∗∗ | 30.08∗∗∗ | 0.043 | |
| MBD | 53.01∗∗∗ | 49.13∗∗∗ | 0.028 | |
| EO + MBD | 42.67∗∗∗ | 40.12∗∗∗ | 0.031 | |
Mean values of triplicate individual experiments and SDs are shown. ∗∗∗Indicates the statistical significance (P < 0.05).
Examination of Treated and Untreated P. aeruginosa Biofilm by SEM
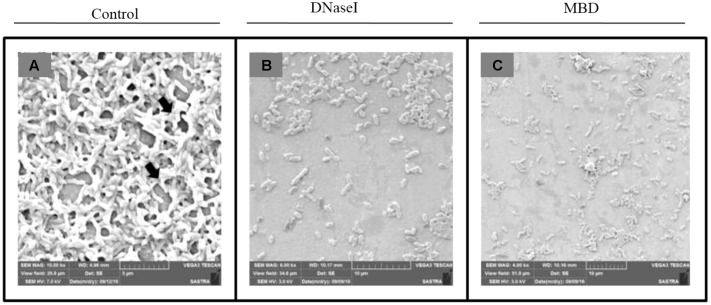
To analyze the antibiofilm potential of DNaseI and MBD on P. aeruginosa biofilm, SEM was performed. From the SEM results it is depicted that untreated P. aeruginosa (Figure 6A) has a thick layer of EPS, whereas there is a disruption in the EPS matrix of biofilm treated with DNaseI and MBD (Figures 6B,C).
FIGURE 6.
Scanning Electron Microscopic image of P. aeruginosa biofilm (A) Control, (B) DNaseI treated, (C) MBD treated. Black arrow in (A) control indicates the EPS forming matrix.
Visualization of eDNA and effect of DNases on P. aeruginosa Biofilm
To detect the presence of eDNA, P. aeruginosa biofilms were stained with PI (which specifically stains eDNA) and was visualized under a fluorescent microscope. From the fluorescent micrograph results it is evident that untreated biofilm has high accumulation of eDNA (Figure 7A) whereas treated has lesser eDNA accumulation (Figures 7B,C).
FIGURE 7.
Fluorescent microscopy image of P. aeruginosa eDNA. Propidium iodide (PI) stained eDNA in red. (A) Control, (B) DNaseI treated, (C) MBD treated.
Virulence Assay
Inhibition of Alginate and EPS Production
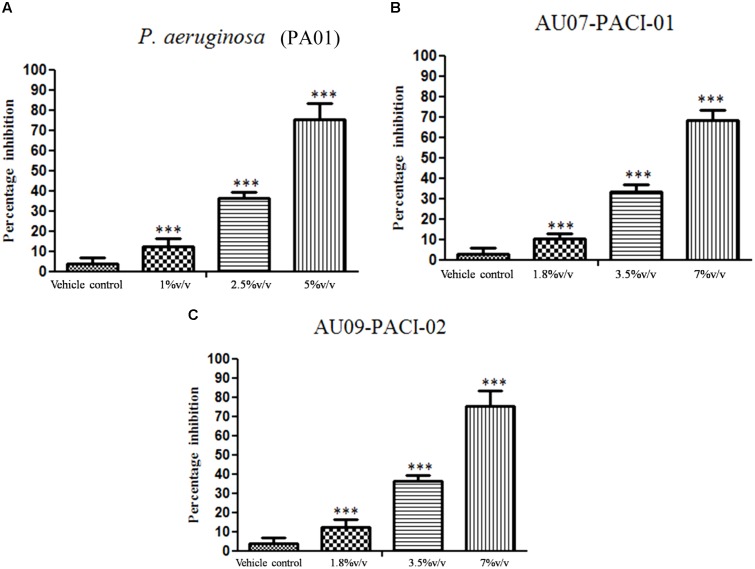
Essential oil inhibits the production of EPS, a maximum reduction in OD was observed with P. aeruginosa treated with sub-MIC (5% v/v) (Figure 8). Similarly, EO exhibited a concentration dependent inhibitory effect in the production of alginate by PAO1. At sub-MIC (5% v/v) the alginate production was reduced to the maximum of 71% ± 4.38 whereas 1% and 2.5% v/v had lesser inhibitory effect on alginate production (Figure 9A). The EO at its sub-MIC (7% v/v) inhibited the alginate production of clinical isolate AU07 by 65% ± 3.49 and 61% ± 3.58 for AU09 (Figures 9B,C). Likely, cinnamaldehyde inhibits the alginate production at its sub-MIC (0.18 mg/ml) of about 69% ± 2.75 for P. aeruginosa, 63.5% ± 4.63 for AU07 PACI-01 at its sub-MIC (1 mg/ml) and 59.5% ± 2.43 for AU09 PACI-02 at its sub-MIC (1 mg/ml) (Supplementary Figure S1). Linalool, another component of EO, inhibits alginate of about 67% ± 2.99 for P. aeruginosa, 63% ± 1.99 for AU07 PACI-01 and 60% ± 3.12 for AU09 PACI-02 (Supplementary Figure S2).
FIGURE 8.

Inhibitory Effect of EO at sub MIC (5% v/v) on EPS production of P. aeruginosa biofilm. Mean values of triplicate independent experiments ± SD are shown. One-way ANOVA test demonstrates significant difference between the control and the test. Double asterisk indicates significant at P ≤ 0.01.
FIGURE 9.
Inhibition of alginate production (A) P. aeruginosa biofilm in the presence of EO (1–5% v/v), (B) AU07-PACI-01 biofilm in the presence of EO (1.8–7% v/v), (C) AU09-PACI-02 biofilm in the presence of EO (1.8–7% v/v). Mean values of triplicate independent experiments ± SD are shown. One-way ANOVA test demonstrates significant difference between the control and the test. Single asterisk indicates significant at P ≤ 0.025 and triple asterisk indicates significant at P ≤ 0.005.
Effect of EO on LasA Staphylolytic Activity
Supernatant of PAO1 treated with EO showed significant reduction in LasA staphylolytic activity in a concentration dependent manner. A maximum reduction of about 76% ± 8.06 was observed at sub-MIC (5% v/v) treated (Figure 10A). The EO at its sub-MIC (7% v/v) reduces the LasA staphylolytic activity for AU07 by 68% ± 4.95 and 75% ± 7.93 for AU09 (Figures 10B,C). The reduction of LasA staphylolytic activity was found to be 70% ± 5.26 for P. aeruginosa at its sub-MIC (0.18 mg/ml), 62.5% ± 4.22 for AU07 PACI-01 and 60% ± 3.99 for AU09 PACI-02 at its sub-MIC (1 mg/ml) (Supplementary Figure S3). For Linalool the reduction was found to be 67% ± 4.56 for P. aeruginosa, 63% ± 3.65 for P. aeruginosa and 60% ± 3.42 for AU09 PACI-02 (Supplementary Figure S4).
FIGURE 10.
Inhibition of staphylolytic activity (A) P. aeruginosa biofilm by EO treated (1–5% v/v), (B) AU07-PACI-01 biofilm in the presence of EO (1.8–7% v/v), (C) AU09-PACI-02 biofilm in the presence of EO (1.8–7% v/v). Mean values of triplicate independent experiments ± SD are shown. One-way ANOVA test demonstrates significant difference between the control and the test. Single asterisk indicates significant at P ≤ 0.025 and triple asterisk indicates significant at P ≤ 0.005.
Effect of EO on Pyocyanin Production
The production of pyocyanin a blue green phenazine pigment in P. aeruginosa is regulated by QS system. P. aeruginosa biofilm treated with EO showed a maximum reduction of pyocyanin production of about 72% ± 4.65 at its sub-MIC (5%v/v) when compared to the control (Figure 11A). The EO at its sub-MIC (7% v/v) reduces the pyocyanin production by 68% ± 3.95 for AU07 and 62% ± 3.90 for AU09 (Figures 11B,C). Pyocyanin was reduced significantly of about 78% ± 4.70 at its sub-MIC (0.18 mg/ml) for P. aeruginosa treated with cinnamaldehyde and 67% ± 0.87 for AU07 PACI-01 at its sub-MIC (1 mg/ml) and 63% ± 2.01 for AU09 PACI-02 at its sub-MIC (1 mg/ml) (Supplementary Figure S5). Likely, linalool significantly reduces pyocyanin pigment of about 70% ± 2.94 for P. aeruginosa, 67% ± 1.30 for AU07 PACI-01 and 65% ± 2.63 for AU09 PACI-02 (Supplementary Figure S6).
FIGURE 11.
Inhibition of pyocyanin (A) P. aeruginosa biofilm by EO treated (1–5% v/v), (B) AU07-PACI-01 biofilm in the presence of EO (1.8–7% v/v), (C) AU09-PACI-02 biofilm in the presence of EO (1.8–7% v/v). Mean values of triplicate independent experiments ± SD are shown. One-way ANOVA test demonstrates significant difference between the control and the test. Single asterisk indicates significant at P ≤ 0.025, double asterisk indicates significant at P ≤ 0.01, and triple asterisk indicates significant at P ≤ 0.005.
Effect of EO on P. aeruginosa Swarming Motility
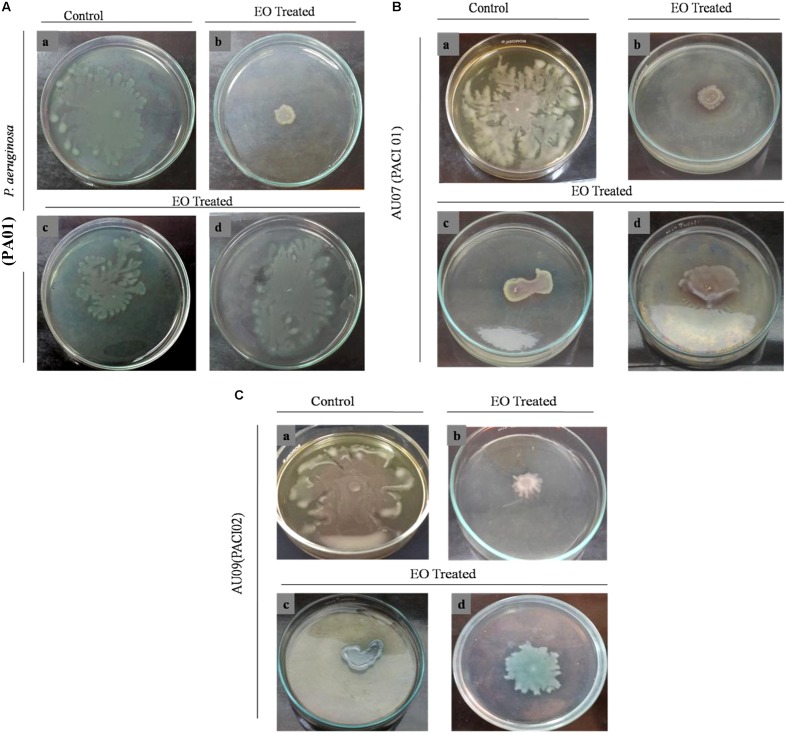
To explore the anti-QS potential of the EO of C. tamala, swarming assay was done against P. aeruginosa in a concentration dependent manner. Maximum reduction of swarming motility was observed in the swarming plates treated with EO at its sub-MIC (5% v/v) concentration (Figure 12). From the results, it is envisaged that EO at its BIC (5% v/v) has the ability to interfere in the Las and Rhl mediated QS system. The individual component of EO such as cinnamaldehyde (Supplementary Figure S7) and linalool (Supplementary Figure S8) inhibit swarming motility of P. aeruginosa and two clinical isolates such as AU07 PACI-01 and AU09 PACI-02 at its sub-MIC. Earlier studies which report about the anti-QS activity of cinnamon oil cite that cinnamaldehyde was the major component of the oil and it was responsible for the anti-QS activity (Brackman et al., 2008; Yap et al., 2015). However, a recent study which report about the anti-QS activity of Ceylon-type whole cinnamon oil had eugenol as its major component (Kalia et al., 2015). In similar lines, the EO from C. tamala had linalool (42%) as its major component followed by cinnamaldehyde (31%). So, we fully concur with the view that the exhibited QSI activity might be due to the synergistic action of the major components present in the EO (Kalia et al., 2015). The potency of an EO form an underexplored Cinnamomum sp. in attenuating QS dependent swarming motility provides a new insight into the anti-virulent potential of the EO and opens up a new avenue for bioprospecting EOs from several underexplored wild Cinnamomum sp. present in the Himalaya range.
FIGURE 12.
Effect of EO on swarming motility of (A) P. aeruginosa in a concentration dependent manner. (a) Control, (b) EO Treated (1.25% v/v), (c) (2.5% v/v), (d) (5% v/v). (B) AU07-PACI-01 (a) Control, (b) EO Treated (1.8% v/v), (c) (3.5% v/v), (d) (7% v/v). (C) AU09-PACI-02 (a) Control, (b) EO Treated (1.8% v/v), (c) (3.5%v/v), (d) (7% v/v).
Discussion
As biofilms are resilient and polymicrobial (Stewart, 2015), a combination of approaches is needed for the development of antibiofilm drugs. Owing to their different modes of action, combination strategies open up new avenues for novel antibiofilm pharmaceuticals. In the present study, we show that EOs when combined with DNase show enhanced disruption of biofilms. A recent study reports that the sole use of EO (C. zeylanicum) alone showed very low efficacy in reducing P. aeruginosa biofilms (Kalia et al., 2015). The present study demonstrated that the synergistic effect of EO and DNase inhibit biofilms to a greater extent. Several synergistic studies have been carried out by using DNase and antibiotics in tandem which show that the removal of eDNA weakens the biofilm matrix (Conover et al., 2011) and thereof increases the susceptibility of both Gram-positive and -negative pathogens to antibiotics (Tetz et al., 2009; Nguyen and Burrows, 2014). Similarly, the synergistic effects of various EOs and antibiotics have also shown enhanced biofilm removal (Duarte et al., 2012; Coelho and Pereira, 2013; Lang et al., 2016). On this basis, the present study shows that synergism of the EO of C. tamala with DNases can also be an effective means to treat P. aeruginosa biofilm infections. The MBD was also more efficient in disrupting the mature biofilms than the EO alone. This shows that EOs have limited penetration into the biofilms and when the biofilm scaffold which includes eDNA is loosened due to the degradation of eDNA by the action of DNAses it enhances the action of EOs. Chronic infection caused by biofilm forming strains of P. aeruginosa during cystic fibrosis (CF) mediates a neutrophil dominated host immune response. During this process a very high concentration of e-DNA gets released as neutrophils disintegrate which leads to increased sputum accumulation (Bayes et al., 2016). Recombinant DNase is reported to reduce this copious amount of viscous sputum, thereby clearing the lower airway secretions (Pressler, 2008). On this basis, the present study shows that synergism of EO of C. tamala with DNases can be an effective way to disrupt P. aeruginosa biofilm associated infections. Alginate is one of the major compounds present in the EPS layer of P. aeruginosa biofilms which helps to maintain the structural integrity of biofilms. In the present study, we observed that C. tamala significantly inhibited alginate production.
Of late, EOs of different plants has been documented to have QS inhibitory activity (Khan et al., 2009; Kalia et al., 2015; Pekmezovic et al., 2016). In the present study, we observed that the EO from C. tamala effectively suppressed several QS mediated virulence factors related to P. aeruginosa. It was interesting to note that the EO was also able inhibit the virulence factors of even the clinical isolates of P. aeruginosa which showcases the anti-infective ability of EOs from native plants and stresses the need for bioprospecting native plant species for anti-infectives. P. aeruginosa possesses two distinct, but interacting, well-characterized QS systems namely las (LasI, LasR) and rhl which coordinates several extracellular virulence factors (Krishnan et al., 2012). From the results, it is suggested that inhibition of pyocyanin production might be due to the presence of LasR or rhl inhibitor in EO (Figure 11). Swarming motility is a key virulent trait in P. aeruginosa which is mediated by Las and Rhl QS system (Pesci et al., 1997; Kumar et al., 2009) which plays a crucial role in dissemination of the pathogen. From the results it was evident that the EO was able to disrupt the dissemination or group motility of the pathogen a crucial factor in biofilm formation. We further show that cinnamaldehyde and linalool, the major compounds in the EO of C. tamala suppressed the QS-based virulence factors in P. aeruginosa PAO1 as well as the clinical isolates. Cinnamaldehyde is already reported as a QS inhibitor in V. harveyi probably by inhibiting/degrading the QS molecule 3-hydroxy-butanoyl homoserine lactone. On the other hand 3-oxo-C12HSL and C4HSL are the major QS molecules in P. aeruginosa (Kalia et al., 2015). So it is envisaged that the QS inhibitory mechanism by cinnamaldehyde and linalool is possibly mediated by acting upon the major QS molecules in P. aeruginosa.
Though several EOs have been explored for their antibiofilm property, to best of our knowledge, the present study forms a first report about the QS and biofilm inhibitory potential of an underexplored EO of C. tamala and the synergistic combination of EO of C. tamala and DNases has increased efficacy in disrupting biofilms. The synergistic use of EO with a DNase of marine bacterial origin might result in a cost effective therapy and its use as a potential antibiofilm agent for the treatment of biofilm device associated infections warrants further investigations.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication. PN designed overall research and wrote the paper.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This research was supported by the DST-SERB sponsored Start Up Research Grant (Young Scientists) (SB/YS/LS-284/2013) and (SB/FT/LS-300/2012) awarded to PN and RM. SF is grateful to the University Grant Commission, New Delhi for providing Research fellowship under Maulana Azad National Fellowship (MANF-2015-17-TAM-55961) and the support is duly acknowledged. Fellowship to DR in the form of JRF by DST-SERB is thankfully acknowledged. DST-FIST funding (No: SR/FST/ETI-331/2013) provided by DST, Govt. of India to SCBT, SASTRA University is greatly acknowledged. We sincerely thank the SASTRA University and its management for providing us the infrastructure needed to carry out our research work.
Footnotes
Funding. This work was financially supported by Science and Engineering Research Board, Department of Science and Technology, New Delhi (SB/YS/LS-284/2013 and SB/FT/LS-300/2012) awarded to PN and RM.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmicb.2017.01144/full#supplementary-material
References
- Bassole I. H., Juliani H. R. (2012). Essential oils in combination and their antimicrobial properties. Molecules 17 3989–4006. 10.3390/molecules17043989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayes H. K., Ritchie N., Irvine S., Evans T. J. (2016). A murine model of early Pseudomonas aeruginosa lung disease with transition to chronic infection. Sci. Rep. 6:35838 10.1038/srep35838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackman G., Defoirdt T., Miyamoto C., Bossier P., Calenbergh S. V., Nelis H., et al. (2008). Cinnamaldehyde and cinnamaldehyde derivatives reduce virulence in Vibrio spp. By decreasing the DNA-binding activity of the quorum sensing response regulator LuxR. BMC Microbiol. 8:149 10.1186/1471-2180-8-149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung P. Y., Toh Y. S. (2014). Anti-biofilm agents: recent breakthrough against multi-drug resistant Staphylococcus aureus. Pathog. Dis. 70 231–239. 10.1111/2049-632X.12141 [DOI] [PubMed] [Google Scholar]
- Clinical and Laboratory Standards Institute (2006). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. Clinical and Laboratory Standards Institute document M7-A7 7th Edn Wayne, PA: Clinical and Laboratory Standards Institute. [Google Scholar]
- Coelho F. A. B. L., Pereira M. O. (2013). “Exploring new treatment strategies for Pseudomonas aeruginosa biofilm based infections based on plant essential oils,” in Microbial Pathogens and Strategies for Combating Them: Science, Technology and Education Vol. 1 ed. Mendez-Vilas A. (Badajoz: Formatex; ), 83–89. [Google Scholar]
- Cole S. J., Records A. R., Orr M. W., Linden S. B., Lee V. T. (2014). Catheter associated urinary tract infection by Pseudomonas aeruginosa is mediated by exopolysaccharide-independent biofilms. Infect. Immun. 82 2048–2058. 10.1128/IAI.01652-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover M. S., Mishra M., Deora R. (2011). Extracellular DNA is essential for maintaining Bordetella biofilm integrity on abiotic surfaces and in the upper respiratory tract of mice. PLoS ONE 11:e16861 10.1371/journal.pone.0016861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagli N., Dagli R., Mahmoud R. S., Baroudi K. (2015). Essential oils, their therapeutic properties and implication in dentistry: a review. J. Int. Soc. Prev. Commun. Dent. 5 335–340. 10.4103/2231-0762.165933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das T., Manefield M. (2012). Pyocyanin promotes extracellular DNA release in Pseudomonas aeruginosa. PLoS ONE 7:e46718 10.1371/journal.pone.0046718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies D. (2003). Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2 114–122. 10.1038/nrd1008 [DOI] [PubMed] [Google Scholar]
- Duarte A., Ferreira S., Silva F., Domingues F. C. (2012). Synergistic activity of coriander oil and conventional antibiotics against Acinetobacter baumannii. Phytomedicine 19 236–238. 10.1016/j.phymed.2011.11.010 [DOI] [PubMed] [Google Scholar]
- Essar D. W., Eberly L., Hadero A., Crawford I. P. (1990). Identification and characterization of genes for a second anthranilate synthesis in Pseudomonas aeruginosa: interchangeability of the two anthranilate synthases aand evolutionary implications. J. Bacteriol. 172 884–900. 10.1128/jb.172.2.884-900.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frieri M., Kumar K., Boutin A. (2016). Antibiotic resistance. J. Infect. Public Health 10.1016/j.jiph.2016.08.007 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Gupta A., Duhan J., Tewari S., Sangwan P., Yadav A., Singh G., et al. (2013). Comparative evaluation of antimicrobial efficacy of Syzygium aromaticum, Ocimum sanctum and Cinnamomum zeylanicum plant extracts against Enterococcus faecalis: a preliminary study. Int. Endod. J. 46 775–783. 10.1111/iej.12058 [DOI] [PubMed] [Google Scholar]
- Herman A., Tambor K., Herman A. (2016). Linalool affects the antimicrobial efficacy of essential oils. Curr. Microbiol. 72 165–172. 10.1007/s00284-015-0933-4 [DOI] [PubMed] [Google Scholar]
- Heydorn A., Nielsen A. T., Hentzer M., Sternberg C., Givskov M., Ersbøll B. K., et al. (2000). Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology 146 2395–2407. 10.1099/00221287-146-10-2395 [DOI] [PubMed] [Google Scholar]
- Kalia M., Yadav V. K., Singh P. K., Sharma D., Pandey H., Narvi S. S., et al. (2015). Effect of cinnamon oil on quorum sensing-controlled virulence factors and biofilm formation in P. aeruginosa. PLoS ONE 10:e0135495 10.1371/journal.pone.0135495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler E., Safrin M., Olson J. C., Ohman D. E. (1993). Secreted LasA of Pseudomonas aeruginosa is a staphylolytic protease. J. Biol. Chem. 268 7503–7508. [PubMed] [Google Scholar]
- Khan M. S. A., Zahin M., Hasan S., Husain F. M., Ahmad I. (2009). Inhibition of quorum sensing regulated bacterial functions by plant essential oils with special reference to clove oil. Lett. Appl. Microbiol. 49 354–360. 10.1111/j.1472-765X.2009.02666.x [DOI] [PubMed] [Google Scholar]
- Koh C. L., Sam C. K., Yin W. F., Tan L. Y., Krishnan T., Chong Y. M., et al. (2013). Plant derived natural product as sources of antiquorum sensing compounds. Sensors 13 6217–6228. 10.3390/s130506217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreth J., Vu H., Zhang Y., Herzberg M. C. (2009). Characterization of hydrogen peroxide –induced DNA release by Streptococcus sanguinis and Streptococcus gordonii. J. Bacteriol. 191 6281–6291. 10.1128/JB.00906-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan T., Yin W. F., Chan K. G. (2012). Inhibition of quorum sensing controlled virulence factor production in Pseudomonas aeruginosa PAO1 by Ayurveda spice clove (Syzygium aromaticum) bud extract. Sensors 12 4016–4030. 10.3390/s120404016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R., Chhibber S., Harjai K. (2009). Quorum sensing is necessary for the virulence of Pseudomonas aeruginosa during urinary tract infection. Kidney Int. 76 286–292. 10.1038/ki.2009.183 [DOI] [PubMed] [Google Scholar]
- Lang M., Rodrigues S., Boulho R., Duteil E., Bazire A., Bedoux G. (2016). An essential oil blend prevents P. aeruginosa PA01 from forming biofilms. J. Bacteriol. Parasitol. 7:268. [Google Scholar]
- Matan N., Rimkeeree H., Mawson A. J., Chompreeda P., Haruthaithanasan V., Parker M. (2006). Antimicrobial activity of cinnamon and clove oils under modified atmosphere conditions. Int. J. Food Microbiol. 15 180–185. 10.1016/j.ijfoodmicro.2005.07.007 [DOI] [PubMed] [Google Scholar]
- Millezi A. F., Cardoso M. D., Alves E., Piccoli R. H. (2013). Reduction of Aeromonas hydrophila biofilm on stainless stell surface by essential oils. Braz. J. Microbiol. 44 73–80. 10.1590/S1517-83822013005000015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musthafa K. S., Saroja V., Pandian S. K., Ravi A. V. (2011). Antipathogenic potential of marine Bacillus sp. SS4 on N-acyl-homoserine-lactone-mediated virulence factors production in Pseudomonas aeruginosa (PAO1). J. Biosci. 36 55–67. 10.1007/s12038-011-9011-7 [DOI] [PubMed] [Google Scholar]
- Nazzaro F., Fratianni F., De Martino L., Coppola R., De Feo V. (2013). Effect of essential oils on pathogenic bacteria. Pharmaceuticals 6 1451–1474. 10.3390/ph6121451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen U. T., Burrows L. L. (2014). DNaseI and Proteinase K impair Listeria monocytogenes biofilm formation and induce dispersal of pre-existing biofilm. Int. J. Food Microbiol. 187 26–32. 10.1016/j.ijfoodmicro.2014.06.025 [DOI] [PubMed] [Google Scholar]
- Nijland R., Hall M. J., Burgess J. G. (2010). Dispersal of biofilms by secreted, matrix degrading, bacterial DNase. PLoS ONE 12:e15668 10.1371/journal.pone.0015668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nithya C., Devi M. G., Pandian S. K. (2011). A novel compound from the marine bacterium Bacillus pumilus S6-15 inhibits biofilm formation in Gram-positive and Gram-negative species. Biofuling 27 519–528. 10.1080/08927014.2011.586127 [DOI] [PubMed] [Google Scholar]
- Nithyanand P., Shafreen R. M., Muthamil S., Pandian S. K. (2015a). Usnic acid inhibits biofilm formation and virulent morphological traits of Candida albicans. Microbiol. Res. 179 20–28. 10.1016/j.micres.2015.06.009 [DOI] [PubMed] [Google Scholar]
- Nithyanand P., Shafreen R. M., Muthamil S., Pandian S. K. (2015b). Usnic acid, a lichen secondary metabolite inhibits Group A Streptococcus biofilms. Antonie. Von. Leeuwenhoek 107 263–272. 10.1007/s10482-014-0324-z [DOI] [PubMed] [Google Scholar]
- Nithyanand P., Shafreen R. M., Muthamil S., Murugan R., Pandian S. K. (2015c). Essential oils from commercial and wild patchouli modulate Group A Streptococcal biofilms. Ind. Crops Prod. 69 180–186. 10.1016/j.indcrop.2015.02.022 [DOI] [Google Scholar]
- Ogunwande I. A., Olawore N. O., Ekundayo O., Walter T. M., Schimidt J. M., Setzer W. N. (2005). Studies on the essential oils composition, antibacterial and cytotoxicity of Eugenia uniflora L. Int. J. Aromather. 2 147–152. 10.1016/j.ijat.2005.07.004 [DOI] [Google Scholar]
- Okshevsky M., Meyer R. L. (2015). The role of extracellular DNA in the establishment, maintenance and perpetuation of bacterial biofilms. Crit. Rev. Microbiol. 41 341–352. 10.3109/1040841X.2013.841639 [DOI] [PubMed] [Google Scholar]
- O’May C., Tufenkji N. (2011). The swarming motility of Pseudomonas aeruginosa is blocked by cranberry proanthocyanidins and other tannin-containing materials. Appl. Environ. Microbiol. 77 3061–3067. 10.1128/AEM.02677-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owlia P., Rasoolib I., Saderia H., Aliahmadi M. (2007). Retardation of biofilm formation with reduced productivity of alginate as a result of Pseudomonas aeruginosa exposure to Matricaria chamomilla essential oil. Phcog. Mag. 3 83–89. [Google Scholar]
- Packiavathy I. A., Sasikumar P., Pandian S. K., Veeraravi A. (2013). Prevention of quorum-sensing-mediated biofilm development and virulence factors production in Vibrio spp. by curcumin. Appl. Microbiol. Biotechnol. 97 10177–10187. 10.1007/s00253-013-4704-5 [DOI] [PubMed] [Google Scholar]
- Pekmezovic M., Aleksic I., Barac A., Arsic-Arsenijevic V., Vasiljevic B., Nikodinovic-Runic J., et al. (2016). Prevention of polymicrobial biofilms composed of Pseudomonas aeruginosa and pathogenic fungi by essential oils from selected Citrus species. Pathog. Dis. 74:ftw102. [DOI] [PubMed] [Google Scholar]
- Pesci E. C., Pearson J. P., Seed P. C., Iglewski B. H. (1997). Regulation of las and rhl quorum sensing in Pseudomonas aeruginosa. J. Bacteriol. 179 3127–3132. 10.1128/jb.179.10.3127-3132.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressler T. (2008). Review of recombinant human deoxyribonuclease (rhDNase) in the management of patients with cystic fibrosis. Biologics 4 611–617. 10.2147/btt.s3052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendran R., Williams C., Lappin D. F., Millington O., Martins M., Ramage G. (2013). Extracellular DNA release acts as an antifungal resistance mechanisms in mature Aspergillus fumigatus biofilms. Eukaryot. Cell. 3 420–429. 10.1128/EC.00287-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasamiravaka T., Labtani Q., Duez P., El Jaziri M. (2015). The formation of biofilms by Pseudomonas aeruginosa: a review of the natural and synthetic compounds interfering with control mechanisms. Biomed. Res. Int. 10:759348 10.1155/2015/759348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regina V. R., Lokanathan A. R., Modrzynski J. J., Sutherland D. S., Meyer R. L. (2014). Surface physicochemistry and ionic strength affects e-DNA’s role in bacterial adhesion to abiotic surfaces. PLoS ONE 9:e105033 10.1371/journal.pone.0105033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro S. M., Felicio M. R., Boas E. V., Goncalves S., Costa F. F., Samy R. P., et al. (2016). New frontiers for antibiofilm drug development. Pharmacol. Ther. 160 133–144. 10.1016/j.pharmthera.2016.02.006 [DOI] [PubMed] [Google Scholar]
- Sethupathy S., Prasath K. G., Ananthi S., Mahalingam S., Balan S. Y., Pandian S. K. (2016). Proteomic analysis reveals modulation of iron homeostasis and oxidative stress response in Pseudomonas aeruginosa PAO1 by curcumin inhibiting quorum sensing regulated virulence factors and biofilm production. J. Proteomics 145 112–126. 10.1016/j.jprot.2016.04.019 [DOI] [PubMed] [Google Scholar]
- Sriramavaratharajan V., Stephan J., Sudha V., Murugan R. (2016). Leaf essential oil of Cinnamomum agasthyamalayanum from the Western Ghats, India – A new source of camphor. Ind. Crops Prod. 86 259–261. 10.1016/j.indcrop.2016.03.054 [DOI] [Google Scholar]
- Stewart P. S. (2015). Prospects for antibiofilm pharmaceuticals. Pharmaceuticals 8 504–511. 10.3390/ph8030504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sydnor E. R. M., Perl T. M. (2011). Hospital epidemiology and infection control in acute care setting. Clin. Microbiol. Rev. 24 141–173. 10.1128/CMR.00027-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetz G. V., Artemenko N. K., Tetz V. V. (2009). Effect of DNase and antibiotics on biofilm characteristics. Antimicrob. Agents Chemother. 53 1204–1209. 10.1128/AAC.00471-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thenmozhi R., Nithyanand P., Rathna J., Pandian S. K. (2009). Antibiofilm activity of coral-associated bacteria against different clinical M serotypes of Streptococcus pyogenes. FEMS Immunol. Med. Microbiol. 57 284–294. 10.1111/j.1574-695X.2009.00613.x [DOI] [PubMed] [Google Scholar]
- Utchariyakiat I., Surassmo S., Jaturanpinyo M., Khuntayaporn P., Chomnawang M. T. (2016). Efficacy of cinnamon bark oil and cinnamaldehyde on anti-multidrug resistant Pseudomonas aeruginosa and the synergistic effects in combination with other antimicrobial agents. BMC. Complement. Alternate. Med. 16:158 10.1186/s12906-016-1134-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap P. S., Krishnan T., Chan K. G., Lim S. H. (2015). Antibacterial mode of action of Cinnamomum verum bark essential oil, alone and in combination with piperacillin against a multi drug resistant Escherichia coli strain. J. Microbiol. Biotechnol. 25 1299–1306. 10.4014/jmb.1407.07054 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.