Abstract
Locally recurrent rectal cancer (LRRC) is a complex disease with far-reaching implications for the patient. Until recently, research was limited regarding surgical techniques that can increase the ability to perform an en bloc resection with negative margins. This has changed in recent years and therefore outcomes for these patients have improved. Novel radical techniques and adjuncts allow for more radical resections thereby improving the chance of negative resection margins and outcomes. In the past contraindications to surgery included anterior involvement of the pubic bone, sacral invasions above the level of S2/S3 and lateral pelvic wall involvement. However, current data suggests that previously unresectable cases may now be feasible with novel techniques, surgical approaches and reconstructive surgery. The publications to date have only reported small patient pools with the research conducted by highly specialised units. Moreover, the short and long-term oncological outcomes are currently under review. Therefore although surgical options for LRRC have expanded significantly, one should balance the treatment choices available against the morbidity associated with the procedure and select the right patient for it.
Keywords: Recurrent rectal cancer, Sacrectomy, Pelvic exenteration, Pelvic sidewall, Radical resection
Core tip: This article provides an up-to-date review of the current international trends in surgical approaches for locally recurrent rectal cancer (LRRC), specifically highlighting the novel radical techniques that are now used in cases previously deemed unresectable. We have described these approaches according to anatomical locations of the recurrences and reviewed the respective oncological and functional outcomes. In addition, laparoscopic surgeries for LRRC are discussed and their outcomes are outlined.
INTRODUCTION
The incidence of locally recurrent rectal cancer (LRRC) has undergone a dramatic decline since the application of total mesorectal excision and addition of preoperative radiotherapy[1-4]. Current studies have reported 5-year recurrence rates of between 5%-10% as compared to previously published figures of 20%-30%[5-7]. The majority of these recurrences occur within the first 2 years after surgery[8,9]. Without subsequent treatment these patients have an extremely poor prognosis. A recurrence has the capacity to significantly reduce a patient’s quality of life by causing refractory pain as well as potentially resulting in a malodorous, fungating or fistulating mass. Non-operative approaches such as radiotherapy or chemoradiotherapy have been shown to offer little symptomatic relief, or survival benefit, and are often associated with significant side-effects[10-12]. Surgery offers the best hope in providing improved survival rates or as the best form of palliation.
In order to achieve clear resection margins radical surgery for pelvic recurrences often requires extended resection involving at least one organ that is adjacent to or involved by the tumour. Due to the morbidity associated with these procedures, LRRC cases previously received scant attention from the surgical world, except for a couple of notable exceptions[13,14]. Consequently, patients would often resort to palliative care either via systemic chemotherapy or a few boosts of radiotherapy. Since then, more specialised centres have taken the effort to provide and refine the provision of radical surgery for this condition. These together with improvements in perioperative intervention, extended resection or exenterative surgeries are now accepted management options in order to achieve a cure[15]. Results from several of these centres have demonstrated a 5-year survival rate of 35% to 50% after surgery for LRRC[16].
It should be noted that the surgical approach during resection of LRRC depends on the site of recurrence. There is significant variation in both techniques and outcomes. Although there is no standard classification system for recurrent rectal cancer, the general consensus amongst colorectal cancer surgeons generally describes a pattern of pelvic invasion based on the anatomic region of the pelvis involved with the disease (Figure 1). (1) Central: Tumour is localised to pelvic organ(s) and adjacent soft tissue structures without adherence to bone; (2) Sacral: Tumour presents in the presacral space and abuts or invades the sacrum or coccyx; and (3) Lateral pelvic sidewall: Tumour involves the soft tissue and structures of the pelvic sidewall (iliac vessels, lateral pelvic lymph nodes, pelvic ureters, pelvic nerves, sidewall musculature) and lateral bony pelvis.
Figure 1.
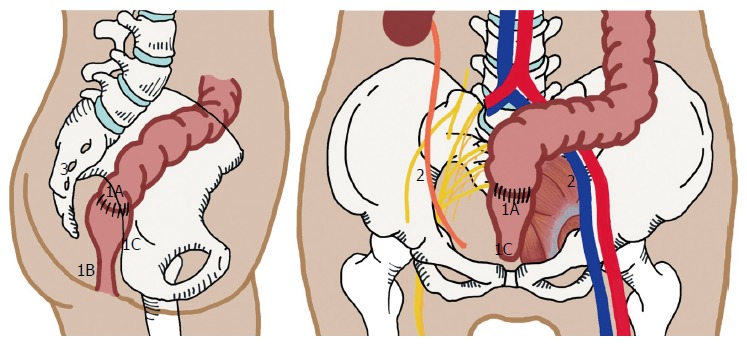
Patterns of pelvic recurrence. 1: Central; 1A: Anastomotic site; 1B: Perineal region, seen after abdominal perineal resection; 1C: Invasion to adjacent soft tissue involving genitourinary organs, or to pubic bone; 2: Lateral Pelvic Side Wall; 3: Posterior/Sacral Recurrence.
Central recurrences have the most favourable prognosis as these recurrences have the highest chance of achieving clear margin resections[17,18]. If the recurrence has spread beyond the soft tissue structures and becomes adherent to or invades into the bony structures (sacrum, ischium, pubic ramus) or extends into the pelvic sidewall structures then en bloc resection becomes more challenging.
In recent times, there has been a steady increase in the number of centres worldwide having vested interest in LRRC. These centres are exploring new techniques in order to improve the likelihood of adequate oncological resection. Due to this peaked interest in LRRC, previously deemed contraindications[19] (Table 1) are now proven by certain specialized centres to be feasible and safe for so called “ultra-radical resection[15]”. In addition, improved reconstructive techniques allow for better functional outcomes. Therefore the surgical approach to LRRC is currently limited by the surgeon’s ability to achieve R0 resection margins with acceptable morbidity, and the patient’s fitness for surgery. This review aims to present an update on the current international trends in surgical approaches as well as to assess the outcome and/or success of such radical procedure with emphasis on ultra-radical resections.
Table 1.
Conditions previously deemed contraindication to surgery for recurrent rectal cancer
| Distant metastases |
| Stage IV primary disease |
| Sacral invasion above S2-S3 |
| Diffuse/circumferential pelvic sidewall involvement resulting in hydronephrosis |
| Encasement (> 180°)of external iliac vessels |
| Invasion to anterior pubic bone |
| Extension of tumour through the sciatic notch |
PREOPERATIVE IMAGING
Surgical planning includes preoperative review of imaging by a specialized multidisciplinary team. The roles of preoperative imaging are three-fold: (1) to exclude metastatic disease; (2) to determine resectability of the tumour; and (3) to guide in surgical planning particularly on the extent of margin resection. The metastatic work-up includes dedicated computed tomography (CT) scan of the chest, abdomen and pelvis, and more recently positron emission tomography (PET) scan. PET/CT can be used also to assess for uptake in areas of concern especially in cases of equivocal recurrence. Next, magnetic resonance (MR) imaging is used to assess resectability of the tumour, owing to its excellent delineation of soft tissue structures. It serves as a road map for the surgical procedure and increases the chances of margin clearance. The extent of resection is decided on the basis of organs, pelvic sidewall, and pelvic floor involvement, which MR imaging has been shown to accurately demonstrate[20,21]. The primary challenge would be to distinguish between fibrotic tissues from actual tumour invasion, particularly at the pelvic sidewalls. PET/MR hybrid, which is not widely available yet, is likely to be the modality of choice in future as it has been shown to improve interpretation of the radiologists in detecting bladder or pelvic sidewall invasion in gynaecologic malignancy[22].
MULTIMODALITY TREATMENT APPROACH
In general, cases of LRRC are discussed in a multidisciplinary team (MDT) meeting which consists of surgeons, radiation oncologists, medical oncologists, pathologists, and radiologists. The important key aspects discussed are: the likelihood of obtaining R0 resection; the need for neoadjuvant therapies, and the appropriate approaches. In resectable, isolated local disease with previous history of pelvic radiotherapy, it is recommended to proceed straight to surgery. In radiotherapy naïve patients, one should consider preoperative chemoradiation and subsequently proceed with surgery 6-wk following completion of preoperative chemoradiation to allow maximum tumour response.
About 50% of patients with LRRC have synchronous distant disease[23], but this does not necessarily preclude surgical intervention. As experience has grown with the management of metastasectomy in primary disease, curative surgeries are increasingly being offered to recurrent cases. Hartley et al[24] reported on the outcome in a series of 42 patients with LRRC and synchronous resectable metastasis. Thirteen (13) patients had synchronous resection, and 9 patients had staged resection, resulting in a total of 22 patients rendered disease free (R0). When R0 is achieved (52% of patients), the median survival is 23 mo, compared to 7-mo in the non-RO group. One must take note that such benefits may be seen only in highly selected patients. The optimal sequence of surgery in the setting of resectable metastasis and LRRC remains to be clarified.
The use of preoperative chemoradiotherapy (CRT) for treatment of LRRC has been extrapolated from benefits seen in locally advanced primary rectal cancer. However, there is still a lack of good data in the literature, in particular there is a lack of prospective randomised trials, that justify the benefit of preoperative CRT. Yu et al[25] compared radiological response in patients with primary rectal cancer and recurrent rectal cancer after neoadjuvant CRT. Interestingly, their study showed that recurrent rectal cancer is 2.4 times less likely to show a > 50% reduction in tumour size following CRT. They concluded that recurrent rectal cancers appear to be relatively radioresistant compared with primary rectal cancer. The limited response may be due to the use of conventional dosages and methods of delivery via external beam radiotherapy. Recent advances in this aspect is seen in the development of more conformal radiotherapy techniques, such as intensity-modulated radiotherapy (IMRT) or intraoperative brachytherapy (IORT), which aims to deliver more targeted and higher dosage radiotherapy[26,27]. Whether such techniques, with a combination of chemosensitization, would achieve better tumour response in LRRC remains to be seen.
SURGERY FOR LRRC
The exact procedure performed is dictated by the anatomical location of the recurrence. The operating surgeon must be experienced working in anatomic planes outside of those required for conventional resection in order to perform a complete resection in a safe manner. In addition, extended resection often requires expertise from other specialties such as urology, oncoplastic surgery, neurosurgery, vascular surgery, orthopaedic surgery and gynaecology. Aids such as ureteric stents, radiological tattooing of surface markings to identify the level of sacrectomy, and vascular bypass shunts are adjuncts for some of these surgeries. Special consideration should be given to patients with prior irradiation, especially if delivered in the distant past, as the dissection may be difficult due to fibrotic tissue. In cases of bulky tumour, tumours in close proximity to major vessels or when a difficult dissection was anticipated with no possibility of vascular control, preoperative arterial embolization of internal iliac artery can be considered.
In the next section, the different procedures are reviewed according to disease location and novel techniques are described.
SURGICAL APPROACH BASED ON LOCATION OF DISEASE
Central recurrences
The surgical principle for central recurrence is straightforward. It is to resect as wide as possible to avoid positive margins to avoid leaving residual cancer post-resection. If the recurrence is at the anastomosis or in a mesorectal region without involvement of genitourinary organs, the standard operation would be an abdominal perineal resection or a ultralow Hartmann’s procedure[28]. During the pelvic phase, the plane of dissection is directed more laterally than the usual mesorectal plane to provide adequate margins from the centrally located anastomotic recurrence. A re-do anterior resection has been shown to be risky and has only been reported in selected patients with a high rate of recurrence[29-32]. It is usually difficult to accurately determine the extent of tumour spread due to the disruption of normal planes from previous operation(s) and from radiotherapy related fibrosis. Most specialised colorectal surgeons tend to prefer an augmented wide resection margin and will potentially sacrifice the possibility of restoring intestinal continuity in order to obtain a superior oncological resection margin.
If the genitourinary tract is involved, an en bloc resection is recommended[28]. Limited involvement of structures such as dome of bladder and posterior vagina can be treated adequately with partial resection provided negative margins are achieved. Tumours involving the bladder trigone or the prostate in men and cervix in women usually require a total pelvic exenteration (Figure 2). Posterior pelvic exenteration involves en bloc resection of the rectum (or tumour recurrence) and reproductive organs, sparing the bladder and distal ureters. Pelvic exenteration is still reported to have a 2%-14% operative mortality rate and a 33%-75% morbidity rate despite improved surgical techniques and perioperative care[33]. Hence, this procedure should only be attempted if the surgeon concerned is confident of achieving R0 resection margin.
Figure 2.
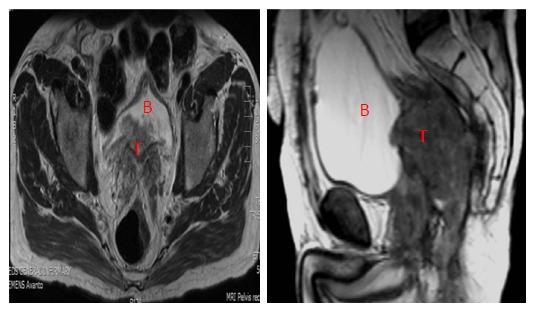
Central recurrence rectal cancer (T) involving the bladder (B).
A recent study by Bhangu et al[34] reviewed cases of pelvic exenteration performed for both locally advanced primary rectal cancer (LAPR) and LRRC done at a specialised colorectal centre with extensive experience in managing LRRC. In this study, R0 resections were achieved in 62% of LRRC cases, compared to 91% of primary rectal cancer cases. The ability to achieve a R0 resection margin status was found to be associated with improved disease-free-survival and local recurrence free survival on multivariate analysis. In the subgroup analysis of cases with R0 resection margin, the survival rate between LRRC and LAPR cases are comparable (79% vs 85%, respectively, P = 0.766). Although LRRC is at higher risk of positive resection margin, a good radical operation can still ensure a significant survival benefit provided negative resection margins are achieved.
To ensure a good functional outcome, after a radical resection, reconstructive measures of resected viscera and soft tissue are often required. Examples of reconstructive surgery after radical en bloc resection include bladder reconstruction, the formation of an ileal conduit and the reconstruction of perineal defects. Local rotation and pedicle myocutaneous flaps are particularly useful in reconstructing the perineum and filling the dead-space in the exenterated pelvis (Figure 3). A vertical rectum abdominis myocutaneous (VRAM) flap would be the more sensible option when patients have been subjected to radiotherapy or after sacrificing the gluteal vessels which significantly compromise the flap perfusion. However these procedures are complex and it is recommended that they are performed by an experienced team[35-37]. It is important to discuss with plastic surgeons preoperatively to decide on the side of the VRAM flap that is best utilised. Most plastic surgeons would prefer to harvest the flap from the right side (unless a prior ileostomy or ileal conduit has been performed) in anticipation of citing a colostomy of the left side. When a locoregional flap is not possible, either due to the presence of infection or an ileo-regional flap is being utilized in an index operation, free flaps such as a latissimus dorsi flap can be considered.
Figure 3.
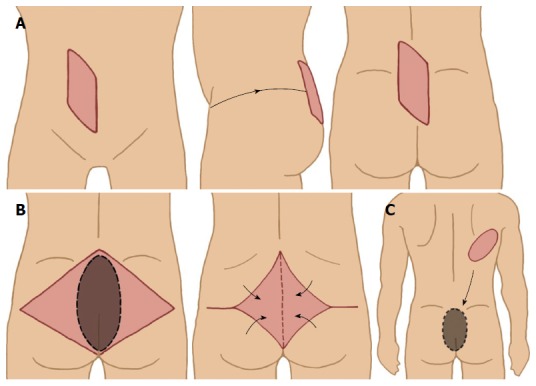
Various myocutaneous flaps used to cover perineal/sacral defect. A: Vertical rectus abdominis myocutaneous flap; B: Gluteal muscle flap; C: Latissimus dorsi free flap.
Anterior involvement of the pubic bone
Tumour recurrences infiltrating anteriorly into the pubic bone are surgically challenging, and in the past were considered inoperable[38,39]. However, Solomon et al[40] have described a surgical approach for composite resection of the anterior pubic bones as part of exenteration procedure. This surgical technique typically involves an abdominal and perineal phase. The key step during abdominal phase is to mobilize the bladder en bloc with the pubic bone and separate the anterior abdominal wall from the symphysis pubis. The level of pubic bone transection delineates the extent of resection inferiorly. The superior and inferior pubic rami are fully exposed during the perineal phase. They are then transected using either an oscillating or Gigli saw. The pelvis is structurally stable even after pubic bone resection and does not require reconstruction. The same group of surgeons have reported an acceptable quality of life after pelvic exenteration with or without en bloc pelvic bone excision[41]. While this technique has proven feasible, the short and long-term oncological outcomes are currently under review.
Sacral invasions
A number of possible treatment options are available when the tumour recurrence extends posteriorly. When sacral involvement is limited to the sacral fascia (Figure 4), an en bloc resection with periosteal elevation may achieve negative margins. However, this method carries an increased risk of intraoperative haemorrhage. A reasonable method to assess the haemorrhage risk is to attempt a trial dissection and to have a low threshold for sacrectomy. As an individual surgeon gains experience with sacrectomies, the intra-operative blood loss can be minimized by resecting the sacrum en bloc with the attached presacral venous plexus[19].
Figure 4.
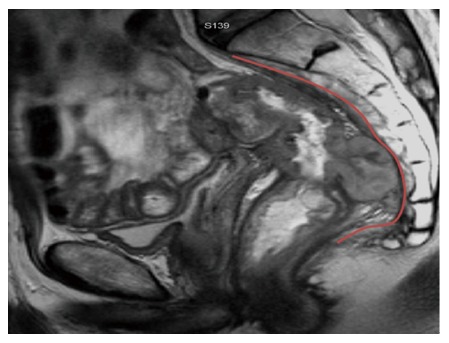
Posterior recurrence involving the presacral fascia (outlined in red).
When bony invasion is present (Figure 5), sacrectomy is mandatory and is performed via a 2-stage composite abdomino-sacral approach. A number of series have reported the safety and feasibility for sacral resection, but sacrectomies were limited to recurrence below the S2/S3 junction. These series reported promising oncological outcomes that translate to a survival benefit (Table 2). In these studies, wound-related complications are the most common and the majority were managed conservatively[42-46]. Postoperative morbidity is reduced by limiting the resection to no higher than the S2/S3 junction. This technique maintains the patient’s urogenital function via preservation of the S2/S3 nerve roots as well as the motor function of S1 nerve roots. Sacral involvement at or above the level of S2 from LRRC was considered by many as a contraindication for resection[47].
Figure 5.
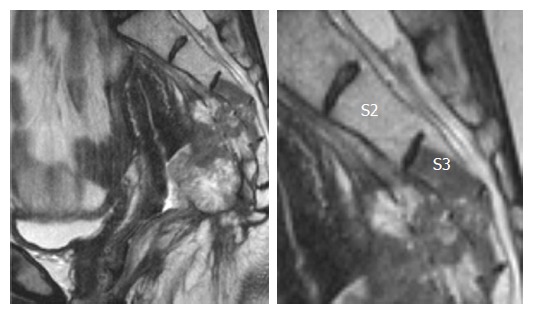
Posterior recurrence invading into distal sacrum.
Table 2.
Results of previous studies of composite abdominal-sacral resection for recurrent rectal cancer
Considering the oncological outcomes of low sacrectomy, there are now considerations made regarding the risk-benefit ratio of a high sacrectomy. This is in spite of the associated high morbidity, particularly neurologic injury and spinopelvic instability[48]. Several publications have demonstrated oncological benefit of high sacral resection for primary sacral tumours[49-51]. Wanebo et al[52] were the first to report on high sacral resection for LRRC. In their series of 53 patients, an R0 resection was achieved in 85% with a 5-year survival of 31%. Subsequently, Dozois et al[53] reported on a series of nine patients that underwent high sacral resection, in which all cases had a R0 resection with a 5-year survival rate of 30%. This represents a promising oncological outcome with regards to high sacrectomies.
More recently, Milne et al[54] compared both high and low sacrectomies. The study found that both have comparable rate of R0 resection (76% high sacrectomy vs 71% low sacrectomy). Interestingly, they found that the level of sacrectomy did not significantly increase the risk of neurological complications (P = 0.112). In addition, high sacrectomies are not associated with higher rate of major complications (43% for high sacrectomies vs 36% for low sacrectomies, P = 0.612)[54].
Sacrectomies are typically completed in the prone jack-knife position. There are few limitations to this approach which includes difficult access to the lateral compartments of the pelvis, inability to gain vascular control of major intra-pelvic vessels, and anaesthetic concerns when ventilating in the prone position. These risks are especially significant for high sacral resection. An abdominal-only approach, first described by Solomon et al[55] in 2014, is an alternative. This route offers the distinct advantage of allowing definitive vascular control and access to major arteries at all times throughout the procedure. However, exposure to the muscular and ligamentous attachments of the sacrum can be limited in this approach. To offset this, the authors suggested placing a rolled towel at the lower back of the patient during pre-operative positioning. This allows for elevation of the lumbar sacral curve towards the operative field and facilitates a safer and more measured dissection of the sacrum during the perineal phase.
One of the technical challenges of a high sacrectomy is that it requires reconstructive surgery to restore pelvic stability. The aim of reconstruction is to ensure a stable fixation between the lumbar spine and the pelvis. This can be achieved with either titanium rod fixation or occasionally via lumbopelvic bony fusion using bone allograft bone fusion[56,57]. Perineal and sacral defects can be quite extensive after en bloc sacral resection of the tumour recurrence. Primary closure may be achieved without tension and may be reinforced with a biological mesh[19,42,58,59]. Alternatively, there are various myocutaneous flaps that can be used for soft tissue reconstruction, as discussed above.
Lateral/pelvic sidewall involvement
Recurrences involving the lateral pelvic side wall often adhere to the bony pelvis or involve key structures such as the ureters, iliac vessels and the sciatic nerve (Figures 6 and 7). This makes it least likely to achieve negative surgical resection margins and therefore results in a poorer prognosis compared to central, anterior and posterior diseases[17-18,31]. Resection of the tumour would encompass en bloc resection of attached structures with subsequent reconstruction. These procedures are technically challenging and their roles are still debatable. Two recent Delphi studies[60,61], have confirmed the views of many world experts that extensive lateral side wall involvement still remains a relative contraindication to curative surgery.
Figure 6.
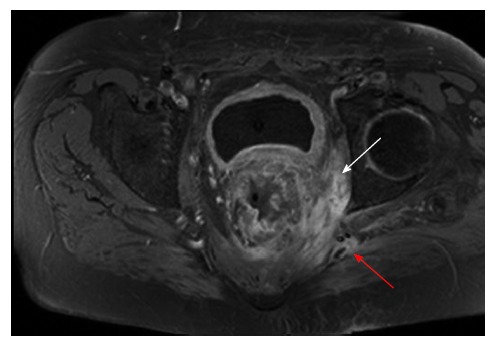
Recurrent rectal cancer in the lower left lateral compartment invading the obturator internus muscle (white arrow) and posteriorly involving the superior gluteal nerve (red arrow).
Figure 7.
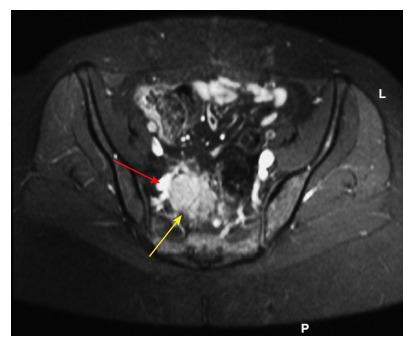
Recurrent rectal cancer at right lateral side wall (yellow arrow), with close proximity to iliac vessels (red arrow); this requires excision of involved vascular segment and reconstruction.
Austin et al[62] from the Sydney group have described a promising approach to extensive lateral sidewall disease, with dissection in the plane between the bony pelvis and the sidewall musculature. The surgery begins with mobilization of the common and external iliac vessels from the main bifurcation of the aorta and vena cava. The ureter is identified and mobilized distally to the vesico-ureteric junction. If the ureter is involved with tumour, en bloc resection is required and the ureter is divided proximal to the level of tumour involvement. The internal iliac artery and vein are ligated and divided at their origins. This will mitigate the risk of heavy pelvic haemorrhage when dissection of the tumour is carried out. The external iliac vessels are mobilized down to the level of the inguinal ligaments.
When the external iliac vessels are involved with either adherence or encasement of the tumour, the involved segment is to be removed en bloc with the tumour to achieve a clear margin. Various vascular reconstruction methods have been described using autologous and synthetic grafts[63,64]. The key is immediate reconstruction to prevent compartment syndrome and thrombosis. Once the major vessels are reflected away, and with medial retraction of the tumour mass, a posterolateral plane of dissection will lead to the lumbar-sacral trunk and sacral nerve roots and then on to the piriformis and obturator muscles as appropriate. An energy device can be used to better achieve hemostasis. If required, lateral recurrence within the greater sciatic notch, the bony ischium, the sacrotuberous and sacrospinatus ligaments could be resected en bloc.
En bloc resection of the sciatic nerve is an established practice for pelvic and lower limb soft tissue sarcoma with acceptable postoperative functional outcomes[65,66]. In the 1990s, Kameyama et al[67] reported on three patients with LRRC involving the sciatic nerve that underwent pelvic exenteration with en bloc sciatic nerve resection. All showed improved quality of life and an ability to walk unassisted with the help of below-the-knee braces.
As expected, the literature is limited regarding the outcomes of patients undergoing surgery for LRRC involving the lateral pelvic side wall. One othe earlier reports from is from Japan. Yamada et al[17] showed no survivors at 5 years for 17 patients with lateral recurrent disease that underwent radical resection. Moore and colleague at Memorial Sloan-Kettering Cancer Centre[18] demonstrated that in cases where pelvic side wall recurrence was suggested preoperatively, there was a 19% rate of R0 resection rate and a similar poor oncological outcome as the study by Yamada. A more recent study, the largest series to date, has reported a hundred cases of pelvic exenteration with lateral pelvic wall excision for LRRC[68]. This was the same group that described the novel approach to lateral pelvic wall excision mentioned above. With the use of this radical technique, they could achieve R0 resection in 62% of patients with LRRC and this has translated to a median overall survival of 35 mo and overall 3-year survival rate of 45%. The authors have observed improved results following experience with this technique and progressive support from multidisciplinary experts.
An alternative approach has been recently described by the St Mark’s group[69]. It involves an initial transgluteal approach to the sciatic notch which is performed with patient in prone position. Once the lateral recurrence has been fully excised, the patient is turned supine to complete the operation via laparotomy. The group successfully achieved R0 resection margin in all their 6 patients. In our opinion, this technique is feasible only in selected patients. It does not permit adequate vascular access and hence would not be suitable for patients with disease involving the iliac vessels.
LAPAROSCOPIC SURGERY FOR LRRC
Open surgery for laparoscopic surgery for LRRC is considered by many as technically challenging, let alone a laparoscopic approach. To date, there are only a few reporting the safety and feasibility of laparoscopic surgery for LRRC (Table 3). The key factors for cases deemed suitable for laparoscopic surgery (all reported cases were non-exenterative surgeries) are (1) centrally located tumour or at most with lateral pelvic nodal recurrence; and (2) surgeons who are skilled in laparoscopic rectal surgery particularly in laparoscopic lateral pelvic node dissection. The authors of these studies[70-74] commented on the advantage of the high-definition magnified view obtained during laparoscopy that facilitates precise dissection in the deep pelvis with distorted anatomical plane. The laparoscopic approach also demonstrated reduced blood loss and earlier return of bowel function[72-74]. From the oncological point of view, all the laparoscopic cases have achieved R0 resection.
Table 3.
Studies on laparoscopic surgery for recurrent rectal cancer
| Ref. | Year | Study design | No. of patients | Site of recurrence | Median blood loss, mL (range) | Median operative time, min (range) | R0 resection rate | Overall postoperative mortality/morbidity rate |
| Lu et al[70] | 2006 | Case series | 7 | Central recurrence: 6 | 200 (109-291) | 211 (198–224) | 100% | NR |
| Presacral: 1 | ||||||||
| Kim et al[71] | 2008 | Case report | 1 | Central/anastomotic recurrence | 50 | 185 | 100% | NR |
| Park et al[72] | 2011 | Comparative study | Lap: 15 | Anastomotic site, ovary and pelvic lateral LN | NR | Lap: 150 (48-460) | Lap: 100% | Lap: 13.3% |
| Open: 26 | Open: 259 (40-514) | Open: 84.6% | Open: 57.7% | |||||
| P = 0.059 | P = NS | P ≤ 0.05 | ||||||
| Nagasaki et al[73] | 2014 | Comparative study | Lap: 13 | Central and Lateral pelvic LN | Lap: 110 (60-800) | Lap: 381 (227-554) | Lap: 100% | Lap: 30.8% |
| Open: 17 | Open: 450 (25-1600) | Open: 241 (125-694) | Open: 94% | Open: 23.5% | ||||
| P = 0.075 | P = 0.024 | P = 0.99 | P = 0.69 | |||||
| Akiyoshi et al[74] | 2015 | Case series | 9 | Lateral pelvic LN | 130 (25-200) | 381 (227-554) | 100% | 33.30% |
CONCLUSION
Management strategies for LRRC cases have evolved over time with more surgeons expanding the criteria in offering curative surgery. With the continuously evolving techniques in radical surgery, as evident in the literature, there has been significant improvement in achieving an R0 margin status. The prospect of survival for these patients has therefore improved significantly. However, due to the complexity of the disease, there is no “one-size-fits-all” surgical approach. LRRC is usually not confined to a single compartment within the pelvis. It is therefore of paramount importance to have a good understanding of the complex anatomy of the pelvis in order to formulate the most appropriate surgical approach. The extent of the radical resection should be tailored to the needs of the patient. A few novel techniques have been developed to encompass a “wider” and “higher” excision, including en bloc resections of the sciatic notch and composite high sacral (S1 and S2) resection. The preliminary data is promising, but the patient pool in each case series is small. In addition, the studies were conducted in highly specialised units. Understandably, there is a lack of substantial research and/or evidence in terms of long-term outcomes. Although surgical options for LRRC have expanded significantly, one should balance the treatment choices available against the morbidity associated with the procedure and select the right patient for it.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Singapore
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: Authors declare no conflict of interests for this article.
Peer-review started: February 4, 2017
First decision: February 23, 2017
Article in press: May 9, 2017
P- Reviewer: Augustin G, Lakatos PL, Piso P, Seow-Choen F S- Editor: Qi Y L- Editor: A E- Editor: Zhang FF
References
- 1.Phillips RK, Hittinger R, Blesovsky L, Fry JS, Fielding LP. Local recurrence following ‘curative’ surgery for large bowel cancer: II. The rectum and rectosigmoid. Br J Surg. 1984;71:17–20. doi: 10.1002/bjs.1800710105. [DOI] [PubMed] [Google Scholar]
- 2.Bokey EL, Ojerskog B, Chapuis PH, Dent OF, Newland RC, Sinclair G. Local recurrence after curative excision of the rectum for cancer without adjuvant therapy: role of total anatomical dissection. Br J Surg. 1999;86:1164–1170. doi: 10.1046/j.1365-2168.1999.01216.x. [DOI] [PubMed] [Google Scholar]
- 3.Cedermark B, Dahlberg M, Glimelius B, Påhlman L, Rutqvist LE, Wilking N. Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med. 1997;336:980–987. doi: 10.1056/NEJM199704033361402. [DOI] [PubMed] [Google Scholar]
- 4.Hansen MH, Kjaeve J, Revhaug A, Eriksen MT, Wibe A, Vonen B. Impact of radiotherapy on local recurrence of rectal cancer in Norway. Br J Surg. 2007;94:113–118. doi: 10.1002/bjs.5576. [DOI] [PubMed] [Google Scholar]
- 5.Shoup M, Guillem JG, Alektiar KM, Liau K, Paty PB, Cohen AM, Wong WD, Minsky BD. Predictors of survival in recurrent rectal cancer after resection and intraoperative radiotherapy. Dis Colon Rectum. 2002;45:585–592. doi: 10.1007/s10350-004-6250-9. [DOI] [PubMed] [Google Scholar]
- 6.Palmer G, Martling A, Cedermark B, Holm T. A population-based study on the management and outcome in patients with locally recurrent rectal cancer. Ann Surg Oncol. 2007;14:447–454. doi: 10.1245/s10434-006-9256-9. [DOI] [PubMed] [Google Scholar]
- 7.Silberfein EJ, Kattepogu KM, Hu CY, Skibber JM, Rodriguez-Bigas MA, Feig B, Das P, Krishnan S, Crane C, Kopetz S, et al. Long-term survival and recurrence outcomes following surgery for distal rectal cancer. Ann Surg Oncol. 2010;17:2863–2869. doi: 10.1245/s10434-010-1119-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gunderson LL, Sosin H. Areas of failure found at reoperation (second or symptomatic look) following “curative surgery” for adenocarcinoma of the rectum. Clinicopathologic correlation and implications for adjuvant therapy. Cancer. 1974;34:1278–1292. doi: 10.1002/1097-0142(197410)34:4<1278::aid-cncr2820340440>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 9.Wanebo HJ, Koness RJ, Vezeridis MP, Cohen SI, Wrobleski DE. Pelvic resection of recurrent rectal cancer. Ann Surg. 1994;220:586–595; discussion 595-597. doi: 10.1097/00000658-199410000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong CS, Cummings BJ, Brierley JD, Catton CN, McLean M, Catton P, Hao Y. Treatment of locally recurrent rectal carcinoma--results and prognostic factors. Int J Radiat Oncol Biol Phys. 1998;40:427–435. doi: 10.1016/s0360-3016(97)00737-2. [DOI] [PubMed] [Google Scholar]
- 11.Ito Y, Ohtsu A, Ishikura S, Boku N, Nihei K, Ogino T, Ikeda H. Efficacy of chemoradiotherapy on pain relief in patients with intrapelvic recurrence of rectal cancer. Jpn J Clin Oncol. 2003;33:180–185. doi: 10.1093/jjco/hyg036. [DOI] [PubMed] [Google Scholar]
- 12.Danjoux CE, Gelber RD, Catton GE, Klaassen DJ. Combination chemo-radiotherapy for residual, recurrent or inoperable carcinoma of the rectum: E.C.O.G. study (EST 3276) Int J Radiat Oncol Biol Phys. 1985;11:765–771. doi: 10.1016/0360-3016(85)90309-8. [DOI] [PubMed] [Google Scholar]
- 13.Wanebo HJ, Gaker DL, Whitehill R, Morgan RF, Constable WC. Pelvic recurrence of rectal cancer. Options for curative resection. Ann Surg. 1987;205:482–495. doi: 10.1097/00000658-198705000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magrini S, Nelson H, Gunderson LL, Sim FH. Sacropelvic resection and intraoperative electron irradiation in the management of recurrent anorectal cancer. Dis Colon Rectum. 1996;39:1–9. doi: 10.1007/BF02048260. [DOI] [PubMed] [Google Scholar]
- 15.Sagar PM. Ultraradical resection for locally recurrent rectal cancer. Dis Colon Rectum. 2014;57:1–2. doi: 10.1097/DCR.0000000000000014. [DOI] [PubMed] [Google Scholar]
- 16.Harris CA, Solomon MJ, Heriot AG, Sagar PM, Tekkis PP, Dixon L, Pascoe R, Dobbs BR, Frampton CM, Harji DP, et al. The Outcomes and Patterns of Treatment Failure After Surgery for Locally Recurrent Rectal Cancer. Ann Surg. 2016;264:323–329. doi: 10.1097/SLA.0000000000001524. [DOI] [PubMed] [Google Scholar]
- 17.Yamada K, Ishizawa T, Niwa K, Chuman Y, Akiba S, Aikou T. Patterns of pelvic invasion are prognostic in the treatment of locally recurrent rectal cancer. Br J Surg. 2001;88:988–993. doi: 10.1046/j.0007-1323.2001.01811.x. [DOI] [PubMed] [Google Scholar]
- 18.Moore HG, Shoup M, Riedel E, Minsky BD, Alektiar KM, Ercolani M, Paty PB, Wong WD, Guillem JG. Colorectal cancer pelvic recurrences: determinants of resectability. Dis Colon Rectum. 2004;47:1599–1606. doi: 10.1007/s10350-004-0677-x. [DOI] [PubMed] [Google Scholar]
- 19.Mirnezami AH, Sagar PM. Surgery for recurrent rectal cancer: technical notes and management of complications. Tech Coloproctol. 2010;14:209–216. doi: 10.1007/s10151-010-0585-0. [DOI] [PubMed] [Google Scholar]
- 20.Messiou C, Chalmers A, Boyle K, Sagar P. Surgery for recurrent rectal carcinoma: The role of preoperative magnetic resonance imaging. Clin Radiol. 2006;61:250–258. doi: 10.1016/j.crad.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 21.Dresen RC, Kusters M, Daniels-Gooszen AW, Cappendijk VC, Nieuwenhuijzen GA, Kessels AG, de Bruïne AP, Beets GL, Rutten HJ, Beets-Tan RG. Absence of tumor invasion into pelvic structures in locally recurrent rectal cancer: prediction with preoperative MR imaging. Radiology. 2010;256:143–150. doi: 10.1148/radiol.10090725. [DOI] [PubMed] [Google Scholar]
- 22.Vargas HA, Burger IA, Donati OF, Andikyan V, Lakhman Y, Goldman DA, Schöder H, Chi DS, Sala E, Hricak H. Magnetic resonance imaging/positron emission tomography provides a roadmap for surgical planning and serves as a predictive biomarker in patients with recurrent gynecological cancers undergoing pelvic exenteration. Int J Gynecol Cancer. 2013;23:1512–1519. doi: 10.1097/IGC.0b013e3182a41e61. [DOI] [PubMed] [Google Scholar]
- 23.Gagliardi G, Hawley PR, Hershman MJ, Arnott SJ. Prognostic factors in surgery for local recurrence of rectal cancer. Br J Surg. 1995;82:1401–1405. doi: 10.1002/bjs.1800821035. [DOI] [PubMed] [Google Scholar]
- 24.Hartley JE, Lopez RA, Paty PB, Wong WD, Cohen AM, Guillem JG. Resection of locally recurrent colorectal cancer in the presence of distant metastases: can it be justified? Ann Surg Oncol. 2003;10:227–233. doi: 10.1245/aso.2003.05.039. [DOI] [PubMed] [Google Scholar]
- 25.Yu SK, Bhangu A, Tait DM, Tekkis P, Wotherspoon A, Brown G. Chemoradiotherapy response in recurrent rectal cancer. Cancer Med. 2014;3:111–117. doi: 10.1002/cam4.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glimelius B. Recurrent rectal cancer. The pre-irradiated primary tumour: can more radiotherapy be given? Colorectal Dis. 2003;5:501–503. doi: 10.1046/j.1463-1318.2003.00501.x. [DOI] [PubMed] [Google Scholar]
- 27.Guo S, Reddy CA, Kolar M, Woody N, Mahadevan A, Deibel FC, Dietz DW, Remzi FH, Suh JH. Intraoperative radiation therapy with the photon radiosurgery system in locally advanced and recurrent rectal cancer: retrospective review of the Cleveland clinic experience. Radiat Oncol. 2012;7:110. doi: 10.1186/1748-717X-7-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mirnezami AH, Sagar PM, Kavanagh D, Witherspoon P, Lee P, Winter D. Clinical algorithms for the surgical management of locally recurrent rectal cancer. Dis Colon Rectum. 2010;53:1248–1257. doi: 10.1007/DCR.0b013e3181e10b0e. [DOI] [PubMed] [Google Scholar]
- 29.Salo JC, Paty PB, Guillem J, Minsky BD, Harrison LB, Cohen AM. Surgical salvage of recurrent rectal carcinoma after curative resection: a 10-year experience. Ann Surg Oncol. 1999;6:171–177. doi: 10.1007/s10434-999-0171-8. [DOI] [PubMed] [Google Scholar]
- 30.Boyle KM, Sagar PM, Chalmers AG, Sebag-Montefiore D, Cairns A, Eardley I. Surgery for locally recurrent rectal cancer. Dis Colon Rectum. 2005;48:929–937. doi: 10.1007/s10350-004-0909-0. [DOI] [PubMed] [Google Scholar]
- 31.Heriot AG, Byrne CM, Lee P, Dobbs B, Tilney H, Solomon MJ, Mackay J, Frizelle F. Extended radical resection: the choice for locally recurrent rectal cancer. Dis Colon Rectum. 2008;51:284–291. doi: 10.1007/s10350-007-9152-9. [DOI] [PubMed] [Google Scholar]
- 32.Yun JA, Huh JW, Kim HC, Park YA, Cho YB, Yun SH, Lee WY, Chun HK. Local recurrence after curative resection for rectal carcinoma: The role of surgical resection. Medicine (Baltimore) 2016;95:e3942. doi: 10.1097/MD.0000000000003942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang TX, Morris DL, Chua TC. Pelvic exenteration for rectal cancer: a systematic review. Dis Colon Rectum. 2013;56:519–531. doi: 10.1097/DCR.0b013e31827a7868. [DOI] [PubMed] [Google Scholar]
- 34.Bhangu A, Ali SM, Brown G, Nicholls RJ, Tekkis P. Indications and outcome of pelvic exenteration for locally advanced primary and recurrent rectal cancer. Ann Surg. 2014;259:315–322. doi: 10.1097/SLA.0b013e31828a0d22. [DOI] [PubMed] [Google Scholar]
- 35.Christensen HK, Nerstrøm P, Tei T, Laurberg S. Perineal repair after extralevator abdominoperineal excision for low rectal cancer. Dis Colon Rectum. 2011;54:711–717. doi: 10.1007/DCR.0b013e3182163c89. [DOI] [PubMed] [Google Scholar]
- 36.Hinojosa MW, Parikh DA, Menon R, Wirth GA, Stamos MJ, Mills S. Recent experience with abdominal perineal resection with vertical rectus abdominis myocutaneous flap reconstruction after preoperative pelvic radiation. Am Surg. 2009;75:995–999. [PubMed] [Google Scholar]
- 37.Anderin C, Martling A, Lagergren J, Ljung A, Holm T. Short-term outcome after gluteus maximus myocutaneous flap reconstruction of the pelvic floor following extra-levator abdominoperineal excision of the rectum. Colorectal Dis. 2012;14:1060–1064. doi: 10.1111/j.1463-1318.2011.02848.x. [DOI] [PubMed] [Google Scholar]
- 38.Yu HH, Leong CH, Ong GB. Pelvic exenteration for advanced pelvic malignancies. Aust N Z J Surg. 1976;46:197–201. doi: 10.1111/j.1445-2197.1976.tb03314.x. [DOI] [PubMed] [Google Scholar]
- 39.Hahnloser D, Nelson H, Gunderson LL, Hassan I, Haddock MG, O’Connell MJ, Cha S, Sargent DJ, Horgan A. Curative potential of multimodality therapy for locally recurrent rectal cancer. Ann Surg. 2003;237:502–508. doi: 10.1097/01.SLA.0000059972.90598.5F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Solomon MJ, Austin KK, Masya L, Lee P. Pubic Bone Excision and Perineal Urethrectomy for Radical Anterior Compartment Excision During Pelvic Exenteration. Dis Colon Rectum. 2015;58:1114–1119. doi: 10.1097/DCR.0000000000000479. [DOI] [PubMed] [Google Scholar]
- 41.Austin KK, Young JM, Solomon MJ. Quality of life of survivors after pelvic exenteration for rectal cancer. Dis Colon Rectum. 2010;53:1121–1126. doi: 10.1007/DCR.0b013e3181e10c46. [DOI] [PubMed] [Google Scholar]
- 42.Sagar PM, Gonsalves S, Heath RM, Phillips N, Chalmers AG. Composite abdominosacral resection for recurrent rectal cancer. Br J Surg. 2009;96:191–196. doi: 10.1002/bjs.6464. [DOI] [PubMed] [Google Scholar]
- 43.Ferenschild FT, Vermaas M, Verhoef C, Dwarkasing RS, Eggermont AM, de Wilt JH. Abdominosacral resection for locally advanced and recurrent rectal cancer. Br J Surg. 2009;96:1341–1347. doi: 10.1002/bjs.6695. [DOI] [PubMed] [Google Scholar]
- 44.Melton GB, Paty PB, Boland PJ, Healey JH, Savatta SG, Casas-Ganem JE, Guillem JG, Weiser MR, Cohen AM, Minsky BD, et al. Sacral resection for recurrent rectal cancer: analysis of morbidity and treatment results. Dis Colon Rectum. 2006;49:1099–1107. doi: 10.1007/s10350-006-0563-9. [DOI] [PubMed] [Google Scholar]
- 45.Moriya Y, Akasu T, Fujita S, Yamamoto S. Total pelvic exenteration with distal sacrectomy for fixed recurrent rectal cancer in the pelvis. Dis Colon Rectum. 2004;47:2047–2053; discussion 2053-2054. doi: 10.1007/s10350-004-0714-9. [DOI] [PubMed] [Google Scholar]
- 46.Weber KL, Nelson H, Gunderson LL, Sim FH. Sacropelvic resection for recurrent anorectal cancer. A multidisciplinary approach. Clin Orthop Relat Res. 2000;(372):231–240. doi: 10.1097/00003086-200003000-00025. [DOI] [PubMed] [Google Scholar]
- 47.Hansen PJ. Effects of coat colour on physiological responses to solar radiation in Holsteins. Vet Rec. 1990;127:333–334. [PubMed] [Google Scholar]
- 48.Wanebo HJ, Marcove RC. Abdominal sacral resection of locally recurrent rectal cancer. Ann Surg. 1981;194:458–471. doi: 10.1097/00000658-198110000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sahakitrungruang C, Chantra K, Dusitanond N, Atittharnsakul P, Rojanasakul A. Sacrectomy for primary sacral tumors. Dis Colon Rectum. 2009;52:913–918. doi: 10.1007/DCR.0b013e3181a0d932. [DOI] [PubMed] [Google Scholar]
- 50.Huth JF, Dawson EG, Eilber FR. Abdominosacral resection for malignant tumors of the sacrum. Am J Surg. 1984;148:157–161. doi: 10.1016/0002-9610(84)90304-0. [DOI] [PubMed] [Google Scholar]
- 51.Fourney DR, Rhines LD, Hentschel SJ, Skibber JM, Wolinsky JP, Weber KL, Suki D, Gallia GL, Garonzik I, Gokaslan ZL. En bloc resection of primary sacral tumors: classification of surgical approaches and outcome. J Neurosurg Spine. 2005;3:111–122. doi: 10.3171/spi.2005.3.2.0111. [DOI] [PubMed] [Google Scholar]
- 52.Wanebo HJ, Antoniuk P, Koness RJ, Levy A, Vezeridis M, Cohen SI, Wrobleski DE. Pelvic resection of recurrent rectal cancer: technical considerations and outcomes. Dis Colon Rectum. 1999;42:1438–1448. doi: 10.1007/BF02235044. [DOI] [PubMed] [Google Scholar]
- 53.Dozois EJ, Privitera A, Holubar SD, Aldrete JF, Sim FH, Rose PS, Walsh MF, Bower TC, Leibovich BC, Nelson H, et al. High sacrectomy for locally recurrent rectal cancer: Can long-term survival be achieved? J Surg Oncol. 2011;103:105–109. doi: 10.1002/jso.21774. [DOI] [PubMed] [Google Scholar]
- 54.Milne T, Solomon MJ, Lee P, Young JM, Stalley P, Harrison JD. Assessing the impact of a sacral resection on morbidity and survival after extended radical surgery for locally recurrent rectal cancer. Ann Surg. 2013;258:1007–1013. doi: 10.1097/SLA.0b013e318283a5b6. [DOI] [PubMed] [Google Scholar]
- 55.Solomon MJ, Tan KK, Bromilow RG, Al-mozany N, Lee PJ. Sacrectomy via the abdominal approach during pelvic exenteration. Dis Colon Rectum. 2014;57:272–277. doi: 10.1097/DCR.0000000000000039. [DOI] [PubMed] [Google Scholar]
- 56.Bederman SS, Shah KN, Hassan JM, Hoang BH, Kiester PD, Bhatia NN. Surgical techniques for spinopelvic reconstruction following total sacrectomy: a systematic review. Eur Spine J. 2014;23:305–319. doi: 10.1007/s00586-013-3075-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kawahara N, Murakami H, Yoshida A, Sakamoto J, Oda J, Tomita K. Reconstruction after total sacrectomy using a new instrumentation technique: a biomechanical comparison. Spine (Phila Pa 1976) 2003;28:1567–1572. [PubMed] [Google Scholar]
- 58.Butler CE, Rodriguez-Bigas MA. Pelvic reconstruction after abdominoperineal resection: is it worthwhile? Ann Surg Oncol. 2005;12:91–94. doi: 10.1245/ASO.2005.11.923. [DOI] [PubMed] [Google Scholar]
- 59.Jacombs AS, Rome P, Harrison JD, Solomon MJ. Assessment of the selection process for myocutaneous flap repair and surgical complications in pelvic exenteration surgery. Br J Surg. 2013;100:561–567. doi: 10.1002/bjs.9002. [DOI] [PubMed] [Google Scholar]
- 60.Chew MH, Brown WE, Masya L, Harrison JD, Myers E, Solomon MJ. Clinical, MRI, and PET-CT criteria used by surgeons to determine suitability for pelvic exenteration surgery for recurrent rectal cancers: a Delphi study. Dis Colon Rectum. 2013;56:717–725. doi: 10.1097/DCR.0b013e3182812bec. [DOI] [PubMed] [Google Scholar]
- 61.Beyond TME Collaborative. Consensus statement on the multidisciplinary management of patients with recurrent and primary rectal cancer beyond total mesorectal excision planes. Br J Surg. 2013;100:1009–1014. doi: 10.1002/bjs.9192. [DOI] [PubMed] [Google Scholar]
- 62.Austin KK, Solomon MJ. Pelvic exenteration with en bloc iliac vessel resection for lateral pelvic wall involvement. Dis Colon Rectum. 2009;52:1223–1233. doi: 10.1007/DCR.0b013e3181a73f48. [DOI] [PubMed] [Google Scholar]
- 63.Brown KG, Koh CE, Solomon MJ, Choy IC, Dubenec S. Spiral saphenous vein graft for major pelvic vessel reconstruction during exenteration surgery. Ann Vasc Surg. 2015;29:1323–1326. doi: 10.1016/j.avsg.2015.01.028. [DOI] [PubMed] [Google Scholar]
- 64.Abdelsattar ZM, Mathis KL, Colibaseanu DT, Merchea A, Bower TC, Larson DW, Dozois EJ. Surgery for locally advanced recurrent colorectal cancer involving the aortoiliac axis: can we achieve R0 resection and long-term survival? Dis Colon Rectum. 2013;56:711–716. doi: 10.1097/DCR.0b013e31827dbcb0. [DOI] [PubMed] [Google Scholar]
- 65.Brooks AD, Gold JS, Graham D, Boland P, Lewis JJ, Brennan MF, Healey JH. Resection of the sciatic, peroneal, or tibial nerves: assessment of functional status. Ann Surg Oncol. 2002;9:41–47. doi: 10.1245/aso.2002.9.1.41. [DOI] [PubMed] [Google Scholar]
- 66.Fuchs B, Davis AM, Wunder JS, Bell RS, Masri BA, Isler M, Turcotte R, Rock MG. Sciatic nerve resection in the thigh: a functional evaluation. Clin Orthop Relat Resp. 2001;382:34–41. doi: 10.1097/00003086-200101000-00007. [DOI] [PubMed] [Google Scholar]
- 67.Kameyama M, Nakamori S, Imaoka S, Hinakawa M, Sasaki Y, Ishikawa O, Kabuto T, Furukawa H, Iwanaga T, Ueda T. [Composite resection of sciatic nerve for local recurrence of rectal cancer] Gan To Kagaku Ryoho. 1993;20:1689–1691. [PubMed] [Google Scholar]
- 68.Solomon MJ, Brown KG, Koh CE, Lee P, Austin KK, Masya L. Lateral pelvic compartment excision during pelvic exenteration. Br J Surg. 2015;102:1710–1717. doi: 10.1002/bjs.9915. [DOI] [PubMed] [Google Scholar]
- 69.Shaikh I, Aston W, Hellawell G, Ross D, Littler S, Burling D, Marshall M, Northover JM, Antoniou A, Jenkins JT. Extended lateral pelvic sidewall excision (ELSiE): an approach to optimize complete resection rates in locally advanced or recurrent anorectal cancer involving the pelvic sidewall. Tech Coloproctol. 2014;18:1161–1168. doi: 10.1007/s10151-014-1234-9. [DOI] [PubMed] [Google Scholar]
- 70.Lu AG, Wang ML, Hu WG, Li JW, Zang L, Mao ZH, Dong F, Feng B, Ma JJ, Zong YP, et al. [Experience of laparoscopic salvage surgery for locally recurrent rectal cancer] Zhonghua Waike Zazhi. 2006;44:597–599. [PubMed] [Google Scholar]
- 71.Kim SH, Neve RS, Joh YG. Multimedia article. Relaparoscopy for salvage surgery in anastomotic recurrence of rectal cancer: feasible and safe. Dis Colon Rectum. 2008;51:1712–1713. doi: 10.1007/s10350-008-9296-2. [DOI] [PubMed] [Google Scholar]
- 72.Park SY, Choi GS, Jun SH, Park JS, Kim HJ. Laparoscopic salvage surgery for recurrent and metachronous colorectal cancer: 15 years’ experience in a single center. Surg Endosc. 2011;25:3551–3558. doi: 10.1007/s00464-011-1756-4. [DOI] [PubMed] [Google Scholar]
- 73.Nagasaki T, Akiyoshi T, Ueno M, Fukunaga Y, Nagayama S, Fujimoto Y, Konishi T, Yamaguchi T. Laparoscopic salvage surgery for locally recurrent rectal cancer. J Gastrointest Surg. 2014;18:1319–1326. doi: 10.1007/s11605-014-2537-x. [DOI] [PubMed] [Google Scholar]
- 74.Akiyoshi T, Nagata J, Nagasaki T, Konishi T, Fujimoto Y, Nagayama S, Fukunaga Y, Ueno M. Laparoscopic salvage lateral pelvic lymph node dissection for locally recurrent rectal cancer. Colorectal Dis. 2015;17:O213–O216. doi: 10.1111/codi.13088. [DOI] [PubMed] [Google Scholar]


