Abstract
AIM
To show outcomes of our series of patients that underwent a total gastrectomy with a robotic approach and highlight the technical details of a proposed solution for the reconstruction phase.
METHODS
Data of gastrectomies performed from May 2014 to October 2016, were extracted and analyzed. Basic characteristics of patients, surgical and clinical outcomes were reported. The technique for reconstruction (Parisi Technique) consists on a loop of bowel shifted up antecolic to directly perform the esophago-enteric anastomosis followed by a second loop, measured up to 40 cm starting from the esojejunostomy, fixed to the biliary limb to create an enteroenteric anastomosis. The continuity between the two anastomoses is interrupted just firing a linear stapler, so obtaining the Roux-en-Y by avoiding to interrupt the mesentery.
RESULTS
Fifty-five patients were considered in the present analysis. Estimated blood loss was 126.55 ± 73 mL, no conversions to open surgery occurred, R0 resections were obtained in all cases. Hospital stay was 5 (3-17) d, no anastomotic leakage occurred. Overall, a fast functional recovery was shown with a median of 3 (3-6) d in starting a solid diet.
CONCLUSION
Robotic surgery and the adoption of a tailored reconstruction technique have increased the feasibility and safety of a minimally invasive approach for total gastrectomy. The present series of patients shows its implementation in a western center with satisfying short-term outcomes.
Keywords: Esophagojejunal anastomosis, Gastric cancer, Total gastrectomy, Robotic surgery, Minimally invasive surgery
Core tip: Minimally invasive surgery is growing interest for gastric cancer. Technology has allowed to increase the safety and feasibility of this approach, even in demanding procedures. Total gastrectomy represents a challenge in this context due to the need to ensure a safe esophagojejunal anastomosis. Leakages can strongly influence the postoperative course of the patient until lead to serious consequences.
INTRODUCTION
Minimally invasive surgery for gastric cancer is growing attention due to its potential advantages in enhancing the postoperative recovery of patients and quality of life[1]. The main limitations are the proper execution of an extended lymphadenectomy, when required, and a safe approach for reconstruction.
The latter is still the object of controversy and most surgeons are concerned about the possibility to perform a totally intracorporeal procedure after a total gastrectomy.
Recent spread of the robotic systems has modified the way we perform minimally invasive surgery and has led to the evolution of traditional laparoscopy. This progress allows surgeons to overcome the limits of laparoscopy through a 3D vision, articulated instruments, filtering of physiological tremor and absence of fulcrum effects, thus increasing dexterity and precision in dissection and suturing movements. These are key factors required for complex and technical demanding reconstructions to restore the digestive continuity.
However, nowadays, few studies[2] have discussed about reconstruction techniques for minimally invasive gastric surgery even if this issue is the most impacted factor on postoperative outcomes.
Hybrid procedures are common described in the literature, in most of cases an extracorporeal reconstruction approach has been adopted because allows to easily overcome the difficulties of an intracorporeal Roux-en-y procedure.
The main limitations surgeons have in minimally invasive reconstruction after total gastrectomy are: reduced freedom of movements in the pneumoperitoneum space, properly identification of the segment of small bowel for the E-J anastomosis and then the level where perform the Jejunojejunal anastomosis. Moreover, traditional laparoscopic instruments cannot enable a hand-sewn anastomosis, thus surgeons have adopted techniques based on the use of mechanical staplers. However, intrinsic limitations of these methods should be considered when approaching an intracorporeal anastomosis involving the esophagus.
This study aims to show outcomes after adopting a new robot-sewn intracorporeal reconstruction technique after total gastrectomy, conceived at our Institution, that can simplify and make friendly this challenging phase.
MATERIALS AND METHODS
Type of study
This is a single-institution observational study, evaluating a new reconstruction approach after total gastrectomy. The study was registered at clinical trials.gov with the registration number: NCT02325453.
Eligibility
Patients with the following characteristics were included: preoperative staging assessment in accordance to international guidelines[3,4], Early Gastric Cancer, Advanced Gastric Cancer, curative surgery, robotic surgical approach. Exclusion criteria were: metastatic disease, palliative resection, synchronous malignancy in other organs, synchronous other major abdominal surgery, high operative risk (ASA > 4).
Data collection
Data of gastrectomies performed from May 2014 to October 2016, at St. Mary’s Hospital of Terni (Italy), were extracted and analyzed.
Data were collected by reviewing medical records and surgeries performed[5]. A tailored web-based protected system was used (https://imigastric.logix-software.it/).
The present study was planned and developed in accordance with the STROBE guidelines and statement[6].
Reported outcomes
Descriptive information on characteristics of patients, details of procedures and tumor findings were reported.
Operative results, data on the postoperative course and assessment of complications were based on the following primary outcomes: Estimated blood loss (EBL, mL), retrieved lymphnodes (No.), hospital stay (d), resumption of a liquid and solid diet (POD), in-hospital complications (No., Type), 30 d readmission (No.).
Secondary outcomes included: the operative time (min), margin status (No. negative/total) and R assessment (No. R0 resections), intraoperative complications and death (No.), resumption of peristalsis (POD).
Statistical analysis
IBM SPSS Statistics V.23 was used in this study and an intention to treat analysis was performed. Continuous variables were reported as mean ± SD or median and range. Numbers and percentages were used to express dichotomous variables.
Surgical technique
A 4-arm Da Vinci SI Robotic Surgical System is used during the procedure (Figure 1). The surgical technique can be divided into six phases: (1) coloepiploic mobilization; (2) ligation of the right gastroepiploic artery; (3) ligation of the right gastric artery and section of the duodenum; (4) lymphadenectomy of major vessels; (5) section of the esophagus; and (6) double-loop reconstruction method (Parisi Technique).
Figure 1.
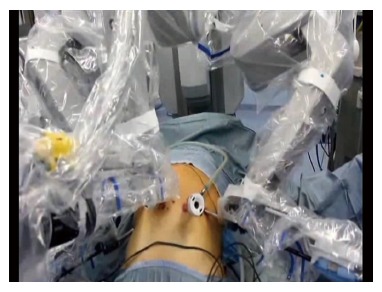
Robotic docking.
The pneumoperitoneum is created with a Veress needle in the peri-umbilical region. The intra-abdominal pressure is set to 12 mmHg.
The mobilization of the stomach can be performed by either traditional laparoscopy or robotic surgery, depending on patient characteristics and surgeon preferences.
First, a complete coloepiploic mobilization is achieved using the harmonic scalpel from right to left and the epiploon retrocavity is opened. During this phase lymph stations no. 4d, 4sa and 4sb are isolated and removed.
During the second phase the superior right colic vein is identified and the trunk of Henle is found. After this step, the gastroepiploic vein is sectioned at its origin. The right gastroepiploic artery is tied between hem-o-locks at its origin and the lymph nodes in station no. 6 are removed.
The first portion of the duodenum is released and the assistant introduces an articulated linear stapler for its section.
The lymphadenectomy of major vessels begins at the level of the proper hepatic artery.
The right gastric artery is identified and sectioned between hem-o-locks, at its origin, thus removing station no. 5. The lesser omentum is also dissected releasing station no. 3.
All the soft tissue along up to hepatic pedicle is removed including station no. 12a. Station no.8 is removed from the common hepatic artery. The dissection continues to the left of side of the celiac trunk to remove the station no. 9. Station no. 7 is dissected and the left gastric artery is sectioned. The splenic artery is followed and station no. 11p is removed, while the tissue on the proximal part of the artery (11p) is cleared based on the tumor and patient characteristics. Station no. 10 is not routinely dissected, because the high risk of major injuries due to anatomical characteristics of western patients that almost always does not allow a safety dissection of that area.
Finally, the soft tissue representing station no. 1 and no. 2 is dissected, thus releasing the esophagus and the anterior and posterior branches of the Vagus nerve which are sectioned.
In the last phase, the digestive continuity is restored with the Parisi Technique (Figure 2). Two stitches are placed to secure the esophagus to the diaphragm pillars (Figure 3). Then, the assistant temporarily removes the robotic arm no. 2 and changes the robotic trocar (8 mm) with a 12 mm trocar in order to introduce the stapler with the correct angle of section.
Figure 2.
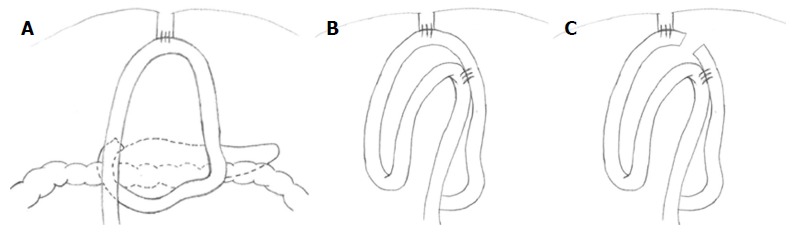
Double loop reconstruction method. A: 1 step: E-J anastomosis; B: 2 step: J-J anastomosis using the second loop; C: 3 step: interruption of continuity between the two anastomoses.
Figure 3.
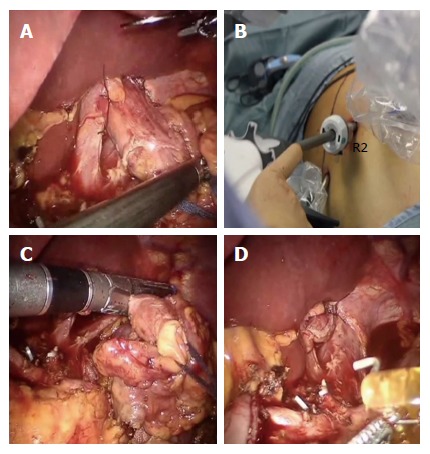
Division of the esophagus. A: After fixing the esophagus to the diaphragm pillars; B: An articulated mechanical linear stapler is introduced by the assistant through a 12 mm trocar; C and D: The esophagus is sectioned and closed.
The esophagus is thereby sectioned and closed (Figure 3), through the mechanical linear stapler, considering the right distance from the tumor and avoiding tensions.
At the beginning the surgeon at the console detects the angle of Treitz to move the bowel loops above the transverse colon close to the sectioned esophagus. The selected portion of jejunum must be free from stretching and twisting, to ensure a successful anastomosis. The selected loop is fixed with two stitches to the posterior wall of the esophagus (Figure 4), placing the biliary side on the esophagus left and the alimentary side on the right. The first loop is prepared. The surgeon performs an end-to-side esophago-jejunal robot-sewn anastomosis (Figure 5). The operator starts performing a first posterior layer with interrupted stitches using a Vicryl 2/0 for each point, joining the jejunal serosal and the muscle layer of the esophagus. Then, both the small intestine and the esophagus are opened. The surgeon performs the internal layer with a running suture, using a 3/0 PDS wire. During each step of the suture both the small intestine wall and the esophageal wall are full-thickness crossed. After this step, the anterior plane is approached. A second running suture joins the posterior one at the anastomosis angles. The anterior plane is completed with interrupted stitches covering the internal layer. The second loop is identified at a distance of about 30 to 40 cm from the E-J anastomosis. This loop is located close to the first anastomosis, on the left side, and it is used to perform the jejunojejunal anastomosis. At this point, the surgeon fixes the chosen intestinal segment (second loop) to the biliary limb of the first loop with two sero-serosal stitches. The assistant fires the stapler (Figure 6) and then the surgeon at the console closes the entry holes of the stapler with two layers of sutures (the first is a running suture and the second layer is made with interrupted stitches). The operation ends with the interruption of the digestive continuity between the two anastomoses (Figure 7) by firing a linear stapler and thus creating a modified Roux-en-Y.
Figure 4.
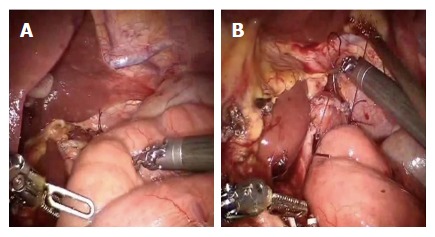
A first loop of small bowel is identified and brought antecolic (A), two stiches are placed to pair it with the esophagus (B).
Figure 5.
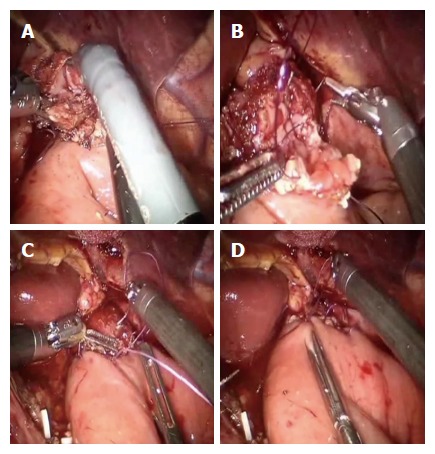
Esophagus and jejunum are opened (A), the posterior layer (B) is first performed followed by the anterior layer (C and D).
Figure 6.
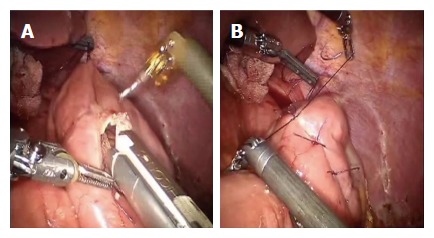
Second loop is identified at 40 cm from the E-J anastomosis, along the alimentary limb, and brought up to the first anastomosis on its left side through a mechanical stapler (A), a side to side jejunojejunal anastomosis is created between the second loop and the biliary limb of the first loop (B).
Figure 7.
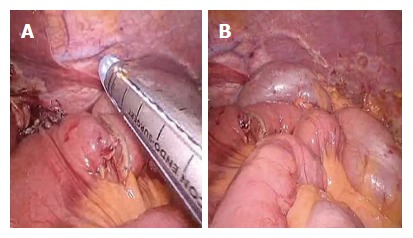
Two anastomoses are easily interrupted by firing the stapler (A and B).
A suction drain is positioned close to the E-J anastomosis, while the naso-enteric tube is not placed. The specimen is removed through a 5 cm Mc Burney incision, in the right iliac fossa.
RESULTS
Data of 55 consecutive patients underwent the robotic double-loop reconstruction method (called Parisi technique) after performing a robotic total gastrectomy for gastric cancer were considered in the present study. Table 1 reported patients’ characteristics. Mean age was 72.56 ± 10.67, 66.04% of patients had one or more comorbidities and the mean BMI was 24.42 ± 72.56. Most tumors were in the middle third of the stomach (54.54%).
Table 1.
Characteristics of patients
| Characteristics | |
| Sex (male/female), no. | 31/24 |
| Age (yr) | 72.56 ± 10.67 |
| BMI (kg/m2) | 24.42 ± 72.56 |
| Comorbidity, n (%) | 35 (66.04) |
| ASA score, n | |
| I | 8 |
| II | 19 |
| III | 28 |
| IV | 0 |
| Tumor location, n | |
| Lower third | 12 |
| Middle third | 30 |
| Upper third | 13 |
If the tumor is localized at the lower third of the stomach, usually we perform a BII subtotal gastrectomy, with a robotic intracorporeal anastomosis. However, in some selected cases we prefer to perform a total gastrectomy.
In 12 of the reported cases, where the tumor was located in between the lower and the middle third of the stomach, we decided, after discussion with the patient, to perform a total than a subtotal gastrectomy for the following reasons: (1) some patients were less than 65 years old and we wanted to reduce the risk of recurrence on the residual limb (5 patients); (2) the lesion appeared to extensively involve the lower two thirds of the stomach (5 patients); and (3) in two cases with diffuse type, there was not only a main pre-pyloric lesion but also a biopsy proven adenocarcinoma at the upper third.
Total operative time was 354.21 ± 68.8 (Table 2). Intraoperative blood loss was 126.55 ± 73. No conversion to open surgery or major intraoperative complications occurred. The median number of retrieved lymph nodes was 35 (95%CI: 15-47). All specimens were evaluated as R0 resections. Histopathological characteristics are shown in Table 3.
Table 2.
Operative results
| Outcomes | |
| Overall operative time, min | 354.21 ± 68.8 |
| Incision for specimen extraction, n | |
| Right McBurney incision | 55 |
| length of minilaparotomy, cm | 5 (4-6) |
| Intraoperative blood loss, Ml | 126.55 ± 73 |
| Intraoperative morbidity, n | 0 |
| Intraoperative Mortality, n | 0 |
| Conversion, n | 0 |
| Extent of lymphadenectomy, n | |
| D1 | 0 |
| D1+ | 5 |
| D2 | 50 |
Table 3.
Clinical outcomes during hospitalization and complications
| Outcomes | |
| Time to peristalsis, d | 1 (1-3)1 |
| Time to resume liquid diet, d | 2 (2-5)1 |
| Time to resume solid intake, d | 3 (3-6)1 |
| Length of hospital stay, d | 5 (3-17)1 |
| Postoperative 30-d complications, n | 0 |
| Reoperations, n | 0 |
| 30-d mortality, n | 0 |
Values are expressed as median (range).
Table 4 summarizes the postoperative findings. The median hospital stay was 5 d. We observed a fast recovery of different levels of food intake after gastrectomy, enabling patients to go through a liquid diet (Median = 2; 95%CI: 2-5) until starting a solid diet (Median = 3; 95%CI: 3-6). No major complications or death occurred. None of the 55 patients experienced anastomotic leakage.
Table 4.
Histopathological data
| Outcomes | |
| Diameter of the tumor, cm | 4.25 (1-8)1 |
| Proximal margin, cm | 6 (2-11)1 |
| Number of harvested lymph nodes, n | 35 (15-47)1 |
| TNM staging, n (%) | |
| Stage 0 | 0 |
| Stage IA | 8 (14.55) |
| Stage IB | 9 (16.37) |
| Stage IIA | 12 (21.82) |
| Stage IIB | 11 (20.00) |
| Stage IIIA | 7 (12.72) |
| Stage IIIB | 6 (10.91) |
| Stage IIIC | 2 (3.63) |
| Stage IV | 0 |
| Residual tumor, n | |
| R0 | 55 |
| R1 | 0 |
| R2 | 0 |
Values are expressed as median (range).
DISCUSSION
The restoration of the digestive tract after total gastrectomy is a technically demanding phase.
Minimally invasive surgery has been developed in recent years even for complex oncological procedures thanks to new available devices and increased surgeons experience[2].
In the literature (Table 5), only 22 studies[5,7-27] from 16 institutions have reported the use of robotic surgery in gastric cancer including total gastrectomy. Eleven studies are comparative[9,11-15,17,18,21-23], others are case series and personal experiences[5,7,8,10,16,19,20,24,27]. Limitations of studies are to provide outcomes including in their analysis different types of gastric resection. In fact, only 5 studies[5,17,22,24,27] reported information on total gastrectomy alone. Others reported data on surgical interventions within a more general analysis including different types of gastric resections as subtotal or proximal gastrectomy. Overall, 466 procedures on total gastrectomy can be detected in the literature. This makes difficult to better understand the real effect of robotic surgery on total gastrectomy that is a different and more complex surgery.
Table 5.
Literature review on robotic total gastrectomy, overall number of reported cases
| Year | Type | Subject | Country | Institution | Period | No. | |
| Present study | 2017 | Prospective CS | RTG | Italy | St. Mary’s Hospital of Terni | 2014-2016 | 55 |
| Jiang et al[24] | 2015 | Retrospective CS | RAG | China | Nanjing University Medical College | 2010-2012 | 65 |
| Kim et al[14] | 2012 | nonRCT | RAG vs LG vs OG | South Korea | Yonsei University College of Medicine | 2005-2010 | 109 |
| Son et al[15] | 2014 | nonRCT | RTG vs LTG | ||||
| 2005-2010 | 51 | ||||||
| Woo et al[11] | 2011 | nonRCT | RAG vs LG | 2005-2009 | 62 | ||
| Song et al[7] | 2009 | Prospective CS | RAG | 2005-2007 | 33 | ||
| Park et al[20] | 2013 | Retrospective CS | RAG | South Korea | National Cancer Center | 2009-2012 | 46 |
| Yoon et al[17] | 2012 | nonRCT | RTG vs LTG | ||||
| 2009-2011 | 36 | ||||||
| Kang et al[13] | 2012 | nonRCT | RAG vs LG | South Korea | Ajou University School of Medicine | 2008-2011 | 16 |
| Hur et al[8] | 2010 | Retrospective CS | RAG | 2010 | 2 | ||
| Hyun et al[18] | 2013 | nonRCT | RAG vs LG | South Korea | Korea University Anam Hospital | 2009-2010 | 9 |
| Son et al[15] | 2012 | nonRCT | RAG vs LG | South Korea | Seoul University Bundang Hospital | 2007-2011 | 1 |
| Junfeng et al[21] | 2014 | nonRCT | RAG vs LG | China | Third Military Medical University | 2010-2013 | 26 |
| Liu et al[19] | 2013 | Prospective CS | RAG | China | Subei People's Hospital of Jiangsu | 2011-2013 | 54 |
| Giulianotti et al[25] | 2003 | Retrospective CS | RAG | Italy | Misericordia Hospital of Grosseto | 2000-2002 | 10 |
| Coratti et al[26] | 2015 | Retrospective CS | RAG | Italy | 2000-2014 | 38 | |
| D'Annibale et al[10] | 2011 | Retrospective CS | RAG | Italy | S. Giovanni Addolorata Hospital | 2004-2009 | 11 |
| Caruso et al[9] | 2011 | nonRCT | RAG vs OG | Italy | Hospital of Spoleto | 2006-2010 | 12 |
| Suda et al[23] | 2014 | nonRCT | RAG vs LG | Japan | Fujita Health University | 2009-2012 | 30 |
| Huang et al[12] | 2012 | nonRCT | RAG vs LG vs OG | Taiwan | Taipei Veterans General Hospital | 2010-2012 | 7 |
| Vasilescu et al[16] | 2012 | Retrospective CS | RAG | Romania | Fundeni Clinical Institute | 2008-2012 | 19 |
| Zawadzki et al[27] | 2014 | CR | RAG | Poland | Wroclaw Medical University | 2014 | 1 |
| Parisi et al[5] | 2015 | Prospective CS | RTG | Italy | St. Mary’s Hospital of Terni | 2014-2015 | 22 |
| Total1 | 466 | ||||||
Excluding the present study.
Moreover, Table 6 shows that most of studies reported limited information. Only eight studies showed data on patient and tumor characteristics or operative and clinical results[2,17,20-24,26].
Table 6.
Literature review on robotic total gastrectomy, details of the reported data
|
Robot - Assistance |
Type | E-J anastomosis | Anastomosis performance | Site of minilaparotomy | Operative and clinical data | Patients and tumor details | |||
| Lymphadenectomy | Stomach mobilization | Reconstruction | |||||||
| Jiang et al[24] | Performed | Performed | Roux-en-Y | EXTRA | Circular stapler | Not provided | Provided | Provided | |
| Performed | Roux-en-Y | INTRA | Robot-sewn | Not provided | |||||
| Kim et al[14] | Not provided | Not provided | Not provided | Roux-en-Y | Not provided | Not provided | Not provided | Lack | Lack |
| Son et al[15] | Performed | Performed | Roux-en-Y | EXTRA | Circular stapler | Upper midline | Provided | Provided | |
| Roux-en-Y | INTRA LAP | Circular stapler | Left lower port | ||||||
| Woo et al[11] | Performed | Performed | Roux-en-Y | EXTRA | Not provided | Not provided | Lack | Lack | |
| Roux-en-Y | INTRA LAP | Not provided | Not provided | ||||||
| Song et al[7] | Performed | Performed | Roux-en-Y | EXTRA | Circular stapler | Upper midline | Lack | Lack | |
| Roux-en-Y | INTRA LAP | Circular stapler | Not provided | ||||||
| Park et al[20] | Performed | Performed | Roux-en-Y | EXTRA | Not provided | Upper midline | Provided | Lack | |
| Yoon et al[17] | Performed | Performed | Roux-en-Y | EXTRA | Circular stapler | Not provided | Provided | Provided | |
| Kang et al[13] | Performed | Performed | Roux-en-Y | EXTRA | Circular stapler | Upper midline | Lack | Lack | |
| Performed | Roux-en-Y | INTRA | Robot-sewn | Not provided | |||||
| Hur et al[8] | Performed | Performed | Performed | Roux-en-Y | INTRA | Robot-sewn | Not provided | Lack | Lack |
| Hyun et al[18] | Performed | Performed | Roux-en-Y | EXTRA | Circular stapler | Upper midline | Lack | Lack | |
| Not provided | Roux-en-Y | INTRA | Not provided | Umbilical port | |||||
| Son et al[15] | Performed | Performed | Roux-en-Y | EXTRA | Not provided | Not provided | Lack | Lack | |
| Junfeng et al[21] | Performed | Performed | Roux-en-Y | EXTRA | Circular stapler | Upper midline | Provided | Provided | |
| Liu et al[19] | Performed | Performed | Performed | Roux-en-Y | INTRA | Robot-sewn | Camera port | Lack | Lack |
| Giulianotti et al[25] | Performed | Performed | Performed | Roux-en-Y | INTRA | Circular stapler | Not provided | Lack | Lack |
| Coratti et al[26] | Performed | Performed | Performed | Roux-en-Y | INTRA | Robot-sewn | Not provided | Provided | Provided |
| D'Annibale et al[10] | Performed | Performed | Performed | Roux-en-Y | INTRA | Circular stapler | Suprapubic | Lack | Lack |
| Caruso et al[9] | Performed | Performed | Performed | Roux-en-Y | INTRA | Circular stapler | Upper midline | Lack | Lack |
| Suda et al[23] | Performed | Performed | Performed | Roux-en-Y | INTRA | Linear stapler | Not provided | Provided | Provided |
| Huang et al[12] | Performed | Performed | Roux-en-Y | INTRA LAP | Circular stapler | Periumbilical | Lack | Lack | |
| Vasilescu et al[16] | Performed | Performed | Performed | Roux-en-Y | INTRA | Circular stapler | Not provided | Lack | Lack |
| Parisi et al[5] | Performed | Performed | Performed | Roux-en-Y | INTRA | Robot-sewn | McBurney | Provided | Provided |
Regarding the technique used, all studies reported the assistance of the robot in the mobilization of the stomach and in lymphadenectomy. Ten authors reported an extracorporeal reconstruction and only eleven studies[2,8-10,13,16,18,19,23,24,26] reported a robotic assistance in this phase and an intracorporeal approach.
There are two main issues to consider: the way to perform the Roux-en-y reconstruction and how to perform it.
In the intracorporeal approach, a circular stapler is generally used but other solutions include the Orvil[16] or the Overlap technique[16,23]. Some authors[10,25] described the use of the robot to perform a manual purse-string around the anvil, but only few reports[5,13,19,24] in the literature reported its use for a complete hand-sewn anastomosis.
Surgeons are generally afraid to perform the latter, because the high surgical skills required in conventional surgery and the risk for leakage if not well performed.
By the other hand and regardless of the approach, using mechanical staplers has standardize the way to perform the reconstruction after total gastrectomy and apparently give more safety. If this is true in open surgery, this is more challenging when adopting an intracorporeal approach. A mechanical trouble when firing the stapler or a leakage for incomplete closing at the E-J level can lead the patient to serious complications until death.
We decided to develop a new technique to overcome the reported limitations of the intracoproreal approach. Particularly we found a feasible and safe way to perform a complete robotic reconstruction without the need to convert the procedure to open or laparoscopic surgery or using others potentially dangerous techniques.
The accuracy of the robotic system, the microsurgical instruments and particularly the endowrist allow the surgeon to perform movements that are even difficult to reproduce in open surgery.
We believe that this technology should be exploited in complex digestive procedures, as in a total gastrectomy and our study demonstrates the usefulness of the robotic system in performing a safe hand-sewn E-J anastomosis.
Although it may appear more complex than other techniques, during our experience, we have gained some tricks: (1) the esophagus should be fixed to both sides at the diaphragmatic pillars to avoid retraction in the chest; (2) a jejunal loop which can be easily approached to the esophagus is essential; (3) two interrupted stiches can help in pairing the esophagus and the jejunum, delimiting the two ends of the E-J anastomosis. Then other two central stitches are placed to complete the posterior external layer. Vicryl is preferable; (4) only at this point the esophagus and the jejunum wall should be opened, we suggest using monopolar curved scissors; (5) two running sutures for the internal layer, the first posterior and the second one anterior are preferable than an interrupted suturing. At the beginning of our experience we performed this step with interrupted stiches but after few cases we decided to move to a running suture because faster and it gives a feeling of safety and high adherence between the visceral walls; and (6) PDS sutures for the internal layer is best, because the combination of an absorbable suture and an extended anastomotic support. This suture gives fluidity and at the same time tightness, when the thread is pulled in tension.
The double loop approach is the other innovative element we reported. This technique can speed up the reconstruction and have several relevant advantages: (1) the loop of bowel can be chosen without tension; (2) there is no confusion between biliary and alimentary tract; and (3) it is not necessary to interrupt the mesentery, reducing the risk of bleeding and internal hernias.
In conclusion, the present study is one of the largest series focused on robotic total gastrectomy in the literature and has shown satisfactory outcomes. The innovative technique adopted has demonstrated feasibility and safety when performing both the E-J and the J-J anastomoses with an intracorporeal robotic approach.
Every method for reconstruction has to tackle the functional problems of blind loop syndrome, or of inadequate transition (e.g., weight loss) through the substitute stomach.
The short-term follow-up didn’t highlight any functional problems until now, while the evaluation on long term results of our approach is ongoing.
COMMENTS
Background
The development of surgical technology during last decades has allowed surgeons to offer patients new approaches and treatment strategies. Several studies have shown the usefulness of robotic systems when performing a gastrectomy for cancer and its potential advantages when compared with traditional laparoscopic devices. However, if it has been well evaluated the role of both laparoscopic and robotic surgery for distal or subtotal gastrectomy, particularly in the eastern population, there is a big debate on safety and feasibility of intracorporeal procedures for reconstruction of the alimentary tract after a total gastrectomy. The present study has addressed this issue in a western center.
Research frontiers
Despite the spread of minimally invasive procedures for complex major abdominal surgeries, few studies have been performed on the totally robotic approach for gastric cancer. Current indications are still object of debate. Moreover, only few centers have tried to find solutions for the reconstruction phase after total gastrectomy.
Innovations and breakthroughs
Robotic systems have revolutionized minimally invasive surgery. Surgeons can overcome the limits of traditional laparoscopy through a three-dimensional vision and articulated instruments. This technological progress should be exploited particularly when performing a demanding phase of a procedure, as in suturing movements. The present study shows a new robotic technique for a reconstructive approach. The double loop method can simplify the way to perform the reconstruction phase after a total gastrectomy and can allow surgeons to overcome the current limitations.
Applications
This study shows the possibility to safely perform a completely intracorporeal anastomosis after a total gastrectomy, considered one of the biggest obstacles in minimally invasive surgery.
Terminology
Robotic systems are used in minimally invasive surgery and allow the primary surgeon to perform the procedure through a remote console.
Peer-review
It is a good observational study who reported the outcome of robotic total gastrectomy in one Institution. Total gastrectomy with minimally invasive technique is quite challenging with either laparoscopic technique or robotic technique. With a series of 55 patients who successfully recovered, they can be convinced by the authors and draw such a conclusion that it can be safe.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study was approved by the region ethics committee (CEAS Umbria).
Informed consent statement: Not required for the present study.
Conflict-of-interest statement: No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Data sharing statement: Technical appendix available from the corresponding author at djdesi85@hotmail.it.
Peer-review started: February 1, 2017
First decision: March 3, 2017
Article in press: May 9, 2017
P- Reviewer: Garcia-Olmo D, Klinge U, Wu AW S- Editor: Qi Y L- Editor: A E- Editor: Zhang FF
References
- 1.Marano A, Choi YY, Hyung WJ, Kim YM, Kim J, Noh SH. Robotic versus Laparoscopic versus Open Gastrectomy: A Meta-Analysis. J Gastric Cancer. 2013;13:136–148. doi: 10.5230/jgc.2013.13.3.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parisi A, Nguyen NT, Reim D, Zhang S, Jiang ZW, Brower ST, Azagra JS, Facy O, Alimoglu O, Jackson PG, et al. Current status of minimally invasive surgery for gastric cancer: A literature review to highlight studies limits. Int J Surg. 2015;17:34–40. doi: 10.1016/j.ijsu.2015.02.021. [DOI] [PubMed] [Google Scholar]
- 3.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3) Gastric Cancer. 2011;14:113–123. doi: 10.1007/s10120-011-0042-4. [DOI] [PubMed] [Google Scholar]
- 4.NCCN. Clinical Practice Guidelines in Oncology: Gastric Cancer. Version I.2014, 2014. Available from: http://wwwnccnorg/professionals/physician_gls/pdf/gastricpdf.
- 5.Parisi A, Ricci F, Trastulli S, Cirocchi R, Gemini A, Grassi V, Corsi A, Renzi C, De Santis F, Petrina A, et al. Robotic Total Gastrectomy With Intracorporeal Robot-Sewn Anastomosis: A Novel Approach Adopting the Double-Loop Reconstruction Method. Medicine (Baltimore) 2015;94:e1922. doi: 10.1097/MD.0000000000001922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014;12:1495–1499. doi: 10.1016/j.ijsu.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 7.Song J, Oh SJ, Kang WH, Hyung WJ, Choi SH, Noh SH. Robot-assisted gastrectomy with lymph node dissection for gastric cancer: lessons learned from an initial 100 consecutive procedures. Ann Surg. 2009;249:927–932. doi: 10.1097/01.sla.0000351688.64999.73. [DOI] [PubMed] [Google Scholar]
- 8.Hur H, Kim JY, Cho YK, Han SU. Technical feasibility of robot-sewn anastomosis in robotic surgery for gastric cancer. J Laparoendosc Adv Surg Tech A. 2010;20:693–697. doi: 10.1089/lap.2010.0246. [DOI] [PubMed] [Google Scholar]
- 9.Caruso S, Patriti A, Marrelli D, Ceccarelli G, Ceribelli C, Roviello F, Casciola L. Open vs robot-assisted laparoscopic gastric resection with D2 lymph node dissection for adenocarcinoma: a case-control study. Int J Med Robot. 2011;7:452–458. doi: 10.1002/rcs.416. [DOI] [PubMed] [Google Scholar]
- 10.D’Annibale A, Pende V, Pernazza G, Monsellato I, Mazzocchi P, Lucandri G, Morpurgo E, Contardo T, Sovernigo G. Full robotic gastrectomy with extended (D2) lymphadenectomy for gastric cancer: surgical technique and preliminary results. J Surg Res. 2011;166:e113–e120. doi: 10.1016/j.jss.2010.11.881. [DOI] [PubMed] [Google Scholar]
- 11.Woo Y, Hyung WJ, Pak KH, Inaba K, Obama K, Choi SH, Noh SH. Robotic gastrectomy as an oncologically sound alternative to laparoscopic resections for the treatment of early-stage gastric cancers. Arch Surg. 2011;146:1086–1092. doi: 10.1001/archsurg.2011.114. [DOI] [PubMed] [Google Scholar]
- 12.Huang KH, Lan YT, Fang WL, Chen JH, Lo SS, Hsieh MC, Li AF, Chiou SH, Wu CW. Initial experience of robotic gastrectomy and comparison with open and laparoscopic gastrectomy for gastric cancer. J Gastrointest Surg. 2012;16:1303–1310. doi: 10.1007/s11605-012-1874-x. [DOI] [PubMed] [Google Scholar]
- 13.Kang BH, Xuan Y, Hur H, Ahn CW, Cho YK, Han SU. Comparison of Surgical Outcomes between Robotic and Laparoscopic Gastrectomy for Gastric Cancer: The Learning Curve of Robotic Surgery. J Gastric Cancer. 2012;12:156–163. doi: 10.5230/jgc.2012.12.3.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim KM, An JY, Kim HI, Cheong JH, Hyung WJ, Noh SH. Major early complications following open, laparoscopic and robotic gastrectomy. Br J Surg. 2012;99:1681–1687. doi: 10.1002/bjs.8924. [DOI] [PubMed] [Google Scholar]
- 15.Son SY, Lee CM, Ahn SH, Lee JH, Park DJ, Kim HH. Clinical Outcome of Robotic Gastrectomy in Gastric Cancer in Comparison with Laparoscopic Gastrectomy: A Case-Control Study. J Minim Invas Surg. 2012;15:27–31. [Google Scholar]
- 16.Vasilescu C, Procopiuc L. Robotic surgery of locally advanced gastric cancer: a single-surgeon experience of 41 cases. Chirurgia (Bucur) 2012;107:510–517. [PubMed] [Google Scholar]
- 17.Yoon HM, Kim YW, Lee JH, Ryu KW, Eom BW, Park JY, Choi IJ, Kim CG, Lee JY, Cho SJ, et al. Robot-assisted total gastrectomy is comparable with laparoscopically assisted total gastrectomy for early gastric cancer. Surg Endosc. 2012;26:1377–1381. doi: 10.1007/s00464-011-2043-0. [DOI] [PubMed] [Google Scholar]
- 18.Hyun MH, Lee CH, Kwon YJ, Cho SI, Jang YJ, Kim DH, Kim JH, Park SH, Mok YJ, Park SS. Robot versus laparoscopic gastrectomy for cancer by an experienced surgeon: comparisons of surgery, complications, and surgical stress. Ann Surg Oncol. 2013;20:1258–1265. doi: 10.1245/s10434-012-2679-6. [DOI] [PubMed] [Google Scholar]
- 19.Liu XX, Jiang ZW, Chen P, Zhao Y, Pan HF, Li JS. Full robot-assisted gastrectomy with intracorporeal robot-sewn anastomosis produces satisfying outcomes. World J Gastroenterol. 2013;19:6427–6437. doi: 10.3748/wjg.v19.i38.6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park JY, Kim YW, Ryu KW, Eom BW, Yoon HM, Reim D. Emerging Role of Robot-assisted Gastrectomy: Analysis of Consecutive 200 Cases. J Gastric Cancer. 2013;13:255–262. doi: 10.5230/jgc.2013.13.4.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Junfeng Z, Yan S, Bo T, Yingxue H, Dongzhu Z, Yongliang Z, Feng Q, Peiwu Y. Robotic gastrectomy versus laparoscopic gastrectomy for gastric cancer: comparison of surgical performance and short-term outcomes. Surg Endosc. 2014;28:1779–1787. doi: 10.1007/s00464-013-3385-6. [DOI] [PubMed] [Google Scholar]
- 22.Son T, Lee JH, Kim YM, Kim HI, Noh SH, Hyung WJ. Robotic spleen-preserving total gastrectomy for gastric cancer: comparison with conventional laparoscopic procedure. Surg Endosc. 2014;28:2606–2615. doi: 10.1007/s00464-014-3511-0. [DOI] [PubMed] [Google Scholar]
- 23.Suda K, Man-I M, Ishida Y, Kawamura Y, Satoh S, Uyama I. Potential advantages of robotic radical gastrectomy for gastric adenocarcinoma in comparison with conventional laparoscopic approach: a single institutional retrospective comparative cohort study. Surg Endosc. 2015;29:673–685. doi: 10.1007/s00464-014-3718-0. [DOI] [PubMed] [Google Scholar]
- 24.Jiang ZW, Liu J, Wang G, Zhao K, Zhang S, Li N, Li JS. Esophagojejunostomy reconstruction using a robot-sewing technique during totally robotic total gastrectomy for gastric cancer. Hepatogastroenterology. 2015;62:323–326. [PubMed] [Google Scholar]
- 25.Giulianotti PC, Coratti A, Angelini M, Sbrana F, Cecconi S, Balestracci T, Caravaglios G. Robotics in general surgery: personal experience in a large community hospital. Arch Surg. 2003;138:777–784. doi: 10.1001/archsurg.138.7.777. [DOI] [PubMed] [Google Scholar]
- 26.Coratti A, Fernandes E, Lombardi A, Di Marino M, Annecchiarico M, Felicioni L, Giulianotti PC. Robot-assisted surgery for gastric carcinoma: Five years follow-up and beyond: A single western center experience and long-term oncological outcomes. Eur J Surg Oncol. 2015;41:1106–1113. doi: 10.1016/j.ejso.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 27.Zawadzki M, Witkiewicz W. Laparoscopic robotic total gastrectomy. Wideochir Inne Tech Maloinwazyjne. 2014;9:650–654. doi: 10.5114/wiitm.2014.45128. [DOI] [PMC free article] [PubMed] [Google Scholar]


