Abstract
The cytolytic toxin cytolysin A (ClyA) from Escherichia coli is probably one of the best-characterized examples of bacterial, α-pore–forming toxins (α-PFTs). Like other PFTs, ClyA exists in a soluble, monomeric form that assembles to an annular, homo-oligomeric pore complex upon contact with detergent or target membranes. Comparison of the three-dimensional structures of the 34 kDa monomer and the protomer in the context of the dodecameric pore complex revealed that ClyA undergoes one of the largest conformational transitions described for proteins so far, in which 55% of the residues change their position and 16% of the residues adopt a different secondary structure in the protomer. Studies on the assembly of ClyA revealed a unique mechanism that differs from the assembly mechanism of other PFTs. The rate-liming step of pore formation proved to be the unimolecular conversion of the monomer to an assembly-competent protomer, during which a molten globule-like off-pathway intermediate accumulates. The oligomerization of protomers to pore complexes is fast and follows a kinetic scheme in which mixtures of linear oligomers of different size are formed first, followed by very rapid and specific association of pairs of oligomers that can directly perform ring closure to the dodecameric pore complex.
This article is part of the themed issue ‘Membrane pores: from structure and assembly, to medicine and technology’.
Keywords: α-pore-forming toxins, cytolysin A, assembly of membrane complexes, assembly kinetics
1. Introduction
Many pathogenic bacteria produce proteins termed pore-forming toxins (PFTs) that either kill target cells or affect target cell function [1–3]. A common feature of all PFTs is their ability to assemble from a soluble, monomeric state into oligomeric, annular membrane complexes. PFTs can be divided into two main classes: α-PFTs and β-PFTs, depending on the type of regular secondary structure elements (α-helix of β-sheet, respectively) with which they span the target membrane [1,4]. Several excellent reviews have recently appeared on the structure and assembly mechanism of PFTs [1,4–7]. There are three well-described types of α-PFTs: the bacterial toxins of the ClyA [8] and colicin [9] families, and actinoporins from sea anemones [10]. This manuscript focuses on the recent progress in understanding the assembly dynamics of cytolysin A from Escherichia coli, the prototype and best characterized member of the ClyA family of α-PFTs [1,5,8].
Cytolysin A (ClyA, also termed HlyE) occurs in numerous pathogenic and non-pathogenic E. coli (including E. coli K12) strains, as well as in strains of Salmonella enterica [11]. ClyA from E. coli is a soluble, monomeric 302-residue protein (33.6 kDa) [12] that is strongly downregulated in vivo [13]. Overproduction of ClyA in E. coli leads to accumulation of ClyA in the periplasmic space [14] and release to the extracellular medium of intact ClyA pore complexes embedded in outer membrane vesicles (OMVs) [15]. Both the mechanism of ClyA release to the medium in OMVs and the transport of ClyA to the periplasm are poorly understood. ClyA transport to the periplasm remains particularly enigmatic, as ClyA lacks a bacterial signal sequence [12–15].
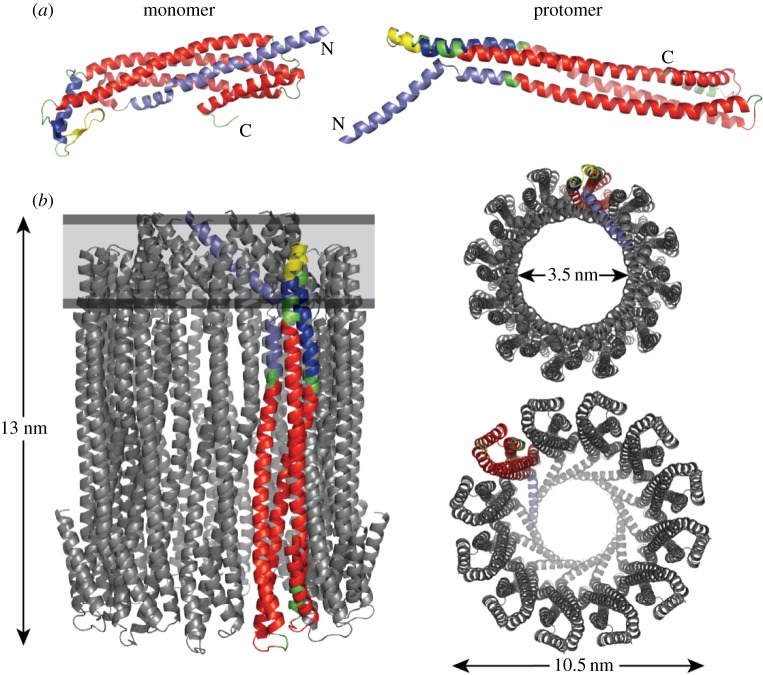
In contrast with this limited knowledge about ClyA secretion and in vivo assembly in OMVs, ClyA is one of the very few PFTs for which high-resolution structural information is available on both the soluble monomer [16] and the annular pore complex [17]. The ClyA monomer consists of two domains: a tail domain composed of a bundle of five α-helices, and a head domain with a small β-hairpin (β-tongue) flanked by two short α-helices (figure 1a). An important prerequisite for solving the structure of the ClyA pore complex proved to be the ability of the ClyA monomer to assemble spontaneously into intact pore complexes upon addition of the detergent n-dodecylmaltoside (DDM) [18]. Initial electron microscopy data indicated that the annular ClyA pore complex consists of 13 protomers [18], but crystallization of the pore complex from DDM solutions yielded a homododecamer [17]. The stoichiometry of the complex may indeed not be exactly defined and could depend on detergent or membrane composition, but it appears that the dodecamer is the predominant form of the complex. The X-ray structure of the dodecameric pore complex (403 kDa) (figure 1b) revealed a hollow cylinder with a height of 130 Å, an outer diameter of 105 Å and a narrowest inner-pore diameter of 35 Å. The inner surface of the pore has a high excess of negative charges, consistent with cation selectivity [19]. The largest part of the pore is located at the extracellular side of the membrane, and only a minor part forms the transmembrane region (figure 1b). Comparison of the structures of the soluble ClyA monomer and the protomer in the pore complex (figure 1a) disclosed one of the largest conformational changes observed in a protein so far, in which 55% of the residues change their position (including a repositioning of the N-terminus by more than 140 Å) and 16% of the residues adopt a different secondary structure in the protomer (figure 1a). The main structural rearrangements occur in the ClyA head domain with the β-tongue, which inserts into the transmembrane region and adopts α-helical structure in the protomer, and the N-terminal α-helix, which swings upward, spans the transmembrane region and forms the iris-like arrangement that defines the constriction of the pore complex in its membrane-spanning region (figure 1b) [17]. The interface between the protomers in the pore complex comprises an area of 2400 Å2, and is mainly hydrophilic and stabilized by a network of 25 inter-molecular hydrogen bonds and 13 inter-molecular salt bridges. In addition, the pore structure showed that soluble ClyA monomers would not be able to form this interface, which agrees with the finding that protomer formation precedes pore complex assembly (see below). The structural differences between the monomer and protomer also suggested a plausible mechanism for the transition from the monomer to the protomer, assuming that the hydrophobic β-tongue inserts first into the membrane, followed by the upward movement of the N-terminal helix [17]. In the following paragraph, we will discuss the experimental evidence for a direct pathway from the monomer to the protomer, which proceeds with a half-life of about 40 s after addition of detergent to the monomer.
Figure 1.
Three-dimensional structures of the soluble ClyA monomer and the ClyA protomer (a) and the ClyA pore complex (b). (a) Comparison of the soluble monomer (left, pdb ID 1QOY) and the protomer (right, pdb ID 2WCD). In the monomer, the N-terminal helix (residues 1–47) is coloured light blue, the short helices in the head domain (residues 163–180 and 196–200) are coloured dark blue and the β-hairpin (residues 185–195) is coloured yellow. The four remaining helices of the tail domain are depicted in red, loops are shown in green. The colour code for residues in the protomer is the same as that in the monomer. Note the β-to-α and loop-to-α transitions upon protomer formation. (b) The dodecameric ClyA pore (pdb ID 2WCD) in side view (left), top view (upper right) and bottom view (lower right). One protomer is coloured according to (a). The membrane-spanning region of ClyA is indicated in the side view (grey band). The dimensions of the pore complex are indicated, as well as the inner diameter of the pore at its narrowest opening in the membrane-spanning region. Note that the largest part of the pore complex is extracellular.
2. The molecular mechanism of cytolysin A assembly
The in vitro assembly mechanism of ClyA pore complexes from soluble ClyA monomers was first studied by initiating pore complex assembly by mixing the soluble monomer at concentrations of 1–10 μM with the detergent DDM and monitoring the reaction via the change in the far-UV circular dichroism (CD) signal [17]. The assembly reaction is characterized by a rapid loss of the negative, α-helical CD signal, followed by the slow recovery of the α-helical signal on the time scale of several minutes to a final value that is more negative than that of the monomer, consistent with the higher α-helix content of the complex compared with the monomer. When pore complex assembly was followed in parallel via the increase in light scattering, the reaction proceeded with the same kinetics as the second, slow phase recorded with far-UV CD. The CD and light-scattering kinetics proved to be independent of the initial ClyA monomer concentration in the range of 1–10 µM, indicating that the rate-limiting step of pore assembly is unimolecular in this concentration range, and that the assembly of protomers to pore complexes is spectroscopically silent in far-UV CD measurements. The data suggested a model of pore assembly in which assembly-competent protomers assemble rapidly to pore complexes, and that the formation of assembly-competent protomers is the rate-limiting step of pore complex formation. In addition, the results demonstrated that a kinetic intermediate with a lower α-helix content than that of the monomer and protomer is populated during protomer formation. However, the ensemble CD measurements did not allow conclusions on the maximum population of the intermediate and its nature, i.e. whether it is on- or off-pathway. In addition, they did not provide information on the kinetic mechanism of protomer assembly, as pore assembly only became rate-limiting at ClyA concentrations below the CD detection limit.
These open questions were addressed by single-molecule Förster resonance energy transfer (FRET) experiments combined with rapid, microfluidic mixing techniques, two-focus fluorescence correlation spectroscopy, stopped-flow CD kinetics and rapid photo–cross-linking experiments [20]. For this purpose, ClyA was site-specifically labelled at residues 56 and 252 with a fluorescence donor and acceptor pair. This pair of residues was chosen because their Cβ–Cβ distance is 11 Å shorter in the protomer compared with the monomer, and because both residues do not interfere with pore complex assembly (figure 2). Single-molecule FRET experiments were then performed under two different sets of conditions: first, the unimolecular reaction from the monomer to the assembly-competent protomer was studied at very low ClyA concentrations (about 100 pM) that guaranteed that protomer assembly did not take place. In the second set of experiments, protomer oligomerization and formation of pore complexes was studied at ClyA concentrations above 5 nM over a wide range of concentrations (5 nM–5 µM). For this purpose, FRET donor/acceptor–labelled ClyA was mixed with a high excess of unlabelled wild-type ClyA, so that ClyA oligomers and pore complexes only contained a single copy of donor/acceptor–labelled ClyA. The complete assembly mechanism could then be clarified based on the fact that the ClyA monomer (M), the monomeric, kinetic intermediate (I), the protomer (P) and the protomer in the context of oligomers or pores (Pn) showed distinct and characteristic FRET efficiencies that allowed the quantitative, time-resolved analysis of their population during protomer formation or pore assembly [20].
Figure 2.
Positions of the FRET pair AlexaFluor 488 (donor, green) and AlexaFluor 594 (acceptor, red) with which ClyA was labelled at positions 56 and 252, respectively, for single-molecule FRET experiments. The Cβ-Cβ distances (d) of the cysteines introduced at positions 56 and 252 for site-specific labelling are indicated for the monomer (top left), protomer (bottom left) and the pore complex (right). The pore is tilted by 10° relative to the straight bottom view orientation (figure 1b) for best visualization of the positions of Cys56 inside the pore and Cys252 on the outer surface of the pore complex. Note that donor/acceptor–labelled ClyA was mixed with excess wild-type ClyA, so that pore complexes statistically only contained a single, labelled protomer. The colour code of secondary structures is the same as that in figure 1.
The single-molecule FRET kinetics of DDM-induced protomer formation at low ClyA concentrations confirmed the existence of a kinetic intermediate. Global fitting of the time-dependent change in the populations of M, I and P revealed that the intermediate is off-pathway (scheme 1). Its maximal population of approximately 80% is reached 20 s after addition of DDM. The results showed that there is indeed a direct reaction from M to P that proceeds with an apparent half-life of about 40 s [20], as proposed in the structural comparison of M and P [17]. In addition, the deduced microscopic rate constants of the I ↔ M ↔ P equilibrium (scheme 1) show that I transiently accumulates during protomer formation because M reacts about 18 times faster to I than to P. The direct pathway from M to P has a half-life of 40 s. The formation of I, however, retards the overall formation of P, which proceeds with a half-life of about 300 s. Notably, in the presence of DDM, M, I and P do not differ strongly in their thermodynamic stability: P is only 8.9 kJ mol−1 more stable than M, and only 4.5 kJ mol−1 more stable than I, so that about 14% of the molecules remain in I after attainment of the I ↔ M ↔ P equilibrium (see also scheme 3). At higher ClyA concentrations, however, protomer oligomerization and pore formation pull P from the equilibrium, so that eventually all molecules can react to pores.
Scheme 1.
DDM-induced protomer formation from ClyA monomers is slowed by formation of the off-pathway intermediate I.
Scheme 3.
Kinetic mechanism of DDM-induced protomer formation in ClyAox with a disulfide bond between the natural cysteine pair Cys87/Cys285 (top), and free energy differences (in kJ mol−1) between the monomer, intermediate and protomer for both ClyA redox states.
The characterization of I with single-molecule FRET revealed a very low FRET efficiency similar to that of unfolded ClyA in the presence of guanidinium chloride, essentially indicating loss of a defined tertiary structure in I. Reconstruction of the far-UV CD spectrum of I with multi-wavelength stopped-flow CD kinetics demonstrated that I has less α-helical secondary structure than M. In addition, I shows specific binding to the fluorescent dye 8-anilino-1-naphthalenesulfonic acid (ANS) that interacts with exposed hydrophobic side chains in protein-folding intermediates [21]. Together, the results show that the off-pathway intermediate I is a molten globule-like state lacking tertiary structure but still possessing α-helical secondary structure elements [20]. The reasons underlying the existence of the off-pathway intermediate remain unknown. Maybe the off-pathway intermediate, which only slows protomer formation but does not prevent the quantitative reaction of monomers to pore complexes, originated as a concession to the fascinating and highly demanding molecular evolution of the ClyA primary structure, which not only needed to be compatible with the strongly diverse tertiary structures of the monomer and the protomer, but also with the assembly competence of the protomers and their ability to form annular complexes with defined stoichiometry.
The kinetic mechanism of pore complex assembly from protomers was analysed at ClyA concentrations above 5 nM where pore complex formation became detectable. Using single-molecule FRET experiments that detected the time course of the fractions of M, I, P and Pn (linear oligomers and pore complexes), two-focus fluorescence correlation spectroscopy monitoring the increase in the average Stokes radii of assembling ClyA oligomers, and photo-cross-linking that allowed the quantification of dodecameric pore complexes after different assembly times with SDS–PAGE analysis, a comprehensive model of pore assembly could be established that proved to be robust over the entire concentration range of 100 pM to 5 µM [20]. The individual steps of the oligomerization of assembly-competent protomers to intact pores are the following (scheme 2).
Scheme 2.
Only two rate constants are required to describe the kinetics of pore (P12) assembly from protomers in the concentration range of 5 nM–5 µM.
First, formation of homodimers (P2), their elongation to larger, linear oligomers by protomer addition, and the association of pairs of small oligomers to larger oligomers shorter than dodecamers occurs fast and can be approximated with a single, uniform rate constant (kelong.) of 1.0 × 105 M−1 s−1 (scheme 2 and figure 3a). Second, a model of chain elongation by sequential monomer addition to linear dodecamers, followed by ring closure, could be excluded because dodecameric pore complexes formed much faster than predicted from this mechanism [20]. Instead, the assembly kinetics were consistent with a reaction scheme in which any pair of linear oligomers that can directly associate with an annular dodecamer (e.g. P3 and P9, P4 and P8, P5 and P7, or P6 and P6, etc.) forms pore complexes (P12) in a very rapid reaction that is more than two orders of magnitude faster than elongation of linear oligomers. In addition, all of the assembly reactions yielding annular P12 dodecamers from any pair of stoichiometrically compatible oligomers could also be approximated with a single rate constant (kpore) of 3.0 × 107 M−1 s−1 (scheme 2 and figure 3b).
Figure 3.
Mechanism of pore complex formation from assembly-competent protomers (filled circles). The entire pore assembly process could be approximated and globally fitted over a wide range of concentrations with only two rate constants: a single rate contant (kelong.) for dimerization and any reaction that leads to larger oligomers (a), and a single rate constant (kpore) for the association of any pair of oligomers that directly yields the dodecameric pore complex (b). The thick arrows in the lower panel indicate that pore formation by association of oligomers of similar size occurs more frequently than pore formation from oligomers of different size. Note that the indicated, pore-like curvature of linear oligomers is only tentative, but supported by the 300-fold higher value of kpore compared with kelong. (scheme 2). (Online version in colour.)
The above mechanism, established for detergent-induced assembly of ClyA pores from soluble monomers in vitro, raises the question of whether the same mechanism is still valid for pore formation in biological membranes. There is indeed good evidence that the I ↔ M ↔ P mechanism is preserved when ClyA monomers interact with erythrocyte membranes because the same structural rearrangements in ClyA were observed with far-UV CD as those in DDM, i.e. an initial loss of helical secondary structure, followed by slow formation of the protomer-specific CD signal with increased helicity [18]. In addition, the protomer-specific CD signal was formed about 10 times faster in erythrocyte membranes compared with DDM [18]. Whether the mechanism of pore formation from assembly-competent protomers in membranes is indeed the same as that in the presence of DDM remains to be established, in particular because lipid composition plays a critical role for assembly of many PFTs [4,6,22]. It is conceivable that not only the protomers, but also pore complexes are formed faster in membranes than in DDM due to the two-dimensional diffusion of protomers in membranes and a pre-orientation of membrane-embedded protomers favouring protomer oligomerization and ring closure. Recently, two kinetic models for ClyA assembly in erythrocyte membranes were tested by analysing the kinetics of haemoglobin release from erythrocytes lysed by ClyA pores, a linear protomer addition model and a non-sequential addition model [23]. The data fitted better to the linear protomer addition model, but a rate-limiting reaction to assembly-competent protomers after ClyA binding to the membranes was not considered. Importantly, however, it was shown that binding of ClyA to erythrocytes is a very rapid reaction and completed within 10 s after mixing of ClyA with erythrocytes under typical haemolysis assay conditions [23]. The combination of haemoglobin release kinetics and single-molecule FRET experiments in the presence of erythrocyte membranes analogous to those performed in detergent appears to be a promising strategy to clarify the assembly mechanism in cell membranes. Another remaining challenge is the analysis of the dependence of ClyA assembly on the lipid composition of the target membrane. This concerns both ClyA pore assembly in different mammalian target cells and pore assembly in the outer bacterial membrane from which membrane vesicles with intact pore complexes are released into the growth medium [15].
An alternative way to study ClyA pore formation in erythrocyte membranes in a quantitative manner is the recording of the decrease in intact erythrocytes as a function of ClyA concentration via the decrease in the optical density of erythrocyte suspensions [21,24]. Typical lysis curves show a pronounced lag phase that is followed by a rapid decrease in optical density to zero density. Empirically, a linear dependence of maximum lysis velocity (maximum slope of the haemolysis curve at 50% cell lysis) and the inverse lag time of lysis on ClyA concentration in the range of 1–100 nM ClyA was found that allows the determination of the specific lysis activity of ClyA, which is defined as the decrease in erythrocyte density per second and ClyA concentration (ΔOD s−1 nM−1). The results also showed that an about 106-fold excess of ClyA monomers over erythroyctes is required to achieve complete lysis within 100 s at 37°C [21,24]. Although the mechanism underlying the linear dependence of lysis velocity of ClyA concentration is not yet understood, the parameter ‘specific lysis activity’ allows the comparison of the haemolytic activities of different ClyA variants and may also be generally applicable to other PFTs. Recently, a detailed study on the dependence of the velocity and lag phase of erythrocyte lysis on the concentration of the cholesterol-dependent PFT pneumolysin from Streptococcus pneumoniae was reported [25]. Pneumolysin, in contrast with ClyA, forms membrane-associated prepore complexes prior to pores and also shows pore activity of incomplete rings (arcs). The results indicated that the lag depends on oligomerization of pneumolysin, and that the prepore-to-pore transition of arcs and the amount of pneumolysin bound to the membrane determine lysis velocity [25]. In the case of ClyA, the determinants of haemolysis lag and velocity as a function of ClyA concentration still need to be determined, which could be the rates of membrane binding, protomer formation and pore assembly in the membrane and the minimum number of ClyA pores required for lysis.
3. Engineered redox switches in cytolysin A
The finding that formation of assembly-competent protomers from soluble monomers is a prerequisite of ClyA pore complex assembly [17,18,20] opened the possibility to generate redox-controlled, disulfide-trapped ClyA variants that can be activated by reducing agents such as dithiothreitol (DTT). Two variants of soluble ClyA were designed (ClyA CC50/190 and ClyA CC6/264) based on the structures of the ClyA monomer [16] and protomer [17], which lack the natural Cys87/Cys285 pair (see below), but bear an artificial disulfide bond between residues that are in close proximity in the monomer and distant in the protomer (disulfide bond between residues 50 and 190 or residues 6 and 264, respectively; figure 4a,b) [21]. As predicted from the structure of the ClyA monomer, both engineered disulfides indeed prevented protomer formation and trapped ClyA in an assembly-incompetent state. Although both disulfide-trapped variants were unable to form protomers, they still formed a molten globule-like state in the presence of DDM that was very similar to the off-pathway intermediate detected during DDM-induced protomer formation of wild-type ClyA [21]. A reduction in the engineered disulfide bonds in ClyA CC50/190 and ClyA CC6/264 by DTT recovered both pore complex formation in DDM and haemolytic activity. Further analysis showed that the assembly mechanism of both reduced variants was the same as that of wild-type ClyA, and that ClyA CC50/190 and ClyA CC6/264 retained 73 and 17% of the specific haemolytic activity of the wild-type, respectively [21]. ClyA variants with a redox switch offer several interesting applications, such as the control of the kinetics of target cell lysis at constant ClyA concentration by varying the concentration or type of reductant.
Figure 4.
Positions of the engineered cysteine pairs in the redox switch variants ClyA CC6/264 (a) and CC50/190 (b). Formation of an intramolecular disulfide bond between each cysteine pair in the respective, soluble monomer (top panels) prevents the conformational transition to assembly-competent protomers (bottom panels) in detergent or target membranes. The engineered disulfide bond in the monomer is highlighted by a black dashed box, and depicted in detail (top right panels). The positions of the introduced cysteines in the modelled structures of the reduced protomers are indicated by yellow spheres. The crystal structure of the disulfide-bonded monomer of ClyA CC6/264 was solved (pdb ID 4PHO), while the structure of the oxidized monomer of ClyA CC50/190 was modelled based on the structure of the soluble ClyA wild-type monomer (pdb ID 1QOY).
4. The role of the conserved Cys87/Cys285 cysteine pair in cytolysin A assembly
Assembled ClyA pore complexes are released to the extracellular space by E. coli in OMVs that contain lipids from the outer bacterial membrane. Therefore, it is likely that ClyA pores in OMVs assemble from ClyA monomers secreted to the oxidizing environment of the bacterial periplasm [15]. It is, however, still unknown how ClyA monomers reach the periplasm, as ClyA lacks a bacterial signal sequence and its export to the periplasm does not require cytolytic activity. ClyA contains a conserved pair of cysteines (Cys87 and Cys285) that are in close vicinity in the three-dimensional structure of both the ClyA monomer and the ClyA protomer in the assembled pore complex [13,15,26]. While the Cys87/Cys285 pair is reduced in ClyA pores isolated from OMVs [15], the Cys87–Cys285 disulfide is formed in soluble ClyA isolated from the periplasm [16,17]. In addition, E. coli strains deficient in disulfide bond formation and lacking either the periplasmic dithiol oxidase DsbA show a ClyA-dependent haemolytic phenotype in contrast with the corresponding wild-type strain [15]. These results, together with reports on the loss of haemolytic activity upon formation of the Cys87–Cys285 disulfide [14–16,27], suggested that disulfide bond formation inactivates ClyA and prevents its assembly into active pore complexes.
This hypothesis was disproved in a recent study in which the DDM-induced assembly of the disulfide-bonded form of ClyA (ClyAox) was compared with that of the reduced form (ClyAred) under identical experimental conditions [24]. The results showed that ClyAox essentially follows the same assembly mechanism as ClyAred, with protomer formation from monomers as the rate-limiting step prior to fast protomer assembly. ClyAox, however, shows specific features that distinguish it from ClyAred.
Single-molecule FRET experiments at picomolar ClyA concentrations demonstrated that the oxidized protomer (Pox) is even formed about 10 times faster than the reduced protomer in DDM, with a half-life of about 30 s compared with about 300 s, respectively [20,24]. Similar to ClyAred, a molten globule-like off-pathway intermediate (Iox) is populated during formation of (Pox). In contrast with ClyAred, however, where Ired accumulates to 80% after 20 s and is 4.4 kJ mol−1 more stable than the reduced monomer (see above), Iox is even 0.5 kJ mol−1 less stable than the oxidized monomer (Mox) (scheme 3) and does not accumulate to more than 30% in DDM. This, together with a threefold faster, direct pathway (kMP) from the monomer to the protomer (schemes 1 and 3), is the reason why ClyAox forms the protomer faster than ClyAred. Scheme 3 summarizes the stabilities of the intermediate and protomer relative to the monomer for ClyAred and ClyAox. Although the protomer is the most stable conformer in the presence of DDM for both ClyA redox forms, the protomer is not populated to 100% at picomolar ClyA concentrations where oligomerization of protomers is suppressed, as the Pred is only 4.5 kJ mol−1 more stable than Ired, and Pox is only 4.3 kJ mol−1 more stable than Mox. Assembly of pore complexes at higher concentrations, however, pulls the protomers from the I ↔ M ↔ P equilibrium, so that pore assembly can proceed to completion for both ClyAred and ClyAox.
Oxidized and reduced ClyA pores in DDM proved to be indistinguishable in negative-stain electron micrographs [24]. Both redox forms also showed very similar specific activities in lysing horse erythrocytes, with ClyAox exhibiting a 1.2-fold higher specific activity compared with ClyAred. Together, the results show that disulfide bond formation between the natural cysteine pair Cys87/Cys285 in ClyA neither prevents pore assembly in vitro nor decreases the specific haemolytic activity of ClyA [24].
But why was disulfide bond formation in ClyA reported to diminish or abolish the haemolytic activity of ClyA [14–16,27]? An important clue came from the recent observation that ClyA can also form soluble oligomers with defined association states in the absence of detergent [27]. Analytical gel filtration experiments revealed that preparations of soluble ClyA slowly aggregate to two distinct oligomeric species with apparent molecular masses of 1180 and 580 kDa when incubated for several hours at elevated temperatures (37°C). These oligomers were first proposed to be prepore complexes that form active pores after insertion into target membranes in an alternative ClyA assembly mechanism [27]. Further analysis, however, showed that these soluble oligomers are irreversible off-pathway products of pore complex assembly, as they show only about 1% haemolytic activity compared with identical mass concentrations of freshly prepared ClyA monomers [24]. Notably, both reduced and oxidized ClyA monomers can assemble into these oligomeric species during prolonged incubation at 37°C. The kinetics of oligomer formation then demonstrated that ClyAox possesses a much higher tendency of spontaneous inactivation by oligomer formation in the absence of detergent or target membranes: oligomerization of Mox, initiated at monomer concentrations of 5 µM by a temperature shift from 4 to 37°C, proceeded 14 times faster compared with Mred, with half-lives of monomer depletion of 1.7 and 23.5 h for Mox and Mred, respectively. In addition, monomer depletion coincided with loss of haemolytic activity for both ClyA redox forms [15]. Therefore, a plausible explanation for the previously reported loss of haemolytic activity of ClyAox in vitro and in vivo is its spontaneous reaction to inactive oligomers that can no longer be recovered for pore formation.
What is, then, the role disulfide bond formation between the natural Cys87/Cys285 pair in ClyA? The observation that ClyA pore complexes secreted by E. coli in OMVs are reduced [15], together with the rapid inactivation of oxidized ClyA at the optimum E. coli growth temperature, suggests that the reduced form of ClyA is the physiologically active redox form. As reduced ClyA is likely to be recruited from the periplasm for pore complex assembly in OMVs, both ClyA redox forms probably occur in the periplasm. The Cys87/285 thiol pair in the folded, reduced ClyA monomer was shown to be resistant against oxidation by the dithiol oxidase DsbA [24], the only known periplasmic enzyme that can introduce disulfide bonds into periplasmic proteins [28]. Thus, there is possibly a competition between folding of reduced ClyA to the DsbA-resistant monomer and oxidation of unfolded ClyA by DsbA prior to ClyA folding in the periplasm. It also remains to be established whether inactive oligomers of oxidized ClyA cause the loss of a haemolytic phenotype of E. coli strains with active DsbA. At any rate, the mechanism of translocation of ClyA to the periplasm remains one of the most important open questions in the elucidation of the biogenesis of ClyA pore complexes.
5. Outlook: engineering of cytolysin A pores
The efficient, spontaneous assembly of ClyA monomers to pore complexes in the presence of detergent and membranes, together with the solved structures of the monomer and the pore complex, opens the possibility to engineer ClyA pores with novel properties by rational means. N- and C-terminal fusions to ClyA that do not compromise pore assembly have already been applied to generate ClyA pore complexes with novel functions [29,30] and could be used in the future to create membrane- or cargo-specific pores. In addition, ClyA proved to be robust against multiple amino acid replacements in the engineering of novel pore variants. Successful examples include the directed evolution of ClyA towards larger pore sizes (up to 14 protomers) [31] and the encapsulation of folded proteins by engineered ClyA variants [32], demonstrating the enormous potential of ClyA for designing pores and molecular cages with novel characteristics.
Acknowledgements
We particularly thank our collaborators N. Ban, S. Benke, U. Grauschopf, M. Mueller and B. Schuler for their contributions to the characterization of the structure of the ClyA and its assembly mechanism.
Authors' contributions
D.R. and R.G. prepared the figures and wrote the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This project was funded by the ETH Zurich and the Swiss National Science Foundation (SNF) within the framework of the NCCR Structural Biology Program.
References
- 1.Dal Peraro M, van der Goot FG. 2016. Pore-forming toxins: ancient, but never really out of fashion. Nat. Rev. Microbiol. 14, 77–92. ( 10.1038/nrmicro.2015.3) [DOI] [PubMed] [Google Scholar]
- 2.Los FC, Randi TM, Aroian RV, Ratner AJ. 2013. Role of pore-forming toxins in bacterial infectious diseases. Microbiol. Mol. Rev. 77, 173–207. ( 10.1128/MMBR.00052-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bischofberger M, Iacovache I, van der Goot FG. 2012. Pathogenic pore-forming proteins: function and host response. Cell Host Microbe 12, 266–275. ( 10.1016/j.chom.2012.08.005) [DOI] [PubMed] [Google Scholar]
- 4.Gilbert RJ, Dalla Serra M, Froelich CJ, Wallace MI, Anderluh G. 2014. Membrane pore formation at protein–lipid interfaces. Trends Biochem. Sci. 39, 510–516. ( 10.1016/j.tibs.2014.09.002) [DOI] [PubMed] [Google Scholar]
- 5.Cosentino K, Ros U, García-Sáez AJ. 2016. Assembling the puzzle: oligomerization of α-pore forming proteins in membranes. Biochim. Biophys. Acta 1858, 457–466. ( 10.1016/j.bbamem.2015.09.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rojko N, Anderluh G. 2015. How lipid membranes affect pore forming toxin activity. Acc. Chem. Res. 48, 3073–3079. ( 10.1021/acs.accounts.5b00403) [DOI] [PubMed] [Google Scholar]
- 7.Reboul CF, Whisstock JC, Dunstone MA. 2016. Giant MACPF/CDC pore forming toxins: a class of their own. Biochim. Biophys. Acta 1858, 475–486. ( 10.1016/j.bbamem.2015.11.017) [DOI] [PubMed] [Google Scholar]
- 8.Hunt S, Green J, Artymiuk PJ. 2010. Hemolysin E (HlyE, ClyA, SheA) and related toxins. Adv. Exp. Med. Biol. 677, 116–126. ( 10.1007/978-1-4419-6327-7_10) [DOI] [PubMed] [Google Scholar]
- 9.Jakes KS, Cramer WA. 2012. Border crossings: colicins and transporters. Annu. Rev. Genet. 46, 209–231. ( 10.1146/annurev-genet-110711-155427) [DOI] [PubMed] [Google Scholar]
- 10.Kristan KC, Viero G, Dalla Serra M, Macek P, Anderluh G. 2009. Molecular mechanism of pore formation by actinoporins. Toxicon 54, 1125–1134. ( 10.1016/j.toxicon.2009.02.026) [DOI] [PubMed] [Google Scholar]
- 11.Huang LJ, Cui J, Piao HH, Hong Y, Choy HE, Ryu PY. 2010. Molecular cloning and characterization of clyA genes in various serotypes of Salmonella enterica. J. Microbiol. 48, 663–667. ( 10.1007/s12275-010-9268-9) [DOI] [PubMed] [Google Scholar]
- 12.del Castillo FJ, Leal SC, Moreno F, del Castillo I. 1997. The Escherichia coli K-12 sheA gene encodes a 34-kDa secreted haemolysin. Mol. Microbiol. 25, 107–115. ( 10.1046/j.1365-2958.1997.4391813.x) [DOI] [PubMed] [Google Scholar]
- 13.Ludwig A, Völkerink G, von Rhein C, Bauer S, Maier E, Bergmann B, Goebel W, Benz R. 2010. Mutations affecting export and activity of cytolysin A from Escherichia coli. J. Bacteriol. 192, 4001–4011. ( 10.1128/JB.01283-09) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atkins A, Wyborn NR, Wallace AJ, Stillman TJ, Black LK, Fielding AB, Hisakado M, Artymiuk PJ, Green J. 2000. Structure–function relationships of a novel bacterial toxin, hemolysin E. The role of αG. J. Biol. Chem. 275, 41 150–41 155. ( 10.1074/jbc.M005420200) [DOI] [PubMed] [Google Scholar]
- 15.Wai SN, et al. 2003. Vesicle-mediated export and assembly of pore-forming oligomers of the enterobacterial ClyA cytotoxin. Cell 115, 25–35. ( 10.1016/S0092-8674(03)00754-2) [DOI] [PubMed] [Google Scholar]
- 16.Wallace AJ, Stillman TJ, Atkins A, Jamieson SJ, Bullough PA, Green J, Artymiuk PJ. 2000. E. coli hemolysin E (HlyE, ClyA, SheA): X-ray crystal structure of the toxin and observation of membrane pores by electron microscopy. Cell 100, 265–276. ( 10.1016/S0092-8674(00)81564-0) [DOI] [PubMed] [Google Scholar]
- 17.Mueller M, Grauschopf U, Maier T, Glockshuber R, Ban N. 2009. The structure of a cytolytic alpha-helical toxin pore reveals its assembly mechanism. Nature 459, 726–730. ( 10.1038/nature08026) [DOI] [PubMed] [Google Scholar]
- 18.Eifler N, et al. 2006. Cytotoxin ClyA from Escherichia coli assembles to a 13-meric pore independent of its redox-state. EMBO J. 25, 2652–2661. ( 10.1038/sj.emboj.7601130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ludwig A, Bauer S, Benz R, Bergmann B, Goebel W. 1999. Analysis of the SlyA-controlled expression, subcellular localization and pore-forming activity of a 34 kDa haemolysin (ClyA) from Escherichia coli K-12. Mol. Microbiol. 31, 557–567. ( 10.1046/j.1365-2958.1999.01196.x) [DOI] [PubMed] [Google Scholar]
- 20.Benke S, Roderer D, Wunderlich B, Nettels D, Glockshuber R, Schuler B. 2015. The assembly dynamics of the cytolytic pore toxin ClyA. Nat Commun. 6, 6198 ( 10.1038/ncomms7198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roderer D, Benke S, Müller M, Fäh-Rechsteiner H, Ban N, Schuler B, Glockshuber R. 2014. Characterization of variants of the pore-forming toxin ClyA from Escherichia coli controlled by a redox switch. Biochemistry 53, 6357–6369. ( 10.1021/bi5007578) [DOI] [PubMed] [Google Scholar]
- 22.Gilbert RJ. 2016. Protein–lipid interactions and non-lamellar lipidic structures in membrane pore formation and membrane fusion. Biochim. Biophys. Acta 1858, 487–499. ( 10.1016/j.bbamem.2015.11.026) [DOI] [PubMed] [Google Scholar]
- 23.Vaidyanathan MS, Sathyanarayana P, Maiti PK, Visweswariah SS, Ayappa KG. 2014. Lysis dynamics and membrane oligomerization pathways for Cytolysin A (ClyA) pore-forming toxin. RSC Adv. 4, 4930–4942. ( 10.1039/C3RA45159C) [DOI] [Google Scholar]
- 24.Roderer D, Benke S, Schuler B, Glockshuber R. 2016. Soluble oligomers of the pore-forming toxin Cytolysin A from Escherichia coli are off-pathway products of pore assembly. J. Biol. Chem. 291, 5652–5663. ( 10.1074/jbc.M115.700757) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilbert JC, Sonnen AFP. 2016. Measuring kinetic drivers of pneumolysin pore structure. Eur. Biophys. J. 45, 365–376. ( 10.1007/s00249-015-1106-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oscarsson J, Mizunoe Y, Li L, Lai XH, Wieslander A, Uhlin BE. 1999. Molecular analysis of the cytolytic protein ClyA (SheA) from Escherichia coli. Mol. Microbiol. 32, 1226–1238. ( 10.1046/j.1365-2958.1999.01435.x) [DOI] [PubMed] [Google Scholar]
- 27.Fahie M, Romano FB, Chisholm C, Heuck AP, Zbinden M, Chen M. 2013. A non-classical assembly pathway of Escherichia coli pore-forming toxin cytolysin A. J. Biol. Chem. 288, 31 042–31 051. ( 10.1074/jbc.M113.475350) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hatahet F, Boyd D, Beckwith J. 2014. Disulfide bond formation in prokaryotes: history, diversity and design. Biochim. Biophys. Acta 1844, 1402–1414. ( 10.1016/j.bbapap.2014.02.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim JY, Doody AM, Chen DJ, Cremona GH, Shuler ML, Putnam D, DeLisa MP. 2008. Engineered bacterial outer membrane vesicles with enhanced functionality. J. Mol. Biol. 380, 51–66. ( 10.1016/j.jmb.2008.03.076) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soskine M, Biesemans A, Moeyaert B, Cheley S, Bayley H, Maglia G. 2012. An engineered ClyA nanopore detects folded target proteins by selective external association and pore entry. Nano Lett. 12, 4895–4900. ( 10.1021/nl3024438) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soskine M, Biesemans A, De Maeyer M, Maglia G. 2013. Tuning the size and properties of ClyA nanopores assisted by directed evolution. J. Am. Chem. Soc. 135, 13 456–13 463. ( 10.1021/ja4053398) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soskine M, Biesemans A, Maglia G. 2015. Single-molecule analyte recognition with ClyA nanopores equipped with internal protein adaptors. J. Am. Chem. Soc. 137, 5793–5797. ( 10.1021/jacs.5b01520) [DOI] [PMC free article] [PubMed] [Google Scholar]