Abstract
The rational (de novo) design of membrane-spanning proteins lags behind that for water-soluble globular proteins. This is due to gaps in our knowledge of membrane-protein structure, and experimental difficulties in studying such proteins compared to water-soluble counterparts. One limiting factor is the small number of experimentally determined three-dimensional structures for transmembrane proteins. By contrast, many tens of thousands of globular protein structures provide a rich source of ‘scaffolds’ for protein design, and the means to garner sequence-to-structure relationships to guide the design process. The α-helical coiled coil is a protein-structure element found in both globular and membrane proteins, where it cements a variety of helix–helix interactions and helical bundles. Our deep understanding of coiled coils has enabled a large number of successful de novo designs. For one class, the α-helical barrels—that is, symmetric bundles of five or more helices with central accessible channels—there are both water-soluble and membrane-spanning examples. Recent computational designs of water-soluble α-helical barrels with five to seven helices have advanced the design field considerably. Here we identify and classify analogous and more complicated membrane-spanning α-helical barrels from the Protein Data Bank. These provide tantalizing but tractable targets for protein engineering and de novo protein design.
This article is part of the themed issue ‘Membrane pores: from structure and assembly, to medicine and technology’.
Keywords: α-helical barrel, coiled coil, de novo protein design, transmembrane proteins
1. Background
Membrane-spanning protein channels and pores play critical roles in cellular functions, transporting ions and small molecules across biological membranes [1–3]. Many of these form bundled and barrel-like structures, which are generally based on either α-helical or β-hairpin units, respectively [4]. The latter dominate bacterial outer membranes (OMPs, aka porins [5]), whereas α helices are the major structure found in both prokaryotic and eukaryotic cell membranes such as voltage-gated ion channels [1,6–8], G-protein coupled receptors [9–11] and ABC transporters [3,12,13] (figure 1). Compared with β-barrel proteins and what might be termed the α-helical bundles above, however, so-called α-helical barrels are relatively unexplored, as examples and high-resolution structures are limited. Here, we define α-helical barrels as bundles of five or more α helices with central accessible channels that are usually highly symmetric. Given the many transport functions of membrane-spanning β-barrels, the successful rational design and engineering of membrane-spanning α-helical–barrel proteins could have significant impact in bionanotechnology and synthetic biology. This would be aided considerably if we could learn from and translate the growing information on water-soluble α-helical barrels and related coiled coils. In this review, we describe membrane-spanning α-helical–barrel structures that we have identified in the Protein Data Bank, and suggest how their untapped potential for designing new protein channels and pores might be explored.
Figure 1.
Examples of three different families of natural membrane-spanning α-helical bundles. The transmembrane α-helical bundles are shown in red. (a) A heteromeric ABC transporter (3QF4) [13]. (b) A G-protein–coupled receptor, adenosine A2A receptor (5G53) [11]. (c) A bacterial voltage-gated sodium channel (3RVY) [6].
While not mature, the field of de novo protein design is advancing rapidly, and it is now possible to design a range of water-soluble proteins rationally using rules of thumb and, increasingly, using computational design methods [14,15]. Broadly speaking, there are two approaches in protein engineering and design: (i) the redesign (or engineering) of natural structural scaffolds; and (ii) the completely de novo design of entirely new proteins, which usually uses structural constraints and sequence-to-structure relationships learnt from natural proteins [16,17].
In the area of redesigning membrane-spanning α-helical barrels, Franceschini et al. have engineered Cytolysin A (ClyA, PDB accession code 2WCD) from Salmonella typhi [18], which forms a membrane-spanning dodecameric α-helical barrel stabilized by a large soluble subunit. The engineered ClyA forms a discrete pore in a planar lipid bilayer, which can translocate dsDNA. Another example is the demonstration that a short peptide from the much larger Wza protein (2J58) autonomously inserts and spans membranes to form active channels [19] (figure 2). So-called cWza is a 35–amino-acid α-helical peptide based on the C-terminal D4 domain of the E. coli polysaccharide transporter Wza [21]. cWza peptides form monodisperse barrels in synthetic membranes that accord with the octameric crystal structure of the whole Wza protein. These engineered α-helical barrels have potential for use in applications for nanopore technology.
Figure 2.
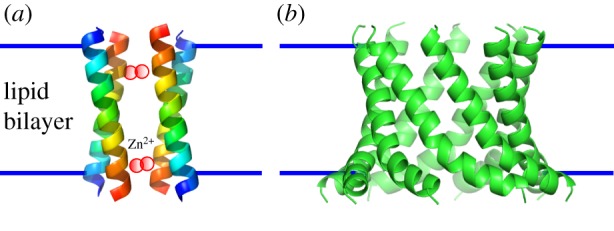
Structural models of ROCKER [20] (a), and cWza pore [19] (b).
In terms of de novo design of a helical bundle, and inspired by the multidrug transporter EmrE (3B5D) [22], DeGrado and colleagues report a membrane-spanning four-helix bundle named ROCKER (2MUZ) that transports zinc ions and antiports protons across membranes [20] (figure 2). The peptide assembly forms a loose dimer of tight helix dimers. The tight interface is stabilized by an array of closely packed alanine residues. DeGrado also presents designs for possible α-helical barrels, comprising five or more helices that span membranes and make ion channels, although stoichiometry and high-resolution structural data have not been reported [23].
In addition to structural inspiration and targets, protein designers require sequence-to-structure relationships, or at least computational methods that capture these, to guide and complete the design process. For membrane-spanning proteins, and particularly for α-helical structures, such relationships are hard to garner because of the preponderance of hydrophobic residues in membrane-spanning regions, making it difficult to identify signals for helix–helix assembly and packing. One of the most intensely studied sequence motifs that does direct such interactions in transmembrane proteins is [GAS]xxx[GAS]. This drives the packing of two helices tightly at ‘pockets’ of two small (Gly, Ala or Ser) amino acid residues [24]. Two-helix dimers designed using this motif have been reported [25,26]. The motif has recently been found also in helix trimers [27], which opens up further design strategies for helix bundles. In terms of α-helical barrels with five or more helices, however, continuous [GAS]xxx[GAS] motifs are not common and may not be the solution.
The coiled coil is another well-known protein-folding motif. It is found in both water-soluble and membrane proteins, and provides a strong basis for all aspects of rational protein design [14,15]. α-Helical barrels are a subset of coiled-coil structures. Moreover, and at least for water-soluble assemblies, α-helical barrels with five to seven helices have been designed and delivered through parametric design in a small number of groups [15,28,29]. There is considerable scope and potential for the design and engineering of membrane-soluble analogues of these α-helical barrels [30]. With this in mind, we focus here on identifying and classifying structures of membrane-spanning coiled-coil–based α-helical barrels in the RCSB Protein Data Bank (PDB). We hope that our findings will aid future protein engineering and design studies of these inspiring and potentially useful design targets.
2. Results
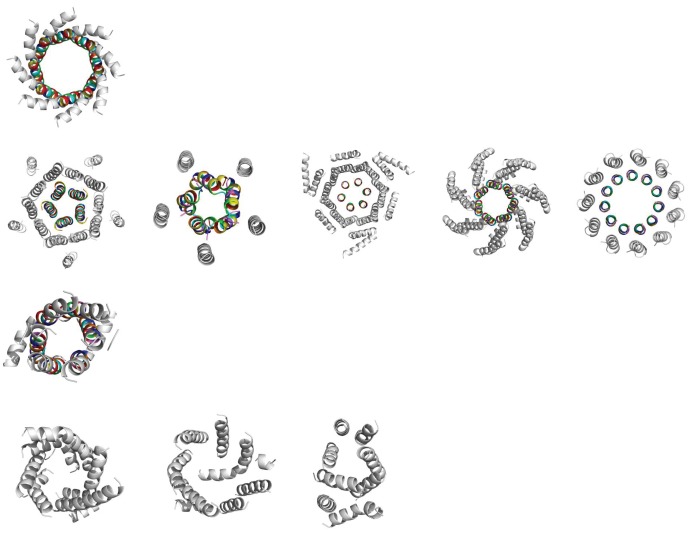
We searched the Protein Data Bank of Transmembrane Proteins [31,32] for structures that were classified as non-redundant and α-helical, and found 396 structures in total. The membrane-spanning regions of these structures, as determined using the TMDET algorithm [31,32], were then examined using SOCKET [33,34]. SOCKET identifies knobs-into-holes packing between side chains of neighbouring α-helices, which is the hallmark of coiled-coil structures. In this way, we found 10 coiled-coil–based α-helical barrels (figure 3). We classified these according to the arrangement of helices within them, considering three factors: the symmetry of the barrel; the presence or absence of surrounding barrels; and whether the helices are arranged in a parallel or antiparallel fashion. This resulted in four classes (figure 3).
Figure 3.
The 10 barrels in their four categories, with each row representing a class. The transmembrane region for each structure, as determined by TMDET, is shown in cartoon form. The coiled-coil regions are shown in rainbow colours, according to the assigned heptad register, with a residues in red and d residues in green. Row 1: symmetric, parallel, autonomous (2J58). Row 2: symmetric, parallel, buttressed (3UQ4, 4EV6, 4HKR, 4HW9, 4BEM). Row 3: symmetric, antiparallel, autonomous (4QND). Row 4: asymmetric, antiparallel, autonomous (4C9 J, 4IL3, 4CAD).
Class 1 contains only Wza (2J58) [21], which is the single example of a symmetric, parallel and autonomous barrel.
Class 2 has five structures that are symmetric, parallel and surrounded, or buttressed by secondary barrels. These are ELIC (ligand-gated ion channel from Erwinia chrisanthemi, 3UQ4) [35]; CorA (a bacterial magnesium transporter, 4EV6) [36]; Orai (a bacterial calcium release-activated calcium (CRAC) channel, 4HKR) [37]; MscS (a bacterial mechanosensitive channel, 4HW9) [38]; and the Na+-driven membrane rotor ring of a bacterial ATP synthase (4BEM) [39].
Class 3 has a sole member, semiSWEET (a bacterial sugar transporter, 4QND) [40], which is a symmetric, antiparallel and autonomous barrel.
Class 4 has three structures that are asymmetric, antiparallel and autonomous: Aac3p (a mitochondrial ADP/ATP carrier, 4C9 J) [41]; Ste24p (a yeast CAAX protease, 4IL3) [42]; and Rce1 (a bacterial Ras and A-factor–converting enzyme, 4CAD) [43].
Coiled coils can be defined parametrically, which is useful for further classifying these structures and for parametric computational designs. With this in mind, we determined the four main coiled-coil structural parameters—oligomeric state, supercoil radius, alpha angle and pitch—for the 10 identified transmembrane α-helical barrels (table 1). With currently available modelling tools [44], these parameters could be used straightforwardly to generate models for these and closely related structural targets, and, hence, as a basis for design. We note, as in water-soluble barrels [28,29], that there is an approximately linear relationship between the number of helices in the transmembrane barrels and the radius of the pores. That said, Wza (2J58), with eight helices, has a larger radius (14.82 Å) than the bacterial ATP synthase (4BEM) (11 helices, 12.86 Å). The central helices of Orai (4HKR) and the ATP synthase rotor ring (4BEM) are notably straight, resulting in very high pitch values (more than 1800 Å) for these assemblies.
Table 1.
Coiled-coil parameters for the classified transmembrane α-helical barrels. For each PDB code, the category is given along with the number of helices in the central barrel. The coiled-coil parameters [44] are given for each structure in Classes 1–3.
| PDB code | class | no. helices | radius (Å) | alpha ( ° ) | pitch (Å) |
|---|---|---|---|---|---|
| 2J58 | 1 | 8 | 14.82 ± 0.65 | 33.50 ± 0.89 | 140.71 |
| 3UQ4 | 2 | 5 | 8.26 ± 0.36 | 11.22 ± 2.25 | 261.65 |
| 4EV6 | 2 | 5 | 8.27 ± 1.21 | 22.15 ± 4.64 | 127.61 |
| 4HKR | 2 | 6 | 9.24 ± 0.12 | 1.76 ± 1.25 | 1892.69 |
| 4HW9 | 2 | 6 | 11.72 ± 0.45 | 21.45 ± 3.90 | 187.48 |
| 4BEM | 2 | 11 | 12.86 ± 0.78 | 2.56 ± 1.86 | 1805.40 |
| 4QND | 3 | 6 | 10.36 ± 1.00 | 60.50 ± 1.54 | 36.82 |
| 4C9 J | 4 | 6 | — | — | — |
| 4IL3 | 4 | 7 | — | — | — |
| 4CAD | 4 | 8 | — | — | — |
To benefit fully from the information that these natural structures offer, their sequences need to be examined, in particular residues involved in helix–helix packing and those directed towards the lumens of the channels. Though not a hard and fast rule, these are usually residues found at a and d sites of so-called heptad (a–g) repeats that are the signature of coiled-coil sequences. As a start, and in representative sequences from the coiled-coil regions of the six structures in Classes 1 and 2, we observe that 5 of 13 residues at a positions are polar (38.5%), while at d positions this increases to 9 out of 15 residues (60.0%). This trend is consistent with α-helical barrels, since the d positions project directly into the lumen of the barrel, and the a sites are directed towards an adjacent helix. Further insights, from larger analysis of related protein sequences as more sequences and structures become available, will provide the basis for sequence-to-structure relationships to guide the design of membrane-spanning α-helical barrels.
3. Discussion and conclusion
The rational design of membrane-spanning proteins represents a considerable challenge in structural molecular biology, which lags behind advances being made in designing water-soluble proteins.
We did test various topology prediction methods (TOPCONS [45], Philius [46], MEMSAT3 [47] and MEMSAT-SVM [48]) with the amino acid sequences of monomer subunits of Wza (Class 1) and Orai (Class 2). Apart from MEMSAT-SVM for Wza and Philius for Orai, these methods did not predict the structurally determined amphipathic membrane-spanning helices that form the barrels. This emphasizes the challenge in designing the transmembrane α-helical barrels, and, indeed, transmembrane proteins in general.
Nonetheless, an ability to design new transmembrane proteins would probably have considerable impact across biotechnology and synthetic biology, particularly in sensing and nanopore technologies. To help address this we have identified and classified a small clutch of protein structures that harbour membrane-spanning α-helical barrels.
The classes of membrane barrels presented offer increasingly difficult challenges to designers. There has already been success in engineering a Class 1 structure [19]. Recent improvements in our understanding of, and our ability to design, large soluble barrels [28,29] provides confidence that similar breakthroughs may now be possible for their membrane structural analogues.
The structures in Class 2, with their secondary or buttressing barrels may be more difficult to design; along with understanding intra-barrel helix–helix interactions, those between the inner and outer barrels will also have to be understood. This challenge may be offset; however, these interactions may confer stability on the structures and thus make them more amenable to design. Again, we can take encouragement from the successful design of analogous water-soluble structures [49].
Class 3 is particularly interesting as the structure comprises a symmetric dimer of single-chain trimers, and represents the further challenge of designing antiparallel assemblies. There has not been as much design success for such structures in aqueous solution, and to facilitate these designs there will be a need to design against the alternative, parallel conformations. There is an abundance of natural antiparallel coiled coils [50], which, potentially, provides data to guide the design of Class 3 structures.
The asymmetric structures in Class 4 perhaps present the most daunting design prospect. The successful design of such a structure would be a clear demonstration of detailed understanding. It is possible that we will obtain such a structure serendipitously through a ‘failed’ or collapsed design of a Class 3 structure.
Clearly, the number of large transmembrane α-helical barrels is small, which limits the understanding that we can apply for design strategies. However, improving techniques for protein modelling and design, combined with recent advances in both membrane- and water-soluble barrels suggest that there is cause for optimism.
4. Methods
SOCKET identifies knob-into-hole interactions between side-chains within a given packing cut-off. For α-helices in solution, it has been determined that a cut-off of 7–7.5 Å is an appropriate choice [33]. We increased this to 9.5 Å in order to capture barrels with less-tight packing.
The SOCKET output files were processed automatically using in-house Python scripts to search for simple interaction cycles between groups of helices that would indicate α-helical barrel structures. This yielded 15 structures, which were then studied in more detail individually, with 10 structures confirmed as barrels.
This final confirmation step included verification of the transmembrane regions of the proteins by making qualitative comparison between the regions determined by TMDET and those identified in the Orientations of Proteins in Membranes (OPM) database [51]. The transmembrane regions in the OPM database agreed with those determined by TMDET.
The radius (r) and alpha (α) values were each calculated using in-house Python scripts; the pitch (p) was derived from these values using the relationship 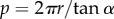 .
.
Heptad register positions were assigned for each residue in the barrels according to their interface angle [44]. We then examined the register for the coiled-coil regions of a representative helix in each of the six symmetric, parallel barrels in Classes 1 and 2.
Authors' contributions
D.N.W., A.N. and J.W.H. conceived the study. J.W.H. carried out the PDB search and structure and sequence analyses. A.N., J.W.H., K.F., A.R.T. and D.N.W. wrote the manuscript.
Competing interests
We declare we have no competing interests.
Funding
A.N. and D.N.W. were funded by the BBSRC (BB/J009784/1); all authors are funded by a European Research Council Advanced Grant to D.N.W. (340764); and D.N.W. holds a Royal Society Wolfson Research Merit Award (WM140008).
References
- 1.Yu FH, Catterall WA. 2004. The VGL-chanome: a protein superfamily specialized for electrical signaling and ionic homeostasis. Sci. STKE 2004, re15 ( 10.1126/stke.2532004re15) [DOI] [PubMed] [Google Scholar]
- 2.Dingledine R, Borges K, Bowie D, Traynelis SF. 1999. The glutamate receptor ion channels. Pharmacol. Rev. 51, 7–61. [PubMed] [Google Scholar]
- 3.Higgins CF. 1992. ABC transporters—from microorganisms to man. Annu. Rev. Cell Biol. 8, 67–113. ( 10.1146/annurev.cb.08.110192.000435) [DOI] [PubMed] [Google Scholar]
- 4.Luckey M. 2008. Membrane structural biology: with biochemical and biophysical foundations. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 5.Koebnik R, Locher KP, Van Gelder P. 2000. Structure and function of bacterial outer membrane proteins: barrels in a nutshell. Mol. Microbiol. 37, 239–253. ( 10.1046/j.1365-2958.2000.01983.x) [DOI] [PubMed] [Google Scholar]
- 6.Payandeh J, Scheuer T, Zheng N, Catterall WA. 2011. The crystal structure of a voltage-gated sodium channel. Nature 475, 353–358. ( 10.1038/nature10238) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uysal S, Vasquez V, Tereshko V, Esaki K, Fellouse FA, Sidhu SS, Koide S, Perozo E, Kossiakoff A. 2009. Crystal structure of full-length KcsA in its closed conformation. Proc. Natl Acad. Sci. USA 106, 6644–6649. ( 10.1073/pnas.0810663106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi N, Ye S, Alam A, Chen L, Jiang Y. 2006. Atomic structure of a Na+- and K+-conducting channel. Nature 440, 570–574. ( 10.1038/nature04508) [DOI] [PubMed] [Google Scholar]
- 9.Rosenbaum DM, Rasmussen SGF, Kobilka BK. 2009. The structure and function of G-protein-coupled receptors. Nature 459, 356–363. ( 10.1038/nature08144) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang DD, Zhao Q, Wu BL. 2015. Structural studies of G protein-coupled receptors. Mol. Cells 38, 836–842. ( 10.14348/molcells.2015.0263) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carpenter B, Nehmé R, Warne T, Leslie AG, Tate CG. 2016. Structure of the adenosine A2A receptor bound to an engineered G protein. Nature 536, 104–107. ( 10.1038/nature18966) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beis K. 2015. Structural basis for the mechanism of ABC transporters. Biochem. Soc. Trans. 43, 889–893. ( 10.1042/bst20150047) [DOI] [PubMed] [Google Scholar]
- 13.Hohl M, Briand C, Grutter MG, Seeger MA. 2012. Crystal structure of a heterodimeric ABC transporter in its inward-facing conformation. Nat. Struct. Mol. Biol. 19, 395–402. ( 10.1038/nsmb.2267) [DOI] [PubMed] [Google Scholar]
- 14.Woolfson DN, Bartlett GJ, Bruning M, Thomson AR. 2012. New currency for old rope: from coiled-coil assemblies to α-helical barrels. Curr. Opin. Struct. Biol. 22, 432–441. ( 10.1016/j.sbi.2012.03.002) [DOI] [PubMed] [Google Scholar]
- 15.Woolfson DN, Bartlett GJ, Burton AJ, Heal JW, Niitsu A, Thomson AR, Wood CW. 2015. De novo protein design: how do we expand into the universe of possible protein structures? Curr. Opin. Struct. Biol. 33, 16–26. ( 10.1016/j.sbi.2015.05.009) [DOI] [PubMed] [Google Scholar]
- 16.Bayley H, Jayasinghe L. 2004. Functional engineered channels and pores. Mol. Membr. Biol. 21, 209–220. ( 10.1080/09687680410001716853) [DOI] [PubMed] [Google Scholar]
- 17.Simms J, Booth PJ. 2013. Membrane proteins by accident or design. Curr. Opin. Chem. Biol. 17, 976–981. ( 10.1016/j.cbpa.2013.10.005) [DOI] [PubMed] [Google Scholar]
- 18.Franceschini L, Soskine M, Biesemans A, Maglia G. 2013. A nanopore machine promotes the vectorial transport of DNA across membranes. Nat. Commun. 4, 2415 ( 10.1038/ncomms3415) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahendran KR, Niitsu A, Kong L, Thomson AR, Sessions RB, Woolfson DN, Bayley H. 2017. A monodisperse alpha-helical peptide barrel. Nat. Chem. 9, 411–419. ( 10.1038/nchem.2647) [DOI] [PubMed] [Google Scholar]
- 20.Joh NH, Wang T, Bhate MP, Acharya R, Wu Y, Grabe M, Hong M, Grigoryan G, DeGrado WF. 2014. De novo design of a transmembrane Zn2+-transporting four-helix bundle. Science 346, 1–6. ( 10.1126/science.1261172) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong C, Beis K, Nesper J, Brunkan-Lamontagne AL, Clarke BR, Whitfield C, Naismith JH. 2006. Wza the translocon for E. coli capsular polysaccharides defines a new class of membrane protein. Nature 444, 226–229. ( 10.1038/nature05267) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morrison EA, DeKoster GT, Dutta S, Vafabakhsh R, Clarkson MW, Bahl A, Kern D, Ha T, Henzler-Wildman KA. 2012. Antiparallel EmrE exports drugs by exchanging between asymmetric structures. Nature 481, 45–50. ( 10.1038/nature10703) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lear JD, Wasserman ZR, DeGrado WF. 1988. Synthetic amphiphilic peptide models for protein ion channels. Science 240, 1171–1181. ( 10.1126/science.2453923) [DOI] [PubMed] [Google Scholar]
- 24.Senes A, Gerstein M, Engelman DM. 2000. Statistical analysis of amino acid patterns in transmembrane helices: the GxxxG motif occurs frequently and in association with beta-branched residues at neighboring positions. J. Mol. Biol. 296, 921–936. ( 10.1006/jmbi.1999.3488) [DOI] [PubMed] [Google Scholar]
- 25.Yin H, et al. 2007. Computational design of peptides that target transmembrane helices. Science 315, 1817–1822. ( 10.1126/science.1136782) [DOI] [PubMed] [Google Scholar]
- 26.Cordova JM, Noack PL, Hilcove SA, Lear JD, Ghirlanda G. 2007. Design of a functional membrane protein by engineering a heme-binding site in glycophorin A. J. Am. Chem. Soc. 129, 512–518. ( 10.1021/ja057495i) [DOI] [PubMed] [Google Scholar]
- 27.Feng X, Barth P. 2016. A topological and conformational stability alphabet for multipass membrane proteins. Nat. Chem. Biol. 12, 167–173. ( 10.1038/nchembio.2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomson AR, Wood CW, Burton AJ, Bartlett GJ, Sessions RB, Brady RL, Woolfson DN. 2014. Computational design of water-soluble alpha-helical barrels. Science 346, 485–488. ( 10.1126/science.1257452) [DOI] [PubMed] [Google Scholar]
- 29.Huang PS, et al. 2014. High thermodynamic stability of parametrically designed helical bundles. Science 346, 481–485. ( 10.1126/science.1257481) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y, Kulp DW, Lear JD, DeGrado WF. 2009. Experimental and computational evaluation of forces directing the association of transmembrane helices. J. Am. Chem. Soc. 131, 11 341–11 343. ( 10.1021/ja904625b) [DOI] [PubMed] [Google Scholar]
- 31.Tusnady GE, Dosztanyi Z, Simon I. 2004. Transmembrane proteins in the Protein Data Bank: identification and classification. Bioinformatics 20, 2964–2972. ( 10.1093/bioinformatics/bth340) [DOI] [PubMed] [Google Scholar]
- 32.Tusnady GE, Dosztanyi Z, Simon I. 2005. PDB_TM: selection and membrane localization of transmembrane proteins in the protein data bank. Nucleic Acids Res. 33, D275–D278. ( 10.1093/nar/gki002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walshaw J, Woolfson DN. 2001. SOCKET: a program for identifying and analysing coiled-coil motifs within protein structures. J. Mol. Biol. 307, 1427–1450. ( 10.1006/jmbi.2001.4545) [DOI] [PubMed] [Google Scholar]
- 34.Walshaw J, Woolfson DN. 2003. Extended knobs-into-holes packing in classical and complex coiled-coil assemblies. J. Struct. Biol. 144, 349–361. ( 10.1016/j.jsb.2003.10.014) [DOI] [PubMed] [Google Scholar]
- 35.Gonzalez-Gutierrez G, Lukk T, Agarwal V, Papke D, Nair SK, Grosman C. 2012. Mutations that stabilize the open state of the Erwinia chrisanthemi ligand-gated ion channel fail to change the conformation of the pore domain in crystals. Proc. Natl Acad. Sci. USA 109, 6331–6336. ( 10.1073/pnas.1119268109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guskov A, Nordin N, Reynaud A, Engman H, Lundback AK, Jong AJO, Cornvik T, Phua T, Eshaghi S. 2012. Structural insights into the mechanisms of Mg2+ uptake, transport, and gating by CorA. Proc. Natl Acad. Sci. USA 109, 18 459–18 464. ( 10.1073/pnas.1210076109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hou X, Pedi L, Diver MM, Long SB. 2012. Crystal structure of the calcium release-activated calcium channel Orai. Science 338, 1308–1313. ( 10.1126/science.1228757) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lai JY, Poon YS, Kaiser JT, Rees DC. 2013. Open and shut: crystal structures of the dodecylmaltoside solubilized mechanosensitive channel of small conductance from Escherichia coli and Helicobacter pylori at 4.4 angstrom and 4.1 angstrom resolutions. Protein Sci. 22, 502–509. ( 10.1002/pro.2222) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matthies D, Zhou WC, Klyszejko AL, Anselmi C, Yildiz O, Brandt K, Müller V, Faraldo-Gómez JD, Meier T. 2014. High-resolution structure and mechanism of an F/V-hybrid rotor ring in a Na+-coupled ATP synthase. Nat. Commun. 5, 14 ( 10.1038/ncomms6286) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu Y, Tao Y, Cheung LS, Fan C, Chen LQ, Xu S, Perry K, Frommer WB, Feng L. 2014. Structures of bacterial homologues of SWEET transporters in two distinct conformations. Nature 515, 448–452. ( 10.1038/nature13670) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruprecht JJ, Hellawell AM, Harding M, Crichton PG, McCoy AJ, Kunji ERS. 2014. Structures of yeast mitochondrial ADP/ATP carriers support a domain-based alternating-access transport mechanism. Proc. Natl Acad. Sci. USA 111, E426–E434. ( 10.1073/pnas.1320692111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pryor EE, et al. 2013. Structure of the integral membrane protein CAAX protease Ste24p. Science 339, 1600–1604. ( 10.1126/science.1232048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manolaridis I, et al. 2013. Mechanism of farnesylated CAAX protein processing by the intramembrane protease Rce1. Nature 504, 301–305. ( 10.1038/nature12754) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wood CW, Bruning M, Ibarra AA, Bartlett GJ, Thomson AR, Sessions RB, Brady RL, Woolfson DN. 2014. CCBuilder: an interactive web-based tool for building, designing and assessing coiled-coil protein assemblies. Bioinformatics 30, 3029–3035. ( 10.1093/bioinformatics/btu502) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsirigos KD, Peters C, Shu N, Kall L, Elofsson A. 2015. The TOPCONS web server for consensus prediction of membrane protein topology and signal peptides. Nucleic Acids Res. 43, W401–W407. ( 10.1093/nar/gkv485) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reynolds SM, Kall L, Riffle ME, Bilmes JA, Noble WS. 2008. Transmembrane topology and signal peptide prediction using dynamic Bayesian networks. PLoS Comput. Biol. 4, e1000213 ( 10.1371/journal.pcbi.1000213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jones DT. 2007. Improving the accuracy of transmembrane protein topology prediction using evolutionary information. Bioinformatics 23, 538–544. ( 10.1093/bioinformatics/btl677) [DOI] [PubMed] [Google Scholar]
- 48.Nugent T, Jones DT. 2009. Transmembrane protein topology prediction using support vector machines. BMC Bioinformatics 10, 159 ( 10.1186/1471-2105-10-159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Doyle L, Hallinan J, Bolduc J, Parmeggiani F, Baker D, Stoddard BL, Bradley P. 2015. Rational design of α-helical tandem repeat proteins with closed architectures. Nature 528, 585–588. ( 10.1038/nature16191) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Testa OD, Moutevelis E, Woolfson DN. 2009. CC+: a relational database of coiled-coil structures. Nucleic Acids Res. 37, D315–D322. ( 10.1093/nar/gkn675) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lomize MA, Lomize AL, Pogozheva ID, Mosberg HI. 2006. OPM: orientations of proteins in membranes database. Bioinformatics 22, 623–625. ( 10.1093/bioinformatics/btk023) [DOI] [PubMed] [Google Scholar]