Abstract
Apoptotic cell death via the mitochondrial pathway occurs in all vertebrate cells and requires the formation of pores in the mitochondrial outer membrane. Two Bcl-2 protein family members, Bak and Bax, form these pores during apoptosis, and how they do so has been investigated for the last two decades. Many of the conformation changes that occur during their transition to pore-forming proteins have now been delineated. Notably, biochemical, biophysical and structural studies indicate that symmetric homodimers are the basic unit of pore formation. Each dimer contains an extended hydrophobic surface that lies on the outer membrane, and is anchored at either end by a transmembrane domain. Membrane-remodelling events such as positive membrane curvature have been reported to accompany apoptotic pore formation, suggesting Bak and Bax form lipidic pores rather than proteinaceous pores. However, it remains unclear how symmetric dimers assemble to porate the membrane. Here, we review how clusters of dimers and their lipid-mediated interactions provide a molecular explanation for the heterogeneous assemblies of Bak and Bax observed during apoptosis.
This article is part of the themed issue ‘Membrane pores: from structure and assembly, to medicine and technology’.
Keywords: apoptosis, Bak, Bax, heterogeneity, membrane bilayer, mitochondrial pore
1. Introduction
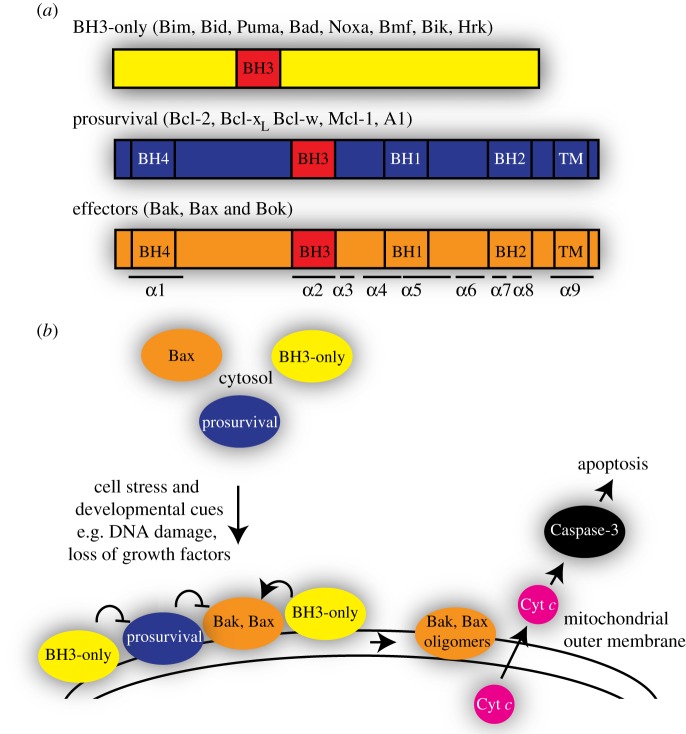
Apoptosis is essential for normal development and tissue homeostasis, and its perturbed regulation contributes to numerous pathological conditions, including cancer and autoimmune and degenerative diseases [1]. Apoptosis is regulated principally by interactions within the Bcl-2 family of proteins, whose members fall into three subclasses (figure 1a). The eight or more pro-apoptotic BH3-only proteins (e.g. Bid and Bim) act as sensors of specific types of cellular stress, and signal by engaging other family members. The pro-survival proteins (e.g. Bcl-2 and Mcl-1) act by sequestering the pro-apoptotic members. Finally, pro-apoptotic Bak and Bax act as critical effectors of apoptosis, as they are required for mitochondrial permeabilization in cells and in mice [2,3]. As illustrated in figure 1b, upon receiving an apoptotic stimulus, upregulated BH3-only proteins bind to Bak and Bax to induce major conformation changes, resulting in Bak and Bax oligomerization and subsequent outer membrane permeabilization. This leads to the release of mitochondrial proteins including cytochrome c, which in turn triggers caspase-driven cell demolition (reviewed in [4]).
Figure 1.
Bcl-2 proteins regulate the mitochondrial pathway of apoptotic cell death. (a) Three subfamilies of Bcl-2 proteins. (b) Bak and Bax activation by the BH3-only proteins is followed by their oligomerization in the mitochondrial outer membrane to release cytochrome c and induce apoptosis.
The three-dimensional structures of non-activated Bak and Bax resemble those of the pro-survival proteins, comprising nine α-helices that form a tight globular bundle (figure 2). Two important features are a surface hydrophobic groove (α2–α5) and a buried BH3 domain in α2 that mediate contact with other family members. A major distinction between Bak and Bax is that Bak is mostly inserted into the mitochondrial outer membrane in healthy cells, whereas Bax is mostly cytosolic and translocates to mitochondria following apoptotic stimuli (figure 1b). Bax translocation is triggered by binding of BH3-only proteins, which releases α9 from the hydrophobic groove [5]. Once the released α9 inserts as a transmembrane domain into the mitochondrial outer membrane, Bax has the same topology as non-activated Bak [6].
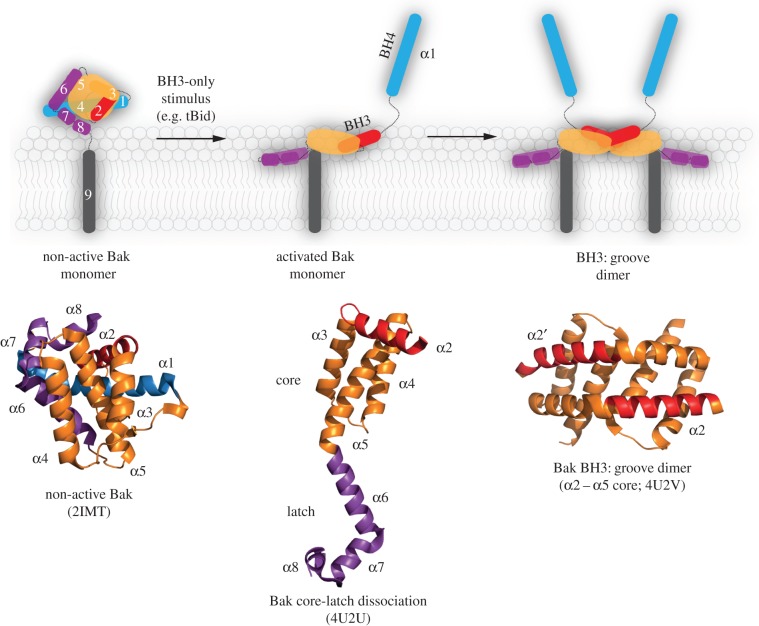
Figure 2.
Bak activation and conformation change results in symmetric homodimers. A schematic showing that Bak unfolds by the N-terminus (α1, blue) and the C-terminal latch (α6–α8, magenta) separating from the α2–α5 core (orange, red). Hydrophobic regions of the core and latch then collapse onto the membrane, while the exposed BH3 domain (in α2) binds to the hydrophobic groove in another activated Bak molecule. Reciprocal BH3:groove binding results in symmetric homodimers. The indicated crystal structures demonstrate the major conformation changes involved. Equivalent changes are observed for Bax.
2. Conversion of Bak and Bax into symmetric homodimers with flexible extremities
Bak and Bax undergo major conformation changes as they convert into pore-forming proteins (figure 2; reviewed in [4]). The changes are triggered by the binding of BH3-only proteins to a hydrophobic surface groove, which generates a cavity underneath both the N- and C-termini [7–9]. Destabilization allows the protein to unfold as three segments: the α1-helix dissociates [10], and the core (α2–α5) separates from the latch (α6–α9) [7,8]. Several newly exposed hydrophobic regions then associate with the mitochondrial outer membrane to lie in-plane (figure 2, activated Bak monomer) [11,12]. The core remains largely folded, but within it the newly exposed hydrophobic BH3 domain (in α2) then binds to the hydrophobic groove of another activated Bak or Bax molecule in a reciprocal manner to form symmetric homodimers (figure 2, BH3:groove dimer). Evidence for symmetric homodimers originated from biochemical studies in mitochondria [13–15] and is supported by X-ray structures of the α2–α5 dimers [7,8] and biophysical studies[12,16–20].
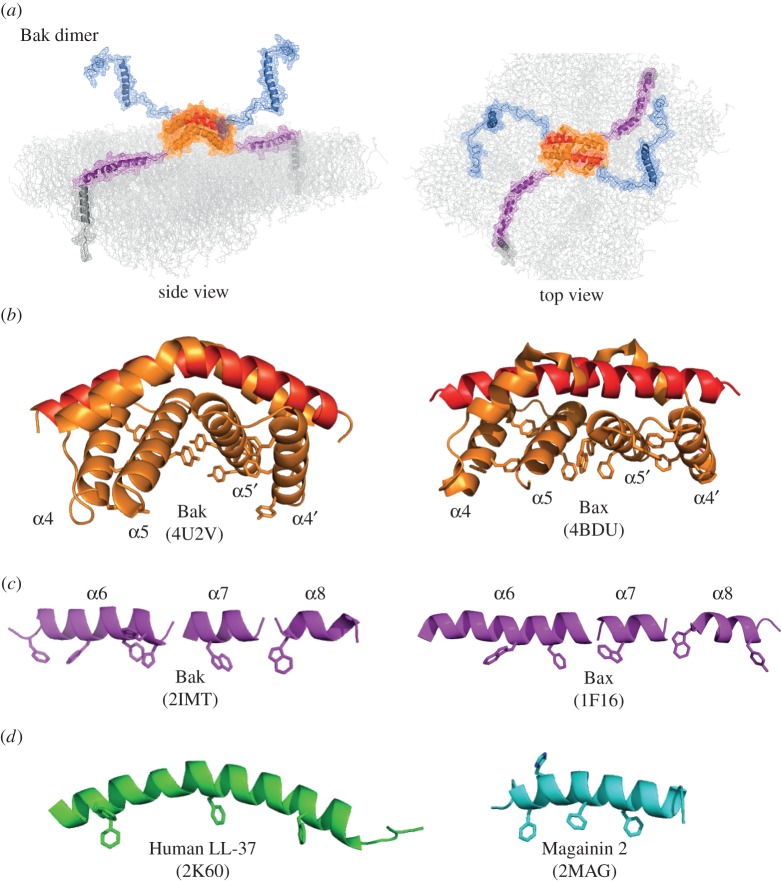
Together these studies support the in-plane model of a Bak dimer (figure 3a) [11,22]. The region in contact with the membrane (α2–α9) resembles an extended flexible amphipathic polypeptide anchored at either end with a transmembrane domain—an unusual structure for a pore-forming protein. Several helices may embed into the outer leaflet of the membrane, encouraged by aromatic residues on one surface of the α2–α5 core dimer (figure 3b) and on one face of the α6–α8 helices (figure 3c). At the N-terminus, the first 70 residues become exposed and do not re-engage with either membrane or protein [10,22,23]. Bax dimers also display aromatic residues on one surface (figure 3b,c) and a similar membrane topology [6,11,16,19], although complete solvent exposure of the N-terminus has not yet been shown. Thus, Bak and Bax homodimers show several features of antimicrobial peptides such as human LL-37 and magainin 2 (figure 3d) that are proposed to form toroidal pores, rather than of the α-helical ClyA and actinoporin proteins that form more structured proteinaceous pores [24,25].
Figure 3.
Membrane topology of the Bak dimer. (a) The in-plane model of the Bak dimer. The N-terminal regions become solvent-exposed while the remainder of the Bak dimer resembles a flexible extended amphipathic peptide that lies in-plane with the membrane, anchored at either end by transmembrane domains. Note that α1 may unfold after it dissociates, decreasing the hydrophobicity of the BH4 structural motif (VFrsYV) therein [10,21]. Images were assembled in PyMol using the structures of Bak (2IMT) and the Bak dimer (4U2V), and represented as cartoon and mesh. (b) Aromatic residues are concentrated on the bent surface of the Bak and Bax α2–α5 core dimers. (c) Aromatic residues can position on one edge of the flexible α6–α8 latch. (d) Examples of antimicrobial peptides thought to form lipidic pores, with aromatic residues indicated. Colour coding as in figure 2.
There are few examples of a homodimer as the building block of a pore that might provide insight into pore formation by Bak and Bax. One such example is the plant defensin NaD1, whose structure comprises seven antiparallel dimers [26]. However, unlike Bak and Bax, the NaD1 complexes are not promoted by major conformation change but by binding of the PIP2 phospholipid, and the oligomers lack transmembrane domains and flexible membrane-associated regions. Members of the colicin family of pore-forming proteins may also assemble as multiples of dimers to form small ring-shaped oligomers (approx. 8 nm in diameter) [25,27,28]. Curiously, the first structure of a Bcl-2 protein and its similarity to the colicins and diphtheria toxin prompted the idea that, as proposed for those proteins, Bak and Bax might form channels or pores by inserting a helical hairpin (α5/α6) through the membrane [29–31]. Notably, hairpin insertion is not consistent with the in-plane model in which α5 remains with the α2–α5 dimer and α6 lies in-plane in the outer membrane (figures 2, 3 and 4) [11,12]. A recent study proposed that Bax α2–α5 dimers can progress to α2–α3–α4 dimers after separation of α5 to allow α5/α6 insertion [16]. However, it was not clear whether those dimers were functional [16], and in other studies separation of Bax α5 from α4 was not required for cytochrome c release [7]. Even so, further comparison of pore formation by the Bcl-2 and colicin families may prove informative.
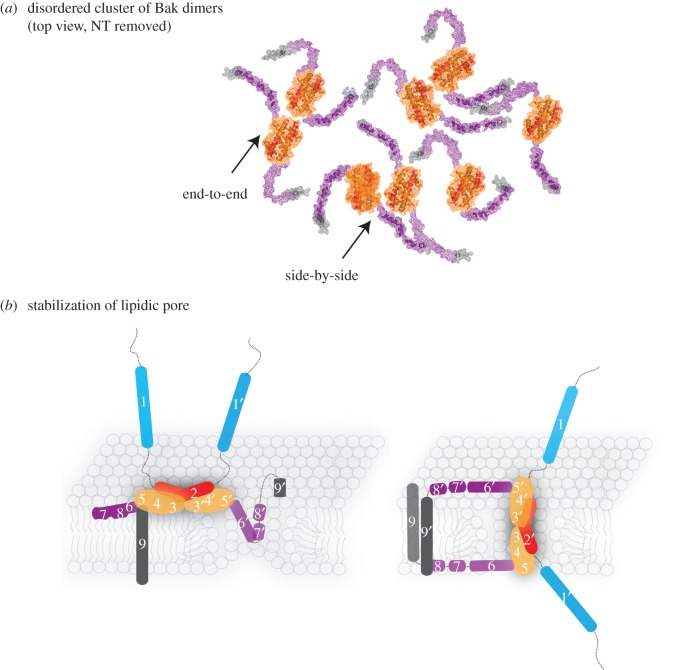
Figure 4.
Possible mechanisms involved in lipidic pore formation and stabilization by homodimers of Bak or Bax. (a) Schematic of Bak dimers forming a disordered cluster on the mitochondrial outer membrane, encouraged by flexibility of the α6–α8 latch. Note that end-to-end or side-by-side contact between the core regions is possible. Images were assembled as in figure 3a. (b) Parts of the dimer may line a lipidic pore. The flexible amphipathic latch may slide into a nascent pore to partially line and stabilize the pore (left; in-plane model [4]). The amphipathic core dimer (α2–α5) may also line the pore generating antiparallel α9-helices (right; clamp model [19]). Colour coding as in figure 2.
3. Dimer–dimer interactions are not via a single protein–protein interface
It is thought that homodimers of Bak or Bax must then associate to higher order oligomers to porate the mitochondrial outer membrane. Such oligomers of Bak and of Bax are generated in mitochondria during apoptosis, as evident by gel filtration, blue native PAGE and linkage studies [32–34]. In addition, recombinant Bak and Bax form high order oligomers in liposome experiments (table 1). High order oligomers observed biochemically correlate with the clusters observed in early microscopy studies (table 1). And these clusters are sometimes apparent at points of mitochondrial fission/fusion [59]. Higher-resolution microscopy shows Bak and Bax complexes of various shapes and sizes in liposomes and mitochondria, including clusters, rings and arcs (table 1). Thus, there is strong correlation between high order oligomers and pore formation. Nevertheless, it is yet to be shown that specifically inhibiting dimer–dimer interaction blocks pore formation.
Table 1.
Heterogeneity of Bak and Bax complexes and pores. AFM, Atomic Force Microscopy; CD, Circular Dichroism; Cryo-EM, Cryo-Electron Microscopy; ΔC, C-terminally truncated; FCS, Fluorescence Correlation Spectroscopy; GUV, Giant Unilamellar Vesicles; IVT, In Vitro Translated; LUV, Large Unilamellar Vesicles; OG, Octyl Glucoside; OMV, Outer Membrane Vesicles; PALM, Photo-activated Localization Microscopy; TEM, Transmission Electron Microscopy; TIRF, Total Internal Reflection Fluorescence Microscopy; SMLM, Single Molecule Localization Microscopy; STED, Stimulated Emission Depletion microscopy.
| Bak/Bax | membrane type | resolution method | characteristics of complexes and pores, including effect on membranes | references | |
|---|---|---|---|---|---|
| peptides | Bax α5, α6 | LUV planar bilayer |
AFM CD spectroscopy confocal X-ray diffraction |
toroidal pores of diameter approximately 5.8 nm decreased membrane line tension at pore rim lipids with positive intrinsic curvature enhance pore formation lipid transbilayer redistribution activity for Bax α5 |
Garcia-Saez 2005, 2006, 2007; Qian 2008 [35–38] |
| recombinant proteins | BaxΔC Bax |
liposome planar bilayer |
patch clamping Fl-dextran release |
Bax forms pH dependent ion-conduction channels/pores maximum of 4 molecules, pore diameter approximately 2.2 nm |
Antonsson 1997, Schlesinger 1997, Saito 2000 [39–41] |
| BaxΔC IVT-Bax Bax (bovine) |
LUV liposome planar bilayer |
voltage clamp membrane lifetime measurements |
decrease in membrane lifetime and linear tension increase in positive monolayer curvature stress lipid transbilayer redistribution |
Basanez 1999, Basanez 2002, Terrones 2004, Landeta 2011 [42–45] | |
| Bax BaxΔC |
LUV | AFM | toroidal pores of diameter approximately 100–300 nm small ring-like structures on bilayer surface structures contain clusters of approximately 22 Bax monomers lipid transbilayer redistribution |
Epand 2002, 2003 [46,47] | |
| Bax OG-Bax |
liposome OMV |
TEM dextran release |
supramolecular lipid pores allowed release of approximately 2000 kDa dextran |
Kuwana 2002 [48] | |
| Bax BakΔC |
LUV GUV mitochondria |
Cryo-EM FCS |
time and protein concentration dependent lipid pores pores of diameter approximately 3–140 nm induction/stabilization of curved membrane structures reduction in vesicle size due to budding, fission, tethering |
Bleicken 2013a, 2013b, 2016 [49–51] | |
| Bax | bilayer nanodiscs | Cryo-EM | active Bax monomers form pores of diameter approximately 3.5 nm | Xu 2013 [52] | |
| Bax Nanogold labelled Bax |
liposome LUV OMV |
TEM Cryo-EM |
pore-like openings with diameter approximately 25–100 nm growing pores in the range of approximately 100–300 nm solitary dynamic pores with negative curvature at edges complete rings of Bax exclusively associated with pore rims |
Schafer 2009, Gillies 2015, Kuwana 2016 [53–55] | |
| Bax | LUV planar bilayer |
AFM TIRF |
multiple oligomer species of dimers round heterogeneous pores of diameter approximately 24–176 nm Bax along pore rim |
Subburaj 2015, Salvador-Gallego 2016 [17,56] | |
| cells | GFP-Bax | HeLa Cos-7 |
TEM confocal |
large clusters containing 1000–20 000 Bax molecules | Nechushtan 2001 [34] |
| CFP-Bax | HeLa | quantitative fluorescence imaging |
Bax complexes with approximately 150–1000 molecules per cluster 620 nm average cluster size | Zhou 2008 [32] | |
| mEos3-Bak | MEF | PALM | heterogeneous Bak clusters of diameter approximately 70–600 nm each cluster contains approximately 20–2000 Bax molecules no pores evident in clusters (using resolution of 20 nm) |
Nasu 2016 [57] | |
| Endog. Bax | HeLa U2OS HT1080 SH-SY5Y CV-1 |
STED | large compact clusters rings up to 400 nm diameter |
Grosse 2016 [58] | |
| GFP-Bax | HeLa HCT-116 |
TEM STED SMLM |
heterogenous distribution of: clusters rings (35 nm diameter) arcs (100–500 nm diameter) lines |
Salvador-Gallego 2016 [56] |
While either Bak or Bax is sufficient to form pores (table 1), the two proteins locate to the same complexes in apoptotic cells [32,34], suggesting that mixtures of the two proteins may be able to generate pores. The mixtures may include heterodimers of Bak and Bax, although heterodimers form only a minor population compared with homodimers [13,60]. The low frequency of heterodimers may be explained by a degree of incompatibility due to the limited sequence similarity of the BH3 domains and grooves of the two proteins. Mixtures may also include homodimers of Bak and of Bax, as Bax is able to intermingle with pre-formed Bak dimers [22]. If mixtures of Bak and Bax homodimers can actually generate pores, this would provide further evidence that protein–protein interactions between dimers are not important for high order oligomers or pore formation.
Molecular structures of Bak or Bax as high order oligomers or pore complexes are currently not available. However, a range of biochemical approaches have been used to examine how activated Bak and Bax interact to generate pores. Most prominent have been linkage studies showing that homodimers can associate via interactions at α-helices 1, 3, 5, 6 and 9 [6,12,13,15,16,19,61–64]. Our initial studies showed that linkage between the α6-helices could link dimers of Bak and Bax [13,15], suggesting that an α6:α6 interface may drive high order oligomers and pore formation. However, there was no evidence that mutations in α6 could block apoptosis [4,65], and several groups reported linkage between additional regions. Thus, some or all of these linkages may be due to collisions rather than to stable complex formation. Flexibility of the N- and C-termini, as depicted in figure 4a, may allow linkage between multiple regions, and would also limit interaction between the α2–α5 core dimers [8,22]. Based on the linkage pattern throughout the full-length of Bak, we recently proposed that dimers form disordered clusters during apoptosis (figure 4a), and this was supported by mathematical simulation of linkage within the whole population of Bak dimers in the sample [22]. It is yet to be determined if only a small subpopulation of dimers directly participates in a pore complex. If so, within this subpopulation the core dimers may adopt an ordered arrangement, e.g. end-to-end or side-by-side.
4. Formation of lipidic (toroidal) pores
Several lines of evidence indicate that Bak and Bax form lipidic rather than proteinaceous pores (table 1). Amphipathic peptides based on the Bak and Bax α5 and α6 helices can permeabilize membranes (table 1), as do amphipathic antimicrobial peptides that act via forming lipidic pores. As the Bak and Bax dimers resemble a flexible amphipathic polypeptide (figure 3a), their shallow insertion into the outer leaflet [11,18,19] may destabilize the lamellar structure of the bilayer to induce lipidic pores. This mechanism of pore formation may be related to the ‘carpet’ model proposed for antimicrobial peptides. In the Shai–Matsuzaki–Huang version of the carpet model [66–68], peptides can disrupt membranes without disintegrating the membranes in a detergent-like manner [69]. The peptides insert close to the membrane surface to promote a convex curvature of the outer leaflet. As the peptide concentration increases, membrane defects occur, and in some cases may be resolved by peptide (or phospholipid) equilibrating across the bilayer. As Bak and Bax are unlikely to equilibrate across the bilayer due to their size and transmembrane domains, the membrane defects may progress to pore formation. There is evidence that pore formation is associated with lipid transbilayer movement [35,42,46].
During pore formation, parts of the dimer may line and stabilize the pore (figure 4b) [11,19,70]. According to the clamp model (figure 4b, right) [19] the core dimer positions roughly perpendicular in a circle to line the pore. Several features of this model are attractive. The length of the core dimer (approx. 4 nm) is the approximate width of the MOM, and the bend observed in the structures (Bak and Bax α2–α5 dimers; figure 3b) may be accommodated by the curved edge of the pore. In this position, the α6–α8 latch would disturb the outer and inner leaflets equally, and the core dimer could contribute a large surface area to stabilize the pore. In addition, the core dimers could pack tightly side by side around the pore. However, one side-by-side orientation of Bak α2–α5 dimers observed in a crystal structure was not supported by linkage studies in mitochondria [8,22]. Moreover, the clamp model suggests that the α9 transmembrane domains become antiparallel within a dimer, presumably after the charged residues (e.g. RRFFKS in human Bak) at the far C-terminus of one activated molecule flip through the hydrophobic bilayer. While such flipping may be similar to insertion of the transmembrane domains of non-activated Bak (and Bax), direct evidence of antiparallel α9-helices in Bax or Bak oligomers is required to support this model.
5. Heterogeneity of Bak and Bax complexes and pores
Consistent with forming a lipidic pore, significant heterogeneity is observed in the characteristics of the Bak and Bax complexes and the actual pores formed by Bak and Bax (table 1). Differences may be due to the levels of Bak and Bax (and pro-survival Bcl-2 proteins), lipid composition and diameter of the mitochondria or liposome, and even the presence or absence of the mitochondrial inner membrane and matrix. Detectable pores in the membrane were not always evident in the clusters, suggesting that at least some clusters may form upstream or downstream of pore formation. Notably, Bax pore size in liposomes increased with higher protein concentration and over time [39,49,53,71], and pore size in Xenopus laevis mitochondria increased in response to a cytosolic factor that was more potent in the presence of caspase inhibitors [72]. Thus, both protein and lipid appear able to enlarge apoptotic pores, a process that would ensure rapid cell death. It will be interesting to determine the role of pores that are not detectable by microscopy. Can a pore stay small, and what is the composition of such a ‘minimal’ pore? Are there multiple small pores in a mitochondrion, perhaps even in a single cluster? Might a single small pore in each mitochondrion be sufficient for apoptosis? Answers to these questions may help to regulate apoptosis at the step of pore formation, including reversing the process.
6. Concluding remarks
Increasing evidence indicates that symmetric homodimers of Bak and of Bax form the structural building block of the apoptotic pore. As there is a strong correlation of higher order oligomers with pore formation, it is important to understand how symmetric dimers can form these oligomers. Within each dimer, the N-terminus is solvent-exposed and flexible, implicating the membrane-associated regions (α2–α9) in driving pore formation. Flexibility of the latch (α6–α9) implies several arrangements of dimers may occur and contribute to the heterogeneity of clusters and pores observed. Notably, insertion of the core and latch into the outer leaflet may remodel the bilayer to form a small lipidic pore, which may then grow considerably. Obtaining structures of small apoptotic pores may yet be possible, and these structures may be the key to understanding how this central step of cell death might be regulated.
Authors' contributions
The authors contributed equally to the planning, writing and illustrating of this article.
Competing interests
We declare we have no competing interests.
Funding
Our work is supported by NHMRC grant nos. (637337, 1008434 and 1016701 to R.M.K.) and by the Victorian State Government Operational Infrastructure Support and the Australian Government NHMRC IRIISS.
References
- 1.Czabotar PE, Lessene G, Strasser A, Adams JM. 2014. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat. Rev. Mol. Cell Biol. 15, 49–63. ( 10.1038/nrm3722) [DOI] [PubMed] [Google Scholar]
- 2.Lindsten T, et al. 2000. The combined functions of proapoptotic Bcl-2 family members Bak and Bax are essential for normal development of multiple tissues. Mol. Cell 6, 1389–1399. ( 10.1016/S1097-2765(00)00136-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei MC, et al. 2001. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292, 727–730. ( 10.1126/science.1059108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Westphal D, Kluck RM, Dewson G. 2014. Building blocks of the apoptotic pore: how Bax and Bak are activated and oligomerize during apoptosis. Cell Death Differ. 21, 196–205. ( 10.1038/cdd.2013.139) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walensky LD, Gavathiotis E. 2011. BAX unleashed: the biochemical transformation of an inactive cytosolic monomer into a toxic mitochondrial pore. Trends Biochem. Sci. 36, 642–652. ( 10.1016/j.tibs.2011.08.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iyer S, Bell F, Westphal D, Anwari K, Gulbis J, Smith BJ, Dewson G, Kluck RM. 2015. Bak apoptotic pores involve a flexible C-terminal region and juxtaposition of the C-terminal transmembrane domains. Cell Death Differ. 22, 1665–1675. ( 10.1038/cdd.2015.15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Czabotar PE, et al. 2013. Bax crystal structures reveal how BH3 domains activate Bax and nucleate its oligomerization to induce apoptosis. Cell 152, 519–531. ( 10.1016/j.cell.2012.12.031) [DOI] [PubMed] [Google Scholar]
- 8.Brouwer JM, et al. 2014. Bak core and latch domains separate during activation, and freed core domains form symmetric homodimers. Mol. Cell 55, 938–946. ( 10.1016/j.molcel.2014.07.016) [DOI] [PubMed] [Google Scholar]
- 9.Moldoveanu T, Grace CR, Llambi F, Nourse A, Fitzgerald P, Gehring K, Kriwacki RW, Green DR. 2013. BID-induced structural changes in BAK promote apoptosis. Nat. Struct. Mol. Biol. 20, 589–597. ( 10.1038/nsmb.2563) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alsop AE, Fennell SC, Bartolo RC, Tan IK, Dewson G, Kluck RM. 2015. Dissociation of Bak alpha1 helix from the core and latch domains is required for apoptosis. Nat. Commun. 6, 6841 ( 10.1038/ncomms7841) [DOI] [PubMed] [Google Scholar]
- 11.Westphal D, et al. 2014. Apoptotic pore formation is associated with in-plane insertion of Bak or Bax central helices into the mitochondrial outer membrane. Proc. Natl Acad. Sci. USA 111, E4076–E4085. ( 10.1073/pnas.1415142111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aluvila S, Mandal T, Hustedt E, Fajer P, Choe JY, Oh KJ. 2014. Organization of the mitochondrial apoptotic BAK pore: oligomerization of the Bak homodimers. J. Biol. Chem. 289, 2537–2551. ( 10.1074/jbc.M113.526806) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dewson G, Ma S, Frederick P, Hockings C, Tan I, Kratina T, Kluck RM. 2012. Bax dimerizes via a symmetric BH3:groove interface during apoptosis. Cell Death Differ. 19, 661–670. ( 10.1038/cdd.2011.138) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dewson G, Kratina T, Sim HW, Puthalakath H, Adams JM, Colman PM, Kluck RM. 2008. To trigger apoptosis Bak exposes its BH3 domain and homo-dimerizes via BH3:grooove interactions. Mol. Cell 30, 369–380. ( 10.1016/j.molcel.2008.04.005) [DOI] [PubMed] [Google Scholar]
- 15.Dewson G, Kratina T, Czabotar P, Day CL, Adams JM, Kluck RM. 2009. Bak activation for apoptosis involves oligomerization of dimers via their alpha6 helices. Mol. Cell 36, 696–703. ( 10.1016/j.molcel.2009.11.008) [DOI] [PubMed] [Google Scholar]
- 16.Zhang Z, et al. 2016. BH3-in-groove dimerization initiates and helix9 dimerization expands Bax pore assembly in membranes. EMBO J. 35, 208–236. ( 10.15252/embj.201591552) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Subburaj Y, Cosentino K, Axmann M, Pedrueza-Villalmanzo E, Hermann E, Bleicken S, Spatz J, Garcia-Saez AJ. 2015. Bax monomers form dimer units in the membrane that further self-assemble into multiple oligomeric species. Nat. Commun. 6, 8042 ( 10.1038/ncomms9042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oh KJ, et al. 2010. Conformational changes in BAK, a pore-forming proapoptotic Bcl-2 family member, upon membrane insertion and direct evidence for the existence of BH3-BH3 contact interface in BAK homo-oligomers. J. Biol. Chem. 285, 28 924–28 937. ( 10.1074/jbc.M110.135293) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bleicken S, Jeschke G, Stegmueller C, Salvador-Gallego R, Garcia-Saez AJ, Bordignon E. 2014. Structural model of active Bax at the membrane. Mol. Cell 56, 496–505. ( 10.1016/j.molcel.2014.09.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bleicken S, Classen M, Padmavathi PV, Ishikawa T, Zeth K, Steinhoff HJ, Bordignon E. 2010. Molecular details of Bax activation, oligomerization, and membrane insertion. J. Biol. Chem. 285, 6636–6647. ( 10.1074/jbc.M109.081539) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kvansakul M, Yang H, Fairlie WD, Czabotar PE, Fischer SF, Perugini MA, Huang DC, Colman PM. 2008. Vaccinia virus anti-apoptotic F1 L is a novel Bcl-2-like domain-swapped dimer that binds a highly selective subset of BH3-containing death ligands. Cell Death Differ. 15, 1564–1571. ( 10.1038/cdd.2008.83) [DOI] [PubMed] [Google Scholar]
- 22.Uren RT, O'Hely M, Iyer S, Bartolo R, Shi MX, Brouwer JM, Alsop AE, Dewson G, Kluck RM. 2017. Disordered clusters of Bak dimers rupture mitochondria during apoptosis. eLife 6, e19944 ( 10.7554/eLife.19944) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weber K, Harper N, Schwabe J, Cohen GM. 2013. BIM-mediated membrane insertion of the BAK pore domain is an essential requirement for apoptosis. Cell Rep. 5, 409–420. ( 10.1016/j.celrep.2013.09.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilbert RJ. 2016. Protein-lipid interactions and non-lamellar lipidic structures in membrane pore formation and membrane fusion. Biochim. Biophys. Acta 1858, 487–499. ( 10.1016/j.bbamem.2015.11.026) [DOI] [PubMed] [Google Scholar]
- 25.Dal Peraro M, van der Goot FG. 2016. Pore-forming toxins: ancient, but never really out of fashion. Nat. Rev. Microbiol. 14, 77–92. ( 10.1038/nrmicro.2015.3) [DOI] [PubMed] [Google Scholar]
- 26.Poon I, et al. 2014. Phosphoinositide-mediated oligomerization of a defensin induces cell lysis. Elife 3, e01808 ( 10.7554/eLife.01808) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greig SL, Radjainia M, Mitra AK. 2009. Oligomeric structure of colicin ia channel in lipid bilayer membranes. J. Biol. Chem. 284, 16 126–16 134. ( 10.1074/jbc.M900292200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dunkel S, Pulagam LP, Steinhoff HJ, Klare JP. 2015. In vivo EPR on spin labeled colicin A reveals an oligomeric assembly of the pore-forming domain in E. coli membranes. Phys. Chem. Chem. Phys. 17, 4875–4878. ( 10.1039/c4cp05638h) [DOI] [PubMed] [Google Scholar]
- 29.Muchmore SW, et al. 1996. X-ray and NMR structure of human Bcl-xL, an inhibitor of programmed cell death. Nature 381, 335–341. ( 10.1038/381335a0) [DOI] [PubMed] [Google Scholar]
- 30.Antignani A, Youle RJ. 2006. How do Bax and Bak lead to permeabilization of the outer mitochondrial membrane? Curr. Opin. Cell Biol. 18, 685–689. ( 10.1016/j.ceb.2006.10.004) [DOI] [PubMed] [Google Scholar]
- 31.Annis MG, Soucie EL, Dlugosz PJ, Cruz-Aguado JA, Penn LZ, Leber B, Andrews DW. 2005. Bax forms multispanning monomers that oligomerize to permeabilize membranes during apoptosis. EMBO J. 24, 2096–2103. ( 10.1038/sj.emboj.7600675) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou L, Chang DC. 2008. Dynamics and structure of the Bax-Bak complex responsible for releasing mitochondrial proteins during apoptosis. J. Cell Sci. 121, 2186–2196. ( 10.1242/jcs.024703) [DOI] [PubMed] [Google Scholar]
- 33.Wei MC, Lindsten T, Mootha VK, Weiler S, Gross A, Ashiya M, Thompson CB, Korsmeyer SJ. 2000. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev. 14, 2060–2071. [PMC free article] [PubMed] [Google Scholar]
- 34.Nechushtan A, Smith CL, Lamensdorf I, Yoon SH, Youle RJ. 2001. Bax and Bak coalesce into novel mitochondria-associated clusters during apoptosis. J. Cell Biol. 153, 1265–1276. ( 10.1083/jcb.153.6.1265) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garcia-Saez AJ, Coraiola M, Serra MD, Mingarro I, Muller P, Salgado J. 2006. Peptides corresponding to helices 5 and 6 of Bax can independently form large lipid pores. FEBS J. 273, 971–981. ( 10.1111/j.1742-4658.2006.05123.x) [DOI] [PubMed] [Google Scholar]
- 36.Qian S, Wang W, Yang L, Huang HW. 2008. Structure of transmembrane pore induced by Bax-derived peptide: evidence for lipidic pores. Proc. Natl Acad. Sci. USA 105, 17 379–17 383. ( 10.1073/pnas.0807764105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garcia-Saez AJ, Coraiola M, Dalla Serra M, Mingarro I, Menestrina G, Salgado J. 2005. Peptides derived from apoptotic Bax and Bid reproduce the poration activity of the parent full-length proteins. Biophys. J. 88, 3976–3990. ( 10.1529/biophysj.104.058008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garcia-Saez AJ, Chiantia S, Salgado J, Schwille P. 2007. Pore formation by a Bax-derived peptide: effect on the line tension of the membrane probed by AFM. Biophys. J. 93, 103–112. ( 10.1529/biophysj.106.100370) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saito M, Korsmeyer SJ, Schlesinger PH. 2000. BAX-dependent transport of cytochrome c reconstituted in pure liposomes. Nat. Cell Biol. 2, 553–555. ( 10.1038/35019596) [DOI] [PubMed] [Google Scholar]
- 40.Schlesinger PH, Gross A, Yin XM, Yamamoto K, Saito M, Waksman G, Korsmeyer SJ. 1997. Comparison of the ion channel characteristics of proapoptotic BAX and antiapoptotic BCL-2. Proc. Natl Acad. Sci. USA 94, 11 357–11 362. ( 10.1073/pnas.94.21.11357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Antonsson B, et al. 1997. Inhibition of Bax channel-forming activity by Bcl-2. Science 277, 370–372. ( 10.1126/science.277.5324.370) [DOI] [PubMed] [Google Scholar]
- 42.Terrones O, Antonsson B, Yamaguchi H, Wang HG, Liu J, Lee RM, Herrmann A, Basanez G. 2004. Lipidic pore formation by the concerted action of proapoptotic BAX and tBID. J. Biol. Chem. 279, 30 081–30 091. ( 10.1074/jbc.M313420200) [DOI] [PubMed] [Google Scholar]
- 43.Landeta O, Landajuela A, Gil D, Taneva S, Di Primo C, Sot B, Valle M, Frolov VA, Basanez G. 2011. Reconstitution of proapoptotic BAK function in liposomes reveals a dual role for mitochondrial lipids in the BAK-driven membrane permeabilization process. J. Biol. Chem. 286, 8213–8230. ( 10.1074/jbc.M110.165852) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Basanez G, Sharpe JC, Galanis J, Brandt TB, Hardwick JM, Zimmerberg J. 2002. Bax-type apoptotic proteins porate pure lipid bilayers through a mechanism sensitive to intrinsic monolayer curvature. J. Biol. Chem. 277, 49 360–49 365. ( 10.1074/jbc.M206069200) [DOI] [PubMed] [Google Scholar]
- 45.Basanez G, et al. 1999. Bax, but not Bcl-xL, decreases the lifetime of planar phospholipid bilayer membranes at subnanomolar concentrations. Proc. Natl Acad. Sci. USA 96, 5492–5497. ( 10.1073/pnas.96.10.5492) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Epand RF, Martinou JC, Montessuit S, Epand RM. 2003. Transbilayer lipid diffusion promoted by Bax: implications for apoptosis. Biochemistry 42, 14 576–14 582. ( 10.1021/bi035348w) [DOI] [PubMed] [Google Scholar]
- 47.Epand RF, Martinou JC, Montessuit S, Epand RM, Yip CM. 2002. Direct evidence for membrane pore formation by the apoptotic protein Bax. Biochem. Biophys. Res. Commun. 298, 744–749. ( 10.1016/S0006-291X(02)02544-5) [DOI] [PubMed] [Google Scholar]
- 48.Kuwana T, Mackey MR, Perkins G, Ellisman MH, Latterich M, Schneiter R, Green DR, Newmeyer DD. 2002. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell 111, 331–342. ( 10.1016/S0092-8674(02)01036-X) [DOI] [PubMed] [Google Scholar]
- 49.Bleicken S, Landeta O, Landajuela A, Basanez G, Garcia-Saez AJ. 2013. Proapoptotic Bax and Bak proteins form stable protein-permeable pores of tunable size. J. Biol. Chem. 288, 33 241–33 252. ( 10.1074/jbc.M113.512087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bleicken S, Wagner C, Garcia-Saez AJ. 2013. Mechanistic differences in the membrane activity of Bax and Bcl-xL correlate with their opposing roles in apoptosis. Biophys. J. 104, 421–431. ( 10.1016/j.bpj.2012.12.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bleicken S, Hofhaus G, Ugarte-Uribe B, Schroder R, Garcia-Saez AJ. 2016. cBid, Bax and Bcl-xL exhibit opposite membrane remodeling activities. Cell Death Dis 7, e2121 ( 10.1038/cddis.2016.34) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu XP, Zhai D, Kim E, Swift M, Reed JC, Volkmann N, Hanein D. 2013. Three-dimensional structure of Bax-mediated pores in membrane bilayers. Cell Death Dis 4, e683 ( 10.1038/cddis.2013.210) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gillies LA, Du H, Peters B, Knudson CM, Newmeyer DD, Kuwana T. 2015. Visual and functional demonstration of growing Bax-induced pores in mitochondrial outer membranes. Mol. Biol. Cell 26, 339–349. ( 10.1091/mbc.E13-11-0638) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schafer B, Quispe J, Choudhary V, Chipuk JE, Ajero TG, Du H, Schneiter R, Kuwana T. 2009. Mitochondrial outer membrane proteins assist Bid in Bax-mediated lipidic pore formation. Mol. Biol. Cell 20, 2276–2285. ( 10.1091/mbc.E08-10-1056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kuwana T, Olson NH, Kiosses WB, Peters B, Newmeyer DD.. 2016. Pro-apoptotic Bax molecules densely populate the edges of membrane pores. Sci. Rep. 6, 27299 ( 10.1038/srep27299) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Salvador-Gallego R, Mund M, Cosentino K, Schneider J, Unsay J, Schraermeyer U, Engelhardt J, Ries J, Garcia-Saez AJ. 2016. Bax assembly into rings and arcs in apoptotic mitochondria is linked to membrane pores. EMBO J. 35, 389–401. ( 10.15252/embj.201593384) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nasu Y, Benke A, Arakawa S, Yoshida GJ, Kawamura G, Manley S, Shimizu S, Ozawa T.. 2016. In Situ Characterization of Bak clusters responsible for cell death using single molecule localization microscopy. Sci. Rep. 6, 27505 ( 10.1038/srep27505) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grosse L, Wurm CA, Bruser C, Neumann D, Jans DC, Jakobs S. 2016. Bax assembles into large ring-like structures remodeling the mitochondrial outer membrane in apoptosis. EMBO J. 35, 402–413. ( 10.15252/embj.201592789) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Karbowski M, et al. 2002. Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J. Cell Biol. 159, 931–938. ( 10.1083/jcb.200209124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mikhailov V, Mikhailova M, Degenhardt K, Venkatachalam MA, White E, Saikumar P. 2003. Association of Bax and Bak homo-oligomers in mitochondria. Bax requirement for Bak reorganization and cytochrome c release. J. Biol. Chem. 278, 5367–5376. ( 10.1074/jbc.M203392200) [DOI] [PubMed] [Google Scholar]
- 61.Zhang Z, et al. 2010. Bax forms an oligomer via separate, yet interdependent, surfaces. J. Biol. Chem. 285, 17 614–17 627. ( 10.1074/jbc.M110.113456) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pang YP, Dai H, Smith A, Meng XW, Schneider PA, Kaufmann SH. 2012. Bak conformational changes induced by ligand binding: insight into BH3 domain binding and Bak homo-oligomerization. Sci. Rep. 2, 257 ( 10.1038/srep00257) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ma S, Hockings C, Anwari K, Kratina T, Fennell S, Lazarou M, Ryan MT, Kluck RM, Dewson G. 2013. Assembly of the Bak apoptotic pore: a critical role for the Bak protein alpha6 helix in the multimerization of homodimers during apoptosis. J. Biol. Chem. 288, 26 027–26 038. ( 10.1074/jbc.M113.490094) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gahl RF, He Y, Yu S, Tjandra N. 2014. Conformational rearrangements in the pro-apoptotic protein, Bax, as it inserts into mitochondria: a cellular death switch. J. Biol. Chem. 289, 32 871–32 882. ( 10.1074/jbc.M114.593897) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Westphal D, Dewson G, Czabotar PE, Kluck RM. 2011. Molecular biology of Bax and Bak activation and action. Biochim. Biophys. Acta 1813, 521–531. ( 10.1016/j.bbamcr.2010.12.019) [DOI] [PubMed] [Google Scholar]
- 66.Gazit E, Miller IR, Biggin PC, Sansom MS, Shai Y. 1996. Structure and orientation of the mammalian antibacterial peptide cecropin P1 within phospholipid membranes. J. Mol. Biol. 258, 860–870. ( 10.1006/jmbi.1996.0293) [DOI] [PubMed] [Google Scholar]
- 67.Ludtke SJ, He K, Heller WT, Harroun TA, Yang L, Huang HW. 1996. Membrane pores induced by magainin. Biochemistry 35, 13 723–13 728. ( 10.1021/bi9620621) [DOI] [PubMed] [Google Scholar]
- 68.Matsuzaki K, Murase O, Tokuda H, Funakoshi S, Fujii N, Miyajima K. 1994. Orientational and aggregational states of magainin 2 in phospholipid bilayers. Biochemistry 33, 3342–3349. ( 10.1021/bi00177a027) [DOI] [PubMed] [Google Scholar]
- 69.Lee MT, Hung WC, Chen FY, Huang HW. 2008. Mechanism and kinetics of pore formation in membranes by water-soluble amphipathic peptides. Proc. Natl Acad. Sci. USA 105, 5087–5092. ( 10.1073/pnas.0710625105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mandal T, Shin S, Aluvila S, Chen HC, Grieve C, Choe JY, Cheng EH, Hustedt EJ, Oh KJ. 2016. Assembly of Bak homodimers into higher order homooligomers in the mitochondrial apoptotic pore. Sci. Rep. 6, 30763 ( 10.1038/srep30763) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Korsmeyer SJ, Wei MC, Saito M, Weiler S, Oh KJ, Schlesinger PH. 2000. Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ. 7, 1166–1173. ( 10.1038/sj.cdd.4400783) [DOI] [PubMed] [Google Scholar]
- 72.Kluck RM, et al. 1999. The pro-apoptotic proteins, Bid and Bax, cause a limited permeabilization of the mitochondrial outer membrane that is enhanced by cytosol. J. Cell Biol. 147, 809–822. ( 10.1083/jcb.147.4.809) [DOI] [PMC free article] [PubMed] [Google Scholar]